Abstract
Olfactory neuroblastoma (ONB) is an unusual malignant neoplasm originating from the olfactory neuroepithelium. Secretion of adrenocorticotropic hormone (ACTH) from this tumor has been exceptionally reported. We describe a young man with resistant hypertension and a cushingoid phenotype. After hormonal confirmation of an ACTH-dependent Cushing syndrome, non-invasive dynamic tests were carried out to evaluate the cause of the ACTH source. Plasma cortisol decrease after a high-dose dexamethasone suppression test and cortisol increase after a desmopressin (DDAVP) stimulation test suggested a Cushing disease. A magnetic resonance image (MRI) of the brain and an Indium-111 octreotide scan revealed a large mass centered in the sphenoid sinus with lateral and posterior extension. An ACTH secreting ONB was confirmed with a trasnasal biopsy. Patient was offered a combined therapy with surgical resection and radiotherapy but refused surgery. The neoplasm was treated with neoadjuvant cisplatin-based chemotherapy followed by fractionated radiotherapy. Hypercortisolism initially improved with metyrapone but normocortisolism was only achieved after local control of the tumor with radiotherapy. Clinical presentation of ONB is usually related to local symptoms (as nasal obstruction and epistaxis) dependent on its ubication and extension. Cushing syndrome from ACTH production is a rare manifestation of ONB. This case also underlies the difficulties related to the interpretation of dynamic endocrine tests in Cushing syndrome.
Keywords: Cushing syndrome, ectopic ACTH syndrome, olfactory neuroblastoma, dexamethasone suppression test, desmopressin stimulation test
Introduction
Olfactory neuroblastoma (ONB) is a rare neural crest-derived malignancy1 (accounting for less than 6% of all sinonasal malignant neoplasms) originating from the olfactory neuroepithelium. This neuroectodermal origin could explain certain morphologic features as well as the possibility of hormonal secretion. Adrenocorticotropic hormone (ACTH) ectopic Cushing syndrome attributable to ONB is exceptional with only 17 histologically confirmed cases reported in the literature to the best of our knowledge.2
Clinical Presentation
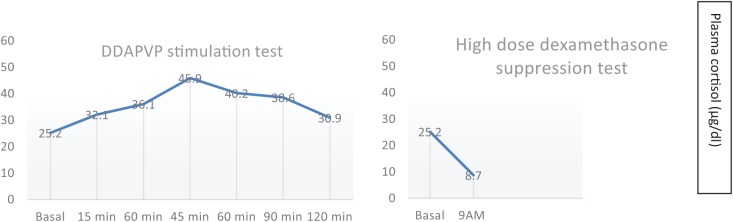
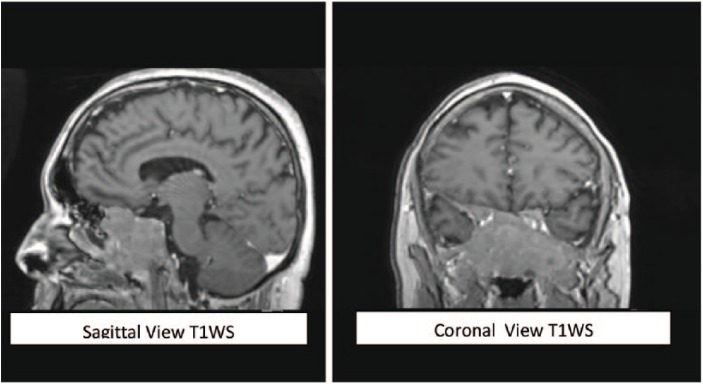
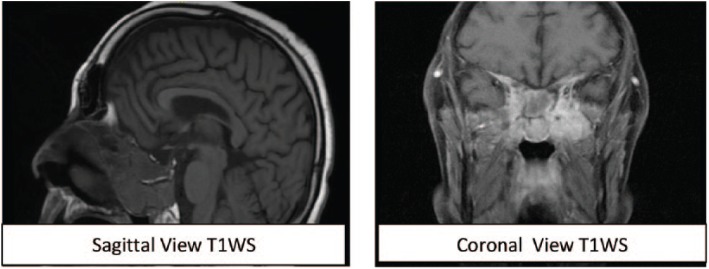
In February 2017, a 31-year-old Caucasian male presented with a 2-year history of resistant hypertension requiring 4 antihypertensive drugs. Typical Cushing appearance on physical examination (round face, dorsal fat pat, wide purple striae, proximal muscle weakness) and rapid weight gain lead to an endocrinologic evaluation. Routine laboratory tests are summarized in Table 1. Besides cushingoid phenotype, he did not complain of headaches or local symptoms. ACTH-dependent Cushing syndrome was confirmed with urinary free cortisol determinations above normal range on two occasions (230 μg/24 h and 301 μg/24 h, reference range below 200 μg/24 h), lack of plasma cortisol response to low-dose dexamethasone suppression test and high plasmatic ACTH levels (164 pg/mL; reference range: 10 to 60 pg/mL). To establish the etiology of the ACTH source (Cushing disease or an ectopic tumor),the patient underwent two non-invasive dynamic tests consistent in a DDAVP (desmopressin) stimulation test and a high-dose dexamethasone suppression test as shown in Table 2.Plasma cortisol increases after the DDAVP test and cortisol decreases after 8 mg of nocturnal dexamethasone suggested Cushing disease (Figure 1). A pituitary magnetic resonance image (MRI) was obtained revealing a large mass (36 × 46 × 46 mm) in the skull base centered in the sphenoid sinus with caudal extension in the nasopharynx and posterior extension in the clivus. The mass completely involved the sella (preventing pituitary gland identification) although without compromising the pituitary stalk and the optic chiasm (Figure 2). The mass was also identified with an Indium-111 octreotide scan confirming somatostatin receptor expression. A trasnasal biopsy of the lesion demonstrated an ACTH secreting ONB. Neoplastic cells showed a lobular architecture and were small with speckled nuclear chromatin and mild nuclear pleomorphism in a dense fibrillary stroma. Positive inmunoreactivity for synaptophysin, CD56, chromogranin, and neuron-specific enolase confirmed the neural tumor origin. Focal S100 staining was also found at the periphery and ACTH positivity was demonstrated in more than 50% of cells. The tumor was staged histologically as Hyams grade I (based on histological architecture and mitotic activity) and clinically as Kadish stage C (based on tumor extension). Patient refused surgery and was treated with neoadjuvant chemotherapy (etoposide and cisplatin for three cycles) followed by a full course of fractionated radiotherapy (50 Gy). Ketoconazole was started for hypercortisolism control but had to be discontinued after 6 weeks of therapy (800 mg daily) because of hepatotoxicity and was changed to metyrapone at increasing doses up to 3000 mg daily in divided doses. Despite the use of steroidogenesis enzyme inhibitors, eucortisolism (defined by normal free urinary cortisol level on two consecutive determinations) was not achieved until 1 month after radiotherapy. At that moment a dramatic clinical improvement was observed and antihypertensive drugs could be withdrawn. A new MRI revealed mass stability persisting in the last follow-up 1 year after radiotherapy (Figure 3).
Table 1.
Initial routine blood tests and hormonal values.
| Normal range | Patient results | ||
|---|---|---|---|
| Complete blood cell count | WBC | 4000-10 500 | 11 200 |
| Neutrophils | 1500-6600 | 8400 | |
| Lymphocytes | 1500-3500 | 1900 | |
| Hb | 13.5-18 | 14.1 | |
| Hc | 42-55 | 41.7 | |
| Liver function tests | ALT (U/L) | 5-40 | 27 |
| AST (U/L) | 5-40 | 26 | |
| Renal function tests | Creatinine (mg/dL) | 0.6-1.1 | 0.9 |
| Urea (mg/dL) | 0-50 | 74 | |
| CKD-EPI (mL/min) | 60-140 | 79.3 | |
| Glucose | mg/dL | 60-100 | 116 |
| Sodium | mmol/L | 135-145 | 142 |
| Potassium | mmol/L | 3.4-5.5 | 3.9 |
| LDH | U/L | 240-480 | 813 |
| Hormonal values | LH (IU/L) | 1.2-8.6 | 2 |
| FSH (IU/L) | 1.3-19.3 | 5.1 | |
| Testosterone (nmol/L) | 6-27 | 8.2 | |
| DHEAS (ng/mL) | 6.6-3.1 | 5.1 | |
| GH (ng/dL) | 0.05-3 | 0.05 | |
| IGF1 (ng/mL) | 41-246 | 92.5 | |
| TSH (uUI/mL) | 0.4-5.3 | 0.22 | |
| Free T4 (pg/mL) | 5.8-16.4 | 7.2 |
Abnormal values are marked in bold.
Abbreviations: WBC, White Blood Cells count; ALT, Alanine Aminotransferase; AST, Apartate aminotransferase; CKD_EPI, chronic kidney disease epidemiology collaboration (ckd_epi) creatinine equation; LDH, Lactate dehydrogenase; LH, Luteinizing hormone; FSH, Follicule stimulating hormone; DHEAS, dehydroepiandrosterone sulfate; GH, Growth Hormone; IGF1, Insuline like factor1; TSH, Thyroid stimulating hormone.
Table 2.
Dynamic endocrine tests of ACTH-dependent Cushing syndrome.
| High-dose dexamethasone suppression test (8 mg given orally at 23 hPM the day before) | |||||||
| Basal | 9 AM | ||||||
| Cortisol (μg/dL) | 25.2 | 8.7a | |||||
| DDAVP stimulation test (10 μg of desmopressin given intravenously) | |||||||
| Basal | 15 min | 30 min | 45 min | 60 min | 90 min | 120 min | |
| Cortisol (μg/dL) | 25.2 | 32.1 | 36.1 | 45.9b | 40.2 | 38.6 | 30.9 |
| ACTH (pg/mL) | 141 | 987c | 493 | 305 | 257 | 144 | 116 |
Abbreviation: ACTH, adrenocorticotropic hormone.
75% of suppression.
82% of increase.
600% of increase.
Figure 1.
Changes in plasma cortisol after dynamic endocrine tests.
Figure 2.
A contrast-enhanced magnetic resonance image of the brain showing a large mass in the skull base involving the sella and extending in the nasopharynx the clivus.
Figure 3.
A magnetic resonance image of the brain 1 year after radiotherapy showing the stability of the tumor size.
Discussion
ONB is a rare malignancy3 with an annual incidence of 0.4-1 in 1 000 000 most commonly diagnosed during the second and sixth decade of life by local symptoms (nasal congestion, anosmia, epistaxis) with an equal sex distribution. Histologically, it shares morphologic features with other small round blue cells neoplasms and the differential diagnosis is not only based on morphologic cytology and architecture but also on inmunohistochemical stains.4 The optimal treatment has not been established due to the disease rarity but observational studies suggest that a combination of surgery and radiotherapy yields lower mortality and recurrence rates than using one modality alone5,6 (5 years survival rates of 65% for the combined treatment and below 50% with surgery or radiotherapy alone). Globally, recurrence disease occurs in almost half of the patients with a median time to recurrence of 7 years.7 Prognosis is also related to the initial mass extension with the best outcomes for tumors limited to the nasal fossa (Kadish Stage A)8 and higher recurrence and mortality rates for Kadish Stage B (extension to the paranasal sinus), Kadish Stage C (extension beyond the paranasal sinus and nasal cavity), and Kadish Stage D (lymph nodes or distant metastasis). Radiotherapy alone has been used in Kadish Stage A and in patients with irresectable neoplasms or who refused surgery as in our case.9 Expected results in Stage C are limited. Chemotherapy as part of a multimodality treatment is reserved for patients with advanced or metastatic disease10 with cisplatin-based regimens preferred at some institutions and is associated with few successful outcomes. Ectopic ACTH Cushing syndrome due to ONB is exceptional with 17 histologically confirmed cases found in the literature.3,11–20 The diagnosis of an ectopic ACTH syndrome requires the exclusion of the most prevalent ACTH-dependent Cushing from pituitary disease. For the distinction between the two entities, dynamic endocrine tests are recommended including suppressive tests (high-dose dexamethasone suppression tests) and stimulation tests (as corticotropin-releasing hormone [CRH] test or DDAVP). In this case, both tests incorrectly suggested an ACTH pituitary source highlighting the low specificity of these tests for the differential diagnosis of Cushing disease and ectopic ACTH syndrome.21,22 In some reported ectopic ACTH Cushing syndromes due to an ONB,12,16 plasma cortisol failed to suppress after high-dose dexamethasone test and did not rise after CRH test suggesting a true ectopic ACTH syndrome. Nevertheless, in other cases, where dynamic test was registered, a variety of responses have been observed. A publication of Kanno et al14 described a woman with non-suppressible hypercortisolism to dexamethasone but hyperresponsive to CRH leading to an inferior petrosal sinus sampling for ACTH whose result still falsely supported a pituitary disease. A hypothetical explanation in this case could be a cyclic Cushing syndrome with the dynamic tests done during an episode of low or absent ACTH neoplastic secretion so the responses could be similar to those expected with a normal hypothalamic-pituitary-adrenal axis. In the few cases reported in the literature, adrenalitic drugs (as ketoconazole and metyrapone) usually decreased cortisol but normocortisolism only was reached once local control tumor was achieved with surgery and/or radiotherapy.14,16-18 In one case,20 a bilateral adrenalectomy was necessary for hormonal improvement before a definitive tumor resection could be performed.
Conclusions
Ectopic ACTH production is a rare manifestation of ONB. We report another case of this uncommon tumor. This case also illustrates the difficulties related to the interpretation of dynamic endocrine tests in Cushing syndrome.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: CF wrote the first draft of the manuscript. AA and AA jointly developed the structure and arguments of the paper and made critical revisions and approved the final version of the manuscript. Both authors reviewed and approved the final manuscript.
References
- 1. Broich G, Pagliari A, Ottaviani F. Esthesioneuroblastoma: a general review of the cases published since the discovery of the tumour in 1924. Anticancer Res. 1996;17:2683–2706. [PubMed] [Google Scholar]
- 2. Kunc M, Gabrych A, Czapiewski P, Sworczak K. Paraneoplastic syndromes in olfactory neuroblastoma. Contemp Oncol (Pozn). 2015;19:6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thompson LD. Olfactory neuroblastoma. Head Neck Pathol. 2009;3:252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hirose T, Scheithauer BW, Lopes MB, et al. Olfactory neuroblastoma: an immunohistochemical, ultrastructural, and flow cytometric study. Cancer. 1995;76:4–19. [DOI] [PubMed] [Google Scholar]
- 5. Dulguerov P, Allal AS, Calcaterra TC. Esthesioneuroblastoma: a meta-analysis and review. Lancet Oncol. 2001;2:683–690. [DOI] [PubMed] [Google Scholar]
- 6. Ozsahin M, Gruber G, Olszyk O, et al. Outcome and prognostic factors in olfactory neuroblastoma: a rare cancer network study. Int J Radiat Oncol Biol Phys. 2010;78:992–997. [DOI] [PubMed] [Google Scholar]
- 7. Ow TJ, Hanna EY, Roberts DB, et al. Optimization of long-term outcomes for patients with esthesioneuroblastoma. Head Neck. 2014;36:524–530. [DOI] [PubMed] [Google Scholar]
- 8. Morita A, Ebersold MJ, Olsen KD. Esthesioneuroblastoma: prognosis and management. Neurosurgery. 1993;32:706–714. [DOI] [PubMed] [Google Scholar]
- 9. Benfari G, Fusconi M, Ciofalo A, et al. Radiotherapy alone for local tumour control in esthesioneuroblastoma. Acta Otorhinolaryngol Ital. 2008;28:292–297. [PMC free article] [PubMed] [Google Scholar]
- 10. Gupta S, Husain N, Sundar S. Esthesioneuroblastoma chemotherapy and radiotherapy for extensive disease: a case report. World J Surg Oncol. 2011;5:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arnesen MA, Scheithauer BW, Freeman S. Cushing’s syndrome secondary to olfactory neuroblastoma. Ultrastruct Pathol. 1994;18:61–68. [DOI] [PubMed] [Google Scholar]
- 12. Yu J, Koch CA, Patsalides A, et al. Ectopic Cushing’s syndrome caused by an esthesioneuroblastoma. Endocr Pract. 2004;10:119–124. [DOI] [PubMed] [Google Scholar]
- 13. Fish S, Harish S, Tapino E. Ectopic ACTH syndrome caused by olfactory neuroblastoma. Resid Staff Physician. 2005;51:30–33. [Google Scholar]
- 14. Kanno K, Morokuma Y, Tateno T, et al. Olfactory neuroblastoma causing ectopic ACTH syndrome. Endocr J. 2005;52:675–681. [DOI] [PubMed] [Google Scholar]
- 15. Josephs L, Jones L, Marenette L, McKeever P. Cushing’s syndrome: an unusual presentation of olfactory neuroblastoma. Skull Base. 2008;18:73–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koo BK, An JH, Jeon KH, et al. Two cases of ectopic adrenocorticotropic hormone syndrome with olfactory neuroblastoma and literature review. Endocr J. 2008;55:469–475. [DOI] [PubMed] [Google Scholar]
- 17. Hodish I, Giordano TJ, Starkman MN, Schteingart DE. Location of ectopic adrenocortical hormone-secreting tumors causing Cushing’s syndrome in the paranasal sinuses. Head Neck. 2009;31:699–706. [DOI] [PubMed] [Google Scholar]
- 18. Mintzer DM, Zheng S, Nagamine M, Newman J, Benito M. Esthesioneuroblastoma (Olfactory Neuroblastoma) with Ectopic ACTH Syndrome: a multidisciplinary case presentation from the Joan Karnell cancer center of Pennsylvania Hospital. Oncologist. 2010;15:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Galioto S, Di Petrillo A, Pastori M, Arecchi A. Metastatic esthesioneuroblastoma secreting adrenocorticotropic hormone in pediatric patients. J Craniofac Surg. 2011;22:1924–1929. [DOI] [PubMed] [Google Scholar]
- 20. Han JY, Mirsadraei L, Yeh MW, et al. Bilateral adrenalectomy: lifesaving procedure in severe Cushing syndrome. Endocr Pract. 2012;18: e85–e90. [DOI] [PubMed] [Google Scholar]
- 21. Aron DC, Raff H, Findling JW. Effectiveness versus efficacy: the limited value in clinical practice of high dose dexamethasone suppression testing in the differential diagnosis of adrenocorticotropin-dependent Cushing’s syndrome. J Clin Endocrinol Metab. 1997;82:1780–1785. [DOI] [PubMed] [Google Scholar]
- 22. Pecori Giraldi F, Invitti C, Cavagnini F; Study Group of the Italian Society of Endocrinology on the Pathophysiology of the Hypothalamic-Pituitary-Adrenal Axis. The corticotropin-releasing hormone test in the diagnosis of ACTH-dependent Cushing’s syndrome: a reappraisal. Clin Endocrinol (Oxf). 2001;54:601–607. [DOI] [PubMed] [Google Scholar]