Abstract
Random-pattern skin flap transplantation is a common procedure in plastic surgery, but its distal area usually incurs ischemia and necrosis. Resveratrol (Rev), a natural polyphenol primarily found in peanuts, grapes, and red wine, which exerts multi-bioactivity. In this study, forty-eight rats with the modified “McFarlane flap” model were divided into Control and Rev groups, which were treated with vehicle Control and Rev, respectively. After 7 days of continuous treatment and observation, ischemic flap tissues were harvested to evaluate angiogenesis, apoptosis, oxidative stress, and autophagy. It was observed a greater survival area of flaps, accompanied with reduced water content and stronger blood supply, in the Rev group than in the Control group. In addition, Rev upregulated the expression of MMP9, VEGF, and Cadherin5, indicating that Rev promotes angiogenesis in ischemic flaps. Moreover, Rev decreased the levels of Bax, CYC, and Caspase3, suggesting that it inhibits apoptosis. Besides, Rev increased the expression of SOD1, eNOS, HO1, the activities of SOD and GSH, and reduced the levels of MDA, which uncovers that it depresses oxidative stress in ischemic flaps. Finally, it increased the expression of Beclin1, LC3II, VPS34, and CTSD, and decreased SQSTM1/p62 levels, which reveals that it activates autophagy in the flaps. These results suggest that Rev promotes random skin flap survival through proangiogenic, antiapoptotic, and antioxidative effects; moreover, autophagy is activated in the process, which might be another underlying mechanism for the flap survival.
Keywords: Resveratrol, random-pattern skin flap, angiogenesis, apoptosis, oxidative stress, autophagy
Introduction
Random-pattern skin flap transplantation is a common procedure in plastic surgery or the restoration of cutaneous defects [1-4]. However, ischemia and subsequent necrosis of skin flaps, one of the most frequent postoperative complications, which occurs in distal areas, limits the length to width ratio of flaps to less than 1.5 or 2 [1,5]. The complications markedly restrict the clinical application of random skin flaps [6]. Postoperative survival of random flaps relies on an adequate blood supply [3]. After random flap operation, angiogenesis occurs from the pedicle bed of the flap towards the distal end, bringing a blood supply to the corresponding areas of flap tissue [7]. Generally, angiogenesis is infrequent in the distal parts of the flaps, and tissue necrosis in this area is mainly caused by a lack of angiogenesis. Moreover, after vascular regeneration in cutaneous flaps, restoration and reperfusion of the blood supply results in ischemia-reperfusion injury (IRI). Apoptosis and oxidative stress, two major mechanisms of IRI, are also crucial factors in the necrosis of random-pattern flaps [8-10]. Therefore, increasing angiogenesis, as well as alleviating oxidative stress and apoptosis, can effectively ameliorate ischemic necrosis of random skin flaps.
Autophagy, a highly conserved eukaryotic cellular recycling process for the degradation of cytosolic macromolecules and organelles [11], is reported to play a key role in angiogenesis, oxidative stress, and apoptosis. Activation of autophagy regulates the expression of multiple endothelial cell receptors, including neuropilin1 degradation, and then promotes angiogenesis [12]. Moreover, it alleviates liver IRI through the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinases (ERK)/mammalian target of rapamycin (mTOR) pathway [13]. Interestingly, calcitriol alleviates IRI and promotes angiogenesis by activating autophagy, which enhances the viability of random flaps [14]. Therefore, the survival of random skin flaps can be enhanced by an agent that promotes angiogenesis and autophagy and reduces apoptosis and oxidative stress.
Resveratrol (3,4’,5-tri-hydroxy-trans-stilbene; Rev) is a natural polyphenol primarily found in peanuts, grapes, and red wine [15]. Previous studies have reported its pharmacological effects involving pro-angiogenesis, anti-apoptosis, anti-oxidative stress, and pro-autophagy [16]. In human endothelial cells, Rev promotes angiogenesis through the glycogen synthase kinase 3 beta (GSK3β)/β-catenin/T-cell factor (TCF)-dependent pathway [17]. It also enhances angiogenesis in ischemic myocardium through the thioredoxin 1 (Trx1)/heme oxygenase 1 (HO1)/vascular endothelial growth factor (VEGF) pathway [18]. Moreover, it enhances cell autophagy through the AMP-activated protein kinase (AMPK)/mTOR pathway, and inhibits cell apoptosis and oxidative stress through the phosphatidylinositide 3-kinase (PI3K)/nuclear factor-like 2 (Nrf2)/HO1 signaling pathway [19]. Hence, we hypothesized that Rev may exerts beneficial functions for random pattern skin flap survival via the above mechanism.
In this study, the effects of Rev on random flap survival were explored in rats. What’s more, its underlying mechanism involved in the properties of angiogenesis enhancement, along with its ability to decrease apoptosis, depress oxidative stress, and up-regulate the level of autophagy, will be evaluated within the context of histological and protein analyses.
Materials and methods
Experimental reagents
Rev (3,4’,5-tri-hydroxy-trans-stilbene, C14H12O3) was purchased from Sigma (St. Louis, MO, USA). Pentobarbital sodium, Diaminobezidin (DAB) developer, Hematoxylin&eosin (H&E) staining Kit were acquired from Solarbio Science & Technology (Beijing, China). Superoxide dismutase (SOD), glutathione (GSH), and malondialdehyde (MDA) assay kits were from Jiancheng Technology (Nanjing, China). And other reagents and antibodies were from the following companies: rabbit monoclonal anti-GAPDH (AP0063; Biogot Technology, Shanghai, China). rabbit monoclonal anti-Matrix metallopeptidase9 (MMP9), anti-VEGF, anti-SOD1, anti-HO1, anti-caspase3 (CAPS3), anti-Phosphoinositide 3 kinase (VPS34) and anti-cathepsin D (CTSD) (19003-1, 10269-1, 12452-1, 10375-2, 10701-1, 213-27-1, and 19677-1; Proteintech Group, Chicago, IL, USA); rabbit monoclonal anti-cadherin5 (A0-2632-2; Boster Biological Technology, Wuhan, China). rabbit monoclonal anti-endothelial nitric oxide synthase (eNOS), anti-cytochrome c (CYC) and anti-Bax (11940S, 14796 and 32027; Cell Signaling Technology, Beverly, MA, USA); rabbit monoclonal anti-Microtubule-associated 1 protein light chain 3 (LC3) (L7543; Sigma-Aldrich Chemical Company, Milwaukee, WI, USA); mouse monoclonal anti-SQSTM1/p62 (ab56416; Abcam, Cambridge, UK); horseradish peroxidase (HRP)-conjugated IgG secondary antibody (Santa Cruz Biotechnology, Dallas, TX, USA); the BCA Kit (Thermo Fisher Scientific, Rockford, IL, USA); the Electrochemiluminescence (ECL) Plus Reagent Kit (PerkinElmer Life Sciences, Waltham, MA, USA); fluorescein isothiocyanate (FITC)-conjugated IgG secondary antibody (Boyun Biotechnology, Nanjing, China); 4’,6-Diamidino-2-phenylindole (DAPI) solution (Beyotime Biotechnology, Jiangsu, China).
Ethics statement
All procedures involving animals follow the Guide for the Care and Use of Laboratory Animals of the China National Institutes of Health, which were approved by the Animal Care and Use Committee of Wenzhou Medical University (wydw 2017-0022). The suffering of rats was minimized with our best effort.
Animals and experimental model
Healthy Sprague-Dawley rats (male, 250-300 g), from Wenzhou Medical University (License No. SCXK [ZJ] 2005-0019), were individually kept in a standard experimental cage in an environment-controlled room (a temperature of 23°C ± 2°C, a relative humidity of 50% ± 5%, artificial lighting from 07:00-19:00 h), and they were provided food and water ad libitum. Before surgery, 40 mg/kg pentobarbital sodium 2% (w/v) was injected interperitoneally for anesthetization of rats. The modified “McFarlane flap” model in the rat dorsum (in the same position in each rat) was performed as published studies [20,21]. A caudal skin flap of 3 × 9 cm was separated from subcutaneous deep fascia on the back of each rat. Subsequently, both sacral arteries were sectioned. Finally, the flaps were sutured to the donor bed by 4-0 silk. The random skin flap area was equally divided into three parts: area I, area II, and area III, from proximal to distal.
Group assignment and drug administration
Rev was dissolved in 96% ethanol and further diluted in distilled water for a final concentration of 2 mg/ml. Forty-eight rats were randomly divided into Control and Rev groups (n = 24 each). Individual rat in the Rev group received a dose of 2 mg/kg/day Rev daily administered by gavage for 7 days after surgery. The Control group (n = 24) received an equal volume of 0.05% (v/v) ethanol in distilled water daily for 7 days. All rats were euthanized by an overdose of pentobarbital sodium and tissue samples were harvested for analysis.
Macroscopic observation of flap survival
We observed macroscopic development of random skin flap for 7 days, and characteristics of appearance, color, texture, hair condition of the flaps were noted. At 7 days postoperatively, survival areas of the random skin flaps were measured by superimposition of photographs on graph paper, with calculation of the percentages as: extent of viable area × 100% ÷ total area.
Laser doppler blood flow (LDBF) imaging
Blood supply in the flaps was evaluated using LDBF imaging on day 7 [22,23]. Six rats per group were anesthetized and scanned by Laserflo BPM (Vasamedic, St. Paul, MN, USA). LDBF imaging generally offers deep penetration enabling enhanced visualization of small vessels below the tissue surface, perfect for angiogenesis modeling. The scanning was performed with 15 cm × 15 cm area and 256 × 256 pixels. Blood supply was marked by the LDBF signal (green, yellow, and red, from weak to strong) and the skin area without blood supply was marked by blue signal, the areas of which were measured by Image J software (NIH, Bethesda, MD, USA). The percentage of the blood supply area was calculated as: LDBF signal area (green, yellow, and red) × 100%, total area.
Assessment of tissue edema
Tissue edema was evaluated according to the water content of the flaps on day 7. After euthanasia, six flap tissues of each group were harvested, dehydrated in an autoclave at 50°C, and weighed daily until constant weight over 2 days. The water content was calculated as: ([wet weight - dry weight]/wet weight) × 100%.
H&E staining
On day 7 after surgery, animals were sacrificed, and three samples (0.5 × 0.5 cm) of middle tissue in area II were harvested. The samples were fixed in 4% neutral buffered formalin for 24 h and embedded in paraffin using standard procedures. Paraffin-embedded sections were cut into 4 μm thickness and were subsequently stained with H&E staining. Under a light microscope (20 × magnification), the angiogenesis, edema, and thickness of granulation tissue were assessed. Moreover, the number of microvessels per unit area (/mm2) was counted manually for assessment of angiogenesis.
Assays of MDA, SOD, and GSH levels
MDA, SOD and GSH assays were performed to assess the oxidative stress of ischemic flaps. On day 7 after operation, tissue samples (0.5 × 0.5 cm) were obtained from area II (n = 6 per group), weighed, homogenized, and diluted to 10% (v/v) in ice bath. Subsequently, MDA content was measured via reaction with thiobarbituric acid (TBA) at 90-100 °C, SOD activity was evaluated by the xanthine oxidase method, and GSH level was determined using modified 5,5’-dithiobis [2-nitrobenzoic acid] method.
Immunohistochemistry (IHC)
Six sections of the middle part of flap area II were deparaffinized in xylene and rehydrated through a graded ethanol series. After washing, the sections were immersed in a 3% hydrogen peroxide solution for 10 min to block endogenous peroxidase. Next, 10 mM/L citrate buffer solution (pH 6.0) was applied for antigen retrieval (20 min, 95°C). After blocking in 10% (w/v) bovine serum albumin for 10 min, the sections were incubated with monoclonal antibodies anti-VEGF (1:300), anti-CD34 (1:100), anti-CASP3 (1:200), anti-SOD1 (1:100) and anti-cadherin5 (1:100) overnight at 4°C. Subsequently, the sections were incubated with HRP-conjugated secondary antibody, counterstained using hematoxylin, and visualized at 20 × magnification using the DP2-TWAN image-acquisition system (Olympus Corp., Tokyo, Japan). Analysis of the images was performed using Image-Pro Plus software (Media Cybernetics, Rockville, MD, USA) for the quantification of VEGF-, CASP3-, SOD1-, CTSD-, and cadherin5- integral absorbance and CD34-positive blood vessels. Six random fields of three random sections from each tissue sample were determined.
Immunofluorescence
Six sections of area II were deparaffinized in xylene and rehydrated through a graded ethanol series. After washing, the slides were treated with 10.2 mM sodium citrate buffer for antigen retrieval (20 min, 95°C), and permeabilized with 0.1% (v/v) PBS-Triton X-100 for 30 min. After blocking in 10% (v/v) bovine serum albumin in PBS for 1 h, the sections were incubated with a monoclonal antibody anti-LC3II (1:200) at 4°C overnight. Followed by, the slides were washed thrice for 10 min each and then incubated with FITC-conjugated secondary antibody (1 h, room temperature, and dark room). The slides were visualized under a fluorescence microscope (Olympus). The percentage of LC3II-positive cells in the dermal layer was counted in six random fields of three random sections from each sample.
Western blotting
Tissue samples (0.5 × 0.5 cm) from the middle of flap area II (n = 6) were harvested, homogenized, and the protein concentration was measured using the BCA assay with BSA as the standard. Proteins (60 μg) were separated in 12% (w/v) gels and electro-transferred to polyvinylidene difluoride membranes. After blocking with 5% skimmed milk, blots were probed with primary antibodies at 4°C overnight: MMP9 (1:1,000), VEGF (1:1,000), Cadherin5 (1:1,000), Bax (1:1000), CYC (1:1,000), CASP3 (1:1,000), HO1 (1:1,000), eNOS (1:1,000), SOD1 (1:1,000), Beclin1 (1:1,000), LC3B (1:500), VPS34 (1:1,000), CTSD (1:1,000), p62 (1:1,000) and GAPDH (1:1,000). After washing with TBS-T, the membranes were treated with HRP-conjugated secondary antibodies (dilution 1:5,000) for 2 h and visualized using the ECL Plus Reagent Kit. The band intensities were measured using Image Lab 3.0 software (Bio-Rad, Hercules, CA, USA).
Statistical analyses
Data were expressed as means ± SEM. Statistical analysis was performed using SPSS ver. 19 software (Chicago, IL, USA). Analysis of variance between two groups was performed using the Student’s t-test. Probability values were considered significant at P < 0.05.
Results
Resveratrol increased survival area of random skin flaps, decreased tissue edema and improved angiogenesis in the flaps
On day 7 after the modified “McFarlane flap” model was established, the necrosis was obvious in area III of each group, as evidenced by scabbing, hardening and a dark and brown nidus, and spread to area II, whereas area I survived (Figure 1A). After quantification and analysis, mean survival area percentages was significantly larger in the Rev group than in the Control group (72.18 ± 6.80% and 50.32 ± 10.30%, respectively; P = 0.001; Figure 1B). As shown in Figure 1C, in the Control group, the distal part of the flaps was swollen and bruised, with venous blood stasis covering subcutaneous tissue. These signs were less obvious in the Rev group than that in the Control group. Percentages of mean water content in flaps of the Rev group (48.27 ± 6.44%) was much lower, compared with the Control group (59.44 ± 8.52%; P = 0.028; Figure 1D). Under LDBF imaging, the Rev group showed a significantly larger area of vascular flow in random skin flaps than the Control group (Figure 1E). Percentages of vascular flow area by LDBF imaging was increased in the Rev group (49.05 ± 10.60%), compared with the Control group (24.58 ± 10.93%; P = 0.003; Figure 1F). As shown in Figure 1G, the Rev group showed more microvessels than that of the Control group. The mean vessel densities (MVDs) were counted to quantify angiogenesis from the observation of H&E staining. The MVDs of area II in the Rev group was 29.40 ± 9.37/mm2, which was significantly greater than that in the Control group (17.59 ± 5.97/mm2; P = 0.026; Figure 1H). For further quantification of the MVDs, IHC for CD34 is performed to label endothelial cells in vessels. As shown in Figure 1I, 1J, The MVDs is larger in ischemic flap of the Rev group (30.63 ± 8.08/mm2) than the Control group (17.18 ± 2.88/mm2; P = 0.003).
Figure 1.
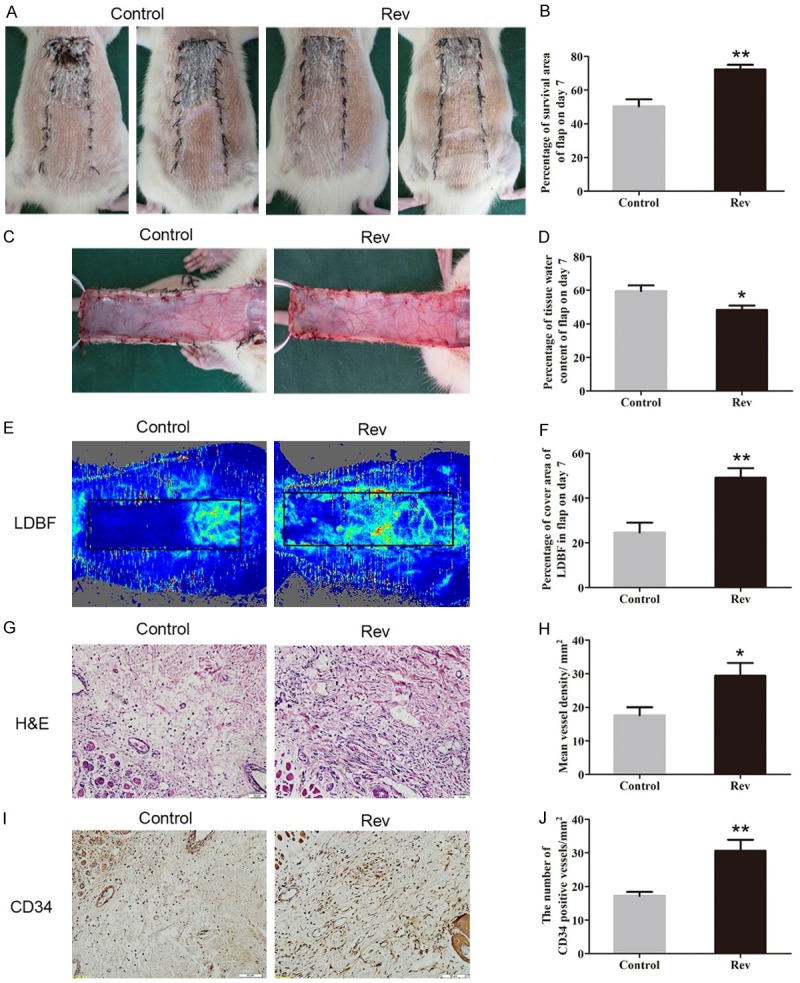
Resveratrol increased survival area of random skin flaps, decreased tissue edema and improved angiogenesis in the flaps. After the modified “McFarlane flap” model was established, the cutaneous appearance and edema of skin flaps were observed and recorded, and neovascularization was assessed by the number of micro vessels in dermis layer. A. Digital photographs of flap survival in the Control and Rev groups were taken on day3 and day7. B. The percentages of survival area in two groups were quantified and analyzed. C. Digital photographs of the inner side of the skin flap were taken from the Control and Rev groups to show the degree of tissue edema. D. Histogram of percentage of tissue water content in each group. E. Laser doppler blood flow (LDBF) imaging of flaps in each group to show the blood supply in the tissue. F. The percentages of area with blood flow in flaps were quantified and analyzed. G. H&E staining to show vessels in area II of flap in the Control group and the Rev group (original magnification × 200; scan bar, 50 μm). H. The MVDs in each group were quantified and analyzed. I. IHC for CD34 to present vessels in area II in each group. J. The CD34-positive vessel densities in the two groups were quantified and analyzed. Significance: *P < 0.05 and **P < 0.01 vs. the Control group. Data were expressed as means ± SEM, n = 6 per group.
Resveratrol promoted angiogenesis in ischemic area of the flaps
IHC for VEGF and cadherin5 expression was performed in random skin flaps to quantify the capacity of neovascularization in both groups. As shown in Figure 2A, VEGF mainly expressed in vessels and stromal cells in area II of random skin flap, which was showed higher level in the Rev group than the Control group (P = 0.005; Figure 2B). Cadherin5, the protein which mainly expressed in similar cells and tissue (Figure 2C), was also higher in the Rev group than in the Control group (P = 0.025; Figure 2D). The levels of angiogenesis related proteins, including MMP9, VEGF, and cadherin5, in area II of random skin flaps were aslo measured by Western blotting (Figure 2E-G). Results showed that the optical density values of all of these proteins were increased in the Rev group compared with the Control group (P = 0.042, 0.030, and 0.037, respectively; Figure 2H, 2J).
Figure 2.
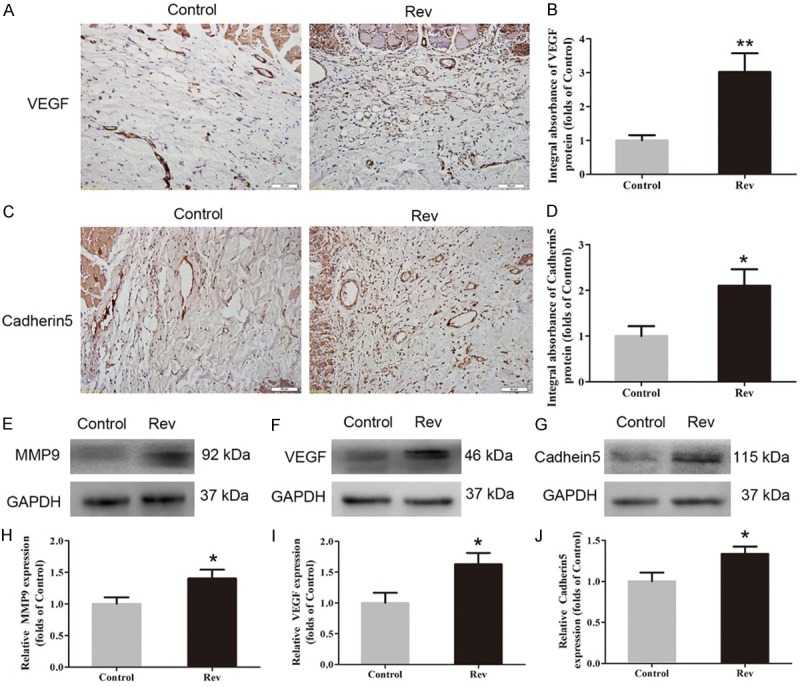
Resveratrol promoted angiogenesis in ischemic area of the flaps. On day 7 after operation, expression of angiogenesis in flap area II was evaluated using IHC for VEGF and cadherin5, and Western blotting for VEGF, MMP9 and cadherin5 expressions. A. IHC for VEGF expression in the ischemic flaps of the Control and Rev groups (original magnification, × 200; scan bar, 100 μm). B. Optical density values of VEGF expressions were quantified and analyzed in each group C. IHC for Cadherin5 expression in the ischemic flaps of the Control group and the Rev group (original magnification, × 200; scan bar, 100 μm). D. Optical density values of Cadherin5 expressions were quantified and analyzed in each group. E-G. Western blotting for MMP9, VEGF and Cadherin5 expressions in Area II flaps in the Control group and the Rev group. The gels were run under the same experimental conditions, and cropped blots were used here. The Original images are available in Figure S1A. H-J. Optical density values of MMP9, VEGF and Cadherin5 were quantified and analyzed in each group, Significance: *P < 0.05 and **P < 0.01 vs. the Control group. Data were presented as means ± SEM, n = 6 per group.
Resveratrol depressed apoptosis in ischemic area of the flaps
IHC was conducted to determine CASP3 level in the dermis layer of area II of each group. Lower CASP3 level was detected in vessels and stromal cells of the Rev group than the Control group (Figure 3A), with a lower integral absorbance in the Rev group (P = 0.026; Figure 2B). Western blot analysis was performed to detected the expressions of Bax, CYC and CASP3 in the ischemic flap tissues (Figure 3C-E). The results showed that the optical density values of Bax, CYC and CASP3 were significantly down-regulated in the Rev group compared with the Control group (P = 0.024, 0.007 and < 0.001, respectively; Figure 3F-H).
Figure 3.
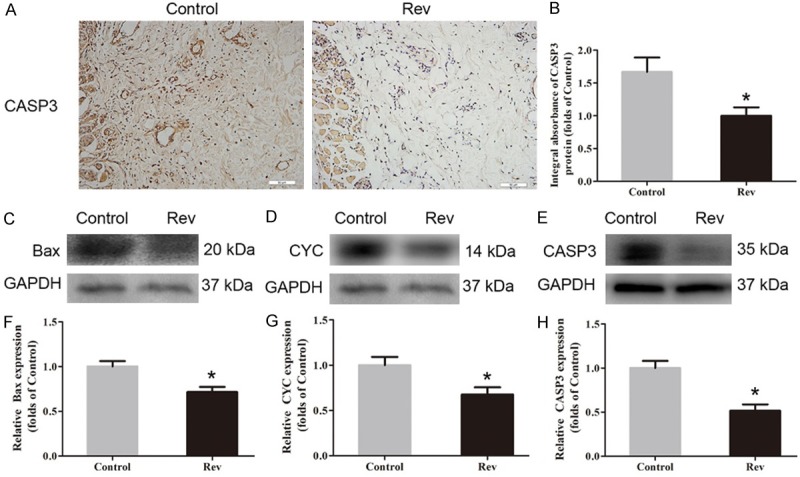
Resveratrol depressed apoptosis in ischemic area of the flaps. On day 7 after operation, apoptosis in area II of flap was evaluated via IHC for CASP3 and Western blotting for the expression of Bax, CYC and CASP3. A. IHC for CASP3 expression in the ischemic flaps of the Control and Rev groups (original magnification, × 200; scan bar, 100 μm). B. Optical density values of CASP3 are quantified and analyzed in each group. C-E. Western blotting for Bax, CYC and CASP3 in Area II flaps in the Control group and the Rev group. The gels were run under the same experimental conditions, and cropped blots are used here. The Original images are available in Figure S1B. F-H. Optical density values of Bax, CYC and CASP3 were quantified and analyzed in each group. Significance: *P < 0.05 and **P < 0.01 vs. the Control group. Data were shown as means ± SEM, n = 6 per group.
Resveratrol attenuated oxidative stress in ischemic area of the flaps
IHC was performed for SOD1 expression to indicate oxidative stress in area II of flaps. As shown in Figure 4A, higher level of SOD1 was observed in vessels and stromal cells of the Rev group, compared with the Control group. The integral absorbance of SOD1 was larger in the Rev group than in the Control group (P = 0.024; Figure 4B). Western blotting was also performed for SOD1, eNOS and HO1 expressions in the flaps. (Figure 4C-E). The results showed that the Rev group expressed more SOD1, eNOS and HO1 than the Control group (P = 0.020, 0.028, and 0.035, respectively; Figure 4F-H). In addition, the SOD activity tested via the xanthine oxidase method, MDA content detected via the modified TBA test and GSH level determined via the modified 5,5’-dithiobis [2-nitrobenzoic acid] method was performed. The results showed that the Rev group had a much higher level of SOD (59.33 ± 16.60 U·mg-1·protein-1) than the Control group (33.67 ± 11.66 U·mg-1·protein-1; P = 0.011; Figure 4I); the GSH level in the Rev group was 2.23 ± 0.44 nM·mg-1·protein-1, which was significantly higher than 1.50 ± 0.33 nM·mg-1·protein-1 in the Control group (P = 0.005; Figure 4J); the mean MDA content in the Rev group was 37.17 ± 4.75 nM·mg-1·protein-1 compared with 57.33 ± 9.27 nM·mg-1·protein-1 in the Control group, with a significant difference (P < 0.001; Figure 4K).
Figure 4.
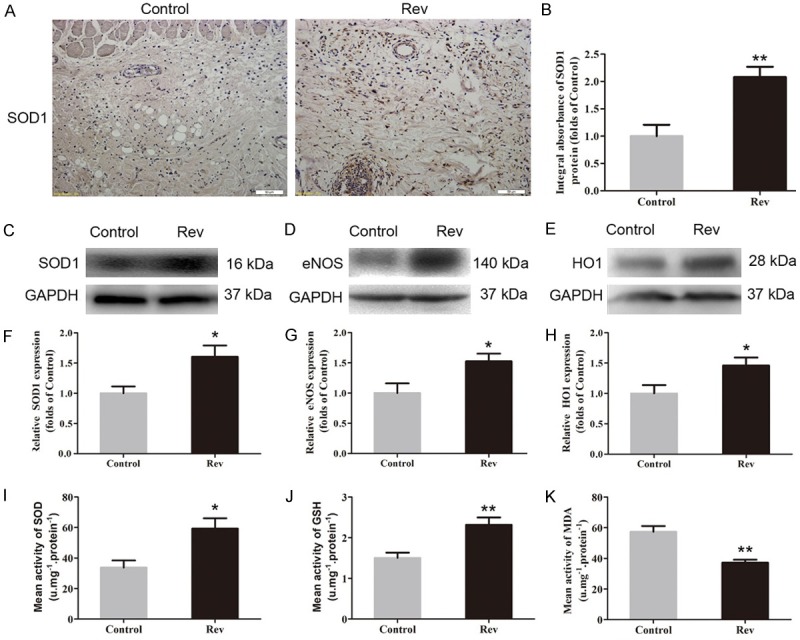
Resveratrol attenuated oxidative stress in ischemic area of the flaps. The oxidative stress in flap area II was assessed by IHC for SOD1, Western blotting for SOD1, eNOS and HO1, and assays for SOD, GSH and MDA activities on day 7. A. IHC for SOD1 expression in area II of random skin flaps in the Control and Rev groups (original magnification, × 200; scan bar, 100 μm). B. Optical density values of SOD1 expression were quantified and analyzed in each group. C-E. Western blotting for the expression of SOD1, eNOS and HO1 in Area II flaps in the Control group and the Rev group. The gels were run under the same experimental conditions, and cropped blots are used here. The Original images are available in Figure S1C. F-H. Optical density values of the expression of SOD1, eNOS and HO1 were quantified and analyzed in each group. I-K. Values of SOD activities, GSH levels and MDA content in flap area II of each group via xanthine oxidase method, the modified 5,5’-dithiobis method and the modified TBA test were quantified and analyzed, respectively. Significance: *P < 0.05 and **P < 0.01 vs. the Control group. Data were expressed as means ± SEM, n = 6 per group.
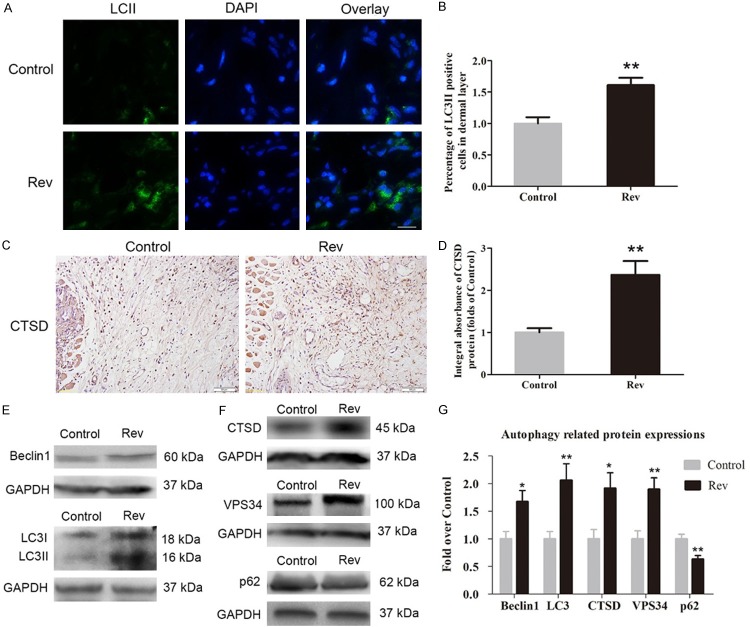
Resveratrol upregulated the level of autophagy in ischemic area of the flaps
Immunofluorescence for LC3II was carried out to display autophagosomes in cells in Area II of flaps. As shown in Figure 5A, autophagosomes were labeled as LC3II punctate dots (green), and nuclei were labeled with DAPI (blue) in the Rev and Control groups (Figure 5A). The densities of LC3II-positive cells in the dermis in the Rev group was much higher than that in the Control group (P = 0.003, Figure 5B). IHC for CTSD was performed to detect lysosome activity in cells in Area II of flaps. As shown in (Figure 5C), higher level of CTSD was observed in vessels and stromal cells of the Rev group, compared with the Control group. The integral absorbance of CTSD was significantly higher in the Rev group than in the Control group (Figure 5D; P = 0.003). Western blotting was also performed for Beclin1, LC3II, VPS34, CTSD and p62 expressions in each group (Figure 5E, 5F). As a result, optical density values of Beclin1, LC3II, VPS34, CTSD were significantly higher in the Rev group than in the Control group (P = 0.020, 0.009, 0.005 and 0.019, respectively), with a lower level of p62 in the Rev group (P = 0.007; Figure 5G).
Figure 5.
Resveratrol upregulated the level of autophagy in ischemic area of the flaps. On day 7, the level of autophagy in area II of flap was assessed by immunofluorescence for LC3II, IHC for CTSD, Western blotting for Beclin1, LC3II/I, CTSD, VPS34 and p62. A. Immunofluorescence for LC3II punctate dots in random skin flaps of the Control group and Rev group. Autophagosomes were labeled with LC3II (green), nuclei were labeled with DAPI (blue) (scale bar, 15 μm). B. LC3II-positive cell densities were quantified and analyzed in ischemic area in each group. C. IHC for CTSD expression in area II of flap in the Control group and Rev group (original magnification, × 200; scan bar, 50 μm). D. Optical density values of CTSD expression were quantified and analyzed in each group. E, F. Western blotting was performed for Beclin1, LC3II/I CTSD, VPS34 and p62 in the Area II tissue in the Control and Rev groups. The gels were run under the same experimental conditions, and cropped blots are used here. The Original images are available in Figure S1D. G. Optical density values of the expression of Beclin1, LC3II, CTSD, vps34 and p62 were quantified and analyzed in each group. Significance: *P < 0.05 and **P < 0.01 vs. the Control group. Data were expressed as means ± SEM, n = 6 per group.
Discussion
Rev, a type of polyphenolic phytoalexin, occurs naturally in numerous plant species and in red wine [15,24]. This agent has received a great deal of attention because many studies have highlighted its benefits in various human disease models, such as cardio- and neuroprotection, cancer prevention and therapy, and immune regulation [25-28]. It is also commonly used to treat ischemic heart disease because it enhances neovascularization and inhibits oxidative stress and apoptosis [25]. Random skin flap necrosis is a common complication after reconstructive or plastic surgery owing to poor blood supply in the distal parts of flaps. Therefore, Rev may be a potential therapeutic agent to improve the survival of random skin flaps. However, few studies have highlighted the therapeutic functions of Rev in the context of random-pattern skin flap transplantation. The present study demonstrated that Rev significantly improves the survival of such flaps. Moreover, its relative pharmacological mechanism involves enhancing angiogenesis, reducing oxidative stress and apoptosis, and activating autophagy.
Rev enhances the expression of VEGF and promotes angiogenesis in human endothelial cells in vitro [17]. Similarly, it has pro-angiogenic effects in an infarcted rat myocardium model [18]. Random skin flap survival can be enhanced by improving angiogenesis, accompanied with increased blood supply. In the present study, the mean vessel density as observed by H&E staining demonstrated that Rev promotes angiogenesis in random skin flaps, and CD34 expression assessed by IHC showed the same result. Furthermore, IHC indicated that Rev upregulates the expression of VEGF and Cadherin5 in the dermal layer of ischemic flaps. Western blotting also showed increased levels of VEGF, MMP9, and Cadherin5 in the flaps of the Rev-treated group. Angiogenesis involves the destruction of preexisting cell connections, mitosis, sprouting of endothelial cells, and maturation of new capillaries [29]. MMP9 degrades proteins related to vessel wall stability [30], VEGF is responsible for inducing mitosis in endothelial cells [31], and Cadherin5 is secreted to form intercellular junctions. In short, we hypothesize that Rev enhances angiogenesis in random skin flaps by enhancing MMP9, VEGF, and Cadherin5 expression.
Tissue damage and necrosis are partly caused by IRI. When the blood supply is restored after angiogenesis, oxygen returns to the ischemic flap, producing an excess of oxygen free radicals (OFRs), resulting in oxidative stress and apoptosis [32]. In the process of oxidative stress, the interaction between OFRs and lipids in the cell membrane leads to lipid peroxidation, followed by the destruction of cells and tissues. MDA, a product of lipid peroxidation, can indicate the level of oxidative stress [33]. On the other hand, bodies synthesize more antioxidant substances such as GSH and superoxide dismutase (SOD, including SOD1, to ameliorate injury during the oxidative stress response [34]. eNOS and HO1 enzymes are also induced and show antioxidant activity [35]. Rev depresses oxidative stress via the PI3K/Nrf2/HO1 signaling pathway, protecting tissues from acute lung injury in a septic rat model [19]. In our study, IHC results demonstrated that Rev improves SOD1 expression in the dermal layer of ischemic flaps. Western blotting showed that Rev treatment upregulates the levels of SOD1, eNOS, and HO1 in random skin flaps. Furthermore, it enhances the expression of GSH and SOD and reduces MDA levels in ischemic flaps. These results suggest that Rev inhibits oxidative stress in random skin flap.
Previous studies have shown that Rev alleviates apoptosis in infarcted rat myocardium and radiation-induced intestinal injury [18]. Thus, we investigated whether it promotes random skin flap survival by inhibiting apoptosis. The swelling and dysfunction of mitochondria play a crucial role in apoptosis [36]. In the process of apoptosis, permeabilization of the mitochondrial outer membranes is obviously increased by the actions of Bax, leading to mitochondrial swelling [37]. Then CYC is released from the mitochondria and helps to form the apoptosome, which activates caspase-3 through a cascade reaction [36]. CASP3 plays a role as an apoptosis executor. In the present study, Bax, CYC, and CASP3 expression levels were determined to analyze the level of apoptosis. IHC results showed that CASP3 expression in ischemic flaps markedly decreased in the Rev group. Western blotting indicated that Rev treatment obviously inhibited the expression of Bax, CYC, and CASP3 compared to the Control group. Therefore, we conclude that Rev alleviates apoptosis in random skin flap.
Recent studies have reported that Rev has cell protective properties by enhancing autophagy, such as increasing the degradation of amyloid-β peptides and then alleviating liver IRI [38]. Autophagy is a highly conserved eukaryotic cellular recycling process for the degradation of cytosolic macromolecules and organelles [11]. Through the circulation of breakdown products in cells and tissues, autophagy plays a vital role in cell survival and protection against metabolic stress [39]. Growing evidence has demonstrated that autophagy promotes survival in vascular disease [45]. For example, autophagy ameliorates cerebral small blood vessel disease and acute myocardial infarction by inhibiting oxidative stress and apoptosis or promoting angiogenesis [39,46]. Rev has been demonstrated to upregulate autophagy levels [40]. Therefore, we evaluated the level of autophagy in random skin flaps after Rev treatment. Autophagy is divided into three steps: autophagosome formation, autolysosome formation, and substrate degradation. Thus, we detected Beclin1, LC3II, and VPS34, which participate in autophagosome formation [41]; CTSD, a marker of autolysosomes [42]; and p62, a product of autophagic degradation [43]. Immunofluorescence showed that LC3II expression increased after Rev treatment. IHC showed that CTSD was more highly expressed after Rev treatment. Moreover, Western blotting showed that Beclin1, LC3II, and VPS34 levels were increased, which indicates an increase in autophagosomes in ischemic skin flaps. Moreover, the expression of CTSD was greater in ischemic tissue from the Rev group, whereas that of p62 was lower, which indicates that autophagic flux was promoted by Rev treatment. Therefore, Rev activates autophagy in random skin flap.
However, the effects of autophagy on cell survival are a “double-edged sword”, as autophagy protects cells in some diseases but serves as a mechanism of programmed cell death in other pathophysiological processes [44]. Therefore, whether Rev promotes the survival of random skin flaps by promoting autophagy remains uncertain. According to previous studies, when OFRs are produced by damaged mitochondria under oxidative stress, autophagy is activated to degrade the mitochondria and OFRs, increasing cell survival. On the other hand, autophagy inhibits apoptosis by reducing the diffusion of cytochrome and apoptosis-induced factors, by separating mitochondria, which also ameliorates tissue necrosis. Our results indicate that autophagy improvement was accompanied by the reduction of oxidative stress and apoptosis in the Rev group. Thus, we suggest that Rev may depress oxidative stress and apoptosis by promoting autophagy in random skin flaps. However, autophagy was also found to be a mechanism of programmed cell death, termed autophagic cell death. Therefore, the role of the activated autophagy by Rev should be confirmed in further investigation. For this point, autophagy inhibitor, such as 3-Methyladenine (3MA) or Chloroquine (CQ), can be used in combination with the treatment of Rev for the flap.
In conclusion, Rev promotes the survival of random-pattern skin flaps by enhancing angiogenesis and inhibiting oxidative stress and apoptosis. Moreover, autophagy is activated in the process, which might be another underlying mechanism for the flap survival.
Acknowledgements
This work was supported by grants from Natural Science Foundation of China (No. 81601705 to Kailiang Zhou, No. 81801930 to Jian Ding, No. 81572227, and No. 81873992 to Huazi Xu); Zhejiang Provincial Medicine and Health Technology Project (No. 2017KY472 to Kailiang Zhou); Wenzhou Science and Technology Bureau Foundation (No. 2016Y0350 to Jian Ding, No. 2015Y 0060 to Shi Li).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Lu F, Mizuno H, Uysal CA, Cai X, Ogawa R, Hyakusoku H. Improved viability of random pattern skin flaps through the use of adipose-derived stem cells. Plast Reconstr Surg. 2008;121:50–58. doi: 10.1097/01.prs.0000293876.10700.b8. [DOI] [PubMed] [Google Scholar]
- 2.Cherry GW, Austad E, Pasyk K, McClatchey K, Rohrich RJ. Increased survival and vascularity of random-pattern skin flaps elevated in controlled, expanded skin. Plast Reconstr Surg. 1983;72:680–687. doi: 10.1097/00006534-198311000-00018. [DOI] [PubMed] [Google Scholar]
- 3.Yan X, Zeng B, Chai Y, Luo C, Li X. Improvement of blood flow, expression of nitric oxide, and vascular endothelial growth factor by low-energy shockwave therapy in random-pattern skin flap model. Ann Plast Surg. 2008;61:646–653. doi: 10.1097/SAP.0b013e318172ba1f. [DOI] [PubMed] [Google Scholar]
- 4.Yang M, Sheng L, Li H, Weng R, Li QF. Improvement of the skin flap survival with the bone marrow-derived mononuclear cells transplantation in a rat model. Microsurgery. 2010;30:275–281. doi: 10.1002/micr.20779. [DOI] [PubMed] [Google Scholar]
- 5.Karaçal N, Ambarcioğlu Ö, Topal U, Mamedov T, Kutlu N. Enhancement of dorsal random-pattern skin flap survival in rats with topical lidocaine and prilocaine (EMLA): enhancement of flap survival by EMLA. J Surg Res. 2005;124:134–138. doi: 10.1016/j.jss.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 6.Mittermayr R, Hartinger J, Antonic V, Meinl A, Pfeifer S, Stojadinovic A, Schaden W, Redl H. Extracorporeal shock wave therapy (ESWT) minimizes ischemic tissue necrosis irrespective of application time and promotes tissue revascularization by stimulating angiogenesis. Ann Surg. 2011;253:1024–1032. doi: 10.1097/SLA.0b013e3182121d6e. [DOI] [PubMed] [Google Scholar]
- 7.Bayati S, Russell RC, Roth AC. Stimulation of angiogenesis to improve the viability of prefabricated flaps. Plast Reconstr Surg. 1998;101:1290–1295. doi: 10.1097/00006534-199804050-00020. [DOI] [PubMed] [Google Scholar]
- 8.Kaminski KA, Bonda TA, Korecki J, Musial WJ. Oxidative stress and neutrophil activation-the two keystones of ischemia/reperfusion injury. Int J Cardiol. 2002;86:41–59. doi: 10.1016/s0167-5273(02)00189-4. [DOI] [PubMed] [Google Scholar]
- 9.Gujral JS, Bucci TJ, Farhood A, Jaeschke H. Mechanism of cell death during warm hepatic ischemia-reperfusion in rats: apoptosis or necrosis? Hepatology. 2001;33:397–405. doi: 10.1053/jhep.2001.22002. [DOI] [PubMed] [Google Scholar]
- 10.van den Heuvel MG, Buurman WA, Bast A, van der Hulst RR. Review: ischaemia-reperfusion injury in flap surgery. J Plast Reconstr Aesthet Surg. 2009;62:721–726. doi: 10.1016/j.bjps.2009.01.060. [DOI] [PubMed] [Google Scholar]
- 11.Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal. 2014;20:460–473. doi: 10.1089/ars.2013.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sachdev U, Lotze MT. Perpetual change: autophagy, the endothelium, and response to vascular injury. J Leukoc Biol. 2017;102:221–235. doi: 10.1189/jlb.3RU1116-484RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Ni Q, Ye Q, Liu F, Fu Z, Wang Q. Tanshinone IIA activates autophagy to reduce liver ischemia-reperfusion injury by MEK/ERK/mTOR pathway. Pharmazie. 2018;73:396–401. doi: 10.1691/ph.2018.7509. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Kl, Zhang YH, Lin DS, Tao XY, Xu HZ. Effects of calcitriol on random skin flap survival in rats. Sci Rep. 2016;6:18945. doi: 10.1038/srep18945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamaleddin MA. The paradoxical pro- and antiangiogenic actions of resveratrol: therapeutic applications in cancer and diabetes. Ann N Y Acad Sci. 2016;1386:3–15. doi: 10.1111/nyas.13283. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Zhou H, Zou Y, Liu Q, Guo C, Gao G, Shao C, Gong Y. Resveratrol modulates angiogenesis through the GSK3β/β-catenin/TCF-dependent pathway in human endothelial cells. Biochem Pharmacol. 2010;80:1386–1395. doi: 10.1016/j.bcp.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 18.Kaga S, Zhan L, Matsumoto M, Maulik N. Resveratrol enhances neovascularization in the infarcted rat myocardium through the induction of thioredoxin-1, heme oxygenase-1 and vascular endothelial growth factor. J Mol Cell Cardiol. 2005;39:813–822. doi: 10.1016/j.yjmcc.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Wang X, Zhang L, Zhang R. Alleviation of acute lung injury in rats with sepsis by resveratrol via the phosphatidylinositol 3-Kinase/nuclear factor-erythroid 2 related factor 2/Heme Oxygenase-1 (PI3K/Nrf2/HO-1) pathway. Med Sci Monit. 2018;24:3604. doi: 10.12659/MSM.910245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly CP, Gupta A, Keskin M, Jackson IT. A new design of a dorsal flap in the rat to study skin necrosis and its prevention. J Plast Reconstr Aesthet Surg. 2010;63:1553–1556. doi: 10.1016/j.bjps.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Kuo YR, Wu WS, Hsieh YL, Wang FS, Wang CT, Chiang YC, Wang CJ. Extracorporeal shock wave enhanced extended skin flap tissue survival via increase of topical blood perfusion and associated with suppression of tissue pro-inflammation. J Surg Res. 2007;143:385–392. doi: 10.1016/j.jss.2006.12.552. [DOI] [PubMed] [Google Scholar]
- 22.Abraham A, Alabdali M, Alsulaiman A, Breiner A, Barnett C, Katzberg HD, Lovblom LE, Perkins BA, Bril V. Laser doppler flare imaging and quantitative thermal thresholds testing performance in small and mixed fiber neuropathies. PLoS One. 2016;11:e0165731. doi: 10.1371/journal.pone.0165731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campos-Junior PH, Alves TJM, Dias MT, Assunçao CM, Munk M, Mattos MS, Kraemer LR, Almeida BG, Russo RC, Barcelos L. Ovarian grafts 10 days after xenotransplantation: folliculogenesis and recovery of viable oocytes. PLoS One. 2016;11:e0158109. doi: 10.1371/journal.pone.0158109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 25.Hao HD, He LR. Mechanisms of cardiovascular protection by resveratrol. J Med Food. 2004;7:290–298. doi: 10.1089/jmf.2004.7.290. [DOI] [PubMed] [Google Scholar]
- 26.Ates O, Cayli S, Altinoz E, Gurses I, Yucel N, Sener M, Kocak A, Yologlu S. Neuroprotection by resveratrol against traumatic brain injury in rats. Mol Cell Biochem. 2007;294:137–144. doi: 10.1007/s11010-006-9253-0. [DOI] [PubMed] [Google Scholar]
- 27.Athar M, Back JH, Tang X, Kim KH, Kopelovich L, Bickers DR, Kim AL. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol. 2007;224:274–283. doi: 10.1016/j.taap.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng YH, Zhou WL, Wu QL, Li XY, Zhao WM, Zou JP. Low dose of resveratrol enhanced immune response of mice. Acta Pharmacol Sin. 2002;23:893–897. [PubMed] [Google Scholar]
- 29.Nowak-Sliwinska P, Alitalo K, Allen E, Anisimov A, Aplin AC, Auerbach R, Augustin HG, Bates DO, van Beijnum JR, Bender RH. Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis. 2018:1–108. doi: 10.1007/s10456-018-9613-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, Wu C, Shen Y, Wang K, Tang L, Zhou M, Yang M, Pan T, Liu X, Xu W. Ten-eleven translocation 2 demethylates the MMP9 promoter, and its down-regulation in preeclampsia impairs trophoblast migration and invasion. J Biol Chem. 2018;293:10059–10070. doi: 10.1074/jbc.RA117.001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stacker SA, Halford MM, Roufail S, Caesar C, Achen MG. A simple bioassay for the evaluation of vascular endothelial growth factors. J Vis Exp. 2016 doi: 10.3791/53867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koh YH, Park YS, Takahashi M, Suzuki K, Taniguchi N. Aldehyde reductase gene expression by lipid peroxidation end products, MDA and HNE. Free Radic Res. 2000;33:739–746. doi: 10.1080/10715760000301261. [DOI] [PubMed] [Google Scholar]
- 33.Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal Biochem. 2017;524:13–30. doi: 10.1016/j.ab.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 34.He F, Li J, Liu Z, Chuang CC, Yang W, Zuo L. Redox mechanism of reactive oxygen species in exercise. Front Physiol. 2016;7:486. doi: 10.3389/fphys.2016.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun MS, Jin H, Sun X, Huang S, Zhang FL, Guo ZN, Yang Y. Free radical damage in ischemia-reperfusion injury: an obstacle in acute ischemic stroke after revascularization therapy. Oxid Med Cell Longev. 2018;2018:3804979. doi: 10.1155/2018/3804979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei B, Lin Q, Ji YG, Zhao YC, Ding LN, Zhou WJ, Zhang LH, Gao CY, Zhao W. Luteolin ameliorates rat myocardial ischaemia-reperfusion injury through activation of peroxiredoxin II. Br J Pharmacol. 2018 doi: 10.1111/bph.14367. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Kl, Zhou YF, Wu K, Tian NF, Wu YS, Wang Yl, Chen DH, Zhou B, Wang XY, Xu HZ. Stimulation of autophagy promotes functional recovery in diabetic rats with spinal cord injury. Sci Rep. 2015;5:17130. doi: 10.1038/srep17130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu Y, Wang J. Wogonin increases β-amyloid clearance and inhibits tau phosphorylation via inhibition of mammalian target of rapamycin: potential drug to treat Alzheimer’s disease. Neurol Sci. 2015;36:1181–1188. doi: 10.1007/s10072-015-2070-z. [DOI] [PubMed] [Google Scholar]
- 39.Lin C, Liu Z, Lu Y, Yao Y, Zhang Y, Ma Z, Kuai M, Sun X, Sun S, Jing Y. Cardioprotective effect of Salvianolic acid B on acute myocardial infarction by promoting autophagy and neovascularization and inhibiting apoptosis. J Pharm Pharmacol. 2016;68:941–952. doi: 10.1111/jphp.12567. [DOI] [PubMed] [Google Scholar]
- 40.Liu C, Shi Z, Fan L, Zhang C, Wang K, Wang B. Resveratrol improves neuron protection and functional recovery in rat model of spinal cord injury. Brain Res. 2011;1374:100–109. doi: 10.1016/j.brainres.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 41.Wu P, Yuan X, Li F, Zhang J, Zhu W, Wei M, Li J, Wang X. Myocardial upregulation of cathepsin D by ischemic heart disease promotes autophagic flux and protects against cardiac remodeling and heart failure. Circ Heart Fail. 2017;10:e004044. doi: 10.1161/CIRCHEARTFAILURE.117.004044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang Y, Chen Y, Jiang H, Nie D. Short-chain fatty acids induced autophagy serves as an adaptive strategy for retarding mitochondria-mediated apoptotic cell death. Cell Death Differ. 2011;18:602. doi: 10.1038/cdd.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zaffagnini G, Savova A, Danieli A, Romanov J, Tremel S, Ebner M, Peterbauer T, Sztacho M, Trapannone R, Tarafder AK. p62 filaments capture and present ubiquitinated cargos for autophagy. EMBO J. 2018:e98308. doi: 10.15252/embj.201798308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li D, Wang J, Hou J, Fu J, Liu J, Lin R. Salvianolic acid B induced upregulation of miR-30a protects cardiac myocytes from ischemia/reperfusion injury. BMC Complement Altern Med. 2016;16:336. doi: 10.1186/s12906-016-1275-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han J, Pan XY, Xu Y, Xiao Y. Curcumin induces autophagy to protect vascular endothelial cell survival from oxidative stress damage. Autophagy. 2012;8:812–825. doi: 10.4161/auto.19471. [DOI] [PubMed] [Google Scholar]
- 46.Guan ZF, Tao YH, Zhang XM, Guo QL. G-CSF and cognitive dysfunction in elderly diabetic mice with cerebral small vessel disease: preventive intervention effects and underlying mechanisms. CNS Neurosci Ther. 2017;23:462–474. doi: 10.1111/cns.12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.