Abstract
Sodium tanshinone IIA sulfonate (STS), a water-soluble derivative of tanshinone IIA, has been used in traditional Chinese medicine for many years. Many experiments have demonstrated that STS has anti-inflammatory, anti-apoptosis and angiogenesis effects. However, it is unclear whether STS has the same beneficial effects on myocardial infarction in vivo. The aim of our experiments was to investigate whether STS could improve cardiac function and prevent myocardial remodeling after myocardial infarction (MI) in mice. The MI model was established by surgical ligation of the left anterior descending (LAD) coronary artery. Then the mice were randomly divided into STS and untreated groups. The results of treatment for 3 weeks showed that STS could increase the survival rate, reduce the release of some inflammatory cytokines, inhibit cell apoptosis and promote angiogenesis. The study presents a new potential treatment method for ischemic heart disease.
Keywords: Sodium tanshinone IIA sulfonate, myocardial infarction, anti-inflammation, anti-apoptosis, angiogenesis
Introduction
Myocardial infarction (MI) is one of the most frequent causes of mortality and morbidity. Although the death rate of MI has declined, the burden of the disease remains high [1]. An acute MI can produce changes that may result in alterations of cardiac hemodynamics, deterioration of diastolic and systolic function, and a propensity for arrhythmias in the topography of the ventricle. This remodeling can enormously affect cardiac function and prognosis for survival [2,3]. Most patients that survive acute MI inevitably suffer from heart failure (HF) caused by myocardial remodeling, and patients with acute MI have a high risk of dying after hospital discharge [4,5]. In conclusion, it is necessary to search for effective therapies to prevent myocardial remodeling after MI.
Salvia miltiorrhiza Bunge (known as Danshen in China) is a traditional Chinese medicine that has been widely used to treat cardiovascular disease, cerebrovascular disease and tumors [6]. One of its key bioactive components, sodium tanshinone IIA sulfonate (STS), is a water-soluble derivative isolated from Salvia miltiorrhiza Bunge. STS has been reported to possess various medical effects, such as dilating the coronary arteries and anti-inflammatory, anti-apoptosis, and anti-oxidation properties [7]. In recent years, STS has been proven to have a beneficial effect on impaired nerve functions and diabetic neuropathy [8]. Furthermore, it also has cardioprotective effects, such as preventing the toxicity induced by myocardial ischemia/reperfusion injury and radiation [9,10]. The probable mechanism underlying the protective effects of STS has been deduced as reducing oxidative stress-triggered damage and apoptosis [11]. However, it is unknown whether STS can protect myocardial cells through other mechanisms.
Previous studies have proven that myocardial fibrosis, inflammation and apoptosis play important roles in the process of myocardial remodeling after MI [12]. It has been demonstrated that tumor necrosis factor alpha (TNF-α), interleukin 1 beta (IL-1β) and transforming growth factor beta (TGF-β) are essential factors for the pathogenesis of myocardial remodeling. Suppressing the release of these factors is a valid approach to reduce the progression of myocardial remodeling [13,14]. Otherwise, some reports have proven that therapeutic angiogenesis is an effective method for ischemic heart disease. A variety of growth factors, such as vascular endothelial growth factor (VEGF), have important effects on enhancing angiogenesis [15,16]. Alpha smooth muscle actin (α-SMA) and platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) are markers of vascular endothelial cells [17-19]. Several studies have demonstrated that STS has anti-apoptotic, anti-inflammatory, anti-oxidative stress and angiogenesis promoting effects in vitro [20-22]. In our study, we used a mouse model of continuous myocardial ischemia by ligating of the left anterior descending (LAD) coronary artery to investigate whether treatment with STS could improve cardiac function, promote angiogenesis and attenuate myocardial remodeling in vivo after MI.
Materials and methods
Animals
C57BL/6J male mice (weighing 20-25 g, aged 8 weeks) were purchased from the Nanjing Medical University Animal Center. The study was performed in accordance with the Nanjing Medical University Animal Center guide for the care and use of Laboratory Animals and was approved by the Ethics Committee for Lab Animal Use of Nanjing Medical University (Permit Number: IACUC-1707002).
Mouse model of myocardial infarction
The mouse model of continuous myocardial ischemia was established according to previous description [23]. Briefly, anesthesia was induced by an intraperitoneal injection of pentobarbital sodium (50 mg/kg) prior to surgery. The mice were intubated and connected to a ventilator to maintain normal respiration. Then, a left thoracotomy was performed to expose the heart. MI was induced by surgical ligation of the LAD coronary artery by an 8-0 polypropylene suture passed approximately 2-3 mm from the inferior margin of the left auricle. The successful induction of MI was confirmed based on the observation of the pale color of the anterior portion of the left ventricle and electrocardiogram (ECG) ST-segment elevation. The thorax was closed by surgical sutures. Sham operations were done by the same method but without ligating the LAD coronary artery.
Sodium tanshinone IIA sulfonate (STS)
STS was purchased from Aladdin Biochemical Technology Co. Ltd. (Shanghai, China, purity >98%). The operated mice as described above survived for 3 hours were randomized to treatment with STS (30 mg/kg/d, intraperitoneal injection, n=20) or vehicle alone (n=20) for 3 weeks. Sham-operated mice were also given the vehicle (n=10). Mice were sacrificed by carbon dioxide at 3 weeks after ligation.
Echocardiography measurement
The cardiac structure and function were evaluated by echocardiography with a Vevo 2100-a high resolution imaging system (Visual Sonics, Canada) with a MS-250 and 16.0-21.0 MHZ imaging transducer at baseline, week 1 and week 3. All mice were anesthetized by isoflurane before echocardiography examination.
Histopathology
To evaluate the morphological changes and the extent of cardiac fibrosis, the hearts were harvested at 21 days after MI, washed in PBS and fixed in 4% paraformaldehyde overnight and embedded in paraffin. Each heart was cut into 4-μm-thick sections and stained with hematoxylin and eosin (HE) and Masson trichrome. Each section was imaged by a microscope (Nikon, Japan).
Immunohistochemistry staining
For immunohistochemistry staining, paraffin embedded myocardial samples were dewaxed and rehydrated in xylene and ethyl alcohol followed by incubation in 0.3% methanol/H2O2 to block endogenous peroxidases. Antigen retrieval was performed by boiling the slides in a 10 mM citrate pH 6.0 solution for 20 min using a microwave oven. Next, sections were incubated overnight at 4°C with the primary antibodies. A two-step technique (SuperPicture-TM3rd Gen IHC Detection kit; Invitrogen, CA, USA) was used for visualization, with 3,3’-diaminobenzidine (DAB, 0.1 mg/ml, 0.02% H2O2) (Vector Laboratories, Burlingame, USA) as a chromogen and sections were counterstained with hematoxylin. We used average optical density (AOD) to quantify the expression level. Primary antibodies for immunohistochemistry were as follows: TGF-β, TNF-α, IL-1β (Abcam, USA), Bcl-2, Bax, α-SMA, CD31, VEGF (Cell Signaling Technology, USA).
Western blotting analysis
Total protein in vivo was obtained from left ventricular myocardial tissues. After centrifugation, they were followed by sonication and heat denaturation. A total of 20 µg protein lysates were electrophoresed, separated on 6-12% SDS-PAGE and transferred onto nitrocellulose membranes (Bio-Rad, Hercules, USA). The membranes were blocked with 5% skim milk at room temperature for an hour, and then incubated over night at 4°C with primary antibodies including rabbit anti-Bcl-2 (1:1000; Cell Signaling Technology, USA), rabbit anti-Bax (1:1000; Cell Signaling Technology, USA and rabbit anti-GAPDH (1:1000; Cell Signaling Technology, USA). The membranes were then incubated with HRP-conjugated secondary antibodies (1:5000; Abcam, USA) at room temperature for 2 hours. The antigen-antibody complexes were detected by using a Super Signal ECL kit (Thermo, USA) in a Western blotting detection system (Bio-Rad, CA, USA). The results were expressed as density values normalized to GAPDH.
TUNEL staining
The TUNEL protocol was based on TUNEL detection kit. Pretreatment of the myocardium was the same as for HE staining. The fixed tissues were embedded in paraffin and 4-μm-thick sections were deparaffinized by washing in xylene and a descending ethanol series. The sections were subsequently incubated with 20 μg/mL proteinase K for 30 min at 37°C, and endogenous peroxidase was inactivated by 3% H2O2 in methanol for 10 min. They were incubated with 50 μL of a TUNEL reaction mixture on the section for 60 min at 37°C, and then 50 μL converter-POD was added to the sample for 30 min at 37°C. For color development, sections were supplemented with 50 μL DAB substrate for 10 min at room temperature to detect labeled nuclei, and then counterstained by hematoxylin. For each slide, 10 separate fields were examined randomly and digitized by microscopy at a magnification of 400×. The apoptotic index (AI) was calculated as the ratio of TUNEL-positive cells to the total number of myocytes. Apoptotic index = apoptosis cell number/1000 cells*100%.
Oxidative stress index
After the specimens were cut, the weights were recorded, and then the specimens were placed into a homogenate editor. A specific amount of precooling PBS or saline was added, and the specimens were homogenized at 4°C, 2000-3000 r/min, 20 min; the supernatant was then carefully collected. According to the instructions of the superoxide dismutase (SOD), glutathione (GSH), and malondialdehyde (MDA) testing kit, their absorbance was detected and recorded, and the concentrations of SOD, MDA and GSH were calculated.
Statistical analysis
Data from at least three independent experiments were used to calculate the mean ± standard deviation (SD) using SPSS 18.0 statistical software. The overall survival of mice after MI was evaluated using Kaplan-Meier curves survival analysis and compared by the log-rank test. Statistical analyses between groups were performed by unpaired Student’s t-test or one-way ANOVA followed by a post hoc Fisher’s comparison test. A value less than 0.05 was considered to be statistically significant.
Results
STS improved survival rate and cardiac function in MI mice
The survival rate of the STS-treated group was 82% after MI, while that of the untreated group was 56% (Figure 1A). Postmortem examination showed that the main reason for death was heart failure. At 1 week and 3 weeks after MI, the echocardiography showed that treatment with STS promoted the motion of the left ventricular anterior wall, LVEF and LVFS compared with the untreated group (Figure 1B and 1C).
Figure 1.
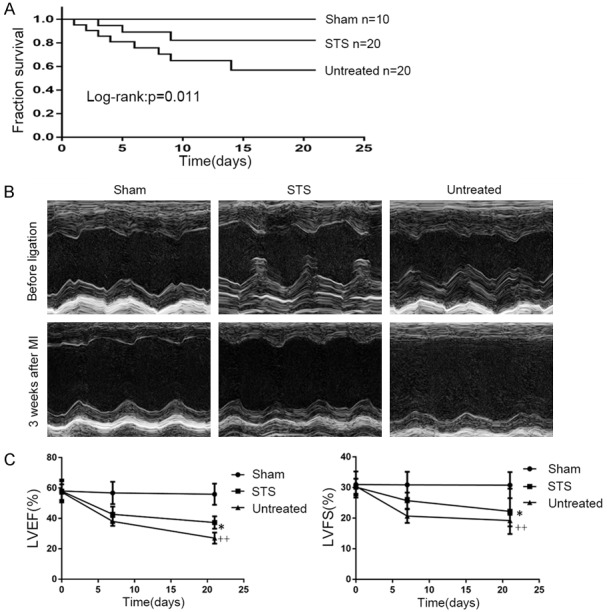
STS improved survival rate and cardiac function in MI mice. A. Survival rates within 3 weeks after MI of the sham, untreated and STS groups. B. Representative M-mode echocardiographic images of mice before ligation, and 3 weeks after MI in each group. C. Analysis of LVEF and LVFS at 7 days and 21 days after MI in each group. *P<0.05 compared to the untreated group, ++P<0.01 compared to the sham group, n=10 per group.
STS improved myocardial pathological changes in MI mice
After 3 weeks, HE staining showed that abundant inflammatory cells infiltrated in the border zones, and the myocardial cells were arranged irregularly in the untreated group. However, in the STS-treated group, most myocardial cells were arranged in an orderly manner, and the area of inflammatory infiltration was smaller (Figure 2A). Masson staining demonstrated that fibrosis and collagen deposition significantly decreased in the STS group compared with those of the untreated group (Figure 2B).
Figure 2.
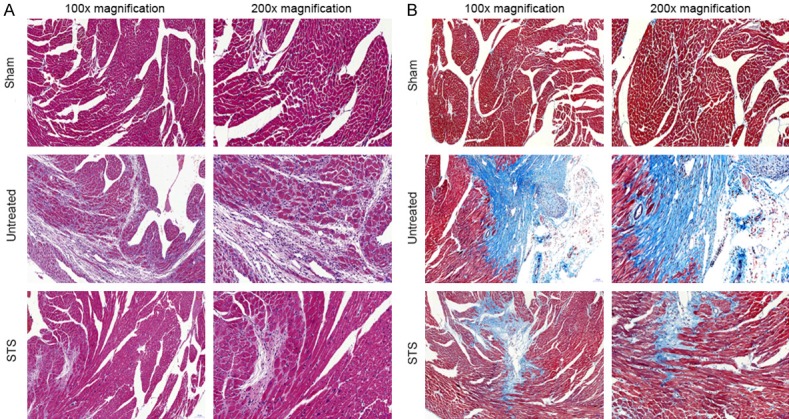
STS improved myocardial pathological changes in MI mice. A. Representative illustration of hematoxylin and eosin staining of infarcted mouse hearts. These photos demonstrated the intense inflammatory response and myocardial cells arranged irregularly after MI. B. Representative images of Masson’s trichrome-staining infarcted hearts in mice. Blue represents region with replacement fibrosis.
STS reduced the release of inflammatory cytokines in MI mice
Immunohistochemical staining and quantitative analysis showed that the expression levels of TNF-α, IL-1β and TGF-β were much higher in the untreated group compared with those in the sham-operated group. The expression levels of these inflammatory cytokines were decreased after STS treatment (Figure 3A and 3B). This indicated that STS might reduce the release of inflammatory cytokines caused by MI.
Figure 3.
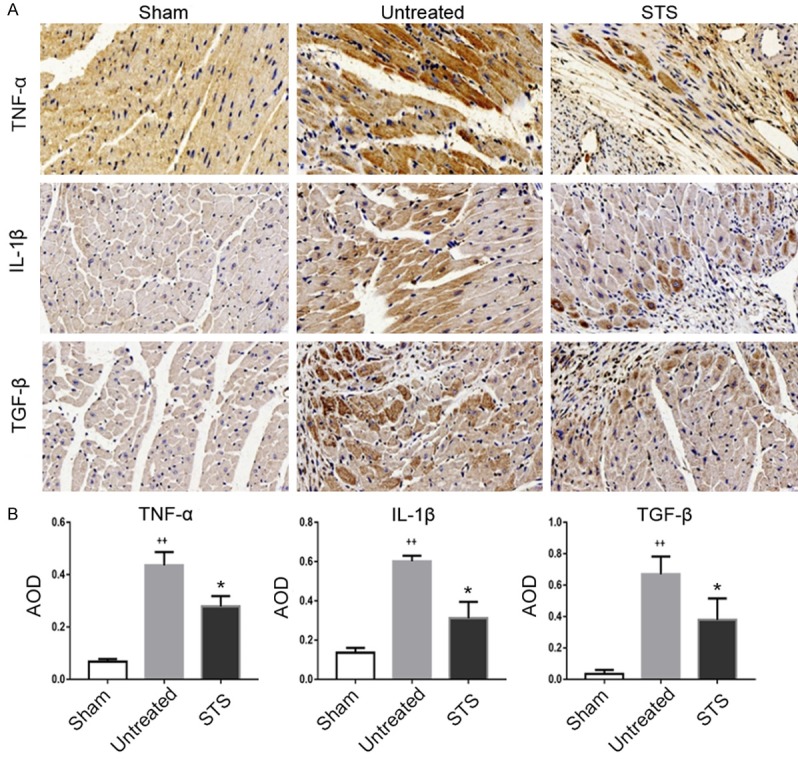
STS reduced the release of inflammatory cytokines in MI mice. A. Immunohistochemical staining for TNF-α, IL-1β and TGF-β in the sham, untreated and STS groups. B. Quantitative analysis of TNF-α IL-1β and TGF-β expression in each group. *P<0.05 compared to the untreated group, ++P<0.01 compared to the sham group.
STS increased resistance to oxidative stress in MI mice
To explore the mechanism underlying the anti-oxidant effect of STS, we evaluated the expression levels of SOD, GSH and MDA in cardiac tissue homogenates. The results showed that the levels of SOD and GSH increased in the STS group compared with those in the untreated group (Figure 4A and 4B), and the MDA level in the STS group was lower than that in the untreated group (Figure 4C).
Figure 4.
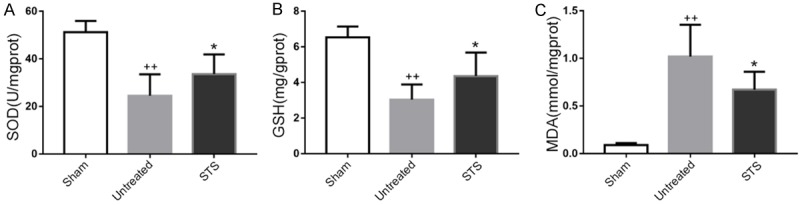
STS increased resistance to oxidative stress in MI mice. The concentrations of SOD (A), GSH (B) and MDA (C) in cardiac tissue homogenates were determined by spectrophotometry in each group. *P<0.05 compared to the untreated group, ++P<0.01 compared to the sham group.
STS resisted myocardial apoptosis in MI mice
Immunohistochemistry and Western blotting were used to determine the apoptosis process. Immunohistochemistry showed TUNEL-positive apoptotic cardiomyocytes exhibiting brown nuclei staining (Figure 5A). The apoptotic index of the untreated group was significantly higher than in the sham-operated group. The number of TUNEL-positive cells in the STS group was lower compared with that in the untreated group (Figure 5B). We determined the expressions of the apoptosis related proteins Bax and Bcl-2 by immunohistochemistry and Western blotting. The expression of Bcl-2 was significantly increased in the STS group compared with that in the untreated group (Figure 5C, 5E and 5F), while the expression of Bax in the STS group was significantly lower than in the untreated group (Figure 5D, 5E and 5G). The Bcl-2/Bax ratio increased in the STS group compared with that in the untreated group (Figure 5H).
Figure 5.
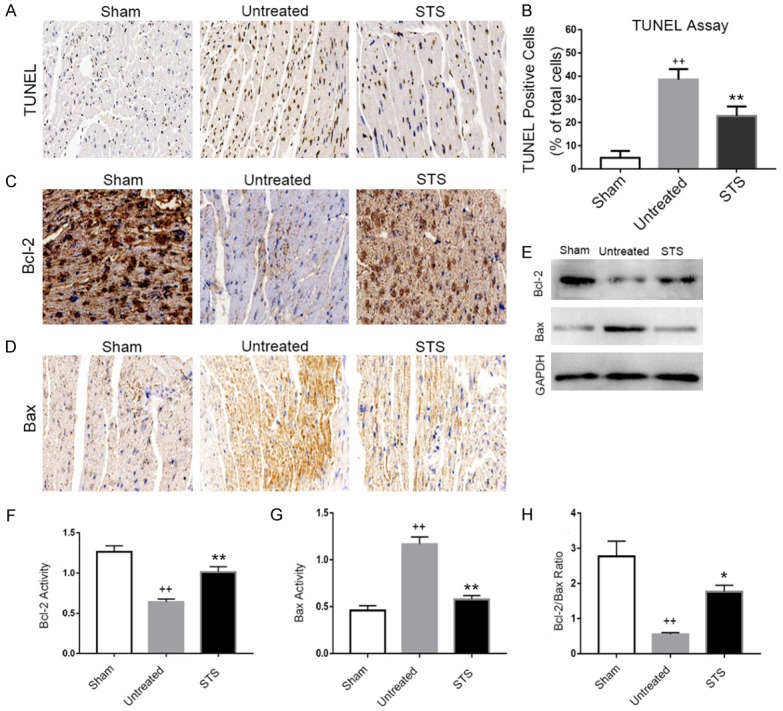
STS resisted myocardial apoptosis in MI mice. (A) Representative photomicrographs of TUNEL staining in each group at 3 weeks after MI. TUNEL-positive cells exhibited brown nuclei staining. (B) The cardiomyocyte apoptosis index was significantly lower in the STS treated group than that in the untreated group. Immunohistochemical staining for Bcl-2 (C) and Bax (D) in the sham, untreated and STS groups. (E) Western blotting analysis of protein expressions of Bcl-2 and Bax in each group. Densitometry for Bcl-2 (F) and Bax (G) normalized to GAPDH. (H) Analysis of the ratio between Bcl-2 and Bax in each group. *P<0.05 compared to the untreated group, **P<0.01 compared to the untreated group, ++P<0.01 compared to the sham group.
STS promoted angiogenesis in MI mice
As shown in Figure 6A and 6B, the expression of VEGF in the STS group was significantly higher than that in the untreated group. α-SMA-positive and CD31-positive vessels were also detected by immunohistochemistry staining. The vascular density in the STS group in the 200× high power field was significantly increased compared with that in the untreated group (Figure 6C-F).
Figure 6.
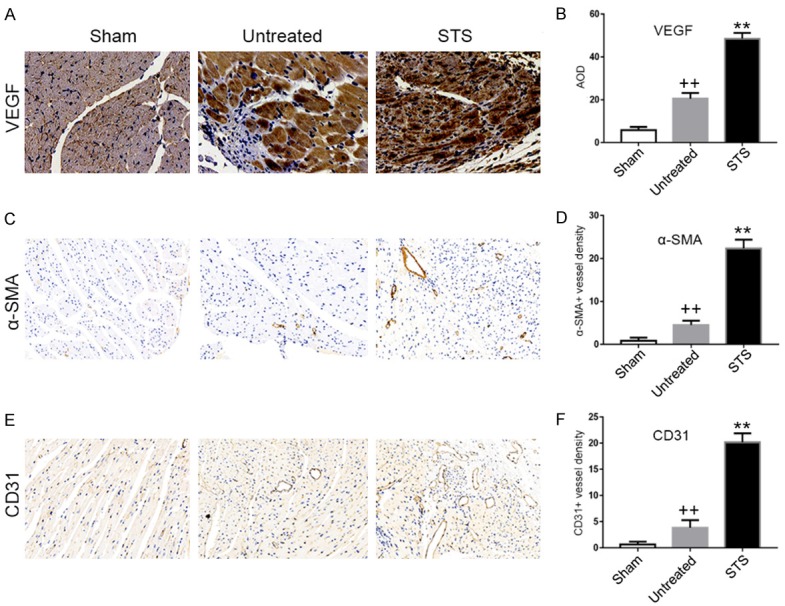
STS promoted angiogenesis in MI mice. Immunohistochemical staining for VEGF (A), CD31 (C) and α-SMA (E) in the sham, untreated and STS groups. (B) Quantitative analysis of VEGF expression by AOD in each group. Quantification of α-SMA-positive vessels (D) and CD31-positive vessels (F) in a 200× high-power field in each group. **P<0.01 compared to the untreated group, ++P<0.01 compared to the sham group.
Discussion
In this study, we observed that treatment with STS could increase the survival rate, enhance heart function and increase anti-oxidant stress in mice after MI. STS could alleviate the progression of myocardial remodeling by attenuating inflammatory release, myocardial fibrosis and apoptosis. Decreasing the expressions of TNF-α, IL-1β, TGF-β, Bax, and MDA and increasing the expressions of Bcl-2, SOD, and GSH might be the potential mechanisms. Otherwise, STS could increase the expression of VEGF and vascular density. The potential mechanisms might be associated with the downregulated expressions of TGF-β, TNF-α, IL-1β, and Bax and the upregulated expression of Bcl-2 to ameliorate myocardial ischemia and enhance cardiac repair. These results indicate that STS could be an effective drug to prevent myocardial remodeling after MI.
After MI, the increased oxidative stress and inflammatory cytokines, such as TNF-α, IL-1β and TGF-β, play crucial roles in the process of myocardial remodeling and fibrosis [24]. Moreover, oxidative stress can promote the release of inflammatory cytokines. The overexpression of inflammatory cytokines leads to cardiac hypertrophy and myocardial remodeling and then heart failure [25,26]. It has been proven that STS has anti-inflammatory and anti-fibrotic effects in some studies [27,28]. In our research, the expression of inflammatory mediators increased after MI, and STS treatment could decrease the expression of TNF-α, IL-1β, and TGF-β. Meanwhile, STS promoted anti-oxidant stress by decreasing the MDA level and increasing the SOD and GSH levels. Our data indicate that STS treatment may reduce myocardial remodeling via anti-inflammatory, anti-fibrotic and anti-oxidant stress.
Apoptosis plays an important role in fundamental biological functions such as growth, differentiation, immunity and death. Excessive apoptosis may be associated with the pathogenesis of myocardial infarction and heart failure [29]. Consistently, apoptotic cells in our study were found to be prominent after MI. Our results also prove that STS treatment can reduce apoptosis in MI mice. The underlying mechanism might include the increased expression of Bcl-2 and the suppressed expression of Bax. Aggrandizing the Bcl-2/Bax ratio has been confirmed to be important in the regulation of cell apoptosis [30]. Our research correlates well with previous studies and further proofs the anti-apoptotic effects of STS in infarct expansion during LV remodeling after MI.
Therapeutic angiogenesis is a new potential treatment for ischemic cardiovascular disease [31]. VEGF, one of the most important angiogenic growth factors, plays a vital role in promoting angiogenesis and collateral vessel formation [32]. The release of VEGF after MI may improve collateral circulation thus decreasing infarction size and leading to a prominent improvement of cardiac function [33,34]. In our study, the vascular density in the STS group was significantly higher than that in the untreated group, in line with the expression of VEGF. These data indicate that STS may contribute to angiogenesis.
Our research comprehensively expounds the effects of STS in improving the prognosis of mice after MI. We demonstrate that STS can improve cardiac function and enhance cardiac repair through anti-inflammatory, anti-apoptosis, anti-oxidation and pro-angiogenesis effects. However, this is a preliminary study and further in-depth studies are warranted to obtain the specific mechanisms of STS in anti-inflammation, anti-apoptosis, anti-oxidation and proangiogenesis.
In conclusion, our study found that STS could ameliorate cardiac function and myocardial remodeling after MI by suppressing inflammation, apoptosis, and oxidative stress and promoting angiogenesis. Therefore, STS could become a promising and effective drug for the treatment of MI.
Acknowledgements
This work was funded by the National High Technology Research and Development Program of China (863 Program), the National Natural Science Foundation of China (No.81170102 & No.81441011 & No.81670328), the Doctoral Scientific Fund Project of the Ministry of Education of China (20123234110015), the Fourth Period Project “333” of Jiangsu Province (BRA2012207), the Chinese Medical Association of the Sunlight Foundation (SCRFCMDA201217), the Collaborative Innovation Center of Nanjing Medical University, and the Natural Science of Jiangsu Province Youth Fund (BK20141020).
Disclosure of conflict of interest
None.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics--2010 update: a report from the American heart association. Circulation. 2010;121:948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 2.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez A, Ravassa S, Beaumont J, Lopez B, Diez J. New targets to treat the structural remodeling of the myocardium. J Am Coll Cardiol. 2011;58:1833–1843. doi: 10.1016/j.jacc.2011.06.058. [DOI] [PubMed] [Google Scholar]
- 4.Coles AH, Fisher KA, Darling C, McManus D, Maitas O, Yarzebski J, Gore JM, Lessard D, Goldberg RJ. Recent trends in post-discharge mortality among patients with an initial acute myocardial infarction. Am J Cardiol. 2012;110:1073–1077. doi: 10.1016/j.amjcard.2012.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen P, Pang S, Yang N, Meng H, Liu J, Zhou N, Zhang M, Xu Z, Gao W, Chen B, Tao Z, Wang L, Yang Z. Beneficial effects of schisandrin B on the cardiac function in mice model of myocardial infarction. PLoS One. 2013;8:e79418. doi: 10.1371/journal.pone.0079418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X, Yan J, Feng J. Treatment with tanshinone IIA suppresses disruption of the blood-brain barrier and reduces expression of adhesion molecules and chemokines in experimental autoimmune encephalomyelitis. Eur J Pharmacol. 2016;771:18–28. doi: 10.1016/j.ejphar.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Wei B, Li WW, Ji J, Hu QH, Ji H. The cardioprotective effect of sodium tanshinone IIA sulfonate and the optimizing of therapeutic time window in myocardial ischemia/reperfusion injury in rats. Atherosclerosis. 2014;235:318–327. doi: 10.1016/j.atherosclerosis.2014.05.924. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Wang L, Li X, Lv C, Feng D, Luo Z. Tanshinone IIA improves impaired nerve functions in experimental diabetic rats. Biochem Biophys Res Commun. 2010;399:49–54. doi: 10.1016/j.bbrc.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 9.Zhang W, Li Y, Li R, Wang Y, Zhu M, Wang B, Li Y, Li D, Xie P, Liu B. Sodium tanshinone IIA sulfonate prevents radiation-induced toxicity in H9c2 cardiomyocytes. Evid Based Complement Alternat Med. 2017;2017:4537974. doi: 10.1155/2017/4537974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan Y, Qian JX, Lu SQ, Chen JW, Zhao XD, Jiang Y, Wang LH, Zhang GX. Protective effects of tanshinone IIA sodium sulfonate on ischemia-reperfusion-induced myocardial injury in rats. Iran J Basic Med Sci. 2017;20:308–315. doi: 10.22038/ijbms.2017.8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu J, Huang H, Liu J, Pi R, Chen J, Liu P. Tanshinone IIA protects cardiac myocytes against oxidative stress-triggered damage and apoptosis. Eur J Pharmacol. 2007;568:213–221. doi: 10.1016/j.ejphar.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 12.Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther. 2009;123:255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Sivasubramanian N, Coker ML, Kurrelmeyer KM, MacLellan WR, DeMayo FJ, Spinale FG, Mann DL. Left ventricular remodeling in transgenic mice with cardiac restricted overexpression of tumor necrosis factor. Circulation. 2001;104:826–831. doi: 10.1161/hc3401.093154. [DOI] [PubMed] [Google Scholar]
- 14.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 15.Yla-Herttuala S, Rissanen TT, Vajanto I, Hartikainen J. Vascular endothelial growth factors: biology and current status of clinical applications in cardiovascular medicine. J Am Coll Cardiol. 2007;49:1015–1026. doi: 10.1016/j.jacc.2006.09.053. [DOI] [PubMed] [Google Scholar]
- 16.Lekas M, Lekas P, Latter DA, Kutryk MB, Stewart DJ. Growth factor-induced therapeutic neovascularization for ischaemic vascular disease: time for a re-evaluation? Curr Opin Cardiol. 2006;21:376–384. doi: 10.1097/01.hco.0000231409.69307.d2. [DOI] [PubMed] [Google Scholar]
- 17.Kastrup J. Therapeutic angiogenesis in ischemic heart disease: gene or recombinant vascular growth factor protein therapy? Curr Gene Ther. 2003;3:197–206. doi: 10.2174/1566523034578366. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Cui X, Zacharek A, Roberts C, Chopp M. eNOS mediates TO90317 treatment-induced angiogenesis and functional outcome after stroke in mice. Stroke. 2009;40:2532–2538. doi: 10.1161/STROKEAHA.108.545095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Besnier M, Galaup A, Nicol L, Henry JP, Coquerel D, Gueret A, Mulder P, Brakenhielm E, Thuillez C, Germain S, Richard V, Ouvrard-Pascaud A. Enhanced angiogenesis and increased cardiac perfusion after myocardial infarction in protein tyrosine phosphatase 1B-deficient mice. FASEB J. 2014;28:3351–3361. doi: 10.1096/fj.13-245753. [DOI] [PubMed] [Google Scholar]
- 20.Wu WY, Wang WY, Ma YL, Yan H, Wang XB, Qin YL, Su M, Chen T, Wang YP. Sodium tanshinone IIA silate inhibits oxygen-glucose deprivation/recovery-induced cardiomyocyte apoptosis via suppression of the NF-kappaB/TNF-alpha pathway. Br J Pharmacol. 2013;169:1058–1071. doi: 10.1111/bph.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu Y, Liang Z, Wang H, Jin J, Zhang S, Xue S, Chen J, He H, Duan K, Wang J, Chang X, Qiu C. Tanshinone IIA protects H9c2 cells from oxidative stress-induced cell death via microRNA-133 upregulation and Akt activation. Exp Ther Med. 2016;12:1147–1152. doi: 10.3892/etm.2016.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen YF, Day CH, Lee NH, Chen YF, Yang JJ, Lin CH, Chen RJ, Rajendran P, Viswanadha VP, Huang CY. Tanshinone IIA inhibits beta-catenin nuclear translocation and IGF-2R activation via estrogen receptors to suppress angiotensin II-Induced H9c2 cardiomyoblast cell apoptosis. Int J Med Sci. 2017;14:1284–1291. doi: 10.7150/ijms.20396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salto-Tellez M, Yung LS, El-Oakley RM, Tang TP, ALmsherqi ZA, Lim SK. Myocardial infarction in the C57BL/6J mouse: a quantifiable and highly reproducible experimental model. Cardiovasc Pathol. 2004;13:91–97. doi: 10.1016/S1054-8807(03)00129-7. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Takemura G, Okada H, Miyata S, Maruyama R, Li L, Higuchi M, Minatoguchi S, Fujiwara T, Fujiwara H. Reduction of inflammatory cytokine expression and oxidative damage by erythropoietin in chronic heart failure. Cardiovasc Res. 2006;71:684–694. doi: 10.1016/j.cardiores.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Hirotani S, Otsu K, Nishida K, Higuchi Y, Morita T, Nakayama H, Yamaguchi O, Mano T, Matsumura Y, Ueno H, Tada M, Hori M. Involvement of nuclear factor-kappaB and apoptosis signal-regulating kinase 1 in G-protein-coupled receptor agonist-induced cardiomyocyte hypertrophy. Circulation. 2002;105:509–515. doi: 10.1161/hc0402.102863. [DOI] [PubMed] [Google Scholar]
- 26.Higuchi Y, Otsu K, Nishida K, Hirotani S, Nakayama H, Yamaguchi O, Matsumura Y, Ueno H, Tada M, Hori M. Involvement of reactive oxygen species-mediated NF-kappa B activation in TNF-alpha-induced cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2002;34:233–240. doi: 10.1006/jmcc.2001.1505. [DOI] [PubMed] [Google Scholar]
- 27.Cheng J, Chen T, Li P, Wen J, Pang N, Zhang L, Wang L. Sodium tanshinone IIA sulfonate prevents lipopolysaccharide-induced inflammation via suppressing nuclear factor-kappaB signaling pathway in human umbilical vein endothelial cells. Can J Physiol Pharmacol. 2018;96:26–31. doi: 10.1139/cjpp-2017-0023. [DOI] [PubMed] [Google Scholar]
- 28.Zhu J, Xu Y, Ren G, Hu X, Wang C, Yang Z, Li Z, Mao W, Lu D. Tanshinone IIA sodium sulfonate regulates antioxidant system, inflammation, and endothelial dysfunction in atherosclerosis by downregulation of CLIC1. Eur J Pharmacol. 2017;815:427–436. doi: 10.1016/j.ejphar.2017.09.047. [DOI] [PubMed] [Google Scholar]
- 29.Crow MT, Mani K, Nam YJ, Kitsis RN. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res. 2004;95:957–970. doi: 10.1161/01.RES.0000148632.35500.d9. [DOI] [PubMed] [Google Scholar]
- 30.Moll UM, Wolff S, Speidel D, Deppert W. Transcription-independent pro-apoptotic functions of p53. Curr Opin Cell Biol. 2005;17:631–636. doi: 10.1016/j.ceb.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Lv YX, Zhong S, Tang H, Luo B, Chen SJ, Chen L, Zheng F, Zhang L, Wang L, Li XY, Yan YW, Pan YM, Jiang M, Zhang YE, Wang L, Yang JY, Guo LY, Chen SY, Wang JN, Tang JM. VEGF-A and VEGF-B Coordinate the arteriogenesis to repair the infarcted heart with vagus nerve stimulation. Cell Physiol Biochem. 2018;48:433–449. doi: 10.1159/000491775. [DOI] [PubMed] [Google Scholar]
- 32.Lin YD, Luo CY, Hu YN, Yeh ML, Hsueh YC, Chang MY, Tsai DC, Wang JN, Tang MJ, Wei EI, Springer ML, Hsieh PC. Instructive nanofiber scaffolds with VEGF create a microenvironment for arteriogenesis and cardiac repair. Sci Transl Med. 2012;4:146ra109. doi: 10.1126/scitranslmed.3003841. [DOI] [PubMed] [Google Scholar]
- 33.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 34.LeCouter J, Kowalski J, Foster J, Hass P, Zhang Z, Dillard-Telm L, Frantz G, Rangell L, DeGuzman L, Keller GA, Peale F, Gurney A, Hillan KJ, Ferrara N. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature. 2001;412:877–884. doi: 10.1038/35091000. [DOI] [PubMed] [Google Scholar]


