Abstract
The hypothalamic paraventricular nucleus (PVN) is a crucial region involved in maintaining homeostasis through the regulation of cardiovascular, neuroendocrine, and other functions. The PVN provides a dominant source of excitatory drive to the sympathetic outflow through innervation of the brainstem and spinal cord in hypertension. We discuss current findings on the role of the PVN in the regulation of sympathetic output in both normotensive and hypertensive conditions. The PVN seems to play a major role in generating the elevated sympathetic vasomotor activity that is characteristic of multiple forms of hypertension, including primary hypertension in humans. Recent studies in the spontaneously hypertensive rat model have revealed an imbalance of inhibitory and excitatory synaptic inputs to PVN pre-sympathetic neurons as indicated by impaired inhibitory and enhanced excitatory synaptic inputs in hypertension. This imbalance of inhibitory and excitatory synaptic inputs in the PVN forms the basis for elevated sympathetic outflow in hypertension. In this review, we discuss the disruption of balance between glutamatergic and GABAergic inputs and the associated cellular and molecular alterations as mechanisms underlying the hyperactivity of PVN pre-sympathetic neurons in hypertension.
Keywords: Hypothalamus, Paraventricular nucleus, Synaptic plasticity, Essential hypertension, Sympathetic nervous system
Introduction
The hypothalamic paraventricular nucleus (PVN) is a heterogeneous nucleus comprising different types of neurons controlling neuroendocrine and autonomic functions. These neurons play important roles in integrating hormone release and neuroendocrine regulation [1, 2], and sympathetic drive under pathological conditions such as hypoxia, heart failure, and hypertension. Using neuronal tracing approaches, the neuroanatomical connections between the PVN and other brain regions involved in cardiovascular regulation have been illustrated. Particularly, the pre-autonomic neurons in the PVN directly project to the intermediolateral column (IML) in the spinal cord [3], the rostral ventrolateral medulla (RVLM) [4], and the nucleus of the solitary tract (NTS) [5], suggesting a critical role of PVN in autonomic regulation. With regard to neuronal function, the excitability of pre-sympathetic PVN neurons is finely tuned by both inhibitory (GABAergic) and excitatory (glutamatergic) synaptic inputs. In hypertension, impaired GABAergic input and/or enhanced glutamatergic input result in hyperactivity of the pre-sympathetic PVN neurons and elevated sympathetic outflow. The cellular and molecular mechanisms involved in the hypothalamic regulation of blood pressure and sympathetic activity in hypertension induced by angiotensin II and a high-salt diet, gene mutation, and obesity include increased activity in the renin-angiotensin system [6–10], enhanced oxidative stress [9, 11, 12], augmented orexin signaling pathways [13], reduced small-conductance Ca2+-activated K+ channel function [14], and activated inflammatory pathways [9]. Based on recent findings in our laboratory, we discuss the neuronal mechanisms underlying the hyperactivity of pre-sympathetic PVN neurons and the contribution of impaired synaptic inputs to the PVN in elevated sympathetic overflow in spontaneously hypertensive rats (SHRs).
Outputs and Inputs of Pre-sympathetic PVN Neurons
The pre-sympathetic PVN neurons, which project directly to the RVLM and IML, are predominantly distributed in the dorsal and medial parvocellular regions of the PVN [15]. These regions contain fibers and terminals from adrenergic/noradrenergic regions in the NTS (A2 and C2 cell groups) and the ventrolateral medulla (A1 and C1 groups) [16, 17], and receive direct inputs from the medial part of the central amygdala [18] and the arcuate nucleus [19]. Moreover, the medial parvocellular region in the PVN is heavily innervated by afferents from the forebrain median preoptic nucleus [20] and the circumventricular organs, especially the subfornical organ and organum vasculosum of the lamina terminalis [21, 22]. Through these afferents, information on the pressure, volume and oxygen level, as well as chemical signals including angiotensin II and hyperosmolality, converges on and is integrated in the PVN [20, 23–26]. In addition, pre-sympathetic PVN neurons receive excitatory and inhibitory synaptic inputs from local neuronal circuits within the PVN. Neuronal tracing studies have demonstrated that pre-sympathetic PVN neurons project to regions involved in the regulation of autonomic functions including the IML in the spinal cord [3], the RVLM [4], and the NTS [5]. The distribution of the pre-sympathetic PVN neurons is topographically related to the target organs. For example, the pre-sympathetic neurons regulating cardiac sympathetic outflow are located more medially in the dorsal division of the PVN [4] and some distance from those that regulate the adrenal gland [27, 28]. Particularly, some pre-sympathetic PVN neurons provide dual projections to sympathetic nerves that control the heart and adrenal glands [29], and some pre-sympathetic neurons send efferents to the RVLM and spinal cord [30]. The majority of pre-sympathetic PVN neurons are parvocellular, and previous studies using a combination of retrograde tracing and either immunohistochemical staining or in situ hybridization have shown that pre-sympathetic neurons also express dynorphin, enkephalin, vasopressin, oxytocin, and corticotropin-releasing factor [31–33]. Together, this anatomical and neurochemical diversity of pre-sympathetic PVN neurons indicates a wide range of activities in the regulation of cardiovascular functions.
Gamma-aminobutyric acid (GABA) and glutamate are the predominant excitatory and inhibitory neurotransmitters in the central nervous system, and the excitability of pre-sympathetic PVN neurons is finely regulated by these synaptic inputs [34, 35]. The inhibitory actions of GABA are mediated primarily through ionotropic GABAA receptors and metabotropic GABAB receptors. When activated by GABA, GABAA receptors increase the conductance for anions such as Cl– and HCO3– to hyperpolarize the membrane potential. Activation of the postsynaptic GABAB receptors increases the outward K+ currents through an activated K+ conductance [36], while activation of presynaptic GABAB receptors decreases the synaptic transmitter release [37]. The excitatory glutamate receptors are divided into ionotropic receptors, which include N-methyl-D-aspartate receptors (NMDARs), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs), and kainate receptors, as well as metabotropic glutamate receptors (mGluRs). In the following section, we review recent findings on synaptic plasticity in the PVN in SHRs, a commonly-used animal model of primary hypertension.
Role of the PVN in Regulating Sympathetic Outflow Under Normotensive and Hypertensive Conditions
Sympathetic outflow is critically regulated by the firing activity of pre-sympathetic PVN neurons, and the activity of these neurons is tightly regulated by inhibitory and excitatory synaptic inputs. Under normotensive conditions, the pre-sympathetic PVN neurons are predominantly innervated by GABAergic inputs [38, 39], because microinjection of a GABAA antagonist such as bicuculline or gabazine significantly increases the mean arterial pressure (MAP) and sympathetic nerve activity (SNA) in a dose-dependent manner in rats [39] and conscious sheep [38]; conversely, muscimol, a GABAA agonist, when microinjected into the PVN, significantly decreases the MAP, renal SNA, and heart rate (HR) in anesthetized Wistar-Kyoto (WKY) [40] and Sprague-Dawley rats [41]. However, microinjection of the ionotropic glutamate receptor antagonist kynurenic acid, an NMDAR antagonist and/or a non-NMDAR antagonist does not significantly change the MAP and SNA in normotensive WKY rats [42]. These findings suggest that GABAergic input is the predominant innervation of pre-sympathetic PVN neurons under physiological conditions.
The SHR is an ideal animal model to investigate the role of neuronal plasticity in elevated sympathetic outflow [43]. In 1991, Kazuo and colleagues [44] found that electrically-induced lesions of the PVN lower the arterial blood pressure in SHRs, and PVN lesions significantly attenuate the depressor effects of ganglionic blockade in SHRs, indicating that the PVN is an important central source driving sympathetic outflow during the development of hypertension in SHRs [44]. In contrast to the synaptic inputs under normotensive condition, glutamatergic inputs to pre-sympathetic PVN neurons are profoundly enhanced, while GABAergic inputs are significantly decreased in SHRs. In this regard, blocking glutamate ionotropic receptors, including NMDARs or non-NMDARs, in the PVN remarkably decreases the lumbar SNA, MAP, and HR in SHRs but not in normotensive WKY rats [42]. Depressor effects have also been reported after PVN microinjection of selective antagonists against mGluR1 or mGluR5 in SHRs but not in WKY rats [45]. Thus, the glutamatergic inputs to the pre-sympathetic PVN neurons are tonically increased in SHR. Furthermore, although inhibition of GABAA receptors in the PVN by gabazine increases the MAP, lumbar activity, and HR in SHRs, the pressor level in SHRs was significantly smaller than that in WKY rats [39], suggesting that the GABAergic inputs to the PVN are decreased in SHRs. Taken together, the imbalance of increased glutamatergic inputs and decreased GABAergic inputs to the pre-sympathetic neurons results in augmented sympathetic drive from the PVN in SHRs.
GABAergic Plasticity in Pre-sympathetic PVN Neurons in SHRs
The GABAA receptor-mediated inhibition is impaired in the PVN of SHRs. The frequency and amplitude of GABAA-mediated inhibitory postsynaptic currents and the evoked GABAA current are significantly lower in the pre-sympathetic PVN neurons of SHRs than in normotensive WKY rats [46]. In addition, blockade of GABAA receptors by bicuculline or gabazine decreases or does not significantly change the firing activity of pre-sympathetic PVN neurons in SHRs, but profoundly increases the firing activity of PVN neurons in WKY rats [46]. These data suggest that the function of GABAA receptors is impaired in the pre-sympathetic PVN neurons of SHRs. The reasons for this impairment may be reduced presynaptic GABA release [34, 47] and/or decreased numbers of GABAA receptors, or loss of GABAergic neurons under hypertensive conditions [48].
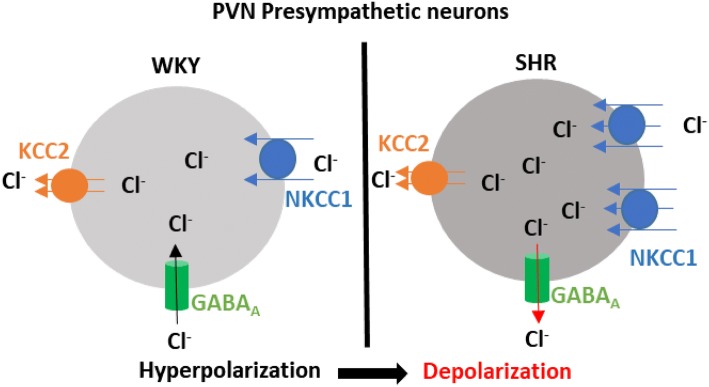
One key determinant of GABAA receptor function is the intracellular Cl– concentration ([Cl–]i) since GABAA receptors are ligand-gated anion channels with a predominant permeability to Cl– and a limited permeability to HCO-3 [49]. Under conditions of high [Cl–]i, GABAA receptor activation induces Cl– outflow to hyperpolarize the cell membrane. When the [Cl–]i is low, GABAA receptor activation depolarizes the cell membrane [49]. The Cl– homeostasis is maintained by both Na+-K+-Cl–-cotransporter-1 (NKCC1) and K+-Cl–-cotransporter-2 (KCC2) in PVN neurons. The [Cl–]i determines the GABA reversal potential (EGABA), which affects the response to the activation of GABAA receptors [49–51]. The mRNA and protein levels of NKCC1, but not KCC2, in the PVN are markedly increased in SHRs [52]. The upregulation of NKCC1 results in an increase in [Cl–]i, which leads to a depolarizing shift of EGABA in pre-sympathetic PVN neurons and impairs GABAergic inhibition in the PVN of SHRs [52] (Fig. 1). Furthermore, NKCC1 protein on the plasma membrane in the PVN of SHRs is highly glycosylated, and inhibiting NKCC1 N-glycosylation normalizes EGABA and restores the GABA inhibition of pre-sympathetic PVN neurons in SHRs. This finding suggests that N-glycosylation is a crucial posttranslational modification for functional NKCC1. In addition, central application of bumetanide, an NKCC1 inhibitor, decreases sympathetic outflow and recovers the GABAA receptor-mediated sympathoinhibitory responses in the PVN of SHRs. In another rat model of hypertension, deoxycorticosterone acetate-salt hypertensive rats, EGABA in the PVN vasopressin neurons is also shifted to depolarization and associated with an upregulation of NKCC1 protein levels in the PVN. Inhibition of NKCC1 by intracerebroventricular injection of bumetanide delays the development of hypertension induced by deoxycorticosterone acetate-salt treatment [53]. These findings suggest that upregulation of NKCC1 in the PVN is responsible for the impaired GABAergic inhibition in hypertension.
Fig. 1.
An increase in intracellular Cl– switches the GABAA-mediated hyperpolarization to depolarization in the pre-sympathetic PVN neurons of SHRs. The homeostasis of intracellular Cl– is balanced by the counterpart Cl– co-transporters NKCC1 and KCC2. NKCC1 imports Cl– to increase [Cl–]i, whereas KCC2 exports Cl– to decrease [Cl–]i. In SHRs, an increase in NKCC1 activity elevates the [Cl–]i, which shifts the GABA reversal potential above the resting membrane potential. Thus, activation of GABAA leads to Cl– outflow to induce GABAA-mediated depolarization rather than hyperpolarization under physiological conditions.
The metabotropic GABAB receptors are distributed in both pre- and postsynaptic sites in the PVN. Recent evidence has demonstrated that GABAB receptor function in the PVN is increased in SHRs, because microinjection of baclofen, the GABAB receptor agonist, into the PVN produces a greater inhibitory effect on sympathetic outflow in SHRs than in WKY rats [34], while the GABAB receptor antagonist CGP55845 increases the firing activity of pre-sympathetic PVN neurons in SHRs but has no effect on the PVN neurons in WKY rats [46]. In addition, the GABAB receptor agonist baclofen induces a larger membrane hyperpolarization and outward currents in the pre-sympathetic PVN neurons in SHRs than in WKY rats [34]. These findings suggest that the GABAB receptor is upregulated in the PVN and tonically activated to regulate the excitability of pre-sympathetic PVN neurons in SHRs [46]. GABAB receptors are distributed in presynaptic terminals and can regulate the release of both glutamate and GABA. Electrophysiological data suggest that the synaptic glutamate release to pre-sympathetic PVN neurons under GABAB receptor control is enhanced, while the GABAergic synaptic inputs under the control of presynaptic GABAB receptors are attenuated in SHRs compared with normotensive control rats [34].
Glutamatergic Plasticity in Pre-sympathetic PVN Neurons in Hypertension
Both in vivo and in vitro evidence has demonstrated that glutamatergic synaptic transmission is enhanced in the PVN in the control of sympathetic outflow under hypertensive conditions. Blocking ionotropic glutamate receptors in the PVN has little effect on sympathetic vasomotor tone in normotensive rats, but profoundly reduces the sympathetic output and MAP in SHRs [42]. In addition to SHRs [54–56], enhanced glutamatergic synaptic input in the PVN has been found in salt-sensitive [57] and angiotensin II-induced hypertension [6, 58]. In brain-slice recording, the frequency of glutamatergic miniature excitatory postsynaptic currents (mEPSCs) in pre-sympathetic PVN neurons is profoundly higher in SHRs than in normotensive WKY rats. Blocking NMDARs with D-2-amino-5-phosphonopentanoate remarkably attenuates this increase in mEPSC frequency in SHRs, suggesting that the NMDAR-mediated presynaptic glutamate release is enhanced in the pre-sympathetic PVN neurons in SHRs [35]. Because blocking NMDARs fails to change the frequency of mEPSCs in PVN neurons in normotensive WKY rats, NMDAR-mediated presynaptic glutamate release to pre-sympathetic PVN neurons is latent under normotensive conditions but becomes tonically activated in SHRs [55, 56].
NMDARs are located presynaptically and postsynaptically in the central nervous system. Postsynaptic NMDAR activity in pre-sympathetic PVN neurons is also enhanced in hypertension. Currents induced by the puff application of NMDA are markedly larger in spinally-projecting PVN neurons in SHRs than in WKY rats [35, 54–56]. Blockade of NMDARs significantly reduces the firing activity of pre-sympathetic PVN neurons in brain-slice preparations and reduces sympathetic vasomotor activity in SHRs [34, 42, 45]. In addition to SHRs, ablation of NMDAR subunit GluN1 in the PVN attenuates angiotensin II-induced hypertension in mice [59], suggesting that NMDARs in the PVN play an important role in angiotensin II-induced hypertension.
Regulation of Ionotropic NMDAR Phosphorylation by Kinase
Phosphorylation and de-phosphorylation, the reciprocal processes produced by kinases and phosphatases, are fundamental mechanisms of regulating NMDAR activity across the central nervous system [60–64]. Tyrosine kinases such as Src kinase [55], and serine/threonine protein kinases such as casein kinase I (CK1) [65], casein kinase II (CK2) [56] and Ca2+/calmodulin-dependent protein kinase II (CaMKII) [54] are critically involved in the enhanced NMDAR activity in pre-sympathetic PVN neurons in SHRs. Src kinase can phosphorylate the Tyr-1325 in the NR2A subunit [66]. The increased Src kinase activity enhances both the presynaptic and postsynaptic NMDAR activity in the PVN neurons that project to the RVLM in SHRs, because inhibition of Src kinase significantly attenuates the increased frequency of mEPSCs and currents elicited by puff application of NMDA, and decreases the firing activity of these neurons [55]. In addition, inhibition of Src kinase decreases the MAP and SNA in SHRs, but not in WKY rats [55].
CK2 and CaMKII phosphorylate NR2B subunits at different residues, Ser-1480 and Ser-1303, respectively [67]. The protein levels of both are increased in the PVN in SHRs [54, 56]. Also, both are involved in the enhanced synaptic NMDAR activity in pre-sympathetic PVN neurons in SHRs [54, 56]. Blocking CK2 or CaMKII activity significantly attenuates the currents elicited by puff application of NMDA and NMDAR-mediated mEPSCs, and decreases the firing activity of sympathetic PVN neurons in SHRs [54, 56]. In anesthetized SHRs, inhibition of CK2 or CaMKII activity in the PVN leads to a greater decrease of MAP and SNA in SHRs than in normotensive WKY rats [54, 56]. It seems that NMDARs are phosphorylated by Src, CK2, and CaMKII at different residues and all phosphorylation leads to a similar increase in NMDAR function in pre-sympathetic PVN neurons in SHRs. Src kinases can phosphorylate the tyrosine residues in the CK2 catalytic subunits to increase CK2 activity [68], thus, inhibition of both Src and CK2 activity produces a similar decrease in NMDAR activity in pre-sympathetic PVN neurons [68].
The phosphorylation levels of NMDAR are tightly regulated by the protein kinases and phosphatases as well as their reciprocal interactions. NMDAR activity can be inhibited by protein phosphatases such as PP1/2A [6] and/or PP2B (calcineurin) [69, 70] and increased by inhibition of these phosphatases. We recently found that a CK1 inhibitor increases the currents elicited by puff application of NMDA, NMDAR-mediated EPSCs, and the firing activity of pre-sympathetic PVN neurons in WKY rats but not in SHRs [65]. Inhibiting PP1/2A or PP2B activity mimics the effect of CK1 inhibition on NMDAR activity in PVN neurons, and the CK1 inhibitor does not produce a further increase in NMDAR activity after inhibition of PP1/2A and PP2B in pre-sympathetic PVN neurons [65]. The protein level of CK1ε in the PVN is significantly decreased in SHRs, and the CK1 inhibitor increases NMDAR activity in the pre-sympathetic PVN neurons of WKY rats but not in SHRs [65], suggesting attenuated CK1 activity in the PVN of SHRs. Decreased CK1 activity in the PVN may result in increased phosphorylation of NMDARs in SHRs. Because CK1 does not directly phosphorylate NMDARs [71], it may potentiate the activity of protein phosphatases such as PP1/2A [6] and/or PP2B to decrease the phosphorylation level of NMDARs [69, 70].
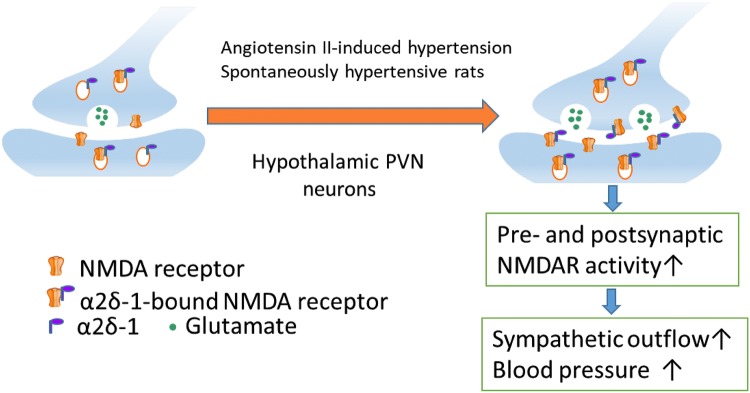
In a recent study, we discovered that α2δ-1-bound NMDARs in the hypothalamus are critically involved in the augmented sympathetic outflow in both angiotensin II-induced hypertension and SHRs [72, 73]. α2δ-1, an auxiliary subunit of the voltage-dependent Ca2+ channel, is a powerful regulator of NMDARs through direct interaction with NMDARs [74]. Increased α2δ-1-bound NMDARs in the hypothalamus in SHRs contribute to increased pre- and postsynaptic NMDAR activity and augmented sympathetic outflow in hypertension [73]. α2δ-1-bound NMDARs also play a role in autonomic dysregulation in the angiotensin II-induced enhancement of NMDAR activity in the PVN. Angiotensin II augments sympathetic vasomotor tone and increases excitatory glutamatergic inputs to pre-sympathetic PVN neurons by stimulating α2δ-1-bound NMDARs at synapses in normal rats and mice [72]. Ablation of the Cacna2d1 gene eliminates the angiotensin II-induced augmentation of NMDARs in the PVN (Fig. 2). The interaction between α2δ-1-bound NMDARs and protein kinases in the regulation of NMDAR activity in hypertension is currently unknown. Because increased phosphorylation can strengthen protein-protein binding complexes, it is possible that certain protein kinases potentiate the phosphorylation of α2δ-1 and/or NMDAR proteins to promote their physical interactions by changing their physicochemical properties, stability, and dynamics.
Fig. 2.
α2δ-1-bound NMDARs in the hypothalamus are critically involved in the augmented sympathetic outflow in both angiotensin II-induced hypertension and SHRs.
Regulation of Ionotropic AMPARs
AMPARs are critical in mediating fast glutamatergic synaptic transmission. AMPARs in the PVN of SHRs undergo a switch to a Ca2+-permeable form. AMPARs without the GluR2 subunit are permeable to Ca2+ and are voltage-dependently blocked by intracellular polyamines [75–77], whereas AMPARs containing the GluR2 subunit are impermeable to Ca2+ [77, 78]. AMPAR-mediated EPSCs display inward rectification at positive holding potentials in spinally-projecting PVN neurons in SHRs [79]. Furthermore, the amplitude of AMPAR-mediated EPSCs and the excitability of spinally projecting PVN neurons are substantially reduced by a selective Ca2+-permeable AMPAR blocker, 1-naphthyl acetyl spermine, in SHRs but not in WKY rats [79], suggesting that increased Ca2+-permeable AMPAR activity contributes to the hyperactivity of pre-sympathetic PVN neurons in SHRs [79]. This increased activity of Ca2+-permeable AMPARs and augmented NMDAR activity can result in increased [Ca2+]i levels in pre-sympathetic PVN neurons in SHRs.
Regulation of Metabotropic Glutamatergic Receptors
Excessive glutamate release activates mGluRs in the PVN of SHRs and regulates sympathetic outflow in hypertension [80]. Group I mGluRs (mGluR1 and mGluR5) are coupled to Gq/11 proteins, and activation of group I mGluRs increases neuronal excitability and synaptic neurotransmitter release via signaling pathways that include protein kinase C. Antagonism of mGluR5 receptors in the PVN has a greater inhibitory effect on SNA and MAP than that of an mGluR1 receptor antagonist in SHRs. These data suggest that mGluR5 receptors play a dominant role in maintaining the elevated sympathetic vasomotor activity in SHRs [80]. In addition, mGluR5 mRNA and protein expression levels in the PVN are markedly higher in SHRs than in WKY rats [45]. Blocking NMDAR activity in the PVN largely attenuates the sympathoexcitatory response to administration of a group I mGluR agonist into the PVN [45], suggesting that activation of group I mGluRs excites pre-sympathetic PVN neurons by activating NMDARs.
In summary, recent findings from in vitro brain-slice studies have revealed the molecular and cellular mechanisms of synaptic plasticity in the pre-sympathetic PVN neurons in animal models of essential hypertension. The imbalance of augmented glutamatergic inputs and diminished GABAergic inputs serves as the cellular and molecular basis of hyperactivity in pre-sympathetic PVN neurons, which leads to the elevated sympathetic outflow in hypertension.
Perspectives
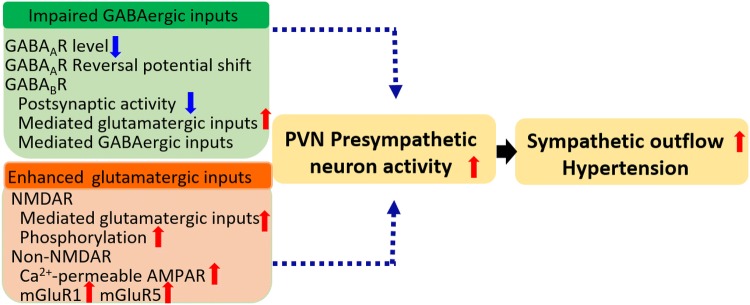
The treatment for essential hypertension is still challenging because the mechanisms have not been fully elucidated. The pre-sympathetic PVN neurons serve as a major source of sympathetic drive. In normotensive animals, these neurons do not contribute to the resting sympathetic nerve activity and blood pressure under normotensive conditions, but do contribute to the increased sympathetic vasomotor activity in hypertension. The increased excitability of pre-sympathetic PVN neurons is a consequence of enhanced glutamatergic inputs and/or impaired GABAergic inputs to these neurons (Fig. 3). Recovery of the balance between these excitatory and inhibitory synaptic inputs may attenuate the sympathetic outflow and decrease the blood pressure in hypertension. However, targets among the signaling mechanisms responsible for the increased NMDAR and Ca2+-permeable AMPAR activity, such as kinase inhibitors, lack specificity. Because glutamate and GABA receptors are crucial for many physiological functions, targeting these receptors produces intolerable adverse effects. Thus, specific protein kinases or NMDAR-interacting proteins responsible for abnormal NMDAR activity in the PVN warrant further study for treating neurogenic hypertension.
Fig. 3.
Imbalance of inhibitory and excitatory synaptic inputs leads to hyperactivity of pre-sympathetic PVN neurons in SHRs.
The impaired GABAergic inputs include down-regulation of GABAA receptors, depolarization of the GABA reversal potential due to enhanced NKCC1 activity, and decreased GABAB receptor activity. The enhanced glutamatergic inputs include increased activity of presynaptic NMDAR-mediated glutamate release and increased postsynaptic NMDAR activity due to phosphorylation by kinases including CK1, CK2, Src, and CaMKII. In addition, the increased proportion of Ca2+-permeable AMPARs and the increased activity of mGluR5 also contribute to the hyperactivity of pre-sympathetic PVN neurons in SHRs. The impaired GABAergic inputs and enhanced glutamatergic inputs tilt the pre-sympathetic PVN neurons to an excitatory state that results in heightened sympathetic outflow and hypertension in SHRs.
Acknowledgements
The studies conducted in the authors’ laboratories were supported by National Institutes of Health Grants HL131161, HL139523, and HL142133.
References
- 1.Gao Y, Zhou JJ, Zhu Y, Kosten T, Li DP. Chronic unpredictable mild stress induces loss of GABA inhibition in corticotrophin-releasing hormone-expressing neurons through NKCC1 upregulation. Neuroendocrinology. 2017;104:194–208. doi: 10.1159/000446114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou JJ, Gao Y, Zhang X, Kosten TA, Li DP. Enhanced hypothalamic NMDA receptor activity contributes to hyperactivity of HPA axis in chronic stress in male rats. Endocrinology. 2018;159:1537–1546. doi: 10.1210/en.2017-03176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saper CB, Loewy AD, Swanson LW, Cowan WM. Direct hypothalamo-autonomic connections. Brain Res. 1976;117:305–312. doi: 10.1016/0006-8993(76)90738-1. [DOI] [PubMed] [Google Scholar]
- 4.Shafton AD, Ryan A, Badoer E. Neurons in the hypothalamic paraventricular nucleus send collaterals to the spinal cord and to the rostral ventrolateral medulla in the rat. Brain Res. 1998;801:239–243. doi: 10.1016/s0006-8993(98)00587-3. [DOI] [PubMed] [Google Scholar]
- 5.Affleck VS, Coote JH, Pyner S. The projection and synaptic organisation of NTS afferent connections with presympathetic neurons, GABA and nNOS neurons in the paraventricular nucleus of the hypothalamus. Neuroscience. 2012;219:48–61. doi: 10.1016/j.neuroscience.2012.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qi J, Zhang DM, Suo YP, Song XA, Yu XJ, Elks C, et al. Renin-angiotensin system modulates neurotransmitters in the paraventricular nucleus and contributes to angiotensin II-induced hypertensive response. Cardiovasc Toxicol. 2013;13:48–54. doi: 10.1007/s12012-012-9184-9. [DOI] [PubMed] [Google Scholar]
- 7.Bardgett ME, Holbein WW, Herrera-Rosales M, Toney GM. Ang II-salt hypertension depends on neuronal activity in the hypothalamic paraventricular nucleus but not on local actions of tumor necrosis factor-alpha. Hypertension. 2014;63:527–534. doi: 10.1161/HYPERTENSIONAHA.113.02429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardinale JP, Sriramula S, Mariappan N, Agarwal D, Francis J. Angiotensin II-induced hypertension is modulated by nuclear factor-kappaBin the paraventricular nucleus. Hypertension. 2012;59:113–121. doi: 10.1161/HYPERTENSIONAHA.111.182154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sriramula S, Xia H, Xu P, Lazartigues E. Brain-targeted angiotensin-converting enzyme 2 overexpression attenuates neurogenic hypertension by inhibiting cyclooxygenase-mediated inflammation. Hypertension. 2015;65:577–586. doi: 10.1161/HYPERTENSIONAHA.114.04691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu Y, Xue BJ, Zhang ZH, Wei SG, Beltz TG, Guo F, et al. Early interference with p44/42 mitogen-activated protein kinase signaling in hypothalamic paraventricular nucleus attenuates angiotensin II-induced hypertension. Hypertension. 2013;61:842–849. doi: 10.1161/HYPERTENSIONAHA.111.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su Q, Qin DN, Wang FX, Ren J, Li HB, Zhang M, et al. Inhibition of reactive oxygen species in hypothalamic paraventricular nucleus attenuates the renin-angiotensin system and proinflammatory cytokines in hypertension. Toxicol Appl Pharmacol. 2014;276:115–120. doi: 10.1016/j.taap.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Yuan N, Zhang F, Zhang LL, Gao J, Zhou YB, Han Y, et al. SOD1 gene transfer into paraventricular nucleus attenuates hypertension and sympathetic activity in spontaneously hypertensive rats. Pflugers Arch. 2013;465:261–270. doi: 10.1007/s00424-012-1173-0. [DOI] [PubMed] [Google Scholar]
- 13.Zhou JJ, Yuan F, Zhang Y, Li DP. Upregulation of orexin receptor in paraventricular nucleus promotes sympathetic outflow in obese Zucker rats. Neuropharmacology. 2015;99:481–490. doi: 10.1016/j.neuropharm.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larson RA, Gui L, Huber MJ, Chapp AD, Zhu J, LaGrange LP, et al. Sympathoexcitation in ANG II-salt hypertension involves reduced SK channel function in the hypothalamic paraventricular nucleus. Am J Physiol Heart Circ Physiol. 2015;308:H1547–H1555. doi: 10.1152/ajpheart.00832.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawchenko PE, Swanson LW. Central noradrenergic pathways for the integration of hypothalamic neuroendocrine and autonomic responses. Science. 1981;214:685–687. doi: 10.1126/science.7292008. [DOI] [PubMed] [Google Scholar]
- 16.Palkovits M, Mezey E, Zaborszky L, Feminger A, Versteeg DH, Wijnen HJ, et al. Adrenergic innervation of the rat hypothalamus. Neurosci Lett. 1980;18:237–243. doi: 10.1016/0304-3940(80)90291-8. [DOI] [PubMed] [Google Scholar]
- 17.Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res. 1982;257:275–325. doi: 10.1016/0165-0173(82)90010-8. [DOI] [PubMed] [Google Scholar]
- 18.Marcilhac A, Siaud P. Identification of projections from the central nucleus of the amygdala to the paraventricular nucleus of the hypothalamus which are immunoreactive for corticotrophin-releasing hormone in the rat. Exp Physiol. 1997;82:273–281. doi: 10.1113/expphysiol.1997.sp004022. [DOI] [PubMed] [Google Scholar]
- 19.Lin L, York DA. Amygdala enterostatin induces c-Fos expression in regions of hypothalamus that innervate the PVN. Brain Res. 2004;1020:147–153. doi: 10.1016/j.brainres.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 20.Llewellyn T, Zheng H, Liu X, Xu B, Patel KP. Median preoptic nucleus and subfornical organ drive renal sympathetic nerve activity via a glutamatergic mechanism within the paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol. 2012;302:424–432. doi: 10.1152/ajpregu.00403.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hubschle T, McKinley MJ, Oldfield BJ. Efferent connections of the lamina terminalis, the preoptic area and the insular cortex to submandibular and sublingual gland of the rat traced with pseudorabies virus. Brain Res. 1998;806:219–231. doi: 10.1016/s0006-8993(98)00765-3. [DOI] [PubMed] [Google Scholar]
- 22.Sawchenko PE, Swanson LW. The organization of forebrain afferents to the paraventricular and supraoptic nuclei of the rat. J Comp Neurol. 1983;218:121–144. doi: 10.1002/cne.902180202. [DOI] [PubMed] [Google Scholar]
- 23.Clement DL, Pelletier CL, Shepherd JT. Role of vagal afferents in the control of renal sympathetic nerve activity in the rabbit. Circ Res. 1972;31:824–830. doi: 10.1161/01.res.31.6.824. [DOI] [PubMed] [Google Scholar]
- 24.Karim F, Kidd C, Malpus CM, Penna PE. The effects of stimulation of the left atrial receptors on sympathetic efferent nerve activity. J Physiol. 1972;227:243–260. doi: 10.1113/jphysiol.1972.sp010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kappagoda CT, Linden RJ, Snow HM. Effect of stimulating right atrial receptors on urine flow in the dog. J Physiol. 1973;235:493–502. doi: 10.1113/jphysiol.1973.sp010399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi P, Stocker SD, Toney GM. Organum vasculosum laminae terminalis contributes to increased sympathetic nerve activity induced by central hyperosmolality. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2279–R2289. doi: 10.1152/ajpregu.00160.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coote JH. A role for the paraventricular nucleus of the hypothalamus in the autonomic control of heart and kidney. Exp Physiol. 2005;90:169–173. doi: 10.1113/expphysiol.2004.029041. [DOI] [PubMed] [Google Scholar]
- 28.Schramm LP, Strack AM, Platt KB, Loewy AD. Peripheral and central pathways regulating the kidney: a study using pseudorabies virus. Brain Res. 1993;616:251–262. doi: 10.1016/0006-8993(93)90216-a. [DOI] [PubMed] [Google Scholar]
- 29.Jansen AS, Nguyen XV, Karpitskiy V, Mettenleiter TC, Loewy AD. Central command neurons of the sympathetic nervous system: basis of the fight-or-flight response. Science. 1995;270:644–646. doi: 10.1126/science.270.5236.644. [DOI] [PubMed] [Google Scholar]
- 30.Pyner S, Coote JH. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience. 2000;100:549–556. doi: 10.1016/s0306-4522(00)00283-9. [DOI] [PubMed] [Google Scholar]
- 31.Coldren KM, Li DP, Kline DD, Hasser EM, Heesch CM. Acute hypoxia activates neuroendocrine, but not presympathetic, neurons in the paraventricular nucleus of the hypothalamus: differential role of nitric oxide. Am J Physiol Regul Integr Comp Physiol. 2017;312:R982–r995. doi: 10.1152/ajpregu.00543.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hallbeck M, Larhammar D, Blomqvist A. Neuropeptide expression in rat paraventricular hypothalamic neurons that project to the spinal cord. J Comp Neurol. 2001;433:222–238. doi: 10.1002/cne.1137. [DOI] [PubMed] [Google Scholar]
- 33.Biag J, Huang Y, Gou L, Hintiryan H, Askarinam A, Hahn JD, et al. Cyto- and chemoarchitecture of the hypothalamic paraventricular nucleus in the C57BL/6J male mouse: a study of immunostaining and multiple fluorescent tract tracing. J Comp Neurol. 2012;520:6–33. doi: 10.1002/cne.22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li DP, Yang Q, Pan HM, Pan HL. Plasticity of pre- and postsynaptic GABAB receptor function in the paraventricular nucleus in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2008;295:H807–H815. doi: 10.1152/ajpheart.00259.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li DP, Yang Q, Pan HM, Pan HL. Pre- and postsynaptic plasticity underlying augmented glutamatergic inputs to hypothalamic presympathetic neurons in spontaneously hypertensive rats. J Physiol. 2008;586:1637–1647. doi: 10.1113/jphysiol.2007.149732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dutar P, Nicoll RA. A physiological role for GABAB receptors in the central nervous system. Nature. 1988;332:156–158. doi: 10.1038/332156a0. [DOI] [PubMed] [Google Scholar]
- 37.Bowery NG, Hill DR, Hudson AL, Doble A, Middlemiss DN, Shaw J, et al. (-)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980;283:92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- 38.Ramchandra R, Hood SG, Frithiof R, McKinley MJ, May CN. The role of the paraventricular nucleus of the hypothalamus in the regulation of cardiac and renal sympathetic nerve activity in conscious normal and heart failure sheep. J Physiol. 2013;591:93–107. doi: 10.1113/jphysiol.2012.236059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li DP, Pan HL. Role of gamma-aminobutyric acid (GABA)A and GABAB receptors in paraventricular nucleus in control of sympathetic vasomotor tone in hypertension. J Pharmacol Exp Ther. 2007;320:615–626. doi: 10.1124/jpet.106.109538. [DOI] [PubMed] [Google Scholar]
- 40.Akine A, Montanaro M, Allen AM. Hypothalamic paraventricular nucleus inhibition decreases renal sympathetic nerve activity in hypertensive and normotensive rats. Auton Neurosci. 2003;108:17–21. doi: 10.1016/j.autneu.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 41.Zahner MR, Pan HL. Role of paraventricular nucleus in the cardiogenic sympathetic reflex in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:420–426. doi: 10.1152/ajpregu.00563.2004. [DOI] [PubMed] [Google Scholar]
- 42.Li DP, Pan HL. Glutamatergic inputs in the hypothalamic paraventricular nucleus maintain sympathetic vasomotor tone in hypertension. Hypertension. 2007;49:916–925. doi: 10.1161/01.HYP.0000259666.99449.74. [DOI] [PubMed] [Google Scholar]
- 43.Okamoto K, Aoki K. Development of a strain of spontaneously hypertensive rats. Jpn Circ J. 1963;27:282–293. doi: 10.1253/jcj.27.282. [DOI] [PubMed] [Google Scholar]
- 44.Takeda K, Nakata T, Takesako T, Itoh H, Hirata M, Kawasaki S, et al. Sympathetic inhibition and attenuation of spontaneous hypertension by PVN lesions in rats. Brain Res. 1991;543:296–300. doi: 10.1016/0006-8993(91)90040-3. [DOI] [PubMed] [Google Scholar]
- 45.Li DP, Zhu LH, Pachuau J, Lee HA, Pan HL. mGluR5 Upregulation increases excitability of hypothalamic presympathetic neurons through NMDA receptor trafficking in spontaneously hypertensive rats. J Neurosci. 2014;34:4309–4317. doi: 10.1523/JNEUROSCI.4295-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li DP, Pan HL. Plasticity of GABAergic control of hypothalamic presympathetic neurons in hypertension. Am J Physiol Heart Circ Physiol. 2006;290:1110–1119. doi: 10.1152/ajpheart.00788.2005. [DOI] [PubMed] [Google Scholar]
- 47.Ichida T, Takeda K, Sasaki S, Nakagawa M, Hashimoto T, Kuriyama K. Age-related decrease of gamma-aminobutyric acid (GABA) release in brain of spontaneously hypertensive rats. Life Sci. 1996;58:209–215. doi: 10.1016/0024-3205(95)02278-3. [DOI] [PubMed] [Google Scholar]
- 48.Kunkler PE, Hwang BH. Lower GABAA receptor binding in the amygdala and hypothalamus of spontaneously hypertensive rats. Brain Res Bull. 1995;36:57–61. doi: 10.1016/0361-9230(94)00164-v. [DOI] [PubMed] [Google Scholar]
- 49.Kaila K, Voipio J, Paalasmaa P, Pasternack M, Deisz RA. The role of bicarbonate in GABAA receptor-mediated IPSPs of rat neocortical neurones. J Physiol. 1993;464:273–289. doi: 10.1113/jphysiol.1993.sp019634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Payne JA, Rivera C, Voipio J, Kaila K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 2003;26:199–206. doi: 10.1016/S0166-2236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- 51.Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, et al. The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- 52.Ye ZY, Li DP, Byun HS, Li L, Pan HL. NKCC1 upregulation disrupts chloride homeostasis in the hypothalamus and increases neuronal activity-sympathetic drive in hypertension. J Neurosci. 2012;32:8560–8568. doi: 10.1523/JNEUROSCI.1346-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim YB, Kim YS, Kim WB, Shen FY, Lee SW, Chung HJ, et al. GABAergic excitation of vasopressin neurons: possible mechanism underlying sodium-dependent hypertension. Circ Res. 2013;113:1296–1307. doi: 10.1161/CIRCRESAHA.113.301814. [DOI] [PubMed] [Google Scholar]
- 54.Li DP, Zhou JJ, Zhang J, Pan HL. CaMKII regulates synaptic NMDA receptor activity of hypothalamic presympathetic neurons and sympathetic outflow in hypertension. J Neurosci. 2017;37:10690–10699. doi: 10.1523/JNEUROSCI.2141-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qiao X, Zhou JJ, Li DP, Pan HL. Src kinases regulate glutamatergic input to hypothalamic presympathetic neurons and sympathetic outflow in hypertension. Hypertension. 2017;69:154–162. doi: 10.1161/HYPERTENSIONAHA.116.07947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ye ZY, Li L, Li DP, Pan HL. Casein kinase 2-mediated synaptic GluN2A up-regulation increases N-methyl-D-aspartate receptor activity and excitability of hypothalamic neurons in hypertension. J Biol Chem. 2012;287:17438–17446. doi: 10.1074/jbc.M111.331165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gabor A, Leenen FH. Cardiovascular effects of angiotensin II and glutamate in the PVN of Dahl salt-sensitive rats. Brain Res. 2012;1447:28–37. doi: 10.1016/j.brainres.2012.01.060. [DOI] [PubMed] [Google Scholar]
- 58.Glass MJ, Wang G, Coleman CG, Chan J, Ogorodnik E, Van Kempen TA, et al. NMDA receptor plasticity in the hypothalamic paraventricular nucleus contributes to the elevated blood pressure produced by angiotensin II. J Neurosci. 2015;35:9558–9567. doi: 10.1523/JNEUROSCI.2301-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Biancardi VC, Campos RR, Stern JE. Altered balance of gamma-aminobutyric acidergic and glutamatergic afferent inputs in rostral ventrolateral medulla-projecting neurons in the paraventricular nucleus of the hypothalamus of renovascular hypertensive rats. J Comp Neurol. 2010;518:567–585. doi: 10.1002/cne.22256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang YT, Salter MW. Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature. 1994;369:233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]
- 61.Lu WY, Xiong ZG, Lei S, Orser BA, Dudek E, Browning MD, et al. G-protein-coupled receptors act via protein kinase C and Src to regulate NMDA receptors. Nat Neurosci. 1999;2:331–338. doi: 10.1038/7243. [DOI] [PubMed] [Google Scholar]
- 62.Chergui K, Svenningsson P, Greengard P. Physiological role for casein kinase 1 in glutamatergic synaptic transmission. J Neurosci. 2005;25:6601–6609. doi: 10.1523/JNEUROSCI.1082-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kimura R, Matsuki N. Protein kinase CK2 modulates synaptic plasticity by modification of synaptic NMDA receptors in the hippocampus. J Physiol. 2008;586:3195–3206. doi: 10.1113/jphysiol.2008.151894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Omkumar RV, Kiely MJ, Rosenstein AJ, Min KT, Kennedy MB. Identification of a phosphorylation site for calcium/calmodulindependent protein kinase II in the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 1996;271:31670–31678. doi: 10.1074/jbc.271.49.31670. [DOI] [PubMed] [Google Scholar]
- 65.Li DP, Zhou JJ, Pan HL. Endogenous casein kinase-1 modulates NMDA receptor activity of hypothalamic presympathetic neurons and sympathetic outflow in hypertension. J Physiol. 2015;593:4439–4452. doi: 10.1113/JP270831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang M, Leonard JP. Identification of mouse NMDA receptor subunit NR2A C-terminal tyrosine sites phosphorylated by coexpression with v-Src. J Neurochem. 2001;77:580–588. doi: 10.1046/j.1471-4159.2001.00255.x. [DOI] [PubMed] [Google Scholar]
- 67.Chung HJ, Huang YH, Lau LF, Huganir RL. Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand. J Neurosci. 2004;24:10248–10259. doi: 10.1523/JNEUROSCI.0546-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Donella-Deana A, Cesaro L, Sarno S, Ruzzene M, Brunati AM, Marin O, et al. Tyrosine phosphorylation of protein kinase CK2 by Src-related tyrosine kinases correlates with increased catalytic activity. Biochem J. 2003;372:841–849. doi: 10.1042/BJ20021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lieberman DN, Mody I. Casein kinase-II regulates NMDA channel function in hippocampal neurons. Nat Neurosci. 1999;2:125–132. doi: 10.1038/5680. [DOI] [PubMed] [Google Scholar]
- 70.Tong G, Shepherd D, Jahr CE. Synaptic desensitization of NMDA receptors by calcineurin. Science. 1995;267:1510–1512. doi: 10.1126/science.7878472. [DOI] [PubMed] [Google Scholar]
- 71.Venerando A, Ruzzene M, Pinna LA. Casein kinase: the triple meaning of a misnomer. Biochem J. 2014;460:141–156. doi: 10.1042/BJ20140178. [DOI] [PubMed] [Google Scholar]
- 72.Ma H, Chen SR, Chen H, Li L, Li DP, Zhou JJ, et al. alpha2delta-1 is essential for sympathetic output and NMDA receptor activity potentiated by angiotensin II in the hypothalamus. J Neurosci. 2018;38:6388–6398. doi: 10.1523/JNEUROSCI.0447-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ma H, Chen SR, Chen H, Zhou JJ, Li DP, Pan HL. alpha2delta-1 couples to NMDA receptors in the hypothalamus to sustain sympathetic vasomotor activity in hypertension. J Physiol. 2018;596:4269–4283. doi: 10.1113/JP276394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen J, Li L, Chen SR, Chen H, Xie JD, Sirrieh RE, et al. The alpha2delta-1-NMDA receptor complex is critically involved in neuropathic pain development and gabapentin therapeutic actions. Cell Rep. 2018;22:2307–2321. doi: 10.1016/j.celrep.2018.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bowie D, Mayer ML. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron. 1995;15:453–462. doi: 10.1016/0896-6273(95)90049-7. [DOI] [PubMed] [Google Scholar]
- 76.Donevan SD, Rogawski MA. Intracellular polyamines mediate inward rectification of Ca(2+)-permeable alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Proc Natl Acad Sci U S A. 1995;92:9298–9302. doi: 10.1073/pnas.92.20.9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Isaac JT, Ashby MC, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54:859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 78.Hollmann M, Hartley M, Heinemann S. Ca2+ permeability of KA-AMPA–gated glutamate receptor channels depends on subunit composition. Science. 1991;252:851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- 79.Li DP, Byan HS, Pan HL. Switch to glutamate receptor 2-lacking AMPA receptors increases neuronal excitability in hypothalamus and sympathetic drive in hypertension. J Neurosci. 2012;32:372–380. doi: 10.1523/JNEUROSCI.3222-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li DP, Pan HL. Increased group I metabotropic glutamate receptor activity in paraventricular nucleus supports elevated sympathetic vasomotor tone in hypertension. Am J Physiol Regul Integr Comp Physiol. 2010;299:552–561. doi: 10.1152/ajpregu.00195.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]