Dear Editor,
Body protection compound (BPC) 157 is a stable gastric pentadecapeptide. Predrag Sikiric’s team has carried out many investigations of its cytoprotective effects in different organs and tissues [1, 2]. Their evidence indicates that BPC157 has potent cytoprotection in neural injury and gastrointestinal (GI) ulcers. Nevertheless, the effects of BPC157 in the GI remain unclear. It is well known that the gut produces 95% of the 5-hydroxytryptamine (5-HT) in the human body. The 5-HT in the intestinal mucosa stimulates the production of pro-inflammatory mediators by immune cells. Enteric 5-HT may be a key player in physiological actions and the pathogenesis of intestinal inflammation [3–5]. Previous evidence has shown that enteric 5-HT plays a prominent role in inflammatory bowel disease [6, 7]. The reports imply that BPC157 significantly modulates the regional synthesis of 5-HT in different areas of the rat brain [8, 9]. So far, the detailed mechanism of action of BPC157 has remained elusive in GI physiology and pathophysiology. Therefore, the aim of the study was to clarify the cytoprotective mechanism of BPC157 in the GI by investigating the release of enteric 5-HT, the survival rate of cultured enteric neurons, the proliferation of cultured enteric glial cells (EGCs), and intestinal motility with and without BPC157. This study was approved by Research Risk and Safety Office and Human Ethics Committee in Jingmen First Hospital (protocol 013R0507 and protocol 013H0688).
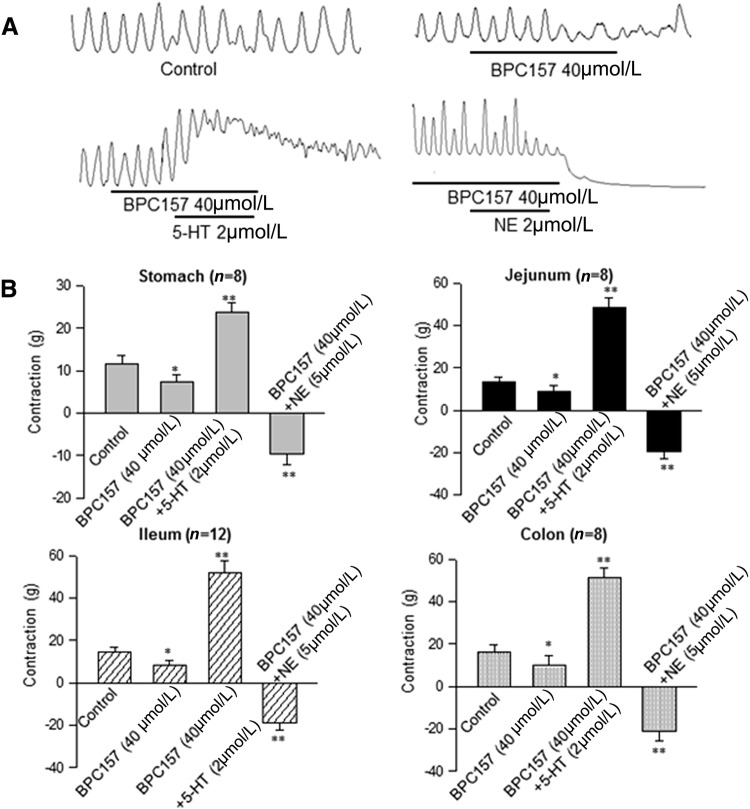
In order to test the effects of BPC157 on intestinal motility, we used a bath organ system (Fig. S1) to measure contractile responses to BPC157 in rat and human intestinal strips. Application of BPC157 suppressed motility in the GI preparations including human intestinal strips (jejunum n = 12, ileum n = 8, and colon n = 8) and rat strips (stomach n = 8, jejunum n = 8, ileum n = 12, and colon n = 8). Pretreatment with 40 µmol/L BPC157 for 10 min did not suppress the tonic contraction evoked by 5-HT or the contractile inhibition induced by norepinephrine (NE) in these strips (Fig. 1). To further examine the effects of BPC157 on the release of enteric 5-HT, we used ELISA to measure the 5-HT concentration in test samples. The results showed that the 5-HT concentration was significantly lower in the BPC157 (40 µmol/L) groups than in the control groups, including the rat ileal and colonic groups (Fig. S2). Nevertheless, similar results were not found in human ileal and colonic samples from gastrointestinal surgery for patients of different genders, ages, and diseases.
Fig. 1.
Effects of BPC157 in human and rat GI muscle strips. A Bath application of 40 µmol/L BPC157 suppressed the amplitude of contraction of a human longitudinal axial muscle strip, but did not alter the contractile effect of 5-HT and the inhibitory effect of NE. B BPC157 suppressed the contractile responses in rat stomach, jejunal, ileal, and colonic muscle strips. Application of BPC157 attenuated the amplitude of contraction, but pre-incubation with BPC157 did not alter the contractile response elicited by 5-HT or NE. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01.
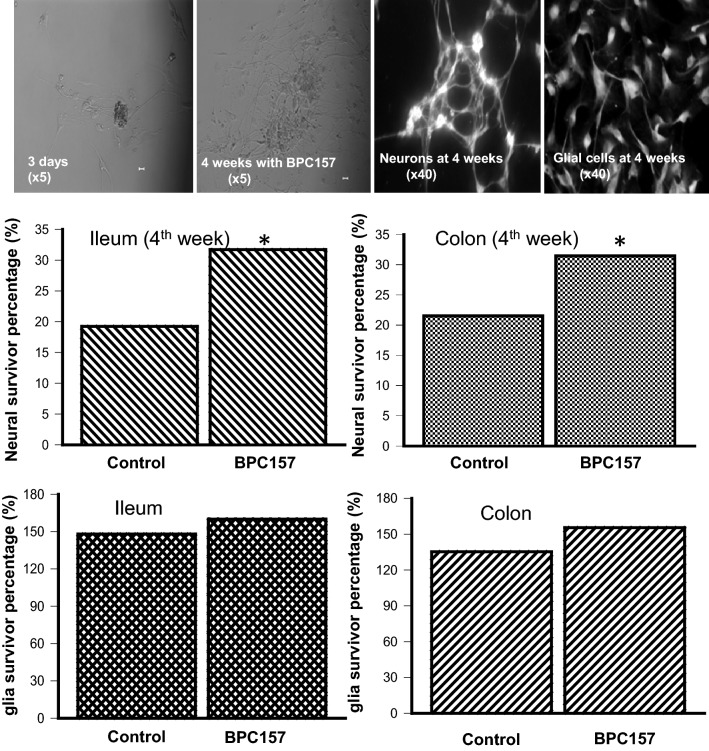
Our evidence showed that the suppression of intestinal motility by BPC157 was not correlated with a direct action of 5-HT on the enteric muscular strips, but BPC157 did suppress the synthesis and release of 5-HT in the GI. To further determine whether BPC157 plays a cytoprotective role in the GI, we assessed its effects in cultured enteric neurons and EGCs. After application of BPC157 (100 µmol/L) to the culture medium of freshly-isolated myenteric neurons and EGCs, their survival was quantified at 4 weeks in culture as the percentage to that at 3 days of culture. The results showed that 31.69% of cultured neurons from rat ileum on the 3rd day survived in the 4th week in the BPC157 group, but only 19.23% of neurons survived in the 4th week in the control group. In cultured EGCs, the glia proliferated up to 159.95% of original number in the 4th week in the BPC157 group, but the proliferation rate was 147.98% in the control group. Similar results were obtained from the colonic group (Fig. 2, Table S1).
Fig. 2.
BPC157 enhances the percentages of cultured enteric neurons and EGCs. Enteric ganglia were freshly isolated from myenteric preparations of rat (n = 4) ileum and colon. The ganglia were separated into 18 culture dishes of which 7 had Krebs solution (control group) and 11 dishes had BPC157 (100 μmol/L). Bars represent the percentages of cells counted at week 4 to those on day 3. *P < 0.05.
In summary, application of BPC157 suppressed the synthesis and release of enteric 5-HT and attenuated GI motility in human and rat enteric samples. BPC157 increased the survival rate of cultured enteric neurons and the proliferation rate of cultured EGCs.
Early emerging evidence has shown that BPC157 has potent anti-ulcer effects in the gut [1, 2, 10–12]. We found that application of BPC157 attenuated the tonic and phasic contractions of ileal and colonic strips from human and rat. However, BPC157 neither antagonized the stimulatory action of exogenous 5-HT nor enhanced the inhibitory action of NE on intestinal motility. The results suggest that BPC157 is not directly involved in activation of receptors in neural and muscular cells to influence bowel motility. The results from 5-HT ELISA showed that application of BPC157 reduced the concentration of 5-HT in rat enterocytes. Likewise, the level of 5-HT was reduced by BPC157 in human bowel samples, but the results were not consistent in the BPC157 and control groups, such that BPC157 did not decrease the 5-HT concentration in the biopsies from patients with colonic cancer. The data from human samples suggested that the concentration of enteric 5-HT was influenced by pathological, age, and gender factors in the donors. Our results imply that a reduction of enteric 5-HT concentration by BPC157 might attenuate intestinal motility. We found that BPC157 increased the survival of cultured enteric neurons and the proliferation of cultured EGCs, which may improve healing of damaged enteric nervous and mucosal structures. Our findings provide a novel understanding of the cytoprotective mechanism and application of BPC157 in potential therapy for enteric neural injury and GI ulcers. However, the detailed cell signaling pathway(s) of BPC157 needs to be further investigated in enteric neurons and enterocytes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We appreciate very much the gift of BPC157 from Dr. Predrag Sikiric in Zagreb, Croatia. This work was supported by a JMEY International collaboration Grant (020002015).
Contributor Information
Junhua Li, Email: 37517075@qq.com.
Guo-Du Wang, Email: gd53wang@hotmail.com.
References
- 1.Sikirić P, Petek M, Rucman R, Seiwerth S, Grabarević Z, Rotkvić I, et al. A new gastric juice peptide, BPC-An overview of stomach/stress/organoprotection hypothesis and BPC beneficial effects. J Physiol (Paris) 1993;87:313–327. doi: 10.1016/0928-4257(93)90038-U. [DOI] [PubMed] [Google Scholar]
- 2.Sikiric P, Seiwerth S, Grabarevic Z, Petek M, Rucman R, Turkovic B, et al. The beneficial effect of BPC 157, a 15 amino acid peptide BPC fragment, on gastric and duodenal lesions induced by restraint stress, cysteaniune and 96% ethanol in rats. A comparative study with H2 receptor antagonists, dopaunine promoters and gut peptides. Life Sci 1994, 54: PL63–68. [DOI] [PubMed]
- 3.Linan-Rico A, Ochoa-Cortes F, Beyder A, Soghomonyan S, Zuleta-Alarcon A, Coppola V, et al. Mechanosensory signaling in enterochromaffin cells and 5-HT release: potential implications for gut inflammation. Front Neurosci. 2016;10:564. doi: 10.3389/fnins.2016.00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Wood JD. Enteric nervous system neuropathy: repair and restoration. Curr Opin Gastroenterol. 2011;27:106–111. doi: 10.1097/MOG.0b013e328342a6ea. [DOI] [PubMed] [Google Scholar]
- 6.Gershon MD. Serotonin is a sword and a shield of the bowel: serotonin plays offense and defense. Trans Am Clin Climatol Assoc. 2012;123:268–280. [PMC free article] [PubMed] [Google Scholar]
- 7.Smith TK, Gershon MD. Cross Talk proposal: 5-HT is necessary for peristalsis. J Physiol. 2015;593:3225–3227. doi: 10.1113/JP270182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boban Blagaic A, Blagaic V, Mirt M, Jelovac N, Dodig G, Rucman R, et al. Gastric pentadecapeptideBPC 157 effective against serotonin syndrome in rats. Eur J Pharmacol. 2005;512:173–179. doi: 10.1016/j.ejphar.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 9.Tohyama Y, Sikirić P, Diksic M. Effects of pentadecapeptide BPC157 on regional serotonin synthesis in the rat brain: alphamethyl-l-tryptophan autoradiographic measurements. Life Sci. 2004;76:345–357. doi: 10.1016/j.lfs.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Sikiric P, Seiwerth S, Rucman R, Kolenc D, Vuletic LB, Drmic D, et al. Brain-gut axis and pentadecapeptide BPC 157: Theoretical and practical implications. Curr Neuropharmacol. 2016;14:857–865. doi: 10.2174/1570159X13666160502153022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, et al. Stable gastric pentadecapeptide BPC 157: novel therapy in gastrointestinal tract. Curr Pharm Des. 2011;17:1612–1632. doi: 10.2174/138161211796196954. [DOI] [PubMed] [Google Scholar]
- 12.Cirillo C, Vanden BP, Tack J. Role of serotonin in gastrointestinal physiology and pathology. Minerva Endocrinol. 2011;36:311–324. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.