Abstract
Dental stem cell biotechnology has been used as a potential method to treat the dental diseases. This study aimed to investigate effects of mechanical stimulation on osteogenic properties of rat dental mesenchymal stem cells (DMSCs). DMSCs were isolated from rat teeth root tissues and identified by detecting vimentin and keratin expression. Flexcell FX4K tension system that mediating cyclic strain was used to treat DMSCs. MTT assay was used to observe DMSCs viability. Alkaline phosphatase (ALP) staining and alizarin red staining were conducted. Osteogenesis-specific biomarkers, such as receptor activator for nuclear factor-kB ligand (RANKL), osteoprotegerin (OPG), dentin sialoprotein (DSP) and bone sialoprotein (BSP), were evaluated using RT-PCR, western blot and immunohistochemistry assay, respectively. Positive ALP staining and alizarin red staining confirmed DMSCs phenotype. There were no significant morphology differences between mechanical stimuli treated cells and normal control cells. MTT results showed no significant differences between normal control cells and mechanically stimulated DMSCs. RT-PCR, western blot and immunohistochemistry assay indicated that 10% cyclic strain could trigger an obvious change of mRNA and protein expression of RANKL, OPG, DSP and BSP, respectively. Mechanical stimulation could trigger relative higher levels of calcium deposition in DMSCs. Mechanical strain triggered bone formation mainly through activating RANKL gene expression. In conclusion, 10% cycle mechanical strain could stimulate higher amounts of ALP and calcium deposition by activating RNAKL, and could trigger dramatically changes of mRNA and protein expression of osteogenesis-specific biomarkers, such as OPG, BSP and DSP.
Keywords: Dental mesenchymal stem cells, mechanical strain, osteogenesis, biomarker
Introduction
In clinical, many risk factors could cause the tooth loss, including dental caries, periodontal disorders, trauma and various kinds of genetic diseases, all of which affect the adults’ lives adversely [1]. In recent years, the tissue engineering and the regenerative drugs have been provided the promising treatment for the dentistry [2]. The previous studies [3-5] reported that the bone mesenchymal stem cells (BMSCs) act as the promising candidates for bone repair and injured tissue therapy by utilizing multi-potent differentiation ability, expandability and the accessibility. Especially, the dental stem cell biotechnology and engineering have been employed to investigate it’s potential for treating the dental diseases and regenerating the living functional teeth [6]. However, the whole tooth structures, such as dentin/pulp complex, enamel, periodontal tissues, have not been successfully regenerated till now, which are urgent for the dental engineering. The teeth root is the most important part for the tooth functions, which could provide a better foundation for the artificial crown and the natural crown [7]. The previous studies [8,9] proved that the dental stem cells are the appropriate cells for the bioengineered tooth root, however, the sources of dental stem cells are always limited. Therefore, the scientists and investigators applied some alterative cells, including allogeneic mesenchymal stem cells, progenitor cells, to treat the dental tissue regeneration [1,10]. In this study, we isolated and identified the dental mesenchymal cells (DMSCs), and investigated the application of DMSCs in dental tissue regeneration biotechnology.
The mechanical strain or stimuli is known as a critical factor for the bone homeostasis regulation, absence of which may lead to the loss of bone mass [11,12]. The previous studies [13,14] also indicated that the remodeling processes always be triggered by mechanical stimuli, which could improve the bone formation, bone mineral accumulation. Sumanasinghe et al [15] found that the mechanical strain could increase the expression of bone morphogenetic protein 2 (BMP2) mRNA in BMSCs. Lohberger et al [10] also proved that the continuous mechanical strain could induce obvious increase of osteogenesis-specific markers, such as type-I collagen (Coll A1), osteopontin (SPP1) and osteocalcin (BGLAP), in the osteogenic differentiated BMSCs.
In this study, the mechanical strain or stimuli (triggered by FX4K tension system) was employed to evaluate the effects of mechanical strain on the expression of osteogenesis-associated transcription factors, including receptor activator for nuclear factor-kB ligand (RANKL) [16], osteoprotegerin (OPG) [16], dentin sialoprotein (DSP) [17] and bone sialoprotein (BSP) [18]. The evaluation for effects of mechanical stimulation on DMSCs may provide a promising potential for mechanism of bone or tooth regeneration, oral and maxillofacial surgery, and the other tooth related diseases.
Materials and methods
Isolation, identification and culture of dental mesenchymal cells (DMSCs)
SD rats (weighting from 225 g to 250 g) were purchased from the Center of Experimental Animal in Third Military Medical University (Chongqing, China). The rat teeth root tissues were obtained from the above rats. Then, the dental mesenchymal cells (DMSCs) were harvested from the teeth root tissues digesting by 0.25% trypsin (Beyotime Biotech, Co. Int, Beijing, China) at 37°C for 30 min. Subsequently, the above isolated DMSCs were cultured in the DMEM (Gibco, Grand Island, NY, USA), supplementing with 100 μg/ml of streptomycin (Beyotime Biotech, Co. Int, Beijing, China), 100 U/ml of penicillin (Beyotime Biotech, Co. Int, Beijing, China) and 10% fetal bovine serum (FBS, Gibco, Grand Island, NY, USA), at 37°C and in 5% CO2. The isolated DMSCs cells always present the typical morphology and express the Vimentin and Keratin positively, according to the immunhistochemistry assay.
The present study was approved by the Ethics Committee of School of Stomatology, Capital Medical University, Beijing, China (Approval No. KQYY-2013082601).
Mechanical strain and stimulation
The mechanical cyclic tensile stretch (strain in this study) was performed by using Flexcell FX-4000 Tension System (FX4K; Flexcell Int. Co., Hillsborough, NC, USA). The Flexcell Tension System mainly plays functions by utilizing a vacuum to strain the DMSCs adhered to flexible silicon membranes (BioFlex plates; Flexcell Int. Co., Hillsborough, NC, USA). The set up for Flexcell Tension System was conducted according to the previous published study [10]. DMSCs were seeded and cultured on the collagen type-I coated BioFlex plates (at the final density of 0.5×105 DMSCs/well). When the density of DMSCs achieves to 70% confluence, the DMSCs were subjected to a series of mechanical stimulation. In this study, we set up the force of strain about 70 g to 80 g, and 2 h for every day, continuing for 7 days. Each cycle of Flexcell Tension System stimulation consists of 30 s relaxation and 10 s strain. For the normal control DMSCs, which were cultured under the same conditions, however, removing the tension system strain protocol.
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay
The mechanical strained and stimulated DMSCs (collected the 1 day, 3 days and 7 days cells undergoing mechanical stimulation) and control DMSCs were cultured in 96-well tissue culture plates. The viability of DMSCs was evaluated by examining the conversion of MTT (Sigma-Aldrich, St Louis, MO, USA) to the formazan product. DMSCs were cultured and harvested at 12 h, 24 h, 48 h, 72 h and 96 h, and treated with MTT at a final concentration of 5 mg/ml in DMEM at 37°C for 4 h. The MTT reaction was terminated by discarding the supernatant, and the formazan products were dissolved by using 150 μl DMSO per well. Finally, the 96-well plates were read at wavelength of 490 nm on ELISA examination equipment (Model: MK3; ThermoFisher Scientific, Waltham, MA, USA). At least 6 wells were repeated for every assay.
Alkaline phosphatase (ALP) activity assay
The mechanical strained and stimulated DMSCs and control DMSCs were seeded onto Ti disks at a density of 2×104 DMSCs/well in 24-well plates, and cultured for 1 day, 3 days and 7 days. Then, the DMSCs were harvested in the ALP activity examination assay buffer (containing 1 mM MgCl2, 1 M diethanolamine-HCl and 10 mM L-Homoarginine, and adjusting the pH value to 9.8). The lysed DMSCs homogenates were used to detect the ALP activity by using colorimetric assay, according to the previous study [19]. In this study, the ALP activity was evaluated by using alkaline phosphatase kit (BD Biosciences, Bedford, MA, USA) dependent on the Fast blue RR salt and napthol AS-MX phosphate, according to the instructions of manufacturer. Briefly, the colorimetric alkaline phosphatase substract was added into the wells. The DMSCs were cultured in 24-well plates and shaken at 37°C for 10 min in humidified atmosphere and 5% CO2. Finally, the images of ALP stained DMSCs were observed and captured under the inverted fluorescence microscope (Model: IX51; Olympus, Japan).
Alizarin red staining
The mechanical strained and stimulated DMSCs and control DMSCs were seeded onto the cover-slip (Nunc, Rochester, NY, USA) at a density of 2×104 DMSCs/well in 24-well plates, and cultured for 1 day, 3 days and 7 days. Then, the DMSCs were harvested in PBS, and washed for 2 min and 2 times. The DMSCs were fixed with 4% paraformaldehyde (Sangon Biotech, Shanghai, China) for 20 min at room temperature. The DMSCs were washed again with the PBS for 2 min and 3 times. All of cells were stained with 0.01% alizarin red (Sigma-Aldrich, St Louis, MO, USA) dissolved in 70% ethanol for 10 min at 37°C [20]. Then, the DMSCs were washed with double distilled water for 5 min and 2 times. Finally, the images of alizarin red stained DMSCs were observed and captured under the inverted fluorescence microscope (Model: IX51; Olympus, Japan).
RNA extraction, reverse transcription and real-time PCR
The total RNAs of DMSCs were extracted by using Trizol kit (Beyotime Biotech, Co. Int, Beijing, China) according to manufacturer’s instruction. Then, the extracted total RNAs were transcribed reversely by using reverse-transcription reagents (Western Biotech. Chongqing, China) to synthesize the complementary DNA (cDNA). The PCR primers for OPG, RANKL, DSP and BSP were synthesized by Western Biotech. Inc. Co. (Chongqing, China) and the related sequences were listed in Table 1. The Sybr Green I real-time PCR kit (Western Biotech. Chongqing, China) was employed for amplifying the above genes according to the instructions of manufacturer. The amplification processes of RT-PCR were conducted on a real-time PCR instrument (Eppendorf, Germany), according to the followings: 4 min at 94°C, followed by 35 cycles of 20 s at 94°C, 30 s at 60°C and 30 s at 72°C, and terminated at 10 min at 72°C. All of the above reactions were conducted at least for triplicate and with the final volume of 50 μl. The 2-ΔCt (2-[(Ct of gene) - (Ct of U6)]) method was utilized for the RT-PCR products analysis.
Table 1.
Primers for the RT-PCR assay
| Gene | Sequences | Length (bp) | Gene bank ID | |
|---|---|---|---|---|
| OPG | Forwards | AAGATCAGCCCAGACGAGATTG | 167 | 25341 |
| Reverse | ACGGTTTTGGGAAAGTGGTATG | |||
| RANKL | Forwards | TGATGGAAGGTTCGTGGCTC | 149 | 117516 |
| Reverse | CTTGGCCCAGCCTCGATC | |||
| DSP | Forwards | ACCAGAAAATCGTCCACAAGG | 113 | 306871 |
| Reverse | GCCCACAGAAGGGACAAGC | |||
| BSP | Forwards | CGAGGAGGCAAGCGTCAC | 171 | 24477 |
| Reverse | ACCGTGCTGCTCTTTCTGG | |||
| Actin | Forwards | CCCATCTATGAGGGTTACGC | 150 | 81822 |
| Reverse | TTTAATGTCACGCACGATTTC | |||
Western blot assay
The DMSCs were harvested by using 0.25% trypsin in PBS solution. The harvested DMSCs were shortly centrifuged and suspended with lysis buffer (containing 0.5% sodium deodycholate, 10 mM Tris-HCl, 10 mM EDTA, 100 mM NaCl and 0.5% Nonidet P-40), and supplementing with complete protease inhibitor (Sigma-Aldrich, St Louis, MO, USA). The DMSCs lysates were separated by using 15% SDS-PAGE and electrotransferred onto the polyvinylidene fluoride (PVDF) membranes. PVDF membranes were blocked with 5% defatted milk (dissolved in PBST, adjusting pH 7.6 and containing 0.05% Tween-20) at 4°C overnight. Then, the PVDF membranes were incubated with the rabbit anti-rat OPG polyclonal antibody (1:500; Catalogue No. ab73400, Abcam, UK), rabbit anti-rat RANKL polyclonal antibody (1:500; Catalogue No. ab62516, Abcam, UK), rabbit anti-rat BSP polyclonal antibody (1:500; Catalogue No. ab52128, Abcam, UK), rabbit anti-rat DSP polyclonal antibody (1:500; Catalogue No. ab109445, Abcam, UK) and rabbit anti-rat actin polyclonal antibody (1:500; Catalogue No. ab8227, Abcam, UK) for 2 h at room temperature. PVDF membranes were washed with PBST for 5 min and 3 times, and incubated with horseradish peroxidase (HRP) conjugated goat anti-rabbit IgG (1:500; Catalogue No. 41155, Sigma-Aldrich, St Louis, MO, USA) for 1 h at 37°C. The reactive protein signals were visualized and detected by using enhanced chemiluminescence (ECL) kit (Catalogue No. 32132; Pierce, ThermoFisher Scientific, Waltham, MA, USA).
Immunohistochemistry
The DMSCs were cultured by using the cover-slip (Nunc, Rochester, NY, USA), and fixed with 4% paraformaldehyde (Sangon Biotech, Shanghai, China) for 15 min. Post-washing with phosphate buffer solution (PBS) for 5 min and 3 times, the endogenous peroxidase was inactivated by incubating with 3% hydrogen peroxide at 25°C for 5 min. DMSCs were blocked by using 5% BSA for 20 min, and washed for 5 min and 3 times. DMSCs were incubated with the rabbit anti-rat vimentin polyclonal antibody (1:1000 in PBS) (Catalogue No. ab45939; Abcam, UK), the rabbit anti-rat keratin monoclonal antibody (1:2000) (Catalogue No. ab185627; Abcam, UK), rabbit anti-rat OPG polyclonal antibody (1:500; Catalogue No. ab73400, Abcam, UK), rabbit anti-rat RANKL polyclonal antibody (1:500; Catalogue No. ab62516, Abcam, UK), rabbit anti-rat BSP polyclonal antibody (1:500; Catalogue No. ab52128, Abcam, UK), rabbit anti-rat DSP polyclonal antibody (1:500; Catalogue No. ab109445, Abcam, UK) and rabbit anti-rat actin polyclonal antibody (1:500; Catalogue No. ab8227, Abcam, UK) at 4°C overnight. Subsequently, DMSCs were washed with PBS for 5 min and 3 times, and incubated with the goat anti-rabbit peroxidase-confugated IgG (1:500 in PBS) (Catalogue No. ab6717, Abcam, UK) at 25°C for 1 h. At last, the DMSCs were washed with PBS for 5 min and 3 times, and the DMSCs were immersed in alkaline phosphatase labeled diaminobenzidine (DAB, ZSGB Bio. Inc. Co., Beijing, China), and rinsed in PBS for 5 min and 3 times. The images of stained DMSCs were observed and acquired by using inverted fluorescence microscope (Model: IX51; Olympus, Japan).
RNA interference for RNAKL
The small interfering RNA targeting RANKL was used to down-regulate the RANKL expression in DMSCs. The interfering RNA (siRNA) gene was synthesized by Western Biotech. (Chongqing, China). The sense sequence for the rat RNAKL siRNA utilized was 5’-CGCGCUGCUUCUACAGAAU-3’, and the anti-sense sequence utilized was 5’-AUUCUGUAGAAGCAGCGCG-3’. The sense sequence for the rat sham siRNA used was 5’-ATCACTGCCACTCAGAAGAC-3’ and the anti-sense siRNA used was 5’-ACATTGGGGTAGGAACAC-3’. The DMSCs were transfected with the siRNA by using Lipofectamine 2000 (Invitrogen Inc., Calsbad, CA, USA) according to the manufacturer’s instructions.
Statistical analysis
All of the data in this study were analyzed by using the SPSS software 18.0 (SPSS Inc., Chicago, Ull, USA). The data were described as mean ± SD, which were also performed at least three independent experiments. Comparison of means among multiple groups was performed by using the post Tukeys’ hoc test after the ANOVA. The P value less than 0.05 was considered to be significant difference.
Results
Isolation and identification for DMSCs
The DMSCs were isolated from the teeth root tissues. The cell morphology of the isolated cells was consistent with the typical morphology of DMSCs (Figure 1A). In order to confirm whether the isolated cells were the DMSCs, the DMSCs specific markers, including vinmentin, keratin, were detected by using immunohistochemistry assay. The results confirmed that the isolated cells positively expressed the vimentin (Figure 1B) and keratin (Figure 1C).
Figure 1.
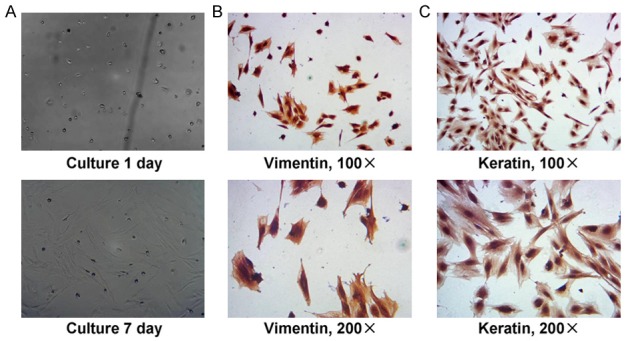
Primary culture and identification for the isolated dental mesenchymal cells (DMSCs). A. Isolated and primary cultured DMSCs cells observed under light microscopy. B. Identification for the isolated DMSCs by detecting Vimentin expression and immunohistochemistry assay. C. Identification for the isolated DMSCs by detecting Keratin expression and immunohistochemistry assay. The cells incubated with rabbit anti-Vimentin polyclonal antibody and rabbit anti-Keratin polyclonal antibody. The amplification has been illustrated in the graphs.
DMSCs morphology and cell growth during mechanical stimulation
Following with the mechanical stimulation 1 day, 3 days and 7 days later, the morphology of DMSCs was observed. The results indicated that there were no significant morphology differences between mechanical stimuli treated cells and normal control cells (Figure 2A). However, the amounts of cells were increased in mechanical stimuli treated cells compared to that in the control cells, but without significant differences (Figure 2A). The MTT results showed that the cell viabilities of mechanical stimuli treated cells were significantly decreased compared to that in the control cells (Figure 2B, P<0.05) at different examining time points (12 h, 24 h, 48 h, 72 h and 96 h). The Flexcell FX-4000 Tension System was illustrated in Figure 2C.
Figure 2.
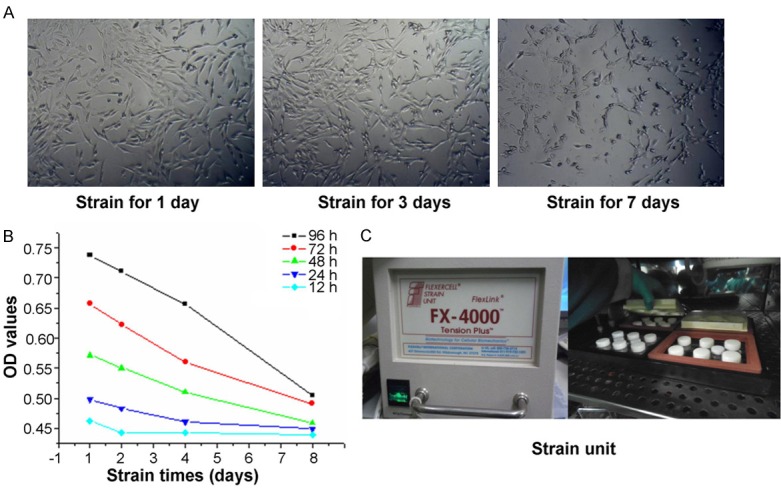
DMSCs morphology observation and the examination of DMSCs cell viability. A. DMSCs morphology observation. B. DMSCs cell viability examination by using MTT assay. C. Image of the Flexcell FX-4000 Tension System.
Mechanical stimulation triggered alkaline phosphatase activation and calcium deposition
In order to identify the osteogenic properties of DMSCs undergoing the mechanical stimulation, the bone formation related biomarkers, such as ALP and alizarin red, were examined and observed under the inverted fluorescence microscope. The results indicated that ALP staining was obviously increased in the mechanical stimulation group compared to that in the blank DMSCs group (Figure 3A). Meanwhile, ALP expression was also increased following with the mechanical stimulation time points. The alizarin red staining results indicated that the alizarin red staining positive DMSCs in mechanical stimulation group were obviously more compared to that in the control DMSCs (Figure 3B), which suggests that the mechanical stimulation could trigger the calcium deposition.
Figure 3.
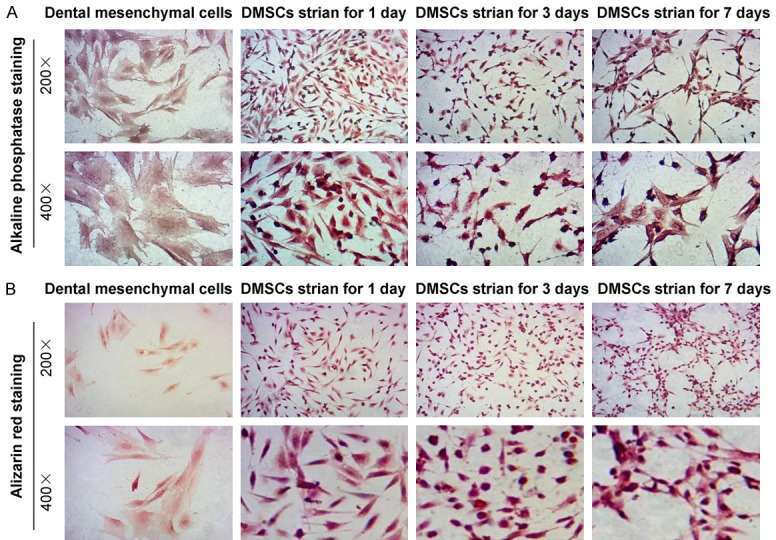
Immunohistochemistry staining for the alkaline phosphatase (ALP) and alizarin red. A. ALP staining for examining the ALP activity. B. Alizarin red staining for evaluating calcium deposition. The amplification has been illustrated in the graphs.
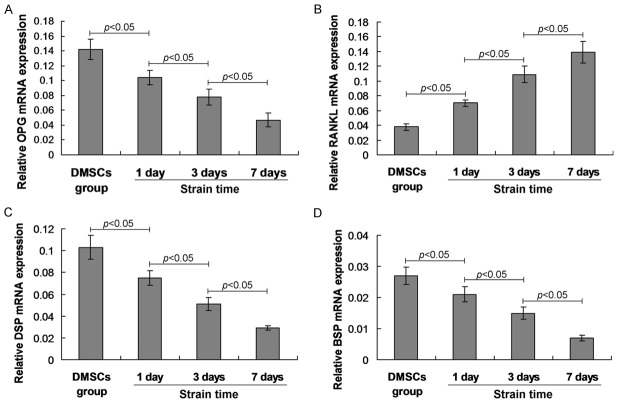
Mechanical stimulation regulated osteogenesis-specific biomarkers’ mRNA expression
To evaluate the effects of cyclin strain on osteogenesis, the DMSCs were mechanically stimulated by Flexcell FX4K tension system at 1 day, 3 days and 7 days post stimulation. The relative mRNA expression of OPG, RANKL, DSP and BSP, were analyzed by using RT-PCR analysis at 1 day, 3 days and 7 days post mechanical stimulation. The OPG mRNA levels illustrated a significant decrease at 1 day, 3 days and 7 days post the mechanical stimulation (Figure 4A, P<0.05). Meanwhile, OPG mRNA expression was also decreased following with the prolong of mechanical stimulation (Figure 4A, P<0.05). The RANKL mRNA expression was significantly increased at 1 day, 3 days and 7 days post mechanical stimulation compared to that of the control DMSCs group (Figure 4B, P<0.05), and which was also increased following with the stimulation time. Furthermore, the mRNA expression of DSP (Figure 4C) and BSP (Figure 4D) were also significantly decreased at 1 day, 3 days and 7 days post mechanical stimulation compared to that of the control DMSCs group (P<0.05).
Figure 4.
Observation for the osteogenesis specific biomarkers undergoing the effects of mechanical stimulation. The mRNA of OPG (A), RANKL (B), DSP (C) and BSP (D) were normalized to the internal control of actin mRNA expression. The statistically significant differences between two groups were illustrated in the graphs.
Mechanical stimulation enhanced RANKL and decreased OPG, DSP and BSP protein expression
The relative expression of OPG, RANKL, DSP and BSP were also analyzed by using western blot assay at 1 day, 3 days and 7 days post mechanical stimulation. The results showed that the mechanical stimulation significantly decreased the OPG, DSP and BSP expression, and increased RANKL expression at 1 day, 3 days and 7 days post mechanical stimulation compared to that of the control DMSCs group (Figure 5, P<0.05).
Figure 5.
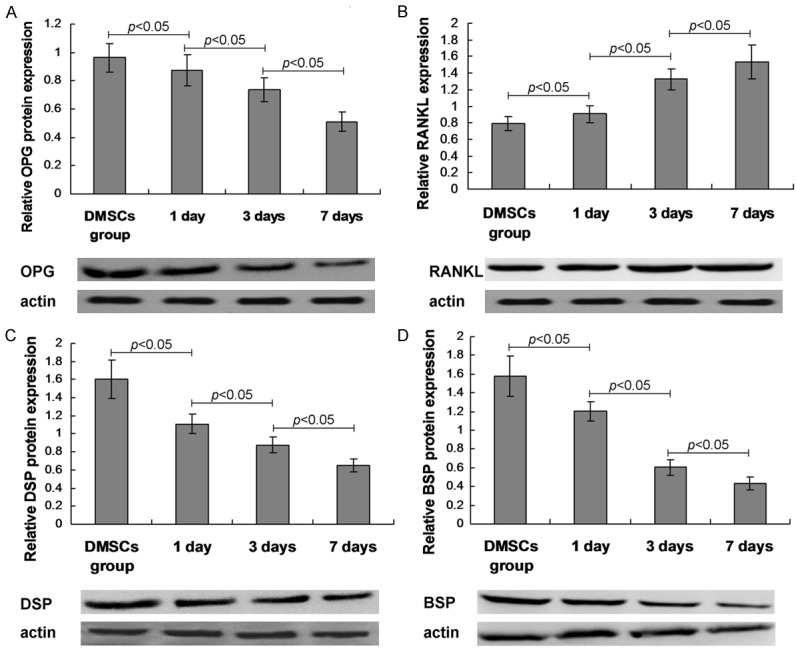
Western bolt assay results for the osteogenesis specific biomarkers. A. OPG expression and statistical analysis. B. RANKL expression and statistical analysis. C. DSP expression and statistical analysis. D. BSP expression and statistical analysis. The amplification has been illustrated in the graphs.
Moreover, the OPG, RANKL, DSP and BSP expression were examined by using immunohistochemistry assay. The immunohistochemical images illustrated that the OPG, DSP and BSP staining positive DMSCs were obviously decreased at 1 day, 3 days and 7 days post mechanical stimulation compared to that in control DMSCs (Figure 6). However, the negative control illustrated no positive staining DMSCs. The statistical analysis data showed that mechanical stimulation significantly decreased OPG, DSP and BSP, and increased RANKL expression post mechanical stimulation compared to that of control DMSCs group (Figure 6, P<0.05).
Figure 6.
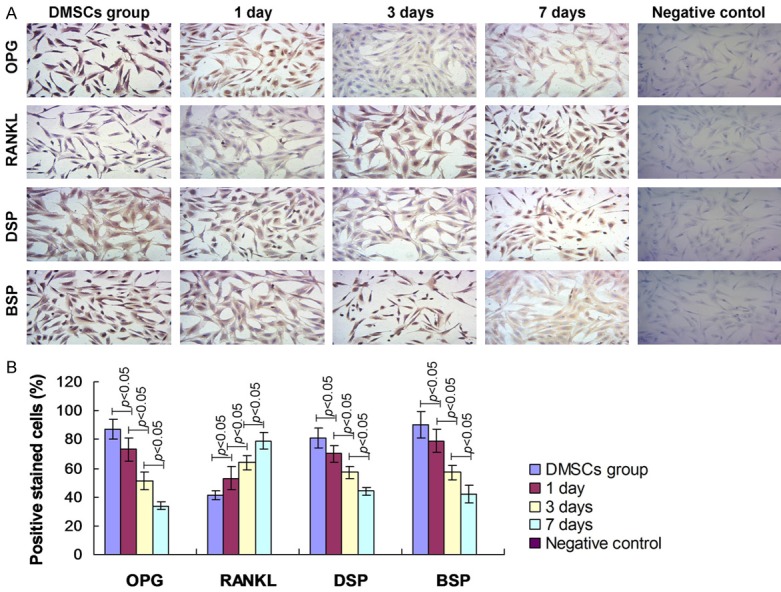
Examination for the osteogenesis-specific biomarkers by using immunohistochemistry. A. OPG, RANKL, DSP and BSP expression determination. B. Statistical analysis for biomarkers.
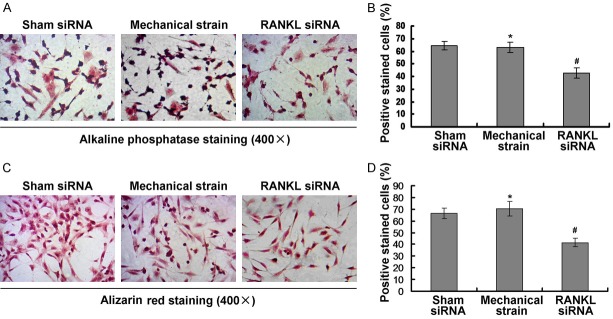
Mechanical stimulation increased ALP activation and calcium deposition
In order to observe the mechanism of mechanical strain affecting on the ALP activation and the calcium deposition, the RANKL siRNA (siRNA) was transfected into the mechanical strain treated DMSCs group. The results indicated that the ALP activity of RANKL siRNA combining mechanical strain treated DMSCs cells were significantly decreased compared to mechanical strain treated DMSCs (Figure 7A, 7B). Meanwhile, the alizarin red staining positive DMSCs in RANKL siRNA combining mechanical strain treated DMSCs were obviously decreased compared to the control mechanical strain treated DMSCs (Figure 7C, 7D).
Figure 7.
Effects of RANKL small interfering RNA (RANKL siRNA) on the alkaline phosphatase (ALP) and alizarin red. A. ALP staining for examining the ALP activity. B. Statistical analysis for ALP activity. C. Alizarin red staining for evaluating calcium deposition. D. Statistical analysis for calcium deposition. The amplification has been illustrated in the graphs.
Discussion
The previous studies [21-23] always applied the bone marrow derived stromal cells to the laboratory investigation and the clinical engineering trials, and also received some interesting findings. However, in recent years, many other cells also illustrated the similar characteristics, including the dental pulp cells, the cells isolated from mandibular and maxillary bone [24]. The present study investigated the function of mechanical stimulation in the osteogenic properties and the bone formation of the dental mesenchymal cells for the first time.
Till now, the mechanical stimulation technology also exists some controversies in the application, just because there were also a relative higher responsiveness for the cells undergoing mechanical stimulation [10,25]. The controversy mainly focuses on the followings, including the tension, fluid shear, compression, which could potentially affect the cells differentiation and bone forming. Actually, the most popularly used mechanical stimulation for the culture cells is the fluid shear flow and the cyclic stretch [26]. In this study, the dental mesenchymal cells were seeded onto the type-I coated BioFlex plates and were mechanically stimulated by using Flexcell Tension System with a frequency of 0.5 Hz. Actually, we have conducted a few pre-experiments for the mechanical loading and the amounts of mechanical strain, which could merit or improve the growth of mesenchymal stem and progenitor cells [27]. Our pre-experiments showed that the 10% cyclic strain or stimulation could keep the proliferation of DMSCs and remain the normal cell morphology.
Firstly, in order to identify the isolated cells, the DMSCs specific markers, vimentin and keratin [28-31], were detected in isolated cells. Consistent with the previous studies [31,32], in our study, the isolated cells illustrated the typical vimentin and keratin expression in cells. Moreover, the cell viability was not affected by the mechanical stimulation according to MTT assay results. The morphology examination results indicated that there were no significant morphology differences between mechanical stimuli treated cells and normal control cells. This result is consistent with Lohberger et al’s [10] findings, and suggests that the mechanical stimulation treatment could keep the morphology of the strained DMSCs cells, which provides the basis for the following experiments.
Meanwhile, we also examined the ALP activity and the alizarin red staining to confirm the osteogenic properties of DMSCs undergoing mechanical stimulation. Similar to the previous studies, the ALP and alizarin red staining results indicated that the ALP expression was significantly increased and the more alizarin red staining cells were observed in the mechanically stimulated groups [10,33]. In order to investigate and confirm the osteogenic properties of mechanically stimulation, the RANKL, OPG, DSP and BSP were examined, all of which were considered as the central biomarker genes within the osteoblast phenotype. Zheng et al [34] found that the changes of the RANKL and OPG expression were correlated with the mesenchymal dental pulp cells, which suggest that the RANKL and OPG are critical for the bone formation. Mehrazarin et al [35] also reported that the subculture-induced dental mesenchymal stem cells could lead to the significantly decreased of BSP and BSP, which also hint that the BSP and DSP act as the important biomarkers for dental mesenchymal stem cells-triggered bone formation. In this study, the RT-PCR assay, western blot assay and immunohistochemistry assay results indicated that levels of RANKL were dramatically increased and levels of OPG, DSP and BSP were significantly decreased undergoing mechanical stimulation. The results showed that the changes of RANKL and OPG were consistent with the Zhang et al’ [36] findings, which proved that mechanical stress regulated the RNAKL/OPG expression and ratio in periodontal ligament stem cells. Wei et al [37] discovered that the mechanical stretching force could induce the BSP and DSP expression in human periodontal ligament stem cells. However, the previous studies have not been investigated the effects of mechanical stress or force on expression of RANKL, OPG, BSP and DSP in the dental mesencymal stem cells till now, and the present study was the first one. Furthermore, may be there are also the other proteins that up-regulate in response to the application of the mechanical stress or strain, which would be explored in the future studies.
Furthermore, in order to investigate the mechanism of mechanical strain caused ALP activity and calcium deposition, the RANKL siRNA was synthesized and transfected to mechanical strain treated DMSCs cells. The results indicated that ALP activity and calcium deposition were significantly decreased compared to mechanical strain treated DMSCs. These results indicated that the mechanical strain triggered the bone formation mainly through activating the RANKL gene expression.
Although some of the interesting and significant results were obtained, there were also a few limitations. Firstly, the data in this study may be insufficient for the role of mechanical strain on dental mesencymal stem cells. In the following study, we would verify the roles of mechanical strain on dental mesenchymal stem cells by using RNA interference experiments. Secondarily, the markers to phenotype DMSCs in this study seems not sufficient, though which have been used by some reports [28-31]. In the future study, we would identify the DMSCs by using some more specific biomarkers. Thirdly, the findings and conclusions in this study have not been verified in the animal models. We would establish the animal model to verify the present results.
In conclusion, 10% cycle mechanical strain could stimulate the higher amounts of ALP and calcium deposition, and trigger the dramatically changes of mRNA and protein expression of osteogenesis-specific biomarkers, such as RANKL, OPG, BSP and DSP in dental mesenchymal stem cells. In the future clinical and basic researches, the mechanical stimulation would act as a regulator of osteogenic-differentiation and bone formation, which could also be beneficial to the orthopaedic tissue engineering and the craniofacial surgery.
Acknowledgements
This study was granted by the National Natural Science Foundation of China (Grant No. 81300849), Beijing Natural Science Foundation (Grant No. 7132067) and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, Ministry of Education of China (2013).
Disclosure of conflict of interest
None.
References
- 1.Wei F, Song T, Ding G, Xu J, Liu Y, Liu D, Fan Z, Zhang C, Shi S, Wang S. Functional tooth restoration by allogeneic mesenchymal stem cell-based bio-root regeneration in swine. Stem Cells Dev. 2013;22:1752–1762. doi: 10.1089/scd.2012.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 3.Kon E, Filardo G, Roffi A, Di Martino A, Hamdan M, De Pasqual L, Merli ML, Marcacci M. Bone regeneration with mesenchymal stem cells. Clin Cases Miner Bone Metab. 2012;9:24–27. [PMC free article] [PubMed] [Google Scholar]
- 4.An Y, Song Y, Wang Z, Wang J, Wu G, Zhu G, Chen L. Effect of low-intensity pulsed ultrasound on the biological behaviors of bone marrow mesenchymal stem cells on titanium with different surface topographies. Am J Transl Res. 2018;10:67–76. [PMC free article] [PubMed] [Google Scholar]
- 5.Clausen C, Hermund NU, Donatsky O, Nielsen H. Characterization of human bone cells derived from the maxillary alveolar ridge. Clin Oral Implants Res. 2006;17:533–540. doi: 10.1111/j.1600-0501.2006.01254.x. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda E, Morita R, Nakao K, Ishida K, Nakamura T, Takano-Yamamoto T, Ogawa M, Mizuno M, Kasugai S, Tsuji T. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc Natl Acad Sci USA. 2009;106:13475–13480. doi: 10.1073/pnas.0902944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eckert SE, Choi YG, Sanchez AR, Koka S. Comparison of dental implant systems: quality of clinical evidence and prediction of 5-year survival. Int J Oral Maxillofac Implants. 2005;20:406–415. [PubMed] [Google Scholar]
- 8.Wang S, Liu Y, Fang D, Shi S. The miniature pig: a useful large animal model for dental and orofacial research. Oral Dis. 2007;13:530–537. doi: 10.1111/j.1601-0825.2006.01337.x. [DOI] [PubMed] [Google Scholar]
- 9.Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, Liu H, Gronthos S, Wang CY, Wang S, Shi S. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006;1:e79. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lohberger B, Kaltenegger H, Stuendl N, Payer M, Rinner B, Leithner A. Effect of cyclic mechanical stimulation on the expression of osteogenesis genes in human intraoral mesenchymal stromal and progenitor cells. Biomed Res Int. 2014;2014:189516. doi: 10.1155/2014/189516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gharibi B, Cama G, Capurro M, Thompson I, Deb S, Di Silvio L, Hughes FJ. Gene expression responses to mechanical stimulation of mesenchymal stem cells seeded on calcium phosphate cement. Tissue Eng Part A. 2013;19:2426–2438. doi: 10.1089/ten.tea.2012.0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armbrecht G, Belavy DL, Backstrom M, Beller G, Alexandre C, Rizzoli R, Felsenberg D. Trabecular and cortical bone density and architecture in women after 60 days of bed rest using high-resolution pQCT: WISE 2005. J Bone Miner Res. 2011;26:2399–2410. doi: 10.1002/jbmr.482. [DOI] [PubMed] [Google Scholar]
- 13.Shah K, Armamento-Villareal R, Parimi N, Chode S, Sinacore DR, Hilton TN, Napoli N, Qualls C, Villareal DT. Exercise training in obese older adults prevents increase in bone turnover and attenuates decrease in hip bone mineral density induced by weight loss despite decline in bone-active hormones. J Bone Miner Res. 2011;26:2851–2859. doi: 10.1002/jbmr.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nilsson M, Ohlsson C, Oden A, Mellstrom D, Lorentzon M. Increased physical activity is associated with enhanced development of peak bone mass in men: a five-year longitudinal study. J Bone Miner Res. 2012;27:1206–1214. doi: 10.1002/jbmr.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sumanasinghe RD, Bernacki SH, Loboa EG. Osteogenic differentiation of human mesenchymal stem cells in collagen matrices: effect of uniaxial cyclic tensile strain on bone morphogenetic protein (BMP-2) mRNA expression. Tissue Eng. 2006;12:3459–3465. doi: 10.1089/ten.2006.12.3459. [DOI] [PubMed] [Google Scholar]
- 16.Zhao JJ, Wu ZF, Wang L, Feng DH, Cheng L. MicroRNA-145 mediates steroid-induced necrosis of the femoral head by targeting the OPG/RANK/RANKL signaling pathway. PLoS One. 2016;11:e0159805. doi: 10.1371/journal.pone.0159805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan C, Yuan G, Luo D, Zhang L, Lin H, Liu H, Chen L, Yang G, Chen S, Chen Z. The dentin sialoprotein (DSP) domain regulates dental mesenchymal cell differentiation through a novel surface receptor. Sci Rep. 2016;6:29666. doi: 10.1038/srep29666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouet G, Bouleftour W, Juignet L, Linossier MT, Thomas M, Vanden-Bossche A, Aubin JE, Vico L, Marchat D, Malaval L. The impairment of osteogenesis in bone sialoprotein (BSP) knockout calvaria cell cultures is cell density dependent. PLoS One. 2015;10:e0117402. doi: 10.1371/journal.pone.0117402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dahl R, Sergienko EA, Su Y, Mostofi YS, Yang L, Simao AM, Narisawa S, Brown B, Mangravita-Novo A, Vicchiarelli M, Smith LH, O’Neill WC, Millan JL, Cosford ND. Discovery and validation of a series of aryl sulfonamides as selective inhibitors of tissue-nonspecific alkaline phosphatase (TNAP) J Med Chem. 2009;52:6919–6925. doi: 10.1021/jm900383s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker MB, Kimmel CB. A two-color acid-free cartilage and bone stain for zebrafish larvae. Biotech Histochem. 2007;82:23–28. doi: 10.1080/10520290701333558. [DOI] [PubMed] [Google Scholar]
- 21.Li W, Wei S, Liu C, Song M, Wu H, Yang Y. Regulation of the osteogenic and adipogenic differentiation of bone marrow-derived stromal cells by extracellular uridine triphosphate: the role of P2Y2 receptor and ERK1/2 signaling. Int J Mol Med. 2016;37:63–73. doi: 10.3892/ijmm.2015.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pang Y, Deng C, Geng S, Weng J, Lai P, Liao P, Zeng L, Lu Z, Zhang J, Du X. Premature exhaustion of mesenchymal stromal cells from myelodysplastic syndrome patients. Am J Transl Res. 2017;9:3462–3468. [PMC free article] [PubMed] [Google Scholar]
- 23.Kon E, Muraglia A, Corsi A, Bianco P, Marcacci M, Martin I, Boyde A, Ruspantini I, Chistolini P, Rocca M, Giardino R, Cancedda R, Quarto R. Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical-size defects of sheep long bones. J Biomed Mater Res. 2000;49:328–337. doi: 10.1002/(sici)1097-4636(20000305)49:3<328::aid-jbm5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 24.Lohberger B, Payer M, Rinner B, Kaltenegger H, Wolf E, Schallmoser K, Strunk D, Rohde E, Berghold A, Pekovits K, Wildburger A, Leithner A, Windhager R, Jakse N. Tri-lineage potential of intraoral tissue-derived mesenchymal stromal cells. J Craniomaxillofac Surg. 2013;41:110–118. doi: 10.1016/j.jcms.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Li R, Liang L, Dou Y, Huang Z, Mo H, Wang Y, Yu B. Mechanical strain regulates osteogenic and adipogenic differentiation of bone marrow mesenchymal stem cells. Biomed Res Int. 2015;2015:873251. doi: 10.1155/2015/873251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mullender M, El Haj AJ, Yang Y, van Duin MA, Burger EH, Klein-Nulend J. Mechanotransduction of bone cells in vitro: mechanobiology of bone tissue. Med Biol Eng Comput. 2004;42:14–21. doi: 10.1007/BF02351006. [DOI] [PubMed] [Google Scholar]
- 27.Jagodzinski M, Breitbart A, Wehmeier M, Hesse E, Haasper C, Krettek C, Zeichen J, Hankemeier S. Influence of perfusion and cyclic compression on proliferation and differentiation of bone marrow stromal cells in 3-dimensional culture. J Biomech. 2008;41:1885–1891. doi: 10.1016/j.jbiomech.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Polioudaki H, Agelaki S, Chiotaki R, Politaki E, Mavroudis D, Matikas A, Georgoulias V, Theodoropoulos PA. Variable expression levels of keratin and vimentin reveal differential EMT status of circulating tumor cells and correlation with clinical characteristics and outcome of patients with metastatic breast cancer. BMC Cancer. 2015;15:399. doi: 10.1186/s12885-015-1386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng QM, Lu JJ, Zhao J, Wei X, Wang L, Liu PS. Periostin facilitates the epithelial-mesenchymal transition of endometrial epithelial cells through ILK-Akt signaling pathway. Biomed Res Int. 2016;2016:9842619. doi: 10.1155/2016/9842619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larouche D, Tong X, Fradette J, Coulombe PA, Germain L. Vibrissa hair bulge houses two populations of skin epithelial stem cells distinct by their keratin profile. FASEB J. 2008;22:1404–1415. doi: 10.1096/fj.07-8109com. [DOI] [PubMed] [Google Scholar]
- 31.Fu CH, Lin RJ, Yu J, Chang WW, Liao GS, Chang WY, Tseng LM, Tsai YF, Yu JC, Yu AL. A novel oncogenic role of inositol phosphatase SHIP2 in ER-negative breast cancer stem cells: involvement of JNK/vimentin activation. Stem Cells. 2014;32:2048–2060. doi: 10.1002/stem.1735. [DOI] [PubMed] [Google Scholar]
- 32.Chang TH, Huang HD, Ong WK, Fu YJ, Lee OK, Chien S, Ho JH. The effects of actin cytoskeleton perturbation on keratin intermediate filament formation in mesenchymal stem/stromal cells. Biomaterials. 2014;35:3934–3944. doi: 10.1016/j.biomaterials.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 33.Osathanon T, Nowwarote N, Manokawinchoke J, Pavasant P. bFGF and JAGGED1 regulate alkaline phosphatase expression and mineralization in dental tissue-derived mesenchymal stem cells. J Cell Biochem. 2013;114:2551–2561. doi: 10.1002/jcb.24602. [DOI] [PubMed] [Google Scholar]
- 34.Zheng Y, Chen M, He L, Marao HF, Sun DM, Zhou J, Kim SG, Song S, Wang SL, Mao JJ. Mesenchymal dental pulp cells attenuate dentin resorption in homeostasis. J Dent Res. 2015;94:821–827. doi: 10.1177/0022034515575347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehrazarin S, Oh JE, Chung CL, Chen W, Kim RH, Shi S, Park NH, Kang MK. Impaired odontogenic differentiation of senescent dental mesenchymal stem cells is associated with loss of Bmi-1 expression. J Endod. 2011;37:662–666. doi: 10.1016/j.joen.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang L, Liu W, Zhao J, Ma X, Shen L, Zhang Y, Jin F, Jin Y. Mechanical stress regulates osteogenic differentiation and RANKL/OPG ratio in periodontal ligament stem cells by the Wnt/beta-catenin pathway. Biochim Biophys Acta. 2016;1860:2211–221. doi: 10.1016/j.bbagen.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Wei FL, Wang JH, Ding G, Yang SY, Li Y, Hu YJ, Wang SL. Mechanical force-induced specific MicroRNA expression in human periodontal ligament stem cells. Cells Tissues Organs. 2014;199:353–363. doi: 10.1159/000369613. [DOI] [PubMed] [Google Scholar]