Abstract
Thoracic aortic dissection (TAD) is a highly lethal vascular disease characterized by medial degeneration. Heat shock protein 90 (HSP90) had been proved as a potential target for a variety of diseases. The aim of this study was to identify the effect of HSP90 inhibitor on TAD progress, and to explore the potential utility of HSP90 inhibitors as therapeutic avenue for TAD. In clinical samples, the elevated HSP90 expression was detected in aortic walls from TAD patients (n=20) by real-time PCR and western blot, and was positively correlated with osteopontin (OPN), a synthetic phenotypic marker of smooth muscle cells (SMCs), while negatively correlated with SM22, a contractile phenotypic marker. In a β-aminopropionitrile fumarate-induced AD mice model, 17-DMAG, a HSP90-inhibitor, effectively reduced the incidence and mortality of TAD. Histological examination confirmed that 17-DMAG significantly alleviated the loss of elastic fibers integrity. Meanwhile, the phenotypic switch of SMCs was significantly suppressed by 17-DMAG, demonstrated by the change of phenotypic markers expression. On the cellular level, 17-DMAG suppressed phenotypic switch of SMCs induced by PDGF-bb, and significantly depressed the excessive proliferation and migration of SMCs. Flow cytometry analysis showed that 17-DMAG induced cell cycle arrest in G1 phase. In summary, 17-DMAG could effectively alleviate the TAD progress by suppressing SMCs phenotypic switch, and inhibition of HSP90 might be a potential avenue for TAD therapy.
Keywords: Thoracic aortic dissection, heat shock protein 90, smooth muscle cells, phenotypic switch, 17-DMAG
Introduction
Thoracic aortic dissection (TAD) is estimated to occur at a rate of 3 cases per 100,000 individuals per year, which is a highly lethal vascular disease [1,2]. Since acute TAD renders the aorta prone to rapid dilation and rupture, overall about 20% of patients died before hospital admission, 30% during hospitalization, and a further 20% over the next 10 years [3]. Currently, dissection formation, progression and rupture cannot be reliably prevented pharmacologically, because the molecular mechanism of TAD pathogenesis are poorly understood [4,5]. However, the exploration of novel drugs effectively alleviating TAD progress never cease.
The key histopathologic feature of TAD is medial degeneration, which is characterized by smooth muscle cells (SMCs) depletion and extracellular matrix degradation [6,7]. SMCs play important roles in maintaining the structure integrity and function of vessels. Under pathologic stimulation, the quiescent contractile SMCs could transform to activated synthetic state, resulting in the increase of SMCs migration and extracellular matrix secretion [8,9]. This de-differentiation process, known as phenotype switch, is closely related to the aortic progressively dilation and ultimately rupture [10].
Heat shock protein 90 (HSP90) is a highly conserved molecular chaperone, which might assemble various types of client proteins and regulate protein folding, signal transduction, translocation and transcription [11,12]. Currently, HSP90 has attracted a great deal of interest as a potential anticancer target. The clinical trials in cancer patients had indicated that combination of HSP90 inhibitors and other drugs could be administered with acceptable toxicity [13,14]. Recent studies had proved that HSP90 could be the potential target for cardiovascular diseases, including abdominal aortic aneurysm and pulmonary arterial hypertension [15,16]. In this study, we aimed to investigate the therapeutic impact of 17-2-dimethylamminoethylamino-17-demethoxygeldanamycin (17-DMAG), an inhibitor of HSP90, on dissection formation, progression and rupture, and explore the potential utility of HSP90 inhibitors as therapeutic avenue for TAD.
Materials and methods
Clinical samples
All clinical aorta samples from TAD patients were collected after aortic replacement surgery at Department of Cardiovascular Surgery, Changhai Hospital. TAD diagnosis was made following clinical history, physical examinations, and computed tomographic angiography of the aorta. Patients with connective tissue diseases (Marfan syndrome, Ehlers-Danlos syndrome, etc.), bicuspid aortic valve malformation, syphilis or family history of aortic diseases, were excluded. All normal aorta samples were collected from cadaver donors.
This study was carried out in accordance to the principles of the Declaration of Helsinki and approved by the Medical Ethics Committee in Shanghai Changhai Hospital. Written informed consent was obtained from all the participants prior to enrollment.
AD mice model
AD mice model was induced by β-aminopropionitrile fumarate (BAPN, Sigma) and angiotensin II (Ang II, Sigma) as described previously [17,18]. Male FVB mice, weighing from 16 to 18 g, were randomly divided into 3 groups (n=40 for each group): Control, BAPN and 17-DMAG groups. Briefly, mice were fed on a regular diet and administered BAPN dissolved in drinking water (1 g/kg per day) for 4 weeks. Then, osmotic mini pumps (Alzet) filled with angiotensin II (Ang II, 1 μg/kg per minute) were subcutaneously implanted for 48 hours. In 17-DMAG group, mice were subcutaneously injected with 17-DMAG (10 mg/kg, Sigma) every 2 days during BAPN administered stage.
The animal work performed in this study was approved by institutional review board of the local university, and the experiment protocols were carried out according to the guidelines for the care and use of laboratory animals established by the US National Institutes of Health.
Cell culture and intervention
Rat aortic smooth muscle cells were isolated by type I collagenase (2 mg/mL, Sigma) digestion of aortas and cultured in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum. SMCs (passage 3-5) were seed in plates, and stimulated by PDGF-bb (10 ng/mL, Peprotech) with or without 17-DMAG (1 μmol/L, Sigma).
Histological analysis
The de-paraffinized and rehydrated sections were stained with hematoxylin-eosin (HE) and Victoria Blue (VB) for histological observation [19]. For immunohistochemical analysis, after antigen retrieval and blocking the endogenous peroxidases, the sections were incubated with the primary antibodies overnight followed by incubation with HRP-conjugated secondary antibody. The staining was developed by using a DAB chromogen kit (Beyotime) according to the instruction.
CCK-8 cell viability assay
Rat aortic smooth muscle cells were seeded in 96-well plates followed by different treatment. After 24 h, cells were stained with 10 μL CCK-8 solution (Beyotime) per well for 2 h. The absorbance was measured at 450 nm using a microplate reader (BioTek).
Flow cytometry analysis
For apoptosis detection, SMCs were harvested and stained by Annexin V/propidium iodide at room temperature for 15 minutes. For cell cycle analysis, SMCs were fixed with cold 70% ethanol, and incubated in staining solution (20 μg/mL propidium iodide and 50 μg/mL RNase A in phosphate-buffered saline) at room temperature for 30 minutes. All samples were analyzed using FACSort flow cytometer and Cell Quest Pro (BD).
Real-time quantitative polymerase chain reaction (Real-time PCR)
Total RNA (200 ng/μL) extracted from SMCs was used to generate cDNA by using PrimeScript RT reagent Kit (TAKARA) with oligo-dT and random primer. Real-time PCR was performed on a LightCycler 480 II quantitative PCR system (Roche) using SYBR Green (TAKARA). β-actin was detected as loading control. The sequences of primers used in real-time PCR were listed in Table S1.
Western blot
Protein was extracted from aorta tissues or SMCs by RIPA buffer plus protease inhibitors. Equal amount of protein (about 30 μg) was separated by SDS-PAGE and transferred to the PVDF membrane. After blocking, the membrane was incubated with diluted primary antibodies overnight followed by incubation with HRP-conjugated secondary antibody. Proteins were visualized by ECL Plus Western Blotting Substrate (Thermo Scientific) on ImageQuant LAS500 (GE). β-actin was detected as loading control.
Statistical analysis
All statistical analyses were performed by SPSS version 17.0. The qualitative data was compared with Chi-square test or Fisher’s exact test when necessary. The quantitative data were analyzed by One-way ANOVA followed by the post-hoc tests of Tukey. The correlation analysis was performed by Spearman test. P-values less than 0.05 were considered statistically significant difference.
Results
Patients characteristics
A total of 20 TAD patients and 8 cadaver control were enrolled in this study. The clinical and laboratory features of the subjects are shown in Table 1. There was no significant difference in clinical characteristics between two groups, except systolic blood pressure. Compared with the normal tissues, the loss of SMCs and fragmentation of elastic fibers were significantly found in aortic walls from TAD patients by histological examination (Figure S1).
Table 1.
Clinical characteristics of patients with TAD and donors control
| Control (n=8) | TAD (n=20) | P | |
|---|---|---|---|
| Ages [Year] | 51.9±9.1 | 49.8±12.8 | 0.680 |
| Male [n (%)] | 6 (75.0%) | 17 (85.0%) | 0.533 |
| Smoking history [n (%)] | 4 (50.0%) | 12 (60.0%) | 0.952 |
| SBP [mmHg] | 124.1±5.1 | 141.7±20.2 | 0.001** |
| DBP [mmHg] | 81.0±5.6 | 83.3±16.6 | 0.590 |
| Blood glucose [mmol/L] | 7.00±1.03 | 6.75±1.56 | 0.675 |
| WBC [×109/L] | 8.24±2.15 | 9.65±2.53 | 0.179 |
| NEUT [%] | 69.4±8.3 | 71.7±12.6 | 0.633 |
| LDL [mmol/L] | 3.20±0.64 | 4.11±1.77 | 0.056 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; WBC, white blood cell; NEUT, neutrophil count; LDL, low-density lipoprotein.
P<0.01.
Elevated expression of HSP90 and synthetic phenotypic markers in aortic walls from TAD patients
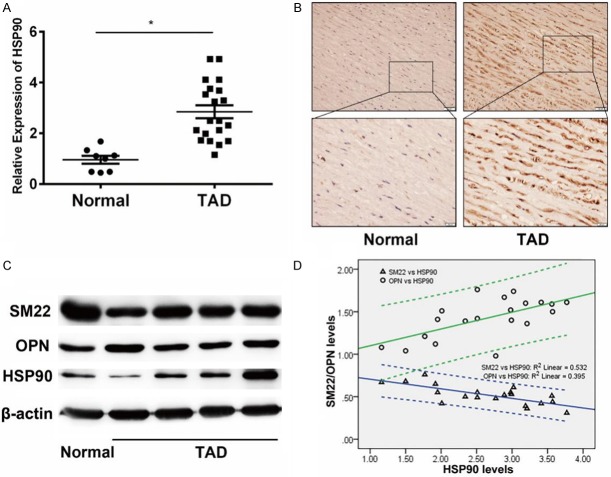
The expression of HSP90 in aortic walls from TAD patients was first detected by real-time PCR. Compared with normal group (n=8), HSP90 was up-regulated in aortic walls from TAD patients (n=20, P<0.05, Figure 1A). Immunohistochemistry showed that strong staining of HSP90, which mainly located in aortic medium, could be detected in 90% (18/20) TAD patients (Figure 1B). Western blot assay further confirmed this result (Figures 1C and S2). Then, Spearman analysis was performed on the expression of HSP90 and SMCs phenotypic markers in TAD patients. Expression of HSP90 was positively correlated (rs=0.573, P<0.01) with OPN, a synthetic phenotypic marker, while negatively correlated (rs=-0.705, P<0.01) with SM22, a contractile phenotypic marker (Figure 1D).
Figure 1.
Elevated expression of HSP90 and synthetic phenotypic markers in aortic walls from TAD patients. A. Detection of HSP90 expression in aortic walls from patients with TAD (n=20) and healthy control (n=8) by real-time PCR. The expression of HSP90 in aortic walls from patients with TAD higher than healthy control. β-actin was used as an internal control. *P<0.05 vs Normal group. B. Representative image of immunohistochemistry of HSP90 staining in aortic walls tissues lesions. The staining of HSP90 in aortic walls tissues of TAD patients was higher than normal group. C. Western blot analysis of SM22, OPN and HSP90 expression in aortic walls tissues. Compared with normal group, the expression of HSP90 and OPN were higher in TAD group, while SM22 was lower expressed. D. Spearman analysis of the correlation between protein expression of HSP90 and SMCs phenotypic markers in TAD patients. The protein levels were determined according the densitometry from western blot assay, which was evaluated by Image J software. The expression of HSP90 was positively correlated with OPN while negatively correlated with SM22.
HSP90 inhibitor effectively reduced the incidence and mortality of TAD in mice
To test whether inhibition of HSP90 impacted the development of AD, we investigated the effect of 17-DMAG on an AD mice model induced by BAPN plus Ang II. In BAPN group, 15% of mice (6/40) died at BAPN administration stage, and 20% of mice (8/40) died at Ang II intervening stage. There were only 5% mice (2/40) died in 17-DMAG group at Ang II intervening stage (Figure 2A). Compared with BAPN group, the mortality was significantly decreased in 17-DMAG group (P<0.01). Compared with control group, the weight gain of mice were significantly decreased in BAPN and 17-DMAG groups (both P<0.01). There was no significant difference between BAPN and 17-DMAG group (P>0.05. Figure 2B). The diastolic blood pressure was significantly reduced in BAPN group, but improved in 17-DMAG group. The systolic blood pressure was not significantly changed among the three groups. Correspondingly, the pulse blood pressure was significantly increased in BAPN group, but improved in 17-DMAG group (P>0.05. Figure 2C). Generally, AD occurred in 85.0% mice (34/40) from BAPN group, including 21 TAD (15 mice died for dissection rupture) and 13 abdominal aortic dissection (AAD), and in 60.0% mice (24/40) from 17-DMAG group, including 9 TAD (2 mice died for dissection rupture) and 15 AAD. There was no significant change on AAD incidence between BAPN and 17-DMAG groups (P>0.05). However, the incidence of TAD was significantly reduced in 17-DMAG group (P<0.01). Meanwhile, the mortality due to TAD rupture was also significantly reduced after 17-DMAG treatment (P<0.01). All the dissection did not rupture in 17-DMAG group (Table 2).
Figure 2.
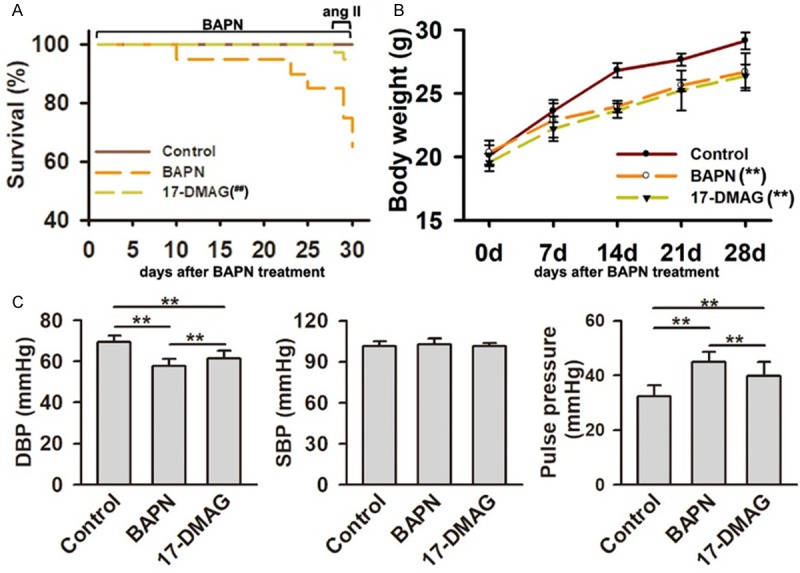
HSP90 inhibitor effectively reduced the incidence and mortality of TAD in mice. A. Survival curves of BAPN-treated mice with or without 17-DMAG treatment (n=40 initially for each group). The mortality of mice in 17-DMAG group was significantly decreased compared with that in BAPN group. ##P<0.01 vs BAPN group. B. The body weight change in BAPN group with or without 17-DMAG treatment. The weight gains of mice in BAPN and 17-DMAG groups were significantly decreased compared with that in control group. No significant difference was detected between BAPN and 17-DMAG groups. **P<0.01 vs control group. C. The blood pressure change in BAPN-induced mice with or without 17-DMAG treatment. DBP, diastolic blood pressure. SBP, systolic blood pressure. **P<0.01.
Table 2.
Incidence and mortality of AD mice with or without 17-DMAG treatment
| Group (n=40) | Incidence (n [%]) | Mortality (n [%]) | ||
|---|---|---|---|---|
|
| ||||
| TAD | AAD | TAD | AAD | |
| BAPN | 21 [52.5%] | 13 [32.5%] | 14 [35.0%] | 0 [0] |
| 17-DMAG | 9 [22.5%] | 15 [37.5%] | 2 [5.0%] | 0 [0] |
| P-value | 0.010** | 0.815 | 0.001** | 1.000 |
TAD, thoracic aortic dissection; AAD, abdominal aortic dissection. Fisher’s exact test was applied for statistical analysis.
P<0.01.
HSP90 inhibitor effectively alleviated the loss of elastic fibers integrity
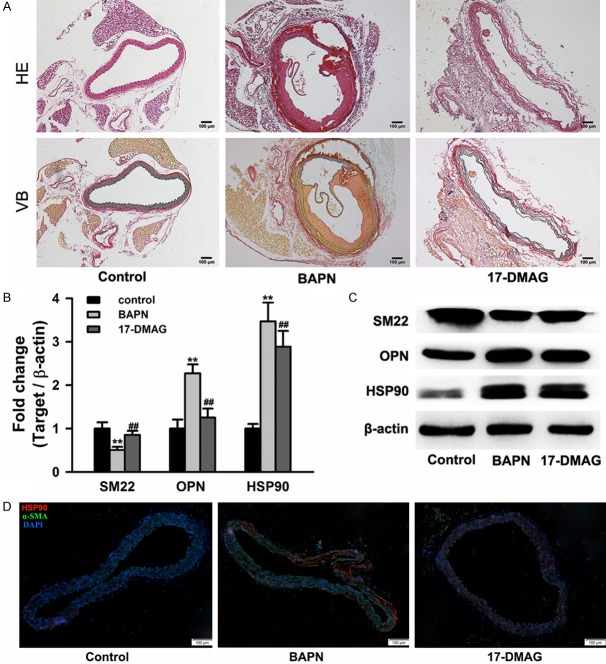
To further investigate the effect of 17-DMAG on medial degeneration, HE and VB staining were performed on paraffin sections of mice aorta. The elastic fibers were regularly arranged in control group, while serious degeneration and fragmentation of elastic fibers could be found in aortic walls from BAPN group. However, the breakage of elastic fibers was significantly alleviated in 17-DMAG group (Figure 3A). Consistent with the results from clinical samples, the increase expression of HSP90 was also detected in aortic walls from BAPN group. The synthetic phenotypic marker (OPN) was significantly upregulated, while the contractile phenotypic marker (SM22) was significantly downregulated in aortic walls from BAPN group. Moreover, the inhibition of HSP90 via 17-DMAG significantly attenuated the upregulation of OPN and downregulation of SM22 (Figures 3B, 3C and S3). Immunofluorescence microscopy assay further confirmed that HSP90 expression was significantly up-regulated in aortic walls from BAPN-treated mice, and mainly located in smooth muscle cells (Figure 3D).
Figure 3.
HSP90 inhibitor 17-DMAG alleviated the loss of elastic fibers integrity and reduction of SMCs contraction. A. Representative image of hematoxylin-eosin (HE) and victoria blue (VB) staining in aortic walls. Serious degeneration and fragmentation of elastic fibers in BAPN group, however the breakage of elastic fibers was significantly alleviated in 17-DMAG group. B and C. The mRNA and protein expression of SM22, OPN and HSP90 in aortic walls tissues. Real-time PCR and western blot assays were performed on aortic walls tissues from control, BAPN and 17-DMAG groups (n=6 for each group) respectively. Treatment of 17-DMAG could significantly attenuate the expression change of SM22, OPN and HSP90. **P<0.01 vs control group, ##P<0.01 vs BAPN group. D. Representative image of immunofluorescence staining with HSP90 antibody (red) and α-SMA antibody (green). HSP90 was highly expressed in aortic walls from BAPN-treated mice, and mainly located in smooth muscle cells. DAPI (blue) was used for nuclei staining.
HSP90 inhibitor suppressed phenotypic switch of SMCs induced by PDGF-bb
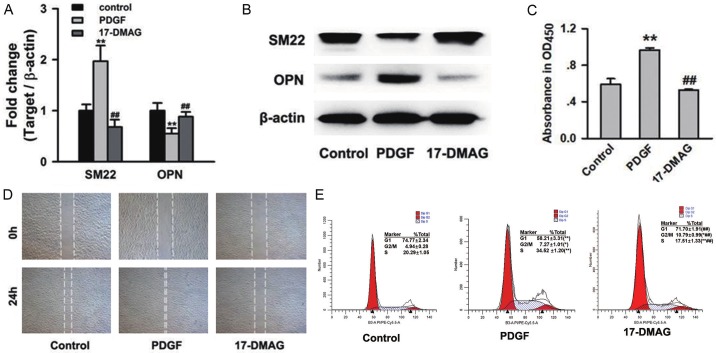
Since abnormal phenotypic switch of SMCs is a hallmark of AD, we investigated the effect of HSP90 inhibitor on phenotypic switch of SMCs induced by platelet derived growth factor-bb (PDGF-bb). Real-time PCR and western blot assays identified that PDGF-bb induced up-regulation of synthetic phenotypic marker (OPN) and down-regulation of contractile phenotypic marker (SM22), but treatment with 17-DMAG significantly attenuated these effects of PDGF-bb (P<0.01, Figures 4A, 4B and S4). CCK-8 cell viability assay identified an increase of ~1.5-fold following 24-hours of PDGF-bb stimulation, whereas 17-DMAG caused a reduction irrespective of stimulation (P<0.01, Figure 4C). Furthermore, 17-DMAG treatment displayed a strong inhibitory effect on SMCs migration (Figure 4D). Result of flow cytometry analysis identified an increasing G1 phase-cell population (58.21±3.31% to 71.70±1.91%, P<0.01), and decreasing S phase-cell population (34.52±1.20% to 17.51±1.33%, P<0.01) in 17-DMAG-treated cells under PDGF-bb-stimulated condition (Figure 4E).
Figure 4.
HSP90 inhibitor 17-DMAG suppressed phenotypic switch of SMCs induced by PDGF-bb. A and B. The mRNA and protein expression of SM22 and OPN in PDGF-bb induced SMCs with or without 17-DMAG treatment. Real-time PCR and western blot assays were repeated for 3 times. Treatment of 17-DMAG could significantly attenuate the expression change of SM22, and OPN induced by PDGF-bb. **P<0.01 vs Control group, and ##P<0.01 vs PDGF group. C, D. The effect of 17-DMAG on proliferation and migration of SMCs. The cell viability was detected by CCK-8 assay. The migration ability of SMCs was detected by wound healing assay. Treatment of 17-DMAG significantly reduced excessive proliferation and migration of SMCs induced by PDGF-bb. **P<0.01 vs control group and ##P<0.01 vs PDGF group. E. The effect of 17-DMAG on cell cycle of SMCs. Flow cytometry analysis proved that treatment of 17-DMAG induced SMCs cell cycle arrest in the G1 phase. *P<0.05 and **P<0.01 vs control group. #P<0.05 and ##P<0.01 vs PDGF group.
Discussion
As the most devastating complication, patients with TAD always need the emergency operation currently. However, over 20% of TAD patients died before reaching hospital because of the rapid dilation and rupture. Thus, it is urgent to seek for the ideal drugs which can alleviate the progress of TAD. Our results confirmed that HSP90 inhibitor 17-DMAG could improve the vascular remodeling induced by BAPN, and significantly reduce the incidence of dissection rupture on the mice model. Therefore, HSP90 inhibitors might be the potential drugs to prevent the dissection formation and progression, even delay or avoid the rupture.
As a highly conserved molecular chaperone, HSP90 would be up-regulated in response to stress of temperature, hypoxia, and other stimulations. The formation and progression of AD might be the inducing factors of HSP90 expression. In our previous study, significant fragmentation and loss of elastic fibers began to be found at 3rd week of BAPN treatment [18]. However, HSP90 expression was significantly increased at 1st week, and maintained at high level during the period of BAPN treatment (Figure S5). Moreover, inhibition of HSP90 could significantly alleviate the breakage of elastic fibers. Therefore, it might be believed that up-regulation of HSP90 was the causal factor for TAD development.
As the potential anticancer target, the inhibitors of HSP90 also aroused a lot of interests in cardiovascular diseases. Several research had proved that 17-DMAG could alleviate several vascular diseases processes. In experimental atherosclerosis development, 17-DMAG could interfere with oxidative stress through reduction in pro-oxidative factors [20]. In cerebral ischemic stroke model, inhibition of HSP90 by 17-DMAG could protect blood-brain barrier integrity via decreasing NF-κB dependent MMP9 expression [21]. Upregulation of MMP9 has been shown to play a key role in the development of AD. In the present study, upregulation of MMP-9 expression was also confirmed in vascular wall tissues from TAD patients (Figure S6). Moreover, treatment of 17-DMAG could significantly reduce the expression of MMP9 in vascular wall tissues from AD mice (Figure S7). It might be the mechanism of 17-DMAG on alleviating the loss of elastic fibers integrity.
Phenotypic switch of aortic SMCs is the causal factor for AD development. The transformation from contractile to synthetic phenotype of SMCs is associated with the increase of proliferation and migration [8,22]. It had been reported that inhibition of HSP90 could decrease migration and proliferation of SMCs by repressing ERK1/2 and Akt signaling pathway [23]. In the present study, the activation of ERK1/2 signaling could also be detected in PDGF-bb-stimulated SMCs, but significantly inhibited by 17-DMAG (Figure S8). Therefore, it might be supposed that 17-DMAG might suppress SMCs phenotypic switch by repressing ERK1/2 signaling pathway.
The systemic activation of the inflammatory system throughout the entire development process of AD, and the infiltration of inflammatory cells is an important cause of AD rupture [24]. Recent reports showed that HSP90 inhibitors could attenuate inflammatory response in atherosclerosis and pulmonary arterial hypertension [16,25]. Our data demonstrated that treatment with 17-DMAG effectively protected from AD rupture. It might also be due to the anti-inflammatory of HSP90 inhibitors.
Many inhibitors of HSP90 had been developed and undergone clinical trials. Geldanamycin (GA), the natural HSP90 inhibitor, could inhibit the ATPase cycle by binding to the N-terminal ATP-binding site of HSP90, but its hepatotoxicity and poor solubility properties limited the clinical application [26]. Therefore, the less toxic GA derivatives, 17-allylamino-17-demethogeldanamycin (17-AAG), 17-DMAG, and retaspimycin (IPI-504) had been developed as potential drugs for a variety of cancers. Recently, specific HSP90 small molecular inhibitors had been demonstrated anti-tumor activity, such as STA-9090, PF-4470296 and PF-3823863 [27,28]. Our study proved that more effective HSP90 inhibitors would be applied in treatment of AD.
Conclusions
The present work has led us to conclude that 17-DMAG may represent an effective treatment avenue for AD. Currently, HSP90 inhibitor therapy is plagued by numerous challenge relating to bioavailability, toxicity and sility. Moreover, the molecular mechanisms and the drug sensitivity genes have not been entirely elucidated. Development of more efficient and specific HSP90 inhibitors would promise new therapeutic strategies for AD in future.
Acknowledgements
National Natural Science Foundation of China (81470592 and 81600010), Project of the Science and Technology Committee of Shanghai (16ZR1400900), Shanghai Municipal Health Planning Commission (201740221), and China Cardiovascular Association CS program. We thank all members of the laboratory for helpful discussions and comments on the manuscript. We also thank Dr. Hongjie Xu for his help on language editing.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Golledge J, Eagle KA. Acute aortic dissection. Lancet. 2008;372:55–66. doi: 10.1016/S0140-6736(08)60994-0. [DOI] [PubMed] [Google Scholar]
- 2.LeMaire SA, Russell L. Epidemiology of thoracic aortic dissection. Nat Rev Cardiol. 2011;8:103–113. doi: 10.1038/nrcardio.2010.187. [DOI] [PubMed] [Google Scholar]
- 3.Olsson C, Thelin S, Stahle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation. 2006;114:2611–2618. doi: 10.1161/CIRCULATIONAHA.106.630400. [DOI] [PubMed] [Google Scholar]
- 4.Chen K, Varon J, Wenker OC, Judge DK, Fromm RE Jr, Sternbach GL. Acute thoracic aortic dissection: the basics. J Emerg Med. 1997;15:859–867. doi: 10.1016/s0736-4679(97)00196-0. [DOI] [PubMed] [Google Scholar]
- 5.Goldfinger JZ, Halperin JL, Marin ML, Stewart AS, Eagle KA, Fuster V. Thoracic aortic aneurysm and dissection. J Am Coll Cardiol. 2014;64:1725–1739. doi: 10.1016/j.jacc.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 6.Wu D, Shen YH, Russell L, Coselli JS, LeMaire SA. Molecular mechanisms of thoracic aortic dissection. J Surg Res. 2013;184:907–924. doi: 10.1016/j.jss.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Wang L, Fu W. Smooth muscle cell phenotypic diversity between dissected and unaffected thoracic aortic media. J Cardiovas Surg. 2013;54:511–521. [PubMed] [Google Scholar]
- 8.Wang L, Zhang J, Fu W, Guo D, Jiang J, Wang Y. Association of smooth muscle cell phenotypes with extracellular matrix disorders in thoracic aortic dissection. J Vasc Surg. 2012;56:1698–1709. doi: 10.1016/j.jvs.2012.05.084. [DOI] [PubMed] [Google Scholar]
- 9.Davis-Dusenbery BN, Wu C, Hata A. Micromanaging vascular smooth muscle cell differentiation and phenotypic modulation. Arterioscler Thromb Vasc Biol. 2011;31:2370–2377. doi: 10.1161/ATVBAHA.111.226670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi JC, LeMaire SA. Thoracic aortic dissection: genes, molecules, and the knife. Tex Heart Inst J. 2012;39:838–839. [PMC free article] [PubMed] [Google Scholar]
- 11.Whitley D, Goldberg SP, Jordan WD. Heat shock proteins: a review of the molecular chaperones. J Vasc Surg. 1999;29:748–751. doi: 10.1016/s0741-5214(99)70329-0. [DOI] [PubMed] [Google Scholar]
- 12.Pearl LH, Prodromou C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu Rev Biochem. 2006;75:271–294. doi: 10.1146/annurev.biochem.75.103004.142738. [DOI] [PubMed] [Google Scholar]
- 13.Tse AN, Klimstra DS, Gonen M, Shah M, Sheikh T, Sikorski R, Carvajal R, Mui J, Tipian C, O’Reilly E, Chung K, Maki R, Lefkowitz R, Brown K, Manova-Todorova K, Wu N, Egorin MJ, Kelsen D, Schwartz GK. A phase 1 dose-escalation study of irinotecan in combination with 17-allylamino-17-demethoxygeldanamycin in patients with solid tumors. Clin Cancer Res. 2008;14:6704–6711. doi: 10.1158/1078-0432.CCR-08-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goetz MP, Toft D, Reid J, Ames M, Stensgard B, Safgren S, Adjei AA, Sloan J, Atherton P, Vasile V, Salazaar S, Adjei A, Croghan G, Erlichman C. Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. J. Clin. Oncol. 2005;23:1078–1087. doi: 10.1200/JCO.2005.09.119. [DOI] [PubMed] [Google Scholar]
- 15.Qi J, Yang P, Yi B, Huo Y, Chen M, Zhang J, Sun J. Heat shock protein 90 inhibition by 17-DMAG attenuates abdominal aortic aneurysm formation in mice. Am J Physiol Heart Circ Physiol. 2015;308:H841–852. doi: 10.1152/ajpheart.00470.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang GK, Li SH, Zhao ZM, Liu SX, Zhang GX, Yang F, Wang Y, Wu F, Zhao XX, Xu ZY. Inhibition of heat shock protein 90 improves pulmonary arteriole remodeling in pulmonary arterial hypertension. Oncotarget. 2016;7:54263–54273. doi: 10.18632/oncotarget.10855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurihara T, Shimizu-Hirota R, Shimoda M, Adachi T, Shimizu H, Weiss SJ, Itoh H, Hori S, Aikawa N, Okada Y. Neutrophil-derived matrix metalloproteinase 9 triggers acute aortic dissection. Circulation. 2012;126:3070–3080. doi: 10.1161/CIRCULATIONAHA.112.097097. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Zhao ZM, Zhang GX, Yang F, Yan Y, Liu SX, Li SH, Wang GK, Xu ZY. Dynamic autophagic activity affected the development of thoracic aortic dissection by regulating functional properties of smooth muscle cells. Biochem Biophys Res Commun. 2016;479:358–364. doi: 10.1016/j.bbrc.2016.09.080. [DOI] [PubMed] [Google Scholar]
- 19.Kim CW, Kumar S, Son DJ, Jang IH, Griendling KK, Jo H. Prevention of abdominal aortic aneurysm by anti-microRNA-712 or anti-microRNA-205 in angiotensin II-infused mice. Arterioscler Thromb Vasc Biol. 2014;34:1412–1421. doi: 10.1161/ATVBAHA.113.303134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madrigal-Matute J, Fernandez-Garcia CE, Gomez-Guerrero C, Lopez-Franco O, Munoz-Garcia B, Egido J, Blanco-Colio LM, Martin-Ventura JL. HSP90 inhibition by 17-DMAG attenuates oxidative stress in experimental atherosclerosis. Cardiovasc Res. 2012;95:116–123. doi: 10.1093/cvr/cvs158. [DOI] [PubMed] [Google Scholar]
- 21.Qi J, Liu Y, Yang P, Chen T, Liu XZ, Yin Y, Zhang J, Wang F. Heat shock protein 90 inhibition by 17-Dimethylaminoethylamino-17-demethoxygeldanamycin protects blood-brain barrier integrity in cerebral ischemic stroke. Am J Transl Res. 2015;7:1826–1837. [PMC free article] [PubMed] [Google Scholar]
- 22.Yan Y, Tan MW, Xue X, Ding XY, Wang GK, Xu ZY. Involvement of Oct4 in the pathogenesis of thoracic aortic dissection via inducing the dedifferentiated phenotype of human aortic smooth muscle cells by directly upregulating KLF5. J Thorac Cardiovasc Surg. 2016;152:820–829. doi: 10.1016/j.jtcvs.2016.05.036. [DOI] [PubMed] [Google Scholar]
- 23.Kim J, Jang SW, Park E, Oh M, Park S, Ko J. The role of heat shock protein 90 in migration and proliferation of vascular smooth muscle cells in the development of atherosclerosis. J Mol Cell Cardiol. 2014;72:157–167. doi: 10.1016/j.yjmcc.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Anzai A, Shimoda M, Endo J, Kohno T, Katsumata Y, Matsuhashi T, Yamamoto T, Ito K, Yan X, Shirakawa K, Shimizu-Hirota R, Yamada Y, Ueha S, Shinmura K, Okada Y, Fukuda K, Sano M. Adventitial CXCL1/G-CSF expression in response to acute aortic dissection triggers local neutrophil recruitment and activation leading to aortic rupture. Circ Res. 2015;116:612–623. doi: 10.1161/CIRCRESAHA.116.304918. [DOI] [PubMed] [Google Scholar]
- 25.Madrigal-Matute J, Lopez-Franco O, Blanco-Colio LM, Munoz-Garcia B, Ramos-Mozo P, Ortega L, Egido J, Martin-Ventura JL. Heat shock protein 90 inhibitors attenuate inflammatory responses in atherosclerosis. Cardiovasc Res. 2010;86:330–337. doi: 10.1093/cvr/cvq046. [DOI] [PubMed] [Google Scholar]
- 26.Bottoni P, Giardina B, Scatena R. Proteomic profiling of heat shock proteins: an emerging molecular approach with direct pathophysiological and clinical implications. Proteomics Clin Appl. 2009;3:636–653. doi: 10.1002/prca.200800195. [DOI] [PubMed] [Google Scholar]
- 27.Mehta PP, Kung PP, Yamazaki S, Walls M, Shen A, Nguyen L, Gehring MR, Los G, Smeal T, Yin MJ. A novel class of specific Hsp90 small molecule inhibitors demonstrate in vitro and in vivo anti-tumor activity in human melanoma cells. Cancer Lett. 2011;300:30–39. doi: 10.1016/j.canlet.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Trepel JB, Neckers LM, Giaccone G. STA-9090, a small-molecule Hsp90 inhibitor for the potential treatment of cancer. Curr Opin Investig Drugs. 2010;11:1466–1476. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.