Abstract
Angiotensin (Ang)-(1–7) is an important biologically-active peptide of the renin-angiotensin system. This study was designed to determine whether inhibition of Ang-(1–7) in the hypothalamic paraventricular nucleus (PVN) attenuates sympathetic activity and elevates blood pressure by modulating pro-inflammatory cytokines (PICs) and oxidative stress in the PVN in salt-induced hypertension. Rats were fed either a high-salt (8% NaCl) or a normal salt diet (0.3% NaCl) for 10 weeks, followed by bilateral microinjections of the Ang-(1–7) antagonist A-779 or vehicle into the PVN. We found that the mean arterial pressure (MAP), renal sympathetic nerve activity (RSNA), and plasma norepinephrine (NE) were significantly increased in salt-induced hypertensive rats. The high-salt diet also resulted in higher levels of the PICs interleukin-6, interleukin-1beta, tumor necrosis factor alpha, and monocyte chemotactic protein-1, as well as higher gp91phox expression and superoxide production in the PVN. Microinjection of A-779 (3 nmol/50 nL) into the bilateral PVN of hypertensive rats not only attenuated MAP, RSNA, and NE, but also decreased the PICs and oxidative stress in the PVN. These results suggest that the increased MAP and sympathetic activity in salt-induced hypertension can be suppressed by blockade of endogenous Ang-(1–7) in the PVN, through modulation of PICs and oxidative stress.
Keywords: High-salt diet, Hypertension, Angiotensin-(1–7), Paraventricular nucleus, Pro-inflammatory cytokines
Introduction
The hypothalamic paraventricular nucleus (PVN) is well known as the control center for arterial blood pressure, and a predominant region for coordinating neural signals regulating blood pressure [1, 2]. The renin-angiotensin system (RAS) also plays a central role in the regulation of cardiovascular activity and the pathogenesis of hypertension. Recent studies have shown that angiotensin (Ang)-(1–7) is an important biologically-active peptide in the RAS family [3]. Ang-(1–7) is formed either directly from Ang II or indirectly from Ang I through angiotensin-converting enzyme 2 [4]. Most of the functions of Ang-(1–7) are mediated by its Mas receptors (MasRs) and can be selectively blocked by the specific antagonist D-Alanine-Ang-(1–7) (A-779) [5, 6]. Previous studies have shown that Ang-(1–7) antagonizes the effects of Ang II in peripheral tissues [7, 8]. However, other groups have shown that microinjection of Ang-(1–7) into the rostral ventrolateral medulla increases the mean arterial pressure (MAP) and renal sympathetic nerve activity (RSNA) in normal rats [9, 10]. It has also been reported that microiontophoretic application of Ang-(1–7) into the PVN augments the excitability of neurons in this region [11, 12], and this effect is selectively blocked by A-779[12, 13]. In addition, there is an abundant increase in immunoreactive staining of Ang-(1–7) in the rat forebrain [14] and in the hypothalamus of rats with aortic coarctation-induced hypertension [15]. More importantly, strong staining for the Ang-(1–7) MasR has also been observed in the PVN. Furthermore, the MasR staining is predominantly present in neurons [14, 16]. These findings indicate a possible role for the neuromodulatory action of Ang-(1–7) in the PVN.
The pro-inflammatory cytokines (PICs) in the PVN, such as tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1beta (IL-1β), contribute to the sympathetic activity and pathophysiology of cardiovascular diseases [17–19]. Reactive oxygen species (ROS) are involved in the pathophysiology of hypertension. However, the effect of microinjection of A-779 into the PVN on the PICs in the PVN during hypertension remains unclear.
The main objective of this study was to test whether endogenous Ang-(1–7) in the PVN contributes to the maintenance of MAP and sympathetic activity in rats with high salt-induced hypertension. We found that blockade of endogenous Ang-(1–7) by microinjection of A-779 into the PVN attenuated hypertensive responses. We further explored the underlying mechanisms by investigating the PICs and oxidative stress in the PVN.
Materials and Methods
Animals
Experiments were performed on male Sprague-Dawley rats weighing 250 g–275 g. They were housed in a climate-controlled room with a 12-h light-dark cycle and allowed access to standard rat chow and tap water ad libitum. These experiments were approved by the Animal Care and Use Committee of Xi’an Jiaotong University. All the experiments conformed to the Guidelines for the Care and Use of Experimental Animals of the United States National Institutes of Health (NIH Publication No. 85-23, revised 1996).
One week after habituation to the new environment, rats were fed with a normal-salt (0.3% NaCl; NS) or a high-salt (8% NaCl; HS) diet for 10 weeks. Then the rats were randomly selected to receive A-779 or vehicle. Therefore, they were divided into 4 groups: (1) HS + vehicle, (2) HS + A-779, (3) NS + vehicle, and (4) NS + A-779. A total of 145 rats were used in the study. The successful rate of bilateral PVN cannulation was about 65%.
Measurement of Mean Arterial Pressure
During the 10-week experimental period, MAP was measured weekly in the HS and NS groups. The AP was noninvasively measured with a tail-cuff (BP100A, Chengdu Techman Software Co., Ltd, China). Awake rats were warmed in an ambient temperature of 30°C by placing them in a holding device mounted on a thermostatically-controlled warming plate. All animals were habituated to the blood pressure measuring system and to the holders daily for one week prior to the initiation of experimental measurements. Each rat was allowed to accommodate to the cuff for 10 min before the pressure measurement. The value was the average of six measurements per day in each rat [22].
At the end of week 10, rats were anesthetized with a ketamine (90 mg/kg) and xylazine (10 mg/kg) mixture via intraperitoneal injection (i.p.). A polyethylene catheter was inserted into the carotid artery for MAP and heart rate (HR) recording [23, 24]. The catheter was pre-filled with 0.1 mL heparinized saline (50 units/mL) and connected to a pressure transducer attached to a digital BP monitor and a polygraph (BL420, Chengdu Techman Software Co., Ltd). MAP and HR recording lasted 30 min and the averages were calculated.
Microinjection of Drugs into the PVN
Microinjection of vehicle or A-779 (3 nmol) into the PVN was carried out in HS and NS rats as described previously [6, 25, 26]. Briefly, each rat was placed in a stereotaxic frame. The stereotaxic coordinates for the PVN are 1.8 mm caudal to bregma, 0.4 mm lateral to the sagittal suture, and 7.9 mm below the skull surface according to the rat atlas of Paxinos and Watson. Microinjection into the PVN was completed in 1 min and the volume was 50 nL in the PVN on each side. At the end of each acute administration, 50 nL of 2% Evan’s Blue was injected into each microinjection site. The sites were histologically verified under a light microscope (Eclipse 80i, Nikon). Rats with sites outside of the PVN were excluded from data analysis. The RSNA, MAP, and HR were recorded before and after, and blood and tissue samples were collected 24h after the microinjections.
Collection of Blood and Tissue Samples
Rats were decapitated under anesthesia (mixture of 90 mg/kg ketamine and 10 mg/kg xylazine, i.p.), and then blood and tissue samples were collected. The PVN was isolated using the microdissection procedure of Palkovits as previously described [17, 19, 27, 28]. Plasma and tissue samples were stored at − 80 °C for the next steps.
Renal Sympathetic Nerve Activity Recording
The rats were anaesthetized with a ketamine (80 mg/kg) and xylazine (10 mg/kg) mixture (i.p.) for RSNA recording. The general methods for recording and analyzing RSNA were as described previously [18, 29–31].
Immunofluorescence and Immunohistochemistry
The immunofluorescence and immunohistochemical methods were performed as described previously to immunolocalize IL-1β, IL-6, gp91phox, and TNF-α expression in the PVN [32, 33]. The primary antibodies against IL-1β (sc-1251, polyclonal goat anti-rat), IL-6 (sc-1265, polyclonal goat anti-rat), gp91phox (sc-5827, polyclonal goat anti-rat), and TNF-α (sc-8301, polyclonal rabbit anti-rat) were from Santa Cruz Biotechnology Inc. (Dallas, TX).
Western Blotting
Western blotting was used to measure gp91phox, IL-1β, IL-6, and MCP-1 expression in the PVN. The methods were as described previously [34, 35]. Protein loading was controlled by probing all blots with β-actin antibody (Thermo Scientific, Waltham, MA) and the protein intensity was normalized to that of β-actin. Band density was analyzed with ImageJ (NIH, Bethesda, MD) [36, 37].
Biochemical Assays
The level of norepinephrine (NE) in plasma and levels of TNF-α, IL-6 in PVN were quantified using rat ELISA kits (Invitrogen, Carlsbad, CA) [21, 22]
Statistical Analysis
All data are presented as mean ± SEM and P<0.05 was considered statistically significant. Statistical analyses were performed using Prism version 5.0 (GraphPad Software Inc., San Diego, CA). Repeated measures ANOVA was used to analyze the MAP of the tail blood pressures. One-way ANOVA with Tukey’s post hoc test was applied to perform the statistical analyses for RNSA, cytokine levels in PVN, plasma NE, the number of positive neurons, fluorescent intensity and Western blotting data. Two-way ANOVA followed by Bonferroni’s post-hoc test was used to analyze cardiovascular and autonomic parameters after A-779 or vehicle (MAP and heart rate before and after application).
Results
Effect of High-Salt Diet on MAP
During the 10 weeks of an HS or NS diet, weekly monitoring of arterial blood pressure was performed using the tail-cuff method. The HS diet induced a significant increase in MAP from 6 to 10 weeks as compared to the beginning (week 0). At the end of 10 weeks, the MAP in the HS rats was 163% of that of the NS rats (156 ± 6.3 vs 95.5 ± 7.8 mmHg, P < 0.05; Fig. 1).
Fig. 1.
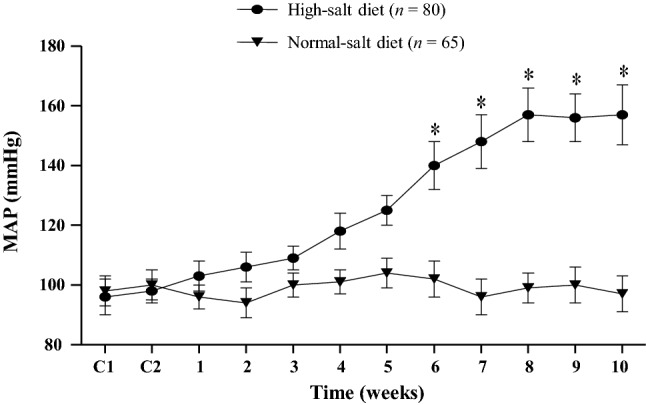
Effect of diet on mean arterial pressure (MAP). MAP increased significantly in rats with a high salt diet (8% NaCl) for 6 weeks and beyond. But the rats with a normal salt (NS) diet (0.3% NaCl) maintained normal MAP. Values are presented as mean ± SEM. *P < 0.05 versus NS rats.
Effect of Microinjection of A-779 into the PVN on MAP and HR
To assess the effect of microinjection of A-779 into the PVN on hypertensive responses in salt-induced hypertension, MAP and HR were monitored using a non-invasive computerized system. The MAP was significantly higher in the HS rats than in the NS rats. Microinjection of A-779 into the PVN caused a significant reduction in MAP in the HS + A779 group while microinjection of vehicle did not change the MAP. In addition, PVN microinjection of A-779 or vehicle in the NS groups (NS + A-779 and NS + vehicle) did not cause any significant change in MAP. And there was no significant difference in HR between the HS and NS groups (Table 1).
Table 1.
Mean arterial pressure (MAP) and heart rate (HR) before and after microinjection of A-779 into the PVN.
| Group | Before | After | ||
|---|---|---|---|---|
| MAP (mmHg) | HR (beats/min) | MAP (mmHg) | HR (beats/min) | |
| HS + PVN A-779 | 145 ± 4* | 369 ± 4 | 126 ± 7*† | 365 ± 6 |
| HS + PVN vehicle | 144 ± 3* | 382 ± 4 | 145 ± 5* | 384 ± 6 |
| NS + PVN A-779 | 103 ± 5 | 358 ± 5 | 99 ± 6 | 355 ± 6 |
| NS + PVN vehicle | 100 ± 6 | 349 ± 7 | 99 ± 5 | 344 ± 4 |
NS, normal salt diet; HS, high salt diet; PVN, paraventricular nucleus. All values are presented as mean ± SEM (n = 7); *P < 0.05 versus NS groups; †P < 0.05 after versus before microinjection of A-779 into the PVN.
Effect of Microinjection of A-779 into the PVN on Superoxide and NAD(P)H Oxidase in the PVN
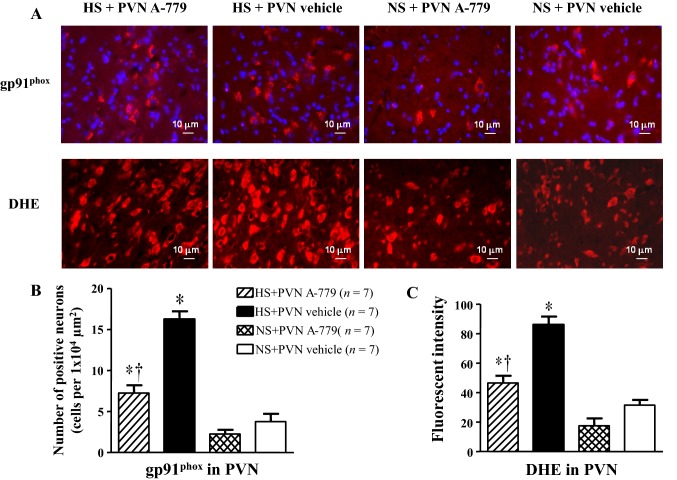
Immunofluorescence studies revealed that the number of gp91phox-positive neurons and superoxide production were higher in the PVN of the HS rats than in the NS rats (P < 0.05). The number of gp91phox-positive neurons and superoxide production in the PVN were reduced after microinjection of A-779 into the bilateral PVN in hypertensive rats from the HS + A-779 group than in the HS+ vehicle group, while both were higher than in the NS groups. The number of gp91phox-positive neurons and superoxide production in the PVN did not significantly differ between the NS + A-779 and NS + vehicle groups (P > 0.05; Fig. 2).
Fig. 2.
Effect of microinjection of A-779 into the PVN on superoxide and gp91phox in the PVN of rats with salt-induced hypertension. Immunofluorescence for the NAD(P)H oxidase subunit gp91phox and dihydroethidium (DHE) was performed on PVN tissue sections. A Immunofluorescence for gp91phox (bright red) and superoxide (bright red, labeled by DHE) in the PVN in different groups. Nuclei were labeled with DAPI (blue). B, C Histograms showing the effects of microinjection of A-779 into the PVN on the number of gp91phox-positive cells (B) and superoxide-positive cells (C) in the PVN of different groups. Values are presented as mean ± SEM; *P < 0.05 versus NS groups (NS + PVN A-779 or NS + PVN vehicle); †P < 0.05 HS + PVN A-779 versus HS + PVN vehicle.
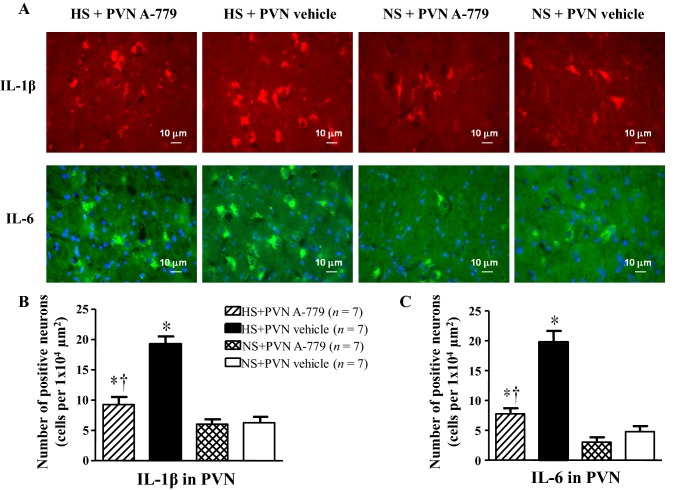
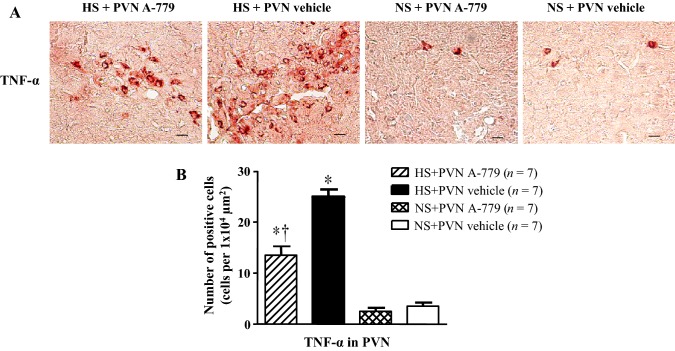
Effect of Microinjection of A-779 into the PVN on Pro-inflammatory Cytokine-Positive Neurons in the PVN
The immunohistochemistry and immunofluorescence results showed that the numbers of neurons positive for IL-1β, IL-6, and TNF-α in salt-induced hypertension were much greater than those in the NS + A-779 and NS + vehicle groups (P < 0.05). The numbers of IL-1β-, IL-6- and TNF-α-positive neurons in the PVN were decreased after microinjection of A-779 into the bilateral PVN in the HS + A-779 group compared to the HS + vehicle group, while they were higher than those in both of the NS groups. The numbers did not significantly differ between the NS + A-779 and NS + vehicle groups (P > 0.05; Figs. 3 and 4).
Fig. 3.
Effect of microinjection of A-779 into the PVN on interleukin-1β (IL-1β) and interleukin-6 (IL-6) in the PVN. Immunofluorescence was used to detect pro-inflammatory cytokines in the PVN in different groups. A IL-1β (bright red) and IL-6 (bright green) were both found in the PVN in the different groups. Nuclei were labeled with DAPI (blue). B, C Histograms showing the numbers of IL-1β-positive (B) and IL-6-positive neurons (C) in the PVN of rats in different groups. Values are presented as mean ± SEM; *P < 0.05 versus NS groups (NS + PVN A-779 or NS + PVN vehicle); †P < 0.05 HS + PVN A-779 versus HS + PVN vehicle.
Fig. 4.
Effect of microinjection of A-779 into the PVN on tumor necrosis factor alpha (TNF-α) in the PVN. A Immunohistochemical staining for TNF-α (bright brown) in the PVN in different groups. Scale bar, 10 µm. B Histogram showing the number of TNF-α-positive neurons in the PVN in different groups. Values are presented as mean ± SEM; *P < 0.05 versus NS groups (NS + PVN A-779 or NS + PVN vehicle); †P < 0.05 HS + PVN A-779 versus HS + PVN vehicle.
Effect of Microinjection of A-779 into the PVN on Protein Levels of gp91phox, IL-1β, MCP-1, and IL-6 in the PVN
Western blotting results showed that the HS rats had higher levels of gp91phox, IL-1β, MCP-1, and IL-6 proteins in the PVN than NS rats (P < 0.05). Microinjectionof A-779 into the PVN prevented these increases, but did not restore them to the levels in the NS rats (P < 0.05). Meanwhile, the levels of these proteins in the PVN did not significantly differ between the NS + A-779 and NS + vehicle groups (P > 0.05; Fig. 5).
Fig. 5.
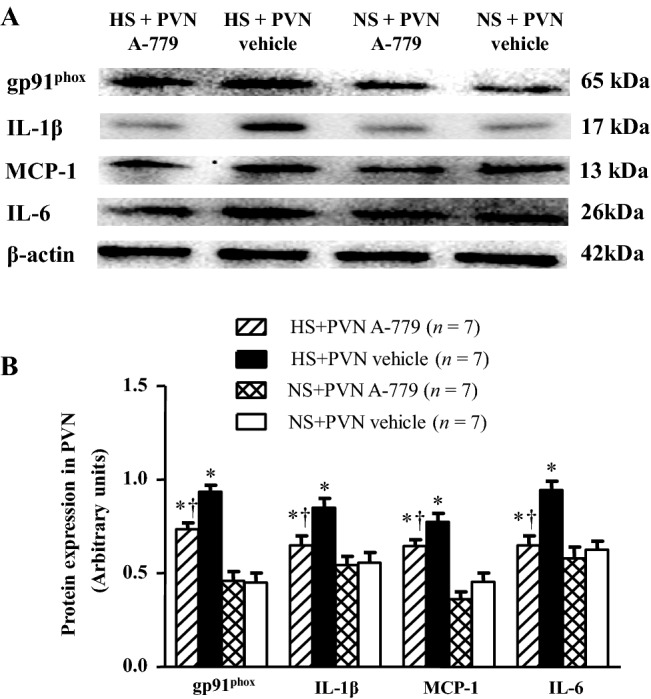
Effects of microinjection of A-779 into the PVN on gp91phox, IL-1β, IL-6, and monocyte chemotactic protein-1 (MCP-1) protein expression in the PVN. Western blotting was used to determine the expression of these proteins. A, B Representative immunoblots (A) and densitometric analysis (B) of protein expression in different groups. Values are presented as mean ± SEM; *P < 0.05 versus NS groups (NS + PVN A-779 or NS + PVN vehicle); †P < 0.05 HS + PVN A-779 versus HS + PVN vehicle.
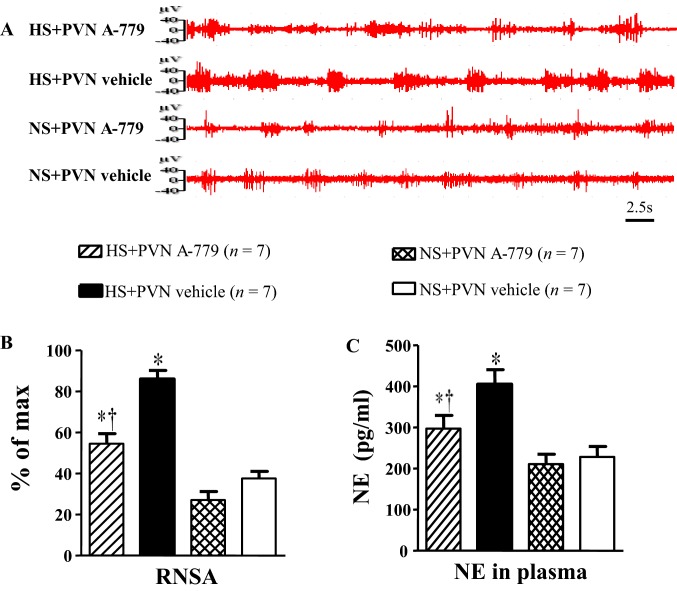
Effect of Microinjection of A-779 into the PVN on Renal Sympathetic Nerve Activity and Plasma Noradrenaline
To evaluate the effect of microinjection of A-779 into the PVN on sympathetic activity in hypertension, we measured the RSNA and plasma NE. Our results showed that the HS diet induced significant increases in both RSNA and plasma NE levels compared with the NS diet (P < 0.05). Microinjection of A-779 into the PVN decreased the RSNA and NE levels in the HS hypertensive rats compared to those microinjected with vehicle, while both remained higher than the NS groups. The RSNA and NE levels in the PVN did not significantly differ between the NS + A-779 and NS + vehicle groups (P > 0.05; Fig. 6).
Fig. 6.
Effect of microinjection of A-779 into the PVN on renal sympathetic nerve activity (RSNA) and the plasma levels of norepinephrine (NE) in the PVN. RSNA was higher in HS rats and this was normalized by microinjection of A-779. A Representative RSNA in rats from different groups. B Bar graph of the maximum values of RSNA in different groups. C Bar graph of plasma NE levels in rats in different groups. Values are presented as mean ± SEM; *P < 0.05 versus NS groups (NS + PVN A-779 or NS + PVN vehicle); †P < 0.05 HS + PVN A-779 versus HS + PVN vehicle.
Effect of Microinjection of A-779 into the PVN on PICs in the PVN
To determine the effect of microinjection of A-779 into the PVN on inflammatory responses in hypertension, we assessed the levels of IL-6 and TNF-α using ELISA. Our results showed that the levels of both in the HS rats were significantly higher than those in the NS rats (P < 0.05). Their levels were decreased after microinjection of A-779 in the hypertensive rats compared to the HS + vehicle group, while remaining higher than the NS groups. The levels of IL-6 and TNF-α did not significantly differ between the NS + A-779 and NS + vehicle groups (P > 0.05) (Table 2).
Table 2.
Effect of microinjection of A-779 into the PVN on pro-inflammatory cytokines in the PVN.
| Group | Protein level in the PVN (pg/mg) | |
|---|---|---|
| TNF-α | IL-6 | |
| HS + PVN vehicle | 8.8 ± 0.6* | 60.3 ± 5.9* |
| HS + PVN A-779 | 5.8 ± 0.5*† | 41.7 ± 4.1*† |
| NS + PVN vehicle | 3.7 ± 0.4 | 29.2 ± 3.4 |
| NS + PVN A-779 | 3.5 ± 0.3 | 26.5 ± 3.2 |
NS, normal salt diet; HS, high salt diet; PVN, paraventricular nucleus. Values are presented as mean ± SEM (n = 7); *P < 0.05 versus NS groups; †P < 0.05 HS + PVN A-779 versus HS + PVN vehicle.
Discussion
The primary findings in the present study were that blockade of endogenous Ang-(1–7) in the PVN not only attenuated MAP and sympathetic activity, but also reduced PICs expression and ROS production in rats with salt-induced hypertension.
Recent studies indicate that RAS and ROS are involved in the pathogenesis of hypertension [38–40]. RAS activation is one of the major factors in the progression of hypertension [41]. Ang-(1–7) is one of the important factors that contribute to the modulation of sympathetic drive and blood pressure in hypertension [10, 42]. Ang-(1–7) in the PVN is as effective as Ang II in enhancing the elevated cardiac sympathetic afferent reflex (CSAR) and increasing sympathetic outflow in hypertension. Ang-(1–7) in the PVN potentiates the effects of Ang II in hypertension, and A-779 abolishes the effects of Ang-(1–7) [10]. It has been found that expression of the MasR protein in the PVN is significantly higher in 2K1C rats than in sham rats. However, there is no significant difference in the Ang-(1–7) level in the PVN between 2K1C and sham rats. These results suggest that the enhanced effects of Ang-(1–7) in the PVN on the RSNA, MAP, and CSAR in 2K1C rats arise from the upregulation of MasRs in the PVN rather than the production or release of Ang-(1–7) in the PVN. This increased MasR expression contributes to the tonic control of RSNA, MAP and CSAR in 2K1C rats [6]. Recently, several researchers have shown that ROS is a vital signaling factor in the PVN, playing an important role in modulating blood pressure and sympathetic activity. Increased superoxide anions in the PVN assist in enhancing RSNA in hypertension [39, 43]. Microinjections of an oxygen free-radical scavenger (Tempol) into the bilateral PVN not only reduce the ROS response and MAP but also attenuate sympathetic activity in hypertension [25]. A recent study from our laboratory showed that microinjections of losartan (an AT1-R antagonist) into the bilateral PVN attenuate the expression of gp91phox in the PVN of rats with heart failure [29]. In this study, bilateral microinjections of the Ang-(1–7) antagonist A-779 into the PVN attenuated MAP and RSNA and also reduced gp91phox expression and superoxide production in rats with hypertension induced by HS. These results indicate that Ang-(1–7) in the PVN contributes to regulating MAP and RSNA by attenuating oxidative stress in high salt-induced hypertension.
Furthermore, the level of plasma NE (a marker of sympathetic activity) was increased in the salt-induced hypertensive rats, and bilateral microinjections of A-779 into the PVN resulted in a substantial decrease in plasma NE, suggesting that excitation of the brain RAS can promote sympathetic activity in hypertension. PICs play an important role in regulating autonomic nervous activity and cardiovascular function in hypertension. TNF-α, IL-1β, and IL-6 can be produced locally in the brain by microglia and neurons, thereby contributing to the pathogenesis of hypertension [44]. Direct microinjection of TNF-α or IL-1β into the PVN has been demonstrated to increase MAP [45], consistent with our finding that increased PICs in the PVN result in sympathoexcitation [20, 21]. Suppression of central angiotensin-converting enzyme has been found to down-regulate the PICs in the heart of the spontaneously hypertensive rat [46, 47]. These results further support the idea that hypertension is an inflammatory state and PICs are involved in the pathogenesis of hypertension. In this study, bilateral microinjections of the Ang-(1–7) antagonist A-779 into the PVN significantly attenuated RSNA, and decreased the expression of MCP-1, IL-1β, TNF-α, and IL-6 in the PVN in rats with hypertension induced by HS. Our results are consistent with the recent report that inhibition of angiotensin-converting enzyme decreases the levels of PICs (IL-1β and IL-6) and increases the level of anti- inflammatory cytokine (IL-10) in the PVN and plasma during Ang II-induced hypertension [47]. These findings suggest that blockade of endogenous Ang-(1–7) in the PVN attenuates MAP and sympathetic activity probably through reducing PICs and attenuating ROS production in HS-induced hypertension (Fig. 7).
Fig. 7.
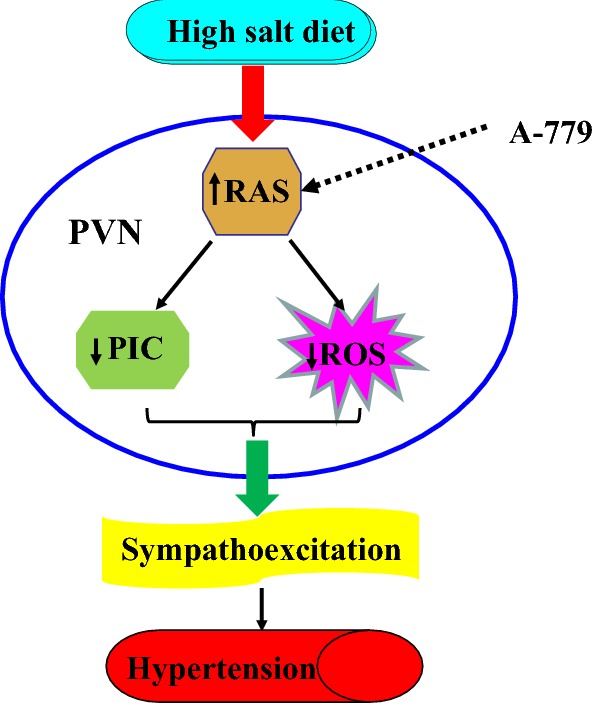
Schematic of the hypothesis. Angiotensin-(1–7) modulates PIC expression and oxidative stress in the paraventricular nucleus (PVN) and contributes to sympathetic nerve activity and blood pressure in salt-induced hypertension. Microinjection of A-779 blocks the function of MasRs, and reduces the effects of angiotensin-(1–7).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81600333, 81770426, 91439120, and 91639105), the China Postdoctoral Science Foundation (2016M602835 and 2016M592802), and the Shaanxi Postdoctoral Science Foundation (2016BSHEDZZ91).
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Joseph Francis, Email: jfrancis@lsu.edu.
Yu-Ming Kang, Email: ykang@mail.xjtu.edu.cn.
References
- 1.Cardinale JP, Sriramula S, Mariappan N, Agarwal D, Francis J. Angiotensin II-induced hypertension is modulated by nuclear factor-kappaB in the paraventricular nucleus. Hypertension. 2012;59:113–121. doi: 10.1161/HYPERTENSIONAHA.111.182154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davisson RL. Physiological genomic analysis of the brain renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol. 2003;285:498–511. doi: 10.1152/ajpregu.00190.2003. [DOI] [PubMed] [Google Scholar]
- 3.Iyer SN, Chappell MC, Averill DB, Diz DI, Ferrario CM. Vasodepressor actions of angiotensin-(1–7) unmasked during combined treatment with lisinopril and losartan. Hypertension. 1998;31:699–705. doi: 10.1161/01.HYP.31.2.699. [DOI] [PubMed] [Google Scholar]
- 4.Kohara K, Brosnihan KB, Chappell MC, Khosla MC, Ferrario CM. Angiotensin-(1–7). A member of circulating angiotensin peptides. Hypertension. 1991;17:131–138. doi: 10.1161/01.HYP.17.2.131. [DOI] [PubMed] [Google Scholar]
- 5.Xu P, Sriramula S, Lazartigues E. ACE2/ANG-(1–7)/Mas pathway in the brain: the axis of good. Am J Physiol Regul Integr Comp Physiol. 2011;300:804–817. doi: 10.1152/ajpregu.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han Y, Sun HJ, Li P, Gao Q, Zhou YB, Zhang F, et al. Angiotensin-(1–7) in paraventricular nucleus modulates sympathetic activity and cardiac sympathetic afferent reflex in renovascular hypertensive rats. PLoS One. 2012;7:e48966. doi: 10.1371/journal.pone.0048966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehlers PI, Nurmi L, Turpeinen AM, Korpela R, Vapaatalo H. Casein-derived tripeptide Ile-Pro-Pro improves angiotensin-(1–7)- and bradykinin-induced rat mesenteric artery relaxation. Life Sci. 2011;88:206–211. doi: 10.1016/j.lfs.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Qi Y, Shenoy V, Wong F, Li H, Afzal A, Mocco J, et al. Lentivirus-mediated overexpression of angiotensin-(1–7) attenuated ischaemia-induced cardiac pathophysiology. Exp Physiol. 2011;96:863–874. doi: 10.1113/expphysiol.2011.056994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou LM, Shi Z, Gao J, Han Y, Yuan N, Gao XY, et al. Angiotensin-(1–7) and angiotension II in the rostral ventrolateral medulla modulate the cardiac sympathetic afferent reflex and sympathetic activity in rats. Pflugers Arch. 2010;459:681–688. doi: 10.1007/s00424-010-0793-5. [DOI] [PubMed] [Google Scholar]
- 10.Sun HJ, Li P, Chen WW, Xiong XQ, Han Y. Angiotensin II and angiotensin-(1–7) in paraventricular nucleus modulate cardiac sympathetic afferent reflex in renovascular hypertensive rats. PLoS One. 2012;7:52557. doi: 10.1371/journal.pone.0052557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ambuhl P, Felix D, Imboden H, Khosla MC, Ferrario CM. Effects of angiotensin analogues and angiotensin receptor antagonists on paraventricularneurones. Regul Pept. 1992;38:111–120. doi: 10.1016/0167-0115(92)90049-Z. [DOI] [PubMed] [Google Scholar]
- 12.Ambuhl P, Felix D, Khosla MC. [7-D-ALA]-angiotensin-(1–7): selective antagonism of angiotensin-(1–7) in the rat paraventricular nucleus. Brain Res Bull. 1994;35:289–291. doi: 10.1016/0361-9230(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 13.Silva AQ, Santos RA, Fontes MA. Blockade of endogenous angiotensin-(1–7) in the hypothalamic paraventricular nucleus reduces renal sympathetic tone. Hypertension. 2005;46:341–348. doi: 10.1161/01.HYP.0000179216.04357.49. [DOI] [PubMed] [Google Scholar]
- 14.Block CH, Santos RA, Brosnihan KB, Ferrario CM. Immunocytochemical localization of angiotensin-(1–7) in the rat forebrain. Peptides. 1988;9:1395–1401. doi: 10.1016/0196-9781(88)90208-2. [DOI] [PubMed] [Google Scholar]
- 15.Gironacci MM, Brosnihan KB, Ferrario CM, Gorzalczany S, Verrilli MA, Pascual M, et al. Increased hypothalamic angiotensin-(1–7) levels in rats with aortic coarctation-induced hypertension. Peptides. 2007;28:1580–1585. doi: 10.1016/j.peptides.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becker LK, Etelvino GM, Walther T, Santos RA, Campagnole-Santos MJ. Immunofluorescence localization of the receptor Mas in cardiovascular-related areas of the rat brain. Am J Physiol Heart CircPhysiol. 2007;293:1416–1424. doi: 10.1152/ajpheart.00141.2007. [DOI] [PubMed] [Google Scholar]
- 17.Kang YM, Ma Y, Zheng JP, Elks C, Sriramula S, Yang ZM, et al. Brain nuclear factor-kappa B activation contributes to neurohumoral excitation in angiotensin II-induced hypertension. Cardiovasc Res. 2009;82:503–512. doi: 10.1093/cvr/cvp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang YM, Wang Y, Yang LM, Elks C, Cardinale J, Yu XJ, et al. TNF-alpha in hypothalamic paraventricular nucleus contributes to sympathoexcitation in heart failure by modulating AT1 receptor and neurotransmitters. Tohoku J Exp Med. 2010;222:251–263. doi: 10.1620/tjem.222.251. [DOI] [PubMed] [Google Scholar]
- 19.Bai J, Yu XJ, Liu KL, Wang FF, Li HB, Shi XL, et al. Tert-butylhydroquinone attenuates oxidative stress and inflammation in hypothalamic paraventricular nucleus in high salt-induced hypertension. Toxicol Lett. 2017;281:1–9. doi: 10.1016/j.toxlet.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Kang YM, Ma Y, Elks C, Zheng JP, Yang ZM, Francis J. Cross-talk between cytokines and renin-angiotensin in hypothalamic paraventricular nucleus in heart failure: role of nuclear factor-kappaB. Cardiovasc Res. 2008;79:671–678. doi: 10.1093/cvr/cvn119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi J, Yu XJ, Shi XL, Gao HL, Yi QY, Tan H, et al. NF-κB blockade in hypothalamic paraventricular nucleus inhibits high-salt-induced hypertension through NLRP3 and Caspase-1. Cardiovasc Toxicol. 2015;16:345–354. doi: 10.1007/s12012-015-9344-9. [DOI] [PubMed] [Google Scholar]
- 22.Li HB, Qin DN, Ma L, Miao YW, Zhang DM, Lu Y, et al. Chronic infusion of lisinopril into hypothalamic paraventricular nucleus modulates cytokines and attenuates oxidative stress in rostral ventrolateral medulla in hypertension. Toxicol Appl Pharmacol. 2014;279:141–149. doi: 10.1016/j.taap.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Liu D, Gao L, Roy SK, Cornish KG, Zucker IH. Role of oxidant stress on AT1 receptor expression in neurons of rabbits with heart failure and in cultured neurons. Circ Res. 2008;103:186–193. doi: 10.1161/CIRCRESAHA.108.179408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braga VA, Colombari E, Jovita MG. Angiotensin II-derived reactive oxygen species underpinning the processing of the cardiovascular reflexes in the medulla oblongata. Neurosci Bull. 2011;27:269–274. doi: 10.1007/s12264-011-1529-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su Q, Qin DN, Wang FX, Ren J, Li HB, Zhang M, et al. Inhibition of reactive oxygen species in hypothalamic paraventricular nucleus attenuates the renin-angiotensin system and proinflammatory cytokines in hypertension. Toxicol Appl Pharmacol. 2014;276:115–120. doi: 10.1016/j.taap.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Sriramula S, Cardinale JP, Francis J. Inhibition of TNF in the brain reverses alterations in RAS components and attenuates angiotensin II-induced hypertension. PLoS One. 2013;8:63847. doi: 10.1371/journal.pone.0063847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang YM, He RL, Yang LM, Qin DN, Guggilam A, Elks C, et al. Brain tumour necrosis factor-alpha modulates neurotransmitters in hypothalamic paraventricular nucleus in heart failure. Cardiovasc Res. 2009;83:737–746. doi: 10.1093/cvr/cvp160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun W, Wang X, Hou C, Yang L, Li H, Guo J, et al. Oleuropein improves mitochondrial function to attenuate oxidative stress by activating the Nrf2 pathway in the hypothalamic paraventricular nucleus of spontaneously hypertensive rats. Neuropharmacology. 2017;113:556–566. doi: 10.1016/j.neuropharm.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Yu XJ, Suo YP, Qi J, Yang Q, Li HH, Zhang DM, et al. Interaction between AT1 receptor and NF-kappaB in hypothalamic paraventricular nucleus contributes to oxidative stress and sympathoexcitation by modulating neurotransmitters in heart failure. Cardiovasc Toxicol. 2013;13:381–390. doi: 10.1007/s12012-013-9219-x. [DOI] [PubMed] [Google Scholar]
- 30.Kang YM, Zhang AQ, Zhao XF, Cardinale JP, Elks C, Cao XM, et al. Paraventricular nucleus corticotrophin releasing hormone contributes to sympathoexcitation via interaction with neurotransmitters in heart failure. Basic Res Cardiol. 2011;106:473–483. doi: 10.1007/s00395-011-0155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bai J, Yu XJ, Liu KL, Wang FF, Jing GX, Li HB, et al. Central administration of tert-butylhydroquinone attenuates hypertension via regulating Nrf2 signaling in the hypothalamic paraventricular nucleus of hypertensive rats. Toxicol Appl Pharmacol. 2017;333:100–109. doi: 10.1016/j.taap.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 32.Kang YM, Gao F, Li HH, Cardinale JP, Elks C, Zang WJ, et al. NF-kappaB in the paraventricular nucleus modulates neurotransmitters and contributes to sympathoexcitation in heart failure. Basic Res Cardiol. 2011;106:1087–1097. doi: 10.1007/s00395-011-0215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller FJ, Jr, Gutterman DD, Rios CD, Heistad DD, Davidson BL. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res. 1998;82:1298–1305. doi: 10.1161/01.RES.82.12.1298. [DOI] [PubMed] [Google Scholar]
- 34.Kang YM, Zhang ZH, Johnson RF, Yu Y, Beltz T, Johnson AK, et al. Novel effect of mineralocorticoid receptor antagonism to reduce proinflammatory cytokines and hypothalamic activation in rats with ischemia-induced heart failure. Circ Res. 2006;99:758–766. doi: 10.1161/01.RES.0000244092.95152.86. [DOI] [PubMed] [Google Scholar]
- 35.Agarwal D, Welsch MA, Keller JN, Francis J. Chronic exercise modulates RAS components and improves balance between pro- and anti-inflammatory cytokines in the brain of SHR. Basic Res Cardiol. 2011;106:1069–1085. doi: 10.1007/s00395-011-0231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mariappan N, Elks CM, Sriramula S, Guggilam A, Liu Z, Borkhsenious O, et al. NF-kappaB-induced oxidative stress contributes to mitochondrial and cardiac dysfunction in type II diabetes. Cardiovasc Res. 2010;85:473–483. doi: 10.1093/cvr/cvp305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Z, You Z, Li M, Pang L, Cheng J, Wang L. Protective effect of resveratrol on the brain in a rat model of epilepsy. Neurosci Bull. 2017;33:273–280. doi: 10.1007/s12264-017-0097-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zimmerman MC, Lazartigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, et al. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res. 2002;91:1038–1045. doi: 10.1161/01.RES.0000043501.47934.FA. [DOI] [PubMed] [Google Scholar]
- 39.Ding L, Zhang LL, Gao R, Chen D, Wang JJ, Gao XY, et al. Superoxide anions in paraventricular nucleus modulate adipose afferent reflex and sympathetic activity in rats. PLoS One. 2013;8:83771. doi: 10.1371/journal.pone.0083771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu GQ, Gao L, Patel KP, Zucker IH, Wang W. ANG II in the paraventricular nucleus potentiates the cardiac sympathetic afferent reflex in rats with heart failure. J Appl Physiol. 1985;2004(97):1746–1754. doi: 10.1152/japplphysiol.00573.2004. [DOI] [PubMed] [Google Scholar]
- 41.Gorbea-Oppliger VJ, Fink GD. Cerebroventricular injection of angiotensin II antagonist: effects on blood pressure responses to central and systemic angiotensin II. J Pharmacol Exp Ther. 1995;273:611–616. [PubMed] [Google Scholar]
- 42.Gomes da Silva AQ, Xavier CH, Campagnole-Santos MJ, Caligiorne SM, Baltatu OC, Bader M, et al. Cardiovascular responses evoked by activation or blockade of GABA(A) receptors in the hypothalamic PVN are attenuated in transgenic rats with low brain angiotensinogen. Brain Res. 2012;1448:101–110. doi: 10.1016/j.brainres.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 43.Han Y, Fan ZD, Yuan N, Xie GQ, Gao J, De W, et al. Superoxide anions in the paraventricular nucleus mediate the enhanced cardiac sympathetic afferent reflex and sympathetic activity in renovascular hypertensive rats. J Appl Physiol. 1985;2011(110):646–652. doi: 10.1152/japplphysiol.00908.2010. [DOI] [PubMed] [Google Scholar]
- 44.Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, et al. Brain microglial cytokines in neurogenic hypertension. Hypertension. 2010;56:297–303. doi: 10.1161/HYPERTENSIONAHA.110.150409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi Z, Gan XB, Fan ZD, Zhang F, Zhou YB, Gao XY, et al. Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiol (Oxf) 2011;203:289–297. doi: 10.1111/j.1748-1716.2011.02313.x. [DOI] [PubMed] [Google Scholar]
- 46.Francis J, Wei SG, Weiss RM, Felder RB. Brain angiotensin-converting enzyme activity and autonomic regulation in heart failure. Am J Physiol Heart Circ Physiol. 2004;287:2138–2146. doi: 10.1152/ajpheart.00112.2004. [DOI] [PubMed] [Google Scholar]
- 47.Kang YM, Zhang DM, Yu XJ, Yang Q, Qi J, Su Q, et al. Chronic infusion of enalaprilat into hypothalamic paraventricular nucleus attenuates angiotensin II-induced hypertension and cardiac hypertrophy by restoring neurotransmitters and cytokines. Toxicol Appl Pharmacol. 2014;274:436–444. doi: 10.1016/j.taap.2013.12.001. [DOI] [PubMed] [Google Scholar]