Abstract
Autonomic disturbances often occur in patients with acute cerebrovascular disease due to damage of the central autonomic network. We summarize the structures of the central autonomic network and the clinical tests used to evaluate the functions of the autonomic nervous system. We review the clinical and experimental findings as well as management strategies of post-stroke autonomic disturbances including electrocardiographic changes, cardiac arrhythmias, myocardial damage, thermoregulatory dysfunction, gastrointestinal dysfunction, urinary incontinence, sexual disorders, and hyperglycemia. The occurrence of autonomic disturbances has been associated with poor outcomes in stroke patients. Autonomic nervous system modulation appears to be an emerging therapeutic strategy for stroke management in addition to treatments for sensorimotor dysfunction.
Keywords: Autonomic disturbance, Autonomic nervous system, Cerebrovascular disease, Stroke
Introduction
Autonomic disturbances often occur in patients with acute cerebrovascular disease due to damage of the central autonomic network [1–4]. The central autonomic network consists of four hierarchal structures at the telencephalic, diencephalic, brainstem, and spinal levels [5]. Normal function of the autonomic nervous system (ANS) is critically dependent on the integrity of the autonomic network [6]. Brain injury in stroke, therefore, can cause various types of central autonomic disturbance depending upon the lesion site, particularly in the frontoparietal areas and brainstem [3]. Emphasis has mainly been laid on the recovery of motor functions; however, dysfunctions of the cardiovascular, gastrointestinal, thermoregulatory, sudomotor, or urinary system are common in stroke patients and can be detected clinically [2, 7, 8]. Emerging evidence has shown that autonomic disturbances after stroke are associated with unfavorable functional outcomes and increased mortality [2]. Thus, ANS modulation is emerging as a new therapeutic strategy for stroke management [9].
In this review, we summarize the anatomical structures and functional output of the central autonomic network as well as the clinical tests for evaluating autonomic function. In particular, we focus on the clinical manifestations and pathophysiological mechanisms of central autonomic disturbances associated with stroke.
Central Autonomic Network
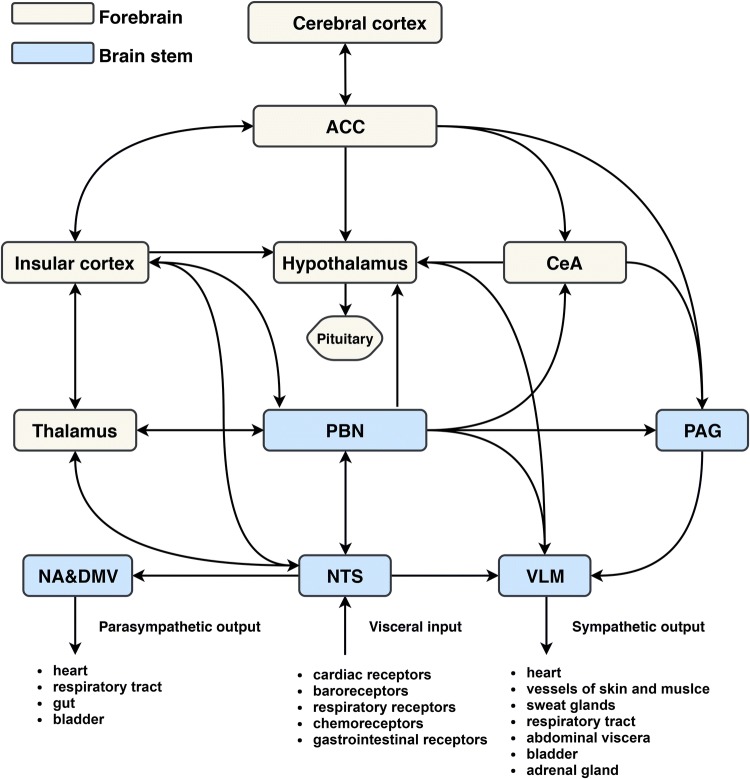
The central autonomic network includes telencephalic, diencephalic, and brainstem structures (Fig. 1) [10].
Fig. 1.
Diagram of the central autonomic network. Visceral information is relayed through the NTS and PBN to forebrain areas such as the hypothalamus, amygdala, thalamus, and insular cortex. The insular cortex has dense reciprocal connections with the ACC, lateral hypothalamic area, NTS, and PBN. These regions are also reciprocally connected. Infarction of the insular cortex may result in the loss of overall autonomic modulation and a decline in parasympathetic tone and baroreflex sensitivity, as well as a shift towards sympathetic dominance. ACC, anterior cingulate cortex; CeA, central amygdala; PBN, parabrachial nucleus; PAG, periaqueductal gray; NA, nucleus ambiguus; DMV, dorsal motor nucleus of the vagus; NTS, nucleus of the solitary tract; VLM, ventrolateral medulla.
Telencephalic Structures
The telencephalic structures consist of insular cortex, anterior cingulate cortex, and amygdala. The insular cortex is the primary interoceptive cortex and integrates visceral, pain, and temperature sensations from gustatory, visceral, muscle, and skin receptors via the thalamus [11–14]. It is also a visceromotor area, controlling both sympathetic and parasympathetic outputs, primarily via a relay in the lateral hypothalamic area [15]. The anterior cingulate cortex initiates autonomic responses related to motivation and goal-directed behavior. It is interconnected with the insular cortex and subdivided into ventral and dorsal regions, the activations of which vary according to experimental task and the associated sympathetic or parasympathetic response [16, 17]. The amygdala provides affective or emotional value to sensory stimuli and is involved in the autonomic and neuroendocrine response to stress. It has widespread connections with the hypothalamus and brainstem, particularly the periaqueductal gray and the medullary reticular formation [18]. Via these projections, the amygdala initiates autonomic and endocrine responses and motor activation that are critical for the expression of emotional responses, particularly the fear response [19].
Diencephalic Structures
The hypothalamus is the main anatomical component of diencephalic structures. The hypothalamus plays a central role in integrating the autonomic and endocrine responses necessary for homeostasis and adaptation [5]. It acts as a visceromotor pattern generator that initiates specific patterns of autonomic responses according to the stimulus, such as a hypoglycemia, body temperature, osmolarity, or external stressors [5]. The hypothalamus modulates not only stress, but also cerebral blood flow, food and sodium intake, glucose metabolism, and thermoregulation, as well as cardiovascular, renal, gastrointestinal, and respiratory functions [12].
Brainstem Structures
The brainstem structures consist of the periaqueductal gray (PAG), parabrachial nucleus (PBN), nucleus of the solitary tract (NTS), ventrolateral medulla (VLM), dorsal motor nucleus of the vagus (DMV), and nucleus ambiguus (NA). The PAG is the interface between the forebrain and the lower brainstem. It consists of different longitudinal columns, and, via their connections to the spinal cord, brainstem, and cortex, participates in the modulation of the cardiovascular, respiratory, thermoregulatory, urinary, reproductive, and pain-control systems [20]. The PBN is a major relay and coordinating center. It receives visceral, nociceptive, and thermoreceptive inputs from the spinal cord and conveys this information to the hypothalamus, amygdala, and thalamus [12]. The PBN also contains several subnuclei involved in taste, salivation, gastrointestinal activity, cardiovascular activity, respiration, osmoregulation, and thermoregulation [12]. The NTS is the first relay station of taste and visceral afferent information [5]. Different subnuclei of the NTS relay the information from baroreceptors and chemoreceptors, as well as cardiac, pulmonary, and gastrointestinal afferents, either directly or via the PBN, to the PAG, hypothalamus, thalamus (and then to the insular cortex), and amygdala [12, 21–23]. The VLM plays a critical role in regulating blood pressure [24]. Neurons in the rostral VLM include C1 adrenaline-synthesizing neurons and glutamatergic neurons that provide the major tonic excitatory input to sympathetic preganglionic neurons innervating resistance vessels of the muscles and visceral organs [21]. In addition, neurons in the caudal VLM include GABAergic and A1 noradrenergic neurons, which mainly mediate several cardiovascular reflexes and hypothalamic functions, respectively [6, 24]. The DMV contains most of the vagal preganglionic parasympathetic neurons; it receives inputs from the NTS and mediates all vagovagal reflexes controlling gastrointestinal motility and secretion [5]. The NA provides primary control of the heart via the cardiac ganglia, the neurons of which are excited by baroreceptor-sensitive NTS neurons and mediate the efferent cardioinhibitory component of the baroreflex [21].
Tests for Evaluation of the Autonomic Nervous System
Tilt Table Testing
Heart rate (HR) and blood pressure (BP) responses to orthostatic standing are well-established tests to assess parasympathetic and sympathetic nervous system activity, respectively [25]. A fall > 30 mmHg in systolic pressure and 15 mmHg in diastolic pressure when changing from the supine to the erect position is defined as abnormal [25]. It should be borne in mind that an abnormal fall in BP may also occur in patients taking antihypertensive drugs and other medications and in those with adrenal insufficiency and hypovolemia. It is suggested that medications should be stopped for five half-lives, and food, coffee, and nicotine should be avoided for 3 h before the test [26]. In resting healthy subjects, HR is determined by the predominantly vagal background autonomic activity. Upon standing, HR increases until it reaches a maximum at about the fifteenth heartbeat, after which it slows to a relatively stable rate at about the thirtieth beat. The 30:15 ratio (R–R interval at beat 30)/(R–R interval at beat 15) has been recommended as an index of cardiovagal function, the magnitude of which decreases with increasing age. In young adults, a ratio of < 1.04 is abnormal [25].
Valsalva Maneuver
The Valsalva maneuver is a useful screening test for evaluating the baroreflex [27]. During and after the maneuver, activations of baroreceptors in the thoracic viscera and arteries change the cardiac vagal efferent and sympathetic vasomotor activity [27]. Lesions of any of these autonomic pathways or of their central connections are likely to result in an abnormal HR response to the Valsalva maneuver. The patient breathes forcefully into a mouthpiece attached to a mercury manometer, maintaining an expiratory pressure of 40 mmHg for 10 or 15 s while an electrocardiographic (ECG) recording of the HR is made. In normal young adults, the ratio of the longest R–R interval to the shortest R–R interval during the maneuver is at least 1.45 [25]. A ratio lower than the age-matched control values usually indicates impaired ANS control of the heart and blood vessels, but low values may also be recorded in patients with heart and lung diseases [25].
Heart Rate Variation
Heart rate variation (HRV) is defined by the variation in heartbeat intervals or correspondingly in the instantaneous heart rate; it has been widely used to assess autonomic dysfunction following stroke [2, 4]. It shows the HR variation around the mean HR and reflects the balance between the sympathetic and parasympathetic nervous systems [26]. This test has become very popular for evaluating ANS function after stroke, because it is noninvasive, easy to perform, and has relatively good reproducibility [2, 28]. The assessment of HRV is generally based on time-domain or frequency-domain analysis. Time-domain analysis uses the mean HR, the standard deviation of normal-to-normal interbeat intervals (SDNN), and the root mean square of square sum of adjacent normal-to-normal interval difference (rMSSD) as the parameters. SDNN reflects overall HR variability, whereas rMSSD is correlated with vagal-mediated control [4]. Time-domain HRV analysis has the widest application in routine clinical evaluation and some of its indices have become well-documented, independent risk factors of cardiovascular events [29]. Frequency analysis splits a signal into its underlying frequencies. Parasympathetic modulation of HR is more pronounced in the frequency range 0.15 Hz–0.5 Hz (high-frequency range), while in the frequency ranging 0.04 Hz–0.15 Hz (low-frequency range), the HRV is controlled by a dual contribution of the sympathetic and parasympathetic nervous systems. The very low-frequency range corresponds to frequencies < 0.04 Hz and reflects the integrative effect of the various controllers, such as humoral effects [4]. The frequency-domain analysis of HRV is much better understood and is also mostly used for research purposes [30].
Baroreflex Sensitivity
The baroreflex is the major neural mechanism for BP control. A fall in BP leads to unloading of the baroreceptors in the carotid sinus and aortic arch, resulting in activation of sympathetic outflow and inhibition of cardiovagal neurons with a resultant increase in HR [26]. Baroreflex sensitivity (BRS) is quantified in milliseconds of R–R interval duration to each mmHg of arterial BP, with a normal value of ~ 15 ms/mmHg and a large interindividual difference [4]. Traditional approaches to test the BRS include pharmacological stimulation and neck suction. More recently, advances in beat-by-beat BP and HR monitoring methods have made it possible to estimate the BRS non-invasively from the R–R interval changes associated with spontaneous fluctuations in BP [27].
Thermoregulatory Sweat Test
The cholinergic part of the ANS can be assessed based on the reaction of sweat glands to various stimuli in the thermoregulatory sweat test [31]. The preganglionic centers that mediate the efferent sympathetic response include the hypothalamus, bulbospinal pathways, the intermediolateral cell column, and white rami [26]. In this test, sweat secretion after raising body temperature by 1 °C–1.4 °C (but below 38 °C) is measured. Various chemical powders that change color on exposure to moisture are used as indicators. The subject is placed in a sweat chamber with the air temperature at 45 °C–50 °C and humidity at 35%–50%. The peak response of sweat glands occurs after 35 min–45 min. Normal subjects should demonstrate generalized perspiration [27].
Plasma Catecholamines
The plasma catecholamine concentration has been accepted as an index of sympathetic activity in studies of cerebral vascular disorders [32, 33]. Noradrenaline is a neurotransmitter in the sympathetic nervous system and is released into the cleft of the nerve terminal-receptor site when the sympathetic nervous system is activated [32]. There is some “spillover” into the plasma on stimulation, so the plasma noradrenaline concentration is usually elevated if the sympathetic activity is increased [32]. However, venous plasma noradrenaline may not provide an accurate index of sympathetic activity as it reflects not only the vesicular release of noradrenaline from sympathetic nerve terminals, but also the effectiveness of junctional clearance. Clinicians should consider the effect of changes in organ/regional blood flow that would impact overall noradrenaline clearance. More recently, the regional noradrenaline spillover rate has permitted the assessment of noradrenaline release from specific organs. The “spillover” in steady-state conditions mirrors the secretion of noradrenaline from the sympathetic nerve terminals and is viewed as the gold standard for quantifying changes in sympathetic nerve activity, especially in human subjects [27].
Autonomic Disturbances After Stroke
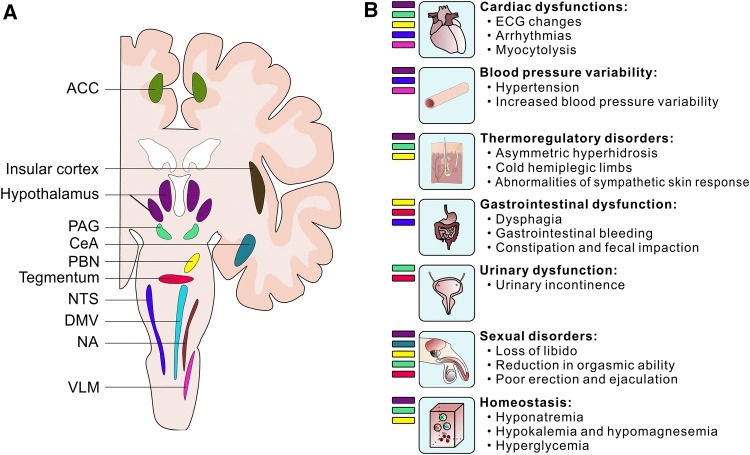
Stroke can damage the anatomical structures of the central autonomic network, leading to autonomic disturbances (Fig. 2).
Fig. 2.
Post-stroke autonomic disturbances caused by damage of the central autonomic network. A Brain structures of the central autonomic network. ACC, anterior cingulate cortex; PAG, periaqueductal gray; CeA, central amygdala; PBN, parabrachial nucleus; NTS, nucleus of the solitary tract; DMV, dorsal motor nucleus of the vagus; NA, nucleus ambiguus; VLM, ventrolateral medulla. B Specific autonomic disturbances correlate with damage of specific brain regions.
Cardiac Dysfunction
The manifestation of cardiac dysfunction following stroke includes ECG changes [34, 35], arrhythmias [36], and myocardial damage [3, 37]. Both experimental and clinical studies have indicated that hemispheric stroke involving the insular cortex or parietal lobe has particularly adverse effects in provoking cardiac consequences [38–40]. Damaged structures of the central autonomic network or connections could cause augmentation or inhibition of one or both divisions of the ANS, resulting in ECG changes without permanent effects on the myocardium. However, the catecholamine elevation associated with increased sympathetic tone could further cause ECG changes and myocardial damage. Cardiac dysfunction following stroke can be identified by ECG, echocardiography, cardiac enzyme levels, and coronary angiography [40].
ECG changes with considerable variation have been frequently reported in patients with acute stroke [34, 41–44]. The most common ECG abnormalities are prolonged QTc-intervals, ST-segment abnormalities, T-wave abnormalities, U-waves, and pathological Q waves (Table 1). Many factors including type of stroke and the localization of the lesion, pre-existing cardiac disorders, and electrolyte disorders may contribute to the variation of ECG abnormalities [34]. QTc prolongation seems to be the most common ECG abnormality reported in subarachnoid hemorrhage (SAH) patients, while patients with ischemic stroke have a higher frequency of T-wave abnormality [34]. Moreover, it seems that patients with history of cardiovascular disease and hypertension are more likely to have ECG alteration after stroke [34]. Although the initial cause of these changes is not fully clear, increased sympathetic output may be primarily responsible for the ischemic, arrhythmic, and repolarization changes of the ECG after stroke [45]. This hypothesis has been confirmed by clinical and experimental reports that insular cortex damage directly or indirectly affects cardiac function [42, 46, 47]. In contrast to ECG changes due to ischemic heart disease, stroke-induced ECG changes tend to appear later, reaching a maximum during the first few days after the onset of cerebrovascular events, and often revert to normal within 2 weeks [3].
Table 1.
ECG changes in stroke.
| Authors | Study population | QTc-interval prolongation | ST-segment abnormalities | U-wave | Pathological Q wave | T-wave abnormalities | Arrhythmias |
|---|---|---|---|---|---|---|---|
| Togha et al. [34] | IS = 303, ICH = 41, SAH = 17 | 117 (32%) | 84 (23%) | 33 (9%) | 57 (16%) | 144 (40%) | 98 (27%) |
| Ramani et al. [35] | IS = 53, ICH = 27, SAH = 19, CVT = 1 | 30 (30%) | 25 (25%) | 13 (13%) | 6 (6%) | 42 (42%) | 30 (30%) |
| van Bree et al. [41] | ICH = 31 | 11 (36%) | 5 (16%) | – | 4 (13%) | 5 (16%) | 13 (42%) |
| Christensen et al. [42] | IS = 162, ICH = 17 | 11 (6%) | 14 (8%) | – | – | 38 (21%) | 87 (49%) |
| Cruickshank et al. [44] | SAH = 40 | 22 (55%) | 16 (40%) | 13 (33%) | 2 (5%) | 29 (73%) | 27 (68%) |
| Goldstein [45] | IS = 68, ICH = 16, SAH = 28, others = 38 | 18 (12%) | 17 (11%) | 14 (9%) | 14 (9%) | 32 (21%) | 25 (17%) |
| Eisalo et al. [48] | SAH = 20 | 11 (55%) | 11 (55%) | 13 (65%) | – | 9 (45%) | 16 (80%) |
| Popescu et al. [49] | ICH = 120 | 60 (50%) | 18 (15%) | – | – | – | 40 (33%) |
| Arruda and de Lacerda [52] | ICH = 55, SAH = 15 | 45 (64%) | 22 (31%) | 8 (11%) | – | 10 (14%) | 14 (20%) |
| Dimant and Grob [54] | IS = 78, ICH = 12, SAH = 10 | 14 (14%) | 44 (44%) | 4 (4%) | 5 (5%) | 31 (31%) | 34 (34%) |
IS Ischemia stroke, ICH intracerebral hemorrhage, SAH subarachnoid hemorrhage, CVT cerebral venous thrombosis.
Cardiac autonomic dysfunction after stroke may also manifest as various forms of cardiac arrhythmia [3]. The incidence of cardiac arrhythmias following stroke has been estimated to be between 17% and 80% [34, 41, 42, 45, 48, 49]. The wide spectrum of arrhythmias in stroke patients includes atrial fibrillation or atrial flutter, sinus tachycardia or sinus bradycardia, premature atrial or ventricular complexes, junctional rhythm, supraventricular tachycardia, asystole, ventricular tachycardia, torsade de pointes, and ventricular fibrillation [45, 50–52]. Such a high heterogeneity of ECG changes reported in different publications might be explained by the different durations and techniques of ECG recording, conflicting definitions of arrhythmias, inclusion or exclusion of patients with pre-existing cardiac diseases, and different locations and types of stroke [30].
In an earlier study of 150 acute stroke patients, Goldstein et al. assessed the incidence of ECG abnormalities as well as the new occurrence rate within 24 h of admission. Arrhythmias of any type occurred in 41/150 (27%) patients with acute stroke, and new arrhythmias occurred in 13/53 (25%) who had prior available tracings. Of all the arrhythmias, atrial fibrillation was the most common, occurring in 21/150 (14%) of patients. Sinus arrhythmia occurred in 10/150 (7%) and was a new finding in 2/53 (4%) of patients. Ventricular arrhythmias occurred in 7/150 (5%) of patients with acute stroke, and new ventricular arrhythmias were found in 4/53 (8%) of patients with prior available tracings [45].
Although the majority of cardiac arrhythmias are benign and do not need urgent therapeutic intervention, clinically significant arrhythmias lead to high mortality and a poor functional outcome [51, 53]. Mortality among acute stroke patients with ventricular tachycardia, ventricular fibrillation, or asystole is significantly greater than that of patients without these ECG changes [45]. In a prospective study of SAH patients, clinically significant arrhythmias after SAH were defined as any rhythm disturbance other than sinus tachycardia, sinus bradycardia, and premature atrial and ventricular beats. In this cohort, 64% of the patients who experienced clinically significant arrhythmia were dead at 3 months [51]. Moreover, the incidence of sudden cardiac death in stroke patients ranges from 2% to 6%, and stroke-induced severe ventricular arrhythmias are responsible for the cardiac arrest [53]. Univariate logistic regression analysis has revealed that age and new injury severity score on admission are independent predictors of cardiac arrhythmia onset within the first 72 h after admission [53]. The risk for clinically significant cardiac arrhythmia after acute stroke is highest during the first 24 h and declines with time during the first 3 days. Therefore, close cardiac monitoring should be performed in the first 24 h, especially for aged patients with a severe neurological deficit [53].
In addition, post-stroke diffuse myocardial damage has been identified in both clinical and experimental studies [54, 55]. This is characterized by subendocardial hemorrhages, myofibrillar degeneration, lipofuscin pigment deposition in myofibrils, and lytic infiltration of a diffuse necrotic area in the heart [3]. This kind of injury was elucidated in an experimental study as myocytolysis, which has been associated with a catecholamine surge due to increased sympathetic hyperactivity [55]. Elevated levels of troponin (TnT), which is a highly sensitive and specific marker for myocardial damage, have been described in 9.6% of stroke patients [43]. A rise in TnT is significantly associated with a poor short-term outcome in stroke patients [43, 56].
Although there are currently no specific management guidelines for the post-stroke ECG changes, assessment of ECG and serum TnT levels is recommended for at least the first 24 h after stroke. This helps to screen for any significant arrhythmia and myocardial injury to prevent sudden cardiac death [57]. As ventricular tachycardia is the leading cause of stroke-induced cardiac arrest, several prospective trials have documented improved survival by applying prophylactic implantable cardioverter-defibrillator therapy in high-risk stroke patients with cardiac diseases and ventricular arrhythmia [53, 58]. Moreover, most of the stroke patients have coexisting electrolyte disturbances such as hypokalemia, which can lead to an increased risk of arrhythmias [59, 60]. Thus, intravenous infusion of potassium-free fluid should be avoided during the first day of stroke onset.
Blood Pressure Variability
The impairment of BP regulation resulting in its elevation and variation has frequently been reported in acute stroke and is associated with an unfavorable outcome [61, 62]. The BP responds to stroke in a time-dependent manner. In a prospective study of 44 first-ever stroke patients, the incidence of high systolic and diastolic BP in patients with arrhythmia was 61.3%, 22.6% and 16.2% at the time of admission, 3 and 7 days after stroke onset, respectively. Similarly, in stroke patients without arrhythmia, an increased systolic and diastolic BP was recorded in 61.6%, 38.4% and 23.2% of patients at the time of admission, 3 and 7 days after stroke onset, respectively [63]. Sykora et al. investigated the BP and BRS of 45 patients with acute intracerebral hemorrhage (ICH) within 72 h from symptom onset. They found that systolic, diastolic, and mean beat-to-beat pressure variability were significantly increased compared with a control group. The BRS gain value, correlated with systolic, diastolic, and mean BP variability, has been proposed as an independent predictor of outcome at 10 days after ICH [64]. In another retrospective study, 98 aneurysmal SAH patients were divided into two groups: VS+ (developing vasospasm) and VS− (not developing vasospasm). Mean, systolic, and diastolic BP values progressed in both the VS− and VS+ cohorts over time. From days 1 to 8, the mean arterial BP increased by 28% in the VS+ group and 3.5% in the VS− group. An elevation of mean arterial BP by > 25% within the first week after SAH is associated with unfavorable outcomes [65].
The pathophysiology of post-stroke BP fluctuation may involve a mixture of various mechanisms, including pre-existing hypertension, activation of the renin–angiotensin–aldosterone axis, and mental stress. Autonomic dysfunction has also been suggested to be an important mechanism, particularly baroreflex dysfunction [9, 66]. The baroreceptors are located in the carotid arteries, cardiac chambers, and aortic arch, and are activated by beat-to-beat fluctuations in systemic BP. The baroreceptors relay information to the NTS and VLM, which is further processed in the insular cortex, medial prefrontal cortex, and hypothalamus. In normal healthy people, an elevated BP activates the baroreceptors and leads to an increase of vagal outflow and a decrease of sympathetic outflow, resulting in a decrease of vascular and cardiac tone [9]. In patients with stroke, an impaired central processing center could lead to baroreflex dysfunction, such as a hypertensive crisis or high BP variability. Of clinical importance, baroreflex impairment has been associated with a less favorable long-term outcome after acute ischemic stroke [61]. In addition, baroreflex failure has also been reported in patients with acute ICH, and this is correlated with beat-to-beat BP variability and independently predicts outcomes at 10 days after ICH [64].
Modulation of baroreflex sensitivity has been suggested to be a new therapeutic target in acute stroke [9]. Baroreflex sensitivity can be actively regulated by certain drugs, especially β-blockers [67, 68]. In a prospective study of 111 stroke patients, the use of β-blockers was independently associated with reduced stroke severity on presentation and better outcomes. Such a neuroprotective effect is most likely due to a sympatholytic effect associated with decreased thrombin, inflammation, and hemoglobin A1C [69]. In addition, pre-treatment with β-blockers before the induction of experimental ischemia leads to a reduction in infarct volume by 40% [67].
Thermoregulatory Dysfunction
Sweating dysfunction after stroke is one of the most obvious and frequently-encountered symptoms of autonomic disturbance [70–72]. In hemiplegic patients, the paretic side of the body usually sweats more profusely and feels colder than the unaffected side [71]. In a prospective study, significant hyperhidrosis on the paretic side was observed in 55% of patients at baseline, and this phenomenon was more pronounced after exposure to heat for 5 min and 10 min [71]. Hyperhidrosis following stroke can occur at all sites (forehead, chest, forearm, hand, leg, and foot), but the face and arm seem to be the most common sites. Hyperhidrosis has been reported in patients with cortical and subcortical lesions, but the response seems to be equal in patients with frontal, parietal, and temporal lesions [71]. The pathogenesis of unilateral hyperhidrosis is still uncertain. The hyperhidrosis can be explained by failure of the suggested sympathoinhibitory pathway that controls sweating of the contralateral face and body. This putative pathway might originate from cortical areas and may follow the pyramidal tract, given that the degree of hyperhidrosis is associated with the severity of hemiparesis [71]. In addition, this hypothesis also explains some of the other manifestations of sympathetic hyperfunction in patients with stroke, such as cold extremities on the paretic side [73]. Although the phenomenon of hyperhidrosis has been reported to be associated with a severe neurological deficit and a poor prognosis [72], future investigation is needed to determine its clinical significance [70, 71].
Cold hemiplegic limbs have also been described in patient with stroke [3, 74, 75]. Although the symptoms can be present in the acute phase, the majority of patients recognize the coldness a few months after stroke onset [74, 75]. The explanation for this phenomenon hinges on the decreased cortical and subcortical inhibitory effect on vasomotor neurons, which subsequently increases the vasoconstrictor tone and reduces the cutaneous blood flow and skin temperature of hemiplegic limbs [3, 4].
The sympathetic skin response (SSR) is a simple method used in clinical practice to assess the reflex activity of the sympathetic pathway [76]. It is based on changes of skin conductance levels in response to various stimuli [76]. SSR amplitude is significantly suppressed in hemispheric infarction compared with controls [77]. The abnormalities may be related to damage in the ascending and descending corticoreticular pathways or the cerebral cortex [3, 78].
Gastrointestinal Dysfunction
Gastrointestinal (GI) complications following stroke are common, with over half of stroke patients presenting with dysphagia, gastrointestinal bleeding, or fecal incontinence [79]. Although most of these manifestations are related to immobilization and the severity of stroke, impaired autonomic innervation of the gastrointestinal tract is a potential underlying mechanism [79]. The incidence of post-stroke dysphagia varies from 37% to 78%, depending on the screening techniques [80]. In a study of 40 post-stroke patients with dysphagia and swallowing difficulties, pharyngeal transit time was increased sixfold compared to controls [81]. The frequency of dysphagia was lowest in patients with unilateral ischemic hemispheric strokes, increased for those with bilateral hemispheric lesions, and greatest for patients with brainstem lesions [79]. In addition, ischemic stroke involving the middle cerebral artery or both hemispheres is associated with a higher incidence and severity of dysphagia. Dysphagia has emerged as an important cause of post-stroke malnutrition and pneumonia, as well as post-stroke mortality [80].
GI bleeding caused by “stress ulcers” mostly occurs 1 week after stroke onset and the incidence varies from 0.1% to 8% [82, 83]. There is a higher frequency of GI bleeding in elderly patients, with severe neurologic deficits, and using nonsteroidal anti-inflammatory drugs. Various studies have shown that multiple sites of brain activity, particularly in the hypothalamus and medulla, influence the secretion of gastric acid, which may be responsible for the GI bleeding after stroke [82–84].
The options available for treating post-stroke dysphagia are limited, but the utility of transcranial magnetic stimulation (TMS) has been evaluated in small studies. In a study of 26 monohemispheric ischemic stroke patients with post-stroke dysphagia, TMS significantly improved dysphagia and this was maintained over 2 months of follow-up [85]. Similar studies have found that anodal transcranial direct current stimulation of the motor cortex is able to improve the Dysphagia Outcome and Severity Scale scores [86, 87]. Considering the risk of aspiration, aggressive screening for post-stroke dysphagia is warranted [84]. The use of routine gastroprotective drugs as prophylaxis for GI bleeding in ischemic stroke patients is controversial, but intravenous administration of antiulcer agents (H2 receptor antagonists) for elderly and severe stroke patients is recommended by some international guidelines [88]. In addition, the selection of antiplatelet agents for secondary stroke prevention is important in order to minimize post-stroke GI bleeding [84]. While Cilostazol seems to be associated with fewer GI bleeds, both the combinations of aspirin-dipyridamole and aspirin-clopidrogrel can increase the risk of GI bleeding [89].
Urinary Incontinence
Urinary incontinence (UI) is a common sequela of acute stroke, affecting more than a third of stroke patients admitted to hospital, with up to a quarter of these patients remaining incontinent at 1 year [90]. It is an involuntary leakage of urine accompanied or immediately preceded by urgency, and urodynamic assessment often reveals uninhibited detrusor contraction [90]. Brittain et al. analyzed the prevalence of UI in stroke patients based on nine hospital-based studies published between 1985 and 1997. They found that the incidence of UI at hospital admission for stroke ranges from 32% to 79% [91]. Kolominsky-Rabas et al. reported an incidence of UI of 35% at 7 days following stroke [92]. Based on data from the UK National Sentinel Stroke Audit between 1998 and 2004, Wilson et al. identified UI rates of 39%–44% at 1 week after stroke [93]. The variation of post-stroke UI incidence among these studies may be due to different definitions of UI, failure to account for the presence of premorbid incontinence, and the different time points of UI assessment.
The regulation of micturition requires connections between many areas in the brain and spinal cord that involve the sympathetic, parasympathetic, and somatic systems [94]. These mechanisms control smooth and striated muscle activity of the urinary bladder, the bladder neck, the urethra, and the urethral sphincter to allow bladder filling and voiding to take place in a coordinated manner. Injury of the suprapontine pathway as a result of a stroke lesion could remove the tonic inhibitory control over the pontine micturition center, resulting in decreased bladder capacity and detrusor overactivity [90, 94].
Current therapeutic options for post-stroke UI include bladder retraining and the use of anticholinergic medications. In addition, environmental and lifestyle interventions need to be considered, such as easy access to the toilet, use of hand-held urinals, access to a call bell, reducing caffeine intake, and changes to medications that exacerbate incontinence [90]. It is important for clinicians to formulate an accurate differential diagnosis of functional incontinence caused by stroke from the overflow incontinence, because the treatments are completely different.
Sexual Disorders
Sexual dysfunction in stroke patients is complex and etiologically multifactorial, including a variety of physical and psychosocial factors [95]. Previous studies have reported that the most common post-stroke sexual dysfunctions manifest as (1) a decline in libido and coital frequency, as well as reduction in vaginal lubrication and orgasmic ability in female patients; and (2) poor or absent erection and ejaculation in male patients [95, 96]. Studies in animals have shown that the media preoptic area, amygdala, paraventricular nucleus, periaqueductal gray, and ventral tegmentum are important integration centers for sexual function and penile erection [97]. Cerebrovascular accidents involving these regions are often associated with erectile dysfunction [97]. In addition to neurologic and cognitive deficits, the quality of sexual life may be impaired due to comorbidity, medication, fear of a new stroke, rejection by the spouse, loss of self-esteem, incontinence, and other urinary/sexual disturbances [3].
Hyperglycemia
Hyperglycemia occurs in 60% of acute stroke patients, of whom 12%–53% did not have the prior diagnosis of diabetes [98]. In non-diabetic stroke patients, the median blood glucose level rises from 5.9 mmol/L at 2.5 h to 6.2 mmol/L at 6 h post-stroke [99]. Two underlying mechanisms contribute to hyperglycemia after stroke: (1) the increased sympathetic activation results in increased output of glucose from the liver and stimulation of glucagon from the pancreas with insulin inhibition [100]; and (2) hypothalamic dysfunction induced by stroke can increase the secretion of cortisone and noradrenaline, which may contribute to the development of insulin resistance [101].
Hyperglycemia in both diabetic and non-diabetic patients has been linked to a poor prognosis both in terms of mortality and functional recovery, independent of the patient’s age, severity of the condition, or stroke type [102, 103]. Hyperglycemia facilitates the development of cellular acidosis in the ischemic penumbra and results in a greater infarct volume, thus promoting the recruitment of potentially salvageable neurons into the infarction [98]. In addition, hyperglycemia is associated with inflammation and oxidative stress as well as increased expression of matrix metalloproteinase (MMP) 9 and MMP-2 [98], which may promote the hemorrhagic transformation of infracted brain tissue. A higher risk of hemorrhagic transformation has been reported in stroke patients with serum glucose levels > 8.4 mmol/L [104]. Substantial reductions in plasma glucose concentrations can be achieved using intensive intravenous insulin. Although it remains controversial, most clinicians consider initiating insulin treatment among stroke patients with a blood glucose level > 11.10 mmol/L [105]. Close monitoring of glucose concentrations and adjusting insulin doses accordingly are strongly recommended to avoid hypoglycemia [105].
Prospective and Conclusions
Since the central autonomic network damage is almost inevitable in acute cerebrovascular disease, autonomic disturbances are important consequences of stroke. Emerging evidence has demonstrated the association of post-stroke autonomic complications with poor outcomes. However, the previous preclinical and clinical publications are mainly observational. Future studies are warranted to better understand the underlying molecular mechanism(s) involved in sympathetic and parasympathetic dysfunction following stroke.
In addition, there is a need to establish new testing modalities for evaluating autonomic disturbances in the setting of acute cerebrovascular events, particularly in aged patients with severe neurological deficits. Given that autonomic functions can be influenced by medication, hospital stress, lifestyle, and, most importantly, pre-existing comorbidity including diabetes, hypertension, and cardiovascular disease, the results of tests should be interpreted carefully in accordance with the clinical manifestations.
It has been reported that optimization of parasympathetic nervous system activation is neuroprotective in both preclinical models of cerebral ischemia and in vitro neuronal hypoxia [106–108]. In a rat model of transient focal cerebral ischemia, Ay et al. demonstrated that vagus nerve stimulation (VNS) significantly decreases infarct size and neurological deficits 24 h after onset of ischemia [109]. The mechanism of the anti-ischemic effect of VNS was suggested to be a reduction in extracellular glutamate level after brain ischemia [110, 111]. Modulation of the ANS has been suggested to be a promising therapeutic direction in the prevention and treatment of autonomic dysfunction related to stroke.
Currently, specific guidelines for the management of post-stroke autonomic disturbances are lacking, even though life-threatening ventricular arrhythmias can occur after stroke. We stress the importance of treating stroke-induced arrhythmias, cardiac injury, and hyperglycemia in a timely manner by clinicians who are experienced in the diagnosis and treatment of these situations. The development of therapeutic strategies targeting the autonomic nervous system dysfunction will improve the overall outcome for stroke patients.
Acknowledgements
This review was supported partly by grants from the National Institutes of Health, USA (NS081740 and NS082184).
Contributor Information
Jianmin Zhang, Email: zjm135@zju.edu.cn.
John H. Zhang, Email: johnzhang3910@yahoo.com
References
- 1.Xiong L, Tian G, Leung H, Soo YOY, Chen X, Ip VHL, et al. Autonomic dysfunction predicts clinical outcomes after acute ischemic stroke: a prospective observational study. Stroke. 2018;49:215–218. doi: 10.1161/STROKEAHA.117.019312. [DOI] [PubMed] [Google Scholar]
- 2.McLaren A, Kerr S, Allan L, Steen IN, Ballard C, Allen J, et al. Autonomic function is impaired in elderly stroke survivors. Stroke. 2005;36:1026–1030. doi: 10.1161/01.STR.0000160748.88374.ce. [DOI] [PubMed] [Google Scholar]
- 3.Korpelainen JT, Sotaniemi KA, Myllylä VV. Autonomic nervous system disorders in stroke. Clin Auton Res. 1999;9:325–333. doi: 10.1007/BF02318379. [DOI] [PubMed] [Google Scholar]
- 4.Al-Qudah ZA, Yacoub HA, Souayah N. Disorders of the autonomic nervous system after hemispheric cerebrovascular disorders: an update. J Vasc Interv Neurol. 2015;8:43–52. [PMC free article] [PubMed] [Google Scholar]
- 5.Cersosimo MG, Benarroch EE. Central control of autonomic function and involvement in neurodegenerative disorders. In: Handbook of Clinical Neurology. 3rd ed. Elsevier, 2013: 45–57. [DOI] [PubMed]
- 6.Benarroch EE. The autonomic nervous system: basic anatomy and physiology. Continuum. 2007;13:13–32. [Google Scholar]
- 7.Bankenahally R, Krovvidi H. Autonomic nervous system: anatomy, physiology, and relevance in anaesthesia and critical care medicine. BJA Educ. 2016;16:381–387. [Google Scholar]
- 8.Alawieh A, Tomlinson S, Adkins D, Kautz S, Feng W. Preclinical and clinical evidence on ipsilateral corticospinal projections: implication for motor recovery. Transl Stroke Res. 2017;8:529–540. doi: 10.1007/s12975-017-0551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sykora M, Diedler J, Turcani P, Hacke W, Steiner T. Baroreflex: a new therapeutic target in human stroke? Stroke. 2009;40:e678–e682. doi: 10.1161/STROKEAHA.109.565838. [DOI] [PubMed] [Google Scholar]
- 10.Benarroch EE. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc. 1993;68:988–1001. doi: 10.1016/s0025-6196(12)62272-1. [DOI] [PubMed] [Google Scholar]
- 11.Craig A. A new view of pain as a homeostatic emotion. Trends Neurosci. 2003;26:303–307. doi: 10.1016/s0166-2236(03)00123-1. [DOI] [PubMed] [Google Scholar]
- 12.Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci. 2002;25:433–469. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- 13.Lu C, Yang T, Zhao H, Zhang M, Meng F, Fu H, et al. Insular cortex is critical for the perception, modulation, and chronification of pain. Neurosci Bull. 2016;32:191–201. doi: 10.1007/s12264-016-0016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reddan MC, Wager TD. Modeling pain using fMRI: from regions to biomarkers. Neurosci Bull. 2018;34:208–215. doi: 10.1007/s12264-017-0150-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagai M, Hoshide S, Kario K. The insular cortex and cardiovascular system: a new insight into the brain-heart axis. J Am Soc Hypertens. 2010;4:174–182. doi: 10.1016/j.jash.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Critchley HD. Psychophysiology of neural, cognitive and affective integration: fMRI and autonomic indicants. Int J Psychophysiol. 2009;73:88–94. doi: 10.1016/j.ijpsycho.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vogt BA, Vogt L, Farber NB, Bush G. Architecture and neurocytology of monkey cingulate gyrus. J Comp Neurol. 2005;485:218–239. doi: 10.1002/cne.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LeDoux J. The amygdala. Curr Biol. 2007;17:R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 20.Misslin R. The defense system of fear: behavior and neurocircuitry. Neurophysiol Clin. 2003;33:55–66. doi: 10.1016/s0987-7053(03)00009-1. [DOI] [PubMed] [Google Scholar]
- 21.Benarroch EE. Central autonomic control. In: Robertson D, Biaggioni I, Burnstock G, Low PA, Paton JFR, editors. Primer on the Autonomic Nervous System. 3. Cambridge: Academic Press; 2012. pp. 9–12. [Google Scholar]
- 22.Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol. 2006;68:279–305. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen L, Li C, Zhai J, Wang A, Song Q, Liu Y, et al. Altered resting-state signals in patients with acute stroke in or under the thalamus. Neurosci Bull. 2016;32:585–590. doi: 10.1007/s12264-016-0064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dampney RAL, Horiuchi J. Functional organisation of central cardiovascular pathways: studies using c-fos gene expression. Prog Neurobiol. 2003;71:359–384. doi: 10.1016/j.pneurobio.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 25.McLeod JG, Tuck RR. Disorders of the autonomic nervous system: part 2. Investigation and treatment. Ann Neurol. 1987;21:519–529. doi: 10.1002/ana.410210602. [DOI] [PubMed] [Google Scholar]
- 26.Low PA. Testing the autonomic nervous system. Semin Neurol. 2003;23:407–422. doi: 10.1055/s-2004-817725. [DOI] [PubMed] [Google Scholar]
- 27.Zygmunt A, Stanczyk J. Methods of evaluation of autonomic nervous system function. Arch Med Sci. 2010;6:11–18. doi: 10.5114/aoms.2010.13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korpelainen JT, Sotaniemi KA, Huikuri HV, Myllylä VV. Abnormal heart rate variability as a manifestation of autonomic dysfunction in hemispheric brain infarction. Stroke. 1996;27:2059–2063. doi: 10.1161/01.str.27.11.2059. [DOI] [PubMed] [Google Scholar]
- 29.Yperzeele L, van Hooff RJ, Nagels G, De Smedt A, De Keyser J, Brouns R. Heart rate variability and baroreceptor sensitivity in acute stroke: a systematic review. Int J Stroke. 2015;10:796–800. doi: 10.1111/ijs.12573. [DOI] [PubMed] [Google Scholar]
- 30.De Raedt S, De Vos A, De Keyser J. Autonomic dysfunction in acute ischemic stroke: an underexplored therapeutic area? J Neurol Sci. 2015;348:24–34. doi: 10.1016/j.jns.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Cohen J, Low P, Fealey R, Sheps S, Jiang NS. Somatic and autonomic function in progressive autonomic failure and multiple system atrophy. Ann Neurol. 1987;22:692–699. doi: 10.1002/ana.410220604. [DOI] [PubMed] [Google Scholar]
- 32.Myers MG, Norris JW, Hachniski VC, Sole MJ. Plasma norepinephrine in stroke. Stroke. 1981;12:200–204. doi: 10.1161/01.str.12.2.200. [DOI] [PubMed] [Google Scholar]
- 33.Naredi S, Lambert G, Edén E, Zäll S, Runnerstam M, Rydenhag B, et al. Increased sympathetic nervous activity in patients with nontraumatic subarachnoid hemorrhage. Stroke. 2000;31:901–906. doi: 10.1161/01.str.31.4.901. [DOI] [PubMed] [Google Scholar]
- 34.Togha M, Sharifpour A, Ashraf H, Moghadam M, Sahraian MA. Electrocardiographic abnormalities in acute cerebrovascular events in patients with/without cardiovascular disease. Ann Indian Acad Neurol. 2013;16:66–71. doi: 10.4103/0972-2327.107710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramani A, Shetty U, Kundaje GN. Electrocardiographic abnormalities in cerebrovascular accidents. Angiology. 1990;41:681–686. doi: 10.1177/000331979004100902. [DOI] [PubMed] [Google Scholar]
- 36.Raad B, Sila C. Cardiac manifestations of neurologic disorders. Continuum. 2011;17:13–26. doi: 10.1212/01.CON.0000394671.36878.ce. [DOI] [PubMed] [Google Scholar]
- 37.Ay H, Koroshetz WJ, Benner T, Vangel MG, Melinosky C, Arsava EM, et al. Neuroanatomic correlates of stroke-related myocardial injury. Neurology. 2006;66:1325–1329. doi: 10.1212/01.wnl.0000206077.13705.6d. [DOI] [PubMed] [Google Scholar]
- 38.Cechetto DF, Hachinski V. Cardiovascular consequence of experimental stroke. Baillieres Clin Neurol. 1997;6:297–308. [PubMed] [Google Scholar]
- 39.Oppenheimer S. The insular cortex and the pathophysiology of stroke-induced cardiac changes. Can J Neurol Sci. 1992;19:208–211. [PubMed] [Google Scholar]
- 40.Rincon F, Dhamoon M, Moon Y, Paik MC, Boden-Albala B, Homma S, et al. Stroke location and association with fatal cardiac outcomes: Northern Manhattan Study (NOMAS) Stroke. 2008;39:2425–2431. doi: 10.1161/STROKEAHA.107.506055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Bree MDR, Roos YWEM, van der Bilt IAC, Wilde AAM, Sprengers MES, Gans K, et al. Prevalence and characterization of ECG abnormalities after intracerebral hemorrhage. Neurocrit Care. 2010;12:50–55. doi: 10.1007/s12028-009-9283-z. [DOI] [PubMed] [Google Scholar]
- 42.Christensen H, Boysen G, Christensen AF, Johannesen HH. Insular lesions, ECG abnormalities, and outcome in acute stroke. J Neurol Neurosurg Psychiatry. 2005;76:269–271. doi: 10.1136/jnnp.2004.037531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fure B, Bruun Wyller T, Thommessen B. Electrocardiographic and troponin T changes in acute ischaemic stroke. J Intern Med. 2006;259:592–597. doi: 10.1111/j.1365-2796.2006.01639.x. [DOI] [PubMed] [Google Scholar]
- 44.Cruickshank JM, Neil-Dwyer G, Stott AW. Possible role of catecholamines, corticosteroids, and potassium in production of electrocardiographic abnormalities associated with subarachnoid haemorrhage. Br Heart J. 1974;36:697–706. doi: 10.1136/hrt.36.7.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goldstein DS. The electrocardiogram in stroke: relationship to pathophysiological type and comparison with prior tracings. Stroke. 1979;10:253–259. doi: 10.1161/01.str.10.3.253. [DOI] [PubMed] [Google Scholar]
- 46.Hachinski VC, Wilson JX, Smith KE, Cechetto DF. Effect of age on autonomic and cardiac responses in a rat stroke model. Arch Neurol. 1992;49:690–696. doi: 10.1001/archneur.1992.00530310032009. [DOI] [PubMed] [Google Scholar]
- 47.Verberne AJM, Owens NC. Cortical modulation of the cardiovascular system. Prog Neurobiol. 1998;54:149–168. doi: 10.1016/s0301-0082(97)00056-7. [DOI] [PubMed] [Google Scholar]
- 48.Eisalo A, Peräsalo J, Halonen PI. Electrocardiographic abnormalities and some laboratory findings in patients with subarachnoid haemorrhage. Br Heart J. 1972;34:217–226. doi: 10.1136/hrt.34.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Popescu D, Laza C, Mergeani A, Bajenaru OA, Antochi FA. Lead electrocardiogram changes after supratentorial intracerebral hemorrhage. Maedica. 2012;7:290–294. [PMC free article] [PubMed] [Google Scholar]
- 50.Estanol BV, Marin OSM. Cardiac arrhythmias and sudden death in subarachnoid hemorrhage. Stroke. 1975;6:382–386. doi: 10.1161/01.str.6.4.382. [DOI] [PubMed] [Google Scholar]
- 51.Frontera JA, Parra A, Shimbo D, Fernandez A, Schmidt JM, Peter P, et al. Cardiac arrhythmias after subarachnoid hemorrhage: risk factors and impact on outcome. Cerebrovasc Dis. 2008;26:71–78. doi: 10.1159/000135711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arruda WO, de Lacerda JFS. Electrocardiographic findings in acute cerebrovascular hemorrhage. Arg Neuropsiquiatr. 1992;50:269–274. doi: 10.1590/s0004-282x1992000300002. [DOI] [PubMed] [Google Scholar]
- 53.Kallmünzer B, Breuer L, Kahl N, Bobinger T, Raaz-Schrauder D, Huttner HB, et al. Serious cardiac arrhythmias after stroke: incidence, time course, and predictors—a systematic, prospective analysis. Stroke. 2012;43:2892–2897. doi: 10.1161/STROKEAHA.112.664318. [DOI] [PubMed] [Google Scholar]
- 54.Dimant J, Grob D. Electrocardiographic changes and myocardial damage in patients with acute cerebrovascular accidents. Stroke. 1977;8:448–455. doi: 10.1161/01.str.8.4.448. [DOI] [PubMed] [Google Scholar]
- 55.Hachinski VC, Smith KE, Silver MD, Gibson CJ, Ciriello J. Acute myocardial and plasma catecholamine changes in experimental stroke. Stroke. 1986;17:387–390. doi: 10.1161/01.str.17.3.387. [DOI] [PubMed] [Google Scholar]
- 56.Kilbourn KJ, Ching G, Silverman DI, McCullough L, Brown RJ. Clinical outcomes after neurogenic stress induced cardiomyopathy in aneurysmal sub-arachnoid hemorrhage: a prospective cohort study. Clin Neurol Neurosurg. 2015;128:4–9. doi: 10.1016/j.clineuro.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 57.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 58.Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death—executive summary. J Am Coll Cardiol. 2006;48:1088–1132. [Google Scholar]
- 59.van den Bergh WM, Algra A, van der Sprenkel JWB, Tulleken CAF, Rinkel GJE. Hypomagnesemia after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2003;52:276–281. doi: 10.1227/01.neu.0000043984.42487.0e. [DOI] [PubMed] [Google Scholar]
- 60.van den Bergh WM, Algra A, Rinkel GJE. Electrocardiographic abnormalities and serum magnesium in patients with subarachnoid hemorrhage. Stroke. 2004;35:644–648. doi: 10.1161/01.STR.0000117092.38460.4F. [DOI] [PubMed] [Google Scholar]
- 61.Robinson TG, Dawson SL, Eames PJ, Panerai RB, Potter JF. Cardiac baroreceptor sensitivity predicts long-term outcome after acute ischemic stroke. Stroke. 2003;34:705–712. doi: 10.1161/01.STR.0000058493.94875.9F. [DOI] [PubMed] [Google Scholar]
- 62.Robinson TG, Potter JF. Postprandial and orthostatic cardiovascular changes after acute stroke. Stroke. 1995;26:1811–1816. doi: 10.1161/01.str.26.10.1811. [DOI] [PubMed] [Google Scholar]
- 63.Orlandi G, Fanucchi S, Strata G, Pataleo L, Pellegrini LL, Prontera C, et al. Transient autonomic nervous system dysfunction during hyperacute stroke. Acta Neurol Scand. 2000;102:317–321. doi: 10.1034/j.1600-0404.2000.102005317.x. [DOI] [PubMed] [Google Scholar]
- 64.Sykora M, Diedler J, Rupp A, Turcani P, Rocco A, Steiner T. Impaired baroreflex sensitivity predicts outcome of acute intracerebral hemorrhage. Crit Care Med. 2008;36:3074–3079. doi: 10.1097/CCM.0b013e31818b306d. [DOI] [PubMed] [Google Scholar]
- 65.Faust K, Horn P, Schneider UC, Vajkoczy P. Blood pressure changes after aneurysmal subarachnoid hemorrhage and their relationship to cerebral vasospasm and clinical outcome. Clin Neurol Neurosurg. 2014;125:36–40. doi: 10.1016/j.clineuro.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 66.Sykora M, Diedler J, Poli S, Rupp A, Turcani P, Steiner T. Blood pressure course in acute stroke relates to baroreflex dysfunction. Cerebrovasc Dis. 2010;30:172–179. doi: 10.1159/000317105. [DOI] [PubMed] [Google Scholar]
- 67.Savitz SI, Erhardt JA, Anthony JV, Gupta G, Li X, Barone FC, et al. The novel β-blocker, carvedilol, provides neuroprotection in transient focal stroke. J Cereb Blood Flow Metab. 2000;20:1197–1204. doi: 10.1097/00004647-200008000-00005. [DOI] [PubMed] [Google Scholar]
- 68.Elghozi JL, Julien C. Sympathetic control of short-term heart rate variability and its pharmacological modulation. Fundam Clin Pharmacol. 2007;21:337–347. doi: 10.1111/j.1472-8206.2007.00502.x. [DOI] [PubMed] [Google Scholar]
- 69.Laowattana S, Oppenheimer SM. Protective effects of beta-blockers in cerebrovascular disease. Neurology. 2007;68:509–514. doi: 10.1212/01.wnl.0000253186.23949.fd. [DOI] [PubMed] [Google Scholar]
- 70.Kim BS, Kim YI, Lee KS. Contralateral hyperhidrosis after cerebral infarction: clinicoanatomic correlations in five cases. Stroke. 1995;26:896–899. doi: 10.1161/01.str.26.5.896. [DOI] [PubMed] [Google Scholar]
- 71.Korpelainen JT, Sotaniemi KA, Myllylä VV. Hyperhidrosis as a reflection of autonomic failure in patients with acute hemispheral brain infarction. Stroke. 1992;23:1271–1275. doi: 10.1161/01.str.23.9.1271. [DOI] [PubMed] [Google Scholar]
- 72.Labar DR, Mohr JP, Nichols FT, Tatemichi TK. Unilateral hyperhidrosis after cerebral infarction. Neurology. 1988;38:1679–1682. doi: 10.1212/wnl.38.11.1679. [DOI] [PubMed] [Google Scholar]
- 73.Korpelainen JT, Sotaniemi KA, Myllylä VV. Asymmetrical skin temperature in ischemic stroke. Stroke. 1995;26:1543–1547. doi: 10.1161/01.str.26.9.1543. [DOI] [PubMed] [Google Scholar]
- 74.Wanklyn P, Forster A, Young J, Mulley G. Prevalence and associated features of the cold hemiplegic arm. Stroke. 1995;26:1867–1870. doi: 10.1161/01.str.26.10.1867. [DOI] [PubMed] [Google Scholar]
- 75.Wanklyn P, Ilsley DW, Greenstein D, Hampton IF, Roper TA, Kester RC, et al. The cold hemiplegic arm. Stroke. 1994;25:1765–1770. doi: 10.1161/01.str.25.9.1765. [DOI] [PubMed] [Google Scholar]
- 76.Kucera P, Goldenberg Z, Kurca E. Sympathetic skin response: review of the method and its clinical use. Bratisl Lek Listy. 2004;105:108–116. [PubMed] [Google Scholar]
- 77.Korpelainen JT, Tolonen U, Sotaniemi KA, Myllylä VV. Suppressed sympathetic skin response in brain infarction. Stroke. 1993;24:1389–1392. doi: 10.1161/01.str.24.9.1389. [DOI] [PubMed] [Google Scholar]
- 78.Linden D, Berlit P. Sympathetic skin responses (SSRs) in monofocal brain lesions: topographical aspects of central sympathetic pathways. Acta Neurol Scand. 1995;91:372–376. doi: 10.1111/j.1600-0404.1995.tb07023.x. [DOI] [PubMed] [Google Scholar]
- 79.Ullman T, Reding M. Gastrointestinal dysfunction in stroke. Semin Neurol. 1996;16:269–375. doi: 10.1055/s-2008-1040984. [DOI] [PubMed] [Google Scholar]
- 80.Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36:2756–2763. doi: 10.1161/01.STR.0000190056.76543.eb. [DOI] [PubMed] [Google Scholar]
- 81.Johnson ER, McKenzie SW, Sievers AE. Dysphagia following stroke: quantitative evaluation of pharyngeal transit times. Arch Phys Med Rehabil. 1992;73:419–423. [PubMed] [Google Scholar]
- 82.Ogata T, Kamouchi M, Matsuo R, Hata J, Kuroda J, Ago T, et al. Gastrointestinal bleeding in acute ischemic stroke: recent trends from the fukuoka stroke registry. Cerebrovasc Dis Extra. 2014;4:156–164. doi: 10.1159/000365245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Davenport RJ, Dennis MS, Warlow CP. Gastrointestinal hemorrhage after acute stroke. Stroke. 1996;27:421–424. doi: 10.1161/01.str.27.3.421. [DOI] [PubMed] [Google Scholar]
- 84.Camara-Lemarroy CR, Ibarra-Yruegas BE, Gongora-Rivera F. Gastrointestinal complications after ischemic stroke. J Neurol Sci. 2014;346:20–25. doi: 10.1016/j.jns.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 85.Khedr EM, Abo-Elfetoh N, Rothwell JC. Treatment of post-stroke dysphagia with repetitive transcranial magnetic stimulation. Acta Neurol Scand. 2009;119:155–161. doi: 10.1111/j.1600-0404.2008.01093.x. [DOI] [PubMed] [Google Scholar]
- 86.Kumar S, Wagner CW, Frayne C, Zhu L, Selim M, Feng W, et al. Noninvasive brain stimulation may improve stroke-related dysphagia: a pilot study. Stroke. 2011;42:1035–1040. doi: 10.1161/STROKEAHA.110.602128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang EJ, Baek SR, Shin J, Lim JY, Jang HJ, Kim YK, et al. Effects of transcranial direct current stimulation (tDCS) on post-stroke dysphagia. Restor Neurol Neurosci. 2012;30:303–311. doi: 10.3233/RNN-2012-110213. [DOI] [PubMed] [Google Scholar]
- 88.Shinohara Y, Yanagihara T, Abe K, Yoshimine T, Fujinaka T, Chuma T, et al. Stroke in general. J Stroke Cerebrovasc Dis. 2011;20:S7–S30. doi: 10.1016/j.jstrokecerebrovasdis.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 89.Malloy RJ, Kanaan AO, Silva MA, Donovan JL. Evaluation of antiplatelet agents for secondary prevention of stroke using mixed treatment comparison meta-analysis. Clin Ther. 2013;35:1490–1500. doi: 10.1016/j.clinthera.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 90.Mehdi Z, Birns J, Bhalla A. Post-stroke urinary incontinence. Int J Clin Pract. 2013;67:1128–1137. doi: 10.1111/ijcp.12183. [DOI] [PubMed] [Google Scholar]
- 91.Brittain KR, Peet SM, Castleden CM. Stroke and incontinence. Stroke. 1998;29:524–528. doi: 10.1161/01.str.29.2.524. [DOI] [PubMed] [Google Scholar]
- 92.Kolominsky-Rabas PL, Hilz M-J, Neundoerfer B, Heuschmann PU. Impact of urinary incontinence after stroke: results from a prospective population-based stroke register. Neurourol Urodyn. 2003;22:322–327. doi: 10.1002/nau.10114. [DOI] [PubMed] [Google Scholar]
- 93.Wilson D, Lowe D, Hoffman A, Rudd A, Wagg A. Urinary incontinence in stroke: results from the UK National Sentinel Audits of Stroke 1998–2004. Age Ageing. 2008;37:542–546. doi: 10.1093/ageing/afn134. [DOI] [PubMed] [Google Scholar]
- 94.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9:453–466. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Korpelainen JT, Kauhanen ML, Kemola H, Malinen U, Myllylä VV. Sexual dysfunction in stroke patients. Acta Neurol Scand. 1998;98:400–405. doi: 10.1111/j.1600-0404.1998.tb07321.x. [DOI] [PubMed] [Google Scholar]
- 96.Jung JH, Kam SC, Choi SM, Jae SU, Lee SH, Hyun JS. Sexual dysfunction in male stroke patients: correlation between brain lesions and sexual function. Urology. 2008;71:99–103. doi: 10.1016/j.urology.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 97.Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am. 2005;32:379–395. doi: 10.1016/j.ucl.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Badiger S, Akkasaligar PT, Narone U. Hyperglycemia and stroke. Int J Stroke. 2013;1:1–6. [Google Scholar]
- 99.Christensen H, Boysen G. Blood glucose increases early after stroke onset: a study on serial measurements of blood glucose in acute stroke. Eur J Neurol. 2002;9:297–301. doi: 10.1046/j.1468-1331.2002.00409.x. [DOI] [PubMed] [Google Scholar]
- 100.Järhult J, Falck B, Ingemansson S, Nobin A. The functional importance of sympathetic nerves to the liver and endocrine pancreas. Ann Surg. 1979;189:96–100. doi: 10.1097/00000658-197901000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang YY, Lin SY, Chuang YH, Sheu WHH, Tung KC, Chen CJ. Activation of hepatic inflammatory pathways by catecholamines is associated with hepatic insulin resistance in male ischemic stroke rats. Endocrinology. 2014;155:1235–1246. doi: 10.1210/en.2013-1593. [DOI] [PubMed] [Google Scholar]
- 102.Sarkar RN, Banerjee S, Basu A. Comparative evaluation of diabetic and non-diabetic stroke–effect of glycaemia on outcome. J Indian Med Assoc. 2004;102:551–553. [PubMed] [Google Scholar]
- 103.Szczudlik A, Slowik A, Turaj W, Wyrwicz-Petkow U, Pera J, Dziedzic T, et al. Transient hyperglycemia in ischemic stroke patients. J Neurol Sci. 2001;189:105–111. doi: 10.1016/s0022-510x(01)00566-4. [DOI] [PubMed] [Google Scholar]
- 104.Demchuk AM, Morgenstern LB, Krieger DW, Chi TL, Hu W, Wein TH, et al. Serum glucose level and diabetes predict tissue plasminogen activator–related intracerebral hemorrhage in acute ischemic stroke. Stroke. 1999;30:34–39. doi: 10.1161/01.str.30.1.34. [DOI] [PubMed] [Google Scholar]
- 105.Radermecker RP, Scheen AJ. Management of blood glucose in patients with stroke. Diabetes Metab. 2010;36:S94–S99. doi: 10.1016/S1262-3636(10)70474-2. [DOI] [PubMed] [Google Scholar]
- 106.Cassidy JM, Cramer SC. Spontaneous and therapeutic-induced mechanisms of functional recovery after stroke. Transl Stroke Res. 2017;8:33–46. doi: 10.1007/s12975-016-0467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cheyuo C, Jacob A, Wu R, Zhou M, Coppa GF, Wang P. The parasympathetic nervous system in the quest for stroke therapeutics. J Cereb Blood Flow Metab. 2011;31:1187–1195. doi: 10.1038/jcbfm.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Egawa N, Lok J, Washida K, Arai K. Mechanisms of axonal damage and repair after central nervous system injury. Transl Stroke Res. 2017;8:14–21. doi: 10.1007/s12975-016-0495-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ay I, Sorensen AG, Ay H. Vagus nerve stimulation reduces infarct size in rat focal cerebral ischemia: an unlikely role for cerebral blood flow. Brain Res. 2011;1392:110–115. doi: 10.1016/j.brainres.2011.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Miyamoto O, Pang J, Sumitani K, Negi T, Hayashida Y, Itano T. Mechanisms of the anti-ischemic effect of vagus nerve stimulation in the gerbil hippocampus. Neuroreport. 2003;14:1971–1974. doi: 10.1097/00001756-200310270-00018. [DOI] [PubMed] [Google Scholar]
- 111.Shimamura N, Katagai T, Kakuta K, Matsuda N, Katayama K, Fujiwara N, et al. Rehabilitation and the neural network after stroke. Transl Stroke Res. 2017;8:507–514. doi: 10.1007/s12975-017-0550-6. [DOI] [PubMed] [Google Scholar]