Abstract
Our previous study demonstrated that the proliferation of human intestinal smooth muscle (ISM) cells was stimulated by butyrate through the yes-associated protein (YAP) pathway in vitro, suggesting a valuable approach for intestinal adaption of short bowel syndrome (SBS). This study was conducted to confirm these findings in vivo. Three-week-old Sprague-Dawley rats were randomly divided into the following groups: Sham group (bowel transection and reanastomosis), SB W group (80% small bowel resection/water ad libitum), and SB Bu group (80% small bowel resection/50 mM sodium butyrate ad libitum). Morphological changes were determined by hematoxylin and eosin staining; the proliferation rate of ISM cells was examined by Ki67 staining, and apoptosis was determined in the TUNEL assay. Changes in the expression of YAP and its downstream genes were evaluated by quantitative-polymerase chain reaction and western blotting. Fourteen days post-operation, a significant increase in ISM thickness was observed in the SB Bu group compared to the SB W group, accompanied by enhanced proliferation of ISM cells and suppression of apoptosis. Notably, YAP expression was also significantly increased in the SB Bu group, with a 6.5-fold increase in the proportion of YAP-positive ISM cells, 2.2-fold increase in YAP mRNA expression, and 3.4-fold increase in protein expression. In conclusion, our results suggest that butyrate promotes ISM adaption through YAP in vivo, which may be a potential therapeutic approach for SBS patients.
Keywords: Short bowel syndrome, intestinal adaptation, intestinal smooth muscle, butyrate, yes-associated protein
Introduction
Short bowel syndrome (SBS), primarily caused by extensive intestinal resection for acquired or congenital gastrointestinal diseases, is a leading cause of intestinal failure. As SBS patients typically require prolonged parenteral nutrition that carries risks of severe complications such as parenteral nutrition-associated liver disease [1,2], it is very important for these patients to begin enteral nutrition to improve intestinal adaption as soon as possible. The length of the remaining small intestine is crucial to intestinal adaptation and the role of intestinal smooth muscle (ISM) has become a focus of this research area. We previously demonstrated that the proliferation of human ISM cells was stimulated by butyrate through the yes-associated protein (YAP) pathway in vitro, suggesting a valuable approach for improving the intestinal adaption of SBS. Therefore, this study was designed to clarify the in vivo effects.
Butyrate, a 4-carbon short-chain fatty acid, is the final product of bacterial fermentation of dietary fibers and is well-known for its role in intestinal homeostasis [3,4]. Butyrate was shown to be the specific short-chain fatty acid responsible for improving intestinal adaptation. Recent studies suggested that the structural and functional indices of intestinal adaptation were significantly increased by supplementation of butyrate in parenteral nutrition in a neonatal piglet model of SBS [5,6]. These studies mainly focused on the mucosal adaptation, while few studies have examined ISM adaptation. Changes within muscular layers are of crucial importance in the residual intestine, as the ISM structure directly influences intestinal motility and thus affects key stages of intestinal digestion and absorption.
YAP is one of the main downstream effectors of the Hippo pathway, exerting its regulatory function by binding to TEA domain (TEAD) family transcription factors [7]. As an evolutionarily conserved transcriptional co-activator, YAP plays a pivotal role in regulating organ development and size. Deletion of YAP in mouse vascular smooth muscle cells resulted in severe vascular abnormalities and defects in cell proliferation [8]. Additionally, Song et al. [9] reported that YAP knockdown suppressed cell proliferation and induced cell apoptosis in endometrial stromal cells; YAP over-expression showed the opposite effects by inducing cell proliferation and suppressing cell apoptosis. We previously demonstrated that YAP mediates the pro-proliferative effect of butyrate on human ISM cells in vitro; in the current study, we hypothesized that supplementation of butyrate may improve the intestinal adaption of SBS by affecting the proliferation and apoptosis of ISM cells through the YAP pathway in vivo.
To this end, 3-week old male Sprague-Dawley (SD) rats with 80% small bowel resection were used as an in vivo SBS model. Our results can be used to optimize treatments for SBS patients.
Materials and methods
Animals
The studies were approved by the Experimental Animal Care and Use Committee of Shanghai Jiao Tong University and conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments. Three-week-old male SD rats (74 ± 4 g) were obtained from Shanghai Laboratory Animal Center (Shanghai, China) and housed individually at 25°C ± 0.5°C and 40-60% relative humidity in sterilized polycarbonate cages (12/12 h-light/dark cycle). Animals were given free access to a fiber-free elemental diet [10] (Xietong, Jiangsu, China) to minimize the effect of residual dietary fiber fermentation in the gastrointestinal tract during the experimental period.
Surgical model and experimental groups
After a 3-day acclimatization, the rats were weighed and anesthetized by an intraperitoneal injection of 2% pentobarbital (30 mg/kg) shortly before surgery. They were randomized into 3 groups (n = 10 in each group): (1) SB W group: Approximately 80% of the small intestine was removed, leaving approximately 10 cm of the terminal ileum and 5 cm of the proximal jejunum by a midline incision. The remaining intestine was anastomosed end-to-end with interrupted 6-0 prolene sutures and the wound was closed in layers with running sutures. After operation, the rats were placed in individual cages and given water ad libitum. (2) SB Bu group: Laparotomy and all surgical manipulations were identical to those in the SB W group, but this group was administered 50 mM sodium butyrate solution ad libitum after the operation. (3) Sham group: The rats underwent the same surgical manipulations without the resection procedure and were given water ad libitum after the operation. A fiber-free elemental diet was reinstated on the second post-operative day. Rats, along with the amount of food and liquid, were weighed each morning before the light was turned off. Food and liquid consumption were determined in grams per 100 g of body weight per day.
Reagents
Sodium butyrate, rabbit anti-TEAD1 (1:1000 for western blotting) and rabbit anti-TEAD4 (1:1000 for western blotting) were purchased from Sigma-Aldrich Company (St. Louis, MO, USA). The following antibodies used in this study were purchased from Cell Signaling Technology (Danvers, MA, USA), including rabbit anti-YAP (1:1000 for western blotting, 1:200 for immunohistochemistry), rabbit anti-BCL2 (1:1000 for western blotting), rabbit anti-BAX (1:1000 for western blotting), rabbit anti-Ki67 (1:200 for immunofluorescence), rabbit anti-proliferating cell nuclear antigen (1:1000 for western blotting), and rabbit anti-β-tubulin (1:1000 for western blotting). The secondary antibodies were the goat anti-rabbit IgG horseradish peroxidase-linked antibody and anti-rabbit IgG (H+L), F(ab’)2 fragment. 4’, 6-Diamidino-2-phenylindole, dihydrochloride (DAPI) was from Cell Signaling Technology.
Morphologic studies
Rats were sacrificed on days 7 and 14 post-operation. A ventral midline incision was made, and the small intestine from the ligament of Treitz through the cecum was removed and placed on ice. Immediately after the removal of this segment, the remaining small intestine was measured with no tension applied, and its total length proximal and distal to the anastomosis was recorded.
Sample collection
Approximately 5 cm segments of the proximal intestinal tissue, approximately 1 cm proximal to the site of anastomosis, were collected on days 7 and 14 after surgery. Thereafter, the intestinal lumen was gently rinsed with 20 mL ice-cold phosphate-buffered saline (PBS) to remove the intestinal contents. Two centimeters of each sample was fixed for 24 h in 4% neutral-buffered formalin at room temperature for histological examination. The remaining 3 cm was cut longitudinally, and the mucosal layer was carefully scraped away. The muscular content was obtained for RNA extraction and western blot analysis by using a cell scraper.
Histology
Samples were stained with hematoxylin and eosin (H&E) and representative photomicrographs were captured with a digital camera-equipped microscope (Leica, Wetzlar, Germany). Microscopic measurements were performed for total villus height and inner circular and outer longitudinal smooth muscle using LAS AF LITE image processing software (Leica).
ISM cell proliferation
The proliferation of smooth muscle cells was assessed by immunofluorescence staining for Ki67. Briefly, the intestinal sections were incubated with xylol and decreasing concentrations of ethanol. The Ki67 antibody was then applied at a dilution of 1:200 overnight in a wet chamber after blocking with 3% bovine serum albumin (BSA) for 60 min at room temperature. The slides were rinsed in PBS and incubated with the secondary antibody conjugated to Alexa Fluor 488 for 60 min at room temperature. The nuclei were counterstained with DAPI. The sections were then visualized using the Leica application suite and a LeicaDFC310 FX Digital Color Camera (Leica). The number of Ki67-positive cells was counted from 10 randomly selected fields for each sample.
Apoptosis assay
An apoptosis assay was performed using an in situ cell death detection kit (TUNEL assay, Roche Diagnostics, Basel, Switzerland) according to the manufacturer’s protocol. Briefly, paraffin-embedded tissue was deparaffinized and then incubated for 20 min with proteinase K (20 g/mL) at 37°C. After blocking with 3% BSA, the specimens were incubated with 100 μL of TdT reaction solution at 37°C for 25 min. The samples were covered with 100 μL of Alexa Fluor 488-conjugated antibody solution and counterstained with propidium iodide. Sections were visualized with an Aperio AT2 slide scanner digital pathology system (Leica Biosystems) and CaseViewer software. The number of labeled cells was estimated by counting TUNEL-positive cells with morphological signs of apoptosis in 10 randomly selected areas.
Immunohistochemistry (IHC)
IHC was performed using the diaminobenzidine chromogen method. Paraffin was removed from the sections with xylene, rehydrated in decreasing concentration ethanol baths, and then rinsed with tap water for 5 min. Antigen retrieval was performed using citrate buffer (pH 6.0), followed by incubation with 0.3% H2O2 for 15 min at room temperature. After blocking, the primary antibodies were applied at 1:200 overnight in a humid chamber at 4°C. The slides were rinsed with PBS and incubated with the secondary antibody for 60 min at room temperature. The slides were rinsed with PBS and counterstained with hematoxylin. The proportions of YAP-positive cells were calculated by microscopic examination (Leica).
Western blotting
Protein extraction was performed by lysing the ISM tissues in the RIPA buffer (Cat# 89900, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with protease inhibitor cocktail (Cat# 1862209, Thermo Fisher Scientific). Protein concentration was determined using the BCA Protein Assay Kit (Cat# 23227, Thermo Fisher Scientific). Equal amounts of total protein were loaded into sodium dodecyl sulfate-polyacrylamide gels for electrophoresis and transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA). The membrane was blocked in 5% BSA in Tris-buffered saline with Tween for 2 h at room temperature before incubation overnight at 4°C with the following primary antibodies: YAP, TEAD1, TEAD4, BCL2, BAX, proliferating cell nuclear antigen, and β-tubulin. After incubation with the primary antibodies, the membranes were washed 3 times for 10 min each time with 1 × Tris-buffered saline with Tween and incubated with goat anti-rabbit IgG horseradish peroxidase-linked antibody at a final dilution of 1:10,000. Immunodetection was performed with an enhanced chemiluminescence detection system (GE Healthcare, Little Chalfont, UK). The density of some specific protein bands was quantified by Image J software (version 1.49v, National Institute of Health, Bethesda, MD, USA).
Quantitative real-time PCR
Total RNA from the ISM layer was extracted using the Trizol isolation method (Cat# 15596, Invitrogen, Carlsbad, CA, USA). The RNA concentration and purity were spectrophotometrically analyzed with a NanoDrop 2000 (Thermo Fisher Scientific). Overall, 500 ng RNA was reverse-transcribed into cDNA using PrimeScriptTM RT Master Mix (Cat# RR036A, Takara, Shiga, Japan) following the manufacturer’s instructions. cDNA was used as a template for quantitative real-time PCR using SYBR Premix Ex Taq II (Cat# RR820A, Takara) in the Thermo Scientific PikoReal Real-Time PCR System (Thermo Fisher Scientific). The amplification program consisted of activation at 95°C for 5 min, followed by 35 amplification cycles, which each consisted of 95°C for 15 s and then 60°C for 1 min. The sequences of the primers used are shown in Table 1. The data was analyzed using PIKO96 software (Thermo Fisher Scientific). GAPDH served as the reference gene. The samples were normalized by calculating ΔCt (Ctgene of interest - CtGAPDH), and gene expression was calculated as 2-ΔCt.
Table 1.
Primer sequences for qRT-PCR analysis
| Gene | RefSeq | Forward primer (5’ to 3’) | Reverse primer (5’ to 3’) |
|---|---|---|---|
| Gapdh | NM_017008.4 | TTCCTACCCCCAATGTATCCG | CATGAGGTCCACCACCCTGTT |
| Tead1 | NM_001198589.1 | AACCATTCTTACAGCGACCCG | CGAACCTCGCATACTCCGTCT |
| Tead4 | XM_006237424.3 | GCCCTCTAATGCCTTCTTCCTT | GGTGGATGCGGTACAAATAGTGA |
| Ctgf | NM_022266.2 | TGTCTTCGGTGGGTCCGTGTA | TGGCTCGCATCATAGTTGGGT |
| Yap1 | NM_001034002.2 | GGCCATGCTCTCCCAACTGAA | GGTTCATGGCAAAACGAGGGT |
| Bax | NM_017059.2 | GGGCCTTTTTGCTACAGGGTTT | AGCAAAGTAGAAAAGGGCAACCAC |
| Bcl2 | NM_016993.1 | TGAGTTCGGTGGGGTCATGTG | AGTTCCACAAAGGCATCCCAG |
Statistical analysis
Values are presented as the mean ± SD. Statistical analysis was performed using the Sigma Stat statistical package (SPSS 19.0, SPSS, Inc., Chicago, IL, USA). The student’s t-test was used to compare two groups, while analysis of variance was used to compare multiple groups. A P-value of less than 0.05 was considered significant.
Results
Oral supplementation of butyrate improves intestinal smooth muscle adaptation in rats with massive small bowel resection
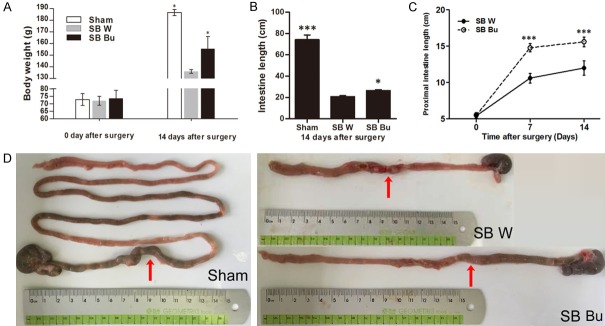
Fourteen days after surgery significant weight loss was observed in the short-bowel rats; however, rats in the SB Bu group exhibited a higher mean body weight compared to those in the SB W group (SB Bu VS SB W: 155.4 ± 10.6 VS 136.0 ± 1.6, P < 0.05, Figure 1A). Moreover, the total residual small intestine was longer in the SB Bu group than in the SB W group (P < 0.05, Figure 1B). Additionally, lengthening of the proximal small bowel in the SB Bu group was more evident than in the SB W group, particularly within the first 7 days after surgery (Figure 1C). Representative images of the residual small intestine are shown in Figure 1D, in which the anastomosis sites are indicated as red arrows. Histological examination (Figure 2A) revealed that at 14 days after surgery, obvious adaptive thickening of the ISM was observed in both the SB W and SB Bu groups. Notably, however, the circular muscle layer in the SB Bu group was up to 72.2% thicker than that in the SB W group (P < 0.001), and the thickness of the longitudinal muscle layer was increased by 17.4% (SB Bu VS SB W, P < 0.05, Figure 2B). Additionally, both villus height (P < 0.001, Figure 2C) and crypt depth (P < 0.05, Figure 2D) were increased significantly in SB Bu rats compared to SB W rats. Collectively, these findings suggest that oral supplementation of butyrate improves ISM adaptation in rats with massive small bowel resection.
Figure 1.
Oral supplementation of butyrate improves intestinal smooth muscle adaptation in rats with massive small bowel resection. A. Body weight change of rats after surgery during the experiment. B. The total length of residual intestine at day 14 after surgery. C. Comparison of proximal small bowel between the SB W and SB Bu groups at days 7 and 14 after surgery. D. Macroscopic view of the residual intestine showing the changes in the length in different groups of rats on postoperative day 14. Arrows show anastomosis sites. Values are the means ± SD, *P < 0.05, ***P < 0.001, vs SB W.
Figure 2.
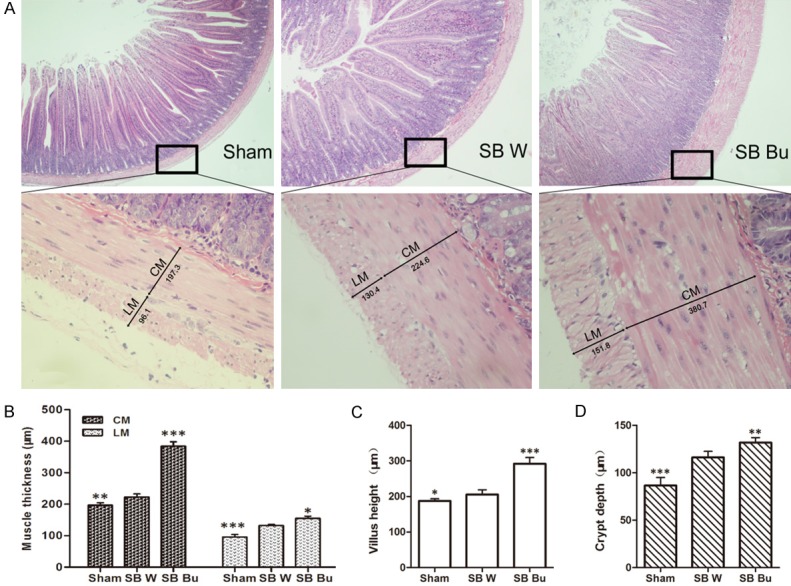
Compensatory and adaptive changes in the proximal small intestine on day 14 post-operation. (A) Histological sections from the proximal small bowel of rats in different groups on postoperative day 14 (upper panel, 40 ×; lower panel, 400 ×). (B) Both circular (CM) and longitudinal muscle (LM) were thicker in SB Bu rats than in SB W rats. The villus height (C) and crypt depth (D) were greater in the SB Bu group. Values are the means ± SD, *P < 0.05, **P < 0.01, ***P < 0.001, vs SB W.
Oral supplementation of butyrate promotes ISM cell proliferation and suppresses apoptosis
Ki67 staining suggested that the effect of butyrate on the adaption of ISM layers may be associated with enhanced cell proliferation. We found that Ki67-positive ISM cells were barely detectable in the Sham group, and only a few scattered positive cells were observed in the SB W group. In contrast, substantial accumulation of Ki67-positive ISM cells was observed in the SB Bu group (Figure 3A). Quantitatively, the proportion of Ki67-positive ISM cells was increased 3.9-fold in the SB Bu group compared to in the SB W group (P < 0.001, Figure 3B). Additionally, the TUNEL assay results suggested that changes in the apoptosis rate were involved in the adaption of ISM layers. As shown in Figure 3C, TUNEL-positive ISM cells were barely detectable in the Sham group. However, robust staining of TUNEL-positive cells was observed along the ISM layer of SB W rats, which was clearly alleviated by supplementation with butyrate. Notably, the apoptotic rate of ISM cells was decreased by 82.1% in the SB Bu group compared to in the SB W group (P < 0.001, Figure 3D). Taken together, these results demonstrate that the beneficial effect of butyrate on ISM adaption is associated with both enhanced cell proliferation and suppressed apoptosis.
Figure 3.
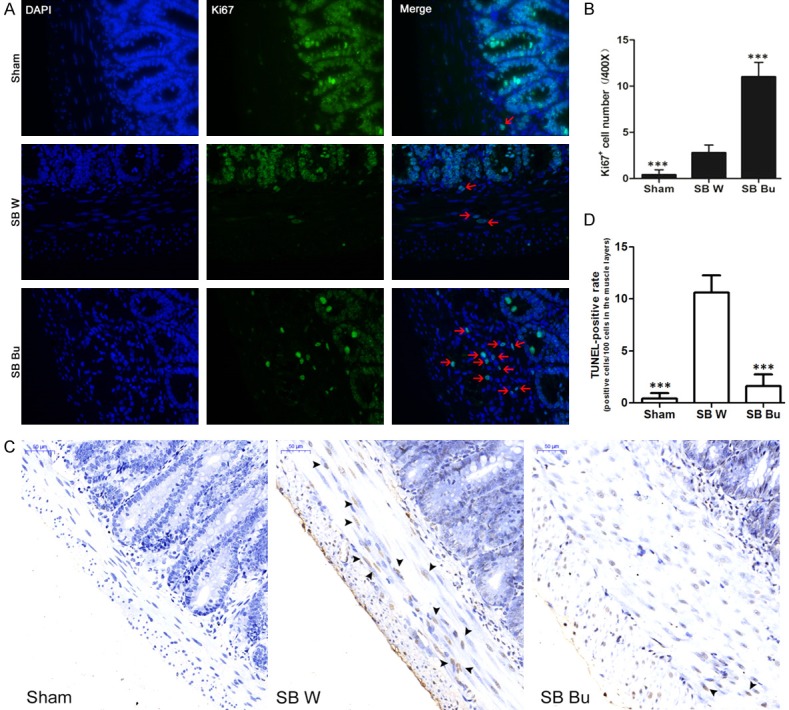
Oral supplementation of butyrate promotes intestinal smooth muscle cell proliferation and suppresses apoptosis. (A) Representative immunofluorescence images (400 ×) for Ki67 (green), showing more Ki67-positive cells (red arrows) in the muscle layers of SB Bu rats. Nuclei are labeled with DAPI (blue). (B) Bar graph showing quantification of Ki67-positive cells in (A). (C) TUNEL staining from proximal small bowel of rats. Black arrows show TUNEL-positive smooth muscle cells. Scale bars, 50 μm. (D) The fraction of TUNEL-positive cells was counted and plotted. Values are the means ± SD, ***P < 0.001, vs SB W.
Effects of butyrate on ISM cells are associated with activation of YAP and its downstream target molecules
Given that YAP has been implicated in the regeneration of mammalian smooth muscle cells [8,11] and the neuromuscular junction [12], we examined the expression of YAP in short-bowel rats. Representative images are shown in Figure 4A. In the Sham group, YAP was diffusely distributed throughout the ISM layers without discernible circumnuclear accumulation. In contrast, YAP-positive cells were scattered in the SB W group and aggregated close to the nucleus in ISM cells of the SB Bu group (Figure 4A). Compared to the SB W group, YAP-positive cells were increased by 6.5-fold in the SB Bu group (P < 0.001, Figure 4B). Furthermore, we analyzed the mRNA levels of Yap1, Tead1, Tead4, Ctgf, Bcl2, and Bax. Among them, Tead1, Tead4, and Ctgf are the downstream target genes of YAP involved in cell proliferation; Bcl2 and Bax are the key cell death regulatory genes identified as downstream targets of the YAP-TEAD1 complex [9,13]. As indicated in quantitative-PCR analysis, the mRNA levels of Yap1, Tead4, and Ctgf were significantly increased in the SB Bu group compared to in the SB W group on day 7 after surgery. Moreover, Bax expression was decreased in the SB Bu group compared to in the SB W group, while Tead1 and Bcl2 remained unchanged. At day 14 after surgery, in addition to Yap1 and Tead4, the expression of Tead1 and Bcl2 was increased in the SB Bu group compared to in the SB W group. Bax remained decreased in the SB Bu group while Ctgf returned to baseline (Figure 5A). Furthermore, western blot analysis was performed to validate the relationship between the expression patterns of these genes and their protein levels. Representative immunoblots and quantitative analysis are shown in Figure 5B and 5C, respectively. Overall, changes in protein levels substantially mirrored those in mRNA levels. Particularly, supplementation with butyrate resulted in an approximately 2.2-fold increase in YAP mRNA levels and 3.4-fold increase in its protein levels. These data suggest that the effects of butyrate on ISM cells are associated with activation of YAP and its downstream target molecules.
Figure 4.
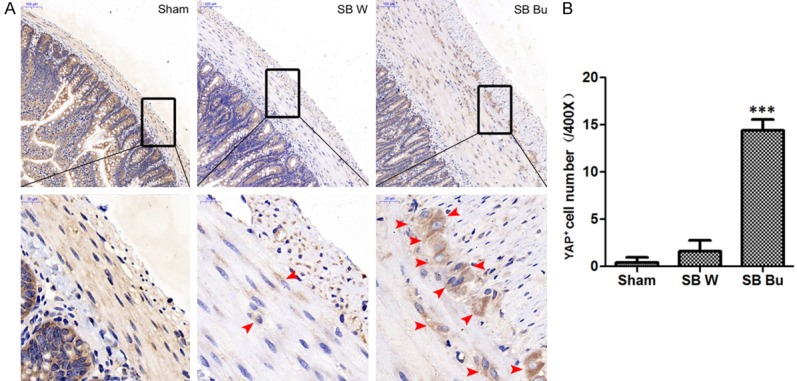
Butyrate activates YAP expression. (A) Representative immunohistochemical results obtained with anti-YAP in the smooth muscle layers of rats on postoperative day 14. Red arrows indicate positive signal to YAP. Scale bars in the upper panel correspond to 100 μm and in the lower panel correspond to 20 μm. (B) Bar graph showing quantification of YAP-positive cells in (A). Values are the means ± SD, ***P < 0.001, vs SB W.
Figure 5.
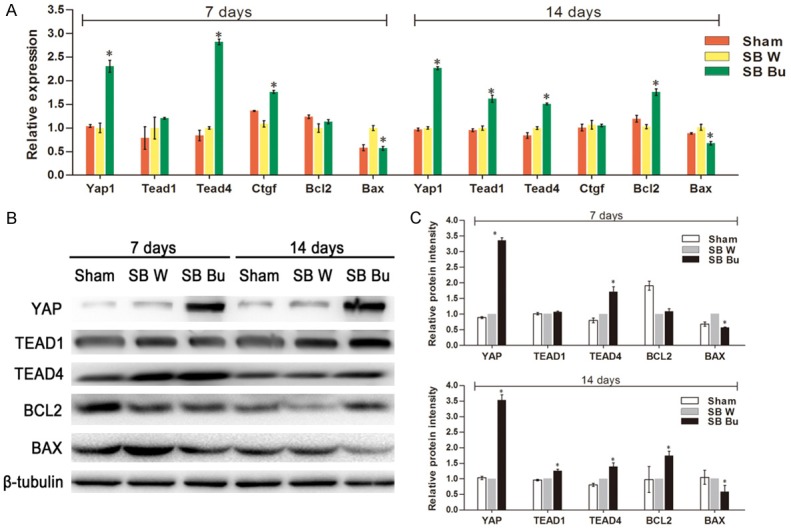
Butyrate activates YAP target downstream molecules. A. The relative mRNA expression levels of Yap1, Tead1, Tead4, Ctgf, Bcl2, and Bax. B. Western blot analysis of intestinal smooth muscle tissue of rats at days 7 and 14 after surgery. β-Tubulin was used as an internal control. C. Relative protein expression of YAP, TEAD1, TEAD4, BCL2, and BAX. Values are the means ± SD, *P < 0.05, vs SB W.
Discussion
SBS commonly arises from massive small bowel resection, which leads to inadequate nutrient intake required for maintenance in adults or growth in children. The primary diseases causing SBS include neonatal necrotizing enterocolitis and congenital intestinal anomalies in children, and Crohn’s disease in adults [14]. Estimating the incidence and prevalence of SBS is difficult because of the multi-factorial etiology and varying residual intestinal length. One of the main goals of the clinical management of patients with SBS is to improve intestinal adaptation as soon as possible using various surgical or medical approaches. Several surgical procedures that directly lengthen the residual intestine have been developed, such as longitudinal intestinal lengthening/tailoring and serial transverse enteroplasty [15,16]. In contrast, medical treatments, such as nutrition support, are dedicated to improving the intestinal absorptive capacity, controlling intestinal motility, and maintaining host-microbiota homeostasis. In this study, we investigated the ISM layers, which support the mucosa and are necessary for mixing and propelling the chyme forward through the intestine. Our results demonstrated that oral supplementation with butyrate promoted adaptation of the residual intestine through its beneficial effects on the ISM cells, characterized by increased muscle layer thickness, small bowel length, and weight gain in SBS rats.
The overall beneficial effect of butyrate is reflected by weight gain (Figure 1A). Although weight gain was observed in both SB W and SB Bu rats shortly after reinstatement of the diet, rats in the SB Bu group gained more weight than those in the SB W group at the end of the experiment. Moreover, stools from the SB Bu group appeared mushy, and were thicker than the watery stools observed in the SB W group. This may be because of enhanced intestinal absorption and improved intestinal transit. Furthermore, our data also indicated more prominent adaptation occurring in the proximal intestine where most of the nutrients are absorbed: the thickness of circular muscle layer and longitudinal layer in the SB Bu group were increased by 72.2% and 17.4%, respectively. However, one of the limitations of this study was that muscular thickness may not be sufficient to fully describe the changes in the SB Bu group. Because the primary response of the ISM to small bowel resection is hypertrophy and longitudinal stretching, alterations in the size, shape, and function of individual ISM cells should also be considered in future studies.
Morphologically, our results also indicate that both cell proliferation and apoptosis in the ISM layers were influenced by butyrate, suggesting a key mediator may be involved that simultaneously regulates proliferation and apoptosis (Figure 3). YAP is an important co-activator of transcription factors in mammalian cells which function as a growth control pathway and determine cell fate. To date, the effects of YAP on cell proliferation and apoptosis have been identified in a variety of cells and tissues [17], including human corneal endothelial cell monolayers [18] and podocytes [19]. Previously, we also reported that butyrate stimulates the proliferation of human ISM cells via the activation and nuclear translocation of YAP in vitro. Taking these findings into account, we examined the changes in the expression of Yap1 and its downstream target genes including Tead1, Tead4, Ctgf, and Bcl2.
Notably, for the delivery of butyrate, the dosages were used according to Stilling’s review [20]. Four dosages (25, 50, 100, and 150 mM) were tested in the preliminary study. Because no significant benefits were observed at the higher dosage (100 mM), including weight gain, residual bowel length, stool shape, and food consumption (g/100 g weight), 50 mM was used in the present study (data not shown). It is reasonable to orally administer butyrate rather than using peripheral routines, as oral delivery is more physiological, simple, and safe, allowing the small intestine to be continuously exposed to butyrate. Additionally, emerging evidence has indicated that oral supplementation of butyrate not only benefits the structural and functional adaptation of the remnant bowel, but also plays a fundamental role in preventing parenteral nutrition-associated complications such as liver dysfunction [21]. In general, the adaptive processes may require several years to reach their maximum effect. In the present study, butyrate was administered immediately after surgery. Butyrate facilitated intestinal adaptation by both augmenting and accelerating intestinal adaptation, thereby shortening the time to reach the plateau. Thus, addition of butyrate through enteral feedings may support the successful transition for patients with SBS. Additionally, oral administration of butyrate facilitates the direct contact of intraluminal butyrate with epithelial cells and subsequent secretion of intestinotrophic factors [22].
In summary, these results highlight the potential use of butyrate in SBS. Oral administration of butyrate contributes to the adaptation of intestinal smooth muscle by activating YAP in vivo. Thus, administration of butyrate may aid the successful transition for patients with SBS.
Acknowledgements
This work was supported by the Shanghai Key Laboratory of Pediatric Gastroenterology and Nutrition (17DZ2272000), the National Natural Science Foundation of China-Key Program (81630039), and the Natural Science Foundation of Shanghai (16ZR1428400).
Disclosure of conflict of interest
None.
References
- 1.Bielawska B, Allard JP. Parenteral nutrition and intestinal failure. Nutrients. 2017;9:466. doi: 10.3390/nu9050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tolga C, Acar A, Tolan KH, Serin S, Tomruk SG, Kilic A, Ezberci F, Unal E. Remaining small bowel length can predict the high rate of total parenteral nutrition complications in intestinal failure? Transplantation. 2017;101:S126–S127. [Google Scholar]
- 3.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–119. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- 4.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 5.Bartholome A, Albin D, Baker D, Holst J, Tappenden K. Supplementation of total parenteral nutrition with butyrate acutely increases structural aspects of intestinal adaptation after an 80% jejunoileal resection in neonatal piglets. JPEN J Parenter Enteral Nutr. 2004;28:210–222. doi: 10.1177/0148607104028004210. [DOI] [PubMed] [Google Scholar]
- 6.Tappenden K. Emerging therapies for intestinal failure. Arch Surg. 2010;145:528–532. doi: 10.1001/archsurg.2010.102. [DOI] [PubMed] [Google Scholar]
- 7.Zhao B, Ye X, Yu J, Li L, Li W, Li S, Lin JD, Wang CY, Chinnaiyan AM. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Hu G, Liu F, Wang X, Wu M, Schwarz JJ, Zhou J. Deletion of yes-associated protein (YAP) specifically in cardiac and vascular smooth muscle cells reveals a crucial role for YAP in mouse cardiovascular development. Circ Res. 2014;114:957–965. doi: 10.1161/CIRCRESAHA.114.303411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song Y, Fu J, Zhou M, Xiao L, Feng X, Chen H, Huang W. Activated hippo/yes-associated protein pathway promotes cell proliferation and anti-apoptosis in endometrial stromal cells of endometriosis. J Clin Endocrinol Metab. 2016;101:1552. doi: 10.1210/jc.2016-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marsman KE, McBurney MI. Dietary fiber increases oxidative metabolism in colonocytes but not in distal small intestinal enterocytes isolated from rats. J Nutr. 1995;125:273–282. doi: 10.1093/jn/125.2.273. [DOI] [PubMed] [Google Scholar]
- 11.Feng X, Liu P, Zhou X, Li MT, Li FL, Wang Z, Meng Z, Sun YP, Yu Y, Xiong Y, Yuan HX, Guan KL. Thromboxane A2 activates YAP/TAZ protein to induce vascular smooth muscle cell proliferation and migration. J Biol Chem. 2016;291:18947–18958. doi: 10.1074/jbc.M116.739722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao K, Shen C, Lu Y, Huang Z, Li L, Rand CD, Pan J, Sun XD, Tan Z, Wang H, Xing G, Cao Y, Hu G, Zhou J, Xiong WC, Mei L. Muscle Yap is a regulator of neuromuscular junction formation and regeneration. J Neurosci. 2017;37:3465–3477. doi: 10.1523/JNEUROSCI.2934-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goulet O, Ruemmele F. Causes and management of intestinal failure in children. Gastroenterology. 2006;130:16–28. doi: 10.1053/j.gastro.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Hommel MJ, Van BR, Haveman JW. Surgical management and autologous intestinal reconstruction in short bowel syndrome. Best Pract Res Clin Gastroenterol. 2016;30:263. doi: 10.1016/j.bpg.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Abuelmagd K. The concept of gut rehabilitation and the future of visceral transplantation. Nat Rev Gastroenterol Hepatol. 2015;12:108–120. doi: 10.1038/nrgastro.2014.216. [DOI] [PubMed] [Google Scholar]
- 17.Mizuno T, Murakami H, Fujii M, Ishiguro F, Tanaka I, Kondo Y, Akatsuka S, Toyokuni S, Yokoi K, Osada H. YAP induces malignant mesothelioma cell proliferation by upregulating transcription of cell cycle-promoting genes. Oncogene. 2012;31:5117. doi: 10.1038/onc.2012.5. [DOI] [PubMed] [Google Scholar]
- 18.Hsueh YJ, Chen HC, Wu SE, Wang TK, Chen JK, Ma DH. Lysophosphatidic acid induces YAP-promoted proliferation of human corneal endothelial cells via PI3K and ROCK pathways. Mol Ther Methods Clin Dev. 2015;2:15014. doi: 10.1038/mtm.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell KN, Wong JS, Gupta R, Asanuma K, Sudol M, He JC, Mundel P. Yes-associated protein (YAP) promotes cell survival by inhibiting proapoptotic dendrin signaling. J Biol Chem. 2013;288:17057–17062. doi: 10.1074/jbc.C113.457390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF. The neuropharmacology of butyrate: the bread and butter of the microbiota-gut-brain axis? Neurochem Int. 2016;99:110–132. doi: 10.1016/j.neuint.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Welters CF, Deutz NE, Dejong CH, Soeters PB, Heineman E. Supplementation of enteral nutrition with butyrate leads to increased portal efflux of amino acids in growing pigs with short bowel syndrome. J Pediatr Surg. 1996;31:526–529. doi: 10.1016/s0022-3468(96)90488-1. [DOI] [PubMed] [Google Scholar]
- 22.Yadav H, Lee J, Lloyd J, Walter P, Rane S. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J Biol Chem. 2013;288:25088–25097. doi: 10.1074/jbc.M113.452516. [DOI] [PMC free article] [PubMed] [Google Scholar]