Abstract
Introduction and Objective:
The main goal of asthma treatment is to achieve and maintain clinical control of the disease. The exhaled fraction nitric oxide (FeNO) level is a biomarker of T-helper cell type 2 (Th2) inflammation of the airways. Our objective was to determine whether the FeNO level can be used to discriminate between patients with controlled, partially controlled, and uncontrolled asthma.
Materials and Methods:
The FeNO level and asthma control were evaluated in a retrospective and analytic cross-sectional study through data collected from asthmatic patients who were assessed by clinical history, asthma control, physical examination, spirometry, and FeNO level. Asthma control was determined by the criteria of the Global Initiative for Asthma and classified as controlled asthma, partially controlled asthma, and uncontrolled asthma. The FeNO values were classified as low (<25 ppb) or intermediate/high (⩾25 ppb) based on the American Thoracic Society recommendations.
Results:
The symptoms of 81 asthmatic patients were classified as controlled (34 [42%] patients), partially controlled (27 [33.3%] patients), and uncontrolled (20 [24.7%] patients). The FeNO level discriminated between the uncontrolled and controlled groups (P = .01) and between the uncontrolled and partially controlled groups (P = .01), but not between the controlled and partially controlled groups (P = .98). An FeNO level >30 ppb was associated with uncontrolled asthma (P = .0001) with an area under the receiver operating characteristic curve of 0.78 (95% confidence interval = 0.65-0.89).
Conclusions:
FeNO level could be helpful in determining asthma control as >30 ppb was associated with uncontrolled asthma.
Keywords: pulmonary and respiratory medicine, anatomy and physiology, primary care/family practice
Introduction
The primary goal of asthma treatment is to achieve control of the disease so that its manifestations are suppressed spontaneously or by treatment.1,2 There is no gold standard to measure the clinical management of asthma. However, functional parameters, evaluation questionnaires of symptoms, airway hyper-responsiveness, and biomarkers of airway inflammation are among the available and validated markers used to evaluate the severity of asthma and are potential ways to monitor disease control. The Global Initiative Guide for Asthma (GINA) recommends symptom assessment questionnaires and spirometry as conventional tests for asthma management because of easiness of implementation and because they are indirect markers of inflammation.3,4 Besides these instruments, the level of asthma control can be assessed by the following four GINA criteria, based on symptoms experienced in the preceding 4 weeks: frequency of asthma symptoms, night-time awakenings due to asthma, limitation of activities, and the need to use drugs for symptom relief. These factors allow asthma to be classified into three distinct groups: controlled asthma, partially controlled asthma, and uncontrolled asthma.2
In recent years, new tools for asthma assessment have been developed. Among them, measurement of the fraction exhaled nitric oxide (FeNO) level has attracted interest because it is an easy technique to use, it provides immediate results, it is noninvasive, and it is a reproducible biomarker of airway inflammation in asthma. Many studies have been conducted to verify the possibility of using the FeNO level as an evaluation tool of asthma control and pulmonary function tests for the management of asthmatic patients.3–9
There are major developments related to FeNO and its relationship with airway inflammation; however, its ability to predict asthma control remains unclear.10 For this reason, this study aimed to verify whether the FeNO level discriminates between patients with uncontrolled asthma, partially controlled asthma, and uncontrolled asthma.
Methods
Study population
A total of 81 asthmatic patients were included in the study. Their FeNO levels were measured after they underwent a medical evaluation at an outpatient clinic specializing in respiratory diseases in the city of Goiania, Goias, Brazil. They were evaluated in the clinic from June 2013 to December 2014.
Study design
This study is an analytical and retrospective cross-sectional study. Patients were eligible to participate if they had their levels of exhaled nitric oxide measured from June 2013 to December 2014 for any reason (ie chronic cough). The inclusion criteria were as follows: (1) patients who had a clinical diagnosis of asthma and (2) patients who had undergone pulmonary function tests. After inclusion in the study, the following data were collected from the medical records: sex, age, weight, height, forced expiratory volume after 1 second (FEV1) pre-bronchodilator (pre-BD) and FEV1 post-bronchodilator (post-BD), FeNO level, clinical diagnosis, smoking status, medication status, exacerbation history, and level of asthma control before the doctor had access to the FeNO results. The following factors were the exclusion criteria: smoking activity (lowers FeNO results), asthma exacerbation treatment before FeNO measurement, presence of respiratory viral infection or other acute respiratory concomitant disease in the 12 weeks before medical care, pregnancy, or breastfeeding.
Measurement of the fraction exhaled nitric oxide levels and pulmonary function test
Before a patient underwent any forced expiratory maneuvers, the FeNO level was measured using an electrochemical portable analyzer (NIOX MINO; Aerocrine AB, Solna, Sweden) that determine exhaled nitric oxide concentration in parts per billion (ppb). The values of FeNO were categorized by an ordinal measurement scale and classified as low or intermediate/high (low, <25 ppb; intermediate/high, ⩾25 ppb) based on the American Thoracic Society criteria.10 The procedure was performed using a nose clip. The patient exhaled air from the lungs for 10 seconds at a constant flow rate of 50 mL/s. Spirometry was performed using a calibrated spirometer (Easyone; ndd Medizintechnik AG, Zurich, Switzerland) based on the American Thoracic Society and European Respiratory Society criteria.11–13
Asthma control
The level of asthma control was assessed by a medical evaluation before a patient underwent FeNO level measurements and spirometry tests. The GINA criteria were used in which patients were evaluated for symptoms they experienced in the preceding 4 weeks by the following factors: weekly frequency of asthma symptoms, nocturnal awakening, limitation in their activity due to asthma, and weekly need of medication for symptom relief. The patients’ asthma symptoms were classified as controlled when they had none of the four criteria; as partially controlled when they had one or two of the four criteria, and as uncontrolled when they had three or more of the four criteria.2
Statistical analysis
Statistical analyses were performed using Stata/SE software (version 13.0; StataCorp, College Station, TX, USA) and SPSS software (version 23) for 64-bit Windows. A significance level of P < .05 was used.
The Shapiro-Wilk test was used to assess the normality of the data. The Pearson chi-square test or the Fisher exact test was used for dichotomous variables. Quantitative variables with normal distribution were expressed as mean and standard deviation and were analyzed using parametric tests. Quantitative variables with non-normal distribution were expressed as median and interquartile range and analyzed using nonparametric tests. A post hoc test was conducted for all statistically significant comparisons and a correlation/regression analysis was conducted between FeNO level and FEV1 pre-BD. The cut-off point of the FeNO measurement as the uncontrolled asthma predictor was calculated using the receiver operating characteristic (ROC) curve.
Ethical aspects
The study was conducted in accordance with the Good Clinical Practice (ICH-G6; ICH, Geneva, Switzerland) and was approved by the Research Ethics Committee of the Federal University of Goias (Goiania, Brazil) under approval number 1228483.
Results
From June 2013 to December 2014, 340 patients performed FeNO level measurements in the outpatient clinic specializing in respiratory diseases and were assessed for eligibility. Of these patients, 241 (70.8%) patients were excluded for not presenting with a diagnosis of asthma. Of the 99 included patients, 18 (18.2%) patients were excluded for not having the asthma classification described in their medical records (Figure 1).
Figure 1.
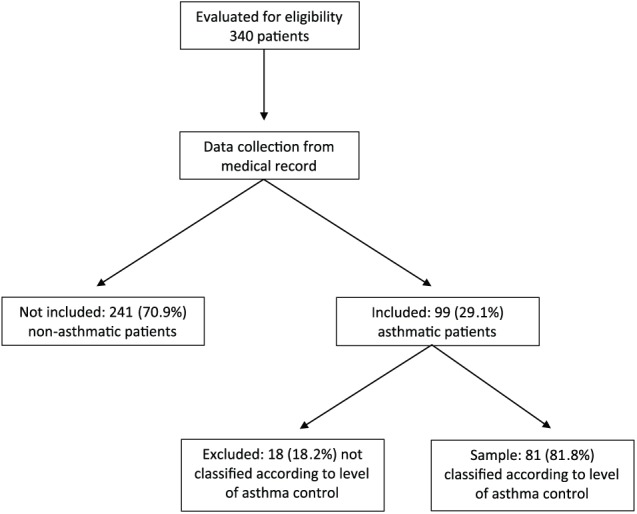
Study design and subjects.
The 81 asthmatic patients included in the study were 48.0 ± 19.4 years of age. There was a predominance of female patients (54 [66.7%] women) in the patient sample, and a predominance of women in the partially controlled and uncontrolled groups (54 [66.7%]) (Table 1). The values for the FeNO level was low (<25 ppb) for 39 (48.1%) individuals and intermediate/high (⩾25 ppb) for 42 (51.9%) individuals.
Table 1.
Clinical and demographic characteristics of 81 asthmatic patients evaluated in an outpatient clinic in the city of Goiania, Goias, Brazil, from June 2013 to December 2014.
| All participants n = 81 |
Controlled group n = 34 (42%) |
Partially controlled group n = 27 (33.3%) |
Uncontrolled group n = 20 (24.7%) |
P-value | |
|---|---|---|---|---|---|
| Sex (F) | 54 (66.7%) | 24 (70.6%) | 17 (63.0%) | 13 (65.0%) | .80 |
| Age (y) | 48 ± 19.4 | 51.9 ± 18.8 | 42.5 ± 19.0 | 48.7 ± 19.8 | .16 |
| BMI (kg/m²) | 27.5 ± 5.9 | 28.2 ± 5.5 | 26.6 ± 5.9 | 27.5 ± 6.5 | .60 |
| Smoking never | 63 (79.7%) | 24 (72.7%) | 24 (88.9%) | 15 (78.9%) | .31 |
| Status former | 16 (20.3%) | 9 (27.3%) | 3 (11.1%) | 4 (21.1%) | |
| Regular IC use | 35 (43.2%) | 13 (38.2%) | 12 (44.4%) | 10 (50.0%) | .69 |
| FeNO (ppb) | 27.0 (14;51)a | 18.0 (13;30)a | 29.0 (18;44)a | 67.5 (33;88)a | .001b |
| FEV1pre (%) | 74.5 ± 19.3 | 77 ± 16.8 | 78.4 ± 20.9 | 64.6 ± 18.7 | .03b |
| FEV1post (%) | 79.2 ± 18.8 | 80.9 ± 17.3 | 82.7 ± 20.1 | 71.4 ± 18.2 | .11 |
Abbreviations: BMI, body mass index; F, female; FeNO, fraction exhaled nitric oxide; FEV1post, forced expiratory volume in 1 second after bronchodilator treatment; FEV1pre, forced expiratory volume in 1 second before bronchodilator treatment; IC, inhaled corticosteroid; ppb: parts per billion.
Values are expressed as median (interquartile range: p25, p75).
Indicates statistical significance (P < .05).
Values are expressed as mean ± standard deviation, unless otherwise indicated. In two subjects, there was no information about previous smoking status, one in controlled group and other in uncontrolled group. The variable “Regular IC use” was based on self-report.
There was a statistically significant difference between the groups regarding the FeNO level and FEV1 pre-BD values (Table 1). The uncontrolled asthma group had significantly higher FeNO levels than the controlled asthma and partially controlled groups. However, there was no difference in the FeNO values between the controlled asthma group and the partially controlled asthma group (Figure 2). Regarding FEV1 post-BD, only the uncontrolled group had a significantly lower FEV1 compared with the partially controlled group (Figure 3). There were no significant differences in age, sex, self-report of inhaled corticosteroid use, smoking history, or body mass index between groups. When all subjects were divided into two groups regarding self-report of regular inhaled corticosteroid use or not, there was no statistical difference between FeNO levels, respectively 30 (18-68) versus 24 (14-44), P = .10.
Figure 2.
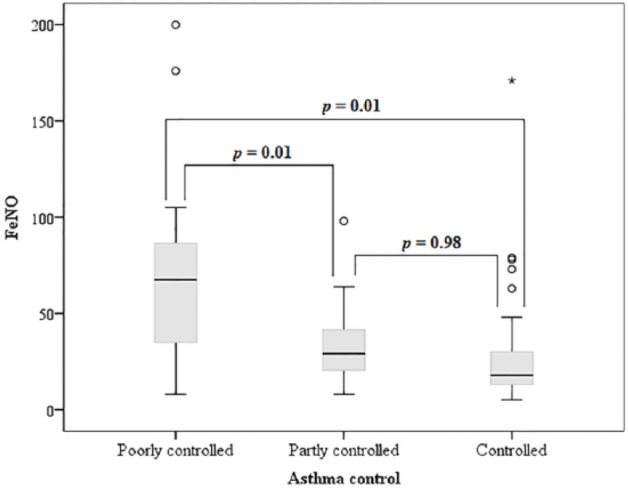
FeNO in asthmatic patients and level of asthma control.
Figure 3.
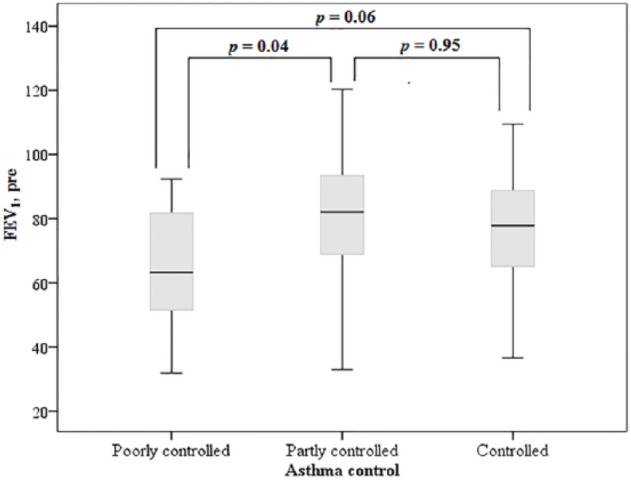
FEV1 in asthmatic patients and level of asthma control.
To evaluate the relationship between FeNO and FEV1, regression analysis was performed for all patients with and without regard to their level of asthma control. The analysis showed low linear correlation coefficient values (r² < 6%), which were not statistically significant.
An ROC curve was generated to predict the identification of uncontrolled individuals using the measurement of exhaled nitric oxide. On comparing the sensitivity, specificity, positive and negative predictive values, and area of the ROC curve, the best combination without a significant loss of sensitivity was an FeNO level >30 ppb. The FeNO value of >30 ppb presented an area under the ROC curve of 0.78 (95% confidence interval [CI] = 0.65-0.88), specificity of 76.47% (95% CI = 58.8-89.3), sensitivity of 80% (95% CI = 56.3-94.3), positive likelihood ratio (+) of 3.40, and negative likelihood ratio of 0.26 (Figure 4).
Figure 4.
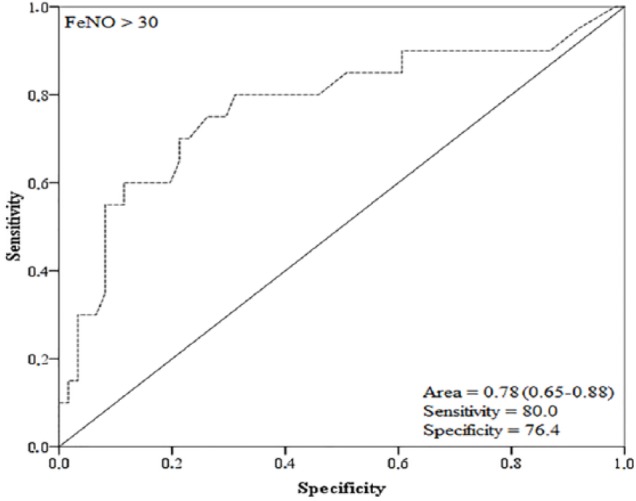
Receiver operating characteristics (ROC) curve of FeNO>30 to predict the identification of uncontrolled asthmatic patients.
Discussion
This study indicated that FeNO level is correlated with asthma symptom control. Inadequate symptom control is strongly associated with high risk of exacerbation,2 that is the reason to use a step-up or step-down of asthma medications, as symptoms could reflect the level of eosinophilic airway inflammation. However, the management-based approach toward symptoms recommended by GINA, which focuses on a direct relationship between eosinophilic inflammation and respiratory symptoms, may be insufficient to manage asthma. There may be a discrepancy between these two factors because of inaccurate reporting of symptoms and because of different types of asthma phenotypes, as allergic early-onset symptom predominant and later onset obese non-eosinophilic high symptomatic asthma could be overtreated and late-onset inflammation predominant oligosymptomatic asthma could be undertreated using the symptom-based aproach.14,15 Within this gap of different asthma phenotypes and the implications for treatment,16 exhaled nitric oxide level measurements can be used. When considered in the overall evaluation of control for treatment and management of asthmatic patients, exhaled nitric oxide could identify subgroups of asthma that the GINA criteria could not. Better identification of these patients would result in better asthma control and would reduce costs by the withdrawal of unnecessary asthma control medications17 and by reducing exacerbations.18 In an observational study19 with a small sample, the estimation of airway inflammation, based on clinical data, was correct in only 50% of patients, and the FeNO results modified the therapeutic decision in more than one-third of patients, notably medication augmentation in 20% and medication decreases in 16%.
Ricciardolo and colleagues verify whether the FeNO measurement could be associated with clinical and functional factors for the evaluation of asthmatic patients in a real-life situation. They also verified that the measurement of FeNO was associated with uncontrolled asthma at the cut-off point of FeNO >29.95 ppb and an area under the ROC curve of 0.70.20 This value was very close to the value in our investigation. Other authors who specifically investigated the association between the FeNO measurement and asthma control, based on the GINA criteria, found no statistically significant difference.21,22 This discrepancy could be explained by the use of different groups of individuals: studies that did not report association between the FeNO measurement and the asthma control used pediatric populations, whereas Ricciardolo and colleagues15 included only adult patients and this study included only 4% of individuals who were younger than 18 years. As agreement regarding asthma control is only moderate when the information provided by adolescents and their caregivers are compared,23 it is likely that the discrepancy between studies concerning association between the FeNO measurement and asthma control could be from methodological differences.24
The data of this study suggest that lung function is an inadequate tool for predicting asthma control. A study that compared the change in FeNO parameters, asthma control questionnaire (ACT) score, and FEV1 levels after completion of an educational program in asthmatic patients showed improved asthma control, decreased FeNO levels, and increased ACT scores; however, there was no significant change in the FEV1 values.4 The updated GINA guidelines 2017 reports that lung function is not strongly correlated with symptoms of asthma, suggesting the use of other instruments of control as they are more important than the functional test, and includes elevated FeNO as a risk factor for poor asthma outcome.2 Although lung function should be always be assessed at diagnosis and during treatment, its role in asthma control is limited not just because of moderate correlation with symptoms, but as much as between-visit variability. Thus, our results reinforce the superiority of inflammatory markers over functional tests regarding prediction of asthma control as the level of airway obstruction is more related to risk of exacerbations than asthma control.2
The use of FeNO measurement for improving the management of asthma in clinical practice has been evidenced in several studies.25–27 Malinovschi and colleagues26 demonstrated a significant increase in disease control after the use of inhaled corticosteroids for 6 weeks in patients with high FeNO values (⩾50 ppb) and intermediate FeNO values (⩾25 ppb and <50 ppb), compared with patients with low FeNO values (⩽25 ppb). Yoon and colleagues27 demonstrated in a randomized trial that anti-inflammatory treatment simultaneously improved the FeNO levels in children with controlled asthma (asymptomatic) when their FeNO levels were >25 ppb. A meta-analysis study found that the measurement of exhaled nitric oxide was important in identifying patients with airway inflammation, who did or did not respond to inhaled corticosteroids, and with the clinical parameters to be associated with reducing the exacerbation rates, compared with management using exclusively clinical parameters.25 The measurement of exhaled nitric oxide is applicable in real-life situations because of the following features: the measurement is very easily performed, compared with bronchial biopsy or eosinophil counts in sputum; it is non-invasive; and it is positively correlated with other inflammatory markers.28 For this reason, the American Thoracic Society recommends measuring the FeNO level to monitor airway inflammation in clinical practice,10 and the American College of Allergy, Asthma and Immunology (Arlington Heights, IL, USA) and the American Academy of Allergy, Asthma and Immunology (Milwaukee, WI, USA) support these recommendations.29
The self-reported regular use of inhaled corticosteroid of 43.2% in this study was in agreement with the literature, as adherence with asthma treatment measures approximately 30%-50%.30 But we could not demonstrate difference in FeNO levels regarding regular inhaled corticosteroid use. As FeNO levels are inversely associated with adherence to inhaled corticosteroids,31 probably the self-reported use was not accurate.
One major benefit of using FeNO for asthma management is to identify patients for whom increased inhaled corticosteroid use will not benefit control because of low eosinophilic inflammation. These symptomatic patients would receive an increased inhaled corticosteroid dose under standard clinical guidelines, but they would be better treated with symptomatic control medications (long-acting beta agonist or long-acting anticholinergic). An example of this approach was demonstrated by Smith et al17 who showed a daily dose of fluticasone of 370 mcg in FeNO group with 45.6% fewer exacerbations versus 641 mcg in the control group. The same could be extrapolated to individuals with comorbidities where the dose of asthma control medication would be adequate, but respiratory symptoms would be persistent due to other diseases (ie gastroesophageal reflux or heart failure). In this case, the finding of a low level of exhaled nitric oxide would support the decision to treat the comorbidities without increasing the dose of inhaled corticosteroids. Although to the best of our knowledge no study specifically evaluated the benefit of this strategy, the negative correlation of FeNO with gastroesophageal reflux disease score in asthmatic patients32 suggests that this would be a reasonable approach. The latest guideline of the National Institute for Health and Care Excellence (NICE) states to consider FeNO measurement as an option to support asthma management in symptomatic patients despite using inhaled corticosteroids.33
The current guideline approach to asthma management that is based on inhaled corticosteroid and beta agonist therapy reflects the lack of ability to define treatment according to specific phenotypic groups.34 Determination of inflammatory phenotypes in asthma (eosinophilic, neutrophilic, mixed, and paucigranulocytic) helps asthma management by showing potential esteroid responsiveness.10 The biomarker approach could possibly prevent the excess use of inhaled corticosteroid in symptomatic patients with non-eosinophilic asthma (neutrophilic or paucigranulocytic), including the later onset obese non-eosinophilic phenotype; prevent an inappropriate (low dose) use of inhaled corticosteroid in severe eosinophilic asthma, which would need a moderate/high dose of inhaled corticosteroid; and also prevent the excess use of high doses of inhaled corticosteroid maintenance therapy associated with long-acting bronchodilators in patients with relatively fixed airflow obstruction. One major advantage of the biomarker approach is the possibility of immediately identifying a situation that would take months of unsuccessful escalating doses of inhaled corticosteroid therapy to stablish.34
The study had several limitations. Hidden biases may have been caused by its retrospective design; some variables were not available on the medical records (atopic status or description or IgE levels); by having been conducted at only one center; and its small sample, which is more prone to variability. Another limitation was the use of a specialized clinic sample in respiratory disease, which may have introduced a selection bias because patients at specialized outpatient clinics tend to have more severe disease and do not represent patients with asthma evaluated by a general practitioner.
The data presented in this study demonstrate that FeNO level measurements correlate with asthma control and would allow a complete characterization of asthmatic patients. Prospective studies are needed to determine and quantify the benefits of a decision-making strategy incorporating symptom assessment and measures of inflammation, based on the FeNO level, and to determine which subgroups or phenotypes this strategy would be more effective in clinical practice.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/ or publication of this article: JLRSJ received training by the Hopkins-Brazil International Clinical Operational Research Training Award, financed by Fogarty International Center/ National Institutes of Health [Grant: USNIH #U2R TW006885 ICOHRTA].
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: JLRSJ and MFR contributed to conceiving this study, participated in the study design, coordination, data interpretation and helped the drafting of the manuscript. FCA contributed to data collection, data interpretation, and drafting of the manuscript. All authors read and approved the final manuscript. JLRSJ is the guarantor of the paper, taking responsibility for the integrity of the work as a whole, from inception to published article.
References
- 1. Cruz AA, Fernandes ALG, Pizzichini E, et al. Diretrizes da Sociedade Brasileira de Pneumologia e Tisiologia para o Manejo da Asma—2012. J Bras Pneumol. 2012;38:S1–S46. [Google Scholar]
- 2. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2018, http://www.ginasthma.org. Accessed June 10, 2018.
- 3. Leblanc A, Botelho C, Coimvra A, Silva JPM, Castro ED, Cernadas JR. Assessment of asthma control: clinical, functional and inflammatory aspects. Eur Ann Allergy Clin Immunol. 2013;45:90–96. [PubMed] [Google Scholar]
- 4. Saito J, Sato S, Fukuhara A, et al. Association of asthma education with asthma control evaluated by asthma control test, FEV1, and fractional exhaled nitric oxide. J Asthma. 2013;50:97–102. [DOI] [PubMed] [Google Scholar]
- 5. Soto-Ramos M, Castro-Rodríguez JA, Hinojos-Gallardo LC, Hernández-Saldaña R, Cisneros-Castolo M, Carrillo-Rodríguez V. Fractional exhaled nitric oxide has a good correlation with asthma control and lung function in Latino children with asthma. J Asthma. 2013;50:590–594. [DOI] [PubMed] [Google Scholar]
- 6. Shiota N, Yokoyama A, Haruta Y, Hattori N, Kohno N. Association of airway inflammation with asthma control level evaluated by the asthma control test. J Asthma. 2011;48:907–913. [DOI] [PubMed] [Google Scholar]
- 7. Ricciardolo FLM. Revisiting the role of exhaled nitric oxide in asthma. Curr Opin Pulm Med. 2014;20:53–59. [DOI] [PubMed] [Google Scholar]
- 8. Papakosta D, Latsios D, Manika K, Porpodis K, Kontakioti E, Gioulekas D. Asthma control test is correlated to FEV1 and nitric oxide in Greek asthmatic patients: influence of treatment. J Asthma. 2011;48:901–906. [DOI] [PubMed] [Google Scholar]
- 9. Scichilone N, Battaglia S, Taormina S, Modica V, Pozzecco E, Bellia V. Alveolar nitric oxide and asthma control in mild untreated asthma. J Allergy Clin Immunol. 2013;131:1513–1517. [DOI] [PubMed] [Google Scholar]
- 10. Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FeNO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. American Thoracic Society/European Respiratory Society. ATS/ERS task force standardisation of lung function testing: standardisation of spirometry. Eur Respir J. 2005;26:319–338.16055882 [Google Scholar]
- 12. American Thoracic Society/European Respiratory Society. ATS/ERS task force standardisation of lung function testing: general considerations for lung function testing. Eur Respir J. 2005;26:153–161. [DOI] [PubMed] [Google Scholar]
- 13. American Thoracic Society/European Respiratory Society. ATS/ERS task force standardization of lung function testing: interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. [DOI] [PubMed] [Google Scholar]
- 14. Haldar P, Pavord ID, Shaw DE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178:218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Desai M, Oppenheimer J. Elucidating asthma phenotypes and endotypes: progress towards personalized medicine. Ann Allergy Asthma Immunol. 2016;116:394–401. doi: 10.1016/j.anai.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 16. Borish L. The immunology of asthma: asthma phenotypes and their implications for personalized treatment. Ann Allergy Asthma Immunol. 2016;117:108–114. doi: 10.1016/j.anai.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith AD, Cowan JO, Brassett KP, Herbison GP, Taylor DR. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med. 2005;352:2163–2173. [DOI] [PubMed] [Google Scholar]
- 18. Peirsman EJ, Carvelli TJ, Hage PY, et al. Exhaled nitric oxide in childhood allergic asthma management: a randomised controlled trial. Pediatr Pulmonol. 2014;49:624–631. doi: 10.1002/ppul.22873. [DOI] [PubMed] [Google Scholar]
- 19. LaForce C, Brooks E, Herje N, Dorinsky P, Rickard K. Impact of exhaled nitric oxide measurements on treatment decisions in an asthma specialty clinic. Ann Allergy Asthma Immunol. 2014;113:619–623. doi: 10.1016/j.anai.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 20. Ricciardolo FLM, Sorbello V, Fontana RB, Schiavetti I, Ciprandi G. Exhaled nitric oxide in relation to asthma control: a real-life survey. Allergol Immunopathol. 2016;44:197–205. doi: 10.1016/j.1ller.2015.05.12. [DOI] [PubMed] [Google Scholar]
- 21. Meena RK, Raj D, Lodha R, Kabra SK. Fractional exhaled nitric oxide for identification of uncontrolled asthma in children. Indian Pediatr. 2016;53:307–310. [DOI] [PubMed] [Google Scholar]
- 22. Waibel V, Ulmer H, Horak E. Assessing asthma control: symptom scores, GINA levels of asthma control, lung function, and exhaled nitric oxide. Pediatr Pulmonol. 2012;47:113–118. doi: 10.1002/ppul.21529. [DOI] [PubMed] [Google Scholar]
- 23. Tibosch M, de Ridder J, Landstra A, et al. Four of a kind: asthma control, FEV1, FeNO, and psychosocial problems in adolescents. Pediatr Pulmonol. 2012;47:933–940. doi: 10.1002/ppul.22514. [DOI] [PubMed] [Google Scholar]
- 24. Green RJ, Klein M, Becker P, et al. Disagreement among common measures of asthma control in children. Chest. 2013;143:117–122. [DOI] [PubMed] [Google Scholar]
- 25. Donohue JF, Jain N. Exhaled nitric oxide to predict corticosteroid responsiveness and reduce asthma exacerbation rates. Respir Med. 2013;107:943–952. doi: 10.1016/j.rmed.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 26. Malinovschi A, Muylem AV, Michiels S, Michils A. FeNO as a predictor of asthma control improvement after starting inhaled steroid treatment. Nitric Oxide. 2014;40:110–116. [DOI] [PubMed] [Google Scholar]
- 27. Yoon JY, Woo S, Kim H, Sun YH, Hahn YS. Fractional exhaled nitric oxide and forced expiratory flow between 25% and 75% of vital capacity in children with controlled asthma. Korean J Pediatr. 2012;55:330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rawy AM, Mansour AI. Fraction of exhaled nitric oxide measurement as a biomarker in asthma and COPD compared with local and systemic inflammatory markers. Egypt J Chest Dis Tuberc. 2015;64:13–20. [Google Scholar]
- 29. AAAAI/ACAAI joint statement of support of the ATS clinical practice guideline: interpretation of exhaled nitric oxide for clinical applications, http://www.aaaai.org/Aaaai/media/MediaLibrary/PDF%20Documents/My%20Membership/FeNOJointStatement3-6-12.pdf. Accessed July 10, 2014. [DOI] [PMC free article] [PubMed]
- 30. Bidwal M, Lor K, Yu J, Ip E. Evaluation of asthma medication adherence rates and strategies to improve adherence in the underserved population at a Federally Qualified Health Center. Res Social Adm Pharm. 2017;13:759–766. doi: 10.1016/j.sapharm.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 31. Wang Z, Pianosi P, Keogh K, et al. The Clinical Utility of Fractional Exhaled Nitric Oxide (FeNO) in Asthma Management. Rockville, MD: Agency for Healthcare Research and Quality; 2017, http://www.ncbi.nlm.nih.gov/books/NBK487497. [PubMed] [Google Scholar]
- 32. Ishizuka T, Hisada T, Kamide Y, et al. The effects of concomitant GERD, dyspepsia, and rhinosinusitis on asthma symptoms and FeNO in asthmatic patients taking controller medications. J Asthma Allergy. 2014;7:131–139. doi: 10.2147/JAA.S67062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management guidance and guidelines, https://www.nice.org.uk/guidance/ng80.
- 34. Pavord ID, Beasley R, Agusti A, et al. After asthma: redefining airways diseases. Lancet. 2018;391:350–400. doi: 10.1016/S0140-6736(17)30879. [DOI] [PubMed] [Google Scholar]


