Abstract
Introduction. Given the lack of independent analyses comparing numerous pharmacotherapies for osteoporosis, the study objective was to identify the optimal osteoporosis treatment based on a woman’s age, fracture history, and ability to tolerate oral bisphosphonates adopting practices recommended in the recently revised Canadian guidelines. Methods. A cost utility analysis from the health care system perspective compared alendronate, etidronate, risedronate, zoledronate, denosumab, and no pharmacotherapy using a Markov model incorporating data on fracture risk and their associated costs, mortality, and disutility and treatment effect. Stratified analysis was conducted based on age, fracture history, and ability to tolerate oral bisphosphonates. Expected lifetime outcomes were obtained through probabilistic analysis with scenario analyses addressing methodological and structural uncertainty. Results. For women able to tolerate oral bisphosphonates, risedronate and etidronate were dominated. Compared to no therapy, alendronate was either dominant or was associated with a low incremental cost per QALY (quality-adjusted life years) gained (ICER)—less than CAN$3,751 based on age and fracture history. In comparison with alendronate, both zoledronate and denosumab were either dominated or associated with a high ICER—greater than CAN$660,000 per QALY. For women unable to tolerate bisphosphonates, dependent on age and fracture history, the ICER for zoledronate versus no therapy ranged from CAN$17,770 to CAN$94,365 per QALY. For all strata, denosumab was dominated by zoledronate or had an ICER greater than CAN$3.0 million. Scenario analyses found consistent findings. Conclusions. Based on a threshold of CAN$50,000 per QALY, alendronate is optimal for osteoporotic women who can tolerate oral bisphosphonates regardless of age or fracture history. For women unable to tolerate oral bisphosphonates, zoledronate is optimal for women with previous fracture or aged 80 to 84 or over 90 with no previous fracture.
Keywords: bisphosphonates, cost-effectiveness, osteoporosis
Osteoporosis is a progressive bone disease characterized by low bone mass that increases bone fragility leading to an increase in fracture risk.1 Osteoporosis can be diagnosed either by the presence of fragility fractures or based on the World Health Organization criteria relating to bone mass: having a bone mineral density (BMD) that is at least 2.5 standard deviations (SD) below the mean peak bone mass of an average young female.2 In Canada, the prevalence of osteoporosis in postmenopausal women increases from approximately 6% in those aged 50 to 59 years to over 40% in those aged over 80.3
The most common fractures associated with osteoporosis are fractures of the hip, vertebrae, or wrist. The major source of morbidity from osteoporosis arises from hip fractures, which are also associated with higher costs and greater mortality.4,5 Alongside the increasing prevalence of osteoporosis, the risk of fracture for an osteoporotic women increases with age.3,6 Fracture risk is also related to previous history of fracture and the degree of low bone mass.2,7
The annual health care cost in 2010 associated with osteoporosis in Canada was CAN$2.3 billion with an additional CAN$1.6 billion for associated use of long-term care facilities.4 The cost of treating hip fractures comprised more than half of the acute care costs associated with osteoporosis.4 Pharmacological treatments for osteoporosis covered in Ontario, the most populous of Canadian provinces, are bisphosphonates (alendronate, etidronate, risedronate, and zoledronate) and the RANKL (receptor activator of nuclear factor kappa-B ligand) inhibitor—denosumab. The annual costs of osteoporosis drug treatments vary considerably although such costs may by partly offset by reducing the economic burden of fracture.
In April 2017, the Canadian Agency for Drugs and Technologies in Health (CADTH) released the 4th edition of the Canadian Guidelines for the Economic Evaluation of Health Technologies.8 The Guidelines represent a major revision from the previous edition, which was published in 2006, to reflect a number of methodological advances that haven taken place in the conduct of economic evaluations and the adoption of a cohesive and appropriate theoretical framework with emphasis on the role of economic evaluation as an input to decision-making processes.9
Within Canada, economic evaluations are conducted primarily to facilitate health care decisions within a publicly funded system. Thus, developers of the guidelines recognized the need to adopt a social decision-making approach as the theoretical paradigm to root the guidelines with the assumption that decision makers within the publicly funded system primarily wish to maximize population health given their budget constraints. Changes within the Guidelines were therefore made to be consistent with the adoption of this paradigm. Significant revisions related to specification of the decision problem, the need for stratified analysis, adopting a theoretically driven discount rate, and the use of probabilistic analysis in the base case: with greater clarity provided in the recommendation for the adoption of a health care system perspective. The objective of this study was to assess the cost-effectiveness of the various pharmacotherapies and identify which treatments are optimal, depending on a woman’s age and fracture history and for the subgroup of women who may not be able to tolerate oral bisphosphonates using methods consistent with the revised Canadian guidelines. Thus, analysis was based on defining the relevant decision problem and then cost-effectiveness was assessed with appropriate consideration of uncertainty and variability.
Methods
Decision Problem
The study was designed to address specific decision problems as required within the recently revised Canadian Guidelines for Economic Evaluation.8 The Guidelines replaced an existing section titled “Study Objective” with a section titled “Decision Problem.” This change recognizes that economic evaluations are primarily designed to inform decision making and specification of a decision problem provides a cohesive basis from which to design the research. The decision problem requires specification of the perspective, interventions, metrics (e.g., costs, outcomes) used to compare the interventions and time horizon.
The decision problem that this analysis addresses is which of the currently available pharmacotherapies for osteoporosis a provincial health ministry, as the payer of prescription medications, should cover within a provincial formulary. Analysis incorporates all pharmacotherapies for osteoporosis currently covered by Canadian provincial formularies (alendronate, denosumab, etidronate, risedronate, and zoledronate). As recommended within the Guidelines, analysis includes a no active pharmacotherapy alternative given that in certain patient populations none of the existing pharmacotherapies may be cost-effective. This, in addition, reflects the previous restrictive listing basis for bisphosphonates in Ontario. For all comparators, patients may in addition be taking calcium and/or vitamin D as is typical in osteoporosis clinical studies. The cost-effectiveness of treatments may vary by patient characteristic. A major change in the Guidelines is in the handling of heterogeneity. The cost-effectiveness of an intervention depends on the characteristics of the population for which it is being evaluated. As the existence of heterogeneity will lead to different conclusions, stratified analysis that requires the population to be parsed into smaller, more homogeneous subgroups is required.10,11 The requirement for stratified analysis directly relates to the adoption of the social decision-making viewpoint. Within this study, there are multiple decision problems relating to various patient strata. For illustration, a base case analysis is presented for a cohort of 70- to 74-year-old osteoporotic women with no previous fracture who are able to tolerate oral bisphosphonates. The analysis is conducted, however, for different patients started based on the cohort’s initial age group (65–69, 70–74, 75–79, 80–84, 85–89, 90+), fracture history (no previous fracture and previous fracture), and whether or not the individual can tolerate oral bisphosphonates. Intolerance or inability to take oral bisphosphonates is based on the definition from the Ontario Drug Benefit program: either hypersensitivity, abnormalities of the esophagus, or an inability to stand or sit upright for at least 30 minutes.
Given the focus on maximizing population, analysis takes the form of a cost utility analysis with a lifetime time horizon, where outcomes are expressed in terms of quality-adjusted life years (QALYs) with analysis presented in terms of the incremental cost per QALY gained (ICER).8
Based on the preferences of the relevant decision makers, analysis adopts the perspective of the health and social care system in that the costs of health, social services, and long-term care (LTC) are included.8 Adoption of a wider perspective would require that health care decision makers would be willing to trade health gains for benefits to other sectors.
For the base case analysis, costs and benefits were discounted at 1.5% per annum.8 Scenario analysis was conducted with discounting at 0%, 3%, and 5%.8 The revised Guidelines recommended adoption of a social discount rate represented by the real rate of interest on provincial government bonds.12 The revised rate is based on the available empirical evidence.12
Model Design
The analysis was conducted using a decision analytic model for osteoporosis developed based on the most recently available data relevant to the Canadian population. The model used is an update of a model employed in several previous analyses and follows guidelines both for the design of economic models in general and for the design of economic models specific to osteoporosis.8,13–16 The major previous use of the model was in a health technology assessment conducted by CADTH, which examined the cost effectiveness of teriparatide compared to bisphosphonates, which informed provincial decisions relating to this product.14 The model was chosen due to it being independent and to its convergent validity in that it replicates the population-level data available from the data sources. Further details of calibration and validation are provided in an online appendix.
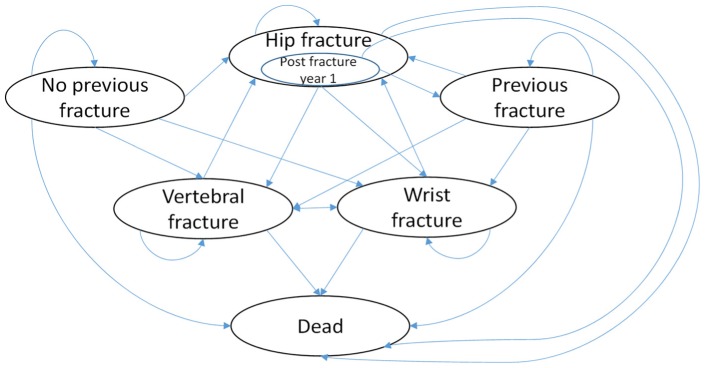
Analysis was conducted using a Markov model with 1-year cycle length and a lifetime horizon. The cycle length was chosen based on the unlikeliness of an individual having more than one hip fracture (the event associated with the greatest cost and disutility) in 1 year. The model incorporates the sequelae associated with osteoporosis (e.g., fracture) and the transition of women through different states related to the development of osteoporosis, history of fracture, and residential status. A schematic of the model is provided in Figure 1.
Figure 1.
Schematic of Markov model. Schematic illustrates all possible transitions form one cycle to the next. Patients with a hip fracture will either enter into the post-fracture state for 1 year, have a repeat hip fracture, or die. Note that the schematic does not illustrate transitions relating to movement in residential status. Patients can transition from living to the community to living in long-term care from any health state.
For the base case the cohort will start the model in the no fracture history state. For each year, a proportion of the cohort can transition into a fracture-related state (hip, wrist, or vertebral), can die, or remain in the no fracture history state. The proportion of the cohort who enter the hip fracture state can in the next cycle either transition into the hip fracture post fracture year 1 state, can die, or can have a repeat hip fracture. The proportion of the cohort in the wrist, vertebral, or hip fracture post year 1 state can transition into any of the fracture event states, die, or enter the previous fracture history state.
The model is populated with relevant transition probabilities and estimates of the costs and utilities associated with each health state3,4,6,7,17–35 (Table 1). Data sources were obtained through review of the available literature and focused on identifying the most recent relevant and appropriate data from a Canadian context.36 Expected values of costs and QALYs for each pharmacotherapy for each patient strata are obtained through probabilistic analysis using Monte Carlo simulation.8 The same technique is used for all strata-specific analyses and all scenario analyses. The model is developed within a Microsoft Excel workbook.
Table 1.
Parameter Estimatesa
| Parameter | Base Value | Probability Distribution | Reference |
|---|---|---|---|
| Natural history data | |||
| Relative risk of wrist fracture for each 1 SD decrease in bone density | 1.4 | Lognormal (1.4, 1.6) | 7 |
| Relative increase of hip fracture for 1 each SD decrease in bone density | 2.6 | Lognormal (2, 3.5) | 7 |
| Relative increase of vertebral fracture for each 1 SD decrease in bone density | 1.8 | Lognormal (1.1, 2.7) | 7 |
| Relative risk of hip fracture given previous fracture | 2 | Lognormal (1.9, 2.2) | 30 |
| Relative risk of wrist fracture given previous fracture | 1.9 | Lognormal (1.3, 2.8) | 30 |
| Relative risk of spine fracture given previous fracture | 2 | Lognormal (1.6, 2.4) | 30 |
| Relative risk of hip fracture given living in LTC | 1.5 | Lognormal (1.3, 1.7) | 31 |
| Relative risk of mortality post hip fracture and living in LTC | 3.24 | Lognormal (2.37, 4.43) | 34 |
| Relative risk of mortality post vertebral fracture | 1.16 | Lognormal (1.03, 1.3) | 35 |
| Relative risk of mortality post hip fracture | 2.87 | Lognormal (2.52, 3.27) | 5 |
| Relative risk of mortality given living in LTC | 1.16 | Lognormal (1.1, 1.2) | 17 |
| Average peak bone mass | 0.857 | Normal (0.857, 0.022) | 3 |
| Bone mass by age | |||
| 50–59 | 0.759 | Normal (0.759, 0.003) | 3 |
| 60–69 | 0.695 | Normal (0.695, 0.003) | 3 |
| 70–79 | 0.661 | Normal (0.661, 0.003) | 3 |
| 80+ | 0.593 | Normal (0.593, 0.006) | 3 |
| Standard deviation for peak bone mass | 0.125 | 3 | |
| Standard deviation of bone mass by age | |||
| 50–59 | 0.119 | 3 | |
| 60–69 | 0.110 | 3 | |
| 70–79 | 0.114 | 3 | |
| 80+ | 0.104 | 3 | |
| Annual probability of vertebral fracture | |||
| 50–59 | 0.0018 | Beta (8.05, 4559.45) | 6 |
| 60–69 | 0.0015 | Beta (4.01, 2626.34) | 6 |
| 70–79 | 0.0039 | Beta (74.44, 18813.8) | 6 |
| 80+ | 0.0076 | Beta (137.16, 17832.5) | 6 |
| Annual probability of wrist fracture | |||
| 50–59 | 0.0031 | Beta (14.08, 4550.05) | 6 |
| 60–69 | 0.0062 | Beta (16.18, 2613.58) | 6 |
| 70–79 | 0.0086 | Beta (309, 35802.22) | 6 |
| 80+ | 0.0076 | Beta (137.16, 17832.5) | 6 |
| Annual probability of hip fracture | |||
| 50–59 | 0.0003 | Beta (2.12, 7215.24) | 6 |
| 60–69 | 0.0018 | Beta (4.84, 2623.84) | 6 |
| 70–79 | 0.0024 | Beta (45.71, 18834) | 6 |
| 80+ | 0.0064 | Beta (115.92, 17861.69) | 6 |
| Proportion of women residing in LTC | |||
| 65–69 | 0.01 | Beta (8,952, 886,248) | 32 |
| 70–74 | 0.023 | Beta (15,235, 647,165) | 32 |
| 75–79 | 0.057 | Beta (29,372, 485,928) | 32 |
| 80–84 | 0.136 | Beta (57,120, 362,880) | 32 |
| >85 | 0.334 | Beta (156,446, 311,954) | 32 |
| Proportion of women who are osteoporotic | |||
| 50–59 | 0.060 | Beta (1,196, 1,273) | 3 |
| 60–69 | 0.183 | Beta (1,505, 1,841) | 3 |
| 70–79 | 0.269 | Beta (991, 1,356) | 3 |
| >80 | 0.413 | Beta (184, 313) | 3 |
| Mortality in general population (females) | |||
| 50–54 | 0.0024 | Beta (1,368,800, 1,372,100) | 33 |
| 55–59 | 0.0036 | Beta (1,248,734, 1,253,300) | 33 |
| 60–64 | 0.0057 | Beta (1,061,242, 1,067,300) | 33 |
| 65–69 | 0.0091 | Beta (887,055, 895,200) | 33 |
| 70–74 | 0.0150 | Beta (652,462, 662,400) | 33 |
| 75–79 | 0.0254 | Beta (502,196, 515,300) | 33 |
| 80–84 | 0.0443 | Beta (401,409, 420,000) | 33 |
| 85–89 | 0.0789 | Beta (265,173, 287,900) | 33 |
| >90 | 0.1772 | Beta (148,523, 180,500) | 33 |
| Proportion of vertebral fractures by treatment requirement | |||
| Hospitalized | 0.09 | Dirichlet (18, 47, 132) | 6 |
| Physician care | 0.24 | 6 | |
| No treatment | 0.67 | 6 | |
| Proportion of wrist fractures requiring hospitalization | 0.10 | Beta (3,697, 33,341) | 4 |
| Treatment effectiveness | |||
| Relative reduction in hip fractures | |||
| Alendronate | 0.59 | Lognormal (0.29, 0.99) | 26 |
| Etidronate | 1.02 | Lognormal (0.12, 3.71) | 26 |
| Risedronate | 0.78 | Lognormal (0.44, 1.31) | 26 |
| Denosumab | 0.67 | Lognormal (0.24, 1.46) | 26 |
| Zoledronate | 0.65 | Lognormal (0.25, 1.33) | 26 |
| Relative reduction in wrist fractures | |||
| Alendronate | 0.93 | Lognormal (0.31, 2.51) | 26 |
| Etidronate | 2.32 | Lognormal (0.26, 8.13) | 26 |
| Risedronate | 0.91 | Lognormal (0.13, 3.06) | 26 |
| Denosumab | 0.84 | Lognormal (0.64, 1.11) | 26 |
| Relative reduction in vertebral fractures | |||
| Alendronate | 0.54 | Lognormal (0.4, 0.7) | 26 |
| Etidronate | 0.64 | Lognormal (0.31, 1.07) | 26 |
| Risedronate | 0.66 | Lognormal (0.48, 0.81) | 26 |
| Denosumab | 0.33 | Lognormal (0.23, 0.47) | 26 |
| Zoledronate | 0.30 | Lognormal (0.21, 0.43) | 26 |
| Treatment continuation rates | |||
| Alendronate (daily) | 0.65 | Beta (65, 35) | 14 |
| Etidronate | 0.57 | Beta (57, 43) | 14 |
| Risedronate (daily) | 0.62 | Beta (62, 38) | 14 |
| Relative reduction in noncompliance | |||
| Once weekly bisphosphonates versus once daily | 0.719 | Lognormal (0.7126, 0.7265) | 27 |
| Denosumab versus bisphosphonates | 0.540 | Lognormal (0.31, 0.93) | 28 |
| Zoledronate versus denosumab | 1.256 | Lognormal (1.15, 1.37) | 29 |
| Cost of health care events | |||
| Hip fracture—living in the community | 50513.75 | Gamma (50,514, 401) | 18,19 |
| Hip fracture—living in LTC | 19582.77 | Gamma (19,583, 403) | 18,19 |
| Hip fracture—women who die following fracture | 12207.83 | Gamma (12,208, 1429) | 18,19 |
| 2nd year post hip fracture | 5134.32 | Gamma (5,134, 210) | 18,19 |
| Wrist fracture—ambulatory | 411.40 | Gamma (411, 4) | 21 |
| Wrist fracture—hospitalized | 8557.40 | Gamma (8,557, 435) | 20 |
| Vertebral fracture—ambulatory | 612.40 | Gamma (612, 10) | 21 |
| Vertebral fracture—hospitalized | 12613.40 | Gamma (12,613, 559) | 20 |
| No fracture—living in LTC | 46301.57 | Gamma (46,302, 953) | 18,19 |
| No fracture—living in community | 9086.38 | Gamma (9,086, 72) | 18,19 |
| Annual drug costs | |||
| Alendronate | 153.66 | Fixed | 22 |
| Etidronate | 122.88 | Fixed | 22 |
| Risedronate | 180.31 | Fixed | 22 |
| Denosumab | 825.67 | Fixed | 22 |
| Zoledronate | 371.06 | Fixed | 22 |
| Utility values | |||
| Women aged 65–69—no fracture | 0.836 | 1 − Lognormal (0.164, 0.004) | 23 |
| Women aged 70–74—no fracture | 0.824 | 1 − Lognormal (0.176, 0.004) | 23 |
| Women aged 75–79—no fracture | 0.792 | 1 − Lognormal (0.208, 0.005) | 23 |
| Women aged >80—no fracture | 0.712 | 1 − Lognormal (0.288, 0.005) | 23 |
| Hip fracture—1st year—utility multiplier | 0.7 | 1 − Lognormal (0.3, 0.033) | 24 |
| Hip fracture—2nd year—utility multiplier | 0.8 | 1 − Lognormal (0.2, 0.071) | 24 |
| Vertebral fracture—hospitalized—utility multiplier | 0.59 | 1 − Lognormal (0.41, 0.094) | 24 |
| Wrist—utility multiplier | 0.956 | 1 − Lognormal (0.044, 0.036) | 24 |
| Vertebral fracture—not hospitalized—utility multiplier | 0.909 | 1 − Lognormal (0.091, 0.043) | 25 |
LTC, long-term care; SD, standard deviation.
Beta and gamma distributions depicted by shape and scale parameters. Dirichlet distribution depicted by concentration parameters. Normal distributions depicted by mean and standard errors. Lognormal distribution depicted by upper and lower bounds of the 95% confidence interval. Costs represent CAN$ in 2017.
Transition Probabilities
Given the structure of the model outlined above, the following transition probabilities are required to allow a simulation of progression through the model: probabilities of hip, wrist, and spine fracture; probability of developing osteoporosis (which is required for calibration purposes as described in the online appendix); probability of being admitted to LTC; and probability of death.
Each of these probabilities will vary by a number of factors. The probability of developing osteoporosis will increase with age.3 The probability of fracture increases with age, residence in LTC, and previous history.6,7,30,31 Admission to LTC increases with age.32 The probability of death increases with age, incidence of fracture, and residence in LTC.5,17,33–35 Specific data on probabilities by risk factors were often unavailable. However, data for alternative parameters were available, which allowed computation of the necessary parameters through calibration of the model. Further details of the required calibration and convergent validity of the model are provided in the online appendix.
Costs
For each particular state within the decision model, there is an associated estimate of costs (adjusted to 2017 Canadian dollars). Consistent with the adopted perspective, costs relate to the management of fractures, osteoporosis, and subsequent admission to LTC. Scenario analysis included the costs of additional non-osteoporosis health care.
The cost of the health care resources associated with the treatment of a hip fracture in Ontario including immediate acute care, rehabilitation, and institutionalization were obtained from an analysis of health care administrative claims and billing data available at the Institute for Clinical Evaluative Sciences.18,19 The costs of treating a woman with vertebral and wrist fractures that required hospitalization were obtained from an analysis of administrative data held by the Ontario Case Costing Initiative.20 Resource use associated with treating a women with vertebral and wrist fractures that do not require hospitalization were assumed to be the same as from a previous Canadian economic study with current costs obtained from a provincial ministry of health.21,37,38 The proportions of women with these fractures requiring hospitalization was derived from the incidence data of fracture from CaMos and a recent burden of illness study.4,6
Drug costs were obtained from the Ontario Drug Benefit Formulary.22 The yearly cost of each product was obtained by summing the acquisition costs of the medication, an additional 8% markup, and the appropriate number of dispensing fees at a cost of CAN$8.83 each (4 for alendronate, etidronate, and risedronate; 2 for zoledronate; and 1 for denosumab).
Utilities
Utility values for women with normal health status were obtained by age from the 2014 Canadian Community Health Survey.23 Utility multipliers associated with hip and wrist fractures and vertebral fractures not requiring hospitalization were derived from a systematic review of utility values for fractures24,25 (Table 1).
Treatment-Specific Parameters
The analysis plan was to adopt the effect of alternative treatments on the risks of fractures from a published network meta-analysis. On review, a number of meta-analyses have been conducted. Given the decision problem that the study is addressing, the meta-analysis would preferably be comprehensive in their inclusion of studies, focused on osteoporotic women and cover at least the comparators of interest. Based on these considerations the most appropriate analysis was conducted by Hopkins and colleagues.26 Analysis adopted estimates derived from a Bayesian indirect treatment comparison with odds ratios converted to relative risks based on prevalence in the placebo groups.26 The Hopkins study covered all major clinical trials in this area; however, it was published prior to the publication of a companion study to the FREEDOM study, which provides the sole evidence of the effect of denosumab on wrist fractures.39,40 This new data were incorporated into the analysis. There is currently no data relating to the impact of zoledronate on wrist fractures, so the same incidence of wrist fractures as with no therapy was assumed.
While the epidemiological data detailed above provide evidence on probabilities across all ages, fracture history, and time; data on treatment effectiveness are restricted to single relative risks relating to the time horizon of the clinical trials. Thus, analysis did make the assumption that there would be a continuance of treatment effect for the duration of therapy; however, given the duration of clinical trials this assumption is likely valid.
Treatment duration was assumed to be a maximum of 5 years. There is evidence that patients experience continued reductions in the risk of fracture after stopping therapy. Thus, the model allows for a fracture set time whereby there is a linear reduction of benefit after the curtailment of therapy up to a set period of time. Base analysis assumed a set time of 2 years with scenario analyses adopting a set time of 0 and 5 years.14,41–43
Adherence was incorporated into the model by assuming that a proportion of patients would stop treatment within 12 months of commencement—those who continue would continue for the maximum duration of treatment specified in the analysis. It is necessary to incorporate differential adherence with pharmacotherapies based primarily on their frequency. Data on 12-month adherence to daily alendronate, etidronate, and risedronate were based on data for Ontario.14 Improved adherence for weekly bisphosphonates versus daily was modelled based on relative adherence rates from an analysis of administrative data.27 Improved adherence with denosumab versus weekly bisphosphonates was modelled based on data from a randomized controlled trial.28 The relative adherence with zoledronate versus denosumab was modelled based on data from a recent retrospective observational study.29 Thus, annual adherence rates adopted within the study were 57% for etidronate, 74.8% for weekly alendronate, 72.7% for weekly risedronate, 86.4% for denosumab, and 82.9% for zoledronate.
Analysis
Analysis is presented according to the recent Canadian guidelines.8 Given the objective of maximizing population health, the Guidelines require that costs and outcomes for each intervention must be obtained through probabilistic analysis given that deterministic analysis gives biased estimates when there are nonlinear relationships among input variables and outputs. The Guidelines, therefore, require the use of probabilistic analysis within the base case rather than as probabilistic sensitivity analysis. Thus, the expected values for costs and QALYs were obtained through probabilistic analysis using Monte Carlo simulation.8 All input parameters except drug costs were assumed to be random variables rather than fixed values. Analysis involves re-running the model employing different values for each data input randomly selected from a probability density function that is characterized by the mean value, a measure of dispersion (standard error), and type of distribution. Standard distributions were used for each data element: beta and Dirichlet distributions for probabilities, 1 − lognormal distributions for utility values, lognormal distributions for relative effects, and gamma distributions for cost data.44 Analysis was based on a Monte Carlo simulation whereby 5,000 estimates of the costs and QALYs for each treatment were obtained, which was sufficient to obtain stable estimates of each outcome.
As recommended by the Canadian guidelines, disaggregated results are presented both discounted and undiscounted and a sequential analysis is conducted that requires, first, exclusion of all dominated alternatives and then, second, the estimation sequentially of the incremental cost per QALY gained (ICER) for a less costly comparator compared to the next most costly comparator.
The probability that a particular therapy is optimal for different threshold values for a QALY is illustrated by both a cost-effectiveness acceptability curve and a cost-acceptability frontier.8
As recommended within the revised Guidelines, scenario analyses were conducted to assess if the interpretation of the study results would change with a number of alternative methodological assumptions detailed above.8 This is distinct from deterministic sensitivity analysis whereby the expected value of particular inputs are changed to assess the impact on the study’s results and conclusions. The revised Guidelines do not recommend the use of such analyses as they ignore the likelihood of the alternative parameter values and, thus, do not demonstrate the likelihood of the alternative results identified.
A further series of scenario analyses were conducted such that they adopted assumptions similar to those employed in the five previous manufacturer sponsored studies that compared denosumab, risedronate, and alendronate.41,45–48 These studies adopted a number of assumptions favorable toward denosumab: selective and outdated choice of effectiveness data more favorable toward denosumab41,45–48; assuming the impact on mortality of vertebral fractures would be equal48 or greater41,45–47 when compared to hip fracture; much higher costs for vertebral fracture41,45–47 (in some cases, exceeding those of hip fracture45,46); a lack of clarity on whether the model was fully calibrated especially with respect to mortality41,45–48; and inclusion of the branded cost for risedronate.45–48 A scenario analysis was conducted for women aged 70 to 74 with previous fracture, which partially assessed the impact of these assumptions by adopting alternative relative effects, no calibration of mortality, assuming the cost of vertebral fractures would be 50% of the costs of hip fractures, assuming the impact of an incident vertebral fracture on mortality would be equal to that of hip fracture, and adopting branded costs of risedronate.
Threshold analysis was conducted to ascertain the necessary price reduction for zoledronate and denosumab that would be required for these products to be considered optimal given an assumed maximum willingness to pay for a QALY of CAN$50,000.49
Results
Analysis for Base Case Population
For the base case population, denosumab was the most effective treatment in terms of QALYs and life years gained (both discounted and undiscounted; Table 2). However, the gain in discounted QALYs was 0.00015 when compared with zoledronate and 0.0016 when compared with alendronate. Alendronate was associated with lowest lifetime fracture costs and the lowest overall costs of all pharmacotherapies. Alendronate was associated with the lowest number of hip fractures, while denosumab was associated with the lowest number of wrist fractures and denosumab and zoledronate the lowest number of vertebral fractures.
Table 2.
Disaggregated Lifetime Results for 70- to 74-Year-Old Osteoporotic Women With No Previous Fracturea
| No Therapy | Alendronate | Etidronate | Risedronate | Denosumab | Zoledronate | ||
|---|---|---|---|---|---|---|---|
| Undiscounted | Total lifetime costs | $7,047 | $7,078 | $8,370 | $7,468 | $9,993 | $8,057 |
| QALYs | 12.158 | 12.170 | 12.139 | 12.163 | 12.171 | 12.171 | |
| Treatment costs | $ 0 | $571 | $363 | $653 | $3,469 | $1,534 | |
| Fracture costs | $7,047 | $6,506 | $8,007 | $6,815 | $6,524 | $6,523 | |
| Hip fracturesb | 126.4 | 117.4 | 141.2 | 122.4 | 118.1 | 117.9 | |
| Wrist fracturesb | 162.5 | 164.4 | 261.1 | 175.4 | 152.2 | 160.4 | |
| Spine fracturesb | 153.8 | 139.9 | 152.9 | 144.8 | 130.6 | 130.5 | |
| Life years | 16.729 | 16.737 | 16.717 | 16.733 | 16.737 | 16.737 | |
| Discounted | Total lifetime costs | $6,048 | $6,087 | $7,297 | $6,459 | $8,928 | $7,044 |
| QALYs | 10.671 | 10.681 | 10.654 | 10.675 | 10.683 | 10.682 | |
| Treatment costs | $ 0 | $556 | $353 | $635 | $3,371 | $1,492 | |
| Fracture costs | $6,048 | $5,532 | $6,943 | $5,823 | $5,557 | $5,552 | |
| Life years | 14.562 | 14.568 | 14.552 | 14.565 | 14.568 | 14.568 |
QALYs (quality adjusted life years), lifetime fractures per 1,000 women. Costs represent CAN$ in 2017.
Per 1000 women.
When compared to no therapy, alendronate was the only therapy associated with an ICER less than CAN$50,000 per QALY (Table 3). The ICER for alendronate versus no therapy was $3,751 per QALY. The ICER for zoledronate versus alendronate was CAN$666,285 per QALY, and the ICER for denosumab versus zoledronate was CAN$12.9 million per QALY. Risedronate and etidronate were all dominated by alendronate as they were associated with fewer QALYs and higher costs. The ICER for denosumab versus alendronate was CAN$1.8 million.
Table 3.
Sequential Cost Utility Analysis for 70- to 74-Year Old Osteoporotic Women With No Previous Fracturea
| Costs | QALYs | Incremental Cost per QALY Gained Versus No Therapy | Sequential ICER ($/QALY Gained) | |
|---|---|---|---|---|
| Nondominated therapies | ||||
| No therapy | $6,048 | 10.671 | ||
| Alendronate | $6,087 | 10.681 | $3,751 | $3,751 |
| Zoledronate | $7,044 | 10.682 | $83,503 | $666,285 |
| Denosumab | $8,928 | 10.683 | $238,523 | $12,958,077 |
| Dominated therapies | ||||
| Etidronate | $7,297 | 10.654 | Dominated by no therapy | Dominated by no therapy, alendronate, risedronate, and zoledronate |
| Risedronate | $6,459 | 10.675 | $85,557 | Dominated by alendronate |
| Subject to extended dominance through no therapy and zoledronate | ||||
ICER, incremental cost per QALY gained; QALY, quality-adjusted life year.
Costs represent CAN$ in 2017.
The interpretation for decision makers of this result would be the following. If their willingness to pay for a QALY was less than CAN$3,751 per QALY, then for this patient cohort, no therapy would be optimal. If their willingness to pay for a QALY was between CAN$3,751 and CAN$666,285 per QALY, alendronate would be optimal. If their willingness to pay for a QALY was between CAN$666,285 and CAN$12.9 million per QALY, zoledronate would be optimal. And finally, if their willingness to pay for a QALY was greater than CAN$12.9 million per QALY, denosumab would be optimal.
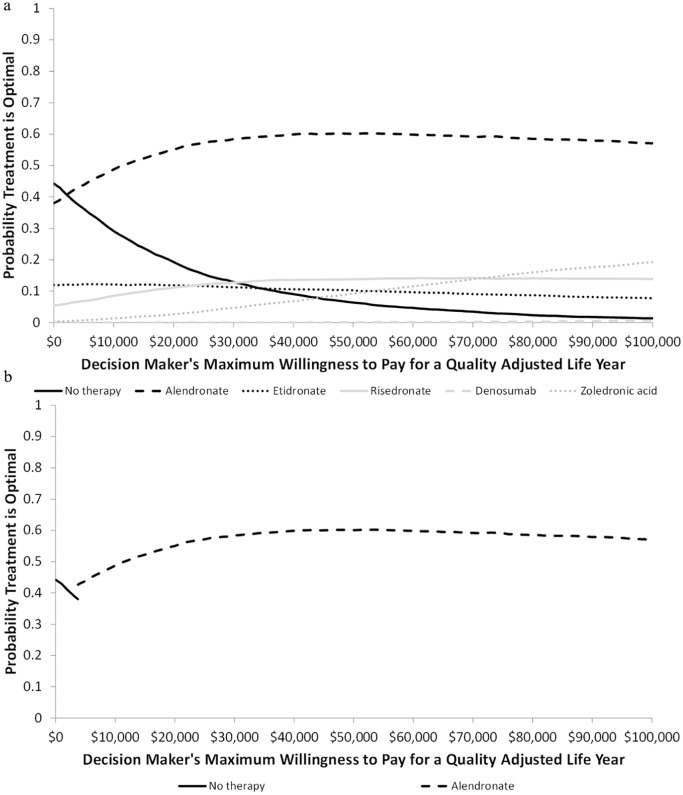
Figure 2a presents the probability that each therapy is optimal based on the threshold value for a QALY ranging from CAN$ 0 to CAN$100,000. For threshold values of a QALY below CAN$2,165, no therapy has the highest probability of being optimal. For values above CAN$2,165, alendronate has the highest probability of being optimal; for values above CAN$12,000 the probability is greater than 50%. At a threshold value of CAN$50,000 per QALY, the probability that each treatment was optimal was 60.1% for alendronate, 13.8% for risedronate, 10.3% for etidronate, 9.3% for zoledronate, 6.4% for no therapy, and 0.1% for denosumab. At threshold value of a CAN$100,000, the probabilities were 59.5% for alendronate, 14.1% for risedronate, 12.9% for zoledronate, 9.4% for etidronate, 3.9% for no therapy, and 0.2% for denosumab.
Figure 2.
Cost-effectiveness acceptability curve and cost-effectiveness frontier for base population: (a) Cost-effectiveness acceptability curve; (b) cost-effectiveness frontier.
Analysis by Patient Strata
Analysis by different strata found consistent results in terms of their interpretation, although estimated ICERs did vary by a woman’s age and fracture history (Table 4). In every strata, alendronate either dominated no therapy or was associated with an ICER of less than CAN$5,000 per QALY. For all strata, alendronate either dominated zoledronate or the ICER for zoledronate versus alendronate was greater than CAN$660,000 per QALY. In most strata, alendronate dominated denosumab. In those strata where denosumab was associated with more QALYs than alendronate, the ICER for denosumab versus alendronate ranged from CAN$1.8 million to CAN$8.0 million per QALY.
Table 4.
Sequential Cost Utility Analysis Results for Alternative Patient Populationsa
| Age | No Previous Fracture | Previous Fracture |
|---|---|---|
| Patients able to tolerate oral bisphosphonates | ||
| 65–69 | ICER for Z v. A = $1.4 million | ICER for Z v. A = $3.1 million |
| ICER for D v. Z = $7.3 million | NT, E, R, and D subject to dominance | |
| NT, E, and R subject to dominance | ||
| 70–74 | ICER for A v. NT = $3,751 | ICER for Z v. A = $1.6 million |
| ICER for Z v. A = $666,285 | NT, E, R, and D subject to dominance | |
| ICER for D v. Z = $13.0 million | ||
| E and R subject to dominance | ||
| 75–79 | ICER for Z v. A = $816,389 | ICER for Z v. A = $5.2 million |
| NT, E, R, and D subject to dominance | NT, E, R, and D subject to dominance | |
| 80–84 | ICER for Z v. A = $1.1 million | A optimal as Z, NT, E, R, and D subject to dominance |
| NT, E, R, and D subject to dominance | ||
| 85–89 | A optimal as Z, NT, E, R, and D subject to dominance | A optimal as Z, NT, E, R, and D subject to dominance |
| 90+ | ICER for A v. NT = $2,721 | A optimal as Z, NT, E, R, and D subject to dominance |
| ICER for Z v. A = $1.3 million | ||
| E, R, and D subject to dominance | ||
| Patients unable to tolerate oral bisphosphonates b | ||
| 65–69 | ICER for Z v. NT = $94,365 | ICER for Z v. NT = $41,374 |
| ICER for D v. Z = $7.3 million | D subject to dominance | |
| 70–74 | ICER for Z v. NT = $83,503 | ICER for Z v. NT = $40,956 |
| ICER for D v. Z = $3.0 million | D subject to dominance | |
| 75–79 | ICER for Z v. NT = $63,263 | ICER for Z v. NT = $20,408 |
| D subject to dominance | D subject to dominance | |
| 80–84 | ICER for Z v. NT = $48,142 | ICER for Z v. NT = $13,484 |
| D subject to dominance | D subject to dominance | |
| 85–89 | ICER for Z v. NT = $51,296 | ICER for Z v. NT = $17,770 |
| D subject to dominance | D subject to dominance | |
| 90+ | ICER for Z v. NT = $46,842 | ICER for Z v. NT= $17,796 |
| D subject to dominance | D subject to dominance | |
A, alendronate; D, denosumab; E, etidronate; ICER, incremental cost per QALY gained; NT, no therapy; QALY, quality-adjusted life year; R, risedronate; Z, zoledronate.
Costs represent CAN$ in 2017.
Comparison of no therapy, denosumab and zoledronate.
For women unable to tolerate oral bisphosphonates for all strata, zoledronate either dominated denosumab or the ICER for denosumab versus zoledronate was greater than CAN$7.3 million per QALY. The ICER for zoledronate versus no therapy was below CAN$50,000 for all women with previous fracture and for those women without a previous fracture aged 80 to 84 and over 90.
Scenario Analysis
The conclusions to be drawn from the results of the scenario analysis did not differ from the base analysis (Table 5). The ICER for alendronate versus no therapy remained less than CAN$8,000 per QALY in all scenarios. The ICER for zoledronate versus alendronate remained greater than $600,000 for all relevant scenarios. In the scenario analysis using assumptions more favorable to denosumab, the ICER for denosumab versus alendronate for those aged 70 to 74 with a previous fracture fell from CAN$8 million to CAN$165,490.
Table 5.
Results of Scenario Analysis for Base Case Populationa
| Scenario | Sequential Result |
|---|---|
| Base case | ICER for A v. NT = $3,751 |
| ICER for Z v. A = $666,285 | |
| ICER for D v. Z = $13.0 million | |
| E and R subject to dominance | |
| Set time = 0 years | ICER for A v. NT = $7,972 |
| ICER for Z v. A = $838,746 | |
| E, R, and D subject to dominance | |
| Set time = 5 years | ICER for Z v. A = $643,327 |
| NT, E, R, and D subject to dominance | |
| Discount rate = 0% | I ICER for A v. NT = $2,577 |
| ICER for Z v. A = $608,211 | |
| ICER for D v. Z = $8.3 million | |
| E and R subject to dominance | |
| Discount rate = 3% | ICER for A v. NT = $5,548 |
| ICER for Z v. A = $800,853 | |
| ICER for D v. Z = $47.1 million | |
| E and R subject to dominance | |
| Discount rate = 5% | ICER for A v. NT = $6,435 |
| ICER for Z v. A = $839,796 | |
| E, R, and D subject to dominance | |
| Inclusion of non-osteoporotic health care costs | ICER for A v. NT = $3,749 |
| ICER for Z v. A = $770,725 | |
| ICER for D v. Z = $4.8 million | |
| E and R subject to dominance | |
| Scenario analysis favoring denosumabb | ICER for D v. A = $165,490 |
| NT and R dominated by A |
A, alendronate; D, denosumab; E, etidronate; ICER, incremental cost per QALY gained; NT, no therapy; QALY, quality-adjusted life year; R, risedronate; Z, zoledronate.
Costs represent CAN$ in 2017.
Analysis compares only no therapy, alendronate, risedronate, and denosumab. Analysis based on assumptions favorable to denosumab relating to calibration, vertebral facture costs, and mortality and treatment effectiveness adopted in previous manufacturer sponsored studies.
Within the base population, based on a threshold of CAN$50,000 per QALY, a price reduction of 90% would be required for denosumab to be optimal. In certain strata, denosumab would not be optimal even if it had zero cost. In other strata, the required price reduction was at least 90%. For zoledronate, the price reduction would have to be 66% for it to be considered optimal. Across all strata, the necessary price reduction ranged from 66% to 85%.
For woman who are intolerant to oral bisphosphonates, zoledronate was optimal in the majority of strata. For zoledronate to be cost-effective in all strata, the necessary price reduction required was 34%. The required price reduction for denosumab to be optimal in this subgroup of women varied by age and fracture history ranging between 63% and 76%.
Discussion
The aforementioned results suggest that for patients who are able to tolerate oral bisphosphonates, alendronate is the optimal treatment regardless of a woman’s age or fracture history. Although alendronate did not dominate denosumab and/or zoledronate in all patient strata, the ICERs for denosumab and zoledronate compared to alendronate were indicative of them not being cost-effective. For patients unable to tolerate oral bisphosphonates, zoledronate can be considered optimal for a proportion of women as it was associated with an ICER of less than CAN$50,000 in the majority of strata (for women with no previous fracture aged 80–84 or over 90 and for all women with a previous fracture). Denosumab was either dominated by zoledronate (for women with no previous fracture aged over 75 and for all women with a previous fracture) or had an ICER compared to zoledronate that was indicative of it not being cost-effective (for women with no previous fracture aged under 75).
Based on the available literature, this is the first economic evaluation comparing such a wide range of osteoporotic treatments that is independent of manufacturer sponsorship. There are a number of publications that have suggested that manufacturer-sponsored economic evaluations may be susceptible to bias.50,51 Five manufacturer-sponsored studies were identified and two independent studies—one comparing denosumab and alendronate and another comparing anabolic drugs (abaloparatide and teriparatide) to no therapy.41,45–48,52,53
The results of this analysis are in contrast to the five previous studies of denosumab funded by the manufacturer. Chau and colleagues reported an incremental cost per QALY gained of $60,266 (2010 CAN$) for denosumab versus alendronate for a base case of 72-year-old women with previous fracture.41 Jönsson and colleagues reported an incremental cost per QALY gained of €27,090 (2009 euros) for 71-year-old women (34% assumed to have a previous fracture).45 Darbà and colleagues reported an incremental cost per QALY gained of €16,294 (2013 euros) for 65-year-old women (28% with previous fracture).46 Parthan and colleagues reported an ICER of $85,060 per QALY for denosumab versus alendronate for a cohort of 72-year-old osteoporotic women—23% with prevalent vertebral fracture (2012 US$). Finally, Hiligsmann and Reginster reported an ICER of €14,166 per QALY for a 70-year-old osteoporotic women with previous vertebral fracture (2009 euros).48
All of the above findings contrast with our result that denosumab was either dominated by alendronate or was associated with a much higher ICER. The difference in results between the studies and the current analysis may be due to four issues. First, it is unclear that the manufacturer-sponsored studies adequately accounted for calibration especially in relation to mortality. Second, the studies used similar estimates of effectiveness that did not incorporate all older clinical trials for oral bisphosphonates. The choice of effectiveness data favored denosumab especially in terms of reductions in hip fractures. Third, the studies made the assumption that the increase in mortality associated with vertebral fractures was either greater or equal to that associated with hip fractures. Finally, the studies assumed much higher costs associated with vertebral fractures, in some instances exceeding those of hip fractures.
A scenario analysis attempted to address these issues by assuming efficacy as used within the manufacturer sponsored studies; equal mortality associated hip and vertebral fractures; higher costs associated with vertebral fractures; and by not calibrating mortality data. This analysis found an ICER for denosumab versus alendronate of $165,490: closer to the various manufacturers’ estimates.
An independent analysis by Karnon and colleagues found that denosumab was not cost-effective compared to alendronate with an estimated ICER for denosumab versus alendronate of $246,749 per QALY (AUS$ unknown date).52 This analysis differed from the current analysis in two ways. First, it modelled the effectiveness of treatment through changes in BMD rather than fracture prevention. Second, it assumed a much lower cost of denosumab based on current Australian funding of denosumab which fixed the cost to be similar to branded alendronate.
A recent independent study from the Institute of Clinical and Economic Review focused on anabolic therapies for postmenopausal osteoporosis.53 Thus, given the comparators, the results are not directly related to the current study. However, the methods adopted and the assumptions made in the current study are consistent with approach adopted by this independent report.
In Ontario under the Ontario Drug Formulary, funding for denosumab is restricted to women who experience significant decline in BMD after 1 year continuous bisphosphonate therapy or are unable to tolerate oral bisphosphonates due to either hypersensitivity or abnormalities of the esophagus. For both criteria, woman must also meet two of three criteria: age over 75, be osteoporotic (a BMD T-score less than or equal to −2.5), and/or have a previous osteoporotic fracture. Funding for zoledronate is restricted to women who are unable to tolerate oral bisphosphonates due to the same reasons and who meet the same criteria as for denosumab. Despite these limited access criteria, expenditure on denosumab in 2015–2016 in Ontario was CAN$34.79 million—suggesting the equivalent of over 47,000 annual users in Ontario.54 For risedronate, annual expenditure of the data suggested 88,000 users. Data for alendronate were unavailable for 2015–2016 but for 2013–2014, the data suggests over 110,000 users.55
There is the potential that provincial drug plans may have negotiated lower prices for zoledronate and denosumab though those prices remain confidential. Threshold analysis was conducted and demonstrated that the costs of denosumab and zoledronate needed to be reduced substantially to allow these therapies to become optimal for treating all osteoporotic women. When limiting these products to those unable to tolerate oral bisphosphonates, the current costs of zoledronate is such that it is cost-effective for treating a high proportion of women who fall into this indication. However, the cost of denosumab would need to be reduced substantially for it to be cost-effective even in this small subgroup of women.
It is unclear what the true incidence of intolerance to oral bisphosphonates is. In one randomized controlled trial of once weekly alendronate compared to placebo, the percentage of patients reporting an upper gastrointestinal tract adverse event was lower for alendronate patients (11%) than for placebo (13%).56 The probability that a patient on alendronate would discontinue due to such an event was 3%. These findings suggest that only a small percentage of osteoporotic women fall in to this subgroup.
The recent Canadian guidelines are consistent in clearly recommending health care system perspective as the primary analysis based on its relevance to the decision problem. Thus, for the current analyses, it would not be appropriate to consider the societal perspective as it has been consistently shown that this is not considered of relevance to provincial decision makers. Adoption of the societal perspective assumes that health care decision makers would be willing to trade health gains for benefits to other sectors. Thus, the choice of the health care system perspective is not a limitation as such. It should be noted that as the population of interest is greater than 65 the likely impacts on productivity will be minimal and results from the societal perspective would likely be similar to current results.
The current study does, however, have a number of limitations. First, there is a lack of head to head clinical trials and thus analysis relied on effectiveness data from an indirect treatment comparison of randomized controlled trials the majority of which were placebo controlled rather than active comparators. It would be beneficial that future novel treatments for osteoporosis are compared with current existing therapies especially oral bisphosphonates, which are available in low-cost generic format.
Second, analysis used fracture prevalence data from 2009. It is assumed that prevalence has not changed greatly but a re-analysis should be conducted if more temporaneous data become available.
Third, a detailed stratified analysis is conducted as per the recent Canadian guidelines for economic evaluation. For many data elements strata-specific data are available but for some the assumption needed to be made that data did not vary by strata. That is a limitation, and should further strata-specific data become available then a reanalysis would be justified.
Fourth, for zoledronate, the effect on wrist fractures is not reported in any clinical trial for postmenopausal women. This is surprising as such data may have been collected within the clinical trials but not reported. Within this analysis it was assumed that the risk of wrist fracture with zoledronate was the same as for no therapy.
Additionally, analysis is based on the assumption that individuals will not undergo sequential treatment with oral bisphosphonates. Thus, the decision problem relates to what would be the optimal treatment for osteoporosis rather than the optimal sequence of treatments. The results of the analysis suggest that the only oral bisphosphonate likely to be cost-effective is alendronate. Furthermore, the analysis suggest that for those unable to tolerate oral bisphosphonates only zoledronate is cost-effective in those with previous fracture. Thus, if sequencing of treatment was necessary for patients who experience a fracture on therapy, the optimal sequence of treatments for postmenopausal osteoporosis is likely to be alendronate followed by zoledronate post fracture.
Finally, costs were based on the most recent available Canadian data, and drug costs were obtained from the Ontario drug formulary. This may be seen as a limitation. Although drug costs may vary by provincial formulary, it is unlikely that list prices would vary substantially enough to alter the conclusions of this analysis.
In conclusion, this study demonstrated that, based on the currently available data, treatment with alendronate is the optimal strategy for women who are able to tolerate oral bisphosphonates. For women unable to tolerate oral bisphosphonates, zoledronate is optimal for a high proportion of women. The required price reductions to lead to denosumab being cost-effective in either group were substantial.
Thus, the policy implications from this analysis is that denosumab should not be covered under provincial drug formularies for the general osteoporotic population unless substantial price reductions can be negotiated. Zoledronate should be covered but this should continue to be restricted to women who are truly intolerant to weekly oral bisphosphonate therapy.
Supplemental Material
Supplemental material, DS_10.1177_2381468318818843 for Cost-Effectiveness of Pharmacological Treatments for Osteoporosis Consistent with the Revised Economic Evaluation Guidelines for Canada by Doug Coyle in MDM Policy & Practice
Footnotes
Supplemental Material: The online supplementary appendix for this article is available on the Medical Decision Making Policy & Practice website at http://journals.sagepub.com/home/mpp.
References
- 1. World Health Organization. The Assessment of Osteoporosis at Primary Health Care Level. Geneva: World Health Organization; 2007. [Google Scholar]
- 2. Qaseem A, Forciea MA, Mclean RM, Denberg TD; Clinical Guidelines Committee of the American College of Physicians. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(11):818–39. doi: 10.7326/M15-1361 [DOI] [PubMed] [Google Scholar]
- 3. Tenenhouse A, Joseph L, Kreiger N, et al. Estimation of the prevalence of low bone density in Canadian women and men using a population-specific DXA reference standard: the Canadian Multicentre Osteoporosis Study (CaMos). Osteoporos Int. 2000;11(10):897–904. [DOI] [PubMed] [Google Scholar]
- 4. Tarride J, Hopkins RB, Leslie WD, et al. The burden of illness of osteoporosis in Canada. Osteoporos Int. 2012;23(11):2591–600. doi: 10.1007/s00198-012-1931-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haentjens P, Magaziner J, Colón-Emeric CS, et al. Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med. 2010;152(6):380–90. doi: 10.1059/0003-4819-152-6-201003160-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lix LM, Azimaee M, Osman BA, et al. Osteoporosis-related fracture case definitions for population-based administrative data. BMC Public Health. 2012;12:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312(7041):1254–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Canadian Agency for Drugs and Technologies in Health. Guidelines for the Economic Evaluation of Health Technologies: Canada. 4th ed. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2017. [Google Scholar]
- 9. Canadian Agency for Drugs and Technologies in Health. Guidelines for the Economic Evaluation of Health Technologies: Canada. 3rd ed. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2006. [Google Scholar]
- 10. Coyle D, Buxton MJ, O’Brien BJ. Stratified cost-effectiveness analysis: a framework for establishing efficient limited use criteria. Health Econ. 2003;12(5):421–7. doi: 10.1002/hec.788 [DOI] [PubMed] [Google Scholar]
- 11. Espinoza MA, Manca A, Claxton K, Sculpher MJ. The value of heterogeneity for cost-effectiveness subgroup analysis: conceptual framework and application. Med Decis Mak. 2014;34(8):951–64. doi: 10.1177/0272989X14538705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Paulden M, Galvanni V, Chakraborty S, Kudinga B, McCabe C. Discounting and the Evaluation of Health Care Programs. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2016. [Google Scholar]
- 13. Waldegger L, Cranney A, Man-son-hing M, Coyle D. Cost-effectiveness of hip protectors in institutional dwelling elderly. Osteoporos Int. 2003;14(3):243–50. doi:10.1007/ s00198-002-1354-3 [DOI] [PubMed] [Google Scholar]
- 14. Coyle D, Tahar AH, Murphy G, et al. Teriparatide and Bisphosphonates for Treatment of Osteoporosis in Women: A Clinical and Economic Analysis. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2006. [Google Scholar]
- 15. Coyle D, Cranney A, Lee KM, Welch V, Tugwell P. Cost effectiveness of nasal calcitonin in postmenopausal women: use of Cochrane Collaboration methods for meta-analysis within economic evaluation. Pharmacoeconomics. 2001;19(5 Pt. 2):565–75. [DOI] [PubMed] [Google Scholar]
- 16. Coyle D, Tosteson AN. Towards a reference case for economic evaluation of osteoporosis treatments. J Rheumatol Suppl. 2003;68:31–6. [PubMed] [Google Scholar]
- 17. Gandjour A, Weyler E. Cost-effectiveness of preventing hip fractures by hip protectors in elderly institutionalized residents in Germany. Value Health. 2008;11(7):1088–95. doi: 10.1111/j.1524-4733.2008.00393.x [DOI] [PubMed] [Google Scholar]
- 18. Nikitovic M. Direct Costs of Hip Fractures Among Seniors in Ontario. Toronto: University of Toronto; 2011. [Google Scholar]
- 19. Nikitovic M, Wodchis W, Krahn M, Cadarette S. Direct health-care costs attributed to hip fractures among seniors: a matched cohort study. Osteoporos Int. 2013;24(2):659–69. doi: 10.1007/s00198-012-2034-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ontario Case Costing Initiative. Health Data Branch web portal. Available from: https://hsim.health.gov.on.ca/hdbportal/
- 21. Nshimyumukiza L, Durand A, Gagnon M, et al. An economic evaluation: simulation of the cost-effectiveness and cost-utility of universal prevention strategies against osteoporosis-related fractures. J Bone Miner Res. 2013;28(2):383–94. doi: 10.1002/jbmr.1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ontario Ministry of Health and Long-Term Care. Ontario Drug Benefit Formulary/Comparative Drug Index. 42nd ed. Toronto: Ontario Ministry of Health and Long-Term Care; 2017. [Google Scholar]
- 23. Statistics Canada. Canadian Community Health Survey—Annual Component (CCHS). Detailed Information for 2014. Ottawa: Statistics Canada; 2015. [Google Scholar]
- 24. Peasgood T, Herrmann K, Kanis JA, Brazier JE. An updated systematic review of health state utility values for osteoporosis related conditions. Osteoporos Int. 2009;20(6):853–68. doi: 10.1007/s00198-009-0844-y [DOI] [PubMed] [Google Scholar]
- 25. Oleksik A, Lips P, Dawson A, et al. Health-related quality of life in postmenopausal women with low BMD with or without prevalent vertebral fractures. J Bone Miner Res. 2000;15(7):1384–92. [DOI] [PubMed] [Google Scholar]
- 26. Hopkins RB, Goeree R, Pullenayegum E, et al. The relative efficacy of nine osteoporosis medications for reducing the rate of fractures in post-menopausal women. BMC Musculoskelet Disord. 2011;12:209. doi: 10.1186/1471-2474-12-209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Emkey RD, Ettinger M. Improving compliance and persistence with bisphosphonate therapy for osteoporosis. Am J Med. 2006;119(4 Suppl. 1):S18–S24. doi: 10.1016/j.amjmed.2005.12.019 [DOI] [PubMed] [Google Scholar]
- 28. Freemantle N, Satram-Hoang S, Tang ET, et al. Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int. 2012;23(1):317–26. doi: 10.1007/s00198-011-1780-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Modi A, Sajjan S, Insinga R, Weaver J, Lewiecki EM, Harris ST. Frequency of discontinuation of injectable osteoporosis therapies in US patients over 2 years. Osteoporos Int. 2017;28(4):1355–63. doi: 10.1007/s00198-016-3886-y [DOI] [PubMed] [Google Scholar]
- 30. Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, 3rd, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15(4):721–39. [DOI] [PubMed] [Google Scholar]
- 31. Crilly RG, Tanner DA, Kloseck M, Chesworth BM. Hip fractures in long-term care: is the excess explained by the age and gender distribution of the residents? J Aging Res. 2010;2010:291258. doi: 10.4061/2010/291258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Statistics Canada. Living Arrangements of Seniors. Families, Households and Marital Status. Structural Type of Dwelling and Collectives, 2011 Census of Population. Ottawa: Statistics Canada; 2013. [Google Scholar]
- 33. Statistics Canada. Life Tables, Canada, Provinces and Territories. Tables: 84-537-X. Ottawa: Statistics Canada; 2017. [Google Scholar]
- 34. Holvik K, Ranhoff AH, Martinsen MI, Solheim LF. Predictors of mortality in older hip fracture inpatients admitted to an orthogeriatric unit in Oslo, Norway. J Aging Health. 2010;22(8):1114–31. doi:10.1177/0898264310378 040 [DOI] [PubMed] [Google Scholar]
- 35. Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 2017;159(11):1215–20. [DOI] [PubMed] [Google Scholar]
- 36. Coyle D, Lee KM, Cooper NJ. Use of evidence in decision models. In: Shemilt I, Mugford M, Vale L, Marsh K, Donaldson C, eds. Evidence-Based Decisions and Economics Health Care, Social Welfare, Education and Criminal Justice. 2nd ed. Oxford: Blackwell; 2010. [Google Scholar]
- 37. Ontario Ministry of Health and Long-Term Care. Ontario Case Costing Initiative (OCCI). Available from: https://www.ontario.ca/data/ontario-case-costing-initiative-occi
- 38. Ontario Ministry of Health and Long-Term Care. Schedule of benefits: physician services under the Health Insurance Act. Available from: http://www.health.gov.on.ca/en/pro/programs/ohip/sob/physserv/sob_master11062015.pdf
- 39. Simon JA, Recknor C, Moffett AH, Jr, et al. Impact of denosumab on the peripheral skeleton of postmenopausal women with osteoporosis: bone density, mass, and strength of the radius, and wrist fracture. Menopause. 2013;20(2):130–7. doi: 10.1097/gme.0b013e318267f909 [DOI] [PubMed] [Google Scholar]
- 40. Cummings S, San Martin J, McClung M, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–75. [DOI] [PubMed] [Google Scholar]
- 41. Chau D, Becker DL, Coombes ME, Ioannidis G, Adachi JD, Goeree R. Cost-effectiveness of denosumab in the treatment of postmenopausal osteoporosis in Canada. J Med Econ. 2012;15(Suppl. 1):3–14. doi:10.3111/13696998 .2012.737393 [DOI] [PubMed] [Google Scholar]
- 42. Tosteson A, Jönsson B, Grima D, O’Brien B, Black D, Adachi J. Challenges for model-based economic evaluations of postmenopausal osteoporosis interventions. Osteoporos Int. 2001;12(10):849–57. [DOI] [PubMed] [Google Scholar]
- 43. Tonino RP, Meunier PJ, Emkey R, et al. Skeletal benefits of alendronate: 7-year treatment of postmenopausal osteoporotic women. Phase III Osteoporosis Treatment Study Group. J Clin Endocrinol Metab. 2017;85(9):3109–15. [DOI] [PubMed] [Google Scholar]
- 44. Edlin R, McCabe C, Hulme C, Hall P, Wright J. Cost Effectiveness Modelling for Health Technology Assessment. Basel: Springer International; 2015. [Google Scholar]
- 45. Jönsson B, Ström O, Eisman J, et al. Cost-effectiveness of denosumab for the treatment of postmenopausal osteoporosis. Osteoporos Int. 2016;22(3):967–82. doi:10.1007/s0 0198-010-1424-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Darbà J, Kaskens L, Vilela FS, Lothgren M. Cost-utility of denosumab for the treatment of postmenopausal osteoporosis in Spain. Clin Outcomes Res. 2015;7:105–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Parthan A, Kruse M, Yurgin N, Huang J, Viswanathan HN, Taylor D. Cost effectiveness of denosumab versus oral bisphosphonates for postmenopausal osteoporosis in the US. Appl Health Econ Health Policy. 2013;11(5):485–97. doi: 10.1007/s40258-013-0047-8 [DOI] [PubMed] [Google Scholar]
- 48. Hiligsmann M, Reginster J. Cost effectiveness of denosumab compared with oral bisphosphonates in the treatment of post-menopausal osteoporotic women in Belgium. Pharmacoeconomics. 2011;29(10):895–911. doi:10.2165/115399 80-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 49. Winquist E, Bell CM, Clarke JTR, et al. An evaluation framework for funding drugs for rare diseases. Value Health. 2012;15(6):982–6. [DOI] [PubMed] [Google Scholar]
- 50. Bell CM, Urbach DR, Ray JG, et al. Bias in published cost effectiveness studies: systematic review. BMJ. 2006;332(7543):699–703. doi: 10.1136/bmj.38737.607558.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Garattini L, Koleva D, Casadei G. Modeling in pharmacoeconomic studies: funding sources and outcomes. Int J Technol Assess Health Care. 2010;26(3):330–3. doi: 10.1017/S0266462310000322 [DOI] [PubMed] [Google Scholar]
- 52. Karnon J, Shafie AS, Orji N, Usman SK. What are we paying for? A cost-effectiveness analysis of patented denosumab and generic alendronate for postmenopausal osteoporotic women in Australia. Cost Eff Resour Alloc. 2016;14:11. doi: 10.1186/s12962-016-0060-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Institute for Clinical and Economic Review. Anabolic Therapies for Osteoporosis in Postmenopausal Women: Effectiveness and Value. Boston: Institute for Clinical and Economic Review; 2017. [Google Scholar]
- 54. Patented Medicine Prices Review Board. NPDUIS CompassRx, 3rd Edition: Annual Public Drug Plan Expenditure Report, 2015/16. Ottawa: Patented Medicine Prices Review Board; 2017. [Google Scholar]
- 55. Patented Medicine Prices Review Board. NPDUIS CompassRx, 2nd Edition: Annual Public Drug Plan Expenditure Report, 2013/14. Ottawa: Patented Medicine Prices Review Board; 2015. [Google Scholar]
- 56. Greenspan S, Field-Munves E, Tonino R, et al. Tolerability of once-weekly alendronate in patients with osteoporosis: a randomized, double-blind, placebo-controlled study. Mayo Clin Proc. 2002;77(10):1044–52. doi: 10.4065/77.10.1044 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_2381468318818843 for Cost-Effectiveness of Pharmacological Treatments for Osteoporosis Consistent with the Revised Economic Evaluation Guidelines for Canada by Doug Coyle in MDM Policy & Practice