Abstract
Distant metastasis is the main cause of death for non-small cell lung cancer (NSCLC) patients, with the bone as a more frequent metastatic site. The expression of various chemokines and their receptors may contribute to the predilection of organ specific metastasis. In this study, we demonstrated that CC chemokine ligand 7 (CCL7) and its receptors CCR1, CCR2 and CCR3 were up-regulated markedly in lung cancer bone metastasis. In addition, CCL7 promoted migration and invasion of lung cancer cells in a dose-dependent pattern but played an insignificant role in cell proliferation mainly through CCR3. Finally, we identified a cohort of critical downstream genes of CCL7 related to cancer metastasis using Illumina deep mRNA sequencing. These findings may help better understand the molecular aspects of bone metastasis in lung cancer, and prefigure a potential clinical value for the prevention of bone recurrences.
Keywords: CCL7, non-small cell lung cancer (NSCLC), migration, invasion, bone metastasis
Introduction
Lung cancer is one of the most common malignant tumors and the leading cause of cancer-related death. The 5-year survival of lung cancer patients is about 16.6% [1,2]. Approximate 85% lung cancer cases are non-small cell lung cancer (NSCLC), whose histopathological types typically consist of adenocarcinoma, squamous cell carcinoma and large cell carcinoma [3]. Bone metastasis occurs in more than 40% NSCLC patients, causing serious consequences in terms of mobility and mortality [4]. Patients with bone metastasis often experience severe pain, pathologic bone fracture, spinal cord compression, and hypercalcemia, which entail poor quality of life, decrease the survival rate, and increase health care costs substantially [5]. However, the pathological mechanism underlying bone metastasis in lung cancer remains poorly understood.
Chemokine ligands (CCLs) belong to the small molecular-weight chemoattractive cytokine family and have been shown to play an important role in tumor biology by affecting tumor growth, invasion and metastasis [6-8]. According to the location of the first two cysteine residues, CCLs are mainly classified as C-C motif (CC) and C-X-C motif (CXC) subfamilies. Chemokine C-C motif ligand 7 (CCL7) is a member of the CC chemokine ligand family. It has been shown to be highly expressed in advanced breast, renal, gastric and colorectal cancers and squamous cells and may play a key regulatory role in cancer invasiveness and migration [7-11]. Meanwhile, CCL7 also has a wide spectrum of activities on different immune cells, including monocytes, T cells, NK cells, basophils, eosinophils, and neutrophils [10,12,13]. However, the role of CCL7 in lung cancer has been rarely reported before, and the downstream pathway of CCL7 remains unclear.
The aim of the present study was to detect mRNA and protein levels of CCL7 and its receptors CCR1, CCR2 and CCR3 in clinical samples of lung cancer bone metastasis and primary lung cancer, in an attempt to clarify the role of CCL7 in lung cancer cell proliferation, migration and invasion, and further explore the possible downstream of CCL7 in lung cancer cells using Illumina deep mRNA sequencing.
Materials and methods
Patients and specimens
Forty normal lung tissue specimens, 40 primary NSCLC tissue specimens and 40 lung cancer bone metastasis tissue specimens were collected from lung cancer patients who received surgical resection in Changzheng Hospital of the Second Military Medical University (Shanghai, China). Written informed consent was obtained before sample collection. The study protocol was approved by the Institutional Review Board of the said university. The tissue specimens were divided into two: one was snap-frozen and stored in liquid nitrogen, and the other was fixed in formalin and paraffin embedded. All the sample treatment procedures were completed within 2 hours after surgical excision.
Cell lines and culture
Human lung cancer cell lines PC9 and A549 were maintained in RPMI-1640 (Gibco, USA) and DMEM (Gibco) respectively, and both supplemented with 10% fetal bovine serum (FBS, Gibco). Cells were maintained in a humid atmosphere with 5% CO2 at 37°C.
RNA extraction and quantitative reverse transcription PCR (qRT-PCR)
Total RNA was extracted using Trizol reagent (Life Technologies, USA), and the first strand cDNA was generated by the Reverse Transcription System (Promega, USA). qRT-PCR for mRNA was performed by TransStart Top Green qPCRSuperMix (TransGen Biotech, China) on 7900HT Fast Real-Time PCR System (Life Technologies Corporation, USA). All PCR primers are listed in Supplementary Table 1.
Tissue microarray and immunohistochemistry (IHC)
The collected specimens were made into tissue microarray and stained by IHC using an indirect immunoperoxidase technique, with the antibodies against CCL7 (AV07048, Sigma, USA), CCR1 (DF2710, Affinity, USA), CCR2 (DF7507, Affinity) and CCR3 (DF10205, Affinity). The IHC stains were assessed independently by three observers who were blind about the specimen characteristics. The expression was recorded after evaluating the staining intensity of positive cells. Results were analyzed by standard light microscopy.
Cell proliferation assay
Cells were seeded in 96-well plates at an initial density of 5 × 103 cells/well. After 12 h, CCL7 protein (Peprotech, USA) was added into the medium and marked as time 0 h. After 12, 24, 48 and 72 h, cell-titer aqueous one proliferation reagent (Bimake, USA) was added to each well and the absorbance was recorded at 450 nm and a standard curve was constructed for each, enabling the cell number to be determined. All the experiments were performed in triplicates.
Cell invasion assay
Cells (1 × 105/well) were suspended in 150 µl FBS free medium and placed in the upper transwell chamber (8 mm pore size with matrigel; 354480, Corning, the Netherlands). The upper chamber was placed in a 24-well culture dish containing 500 µl medium containing 10% FBS with or without CCL7. After 24-h incubation at 37°C, non-migrated cells on the upper membrane were removed with a cotton swab. Migrated cells on the bottom surface were fixed with 4% polyoxymethylene and stained with 0.1% crystal violet. The number of migrated cells was counted. Then the crystal violet was dissolved by acetic acid elution and the absorbance was recorded at 570 nm. All the experiments were performed in triplicates.
Western blot
Whole-cell lysates from PC9 and A549 cells were prepared and 50 μg protein was separated using 10% SDS-PAGE gel. After blocking with TBST containing 3% bovine serum albumin (BSA, Sigma, St. Louis, MO), blots were incubated at 4°C overnight with the primary antibodies against human CCR1 (1:1000), CCR2 (1:1000), CCR3 (1:1000), EPCAM (1:1000, 21050-1-AP, Proteintech, USA), VIM (1:1000, AF0292, Affinity), CD324 (1:1000, AF0131, Affinity), SNAIL (1:1000, AF6032, Affinity), TWIST (1:1000, AF4009, Affinity) and β-actin (1:2000, AF7018, Affinity) respectively. A horseradish peroxidase conjugated IgG was used as secondary antibody according to manufacturer’s instruction. The density of the band of interest was measured using densitometric measurement with Image J (NIH) and normalized to β-actin.
shRNA transfection
CCR3 shRNA and shRNA control plasmids were purchased from GeneChem (Shanghai, China). A total of 2 × 105 cells were plated in 6-well plates, and transfected using DNA Transfection Reagent (biotool, USA) when they grew to 60% confluence according to the instructions.
Bioluminescence imaging
In vivo distant-organ metastasis was monitored by bioluminescent imaging (Caliper Life Sciences, Hopkinton, MA). Briefly, to detect metastasis of A549 cancer cells stably expressing luciferase and infected with a control vector or sh-CCR3 lentivirus at indicated days, D-luciferin potassium salt (AOK Chem, Shanghai) was injected intraperitoneally into the anesthetized mice at a dose of 150 mg/kg. After 10 min, the mice were placed into the IVIS Imaging System. Imagines were recorded with an exposure time of 5 min. Bioluminescence was assayed and photons per second were quantified using software (Living Image 3.2, Caliper).
Illumina deep mRNA sequencing
PC9 cells were harvested after 48-h treatment with recombinant CCL7 (100 nmol/L) and the untreated PC9 cells were used as control. Total RNA was extracted from the cells using TRIzol® Reagent according the manufacturer’s instructions (Invitrogen), and subjected to Illumina deep mRNA sequencing using a service from LC Sciences (Houston, TX, USA). The sequence results were obtained as the FPKM (fragment per kilobase of exons per million reads) for each transcript.
Statistic analysis
SPSS 19.0 statistical software (SPSS Inc., Chicago, IL) was used for statistical analysis. All data are presented as mean ± standard error of the mean (SEM). Statistics were assessed using both Student t test and ANOVA, assuming double-sided independent variance. All experiments were repeated at least three times, and representative experiments are shown. P values of < 0.05 were considered statistically significant.
Results
CCL7 is overexpressed in lung cancer bone metastasis and negatively correlated with the clinical outcome of NSCLC patients
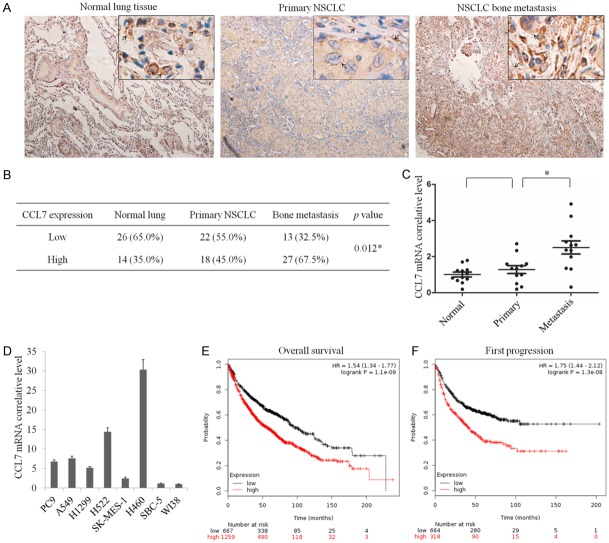
Recent data have shown that chemokines such as CCL7 support the metastasis of colorectal cancer to the liver. However, there is no study reporting the expression of CCL7 in NSCLC. In this study, we identified the protein level of CCL7 in normal lung tissue, primary NSCLC and bone metastasis tissue specimens by IHC staining. The results showed that the expression of CCL7 showed no clear fluctuation between normal lung and primary NSCLC tissues, but CCL7 was obviously overexpressed in bone metastasis specimens compared with the other two groups (Figure 1A and 1B). The mRNA level of CCL7 was examined by qRT-PCR in 12 normal lung tissue, 12 primary NSCLC and 12 bone metastasis tissue specimens. The result showed a similar change in protein level in lung cancer bone metastasis (Figure 1C). Further analysis of qRT-PCR assay exhibited that compared with normal lung fibroblast cells (WI38), the mRNA level of CCL7 was evaluated in squamous carcinoma cells (SK-MES-1), adenocarcinoma cells (PC9, A549, H1299, H522) and large cell lung cancer cells (H460), but not in small cell lung cancer cells (SBC-5) (Figure 1D). To further elucidate the relationship between CCL7 and the clinical outcome of NSCLC patients, we analyzed CCL7 mRNA expression level and the first progression (FP) and overall survival (OS) of NSCLC patients from 2437 lung tumor samples using publicly available datasets (http://kmplot.com). The Kaplan-Meier analyses demonstrated that high CCL7 mRNA expression had a significantly positive correlation with poor OS and FP in NSCLC patients (Figure 1E, 1F).
Figure 1.
CCL7 expression is negatively correlated with the clinical outcome of non-small cell lung cancer (NSCLC) patients. A. Immunohistochemical detection of CCL7 expression in NSCLC bone metastasis, primary NSCLC and normal lung tissues (n = 40). B. Statistics of all samples associated with CCL7 staining. C. qRT-PCR assay of mRNA level of CCL7 in NSCLC bone metastasis, primary NSCLC and normal lung tissues. D. qRT-PCR assay of mRNA level of CCL7 in various lung cancer cell lines. E, F. High CCL7 mRNA expression is positively correlated with poor overall survival (OS) and progression-free survival (FPS) in NSCLC patients. *: P < 0.05.
The expression of CCR1, CCR2 and CCR3 is up-regulated in bone metastasis compared with primary lung cancer
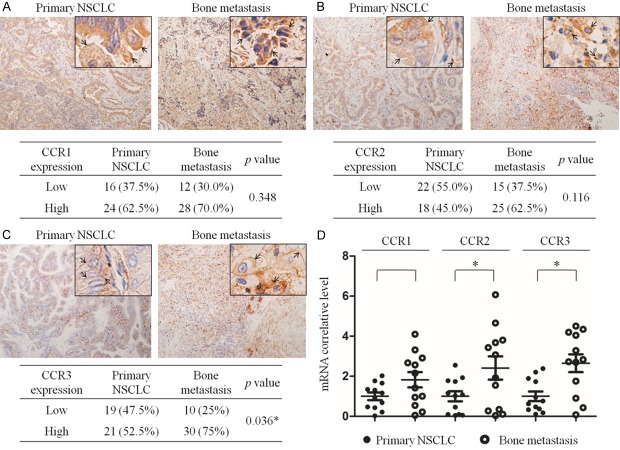
CCL7 is known to act through specific receptors CCR1, CCR2 and CCR3. Thus, IHC staining and qRT-PCR were used to analyze the expression and mRNA levels of CCR1, CCR2 and CCR3 in primary NSCLC and bone metastasis specimens. The results revealed that all the expressions of CCR1, CCR2 and CCR3 in bone metastasis tissues were significantly higher than those in the primary NSCLC tissues, while the mRNA levels of CCR1, CCR2 and CCR3 were also up-regulated in NSCLC bone metastasis (Figure 2). By using publicly available datasets (http://kmplot.com), we found that low mRNA level of CCR2 and CCR3 slightly increased the OS rate and obviously improved free surviva l(FS) of NSCLC patients, but CCR1 mRNA level showed a weak positive correlation with OS and no clear correlation with FS (Supplementary Figure 1A-F).
Figure 2.
Expression of possible target chemokine receptors of CCL7 in primary NSCLC and bone metastasis tissues. A-C. IHC detection of CCR1, CCR2 and CCR3 expression in primary NSCLC and bone metastasis tissues. CCR1, CCR2 and CCR3 were mainly expressed on the cell membrane and in cytoplasm (arrow). D. qRT-PCR measurement of mRNA levels of CCR1, CCR2, CCR3 in primary NSCLC and bone metastasis tissues. *: P < 0.05.
CCL7 plays an insignificant role in lung cancer cell proliferation
To examine the role of CCL7 in lung cancer cells, we stimulated PC9 and A549 cells with different concentrations of recombinant CCL7 (0, 100 and 200 nmol/L) for 72 h and detected the cell proliferation rate by using the CCK8 assay. The results showed that there was no significant difference in absorbance between the three concentration groups at any designated time point (0, 12, 24, 48 and 72 h) (Supplementary Figure 2A and 2B), indicating that CCL7 did little to enhance the growth in either PC9 or A549 cells.
CCL7 significantly increases migration and invasion of lung cancer cells
To further explore the function of CCL7 in lung cancer, we analyzed the cell migration by using scratch assay and stimulated PC9 cells with different concentrations of recombinant CCL7 (0, 100 and 200 nmol/L) for 48 h. The results showed that the cell migration ability of both PC9 and A549 was significantly enhanced and had a positive correlation with the amount of CCL7 (Figure 3A and 3B). Transwell assay was performed to examine the role of CCL7 in lung cancer cell invasion. The result showed that CCL7 significantly increased the migration of PC9 and A549 cells in a dose-dependent manner. Meanwhile, the results of acetic acid elution and absorbance detection showed a similar fluctuation in the number of migrating cells (Figure 3C).
Figure 3.
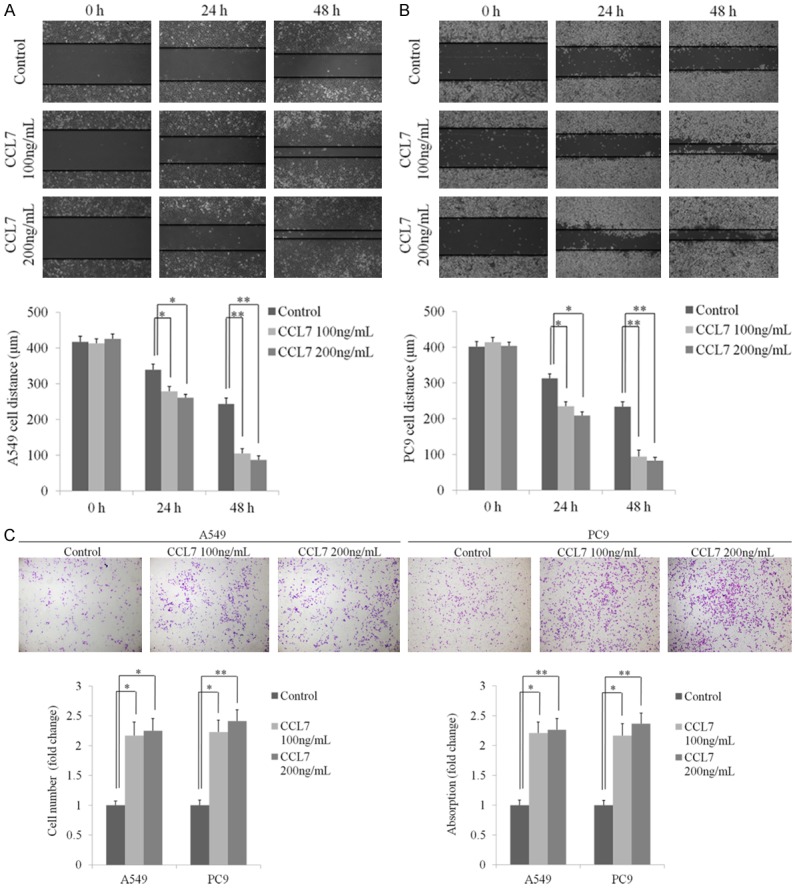
CCL7 promotes migration and invasion of NSCLC cells. A, B. Representative images and statistical analysis of wound healing assay of A549 and PC9 cells incubated with recombinant CCL7 (100 ng/ml, 200 ng/ml) at 24 and 48 h. C. Pictures and bar graphs of transwell invasion assay showing increased invasion of A549 and PC9 cells after treatment with recombinant CCL7 (100 ng/ml, 200 ng/ml). *: P < 0.05; **: P < 0.001.
CCL7 promotes migration and invasion of A549 and PC9 cells via CCR3
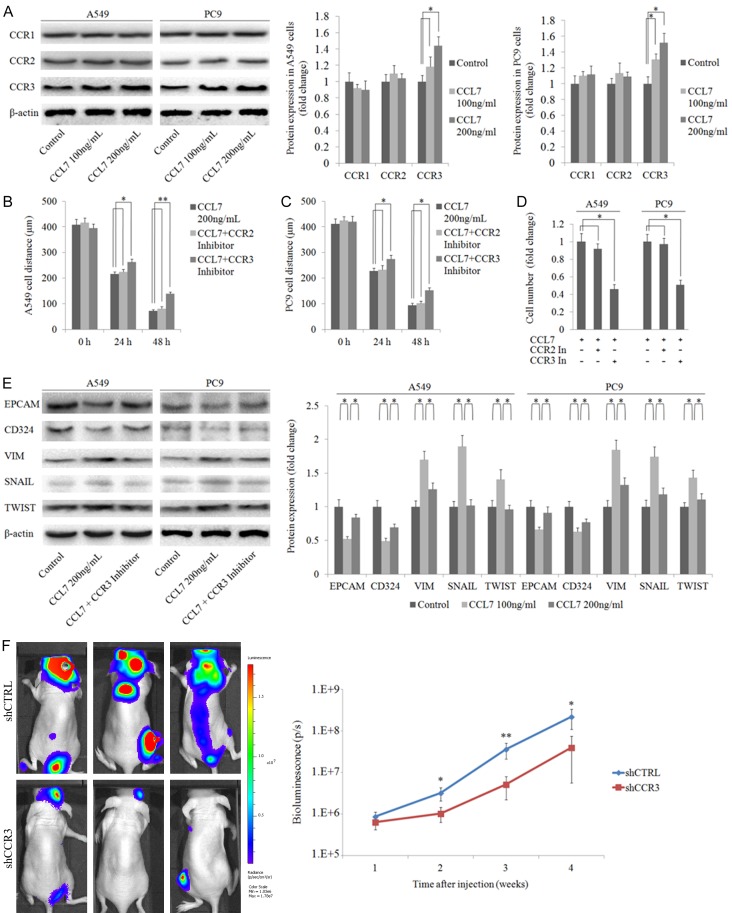
Migration and invasion are important steps in cancer cell metastasis. To further explore the role of CCL7 in NSCLC invasiveness, we stimulated A549 and PC9 cells with various concentrations of recombinant CCL7 (0, 100 and 200 ng/mL) for 48 h. Western blot analysis revealed that only the expression of CCR3 increased in a dose dependent manner (Figure 4A). Then we conducted wound healing assay and invasion assay by treating A549 and PC9 cells with recombinant CCL7 200 ng/mL with or without 400 nmol/L CCR2 inhibitor (ab120812, Abcam, CA, USA) and 20 nmol/L CCR3 inhibitor (691992, Sigma, Saint Louis, USA). Interestingly, the metastasis ability of NSCLC cells induced by CCL7 was markedly reduced in cells added with the CCR3 inhibitor. There was no significant difference between cells with CCR2 inhibitor and cells with CCL7 only samples (Figure 4B-D). We also knocked down CCR3 by shRNAs and obtained the similar result in transwell invasion assay (Supplementary Figure 3). Knowing that the epithelial-to-mesenchymal transition (EMT) process is important in cancer metastasis, we analyzed the protein expression levels of characteristic epithelial and mesenchymal markers such as CD324, EPCAM, VIM and master EMT transcription factors such as SNAIL and TWIST. As anticipated, CCL7 reduced the expression of CD324 and EPCAM, and increased the expression of VIM, SNAIL and TWIST (Figure 4E). These changes could also be blocked by CCR3 inhibitor. In addition, Bioluminescence imaging assay showed that CCR3 knockdown also inhibited A549 cell metastasis (Figure 4F). These data demonstrated that CCR3 was a major activator of CCL7 in inducing cell invasion and migration, the major characteristics of EMT in NSCLC cells.
Figure 4.
CCL7 induces EMT in A549 and PC9 cells via interacting with CCR3. A. Protein expression of CCR1, CCR2 and CCR3 in A549 and PC9 cells with different doses of recombinant CCL7 (100 ng/ml, 200 ng/ml); the result showed that CCL7 had no effect on the expression of CCR. B-D. Wound healing assay and transwell invasion assay of A549 and PC9 cells after co-incubation with recombinant CCL7 and CCR inhibitors respectively; interestingly, inhibition of CCR3 significantly suppressed cell invasion. E. CCL7 increased the expression of mesenchymal markers and decreased the expression of epithelial markers in A549 and PC9 cells via CCR3. F. Bioluminescence imaging showed that CCR3 knockdown inhibited A549 cell metastasis in vivo. *: P < 0.05; **: P < 0.001.
Analysis of CCL7 relevant downstream genes
To further investigate the mechanism by which CCL7 induced NSCLC progression, we performed deep mRNA sequencing to find genes that were responsive to CCL7 stimulation. The results showed that 1607 genes were up-regulated and 1655 genes were down-regulated after treatment of PC9 cells with CCL7 (Figure 5A; Supplementary Table 2). The fold change of part of the genes involved in multi organ metastasis features of NSCLC in mRNA sequencing are listed in Figure 5B. In addition, we performed qRT-PCR to validate the mRNA sequencing results. The results were consistent with the RNA-seq data in all the six selected genes (Figure 5C-H).
Figure 5.
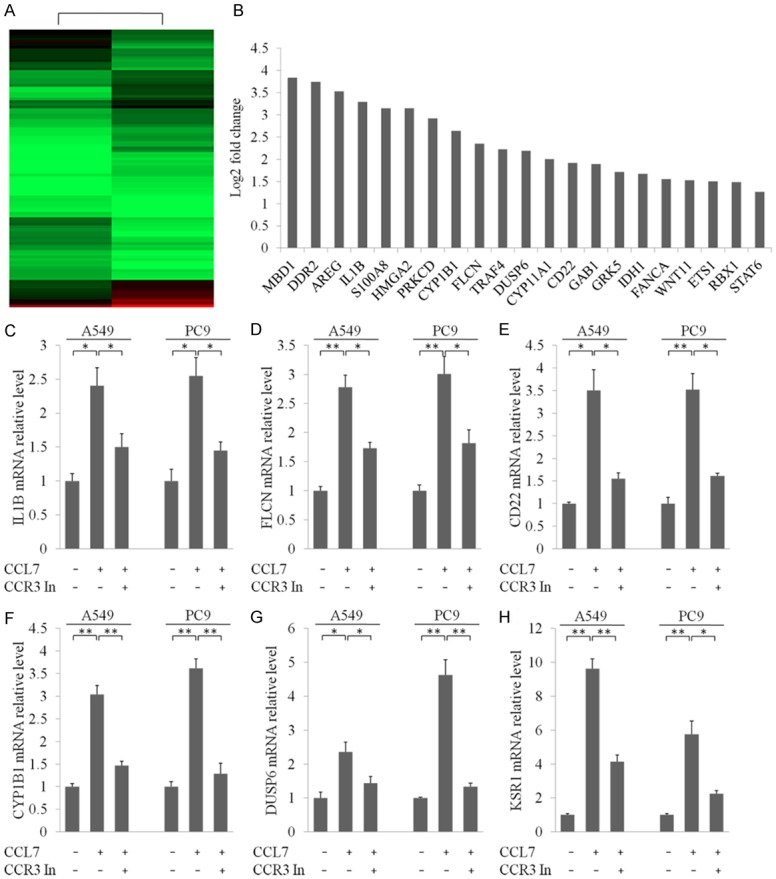
RNA-seq profiling of PC9 cells after incubation with recombinant CCL7. A. Heatmap differentially expressed genes in PC9 cells treated with recombinant CCL7 and negative control. B. List of genes that were involved in multi organ metastasis and underwent significant alterations after treatment with recombinant CCL7. C-H. qRT-PCR measurement to confirm the effect of CCL7 with or without co-incubation with CCR3 inhibitor. *: P < 0.05; **: P < 0.001.
Discussion
CCL7 is one of the most pluripotent chemokines, playing an important role in adhesion, trafficking, homing and angiogenesis of immune cells [14,15]. CCL7 was reported to have an antitumor effect by reducing tumorigenicity and inhibiting tumor growth [16]. Some recent studies demonstrated that CCL7 expression was up-regulated in multiple metastatic cancers [7,9,10]. In addition, the increased expression of CCR2 in tumor cells was also reported to correlate with enhanced metastasis and shorter clinical survival time [17-20]. However, few studies have reported the role of CCL7 in NSCLC. Our study demonstrated that CCL7 and its receptors CCR1, CCR2 and CCR3 were significantly over-expressed in lung cancer bone metastasis compared with primary NSCLC. In addition, there was a negative correlation between CCL7 expression and the clinical outcome of lung cancer patients, suggesting that CCL7 may play an additional role in NSCLC progression.
The direct effect of CCL7 in different tumor cell lines is not consistent between studies. Hu et al [16] reported that ectopic expression of CCL7 could retard tumor growth and inhibit tumor metastasis in colorectal cancer cells. However, Jung et al 7 demonstrated that CCL7 promoted the invasion and migration of oral squamous cell carcinoma. In this study, we found that stimulation of lung adenocarcinoma cell line PC9 and A549 by CCL7 could promote cell migration but not proliferation in vitro. Chemokine mediated organ-specific cancer metastasis is a most recently ascribed mechanism [21]. Our results suggest that lung cancer cells may generate bone specific metastasis by autocrine and paracrine secretion of CCL7.
EMT is the key step in metastasis. The result of our study suggests that CCL7 may provoke NSCLC cell metastasis via the EMT process, mainly through CCR3. Inhibition of CCR3 could markedly decrease the EMT process and metastasis ability in PC9 and A549 cells, suggesting that suppressing communication between CCL7 and CCR3 may be a potential therapeutic strategy for preventing NSCLC bone metastasis. A recent study also demonstrated that the crosstalk between CCL7 and CCR3 promoted colon cell growth and metastasis [22].
At present, there is no convincing explanation about the mechanism and pathway that mediate lung cancer metastasis. To investigate the molecular basis of CCL7-mediated metastasis, we examined changes in CCL7 activated gene expression in lung tumor cells and found that a cohort of genes were significantly up-regulated in stimulated cells compared with control cells. Many genes (MBD1, DDR2, AREG, IL1B, S100A8, HMGA2, TRAF4, DUSP6, CD22, GAB1, GRK5, WNT11, ETS1 and RBX1) were reported to promote tumor migration and metastasis [23-33]. In addition, we found that DDR2, S100A8, IL1B and TRAF4 were involved in the NF-κB signaling pathway, which is believed to be strongly correlated with bone metastasis [34-36]. In addition, CYP11A1 was also reported to be associated with the regulation of bone metabolism [37]. These gene sets are consistent with the high expression level of CCL7 in bone metastatic tumor tissues. Another notable thing is that CCL7 could activate genes both positive (GRK5, WNT11, S100A8, CYP1B1, IDH1, RBX1) and negative (PRKCD, FLCN, DUSP6) regulate cell proliferation in lung cancer cell line [27,30,31,33,35,37-40]. This may partly explain the different effects of CCL7 in different types of tumors. However, further investigations are required to validate the function of CCL7 in lung cancer bone metastasis.
In conclusion, our study demonstrated for the first time that CCL7 was over-expressed in lung cancer bone metastasis compared with primary lung cancer, and played an unfavorable role in the survival of NSCLC patients. As a CC chemokine, CCL7 significantly promoted migration of lung cancer cells in vitro via CCR3. In addition, we identified that CCL7 increased the mRNA level of a large number of genes associated with metastasis and bone dissolution. These results indicate that CCL7 is a novel target in bone metastasis of NSCLC and may have a potential clinical value for the prevention of lung cancer bone metastasis.
Acknowledgements
This work was supported by the grants from the National Natural Science Foundation of China (81501927, 81572641, GJJ15026), and the Training Program for Outstanding Young Talents in Shanghai Health System (XYQ2013099).
Disclosure of conflict of interest
None.
Supporting Information
Supplementary Table 2
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Yu M, Cronin KA. Use of imputed population-based cancer registry data as a method of accounting for missing information: application to estrogen receptor status for breast cancer. Am J Epidemiol. 2012;176:347–356. doi: 10.1093/aje/kwr512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffman PC, Mauer AM, Vokes EE. Lung cancer. Lancet. 2000;355:479–485. doi: 10.1016/S0140-6736(00)82038-3. [DOI] [PubMed] [Google Scholar]
- 4.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 5.Popper HH. Progression and metastasis of lung cancer. Cancer Metastasis Rev. 2016;35:75–91. doi: 10.1007/s10555-016-9618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 7.Jung DW, Che ZM, Kim J, Kim K, Kim KY, Williams D, Kim J. Tumor-stromal crosstalk in invasion of oral squamous cell carcinoma: a pivotal role of CCL7. Int J Cancer. 2010;127:332–344. doi: 10.1002/ijc.25060. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Ji R, Li J, Gu Q, Zhao X, Sun T, Wang J, Li J, Du Q, Sun B. Correlation effect of EGFR and CXCR4 and CCR7 chemokine receptors in predicting breast cancer metastasis and prognosis. J Exp Clin Cancer Res. 2010;29:16. doi: 10.1186/1756-9966-29-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wyler L, Napoli CU, Ingold B, Sulser T, Heikenwälder M, Schraml P, Moch H. Brain metastasis in renal cancer patients: metastatic pattern, tumour-associated macrophages and chemokine/chemoreceptor expression. Br J Cancer. 2014;110:686–694. doi: 10.1038/bjc.2013.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho YB, Lee WY, Choi SJ, Kim J, Hong HK, Kim SH, Choi YL, Kim HC, Yun SH, Chun HK, Lee KU. CC chemokine ligand 7 expression in liver metastasis of colorectal cancer. Oncol Rep. 2012;28:689–694. doi: 10.3892/or.2012.1815. [DOI] [PubMed] [Google Scholar]
- 11.Hwang TL, Lee LY, Wang CC, Liang Y, Huang SF, Wu CM. CCL7 and CCL21 overexpression in gastric cancer is associated with lymph node metastasis and poor prognosis. World J Gastroenterol. 2012;18:1249–1256. doi: 10.3748/wjg.v18.i11.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu LL, McVicar DW, Ben-Baruch A, Kuhns DB, Johnston J, Oppenheim JJ, Wang JM. Monocyte chemotactic protein-3 (MCP3) interacts with multiple leukocyte receptors: binding and signaling of MCP3 through shared as well as unique receptors on monocytes and neutrophils. Eur J Immunol. 1995;25:2612–7. doi: 10.1002/eji.1830250931. [DOI] [PubMed] [Google Scholar]
- 13.Allavena P, Bianchi G, Zhou D, van Damme J, Jílek P, Sozzani S, Mantovani A. Induction of natural killer cell migration by monocyte chemotactic protein-1, -2 and -3. Eur J Immunol. 1994;24:3233–3236. doi: 10.1002/eji.1830241249. [DOI] [PubMed] [Google Scholar]
- 14.McCandless EE, Piccio L, Woerner BM, Schmidt RE, Rubin JB, Cross AH, Klein RS. Pathological expression of CXCL12 at the blood-brain barrier correlates with severity of multiple sclerosis. Am J Pathol. 2008;172:799–808. doi: 10.2353/ajpath.2008.070918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCandless EE, Zhang B, Diamond MS, Klein RS. CXCR4 antagonism increases T cell trafficking in the central nervous system and improves survival from West Nile virus encephalitis. Proc Natl Acad Sci U S A. 2008;105:11270–5. doi: 10.1073/pnas.0800898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu JY, Li GC, Wang WM, Zhu JG, Li YF, Zhou GH, Sun QB. Transfection of colorectal cancer cells with chemokine MCP-3 (monocyte chemotactic protein-3) gene retards tumor growth and inhibits tumor metastasis. World J Gastroenterol. 2002;8:1067–1072. doi: 10.3748/wjg.v8.i6.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zijlmans HJ, Fleuren GJ, Baelde HJ, Eilers PH, Kenter GG, Gorter A. The absence of CCL2 expression in cervical carcinoma is associated with increased survival and loss of heterozygosity at 17q11.2. J Pathol. 2006;208:507–517. doi: 10.1002/path.1918. [DOI] [PubMed] [Google Scholar]
- 18.Yoshidome H, Kohno H, Shida T, Kimura F, Shimizu H, Ohtsuka M, Nakatani Y, Miyazaki M. Significance of monocyte chemoattractant protein-1 in angiogenesis and survival in colorectal liver metastases. Int J Oncol. 2009;34:923–30. doi: 10.3892/ijo_00000218. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Patel L, Pienta KJ. CC chemokine ligand 2 (CCL2) promotes prostate cancer tumorigenesis and metastasis. Cytokine Growth Factor Rev. 2010;21:41–48. doi: 10.1016/j.cytogfr.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soria G, Ofri-Shahak M, Haas I, Yaal-Hahoshen N, Leider-Trejo L, Leibovich-Rivkin T, Weitzenfeld P, Meshel T, Shabtai E, Gutman M, Ben-Baruch A. Inflammatory mediators in breast cancer: coordinated expression of TNFalpha & IL-1beta with CCL2 & CCL5 and effects on epithelial-to-mesenchymal transition. BMC Cancer. 2011;11:130. doi: 10.1186/1471-2407-11-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Datle T, Matsuo T, Yoshimaru T, Kakiuchi S, Goto H, Hanibuchi M, Kuramoto T, Nishioka Y, Sone S, Katagiri T. Identification of genes potentially involved in bone metastasis by genome-wide gene expression profile analysis of non-small cell lung cancer in mice. Int J Oncol. 2012;40:1455–69. doi: 10.3892/ijo.2012.1348. [DOI] [PubMed] [Google Scholar]
- 22.Lee YS, Kim SY, Song SJ, Hong HK, Lee Y, Oh BY, Lee WY, Cho YB. Crosstalk between CCL7 and CCR3 promotes metastasis of colon cancer cells via ERK-JNK signaling pathways. Oncotarget. 2016;7:36842–36853. doi: 10.18632/oncotarget.9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu J, Zhu W, Xu W, Yao W, Zhang B, Xu Y, Ji S, Liu C, Long J, Ni Q, Yu X. Up-regulation of MBD1 promotes pancreatic cancer cell epithelial-mesenchymal transition and invasion by epigenetic down-regulation of E-cadherin. Curr Mol Med. 2013;13:387–400. [PubMed] [Google Scholar]
- 24.Yang Y, Ahn YH, Chen Y, Tan X, Guo L, Gibbons DL, Ungewiss C, Peng DH, Liu X, Lin SH, Thilaganathan N, Wistuba II, Rodriguez-Canales J, McLendon G, Creighton CJ, Kurie JM. ZEB1 sensitizes lung adenocarcinoma to metastasis suppression by PI3K antagonism. J Clin Invest. 2014;124:2696–2708. doi: 10.1172/JCI72171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landvik NE, Hart K, Skaug V, Stangeland LB, Haugen A, Zienolddiny S. A specific interleukin-1B haplotype correlates with high levels of IL1B mRNA in the lung and increased risk of non-small cell lung cancer. Carcinogenesis. 2009;30:1186–1892. doi: 10.1093/carcin/bgp122. [DOI] [PubMed] [Google Scholar]
- 26.Lin Y, Liu AY, Fan C, Zheng H, Li Y, Zhang C, Wu S, Yu D, Huang Z, Liu F, Luo Q, Yang CJ, Ouyang G. MicroRNA-33b inhibits breast cancer metastasis by targeting HMGA2, SALL4 and Twist1. Sci Rep. 2015;5:9995. doi: 10.1038/srep09995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song H, Wu C, Wei C, Li D, Hua K, Song J, Xu H, Chen L, Fang L. Silencing of DUSP6 gene by RNAi-mediation inhibits proliferation and growth in MDA-MB-231 breast cancer cells: an in vitro study. Int J Clin Exp Med. 2015;8:10481–10490. [PMC free article] [PubMed] [Google Scholar]
- 28.Lamontagne M, Timens W, Hao K, Bossé Y, Laviolette M, Steiling K, Campbell JD, Couture C, Conti M, Sherwood K, Hogg JC, Brandsma CA, van den Berge M, Sandford A, Lam S, Lenburg ME, Spira A, Paré PD, Nickle D, Sin DD, Postma DS. Genetic regulation of gene expression in the lung identifies CST3 and CD22 as potential causal genes for airflow obstruction. Thorax. 2014;69:997–1004. doi: 10.1136/thoraxjnl-2014-205630. [DOI] [PubMed] [Google Scholar]
- 29.Bai R, Weng C, Dong H, Li S, Chen G, Xu Z. MicroRNA-409-3p suppresses colorectal cancer invasion and metastasis partly by targeting GAB1 expression. Int J Cancer. 2015;137:2310–2322. doi: 10.1002/ijc.29607. [DOI] [PubMed] [Google Scholar]
- 30.Chakraborty PK, Zhang Y, Coomes AS, Kim WJ, Stupay R, Lynch LD, Atkinson T, Kim JI, Nie Z, Daaka Y. G protein-coupled receptor kinase GRK5 phosphorylates moesin and regulates metastasis in prostate cancer. Cancer Res. 2014;74:3489–3500. doi: 10.1158/0008-5472.CAN-13-2708. [DOI] [PubMed] [Google Scholar]
- 31.Nishioka M, Ueno K, Hazama S, Okada T, Sakai K, Suehiro Y, Okayama N, Hirata H, Oka M, Imai K, Dahiya R, Hinoda Y. Possible involvement of Wnt11 in colorectal cancer progression. Mol Carcinog. 2013;52:207–217. doi: 10.1002/mc.21845. [DOI] [PubMed] [Google Scholar]
- 32.Cao L, Xie B, Yang X, Liang H, Jiang X, Zhang D, Xue P, Chen D, Shao Z. MiR-324-5p suppresses hepatocellular carcinoma cell invasion by counteracting ECM degradation through post-transcriptionally downregulating ETS1 and SP1. PLoS One. 2015;10:e0133074. doi: 10.1371/journal.pone.0133074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X, Wang Y, Zang W, Du Y, Li M, Zhao G. miR-194 targets RBX1 gene to modulate proliferation and migration of gastric cancer cells. Tumour Biol. 2015;36:2393–2401. doi: 10.1007/s13277-014-2849-1. [DOI] [PubMed] [Google Scholar]
- 34.Poudel B, Lee YM, Kim DK. DDR2 inhibition reduces migration and invasion of murine metastatic melanoma cells by suppressing MMP2/9 expression through ERK/NF-kappaB pathway. Acta Biochim Biophys Sin (Shanghai) 2015;47:292–298. doi: 10.1093/abbs/gmv005. [DOI] [PubMed] [Google Scholar]
- 35.Ichikawa M, Williams R, Wang L, Vogl T, Srikrishna G. S100A8/A9 activate key genes and pathways in colon tumor progression. Mol Cancer Res. 2011;9:133–148. doi: 10.1158/1541-7786.MCR-10-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang F, Wang J, Ren HY, Jin J, Wang AL, Sun LL, Diao KX, Wang EH, Mi XY. Proliferative role of TRAF4 in breast cancer by upregulating PRMT5 nuclear expression. Tumour Biol. 2015;36:5901–5911. doi: 10.1007/s13277-015-3262-0. [DOI] [PubMed] [Google Scholar]
- 37.Rodríguez-Sanz M, García-Giralt N, Prieto-Alhambra D, Servitja S, Balcells S, Pecorelli R, Díez-Pérez A, Grinberg D, Tusquets I, Nogués X. CYP11A1 expression in bone is associated with aromatase inhibitor-related bone loss. J Mol Endocrinol. 2015;55:69–79. doi: 10.1530/JME-15-0079. [DOI] [PubMed] [Google Scholar]
- 38.Tan F, Jiang Y, Sun N, Chen Z, Lv Y, Shao K, Li N, Qiu B, Gao Y, Li B, Tan X, Zhou F, Wang Z, Ding D, Wang J, Sun J, Hang J, Shi S, Feng X, He F, He J. Identification of isocitrate dehydrogenase 1 as a potential diagnostic and prognostic biomarker for non-small cell lung cancer by proteomic analysis. Mol Cell Proteomics. 2012;11:M111.008821. doi: 10.1074/mcp.M111.008821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ke G, Liang L, Yang JM, Huang X, Han D, Huang S, Zhao Y, Zha R, He X, Wu X. MiR-181a confers resistance of cervical cancer to radiation therapy through targeting the pro-apoptotic PRKCD gene. Oncogene. 2013;32:3019–3027. doi: 10.1038/onc.2012.323. [DOI] [PubMed] [Google Scholar]
- 40.Hasumi H, Baba M, Hasumi Y, Huang Y, Oh H, Hughes RM, Klein ME, Takikita S, Nagashima K, Schmidt LS, Linehan WM. Regulation of mitochondrial oxidative metabolism by tumor suppressor FLCN. J Natl Cancer Inst. 2012;104:1750–1764. doi: 10.1093/jnci/djs418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.