Orthostatic intolerance (OI) is a series of clinical symptoms that develop during long-term standing in the upright position, with clinical manifestations of frequent, recurrent, or persistent dizziness, fatigue, and heart palpitations with or without syncope. These symptoms are relieved after changing to the supine posture [1]. OI includes vasovagal syncope (VVS), postural tachycardia syndrome, and autonomic nervous dysfunction [2]. The pathogenesis underlying the vasovagal response (VVR) is not well-defined, but is considered to involve changes in the autonomic system and vascular dysfunction [2, 3].
The notion of OI co-morbidity and its importance in chronic fatigue syndrome has gained increasing attention [4, 5]. Recent studies have suggested that the blood coagulation system is activated during orthostatic stress [6, 7]. Induction of VVR leads to changes in blood coagulation factors indicating a hypercoagulable state [8]. Similar changes in the coagulation system have been reported in co-morbid diseases, such as asthma [4], chronic fatigue syndrome [5], and migraine [9]. Significantly, these changes suggest the co-morbidity of OI and coagulation abnormalities.
Here, we review the latest findings of changes in orthostatic hypercoagulability in individuals with a positive orthostatic test result and those with OI, along with the possible mechanisms.
Hemodynamic Response and Coagulation Changes with Orthostatic Tests
Because it is non-invasive and reversible, the lower body negative pressure (LBNP) test has been used for decades to simulate orthostatic stress and the effect of blood loss in humans. LBNP is usually applied in the supine position with negative pressure on the lower regions of the body. Briefly, the protocol includes the application of distinct levels of sub-atmospheric pressure to the lower extremities, which results in pressure gradients during which the blood is forced to flow from the upper parts of the body into the legs and effectively decreases the central blood volume in a pattern similar to acute hemorrhage. Therefore, LBNP provides an alternative test for inducing orthostatic stress and for testing autonomic function. Moreover, LBNP is effective for inducing reversible reflexive hemodynamic responses, such as activation of the renin-angiotensin-aldosterone system (RAAS), the sympathetic nervous system, and hypothalamic-pituitary hormones [10].
The head-up tilt test (HUT) is frequently used to test for orthostatic challenge and diagnose neurally-mediated syncope [1]. Humoral and hemodynamic changes induced by the HUT and LBNP have been widely reported. With the HUT, 12%–20% of the blood is transferred from the upper body to the lower extremities, which results in increased venous volume. As well, ventricular preload and cardiac output and subsequent arterial pressure are decreased, and the baroreceptor reflex is activated, which leads to suppression of the parasympathetic nervous system and activation of the sympathetic nervous system. The heart rate increases, and peripheral vasoconstriction compensates to increase the cardiac output. Healthy individuals demonstrate a slightly increased heart rate, slightly lower systolic blood pressure, increased diastolic blood pressure, and unchanged mean arterial pressure during the early phase of standing still.
Taneja et al. (2007) first studied the regional blood flow and blood volume changes in 10 healthy individuals undergoing graded LBNP and 8 undergoing HUT to understand the difference in circulatory responses to these tests [11]. The LBNP and HUT showed similar hemodynamics and coagulation change patterns for blood flow, peripheral resistance, and blood volume in different parts of the body during upright posture. For instance, LBNP at − 40 mmHg reduced cardiac output by ~ 25%, similar to that with passive HUT. Moreover, combining those two stressors intensified the stress responses, eventually leading to cerebral underperfusion and a presyncopal situation [11, 12]. Table 1 lists the properties of LBNP and HUT as tools to induce orthostatic challenge.
Table 1.
Comparison of LBNP and HUT as tools to induce orthostatic challenge.
| Items | HUT | LBNP |
|---|---|---|
| Lower body pooling | Yes | − 40 mmHg |
| Heart rate increase similar at | 70° | − 50 mmHg |
| Systolic pressure | ↓ | ↓ |
| Diastolic pressure | ↑ | ↓ |
| Gravity-induced | Yes | No |
| Arterial resistance | ↑ | ↑ |
| Thoracic, pelvic, leg blood volumes | ↑ | ↑ |
| Visceral blood volume | ↑ | ↓ |
| Transmural pressure gradient | From the heart to lower body | Throughout lower body subjected to suction |
| Hydrostatic pressure gradient between thorax and abdomen or main arteries | Yes | No |
| Endothelial activation | Release activator | Release activator |
| Shear stress | ↑ | ↑ |
| Hormonal changes | NO↑ H2S↓ |
NO↑ H2S↓ |
| Epinephrine | ↑ | ↑↑ |
↑, increase; ↓, decrease; HUT, head-up tilt test; LBNP, lower limb negative pressure test; NO, nitric oxide; H2S, hydrogen sulfide.
Van Helmond et al. (2015) and Zaar et al. (2009) added some details to the coagulation and hormonal changes occurring during LBNP [13, 14]: coagulation was accelerated, with decreased prothrombin time (PT) and activated partial thromboplastin time (APTT), while platelet count, hemoglobin level, hematocrit, and red blood cell count increased significantly. Epinephrine and norepinephrine levels are both elevated, with the norepinephrine level greatly increased. However, changes in fibrinolysis values during orthostatic tests differ between studies. Crnošija et al. (2017) studied changes during HUT, especially hormonal changes [15]. Their study provided further evidence of increasing norepinephrine levels and found that the dopamine level remained unchanged during HUT. Therefore, both HUT and LBNP lead to significant changes in the coagulation system and some major hormones, and both can be used as orthostatic challenge tests. However, the HUT appears to induce a relatively modest change in platelet count and sympathetic nervous activity (Table 2).
Table 2.
Coagulation changes during HUT and LBNP.
| Items | HUT | LBNP |
|---|---|---|
| PT | ↓ | ↓ |
| APTT | ↓ | ↓ |
| Platelet count | – | ↑ |
| Hemoglobin | ↑ | ↑ |
| Hematocrit | ↑ | ↑ |
| Red blood cell count | ↑ | ↑ |
| Plasma volume | ↑ | ↓ |
| Norepinephrine | ↑ | ↑↑ |
| Fibrinolysis | + | +/–* |
| FVIII | ↑ | ↑ |
| vWF | ↑ | ↑ |
+, positive reaction; –, no significant change; ↑, increase; ↓, decrease.
HUT, head-up tilt test; LBNP, lower limb negative pressure test; PT, prothrombin time; APTT, activated partial thromboplastin time; vWF, von Willebrand factor; FVIII, blood coagulation factor VIII.
*Different results reported.
Updated Information on the Hypercoagulable State in Orthostatic Stress
Coagulation changes during standing have been studied for decades (Table 3). Persson et al. (1996) first found that standing results in a pooling of blood with a subsequent increase in orthostatic pressure in the lower extremities [16]. However, virtually no data are available on the effect of standing still on the coagulation cascade. Masoud et al. (2008) [6] first proposed that standing still significantly activates the coagulation system and named it orthostatic hypercoagulability. These authors found that orthostatic stress causes a significant plasma shift (> 12%) and raises the transmural pressure in the lower extremities, thereby activating the endothelial system, which might be related to the coagulation factor changes in activation of the coagulation system during standing. The authors recruited 18 healthy volunteers who remained at rest in the supine position, then stood at different time intervals. The hemodynamics and plasma fibrinogen level, factors V (FV) and VIII (FVIII) activity, prothrombin fragments 1 and 2 (F1 + 2), endothelial activation-related factors, and the protein C global pathway were measured on resting supine after 15, 30, and 60 min of standing still. Blood pressure and heart rate were stable at different times during standing, but with 30 min of standing, plasma volume decreased and plasma fibrinogen level, FV and FVIII, F1 + 2, tissue factor (TF), and von Willebrand factor (vWF) activity significantly increased. However, the function of the protein C pathway greatly decreased. These findings provide robust evidence of orthostatic hypercoagulability. Furthermore, the TF antigen level significantly increased after 15 min of standing and decreased gradually after 60 min, which suggests that the coagulation cascade is significantly activated after standing still. Two years later, in a new trial, the authors recruited 12 healthy young individuals for two separate visits [17]. Hematocrit, total plasma protein level, coagulation profile, endothelial activation-related factors, and the protein C global pathway were studied in the resting supine position and after 15 and 30 min of standing still. This procedure was repeated after at least a month in the same individuals after intravenous administration of 1.5 L of 0.9% saline. Upon 30 min of standing still with and without fluid loading, the TF level and vWF increased. Also, protein C global activity significantly decreased during 15 and 30 min of stationary standing with and without fluid loading. Thus, intravenous prophylactic rehydration with normal saline results in hemodilution of all coagulation parameters but does not prevent the orthostatic hypercoagulability induced by prolonged standing still [17]. Therefore, the hypercoagulability state of the blood is not completely attributable to hemoconcentration. Both endothelial-activated coagulation and haemoconcentration could be responsible for this physiological phenomenon.
Table 3.
Data from orthostatic hypercoagulability studies.
| Participants | Number of participants (F/M) | Orthostatic provocation tests | Timing | Hypercoagulability | References |
|---|---|---|---|---|---|
| H | 18 (9/9) | Standing test | 0, 15, 30, and 60 min | Y | Masoud et al. 2008 [6] |
| H | 11 (5/6) | Standing test | 0, 15 and 30 min | Y | Masoud et al. 2010 [17] |
| H | 7 (0/7) | HUT plus graded LBNP | 0, 2, 4, 6, 8, 10, 12, 14, 16, 18, and 36 min | Y | Cvirn et al. 2012 [7] |
| H | 21 (7/14) | LBNP | 0, 3, 21, and 26 min | Y | Kraemer et al. 2010 [8] |
| US & OI | 178 (97/81) | HUT | 3 min | Y | Hamrefors et al. 2017 [18] |
| IS & H | 44 (19/25) | STST | 0, and 6 min | Y | Cvirn et al. 2017[19] |
H, healthy individuals; US, unexplained syncope; IS, patients with a history of ischemic stroke; F, female; M, male; HUT, head-up tilt test; LBNP, lower limb negative pressure test; STST, sit-to-stand test.
Both orthostatic stress and the period after terminating the stress have been associated with coagulation changes. In 7 healthy males, Cvirn et al. (2012) [7] combined 70° HUT for 4 min with graded LBNP from − 15 to − 45 mmHg. The men remained supine for 20 min, and presyncope was induced in all of them. Coagulation responses and plasma mass density (for volume changes) were measured before, during, and 20 min after applying orthostatic stress. The increase in blood cell count, prothrombin level, and endogenous thrombin potential during standing still was associated with hemoconcentration. The authors reported a significant increase in values for markers of endothelial activation (TF and tissue plasminogen activator) and thrombin generation (F1 + 2, prothrombin F1 + 2, and thrombin-antithrombin complex [TAT]) beyond hemoconcentration. The values for endothelial activation markers returned to the initial supine values, but those for F1 + 2 and TAT remained elevated at 20 min after the orthostatic stress. These findings indicated an increased coagulability during recovery for as long as 20 min, which might have greater clinical significance than the short-term procoagulant changes that occur during standing. The authors concluded that orthostatic hypercoagulability lasts up to 20 min in individuals with a positive response for presyncope. In addition, some hormone changes were found. The initial elevated levels of catecholamine at 2–3 min post-stress were still high, which suggested its role in the elevated F1 + 2 and TAT levels, at least in the initial stage of recovery from presyncope.
Hormonal changes during orthostatic posture are not surprising because the neuroendocrine system is involved in the pathogenesis of OI [2, 3]. The findings of Cvirn et al. (2012) led us to investigate the association between coagulation and humoral changes during orthostatic posture. To understand the humoral changes and possible mechanisms for the coagulation changes in OI patients, Grasser et al. (2009) [12] studied the hemodynamic and neurohormonal changes in 16 healthy individuals induced by a similar combination of 70° HUT for 5 min and graded LBNP from − 20 to − 80 mmHg. The participants remained supine for 20 min, and presyncope was induced in 6 of them. The peripheral resistance index, plasma volume, plasma levels of norepinephrine and epinephrine, plasma renin activity, and cortisol were measured 1 min before HUT and 1 min after presyncope, immediately after reaching the supine position. All hormones collected in the study showed considerable elevation in values when presyncope was reached. For instance, the plasma level of norepinephrine increased by ~ 50% and epinephrine level > fivefold. Plasma renin activity doubled, while the total plasma cortisol level increased by about half. The cortisol level significantly increased, although this effect may be due to the combination of HUT and LBNP, since it was not as modest as with HUT alone. However, we can still conclude that changes of clearly different magnitudes of the RAAS and the sympathetic nervous system emerge in presyncope induced by orthostatic challenges. We suggest that elevated norepinephrine, epinephrine, plasma renin, and cortisol levels are associated with blood hypercoagulability during standing. These findings provide further evidence that humoral factors may play a key role in OI pathogenesis.
The stress response has been reported to be non-specific and leads to net hypercoagulability. Previous studies have shown elevated plasma vWF and FVIII during orthostatic tests [6, 17]. The specific connection between the VVR and activation of vWF and FVIII needs to be studied systematically. Kraemer et al. (2010) [8] tested LBNP from − 30 to − 90 mmHg in 21 individuals and the VVR in 16, measuring the plasma content of vWF-antigen (vWF:Ag), vWF-ristocetin-cofactor (vWF:RiCo) and FVIII activity, as well as the blood catecholamine level at several stress levels. The first blood sample was collected at the experiment onset. LBNP was then set to − 30 mm Hg and the pressure was reduced by 10 mm Hg every 3 min until − 90 mm Hg was reached. After 5 min, once all circulation parameters returned to normal, the fourth blood sample was taken. Only the group with VVR testing showed a significant increase in plasma vWF:Ag content and FVIII and vWF:RiCo activity. The level of epinephrine was elevated in all participants, but VVR did not develop in 5 who experienced all stress levels, nor were there any changes in clotting factor levels. Thus, the VVR may significantly activate the coagulation system by releasing vWF and FVIII via mechanisms independent of sympathetic activation. Unlike the other studies discussed above, this study related orthostatic stress to coagulation system abnormalities, leading to the co-morbidity hypothesis.
To test the hypothesis that passive orthostasis during HUT evokes pro-coagulatory changes and that these changes differ according to the HUT diagnosis, Hamrefors et al. (2017) [18] used HUT with 178 consecutive patients with syncope and found early changes in coagulation biomarkers. The examination was based on a specially-designed HUT protocol that included supine rest for 15 min and 70° HUT with optional nitroglycerine provocation after 20 min of passive HUT. Blood samples were collected during resting in the supine position and after 3 min of 70° HUT. The levels of plasma fibrinogen, vWF:Ag (vWF:GP1bA), and FVIII activity and APTT with and without activated protein C were determined. Participants were classified by age (cutoff at 65 years), sex, and diagnosis. After 3 min of HUT, vWF:Ag activity increased in all patients regardless of age. Moreover, patients with VVS showed less of an increase in vWF:Ag activity. This study confirmed the existence of orthostatic hypercoagulability in OI patients and supported the hypothesis that VVS is associated with less of an increase in vWF:Ag activity during HUT than in other types of OI. Therefore, measuring coagulation factors might help in the clinical differential diagnosis, medical diagnosis, and management of cardiovascular disease.
Cvirn et al. (2017) [19] presented additional evidence from measuring coagulation factors. The authors used the sit-to-stand test in 44 individuals: 22 after ischemic stroke and 22 healthy controls. Blood samples were collected at baseline and after standing in the upright position for 6 min. PT, APTT, plasma FVIII activity, and other markers of endothelial activation and thrombin generation were measured. In both groups, PT and APTT were significantly shortened and thrombin generation was significantly increased to approximately the same extent during standing compared with baseline. These data consistently demonstrated that orthostatic challenge is associated with activation of the coagulation system. However, effects of orthostatic challenge on FVIII were still not evident. In addition, the level of antidiuretic hormone, a synergistic component of the hypothalamic-pituitary-adrenal axis, is elevated by an orthostatic challenge, and this might induce orthostatic hypercoagulability.
Possible Mechanisms for Coagulation Abnormalities Triggered by OI
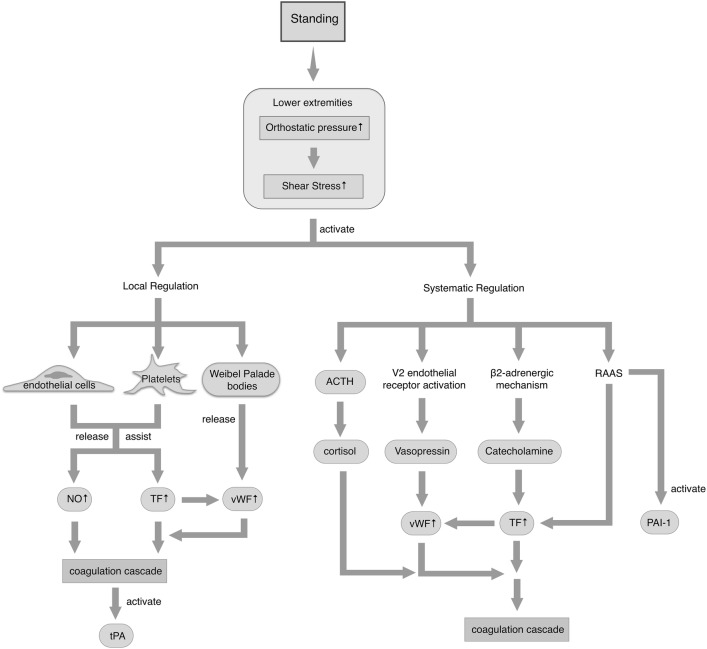
Several mechanisms are involved in OI-triggered coagulation changes (Fig. 1). The shear pressure-induced hemodynamic abnormalities in the circulatory system trigger the start of the entire process, followed by the stimulation of neurohumoral factors that mediate the sympathetic nervous system and the RAAS, thereby triggering the coagulation cascade. Local regulation by endothelial cells, platelets, and Weibel-Palade bodies also plays a key role.
Fig. 1.
Changes in the coagulation system during orthostatic stress. After standing, increased pressure is generated in the lower extremities, and this activates local and systemic regulation. The ellipses represent the small molecules involved. Upward arrows represent increased levels. ACTH, adrenocorticotropic hormone; vWF, von Willebrand factor; TF, tissue factor; tPA, tissue plasminogen activator; PAI-1, plasminogen activator inhibitor-1; NO, nitric oxide.
Standing initially generates orthostatic pressure, which acts as shear stress promoting local regulation and systemic changes, thereby activating the coagulation cascade. Endothelial cells and platelets play a central role in the local regulation of vascular tone during orthostatic challenge. The shear stress of blood flow acts on endothelium and platelets, upregulating TF synthase and the release of vWF, for excessive coagulation activation. Weibel-Palade bodies are also modulated by static shear stress, followed by the release of endothelial vWF [20]. Nitric oxide (NO) released by endothelium is involved in orthostatic hypercoagulability [21, 22]. Vascular endothelium might regulate wall tension by releasing various vasoactive substances such as NO and be involved in amplifying the shear stress effect. The molecules vWF, TF, NO, and other endothelial activators are then released into the circulation and induce a hypercoagulable state [6], followed by an increase in the tissue plasminogen activator level [21].
In addition, orthostatic pressure promotes activation of the sympathetic nervous system via β2-adrenergic receptors and the plasma level of antidiuretic hormone via the V2 endothelial receptor, which leads to vWF release. β2-adrenergic receptors are activated by catecholamine via the sympathetic nervous system. The RAAS is also promoted during standing, which activates the coagulation and fibrinolysis pathways by predominantly increasing the TF and plasminogen activator inhibitor 1 plasma levels [6]. Adrenocorticotropic hormone (ACTH) and cortisol are also involved in this process, indicating a fully-engaged hypothalamic–adrenal stress axis. Because ACTH activation leads to the production of adrenocortical hormones, the hypercoagulable state may be associated with hypercortisolemia [12]. Given that these hormones play important roles in regulating the coagulation system, they may be major contributors to blood hypercoagulability. However, although sex steroid hormones are known to affect endothelial function, they appear to have a limited effect on the endothelial response to orthostatic stress [22]. Further studies are needed to determine whether sex affects susceptibility to OI.
Clinical Implications of Co-morbidity of OI and Coagulation Abnormalities
Experiments suggest that orthostatic stress and syncope lead to hypercoagulability. Kraemer et al. (2010) correlated specifically orthostatic stress and the coagulation system abnormalities demonstrated by elevated vWF and FVIII activity, and Cvirn et al. (2017) found that orthostatic challenge does not affect FVIII activity in healthy individuals. Furthermore, Hamrefors et al. (2017) supported the idea that VVS was associated with less of an increase in vWF:Ag than with other types of OI. In short, vWF and FVIII may play critical roles in OI onset and OI may co-morbid with coagulation abnormalities involving vWF. vWF is a blood glycoprotein involved in hemostasis. It is deficient or defective in von Willebrand disease and is involved in many other diseases, including thrombotic thrombocytopenic purpura, Heyde’s syndrome, and possibly hemolytic-uremic syndrome [23]. Therefore, clinical observations may feature co-morbidity of OI and coagulation abnormalities.
A full understanding of the coagulation state is essential to prevent thrombosis in patients with OI. Although intravenous prophylactic rehydration with normal saline results in hemodilution of all coagulation markers, it has a limited effect in preventing orthostatic hypercoagulability and reducing the risk of VVS [17]. Accordingly, the current concept of fluid intake as a prophylactic intervention or sympatholytic drugs for patients with relapse does not seem sufficiently effective [1]. Considering the changes in the coagulation system in OI patients, hypercoagulability-targeting interventions, such as low-molecular-weight heparin, merit further investigation.
Furthermore, glucocorticoids have a significant effect on retaining sodium and fluid and may help patients with unexplained syncope [24]. However, plasma cortisol and vWF levels are positively correlated [25]. Thus, glucocorticoids may increase the burden of blood hypercoagulability. As discussed above, vWF and FVIII may play important roles in OI onset. The use of beta-blockers reduces activation of the FVIII–vWF complex in plasma, which may be a mechanism for improving the prognosis of patients with syncope [26].
Moreover, even 1 year after a stroke, patients show autonomic dysfunction during orthostatic challenge [19]. However, Li et al. (2016) found that vWF independently predicts all-cause mortality for patients with minor stroke or transient ischemic attack [27]. Thus, in OI, vWF may play a role in the poor prognosis of these patients. The dysfunctional autonomic changes associated with minor stroke or transient ischemic attack may be a novel research direction to help with selecting the thrombolytic intervention and improving the selection of OI patients for additional follow-up [28].
Conclusions
Abnormal coagulation is an important component of OI. Recent studies have reported that orthostatic stress activates the coagulation system and leads to hypercoagulability. During standing, increased pressure is generated in the lower extremities and activates local regulation including an increase in platelet count and Weibel-Palade bodies, activation of the endothelial response, and activation of systemic regulation including the RAAS and the sympathetic nervous system.
This perspective illustrates that patients with OI show abnormal coagulation in terms of vWF and FVIII during the orthostatic state that leads to hypercoagulability, which is absent in healthy individuals. Therefore, clinical observations may feature co-morbidity of OI and coagulation abnormalities. Further studies of the mechanisms of this comorbidity may provide a significant breakthrough in the pathogenesis of OI and its clinical significance.
References
- 1.Writing Committee Members, Shen WK, Sheldon RS, Benditt DG, Cohen MI, Forman DE, et al. ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm 2017, 14(8): e218–e254. [DOI] [PubMed]
- 2.Chen L, Du JB. Advances in studies on orthostatic intolerance in childhood. Chin J Pediatr. 2006;44:904. [PubMed] [Google Scholar]
- 3.Liao Y, Du JB. Intensive reading and interpretation of the guidelines for diagnosis of syncope in children. Chin J Pediatr. 2010;48:262–265. [PubMed] [Google Scholar]
- 4.Liao Y, Zhang QY, Li HX, Wang YL, Liu P, Du JB. Co-morbidity of vasovagal syncope and postural tachycardia syndrome with allergic diseases in children. J Peking Univ. 2017;49:783–788. [PubMed] [Google Scholar]
- 5.Kennedy G, Norris G, Spence V, Mclaren M, Belch JJ. Is chronic fatigue syndrome associated with platelet activation? Blood Coag Fibrinol. 2006;17:89–92. doi: 10.1097/01.mbc.0000214705.80997.73. [DOI] [PubMed] [Google Scholar]
- 6.Masoud M, Saring G, Brenner B, Jacob G. Orthostatic hypercoagulability: a novel physiological mechanism to activate the coagulation system. Hypertension. 2008;51:1545–1551. doi: 10.1161/HYPERTENSIONAHA.108.112003. [DOI] [PubMed] [Google Scholar]
- 7.Cvirn G, Schlagenhauf A, Leschnik B, Koestenberger M, Roessler A, Jantscher A, et al. Coagulation changes during presyncope and recovery. PLoS One. 2012;7:599–602. doi: 10.1371/journal.pone.0042221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kraemer M, Kuepper M, Nebe-Vom SA, Sorgenfrei U, Diehl RR. The influence of vasovagal response on the coagulation system. Clinl Auton Res. 2010;20:105–111. doi: 10.1007/s10286-009-0022-5. [DOI] [PubMed] [Google Scholar]
- 9.Vallejo M, Martínez-Martínez LA, Grijalva-Quijada S, Olguín-Ruvalcaba HM, Salas E, et al. Frequency of migraine in patients with vasovagal syncope. Int J Cardiol. 2014;171:14–15. doi: 10.1016/j.ijcard.2013.11.132. [DOI] [PubMed] [Google Scholar]
- 10.Goswami N, Loeppky JA, Hinghofer-Szalkay H. LBNP: Past protocols and technical considerations for experimental design. Aviation Space Environ Med. 2008;79:459–471. doi: 10.3357/ASEM.2161.2008. [DOI] [PubMed] [Google Scholar]
- 11.Taneja I, Moran C, Medow MS, Glover JL, Montgomery LD, Stewart JM. Differential effects of lower body negative pressure and upright tilt on splanchnic blood volume. Am J Physiol Heart Circ Physiol. 2007;292:1420–1426. doi: 10.1152/ajpheart.01096.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grasser EK, Goswami N, Rössler A, Vrecko K, Hinghofer-Szalkay H. Hemodynamic and neurohormonal responses to extreme orthostatic stress in physically fit young adults. Acta Astronautica. 2009;64:688–696. doi: 10.1016/j.actaastro.2008.11.002. [DOI] [Google Scholar]
- 13.Van Helmond N, Johnson BD, Curry TB, Cap AP, Convertino VA, Joyner MJ. Coagulation changes during lower body negative pressure and blood loss in humans. Am J Physiol Heart Circ Physiol. 2015;309:1591–1597. doi: 10.1152/ajpheart.00435.2015. [DOI] [PubMed] [Google Scholar]
- 14.Zaar M, Johansson PI, Nielsen LB, Crandall CG, Shibasaki M, Hilsted L, et al. Early activation of the coagulation system during lower body negative pressure. Clin Physiol Funct Imaging. 2009;29:427–430. doi: 10.1111/j.1475-097X.2009.00890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crnošija L, Krbot Skorić M, Lovrić M, Junaković A, Miletić V, Alfirev RŠ, et al. Differences in neurohumoral and hemodynamic response to prolonged head-up tilt between patients with high and normal standing norepinephrine forms of postural orthostatic tachycardia syndrome. Auton Neurosci. 2017;205:110–114. doi: 10.1016/j.autneu.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Persson PB. Modulation of cardiovascular control mechanisms and their interaction. Physiol Rev. 1996;76:193–244. doi: 10.1152/physrev.1996.76.1.193. [DOI] [PubMed] [Google Scholar]
- 17.Masoud M, Saring G, Brenner B, Jacob G. Hydration does not prevent orthostatic hypercoagulability. Thromb Haemost. 2010;103:284–290. doi: 10.1160/TH09-06-0370. [DOI] [PubMed] [Google Scholar]
- 18.Hamrefors V, Fedorowski A, Strandberg K, Sutton R, Isma N. Procoagulatory changes induced by head-up tilt test in patients with syncope: observational study. Thromb J. 2017;15:16. doi: 10.1186/s12959-017-0139-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cvirn G, Kneihsl M, Rossmann C, Paar M, Gattringer T, Schlagenhauf A, et al. Orthostatic challenge shifts the hemostatic system of patients recovered from stroke toward hypercoagulability. Front Physiol. 2017;8:12. doi: 10.3389/fphys.2017.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isma N, Sutton R, Hillarp A, Strandberg K, Melander O, Fedorowski A. Higher levels of von Willebrand factor in patients with syncope due to orthostatic hypotension. J Hypertens. 2015;33:1594–1601. doi: 10.1097/HJH.0000000000000595. [DOI] [PubMed] [Google Scholar]
- 21.Pietrucha AZ. Endothelial function in vasovagal syncope. Expert Rev Cardiovasc Ther. 2014;12:1387–1389. doi: 10.1586/14779072.2014.982095. [DOI] [PubMed] [Google Scholar]
- 22.Goswami N, Gorur P, Pilsl U, Anyaehie B, Green DA, Bondarenko AI, et al. Effect of orthostasis on endothelial function: a gender comparative study. PLoS One. 2013;8:e71655. doi: 10.1371/journal.pone.0071655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sadler JE. Biochemistry and genetics of von Willebrand factor. Ann Rev Biochem. 1998;67:395–424. doi: 10.1146/annurev.biochem.67.1.395. [DOI] [PubMed] [Google Scholar]
- 24.Wu X, Wang G, Feng J. Syncope as initial symptom for nephrotic syndrome: a case report. Int J Clin Exp Med. 2015;8:16868–16870. [PMC free article] [PubMed] [Google Scholar]
- 25.Chopra A, Kumar R, Kishore K, Tandon N, Yusuf T, Kumar S, et al. Effect of glucocorticoids on von Willebrand factor levels and its correlation with von Willebrand factor gene promoter polymorphism. Blood Coagul Fibrinolysis. 2012;23:514–519. doi: 10.1097/MBC.0b013e3283548dfc. [DOI] [PubMed] [Google Scholar]
- 26.Boman K, Boman JH, Andersson J, Olofsson M, Dahlöf B. Effects of atenolol or losartan on fibrinolysis and von Willebrand factor in hypertensive patients with left ventricular hypertrophy. Clin Appl Thromb Hemost. 2010;16:146–152. doi: 10.1177/1076029609349501. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Wang Y. Blood biomarkers in minor stroke and transient ischemic attack. Neurosci Bull. 2016;32:463–468. doi: 10.1007/s12264-016-0038-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao Q, Li X, Dong W, Ye M, Cao Y, Zhang M, et al. Factors associated with thrombolysis outcome in ischemic stroke patients with atrial fibrillation. Neurosci Bull. 2016;32:145–152. doi: 10.1007/s12264-016-0019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]