Abstract
The recent development of tools to decipher the intricacies of neural networks has improved our understanding of brain function. Optogenetics allows one to assess the direct outcome of activating a genetically-distinct population of neurons. Neurons are tagged with light-sensitive channels followed by photo-activation with an appropriate wavelength of light to functionally activate or silence them, resulting in quantifiable changes in the periphery. Capturing and manipulating activated neuron ensembles, is a recently-designed technique to permanently label activated neurons responsible for a physiological function and manipulate them. On the other hand, neurons can be transfected with genetically-encoded Ca2+ indicators to capture the interplay between them that modulates autonomic end-points or somatic behavior. These techniques work with millisecond temporal precision. In addition, neurons can be manipulated chronically to simulate physiological aberrations by transfecting designer G-protein-coupled receptors exclusively activated by designer drugs. In this review, we elaborate on the fundamental concepts and applications of these techniques in research.
Keywords: Autonomic regulation, Optogenetics, Calcium sensors, DREADD
Introduction
The pursuit of a solution for the puzzle of complex brain networks started with Cajal and Golgi and continues to this day. The arrangement of axonal and dendritic processes presents an extraordinary challenge for the analysis of how information flows. Electrical stimulation, glutamate uncaging, and high-power laser excitation methods have provided assistance in this endeavor [1, 2]. GFP-tagged retrograde viruses like pseudorabies virus and herpes virus, as well as retrobeads, have led to the identification of neuronal circuitry such as projections from the paraventricular nucleus (PVN) in the midbrain to the spinal cord and then to the periphery, or from the PVN to the rostral ventrolateral medulla (RVLM) or nucleus tractus solitarus in the brainstem [3]. Separately perturbing neuronal activity within nuclei reveal how the brain is compartmentalized to control distinct functions. This has led to the understanding of how nuclei within the brainstem regulate baroreflex sensitivity while the PVN in the hypothalamus is involved in the long-term regulation of blood pressure.
Earlier, to identify downstream changes in autonomic function, a specific brain nucleus would be electrically or chemically stimulated or lesioned. For example, PVN ablation in hypertensive mice can rescue the chronic increase in blood pressure, giving a clue that this nucleus is involved in the maintenance of long-term sympathetic drive [4]. However, the PVN contains a heterogeneous population of neurons and ablation studies do not give information about which types of neurons are involved. Are they excitatory or inhibitory, vasopressinergic or glutamatergic? Retrograde labelling with viruses has helped elucidate neural circuits, but a clear answer regarding their function can be better provided when these neurons are seen in action, in vivo.
Outright electrical ablation of brain regions has provided evidence of how they can be related to a physiological end-point. Ablation of tissue surrounding the anteroventral third ventricle (AV3V) arrests the rise in blood pressure in the one-kidney Grollman model and the Goldblatt model of renal hypertension [5]. However, the tissue encompassing the AV3V contains many nuclei with their respective functions, such as the median pre-optic nucleus (MNPO), sub-fornical organ (SFO), organum vasculosum lamina terminalis (OVLT), and PVN. Ablation of the AV3V provided evidence that this whole region regulates blood pressure. However, identifying the individual function of each nucleus and the stimuli to which they respond requires a more fine-tuned approach. Electrophysiological techniques to patch neurons, both in vivo and in vitro, have enabled deeper insight into brain networks. They have thrown light on circuitry and enabled an understanding of protein interactions that promote or inhibit neuronal excitability. It is possible to identify the functions of two populations of neurons within the same nucleus using patch clamp. For example, a norepinephrine-releasing subpopulation of catecholaminergic neurons in the brainstem responds to the vasoactive peptide Ang-II and is involved in hypertension [6]. These techniques have taken the field forward and enabled the development of newer technology to further explore neural circuits.
Genetic tools have been used to generate conditional neuronal knockouts. This allows specific targeting of neuronal populations within a single heterogeneous nucleus. For example, Agouti-related peptide (AgRP) and pro-opiomelanocortin (POMC) neurons reside in the arcuate nucleus, but can be separately targeted using the Cre-Lox mechanism [7]. The ability to conditionally target a subset of neurons within a nucleus in order to manipulate gene expression has advanced our understanding of several cell-signaling mechanisms, and physiological and behavioral functions. New-age tools such as optogenetics, Capturing and manipulating activated neuronal ensembles (CANE), Ca2+ imaging, and designer receptor exclusively activated by designer drug (DREADD) in combination with older techniques, can manipulate a single neuron and then quantify the resultant end-point physiological function. In this review, we discuss the general principles, delivery strategies, applications in research, and technical challenges of optogenetics, CANE, DREADD, and Ca2+ imaging as well as how they have been used to advance our understanding of brain circuitry.
Optogenetics
Optogenetics is a tool that makes it possible to label and functionally activate or silence specific neurons to modulate behavior or autonomic responses with a high level of control.
General Principles
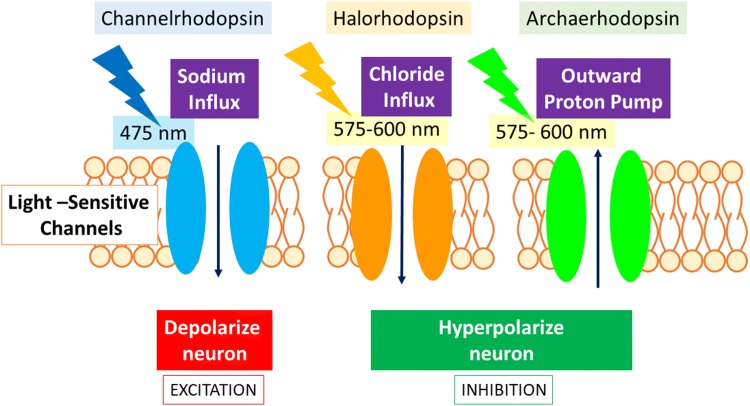
This tool uses light-sensitive ion channels called rhodopsins, isolated from the green algae Chlamydomonas reinhardii. Channelrhodopsin-2 is a light-gated cation channel that opens in response to 475-nm light and allows an influx of sodium ions (Fig. 1). When these channels are transfected into specific neurons, photoactivation by means of an optical fiber depolarizes the neurons [8]. Transfecting neurons with yellow light-sensitive halorhodopsin leads to an influx of chloride ions leading to hyperpolarization [9]. Yellow-green light-sensitive archaerhodopsin activates an outward proton pump [10] which produces hyperpolarizing currents and silences the neuron. These channels respond to light in the range of 575 nm–600 nm.
Fig. 1.
Optogenetics. The excitatory light-gated channel, channelrhodopsin, opens upon excitation with 475-nm light. This allows an influx of sodium ions that depolarizes the neuron. Halorhodopsin and archaerhodopsin silence the neuron by enabling hyperpolarization when they open in response to 575–600-nm light. Upon light activation, halorhodopsin allows chloride influx and archaerhodopsin pumps protons out of the cell, hyperpolarizing the neuron to silence it.
Optogenetic silencing has seen many updates as it involves blocking any depolarization for the entire length of time that the channel is activated. It requires that the neuron can promptly resume its activity as soon as the light is turned off. Archaerhodopsin has the kinetics for such rapid activity.
Delivery Strategies
Light-gated opsins are targeted to specific neurons using a viral construct that uses a neuronal promoter or the Cre-Lox system. Opsins are more often packaged in adeno-associated virus (AAV). The AAV can incorporate limited kilobases of DNA which is sufficient to transfer the specific opsins. Stereotaxically injecting this virus can direct the opsin (driven by a generic neuronal promoter, synapsin) to all neurons in that nucleus. A CamKIIα promoter is used to drive the opsins into glutamatergic neurons, and some studies have shown that a small number of GABAergic neurons are also targeted with this promoter [11]. There are opsin constructs engineered with a double-floxed inverse open-reading frame (DIO) wherein the opsin is inverted in the AAV gene construct. There are two ways of administering the AAV-DIO-opsin to target the neurons of interest. One is to inject AA-DIO-opsin into a transgenic mouse that already expresses Cre-recombinase driven by a specific promoter, and the other is to inject it in combination with an AAV-Cre-promoter to transfect all neurons in that nucleus [12].
Several studies have addressed the effects of incorporating Cre-recombinase into the genome. Does Cre-expression interfere with regular protein expression, does it induce glial cell reactivity in the brain, or does it alter neuronal vulnerability? Extensive data show that Cre-recombinase does not change the neuronal environment, whether it is injected with a virus or constitutively expressed in the genome. In fact, one study used retinal cell structure and function to analyze how Cre-recombinase impacts the neuronal environment. Their model used tamoxifen-induced Cre expression. Using morphometric analysis, immunohistochemical staining, real time-PCR, and angiography they demonstrated that there is no interference by Cre expression [13].
There is more than one way to design experiments to target a neuronal population of interest. Animals with constitutive Cre expression in genetically distinct populations of neurons can be used in several ways. One option is to inject an AAV-DIO-opsin into the nucleus of interest, thus restricting the opsin expression to a specific brain region as well as in a selected population of neurons. Another option is to cross animals with constitutive Cre-recombinase expression with animals that have an opsin knocked-in [14].
Once light-gated opsins are transfected into a neuron, it can be activated by light. Prolonged light exposure and un-inhibited neuronal activity can cause cell damage, along with a heat build-up. Therefore light-pulses of 5 Hz–20 Hz are administered while maintaining a sufficient time-interval between stimulations. The light intensity is carefully optimized to avoid light-induced temperature changes in the tissue [15]
Applications in Research
In an attempt to access deep brain regions and efficiently silence a large volume of neurons, Chuong et al. engineered a red-shifted cruxhalorhodopsin derived from Halobacterium salinarum. This approach was demonstrated in a retinitis pigmentosa mouse model which is characterized by an inability of cone photoreceptor cells to hyperpolarize. Hyperpolarization of the cone photoreceptor is crucial so that it can be reactivated. Transfecting cruxhalorhodopsin channels into cone photoreceptors proved better-suited than other silencing opsins for therapeutic cone reactivation. It achieved greater spike rates in the cone photoreceptors in a more natural frequency range, which could be important for retinal neural coding [16].
Photo-tagging glutamatergic and GABAergic neurons within osmosensitive nuclei such as the MNPO and OVLT made it possible to identify their defining roles in regulating thirst [17]. Turning on glutamatergic neurons with channelrhodopsin and 475-nm light triggers a water-seeking response within milliseconds. Interestingly, water-seeking can be inhibited in dehydrated mice when the GABAergic neurons are optogenetically activated. The rapid relay of information from the brain resulting in a behavioral outcome can be studied because optogenetics can keep pace with the in vivo mechanism.
Injecting an opsin into one nucleus can change the downstream functioning of a connected nucleus. For example, activating catecholaminergic neurons in the RVLM leads to the activation of noradrenergic neurons in the locus coeruleus [18]. In fact, Burke et al. also showed that activating these neurons increases blood pressure in quietly resting rats, confirming the RVLM-locus coeruleus circuit [19].
Technical Challenges
The greatest advantage of this technique is its high spatio-temporal resolution and the ability to selectively activate specific neurons, providing evidence to identify neuronal circuits and their functions in freely-moving animals.
Characteristics that define light-gated opsins are conductance, ion selectivity, kinetics, and desensitization kinetics [20]. The speed with which opsins respond to light-induced activation is controlled by the conductance of the channel. For channelrhodopsin, at 0 mV the channel reaches the reversal potential and there is minimum leak current. The challenge with optogenetics is implanting an optical fiber that must be placed close to the nucleus of interest, with the potential risk of displacing or damaging brain tissue. Prolonged light stimulation can also produce heat damage to cells leading to cell death. Efforts to engineer variants of channelrhodopsin have improved the light sensitivity but slowed the kinetics [21]. A red-shifted variant of channelrhodopsin called ReaChR attempts to eliminate the invasive surgery of lowering an optical fiber into the brain tissue [22]. It allows transcranial stimulation of channelrhodopsin using light in the red to far-red range (600 nm–655 nm). It has proven successful in the motor cortex. However, whether it will be able to stimulate opsins located in deeper brain regions that control autonomic functions is yet to be determined.
CANE
Raising the stakes on optogenetics requires a technique that can identify and capture neurons that functionally respond to a given stimulus in vivo and then photo-tag these neurons. Activating these neurons at a later date, under a neutral circumstance, should elicit the same peripheral or behavioral response, even when the real stimulus is absent. The first step to such a goal is to identify a marker to label activated neurons. cFos, early growth response-1 (Egr1), and activity-regulated cytoskeleton-associated protein (Arc) are expressed by immediate-early genes and are markers of neuronal activation. The cFos expression level reaches a peak within a couple of hours and can be visualized with immunohistochemistry. c-Fos and delta FosB proteins are markers of acute and long-term neuronal activation, respectively [23]. In neurons, cFos is expressed when a build-up of cAMP and Ca2+ activates the CREB/CRE complex. Fos proteins dimerize with transcription factors of the Jun family. This build-up activates another transcription factor called activator protein-1 [24]. The c-Fos promoter light-gated channels can be incorporated into activated neurons. Therefore, in the absence of the original stimulus, shining light on these neurons will still lead to a peripheral response.
This system of capturing activated neurons has seen some refinement. As cFos expression is transient and lost within 6 h, the first challenge was to permanently tag the fos-expressing activated neurons, so that they could be identified on a future date. Consequently, when the same stimulus is presented again, the same ensemble of neurons should be re-activated and their cFos expression is expected to overlap with the previous exposure. This was achieved with a TetTag mouse that combines elements of tetracycline-transactivator (tTA) with neuronal activity-induced activation [25]. In recent years, this system helped identify how a specific memory is encoded by a select population of neurons in the hippocampus [26]. The same neurons light up when a specific memory is revisited in order to recall it. As a result, light-activating those neurons in the absence of a fear stimulus could still instill a fear phenotype, which can be quantified by recording the animal’s freezing response.
For the tetracycline-controlled transcriptional activation to work, the mouse must feed on doxycycline to inhibit cFos-driven tTA expression. Therefore, channelrhodopsin fails to express in activated neurons expressing cFos. To tag neurons that are responsible for making a new memory with channelrhodopsin, tTA must be expressed in the activated neurons that endogenously express cFos. This is possible when the mouse is taken off the doxycycline diet. The withdrawal of doxycycline enables the persistent labelling of activated neurons. cFos expression kinetics involve a peak in expression at 2 h and complete loss by 6 h [23]. However, to allow doxycycline to work, it must be absent from the mouse diet for at least 1–2 days. This allows large amounts of non-specifically activated neurons to express channelrhodopsin. This increases the background and diminishes the specificity of studying the neurons that are responsible for a given behavior. Therefore, a technique that can reduce this time-window so that fewer non-specific neurons are tagged will enhance our ability to identify neurons that are responsible for peripheral physiological changes. This is of great relevance when studying autonomic regulation, as the brain-to-periphery changes are very rapid.
General Principle
CANE is a variation of the cFos-tTa method with additional features. This system uses a knock-in mouse in which neuronal activity-induced Fos expression triggers the co-expression of a destabilized avian receptor (TVA). The TVA receptor is expressed on the neuronal cell membrane for the length of time that cFos expression lasts and thus peaks at the 2-h time point.
Delivery Strategy
TVA is an exceptionally specific receptor for the envelope protein of avian sarcoma and leucosis virus (EnvA). The EnvA-coated virus must be stereotaxically injected within 2 h of an external stimulus, so that it binds to c-Fos-induced TVA receptors. Once the EnvA-coated virus binds to these receptors, it can transfect the neuron with the gene packaged within it. The transfected gene can be a GFP, light-gated opsin, DREADD receptor, Ca2+ sensor, or any other gene of interest. Moreover, the virus coated with EnvA can be a lentivirus or a monosynaptic rabies virus. This feature allows the mapping of inputs received by activated neurons, thus also mapping their projections.
Therefore, CANE enables the persistent and efficient capture of c-Fos-positive neurons when presented with a sensory or autonomic stimulus. It allows the transfection of any gene of interest (e.g., GFP or Cre) within those activated neurons that had responded to the initial stimulus. It also provides for monosynaptic mapping, as the gene of interest can be packaged in a monosynaptic rabies virus and then injected into the brain [27].
Applications in Research
In a classic resident/intruder mouse paradigm, it is possible to distinguish aggressor-specific neurons from social fear neurons with CANE. Both populations of neurons reside within the heterogeneous hypothalamic nucleus. Interestingly, targeting channelrhodopsin in the subset of social fear neurons and shining 475-nm light on them can switch the aggressive response in the resident aggressor mouse into a fear response. Therefore, the aggressor mouse ceases attacking the intruder mouse, moves to the corner of the cage, and freezes [27]. This activity-dependent technology has also led to the identification of nociceptive neurons in the parabrachial nucleus and their connections with the affective pain system [28]. Potentially, this system should be able to capture neurons involved in the autonomic regulation of cardiovascular system and other autonomic processes with greater consistency than somatic processes. This will allow further focused and specific gene modifications to answer questions regarding autonomic regulation and drug targeting.
Technical Challenges
This method requires high precision while stereotaxically injecting the EnvA virus into a nucleus of interest. Moreover, unnecessary neuronal activation leading to cFos expression must be prevented. Certain autonomic and behavioral functions are sensitive to anesthesia and can result in non-specific neuronal labelling.
GFP-Based Genetically Encoded Ca2+ Indicators (GECI)
GECI measures Ca2+ changes within a cell. When a neuron is activated, there is an influx of Ca2+ ions. GECI is a means of imaging neuronal activation within an animal in real time. This is achieved by imaging an increase in intracellular Ca2+in vivo. This technique has been updated from its native form of GCaMP2 to GCaMP8, with improvements in detecting rapid changes in intracellular Ca2+ [29, 30]. GCaMP6–8 have proven to be the most effective indicators of actively firing neurons. Using electrophysiology, several studies have confirmed that GECI faithfully reports Ca2+ surges while a neuron is firing [7, 31].
General Principle
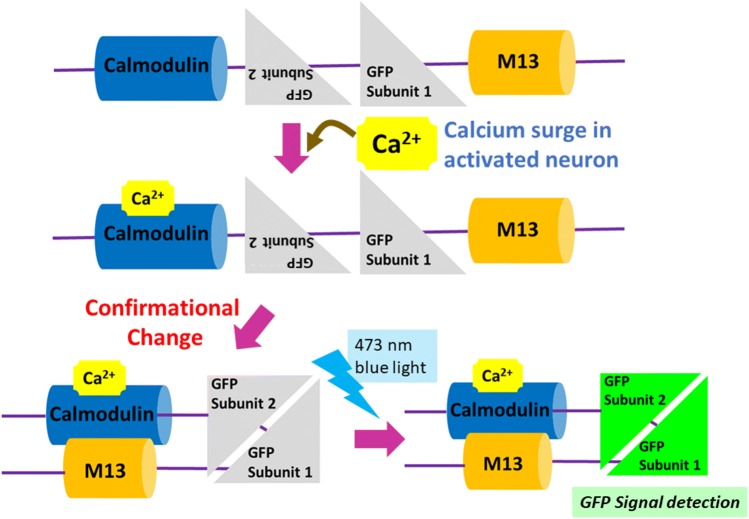
The GECI gene construct translates to a protein that consists of a Ca2+-responsive calmodulin, two green fluorescent protein (GFP) subunits, and a calmodulin binding peptide, M13, which is a component of myosin light chain kinase, in that order. The two GFP subunits are purposely in the wrong order. In this state, they do not fluoresce in response to blue 473-nm light. When Ca2+ surges in the neuron and binds to calmodulin, the calmodulin binds to M13, leading to a conformational change. As illustrated in Fig. 2, the new conformation allows the two GFP subunits to be oriented such that they appropriately dimerize and therefore become functional. Now, shining blue light at 473 nm leads to a GFP signal only where Ca2+ has bound to calmodulin—i.e. in an actively firing neuron [32]—therefore revealing neurons that are activated in response to physiological change.
Fig. 2.
Calcium imaging with GFP-based genetically encoded calcium indicators. Neuronal activation leads to an increase in intracellular Ca2+ which binds to calmodulin. Ca2+-calmodulin binds to M13, therefore re-orienting GFP subunits in the correct order. Now the GFP is detectable upon excitation with 473-nm light, indicating an activation-associated Ca2+ surge.
Delivery Strategy
An AAV-GCaMP cassette can be injected into any nucleus of interest. The GCaMP gene cassette is transfected into the neurons, driven by a specific neuronal promoter or cre-recombinase in the animal. At the time of virus injection, a fiber photometry system is also placed in the same region. An optical fiber connects to a photometry device to capture the fluorescence emitted by GECI. GECI has evolved from being a GFP-detectable system to one that can detect blue, red, and yellow fluorescent proteins [33]. It enables simultaneous 2-color imaging, for example, of neurons and astrocytes [34].
Applications in Research
Real-time activity of feedback neural networks can be recorded with GECI. The hypothalamus has been studied using GCamp6 (GECI) to understand hunger, thirst, and autonomic responses. The hypothalamus contains several populations of neurons that are genetically distinct therefore can be separately targeted. For example, injecting the hunger hormone Ghrelin activates AgRP and inhibits POMC neurons [7]. However, presenting a food pellet to a fasted mouse has an opposite response, mainly because the fasted animal is transitioning from hunger to satiety. It lowers the activity of AgRP neurons, while in POMC neurons it induces heightened firing. Therefore, detecting the precise time of neuronal activation provides clear evidence of what the neurons are reacting to, whether it is hunger, food presentation, food consumption, or satiety. It is also possible to study how neurons have varied responses based upon the caloric quality of food (e.g., peanut butter versus chow). In mice, AgRP and POMC neurons have a quantitatively heightened response to peanut butter compared to regular chow. This intricate neuronal relationship can be recorded because of GECI [7]. GECI has led to understanding the temporal pattern of neuronal firing to control behavior. To understand if it is to rescue a homeostatic aberration or it is in anticipation of a homeostatic aberration, a brilliant example comes from the thirst neurons located in the SFO. Upon food intake, blood tonicity is disturbed, which can be countered by drinking water. Thirst neurons in the SFO start firing to initiate drinking even before blood tonicity is disturbed by food intake. These neurons can predict that a need for excess water will arise in the future, so blood tonicity can be maintained. Moreover, with GECI it is possible to visualize the neuronal firing profile in real time: as the mouse responds with a drinking response, as soon as it makes the first lick of water, there is a rapid reduction in neuronal firing [31].
Technical Challenges
GECI has evolved from GCamp2 to GCaMP6–8, where the temporal resolution is remarkable. It can detect changes in neuronal firing within a specific nucleus in freely-moving and behaving mice. On the other hand, it cannot provide a snapshot of whole-brain function. It cannot answer questions that involve anything beyond the area in question.
DREADD
DREADD is a chemogenetic tool that includes an excitatory or inhibitory G-protein-coupled receptor that can be activated by an exogenous ligand or drug. Theoretically, this ligand is pharmacologically inert and can only bind to the designer receptor. Therefore, the DREADD receptor cannot be activated by acetylcholine or any other endogenous neurotransmitter. It is a ‘remote-control’ technology to chronically activate or silence a neuronal circuit, leading to sustainable and quantifiable changes in physiology.
General Principle
hM3Dq is a Gq protein-coupled receptor that can be activated by clozapine-n-oxide (CNO) (Fig. 3). CNO-activated hM3Dq leads to Ca2+ influx into the cell. When hM3Dq is transfected into a neuron, CNO-induced activation leads to its depolarization [35]. On the other hand, when a Gi-DREADD (hM4Di) is transfected into a neuron, it causes chemogenetic inactivation. hM4Di harnesses the G-protein-coupled inwardly-rectifying K+ channel response. Upon activation with CNO, the neuron hyperpolarizes and is therefore silenced [36].
Fig. 3.
DREADD: Designer receptor engineered for designer drugs. Receptors that mimic Gq protein-coupled (excitatory in neurons) and Gi protein-coupled (inhibitory in neurons) receptors can be selectively activated by the designer drug clozapine-n-oxide (CNO), leading to chronic activation or deactivation of a selected population of neurons.
Delivery Strategies
DREADD receptors can be transfected into neurons of interest using AAV with expression of the receptor controlled by specific promoters. Cre-recombinase-driven expression of DREADD can target a nucleus of interest. Therefore, depending on the promoter and site of injection within the brain, DREADD can be used to target a specific cell-type, a specific neuronal projection, or a specific nucleus within the brain. As both the receptor and drug are exogenous entities, their function cannot be interrupted by endogenous processes.
Application
Since its discovery, DREADD has become a useful tool with which to study neural control of autonomic regulation and behavior. For example, when excitatory hM3Dq DREADD receptors are transfected into oxytocin neurons in the paraventricular nucleus using a Cre-recombinase driver, they can rescue the hypertension caused by chronic intermittent hypoxia-hypercapnia [37]. Chronic activation (21 days) of this selected population of neurons with CNO attenuates the development of hypertension, indicating a crucial role of these neurons in regulating sympathetic output and blood pressure. Shi et al. described how neuropeptide Y decreases sympathetic activity by activating inhibitory neurons within the arcuate nucleus with hM3Dq and CNO [38]. They also provided evidence of a circuit effect by injecting a neuropeptide Y receptor antagonist, not into the arcuate nucleus but into a downstream nucleus (dorsomedial hypothalamus), to reverse this effect. Interestingly, activating dopaminergic neurons in the midbrain using CNO and hM3Dq regulates motor activity in a dose-dependent manner [39].
Technical Challenges
As DREADD receptor technology is becoming frequently used, an important shortcoming with the designer drug CNO has been revealed. In vivo, CNO cannot cross the blood-brain barrier until it is metabolized to clozapine. Moreover, when tested in vivo, clozapine has been found to have a higher affinity for the designer receptor than the designer drug, CNO. As clozapine itself is an FDA-approved antipsychotic, it must act on its target in the brain. It has been found that directly administering clozapine also activates DREADD receptors. To eliminate a possible outcome due to the anti-psychotic agent, it is crucial to control every DREADD experiment with a group that receives CNO without the receptor transfection [40].
Summary
Traditional techniques for functional imaging of the brain with fMRI or PET scans lack precision in interpreting data because they cannot identify or characterize the neurons involved. Recent advances allow the labelling of a neuron, control of its activity, and therefore manipulation of the end-point physiology. We can address which neurons are affected by hunger and to what extent. If the appropriate population of neurons is manipulated, it is possible to stop a dehydrated mouse from drinking by inducing a false feeling of satiety.
Photo-labelling allows the acute manipulation of specific cohorts of neurons, while chemo-genetic tools enable long-term manipulation that can be stretched out for days. Genetically-encoded Ca2+-imaging can visualize the extent to which neurons respond to a stimulus with high temporal accuracy. Each of these techniques involves stereotaxic surgery and relies on viral transfection to capture a select population of neurons. Table 1 summarizes the advantages and limitations of these techniques and lists some published applications for the study of the autonomic system. To fine-tune manipulation, one must correctly identify the genetic promoters that target only those neurons within a nucleus that are of interest. Each of these steps can be controlled and cross-checked to confirm the accuracy of the experiment. These techniques involve complex hardware to stimulate, quantify, and image the outcome.
Table 1.
Optogenetics, CANE, GECI, and DREADDs.
| Method | Advantages | Limitations | Application in autonomic system |
|---|---|---|---|
| Optogenetics | Simulates neuronal function in vivo by depolarizing or hyperpolarizing neurons with pulses of light Targets specific nuclei by harnessing genetic difference using the Cre-Lox system and stereotaxic injection Manipulates neurons in vivo and in vitro High spatio-temporal resolution in vivo |
Involves lowering an optical fiber close to the nucleus of interest at the risk of displacing brain tissue Restricted to acute manipulation; chronic manipulation is not possible Prolonged light-pulses into the brain tissue can cause cell damage Involves 5–6-week wait for light-gated opsins to be expressed on the cell membrane |
Photo-activating catecholaminergic neurons in the RVLM of mice with channelrhodopsin increases blood pressure and has adverse autonomic consequences leading to sleep apnea [19] Archaerhodopsin-induced photo-inhibition of left stellate ganglion in dogs using a wireless LED suppresses nerve activity, thus suppressing cardiac ventricular arrhythmias [41] Photo-activating neurons in the locus ceruleus with channelrhodopsin inhibits parasympathetic transmission to cardiac vagal neurons in the brainstem, leading to tachycardia [42] |
| CANE | Labels causative neurons activated by an autonomic function Compared to other techniques, this method has the lowest non-specific labelling Permanently transfects any gene of interest into neurons, like channelrhodopsin, cre-recombinase, DREADD, GCaMP, and GFP Trans-synaptically labels input circuits using pseudorabies virus to package EnvA and gene of interest High spatio-temporal resolution |
Involves time-bound stereotaxic injection to ensure EnvA virus labels associated neurons Some autonomic and behavioral processes are sensitive to pre-surgery anesthesia which leads to cFos activation in neurons, causing non-specific labelling Must wait for weeks before the gene of interest is expressed in neurons |
Can be used to identify neurons responsible for treatment-induced autonomic activity. This information can be harnessed to optogenetically activate neural pathways. It can also be used to map input circuits and knockout genes of interest |
| GECI | Visualizes neuronal activity that regulates or responds to physiological changes in vivo Allows simultaneous 2-color imaging of neurons and astrocytes It is possible to quantitate neuronal activity detected by the system GECI expression is long-lasting, allowing more than one experimental session with a transfected mouse GECI sensors can be expressed in vivo using transgenic mice, thus avoiding stereotaxic injection of virus and confining its expression to the area of interest High spatio-temporal resolution |
Involves invasive stereotaxic surgery to install the detecting probe The GECI must be selected carefully in order to address a hypothesis correctly Can only provide information on neuronal activation in a selected nucleus but not the whole brain |
GCaMP3 sensors indicate how vagal sensory neurons respond to enteric mechanoreceptors and chemoreceptors [43] GCaMP6 sensors expressed in heat-sensitive neurons in the ventromedial preoptic area detect changes in activity in response to temperature which results in an autonomic response [44] GCaMP3 sensors indicate that somatostatin GABAergic neurons in the dorsal motor nucleus of the vagus regulate parasympathetic gastric activity [45] |
| DREADD | Allows chronic long-term control of neurons Persistent neuronal manipulation is possible without damaging the cell. Activated exclusively by intraperitoneal injection of the designer drug, clozapine-n-oxide Transgenic mice that express DREADD receptors using the Cre-Lox mechanism eliminate the need for invasive stereotaxic surgery |
CNO rapidly metabolizes to clozapine, an FDA-approved antipsychotic Clozapine binds to the designer receptor with greater affinity than CNO Therefore, experiments must be designed with appropriate controls, to eliminate any interference caused by clozapine itself |
Activating excitatory hM3Dq DREADD in oxytocinergic neurons in the PVN rescues chronic intermittent hypoxia-hypercapnia-induced hypertension [37] Using hM3Dq DREADD to activate inhibitory neurons in the arcuate nucleus lowers sympathetic activity [38] hM3Dq DREADD-induced activation of astrocytes in mice leads to autonomic changes such as increases in heart rate, blood pressure, and saliva formation [46] |
Wireless optogenetic tools protect post-surgery ferrules and skull caps from possible detachment. Importantly, they allow remote operation through LEDs to turn on or off neurons without connecting wires and fibers. The process of handling an animal to attach optical fibers to the skull cap causes stress and interference. Micro-LEDs are an upgrade to achieve wireless optogenetic manipulation [47].
Optogenetics and Ca2+ sensors are being used to study cells other than neurons, in the periphery. The Ca2+ sensor GCaMP3 has been transfected into urethral smooth muscle cells to characterize the Ca2+ signaling [48]. Based on the principle of Ca2+ sensors like GCaMPs, ANEPPS are voltage-sensitive dyes designed to respond to changes in membrane potential [49]. Like GCaMPs, there is a range of these dyes that target various cells other than neurons with increasing sensitivity. Changes in membrane potential indicate changes in nerve-impulse propagation, muscle contraction, and cell-signaling. Di-4-ANEPPS has been used to study human cardiomyocytes derived from induced pluripotent stem-cells, to determine how these cells respond to a range of drug treatments [50].
Channelrhodopsin-2 has been transfected into cells other than neurons, like muscle tissue, to mimic continuous training with optical stimulation. This has enabled understanding of the vascularization of muscle fiber bundles [51]. Optogenetic stimulation of skeletal muscles has also been attempted as a possible treatment for paralysis [52].
Evolution of the technology to deliver specialized opsins or DREADD receptors into the human brain could change the way we treat human disorders. DREADD receptors are human mutants of muscarinic receptors that can be activated by CNO [36]. CNO is a derivative of clozapine, which is already an FDA-approved drug. It is well-tolerated at low doses that are enough to activate the designer receptors. Given that DREADD receptors mimic G-protein-coupled receptors and are prevalent in almost every cell, they can be of clinical benefit. If a viable delivery strategy for the human brain is found, then specific neuronal circuits can be targeted and silenced or activated by clozapine or its derivatives.
Now more than ever, we have a clear picture of the neurons and circuits involved in models of disease like Parkinson’s as well as those involved in autonomic regulation, and this provides a strong pre-clinical arsenal. It is possible to inject a single nucleus and map its input circuits and projections, identify activated neurons, and functionally manipulate them, thereby manipulating the gross physiology. It is possible to change the course of disease in animal models by functionally activating or silencing a subset of neurons.
Acknowledgements
This review was supported by grants from the National Institutes of Health (HL093178 to EL and CoBRE P30 GM106392) and Louisiana State University Health Sciences Research Enhancement Program.
Conflict of interest
The authors report no conflict of interest.
References
- 1.Katz LC, Dalva MB. Scanning laser photostimulation: a new approach for analyzing brain circuits. J Neurosci Methods. 1994;54:205–218. doi: 10.1016/0165-0270(94)90194-5. [DOI] [PubMed] [Google Scholar]
- 2.Denk W, Delaney KR, Gelperin A, Kleinfeld D, Strowbridge BW, Tank DW, et al. Anatomical and functional imaging of neurons using 2-photon laser scanning microscopy. J Neurosci Methods. 1994;54:151–162. doi: 10.1016/0165-0270(94)90189-9. [DOI] [PubMed] [Google Scholar]
- 3.Duplan SM, Boucher F, Alexandrov L, Michaud JL. Impact of Sim1 gene dosage on the development of the paraventricular and supraoptic nuclei of the hypothalamus. Eur J Neurosci. 2009;30:2239–2249. doi: 10.1111/j.1460-9568.2009.07028.x. [DOI] [PubMed] [Google Scholar]
- 4.Herzig TC, Buchholz RA, Haywood JR. Effects of paraventricular nucleus lesions on chronic renal hypertension. Am J Physiol. 1991;261:H860–867. doi: 10.1152/ajpheart.1991.261.3.H860. [DOI] [PubMed] [Google Scholar]
- 5.Buggy J, Fink GD, Haywood JR, Johnson AK, Brody MJ. Interruption of the maintenance phase of established hypertension by ablation of the anteroventral third ventricle (AV3V) in rats. Clin Exp Hypertens. 1978;1:337–353. doi: 10.3109/10641967809068612. [DOI] [PubMed] [Google Scholar]
- 6.Teschemacher AG, Wang S, Raizada MK, Paton JF, Kasparov S. Area-specific differences in transmitter release in central catecholaminergic neurons of spontaneously hypertensive rats. Hypertension. 2008;52:351–358. doi: 10.1161/HYPERTENSIONAHA.108.114371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Lin YC, Kuo TW, Knight ZA. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell. 2015;160:829–841. doi: 10.1016/j.cell.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 9.Zhao S, Cunha C, Zhang F, Liu Q, Gloss B, Deisseroth K, et al. Improved expression of halorhodopsin for light-induced silencing of neuronal activity. Brain Cell Biol. 2008;36:141–154. doi: 10.1007/s11068-008-9034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ihara K, Umemura T, Katagiri I, Kitajima-Ihara T, Sugiyama Y, Kimura Y, et al. Evolution of the archaeal rhodopsins: evolution rate changes by gene duplication and functional differentiation. J Mol Biol. 1999;285:163–174. doi: 10.1006/jmbi.1998.2286. [DOI] [PubMed] [Google Scholar]
- 11.Wenker IC, Abe C, Viar KE, Stornetta DS, Stornetta RL, Guyenet PG. Blood pressure regulation by the rostral ventrolateral medulla in conscious rats: effects of hypoxia, hypercapnia, baroreceptor denervation, and anesthesia. J Neurosci. 2017;37:4565–4583. doi: 10.1523/JNEUROSCI.3922-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boneva SK, Gross TR, Schlecht A, Schmitt SI, Sippl C, Jagle H, et al. Cre recombinase expression or topical tamoxifen treatment do not affect retinal structure and function, neuronal vulnerability or glial reactivity in the mouse eye. Neuroscience. 2016;325:188–201. doi: 10.1016/j.neuroscience.2016.03.050. [DOI] [PubMed] [Google Scholar]
- 14.Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci. 2012;15:793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin Y, Yoo M, Kim HS, Nam SK, Kim HI, Lee SK, et al. Characterization of fiber-optic light delivery and light-induced temperature changes in a rodent brain for precise optogenetic neuromodulation. Biomed Opt Express. 2016;7:4450–4471. doi: 10.1364/BOE.7.004450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chuong AS, Miri ML, Busskamp V, Matthews GA, Acker LC, Sorensen AT, et al. Noninvasive optical inhibition with a red-shifted microbial rhodopsin. Nat Neurosci. 2014;17:1123–1129. doi: 10.1038/nn.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abbott SB, Machado NL, Geerling JC, Saper CB. Reciprocal control of drinking behavior by median preoptic neurons in mice. J Neurosci. 2016;36:8228–8237. doi: 10.1523/JNEUROSCI.1244-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holloway BB, Stornetta RL, Bochorishvili G, Erisir A, Viar KE, Guyenet PG. Monosynaptic glutamatergic activation of locus coeruleus and other lower brainstem noradrenergic neurons by the C1 cells in mice. J Neurosci. 2013;33:18792–18805. doi: 10.1523/JNEUROSCI.2916-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burke PG, Abbott SB, Coates MB, Viar KE, Stornetta RL, Guyenet PG. Optogenetic stimulation of adrenergic C1 neurons causes sleep state-dependent cardiorespiratory stimulation and arousal with sighs in rats. Am J Respir Crit Care Med. 2014;190:1301–1310. doi: 10.1164/rccm.201407-1262OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Q, Zeng Z, Hu Z. Optogenetics in neuroscience: what we gain from studies in mammals. Neurosci Bull. 2012;28:423–434. doi: 10.1007/s12264-012-1250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin JY. A user’s guide to channelrhodopsin variants: features, limitations and future developments. Exp Physiol. 2011;96:19–25. doi: 10.1113/expphysiol.2009.051961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin JY, Knutsen PM, Muller A, Kleinfeld D, Tsien RY. ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat Neurosci. 2013;16:1499–1508. doi: 10.1038/nn.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ. DeltaFosB: a molecular switch for long-term adaptation in the brain. Brain Res Mol Brain Res. 2004;132:146–154. doi: 10.1016/j.molbrainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Chiu R, Boyle WJ, Meek J, Smeal T, Hunter T, Karin M. The c-Fos protein interacts with c-Jun/AP-1 to stimulate transcription of AP-1 responsive genes. Cell. 1988;54:541–552. doi: 10.1016/0092-8674(88)90076-1. [DOI] [PubMed] [Google Scholar]
- 25.Reijmers LG, Perkins BL, Matsuo N, Mayford M. Localization of a stable neural correlate of associative memory. Science. 2007;317:1230–1233. doi: 10.1126/science.1143839. [DOI] [PubMed] [Google Scholar]
- 26.Ramirez S, Liu X, Lin PA, Suh J, Pignatelli M, Redondo RL, et al. Creating a false memory in the hippocampus. Science. 2013;341:387–391. doi: 10.1126/science.1239073. [DOI] [PubMed] [Google Scholar]
- 27.Sakurai K, Zhao S, Takatoh J, Rodriguez E, Lu J, Leavitt AD, et al. Capturing and manipulating activated neuronal ensembles with CANE delineates a hypothalamic social-fear circuit. Neuron. 2016;9:739–753. doi: 10.1016/j.neuron.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez E, Sakurai K, Xu J, Chen Y, Toda K, Zhao S, et al. A craniofacial-specific monosynaptic circuit enables heightened affective pain. Nat Neurosci. 2017;20:1734–1743. doi: 10.1038/s41593-017-0012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohkura M, Sasaki T, Sadakari J, Gengyo-Ando K, Kagawa-Nagamura Y, Kobayashi C, et al. Genetically encoded green fluorescent Ca2+ indicators with improved detectability for neuronal Ca2+ signals. PLoS One. 2012;7:e51286. doi: 10.1371/journal.pone.0051286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nat Biotechnol. 2001;19:137–141. doi: 10.1038/84397. [DOI] [PubMed] [Google Scholar]
- 31.Zimmerman CA, Lin YC, Leib DE, Guo L, Huey EL, Daly GE, et al. Thirst neurons anticipate the homeostatic consequences of eating and drinking. Nature. 2016;537:680–684. doi: 10.1038/nature18950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmer AE, Tsien RY. Measuring calcium signaling using genetically targetable fluorescent indicators. Nat Protoc. 2006;1:1057–1065. doi: 10.1038/nprot.2006.172. [DOI] [PubMed] [Google Scholar]
- 33.Zhao Y, Araki S, Wu J, Teramoto T, Chang YF, Nakano M, et al. An expanded palette of genetically encoded Ca(2)(+) indicators. Science. 2011;333:1888–1891. doi: 10.1126/science.1208592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akerboom J, Carreras Calderon N, Tian L, Wabnig S, Prigge M, Tolo J, et al. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front Mol Neurosci. 2013;6:2. doi: 10.3389/fnmol.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jameson H, Bateman R, Byrne P, Dyavanapalli J, Wang X, Jain V, et al. Oxytocin neuron activation prevents hypertension that occurs with chronic intermittent hypoxia/hypercapnia in rats. Am J Physiol Heart Circ Physiol. 2016;310:H1549–1557. doi: 10.1152/ajpheart.00808.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi Z, Madden CJ, Brooks VL. Arcuate neuropeptide Y inhibits sympathetic nerve activity via multiple neuropathways. J Clin Invest. 2017;127:2868–2880. doi: 10.1172/JCI92008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang S, Tan Y, Zhang JE, Luo M. Pharmacogenetic activation of midbrain dopaminergic neurons induces hyperactivity. Neurosci Bull. 2013;29:517–524. doi: 10.1007/s12264-013-1327-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, et al. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science. 2017;357:503–507. doi: 10.1126/science.aan2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu L, Zhou L, Cao G, Po SS, Huang B, Zhou X, et al. Optogenetic modulation of cardiac sympathetic nerve activity to prevent ventricular arrhythmias. J Am Coll Cardiol. 2017;70:2778–2790. doi: 10.1016/j.jacc.2017.09.1107. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Pinol RA, Byrne P, Mendelowitz D. Optogenetic stimulation of locus ceruleus neurons augments inhibitory transmission to parasympathetic cardiac vagal neurons via activation of brainstem alpha1 and beta1 receptors. J Neurosci. 2014;34:6182–6189. doi: 10.1523/JNEUROSCI.5093-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, Liberles SD. Sensory neurons that detect stretch and nutrients in the digestive system. Cell. 2016;166:209–221. doi: 10.1016/j.cell.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan CL, Cooke EK, Leib DE, Lin YC, Daly GE, Zimmerman CA, et al. Warm-sensitive neurons that control body temperature. Cell. 2016;167(47–59):e15. doi: 10.1016/j.cell.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewin AE, Vicini S, Richardson J, Dretchen KL, Gillis RA, Sahibzada N. Optogenetic and pharmacological evidence that somatostatin-GABA neurons are important regulators of parasympathetic outflow to the stomach. J Physiol. 2016;594:2661–2679. doi: 10.1113/JP272069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agulhon C, Boyt KM, Xie AX, Friocourt F, Roth BL, McCarthy KD. Modulation of the autonomic nervous system and behaviour by acute glial cell Gq protein-coupled receptor activation in vivo. J Physiol. 2013;591:5599–5609. doi: 10.1113/jphysiol.2013.261289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu F, Stark E, Ku PC, Wise KD, Buzsaki G, Yoon E. Monolithically integrated muLEDs on silicon neural probes for high-resolution optogenetic studies in behaving animals. Neuron. 2015;88:1136–1148. doi: 10.1016/j.neuron.2015.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drumm BT, Rembetski BE, Cobine CA, Baker SA, Sergeant GP, Hollywood MA, et al. Ca(2+) signalling in mouse urethral smooth muscle in situ: role of Ca(2+) stores and Ca(2+) influx mechanisms. J Physiol. 2018;596:1433–1466. doi: 10.1113/JP275719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pucihar G, Kotnik T, Miklavcic D. Measuring the induced membrane voltage with Di-8-ANEPPS. J Vis Exp 2009. [DOI] [PMC free article] [PubMed]
- 50.Hortigon-Vinagre MP, Zamora V, Burton FL, Green J, Gintant GA, Smith GL. The use of ratiometric fluorescence measurements of the voltage sensitive dye Di-4-ANEPPS to examine action potential characteristics and drug effects on human induced pluripotent stem cell-derived cardiomyocytes. Toxicol Sci. 2016;154:320–331. doi: 10.1093/toxsci/kfw171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Osaki T, Sivathanu V, Kamm RD. Crosstalk between developing vasculature and optogenetically engineered skeletal muscle improves muscle contraction and angiogenesis. Biomaterials. 2018;156:65–76. doi: 10.1016/j.biomaterials.2017.11.041. [DOI] [PubMed] [Google Scholar]
- 52.van Bremen T, Send T, Sasse P, Bruegmann T. Spot light on skeletal muscles: optogenetic stimulation to understand and restore skeletal muscle function. J Muscle Res Cell Motil. 2017;38:331–337. doi: 10.1007/s10974-017-9481-9. [DOI] [PubMed] [Google Scholar]