Abstract
Circular RNAs (circRNAs) are a large class of non coding endogenous RNAs in eukaryotic that are formed through 3’-5’ ligation of a single RNA molecule. According to the different sources of the sequences, circRNA can be divided into three types: exon circRNA (ecRNA), intron circRNA (ciRNA), and exon-intron circRNA. Accumulating studies have shown that circRNAs are abundant, diverse, stable, and cell or tissue specific expression, etc. CircRNA plays a regulating role in gene expression, and an essential role in the process of biological development, such as miRNA sponges, endogenous RNAs and biomarkers, as well as critical role in the diagnosis of diseases. Studies have verified the interplay between circRNAs and the development of embryos, sperms, ovarian epithelial tumors, endometrial cancer and preeclampsia, suggesting the potential of circRNAs to become biomarkers or therapeutical targets for human diseases. In this paper, we reviewed the researches on circRNAs’ characteristics, databases of circRNA, high-throughput sequencing of circRNA, and effect on reproductive and gynecological diseases.
Keywords: Circular RNA, database, embryonic development, preeclampsia, reproductive tumor
Introduction
Noncoding RNAs (ncRNAs), making up more than 98% of human genomes, play an essential role in gene expression and regulation. Besides those familiar faces (like rRNA, tRNA, snRNA, siRNA), new members have just joined the family of ncRNAs, represented by circRNA. Already found in viroids, yeasts and human gene transcript, circRNAs have not been well studied and only regarded as low-abundance RNAs abnormally spliced in transcript. With high-throughput sequencing technologies, researchers have confirmed their universality in eukaryocytes, and participation in human diseases. It can be said that circRNAs are lying in the focus of RNA research field.
Origin and characteristics of circRNAs
Origin of circRNAs
Bearing no 5’ and 3’ poly(A) ends but a closed covalent circle formed during “back-splice” reaction, circRNA arises from protein-coding genes, intergenic spacer between tRNA and Lnc RNA, or antisence transcript [1,2]. According to their sequences, circRNAs are classified into three types: (1) exonic circRNA, composed of only exons and mainly located in cytoplasm [3]; (2) intronic circRNA, composed of only introns and mainly located in nuclei; (3) exon-intronic circRNA (EIciRNA), composed of introns at the region between exons and mainly located in nuclei [5] (Table 1).
Table 1.
Characteristics of different types of circRNAs
| Name | Type | Location | Joint site | Sequence feature | Function |
|---|---|---|---|---|---|
| ciRNA | Intron | Nucleus | 2’-5’ phosphodiester bond | Enrichment of 5’ splice site containing 7 GU motif, enrichment of 3’ branch site containting 11 C motif | Regulation of gene transcription |
| EIciRNA | Exon-intron | Nucleus | 3’-5’ phosphodiester bond | Circularization exons containing the reverse complementary sequence of introns and selective circularization | Regulation of gene transcription |
| ecRNA | Exon | Cytoplasms | 3’-5’ phosphodiester bond | Circularization exons containing the reverse complementary sequence of introns and selective cyclization | MiRNA sponges; interaction with RBP; translation |
ecRNA: Exonic circRNA; ciRNA: Intronic circRNA; EIciRNA: Exon-intronic circRNA.
The circularization of exons and introns forms circRNA. Exonic circularization can be either lariat-driven or intron-pairing-driven [6]. In lariat-driven circularization, axon skipping brings about a lariat, and after this lariat is spliced from the inside, a circular RNA comes into being. The other fashion of larial-driven circularization does not begin with axon skipping. In this process, depending on the secondary structures of RNAs and the reverse complementary pairing of axon-contained ALUs, the introns flanking the circularized axon joins the donor splice site and the acceptor splice site, resulting in the final circularization. According to recent researches, the complementary pairing between the repeated sequences of introns flanking the axon favors the buildup of circRNA, and multiple circRNAs can be produced via the selective pairing and competition between inverted ALU repeats (the recognition sequences of restiriction endonuclease) in one gene [7,8]. Self-circularization of introns begets intronic circRNA [4].
Most circRNAs are produced during the later phase of transcription. Circularization competes with splicing, and this competition may be regulated by some splicing factors [9]. The level of primary circRNAs rises with RNA-pol TER (RNA-pol transcription elongation rate) [10]. The production of circRNAs can be positively regulated by Quaking (QKI), a muscleblind-like (MBL) protein binding RNA [11], and inhibited by RNA-editing adenosine deaminase (ADAR) [12]. Besides, this production may be co-regulated by hnRNP, serine/arginine-rich protein (SR) and intronic repeats (Figure 1).
Figure 1.
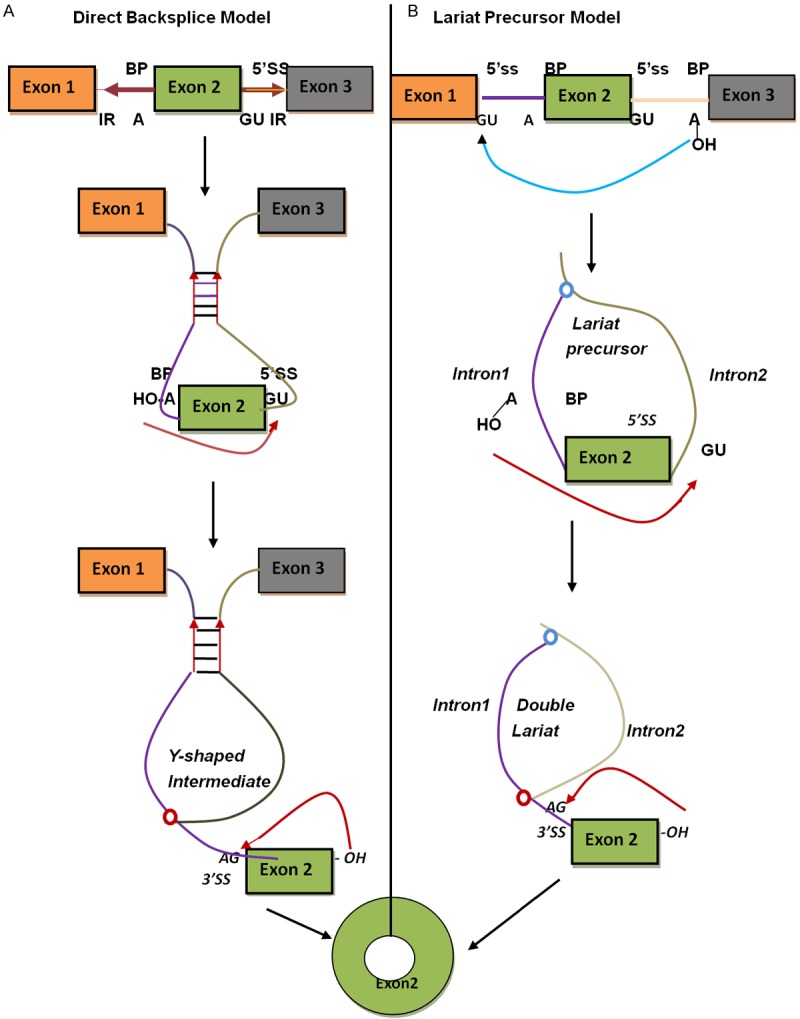
Barrett SP et al. Models for the production of circularRNA [13]. A. Intron-pairing-driven circularzation. Intron1 and intron3 are formed circular structure via base-pairing. Then introns are removed or retained to form circRNA or ElciRNA. B. Lariat-driven circularization. That splice donor in 3’ end of exon1 covalently splices to splices to splice acceptor in 5’ end of exon 4 forms a lariat via exon skipping. Then the introns are removed via splicesome. CircRNA finally is formed.
Characteristics of circRNAs
Compared to a linear RNAs, a circRNA has the following characteristics: (1) Predominantly existing in the cytoplasm, occasionally in the nucleus [14]; (2) Regulating the target gene expression via the response element -mediated interaction with miRNA [15]; (3) Mostly developing from the exons, occasionally from the introns or the intronic segment; (4) Mostly regulating the expression of endogenous ncRNA; (5) Mostly functioning before and after transcription, occasionally during the transcription [16]; (6) Tissue specific and developmental-stage specific [17]; (7) Often found in extracellular fluid (like saliva, blood and urine) with an expression level ten times higher than that of linear mRNAs [18]; (8) Evolutionary conservation in various species [19]; (9) Having a covalently closed loop, withoug 5’ and 3’ ends and poly(A) tail, that is highly resistant to RNA exonuclease or RNase R [20]. These characteristics endow, circRNAs enjoy a stable biological function. The average half-life period of circRNAs in most species is much longer than that of mRNAs (10 h). To date, more than 400 strains of circRNAs have been found in cellfree saliva (CFS) [21].
Biological functions of circRNA
CircRNA can act as miRNA sponge or RNA-binding protein, and be translated into protein (Figure 2).
Figure 2.
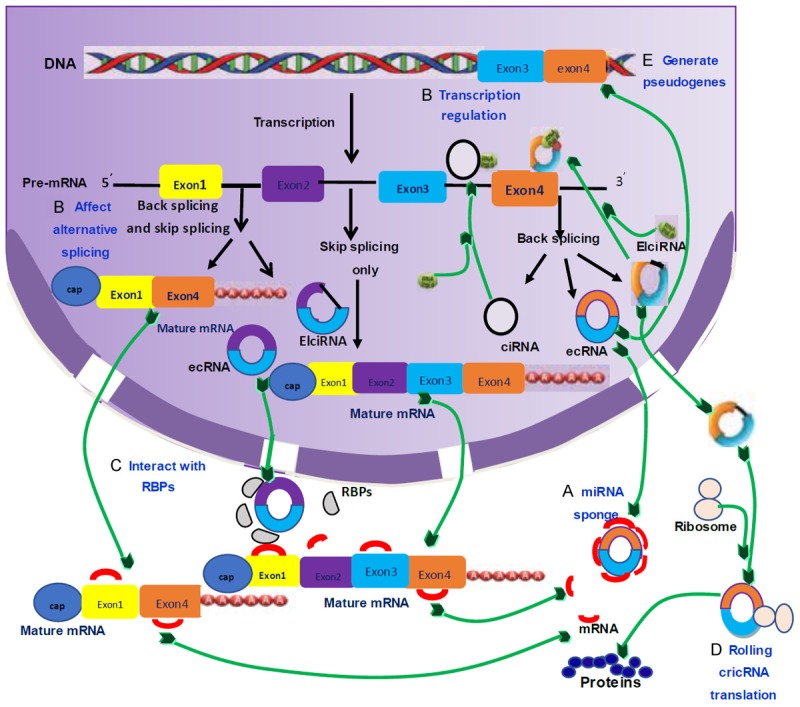
Liu J, Liu T, Wang XM, et al. Functions of the CircRNAs [39]. A. miRNA sponge: circRNA binds miRNA to affect its biological function and regulate miRNA target gene activity; B. Regulating selective splicing or transcription: stable ciRNAs and EIciRNAs are located in the nucleus, bind to RNAs and promote transcription; circRNAs compete with pre-mRNA splicing to reduce linear mRNA and exclude specificity from pre-mRNA to change the composition of processed mRNA; C. Interaction with RBPs: circRNA binds RBPs and ribonucleoprotein complexes to prevent these factors from functioning and play a “storage” function; D. Rolling circle translation: some in vitro circRNAs can be translated into proteins by means of a roll loop amplification mechanism. E. Generate pseudogenes: some circRNAs may be reverse transcribed into cDNA and integrated into the genome, and integration is not yet clear.
As miRNA sponge
The interaction between circRNAs (mainly SrycircRNA and CiRS-7) and miRNAs is catching more researchers’ eyes. Capel et al. found that Sry, a sex-deciding gene on Y chromosome of the mouse, could be transcribed into circRNA [22]. Hansen et al. further verified SrycricRNA, functioned as a miRNA sponge, and contained 16 sites that can bind miR-138 [24-27].
In addition, CDR1as/CiRS-7, a circRNA transcribed from the antisense strand of cerebellar degeneration-related protein 1 (CDR1), is widely found in the brain of humans or mice [23]. CiRS-7, containing 73 miR-7 binding sites, can sponge up miR-7, and simultaneously inhibit its biological function and increase the expression of targeted miR-7. However, in miRNA sponging, circRNA is also split by miRNA. For example, after binding to miRNA-671, CiRS is cleaved and then releases miR-7. Thomas et al. proved that abundant circRNAs sponged up miRNAs, just as miRNAs do for mRNAs. But to date, only a small fraction of these circRNAs and their sponge function have been verified [28].
As RNA-binding protein
RNA-binding proteins (RBP) participate in cellular processes (proliferation, differentiation, transmigration, apoptosis, senescence, oxidative stress response) via post-transcriptional regulation on RNA (selective splicing, transportation, translation) [29]. Researches found that stable RNA-protein complexes (RPCs) were produced as circRNAs sponged up Argonaute (AGO) protein, RNA quaking (QKI) MBL protein, RNA polymerase II (Pol II), eukaryotic initiation factor 4A-III (EIF4A3), to name just a few. These RPS can regulate RBP and interact with linear RNAs [30]. Some circRNAs have been proved to cooperate with RNAs, like protein-binding circ-Foxo3 that interacts with cyclin dependent protein kinase 2 (CKD2) to stop the cell generation cycle at the phase of G1/S [31]. Some circRNAs can change the stability of mRNAs, like CDR1as that cooperates with mRNA to build a stable duplex [32].
In macrophages of mice, RasGEF1B (a circRNA) can increase the stability of intercellular cell adhesion molecule-1 (ICAM-1) [33]. The mechanism through which circRNAs regulate genetic transcription varies. circRNAs mostly found in nucleus first interact with some substances and then bind to miRNAs. For example, in the nuclei of HeLa and HEK293 cells, circ-EIF3J and circ-PAIP2 (two EIciRNAs) can bind to U1snRNP and then interact with RNA PoL II to increase the expression of parent genes [34], suggesting the role of EIciRNAs in positive feedback regulation. This mechanism is also observed by circ-ankrd52 and circ-sirt7 in regulating the transcription of their parent genes. All these findings prove that EIciRNAs and circRNAs regulate genetic transcription in nuclei, but ecircRNAs show their sponge function in cytoplasm.
As a translator in protein production
As a strain of ncRNA, cricRNAs seldom code proteins. But once an internal ribosome entry site (IRES) is inserted into the upstream of a circRNA’s start codon, the coding takes place, producing a transcript functionally differing from a linear transcript. In vivo and in vitro experiments have proven that subunit 40S of the eukaryotic ribosome can bind to an engineering circRNA containing one IRES, thereafter starting the translation [35,36]. Similarly in bacillus coli, transfecting the circRNA carrying an open reading frame (ORF) for green fluorescent protein (GFP) can initiate GFP expression [35]. But until now, only viral circRNAs have been found to code proteins in eukaryocytes. For example, satellite virus HDV of HBV can translate through co-transfecting with HBV in host cells [37]. The translation of viral circRNAs may be associated with specific viral mediators. Although their ability to translate proteins is still obscure, some eukaryotic circRNAs have displayed their potential, as evidenced by the circRNA-synthesized transcript.
Legnini I et al. found that IRES-carrying circ-ZNF609 could translate proteins [38]. Pamudurti N. R et al. found that MBL3 could transcribe proteins in the fly head [39]. Abe N found that without any specific element allowing the entry of internal ribosomes, the artificial circRNA containing multiple FLAG coding sequences could translate proteins through rolling circle amplification (RCA) [40].
High-throughput sequencing of circRNA
Compared with traditional molecular biological methods, twin use of high-throughput sequencing and bioinformatic technology provides a shorter way to explore low-abundance circRNAs. Backsplicing produces circRNAs. Upon its invention, RNA-seq once showed extremely low efficiency in distinguishing backsplicing sites and the resultant circular structure. Thereafter, RNA-seq methods have been improved in a new fashion: 1) comparing the possible circRNA boundaries with genomic sequences if exons can be realigned in a different order [41]; 2) finding circRNAs out of cDNA sequences using multiple splicing methods [42]. The currently used sequencing tools to analyze circRNA include MapSplice, CircSeq, CIRI, CIRCexplorer [43-46], etc. CIRI and its annotations can be used to determine the circRNA transcribed in introns, which is suitable to sequence the eukaryotic organisms annotated or not. Since circRNAs have no poly(A) tails, oligo-dT cannot enrich circRNAs. After knocking out rRNA with ribo-zerokit and linear RNA with RNase R, circRNAs can be obviously enriched [47].
Prediction software and databases of circRNA
CircRNAs are mainly predicted with high-throughput sequencing and bioinformatic technologies. The commonly used prediction software tools include Segemeh, CircRNA finder [49], find-circ, CIRC and PFOR [48-52]. Their accuracy should be compared in the future studies. A reliable prediction software can largely benefit circRNA research. Some new circRNA databases are also available. Circbase (http://www.circbase.org) and Deepbase (http://biocenter.sysu.edu.cn/deepBase) each contains over 150000 circRNA genomes and sequences [53,54]. CirclncRNAnet (http://circnet.mbc.nctu.edu.tw)can analyze the sequencing data automatically selected, and provides the graph structure of functional enrichment analysis and KEGG pathways, RNA-binding protein network and miRNA network [55]. The sequence, genome annotation, expression profile and ceRNA network of circRNAs can be searched in CirclncRNAnet and CircRNAbase [56]. CircRNAIntercome (http://circinteractome.nia.nih.gov), containing the information of a fraction of circRNAs and their PCR products and siRNA sequences, can also be used to predict the RBP sequence of circRNA. Besides, other online circRNA databases using next-generation sequencing include circRNA-finder, CIRCexplorer, DCC, Mapsplice, Segemehl, find-Circ and UROBORUS.
CircRNAs and male reproductive disease
Spermatogenesis, a process during which male reproductive cells proliferate and differentiate into sperms, is powerfully regulated by specific genetic expression, especially the dynamic epigenetic regulation. ncRNA, a major participator in epigenetic regulation, decides the expression level of genes during and after the transcription [57-59]. Studies have shown the role of ncRNA in mammal’s spermatogenesis [60,61]. As a new category of ncRNA, circRNA, like a rising star in the sky, has slipped into the spotlight of transcriptomics [62-65].
Researches have confirmed the tightness between circRNAs and reproductive stem cells. In a transcriptomic analysis on the reproductive cells of mouce, Li et al. found 18822 circRNAs, including 245 uniquely found in males and 676 in females [66]. GO and KEGG analyses demonstrated that the parent genes of these circRNAs parcipated in the differentiation of reproductive stem cells and gender-determination, indicating the regulatory role of circRNAs in the two processes. Using high-throughput sequencing, Dong et al. explored out 15996 circRNAs [67]. GO analysis on the newly discovered circRNAs (67%) among them proved the association between their parent genes and spermatogenesis, sperm motility and fertilization; meanwhile, these circRNAs, either commonly expressed or testicle-specific, could be detected in seminal plasm with a high stability. This stability may be achieved by the circRNA’s closed loop specifically resistant to RNsae R, or the way of binding to seminal proteins [68]. This research has dilled into the function of circRNAs during spermatogenesis, laying a basis for the future work to work out the epigenetic regulation mechanism and noninvasive diagnostic biomarkers for reproductive disease.
CircRNA and female reproductive disease
Expression in granule cells
Granule cells function as nutrient-supplier and transduction signals during the development of oocytes. Their expression profiles can be used to assess the microenvironment surrounding oocytes, which is a beneficial procedure in assisted reproductive technology (ART). Cheng et al. detected the circRNA expression in the granule cells, finding out three circRNAs (circ_103829, circ_103827, circ_104816) upregulated and one circRNA (circ_101889) downregulated in IVF patients of >38 years. After gonadotropin therapy, only two circRNAs (circ_103827, circ_104816) showed the expression level positively correlated with age, and negatively correlated with high-quality embryo number. Bioinformatic analysis indicated that the two circRNAs co-regulated their targets (ANKRD20A90, KCNQ10T1, XIST) through the network “circRNA-miRNA-mRNA” that may be involved in glycometabolism, mitosisand ovary steroid synthesis. Whole-genome association study has proven the close association between ANKRD20A90 expression and life expectancy of Han people in China [69]. KCNQ10T1 is a paternally expressed allele that participates in transcriptional silencing via regulating histone methylation. Any kind of dysregulation can leads to serious biological and hereditary disorders [70]. XIST scarcity causes early-blastula-stage whole-genome dysregulation and post-implantation loss [71]. The epigenetic dysregulation of these circRNAs is essential to the senescence and embryonic abnormal development (Table 2).
Table 2.
Literature summary of circRNAs in the area of reproductive and gynecological diseases
| Journals | Research Content | Field | Num |
|---|---|---|---|
| 2016, Sci Rep | High-throughput sequencing for human testicle tissues. | CircRNAs and male reproductive diseases | 67 |
| 2016, Genome Biology SUPeR | RNA-Seq for human oocytes and preimplantation embryos. | CircRNAs and early embryonic development | 72 |
| 2016, Cellularand Biochemistry | Chip analysis on circRNA expression in preeclampsia and normal preterm-delivery women. | CircRNAs and preeclampsia | 75 |
| 2016, BJOG | Chip analysis on circRNA expression in the blood cells of preeclampsia and normal pregnant women. Establishment of methodology using hsa_circ_10122 combined with plasma protein for disgnose of preeclampsia. | CircRNAs and preeclampsia | 77 |
| 2017, Biomedicine & Pharmacotherapy | Chip analysis on circRNA expression and the diagnostic value of differentially expressed circRNAs in chorionic tissues of normal women and women with recurrent spontaneous abortion. | CircRNAs and RSA | 80 |
| 2016, Oncotarget | RNA sequencing for the expression of mRNAs and circRNAs in primary sites of IIIC epithelial ovarian carcinoma. | CircRNAs and ovarian carcinoma | 81 |
| 2017, RNA Biol | Competitive binding between circPABPN1 and HuR inhibits HuR’s binding to mRNA PANPN1 and the following translation. | CircRNAs and cervical carcinoma | 82 |
| 2017, Gynecol Oncol | MiR-7 as tumor-suppressor in ovarian carcinoma and miR-138 in cervical carcinoma. CiRS-7 stably expressed in HeLa cells. | CircRNAs, ovarian and cervical carcinoma | 87 |
| 2015, Pharmacol Ther | After CDRlas overexpression in HeLa and C33A cells of cervical carcinoma, inhibiting miR-7 activity increases the expression of FAK (target of miR-7). FAK promotes the proliferation, invasion and transmigration of cervical carcinoma cells. | CircRNAs and cervica l carcinoma | 89 |
CircRNA: Circular RNA; RSA: Recurrent spontaneous abortion; CiRS-7: Circular RNA Sponge for miR-7; CDRlas: Cerebellar degeneration-related protein1 antisense; HuR: Hu family of RNA-binding proteins; FAK: Focal adhesion kinase; PABPN1: Poly(A)-binding protein nuclear 1.
Embryonic development
The embryo develops from a germ cell after sperm-egg binding when the genetic transcription of zygote is activated. A stage-dpendent transcriptomic analysis can help verify the molecular mechanism governing the early-stage (especially preimplantation) embryonic development. Unlike the intensely studied mRNA poly(A), circRNA poly(A) is still in the limbo of research field.
Emerging biotechnologies (like deep sequencing, bioinformatic technology) are enriching the genomic data on circRNA [72]. Using SUPeR-seq, Dang et al. sequenced the poly(A)-RNAs in mature ovocytes and preimplantation embryo, finding that numerous circRNAs changed their transcription as the embryo matured. For each gene, the ratio of circRNAs to their transcribed products did not change much (9%) after ovocytic maturation and before cycle 4 started, but began to rise in cycle 8, peaked in morula cycle, and fell gradually thereafter.
For each gene, the ration of circRNAs to linear transcription products is nears 10%. But for others (PRDM2, SETD2, MLLT3, MLLT4, KIT) mainly involved in histone methylization and transcriptional regulation, this ratio exceeds 100%, suggesting the regulatory role of circRNAs in embryonic development. Transcriptomic analysis may be able to screen out the responsible circRNAs and their function, eventually upgrading the network regulating embryonic development. Dang et al. also found some host genes (835/1316, 63%) of mouse circRNAs could generate human circRNAs [73]. Although their molecular mechanism remains unclear, GO analysis suggests that host genes of circRNAs widely exist in human embryo and participate in DNA damage repair, organelle and chromosome development, cell cycle progression, and completion, and cell metabolism (Table 2).
Preeclampsia (PE)
Zhang et al. for the first time tested the expression level of circRNAs in the blood cells in early pregnancy (<20 weeks), and predicted the PE occurrence based on this level plus plasma protein Endoglin (ENG) level (sensitivity, 0.7073; specificity, 0.8049) [74]. According to their findings, the expression level of circRNA 101222 showed obvious difference between PE women and normal women. Qian compared the circRNAs’ expression levels in the placenta tissues of PE women and preterm-delivery women, finding that three circRNAs (hsa_circRNA_100782, hsa_circRNA_102682, hsa_circRNA_104820) were differentially expressed (upregulated in PE women) [75]. Before Qian’s research, Luan Lixia et al. had already verified the highly expressed mir-30a-3p in the placental tissues of PE women. These findings advocate that the ceRNA regulatory network constructed by miRNAs and circRNAs promotes PE development [76].
PE, a severe symptom of pregnancy-induced hypertension, can significantly raise the complication incidence and death rate in both mothers and infants [77]. Early prediction and intervention are effective ways to reduce PE risk. Given its conservation and specificity in tissue development, circRNAs have shown a favorable potential to become a diagnostic marker for PE. This potential is confirmed by Qian et al. who detected the differential expressions of circRNAs in the tissues of PE patients and normal preterm-delivery women [78]. Zhang et al. also combed out 12 differentially expressed circRNAs in the blood cells of pregnant women with PE (FC>5.0, P<0.001) [79]. Of them, hsa_circ_101222 showed an expression level with a 10-FC, and elements responding to PE-associated miR181. So hsa_circ_101222 may be taken as a diagnostic biomarker for PE. Then we tested its efficiency in diagnosing PE. Compared to plasma ENG (sensitivity, 68.3%; specificity, 73.2%), hsa_circ_101222 combined with ENG hoisted this efficiency (sensitivity, 70.7%; specificity, 80.5%) (Table 2).
Recurrent spontaneous abortion (RSA)
Always unexplained and complicated, RSA inflicts victims with profound trauma, either physical or psychological. Using chips prepared with chorionic tissues from RSA women and normal women, Qian et al. found out the circRNAs differentially expressed, including 12 upregulated and 21 downregulated by a >4-FC, indicating their correlation with RPL [80]. ROC analysis confirmed that eight circRNAs (including circRNA_104948, circRNA_104547, hsa_circRNA_101319) could be taken as RSA biomarkers for their favorable sensitivity and specificity. Besides, they also predicted the regulatory pathways of hsa_circRNAs_104792, miRNA_133a and HLA-G (Table 2).
Gynecological diseases
Epithelia ovarian carcinoma
Tumorigenesis is usually triggered by the dysregulation of signaling pathways mediating celluar proliferation and differentiation. The factors include NF-kB, TGF-, ILK regulating EMT and PI3K-AKT-JAKSTAT regulating cell proliferation. Using paired-end RNA sequencing, Ahmed et al. verified that circRNAs were richly expressed in epithelia ovarian carcinoma samples, and more differentially expressed in varying lesions (primary sites, peritoneal and lymph node metastases) compared with mRNAs. At the same time, mRNA’s high expression and circRNA’s downregulation synchronized. miRNA let-7 inversely regulating RAS and MYC (two proto-oncogenes) showed obviously lower expression level in the primary lesion than peritoneal metastases. But the gene that can code circRNAs containing multiple let-7 binding sites was highly expressed in the primary lesion, which may be explained by circRNAs acting as the sponge of miRNAs [81].
Cervical carcinoma
Using human cervical carcinoma HeLa cells, Abdelmohsen et al. verified that circPABPN1 could competitively bind to HuR, thus suppressing HuR’s binding to PABPN1 mRNA and translation [82]. Bachmayr-Heyda et al. found in 13 kinds of human tissues that the global circRNA abundance was negatively correlated with proliferation rate. CDR1as (also called CiRS-7) and SRY (a testis-specific RNA) are two typical circRNAs containing elements binding to suppressor miR-7 in ovarian carcinoma [83-85] and miR-138 in cervical carcinoma [86,87], respectively. CiRS-7 is stably expressed in Hela cells [88]. More in-depth researches should be conducted to verify whether negative regulatory mechanism between CiRS-7 and miR-7, or SRY and miR-138, and how this regulation functions in the development of ovarian carcinoma and cervical carcinoma.
CDRlas demonstrates a higher expression level in cervical carcinoma tissues than in para-carcinoma tissues. But this is opposite to miR-7. In HeLa and C33A cells of cervical carcinoma, overexpressed CDRlas increases the expression of FAK (focal adhesion kinase, target of miR-7) through suppressing the activity of miR-7. FAK overexpression then promotes the proliferation, invasion and metastasis of cervical carcinoma cells, indicating the intricate cooperation between CDRlas, miR-7 and FAK. Therefore molecular targeted treatment based on CiRS-7 regulatory network has potential efficacy in the prevention, diagnosis and treatment of cervical carcinoma [89] (Tables 2, 3).
Table 3.
Function and clinical application of disease related CirRNAs
| Tumor | CirRNA | Function | Role |
|---|---|---|---|
| Expression in granule cells [69] | CircRNA_103827 | Two circRNAs co-regulated their targets (ANKRD20A90, KCNQ10T1, XIST) through the network “circRNA-miRNA-mRNA” | Be involved in glycometabolism, mitosisand ovary steroid synthesis |
| CircRNA_104816 | |||
| Preeclampsia (PE) [76] | Hsa_circ_0029601 | Significantly upregulated, It is combined with plasma protein factor (endoglin) | The prediction accuracy of pre-eclampsia can be improved significantly |
| Hsa_cirRNA_100782 | These findings advocate that the ceRNA regulatory network constructed by miRNAs and circRNAs benefit PE development | ||
| Has_circRNA_102682 | Had already verified the highly expressed mir-30a-3p in the placental tissues of PE women | ||
| Has_circRNA_104820 | |||
| Recurrent spontaneous abortion (RSA) [80] | CircRNA_104948 | Hsa_circRNAs_104792/miRNA-133a/HLA-G | Be taken as RSA biomarkers |
| CircRNA_104547 | |||
| Hsa_circRNA_101319 | |||
| Ovarian carcinoma [90] | Cirs-7 | MIr-7 sponge | CircRNAs may use their circularization to competitively inhibit the linear splicing and sponge function of miRNAs, and modulate the expression of metastasis-evoking genes, all contributing to the metastasis of ovarian carcinoma |
| Cervical carcinoma [88,89] | CDR1as | miRNA sponge (miR-7) | Tumor suppressor was performed by inhibiting the tumorigenic effect of mir-7 |
| CircHIPK3 | miRNA sponge | Regulation of cell growth | |
| Hsa_circ_0018289 | miRNA sponge (miR-497) | Promote tumor cell proliferation, migration and invasion | |
| Breast cancer [93,95] | CDR1as | miRNA sponge (miR-7) | Tumor suppressor was performed by inhibiting the tumorigenic effect of mir-7 |
| Circ-ABCB10 | miRNA sponge (miR-1271) | Promote tumor cell proliferation | |
| Hsa_circ_0001982 | - | Potential tumor biomarkers | |
| Endometrial cancer [98] | Circ-ITCH | miRNA sponge (miRNA-17, miRNA-224) | Circ-ITCH can competitively bind to miRNA-17 and miRNA-224, a process leading to the differential expression of p21 and PTEN (the formers’ targets) |
| Circ-ZNF91 | miRNA sponge (miRNA-23B, miRNA122A2) | The expression level of circ-ZNF91 was found to be negatively correlated with that of miRNA-23B or miRNA122A2 in endometrial cancer tissues, suggesting that circRNAs may serve as miRNA sponge to inhibit the expression of miRNA-23B and miRNA-122A2 |
PE: Preeclampsia; RSA: Recurrent spontaneous abortion; PTEN: Phosphatase and tensin homolog deleted from chromosome 10; CiRS-7: Circular RNA Sponge for miR-7.
Ovarian carcinoma
Ovary plays a dominant part in female reproductive system for its two functions: germinating and releasing oocytes, secreting gonadal hormones to guarantee the development of follicles, estrous cycles and post-pregnancy hormonal level. Females’ biggest health threat, ovarian carcinoma has a high incidence (just next to those of cervical carcinoma and endometrial carcinoma) and mortality (topping all other gynecological carcinomas). Studies have convinced the association between circRNAs and the invasive metastasis of ovarian carcinoma [90]. Using RNA sequencing for the primary site, peritoneal and lymph node metastases of epithelial ovarian carcinoma (stage IIIC), researchers found the upregulated mRNAs and downregulated circRNAs corresponding to NFkB, PI3k/AKT and TGF (both containing multiple miR-24/let-7 binding sites) were upreguated in metastatic carcinoma, indicating that circRNAs may use their circularization to competitively inhibit the linear splicing and sponge function of miRNAs, and modulate the expression of metastasis-evoking genes, all contributing to the metastasis of ovarian carcinoma (Tables 2, 3).
Breast cancer
Serving as the sponge of miR-7, CDR1as can inversely regulate its activity and reduce its suppression on ECF, IRS-1, IRS-2, ak1, Raf1, Ack1 and PIK3CD, which in turn promotes the development of tumors, including breast cancer [91,92]. Besides, once miR-671 binds to CDR1as, an AGO-mediated splitting action is initiated to decrease the expression level of CDR1as, suggesting that miR-671 can indirectly regulate the activity of miR-7 via weakening CDR1as expression [93]. Reddy found miR-7 could inhibit the expression of p21-activated kinase (PAK1), a kinase upregulated in multiple tumors [94]. The expression of miR-7 is positively regulated by its upstream gene HOXD10, the low expression of which can enhance cancer invasiveness. So in the breast cancer cell model, the invasiveness gets stronger as PAK1 level rises, and miR-7 or HOXD10 level drops. Studies found that miR-7 was lowly expressed in tissues of malignant breast cancer, and this expression level was negatively associated with the metastatic ability. Inducing miR-7 expression in the cells lines of invasive breast cancer can inhibit the anchorage-independent proliferation of monolayer cells [95]. MiR-7 can also inhibit epithelial-mesenchymal transition and metastasis via directly regulating the expression of focal adhesion kinase (FAK), an interbody for signals of extracellular matrix integrin and pathways of cellular movement, proliferation and apoptosis. FAK overexpression leads to metastasis and poor prognosis in various tumors, suggesting that miR-7 expression is closely correlated with cancer’s epithelial differentiation: the lower expression, the weak metastatic potency.
Researchers also found that let-7 was lowly expressed in the lymphatic metastasis and proliferation of breast cancer, and could downregulate the expression of some cancer genes (RAS, MYC) and cell-cycle-related genes (Table 3) [96].
Endometrial cancer
Some circRNAs regulate the biological processes of endometrial cancer. But their performance in the cells or tissues engaged in endometrial cancer waits to be unveiled. Recent studies have found that circRNAs may serve as miRNA sponge in endometrial cancer. Phosphatase and tensin homolog deleted from chromosome 10 (PTEN) is a cancer-suppressor with phosphatase activity in that it can inhibit the division (progression of cell cycle), proliferation, invasion and migration, and accelerate the apoptosis of cancer cells; prevent the vascularization in cancer tissues; maintain the defense of immune system. Circ-ITCH can competitively bind to miRNA-17 and miRNA-224, a process leading to the differential expression of p21 and PTEN (the formers’ targets) [97]. Mutations or deletions of PTEN can inactivate its enzymatic activity, then the PTEN loses its ability to inhibit cell proliferation. In this case, the cells show high inclination to malignancy, and endometrial cancer may arise. Chen et al. [99] found the lowly expressed circRNAs (mainly exonic circRNAs) in endometrial cancer tissues than in the normal. In their study, six pairs tissues (endometrial cancer tissues vs. normal tissues) were analyzed. Among them differential circRNA expression was found in 120 pairs, including 22 with upregulated expression and 98 with downregulated expression. Their transcriptional products predispose endometrial tissues to malignancy. In another study, two intronic circRNAs (HSPG2 and RP11255H23.4) were found to be expressed only in the normal endometrial tissues, not in endometrial cancer tissues. But in endometrial tissues, the expression of the miRNAs transcribed from their parent genes increased, indicating that these circRNAs could competitively bind to related miRNAs and play powerful roles in the development of endometrial cancer.
At basilar membrane, HSPG2 binds to growth factors to regulate the growth and regeneration of endothelium. Besides, the expression level of circ-ZNF91 was found to be negatively correlated with that of miRNA-23B or miRNA122A2 in endometrial cancer tissues [98], suggesting that circRNAs may serve as miRNA sponge to inhibit the expression of miRNA-23B and miRNA-122A2 [99]. The behavior of these two miRNAs in human cancers have been reported, and their target genes and roles (either promotive or inhibitive) vary in different types of cancers (Table 3).
Prospect
CircRNAs are major participators in biological processes. Unlike linear RNA, circRNA is a covalently closed continuous loop bearing no 5’ cap and 3’ poly(A) tail and is resistant to exonuclease-mediated degradation. With these techniques, researchers have found that circRNAs are not products of missplicing, but RNAs being endogenous, highly conserved, tissue- and developmental-stage-specific. As a focus in RNA research field, an increasing number of circRNAs differentially expressed in human cells are being dug out with efficient high-throughput sequencing and bioinformatic techniques. Given this huge number, their functions remain to be unveiled. Just like a tip of the hidden iceberg, the current knowledge on circRNAs should be enriched with efforts as many. In-depth research on circRNA expression in granules and ART-achieved preimplantation embryos can provide us a new window into the development of germ cells and embryos.
Meanwhile, the updating databases, testing tools and research skills turn circRNAs into possible non-invasive biomarkers for reproduction and gynecological diseases.
In conclusion, the vibrating circRNA research has opened a doorway for us to enter the genetic regulatory network. Future research will surely reveal the mechanims of circRNAs in the development of reproductive and perinatal diseases.
Acknowledgements
We thank Dr. Chao Liu of Department of Endocrinology, Affiliated Hospital of Integrated Traditional Chinese and Western Medicine, Nanjing University of Chinese Medicine y, who took part with great efforts in revising the paper in English.
Disclosure of conflict of interest
None.
Abbreviations
- CircRNA
Circular RNA
- CiRNA
Circular intronic RNAs
- EIciRNA
Exon-intron circRNAs
- RBP
RNA-binding proteins
- EIF4A3
Eukaryotic initiation factor 4A-III
- PAK1
P21-activated kinase
- FAK
Focal adhesion kinase
- CDR1
Cerebellar degeneration-related protein 1
- PTEN
Phosphatase and tensin ho-molog deleted from chromosome10
References
- 1.Zhu Q, Huang Y, Marton LJ, Woster PM, Davidson NE, Casero RA Jr. Polyamine analogs modulate gene expression by inhibiting lysine - specific demethylase 1 (LSD1) and altering chromatin structure in human breast cancer cells. Amino Acids. 2012;42:887–898. doi: 10.1007/s00726-011-1004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quan G, Li J. Circular RNAs: biogenesis, expression and their potential roles in reproduction. J Ovarian Res. 2018;11:1–12. doi: 10.1186/s13048-018-0381-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y, Peng G, Hsueh EC. Induction of autophagy and apoptosis with polyamine synthesis inhibition and metformin in human melanoma and colon cancer cells. Cancer Res. 2014;74:1418. [Google Scholar]
- 4.Wang Q, Wang YL, Cao CY. Research progress on polyamine metabolism as a target for anti-cancer therapy. Chinese Journal of Clinical Oncology. 2014;41:597–600. [Google Scholar]
- 5.Zhang J, Han Y, Wang YL. Progress in research of the machanism of polyamine regulating cell growth. Chinese Bulletin of Life Sciences. 2014;26:85–90. [Google Scholar]
- 6.Oker A, Ar san ED, Palavan-Ünsal N. Silencing of the polyamine catabolic key enzyme SSAT prevents CDK inhibitor-induced apopto-sis in Caco-2 colon cancer cells. Mol Med Rep. 2012;5:1037–1042. doi: 10.3892/mmr.2012.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang W, Eickhoff JC, Mehraein-Ghomi F, Church DR, Wilding G, Basu HS. Expression of spermidine/spermine N1 - acetyl transferase (SSAT) in human prostate tissues is related to prostate cancer progression and metastasis. Prostate. 2015;75:1150–1159. doi: 10.1002/pros.22996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li HT, Zhou HJ, Tian SM, Zhang XL, Zhao YY. Progresses on regulation roles of polyamines in programmed cell death. Chinese Bulletin of Life Sciences. 2015;19:242–245. [Google Scholar]
- 9.Jang SJ, Wi SJ, Choi YJ, An G, Park KY. Increased polyamine biosynthesis enhances stress tolerance by preventing the accumulation of reactive oxygen species: T-DNA mutational analysis of Oryza sativa lysine decarboxylase-like protein 1. Mol Cells. 2012;34:251–262. doi: 10.1007/s10059-012-0067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karouzakis E, Gay RE, Gay S, Neidhart M. Increased recycling of polyamines is associated with global DNA hypomethylation in rheumatoid arthritis synovial fibroblasts. Arth Rheum. 2014;60:3613–3622. doi: 10.1002/art.34340. [DOI] [PubMed] [Google Scholar]
- 11.Zwighaft Z, Aviram R, Shalev M, Rousso-Noori L, Kraut-Cohen J, Golik M, Brandis A, Reinke H, Aharoni A, Kahana C, Asher G. Circadian clock control by polyamine levels through a mechanism that declines with age. Cell Metab. 2015;22:874–885. doi: 10.1016/j.cmet.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 12.König SG, Öz S, Krämer R. A polyamine-modified near-infrared fluorescent probe for selective staining of live cancer cells. Chem Commun. 2015;51:7360–7363. doi: 10.1039/c5cc01637a. [DOI] [PubMed] [Google Scholar]
- 13.Barrett SP, Wang PL, Salzman J. Circular RNA biogenesis can proceed through an exon-containing lariat precursor. Elife. 2015;4:e07540. doi: 10.7554/eLife.07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danan M, Schwartz S, Edelheit S, Sorek R. Transcriptome-wide discovery of circular RNAs in Archaea. Nucleic Acids Res. 2012;40:3131–3142. doi: 10.1093/nar/gkr1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang C, Wu D, Gao L, Liu X, Jin Y, Wang D, Wang T, Li X. Competing endogenous RNA networks in human cancer: hypothesis, validation, and perspectives. Oncotarget. 2016;7:13479–13490. doi: 10.18632/oncotarget.7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Zhang XO, Chen T, Xiang JF, Yin QF, Xing YH, Zhu S, Yang L, Chen LL. Circular intronic long noncoding RNAs. Mol Cell. 2013;51:792–806. doi: 10.1016/j.molcel.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 17.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, Loewer A, Ziebold U, Landthaler M, Kocks C, le Noble F, Rajewsky N. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 18.Qu S, Yang X, Li X, Wang J, Gao Y, Shang R, Sun W, Dou K, Li H. Circular RNA: a new star of noncoding RNAs. Cancer Lett. 2015;365:141–148. doi: 10.1016/j.canlet.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Rybak-Wolf A, Stottmeister C, Glažar P, Jens M, Pino N, Giusti S, Hanan M, Behm M, Bartok O, Ashwal-Fluss R, Herzog M, Schreyer L, Papavasileiou P, Ivanov A, Öhman M, Refojo D, Kadener S, Rajewsky N. Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol Cell. 2015;58:870–885. doi: 10.1016/j.molcel.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki H, Tsukahara T. A view of pre-mRNA splicing from RNase R resistant RNAs. Int J Mol Sci. 2014;15:9331–9342. doi: 10.3390/ijms15069331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bahn JH, Zhang Q, Li F, Chan TM, Lin X, Kim Y, Wong DT, Xiao X. The landscape of microRNA, Piwi-interacting RNA, and circular RNA in human saliva. Clin Chem. 2015;61:221–230. doi: 10.1373/clinchem.2014.230433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capel B, Swain A, Nicolis S, Hacker A, Walter M, Koopman P, Goodfellow P, Lovell-Badge R. Circular transcripts of thetestis-determining gene Sry in adult mouse testis. Cell. 1993;73:1019–1030. doi: 10.1016/0092-8674(93)90279-y. [DOI] [PubMed] [Google Scholar]
- 23.Hansen TB, Wiklund ED, Bramsen JB, Villadsen SB, Statham AL, Clark SJ, Kjems J. miRNA-dependent gene silencing involving Ago2-mediated cleavage of a circular antisense RNA. EMBO. 2011;30:4414–4422. doi: 10.1038/emboj.2011.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li F, Zhang L, Li W, Deng J, Zheng J, An M, Lu J, Zhou Y. Circular RNA ITCH has inhibitory effect on ESCC by suppressing the Wnt/-catenin pathway. Oncotarget. 2015;6:6001–6013. doi: 10.18632/oncotarget.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng Q, Bao C, Guo W, Li S, Chen J, Chen B, Luo Y, Lyu D, Li Y, Shi G, Liang L, Gu J, He X, Huang S. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Naturecommunications. 2016;7:11215. doi: 10.1038/ncomms11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng L, Chen G, Zhu Z, Shen Z, Du C, Zang R, Su Y, Xie H, Li H, Xu X, Xia Y, Tang W. Circular RNA ZNF609 functions as a competitive endogenous RNA to regulate AKT3 expression by sponging miR-150-5p in Hirschs-prung’s disease. Oncotarget. 2017;8:808–818. doi: 10.18632/oncotarget.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang K, Long B, Liu F, Wang JX, Liu CY, Zhao B, Zhou LY, Sun T, Wang M, Yu T, Gong Y, Liu J, Dong YH, Li N, Li PF. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting mir -223. Eur Heart J. 2016;37:2602–2611. doi: 10.1093/eurheartj/ehv713. [DOI] [PubMed] [Google Scholar]
- 28.Thomas LF, Sætrom P. Circular RNAs are depleted of polymorphisms at microRNA binding sites. Bioinformatics. 2014;30:2243–2246. doi: 10.1093/bioinformatics/btu257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abdelmohsen K, Kuwano Y, Kimh H, Gorospe M. Posttranscriptional gene regulation by RNAbinding proteins during oxidative stress: implications for cellular senescence. Biol Chem. 2008;389:243–255. doi: 10.1515/BC.2008.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glisovic T, Bachorik JL, Yong J, Dreyfuss G. RNA binding proteins and post-transcriptional gene regulation. FEBS Lett. 2008;582:1977–1986. doi: 10.1016/j.febslet.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du WW, Yang W, Liu E, Yang Z, Dhaliwal P, Yang BB. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 2016;44:2846–2858. doi: 10.1093/nar/gkw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansen TB, Wiklund ED, Bramsen JB, Villadsen SB, Statham AL, Clark SJ, Kjems J. miRNA-dependent gene silencing involving Ago2-mediated cleavage of a circular antisense RNA. EMBO J. 2011;30:4414–4422. doi: 10.1038/emboj.2011.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ng WL, Marinov GK, Liau ES, Lam YL, Lim YY, Ea CK. Inducible RasGEF1B circular RNA is a positive regulator of ICAM-1 in the TLR4/LPS pathway. RNA Biol. 2016;13:861–871. doi: 10.1080/15476286.2016.1207036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Z, Huang C, Bao C, Chen L, Lin M, Wang X, Zhong G, Yu B, Hu W, Dai L, Zhu P, Chang Z, Wu Q, Zhao Y, Jia Y, Xu P, Liu H, Shan G. Exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol. 2015;22:256–264. doi: 10.1038/nsmb.2959. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Wang Z. Efficient backsplicing produces translatable circular mRNAs. RNA. 2015;21:172–179. doi: 10.1261/rna.048272.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas LF, Sætrom P. Circular RNAs are depleted of polymorphisms at microRNA binding sites. Bioinformatics. 2014;30:2243–2246. doi: 10.1093/bioinformatics/btu257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kos A, Dijkema R, Arnberg AC, van der Meide PH, Schellekens H. The hepatitis delta (δ) virus possesses a circular RNA. Nature. 1986;323:558. doi: 10.1038/323558a0. [DOI] [PubMed] [Google Scholar]
- 38.Legnini I, Ditmoteo G, Rossi F, Morlando M, Briganti F, Sthandier O, Fatica A, Santini T, Andronache A, Wade M, Laneve P, Rajewsky N, Bozzoni I. Circ ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol Cell. 2017;66:22–37. e9. doi: 10.1016/j.molcel.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu J, Liu T, Wang XM, He AL. Circles reshaping the RNA world: from waste to treasure. Mol Cancer. 2017;16:58. doi: 10.1186/s12943-017-0630-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abe N, Matsumoto K, Nishihar AM, Nakano Y, Shibata A, Maruyama H, Shuto S, Matsuda A, Yoshida M, Ito Y, Abe H. Rolling circle translation of circular RNA in living human cells. Sci Rep. 2015;5:16435. doi: 10.1038/srep16435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salzman J, Chen RE, Olsen MN, Wang PL, Brown PO. Cell-type specific features of circular RNA expression. PLoS Genet. 2013;9:e1003777. doi: 10.1371/journal.pgen.1003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Langenberger D, Christ S, Kunz M, Holdt LM, Teupser D, Hackermüller J, Stadler PF. A multi-split mapping algorithm for circular RNA, splicing, trans-splicing and fusion detection. Genome Biol. 2014;15:R34. doi: 10.1186/gb-2014-15-2-r34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang K, Singh D, Zeng Z, Coleman SJ, Huang Y, Savich GL, He X, Mieczkowski P, Grimm SA, Perou CM, MacLeod JN, Chiang DY, Prins JF, Liu J. MapSplice: accurate mapping of RNA-seq reads for splice junction discovery. Nucleic Acids Res. 2010;38:e178. doi: 10.1093/nar/gkq622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141–157. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao Y, Wang J, Zhao F. CIRI: an efficient and unbiased algorithm for de novo circular RNA identification. Genome Biol. 2015;16:4. doi: 10.1186/s13059-014-0571-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang XO, Wang HB, Zhang Y, Lu X, Chen LL, Yang L. Complementary sequence-mediated exon circularization. Cell. 2014;159:134–147. doi: 10.1016/j.cell.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Ebbesen KK, Kjems J, Hansen TB. Circular RNAs: identification, biogenesis and function. Biochim Biophys Acta. 2016;1859:163–168. doi: 10.1016/j.bbagrm.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 48.Hoffmann S, Otto C, Doose G, Tanzer A, Langenberger D, Christ S, Kunz M, Holdt LM, Teupser D, Hackermüller J. A multi-split mapping algorithm for circular RNA, splicing, trans-splicing, and fusion detection. Genome Biol. 2014;15:R34. doi: 10.1186/gb-2014-15-2-r34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Westholm JO, Miura P, Olson S, Joseph B, Sanfilippo P, Celniker SE, Graveley BR, Lai EC. Genome-wide analysis of drosophila circular RNAs reveals their structural and sequence properties and age-dependent neural accumulation. Cell Rep. 2014;9:1966–1980. doi: 10.1016/j.celrep.2014.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 51.Gao Y, Wang J, Zhao F. CIRI: an efficient and unbiased algorithm for de novo circular RNA identification. Genome Biol. 2015;16:4. doi: 10.1186/s13059-014-0571-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu Q, Wang Y, Cao M, Pantaleo V, Burgyan J, Li WX, Ding SW. Homology-independent discovery of replicating pathogenic circular RNAs by deep sequencing and a new computational algorithm. Proc Natl Acad Sci U S A. 2012;109:3938–3943. doi: 10.1073/pnas.1117815109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.GlaŽar P, Papavasilelou P, Rajewsky N. circBase: a database for circular RNAs. RNA. 2014;20:1666–1670. doi: 10.1261/rna.043687.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng LL, Li JH, Wu J, Sun WJ, Liu S, Wang ZL, Zhou H, Yang JH, Qu LH. Deep base v2.0: identification, expression, evolution and function of small RNAs, lncRNAs and circular RNAs from deep-sequencing data. Nucleic Acids Res. 2016;44:D196–D202. doi: 10.1093/nar/gkv1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu SM, Liu H, Huang PJ, Chang IY, Lee CC, Yang CY, Tsai WS, Tan BC. circlncRNAnet: an integrated web-based resource for mapping functional networks of long or circular forms of noncoding RNAs. Gigascience. 2018;7:1–10. doi: 10.1093/gigascience/gix118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu YC, Li JR, Sun CH, Andrews E, Chao RF, Lin FM, Weng SL, Hsu SD, Huang CC, Cheng C, Liu CC, Huang HD. CircNet: a database of circular RNAsderived from transcriptome sequencing data. Nucleic Acids Res. 2016;44:D209–15. doi: 10.1093/nar/gkv940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pang AL, Johnson W, Ravindranath N, Dym M, Rennert OM, Chan WY. Expression profiling of purified male germ cells: stage-specific expression patterns related to meiosis and postmeiotic development. Physiol Genomics. 2006;24:75–85. doi: 10.1152/physiolgenomics.00215.2004. [DOI] [PubMed] [Google Scholar]
- 58.Rajender S, Avery K, Agarwal A. Epigenetics, spermatogenesis and male infertility. Mutat Res. 2011;727:62–71. doi: 10.1016/j.mrrev.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 59.Mukherjee A, Koli S, Reddy KV. Regulatory non-coding transcripts in spermatogenesis: shedding light on ‘dark matter’. Andrology. 2014;2:360–369. doi: 10.1111/j.2047-2927.2014.00183.x. [DOI] [PubMed] [Google Scholar]
- 60.Chuma S, Nakano T. piRNAe and spermatogenesis in mice. Phil Trans R Soc B. 2013;368:20110338. doi: 10.1098/rstb.2011.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin X, Han M, Cheng L, Chen J, Zhang Z, Shen T, Wang M, Wen B, Ni T, Han C. Expression dynamics, relationships, and transcriptional regulations of diverse transcripts in mouse spermatogenic cells. RNA Biol. 2016;13:1011–1124. doi: 10.1080/15476286.2016.1218588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lasda E, Parker R. Circular RNAs: diversity of form and function. RNA. 2014;20:1829–1842. doi: 10.1261/rna.047126.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jeck WR, Sharpless NE. Detecting and characterizing circular RNAs. Nat Biotechnol. 2014;32:453. doi: 10.1038/nbt.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen L, Huang C, Wang X, Shan G. Circular RNAs in eukaryotic cells. Curr Genomics. 2015;16:312–318. doi: 10.2174/1389202916666150707161554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qu S, Yang X, Li X, Wang J, Gao Y, Shang R, Sun W, Dou K, Li H. Circular RNA: a new star of noncoding RNAs. Cancer Lett. 2015;365:141–148. doi: 10.1016/j.canlet.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 66.Li X, Ao J, Wu J. Systematic identification and comparison of expressed profiles of lncRNAs and circRNAs with associated co-expression and ceRNA networks in mouse germline stem cells. Oncotarget. 2017;8:26573–26590. doi: 10.18632/oncotarget.15719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dong WW, Li HM, Qing XR, Huang DH, Li HG. Identification and characterization of human testis derived circular RNAs and their existence in seminal plasma. Sci Rep. 2016;6:39080. doi: 10.1038/srep39080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheng J, Huang J, Yuan S, Zhou S, Yan W, Shen W, Chen Y, Xia X, Luo A, Zhu D, Wang S. Circular RNA expression profiling of human granulosa cells during maternal aging reveals novel transcripts associated with assisted reproductive technology outcomes. PLoS One. 2017;12:e0177888. doi: 10.1371/journal.pone.0177888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zeng Y, Nie C, Min J, Liu X, Li M. Novel loci and pathways significantly associated with longevity. Sci Rep. 2016;6:21243. doi: 10.1038/srep21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kanduric C. Kcnq1ot1: a chromatin regulatory RNA. Semin Cell Dev Biol. 2011;22:343–350. doi: 10.1016/j.semcdb.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 71.Chu C, Zhang QC, Rocha ST, Flynn RA, Bharadwaj M. Systematic discovery of Xist RNA binding proteins. Cell. 2015;161:404. doi: 10.1016/j.cell.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dang Y, Yan L, Hu B, Fan X, Ren Y, Li R, Lian Y, Yan J, Li Q, Zhang Y, Li M, Ren X, Huang J, Wu Y, Liu P, Wen L, Zhang C, Huang Y, Tang F, Qiao J. Tracing the expression of circular RNAs in human pre-implantation embryos. Genome Biol. 2016;17:130. doi: 10.1186/s13059-016-0991-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fan X, Zhang X, Wu X, Guo H, Hu Y, Tang F, Huang Y. Single-cell RNA-seq transcriptome analysis of linear and circular RNAs in mouse preimplantation embryos. Genome Biol. 2015;16:148. doi: 10.1186/s13059-015-0706-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Y, Yang H, Long Y, Li W. Circular RNA in blood corpuscles combined with plasma protein factor for early prediction of preeclampsia. BJOG. 2016;123:2113–2118. doi: 10.1111/1471-0528.13897. [DOI] [PubMed] [Google Scholar]
- 75.Qian Y, Lu Y, Rui C, Qian Y, Cai M. Potential significance of circular RNA in human placental tissue for patients with preeclampsia. Cell Physiol Biochem. 2016;39:1380. doi: 10.1159/000447842. [DOI] [PubMed] [Google Scholar]
- 76.Luan LX, Zhu XM, Yang YL, Yi Z, Yin JB, Guo W. Expression of mir-30a-3p in the placentas from women with preeclampsia. Journal of Reproductive Medicine. 2013:2253–2256. [Google Scholar]
- 77.López AM, Montalvo V, Vital-Reyes VS, Hinojosa-Cruz JC, Leaños-Miranda A, Martínez-Basila A. Serial determinations of asymmetric dimethylarginine and homocysteine during pregnancy to predict pre-eclampsia: a longitudinal study. BJOG. 2015;122:1586–1592. doi: 10.1111/1471-0528.13516. [DOI] [PubMed] [Google Scholar]
- 78.Qian Y, Lu Y, Rui C, Qian Y, Cai M, Jia R. Potential significance of circular RNA in human placental tissue for patients with preeclampsia. Cell Physiol Biochem. 2016;39:1380–1390. doi: 10.1159/000447842. [DOI] [PubMed] [Google Scholar]
- 79.Zhang YG, Yang HL, Long Y, Li WL. Circular RNA in blood corpuscles combined with plasma protein factor for early prediction opre-eclampsia. BJOG. 2016;123:2113–2118. doi: 10.1111/1471-0528.13897. [DOI] [PubMed] [Google Scholar]
- 80.Qian Y, Wang X, Ruan H, Rui C, Mao PY, Cheng Q, Jia RZ. Circular RNAs expressed in chorionic villi are probably involved in the occurrence of recurrent spontaneous abortion. Biomedicine & Pharmacotherapy. 2017;88:1154–1162. [Google Scholar]
- 81.Ahmed I, Karedath T, Andrews SS, Al-Azwani IK, Mohamoud YA, Querleu D, Rafii A, Malek JA. Altered expression pattern of circular RNAs in primary and metastaticsites of epithelial ovarian carcinoma. Oncotarget. 2016;7:36366–36381. doi: 10.18632/oncotarget.8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abdelmohsen K, Panda AC, Munk R, Grammatikakis I, Dudekula DB, De S, Kim J, Noh JH, Kim KM, Martindale JL, Gorospe M. Identification of HuR target circular RNAs uncovers suppression of PABPN1 translation by CircPABPN1. RNA Biol. 2017;14:361–369. doi: 10.1080/15476286.2017.1279788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bachmayr-Heyda A, Reiner AT, Auer K, Sukhbaatar N, Aust S, Bachleitner-Hofmann T, Mesteri I, Grunt TW, Zeillinger R, Pils D. Correlation of circular RNA abundance with proliferation-exemplified with colorectal and ovarian cancer, idiopathic lung fibrosis, and normal human tissues. Sci Rep. 2015;5:8057. doi: 10.1038/srep08057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou X, Hu Y, Dai L, Dai L, Wang Y, Zhou J, Wang W, Di W, Qiu L. MicroRNA-7 inhibits tumor metastasis and reverses epithelial-mesenchymal transition through AKT/ERK1/2 inactivation by targeting EGFR in epithelial ovarian cancer. PLoS One. 2014;9:e96718. doi: 10.1371/journal.pone.0096718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yeh YM, Chuang CM, Chao KC, Wang LH. MicroRNA-138 suppresses ovarian cancer cell invasion and metastasis by targeting SOX4 and HIF-1alpha. Int J Cancer. 2013;133:867–878. doi: 10.1002/ijc.28086. [DOI] [PubMed] [Google Scholar]
- 86.Hao Z, Yang J, Wang C, Li Y, Zhang Y, Dong X, Zhou L, Liu J, Zhang Y, Qian J. MicroRNA-7 inhibits me-tastasis and invasion through targeting focal adhesion kinase in cervical cancer. Int J Clin Exp Med. 2015;8:480–487. [PMC free article] [PubMed] [Google Scholar]
- 87.Li H, Sheng Y, Zhang Y, Gao N, Deng X, Sheng X. MicroRNA-138 is a potential biomarker and tumor suppressor in human cervical carcinoma by reversely correlated with TCF3 gene. Gynecol Oncol. 2017;145:569–576. doi: 10.1016/j.ygyno.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 88.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 89.Lee BY, Timpson P, Horvath LG, Daly RJ. FAK signaling in human cancer as a target for therapeutics. Pharmacol Ther. 2015;146:132–149. doi: 10.1016/j.pharmthera.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 90.Ikhlak A, Thasni K, Simeon SA. Altered expression pattern of circular RNAs in primary and metastatic sites of epithelial ovarian carcinoma. Oncotarget. 2016;7:36366–36378. doi: 10.18632/oncotarget.8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tang YY, Zhao P, Zou TN, Duan JJ, Zhi R, Yang SY, Yang DC, Wang XL. CircularRNA hsa_circ_0001982 promotes breast cancer cell carcinogenesis through decreasing miR-143. DNA Cell Biol. 2017;36:901. doi: 10.1089/dna.2017.3862. [DOI] [PubMed] [Google Scholar]
- 92.Lü L, Sun J, Shi P, Kong W, Xu K, He B, Zhang S, Wang J. Identification of circular RNAs as a promising new class of diagnostic biomarkers for human breast cancer. Oncotarget. 2017;8:44096–44107. doi: 10.18632/oncotarget.17307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Nature natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 94.Reddy SD, Ohshiro K, Rayala SK, Kumar R. MicroRNA-7, a homeobox D10 target, inhibits p21-Activated kinase 1 and regulates its functions. Cancer Res. 2008;68:8195–8200. doi: 10.1158/0008-5472.CAN-08-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang F, Xia J, Wang N, Zong H. miR-145 inhibits proliferation and invasion of esophageal squamous cell carcinoma in part by targeting c-Myc. Onkologie. 2013;36:754–758. doi: 10.1159/000356978. [DOI] [PubMed] [Google Scholar]
- 96.Xu L, Shi J, Zhang L, Shi S, Qu J, Li Z, Hou K, Qu X, Teng Y. Expression and clinical significance of FAK combined with pAKT in breast cancer. Journal of China Medical University. 2015;44:673–678. [Google Scholar]
- 97.Yang X, Li P, Wang J, Han J, Tao J, Li P, Yang H, Lv Q, Zhang W. Circular RNA circ-ITCH inhibits bladder cancer progression by sponging miR-17/miR-224 and regulating p21, PTEN expression. Mol Cancer. 2018;17:19–31. doi: 10.1186/s12943-018-0771-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hasengaowa , Kodama J, Kusumoto T, Seki N, Nakamura K, Hongo A, Hiramatsu Y. Loss of basement membrane heparan sulfate expression is associated with tumor progression in endometrial cancer. Eur J Gynaecol Oncol. 2005;26:403–406. [PubMed] [Google Scholar]
- 99.Chen BJ, Byrne FL, Takenaka K, Modesitt SC, Olzomer EM, Mills JD, Farrell R, Hoehn KL, Janitz M. Analysis of the circular RNA transcriptome in endometrial cancer. Oncotarget. 2018;9:5786–5796. doi: 10.18632/oncotarget.23534. [DOI] [PMC free article] [PubMed] [Google Scholar]


