Abstract
Long noncoding RNAs (lncRNAs) have been shown to have several functional roles in tumor biology, and they are deregulated in many types of cancer. The role of a novel lncRNA, NORAD, in papillary thyroid carcinoma (PTC) is still unknown. In this study, we demonstrated that NORAD expression was upregulated in PTC cell lines and samples. Ectopic expression of NORAD promoted PTC cell growth, invasion and migration. Overexpression of NORAD promotes epithelial-to-mesenchymal transition (EMT) progression in the PTC cell. Furthermore, overexpression of NORAD suppressed miR-202-5p expression in PTC cells. The data suggested that miR-202-5p expression was downregulated in PTC cell lines and samples and was negatively correlated with NORAD expression in PTC tissues. Overexpression of miR-202-5p suppressed PTC cell growth, invasion and migration. In addition, we demonstrated that elevated expression of NORAD promoted PTC cell growth, invasion and migration by inhibiting miR-202-5p expression. These results suggested that the lncRNA NORAD acts as an oncogene in PTC progression, partly by regulating miR-202-5p expression.
Keywords: Papillary thyroid carcinoma, long noncoding RNA, NORAD, miR-202-5p
Introduction
Thyroid cancer is the most frequent endocrine malignancy, and 80-90% of thyroid malignancies are papillary thyroid carcinoma (PTC) [1-5]. The incidence and prevalence of PTC have rapidly increased over the past twenty years [6,7]. PTC patients in the early stage have excellent prognosis, and the five-year survival rate is approximately 97% [8,9]. However, the 5-year survival rate of PTC cases at later stages is less than 59% [10,11]. Therefore, there is an urgent need to investigate new factors that affect the diagnosis and pathogenesis of PTC.
Long noncoding RNAs (lncRNAs) were previously regarded as transcriptional noise but have emerged as novel modulators in the tumor paradigm [12-17]. Emerging evidence suggests that lncRNA expression is misregulated in numerous tumor types, and aberrant expression of lncRNA may contribute to tumorigenesis [18-21]. Furthermore, lncRNAs plays multiple crucial roles in cellular processes such as cell development, growth, differentiation, invasion, migration and apoptosis [22-24]. Recently, a novel lncRNA, NORAD, was identified [25]. Wu et al. [26] showed that the expression of NORAD was upregulated in esophageal squamous cell carcinoma (ESCC) tissues compared to adjacent normal samples. Li et al. [27] showed that the expression of NORAD was upregulated in pancreatic cancer samples, and higher expression of NORAD was correlated with shorter OS (overall survival). Ectopic expression of NORAD increased pancreatic carcinoma cell invasion and migration. Furthermore, Zhang et al. [28] indicated that NORAD expression was upregulated in colorectal cancer (CRC) samples, and high NORAD expression was correlated with poor prognosis and CRC metastasis. However, the expression and function of NORAD are largely unknown in PTC.
In the present study, we tried to measure the expression of the lncRNA NORAD in PTC cell lines and samples. We demonstrated that NORAD expression was upregulated in PTC cell lines and samples. Ectopic expression of NORAD promoted PTC cell growth, invasion and migration.
Materials and methods
Patient tissues and culture and transfection of cell lines
Forty freshly frozen PTC tissues and adjacent noncancerous samples were collected from PTC patients who underwent tumor resection at the Affiliated Tumor Hospital of Harbin Medical University. All tissues were preserved immediately in liquid nitrogen. All patients signed the informed consent form allowing our research on the collected specimens. Our study was conducted with approval of the Institutional Review Board of the Affiliated Tumor Hospital of Harbin Medical University and followed the Declaration of Helsinki. One normal thyroid cell line (HT-ori3) and four PTC cell lines (K1, BCPAP, TPC1 and NPA187) were purchased from ATCC (American Type Culture Collection; Manassas, VA, USA). All cell lines were kept in DMEM (Gibco, USA) supplemented with fetal bovine serum in the humidified atmosphere with 5% CO2 at 37°C. The PTC cell lines were transfected with pcDNA-NORAD, negative control, miR-202-5p mimic or scramble using Lipofectamine 3000 following the manufacturer’s instructions.
Cell counting Kit-8 assay and invasion and migration assays
Cell growth was analyzed using the CCK8 kit (Cell Counting Kit-8) (Dojindo, Kumamoto, Japan) according to manufacturer’s protocol. Cell growth was measured every day. In brief, ten microliters of CCK8 solution was placed in each cell well, and the plates continued to incubate for 2 hours. The absorbance (OD) at 450 nm was determined on the microplate reader. For cell migration, cells were seeded in 6-well plates and cultured to the appropriate confluence. The cell wound was created with sterile plastic tips. Cells were cultured for 48 hours with serum-free medium. Images were taken at different time points by using a microscope. For the cell invasion assay, transwell chambers were utilized. The upper chamber was coated with Matrigel, and the cells were cultured in the upper chamber with serum-free medium. After 48 hours, the cells were fixed with ethanol, stained with crystal violet and then counted under a microscope (Olympus Corp., Japan).
Quantitative real-time PCR (qRT-PCR)
Total RNA from cells or samples was extracted by using TRIzol (Invitrogen, USA) and then reverse transcribed into cDNA using a reverse transcriptase reagent (Takara Bio, Japan). The lncRNA, miRNA and mRNA expression was analyzed by qRT-PCR by using SYBR green (Takara Biotechnology Co., Dalian, China) on an ABI 7500 Real-Time PCR system (Applied Biosystems, CA, USA). The relative mRNA expression was normalized to GAPDH expression, and miRNA and lncRNA expression was relative to U6 expression. The PCR primer sequences were as follows: NORAD, forward: 5’-TCCTGTTTACAGCGAGGCAA-3’ and reverse: 5’-CCATCTCCATCAACCCAGAAGA-3’; GAPDH, forward: 5’-GGAGCGAGATCCCTCCAAAA-3’ and reverse: 5’-GGCTGTTGTCATACTTCTCATGG-3’. The relative expression was calculated using the 2-ΔΔCT method.
Western blot analysis
Equal Protein was separated on the 10% sodium dodecyl sulfate (SDS)-PAGE gel and transferred to the nitrocellulose membrane (Bio-Rad, China). After treated with non-fat milk, membrane was incubated with primary antibodies (ZEB1, Vimentin and N-cadherin, E-cadherin, Sigma, USA). After being washed for three times, anti-mouse secondary antibodies were added to the membrane. The membrane was then visualized using enhanced chemiluminescence.
Statistical analysis
Statistical analysis was determined by using SPSS software (version 18.0, IBM, Chicago, USA). The significance of differences between groups was analyzed by one-way ANOVA or Student’s t-test. A P value < 0.05 was considered significant.
Results
NORAD was increased in PTC cell lines and samples
To study the role of the lncRNA NORAD in PTC, we first measured the expression of NORAD in PTC samples and cell lines. By qRT-PCR analysis, we demonstrated that NORAD expression was upregulated in four PTC cell lines (NPA187, TPC1, BCPAP and K1) compared to the normal thyroid cell line (HT-ori3) (Figure 1A). As shown in Figure 1B, NORAD expression was higher in PTC samples than in adjacent noncancerous samples. Of the 40 PTC samples, 29 (72.5%) showed upregulation of NORAD expression compared to adjacent tissues (Figure 1C).
Figure 1.
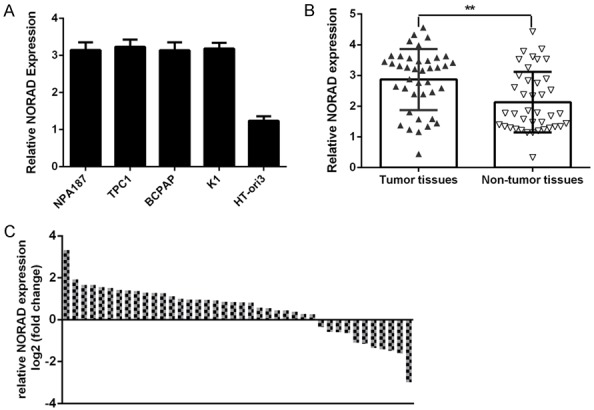
NORAD was increased in PTC cell lines and samples. A. The expression of the lncRNA NORAD in four PTC cell lines (NPA187, TPC1, BCPAP and K1) and in a normal thyroid cell line (HT-ori3) was determined by qRT-PCR analysis. B. The expression of the lncRNA NORAD in PTC tissues was measured by qRT-PCR. C. Of 40 PTC samples, 29 (72.5%) showed upregulation of NORAD expression compared to adjacent tissues. Data are presented as the log2 fold change in PTC tissues relative to nontumor tissues. **P < 0.01.
miR-202-5p was decreased in PTC cell lines and samples
To further investigated the role of miR-202-5p in PTC, we measured the expression of miR-202-5p in PTC samples and cell lines. By qRT-PCR analysis, we demonstrated that miR-202-5p expression was downregulated in four PTC cell lines (NPA187, TPC1, BCPAP and K1) compared to the normal thyroid cell line (HT-ori3) (Figure 1A). As shown in Figure 2B, miR-202-5p expression was lower in PTC samples than in adjacent noncancerous samples. Of the 40 PTC samples, 28 (70%) showed downregulation of miR-202-5p expression compared to adjacent tissues (Figure 2C). Furthermore, we showed that miR-202-5p expression was negatively correlated with NORAD expression in the PTC samples (Figure 2D).
Figure 2.
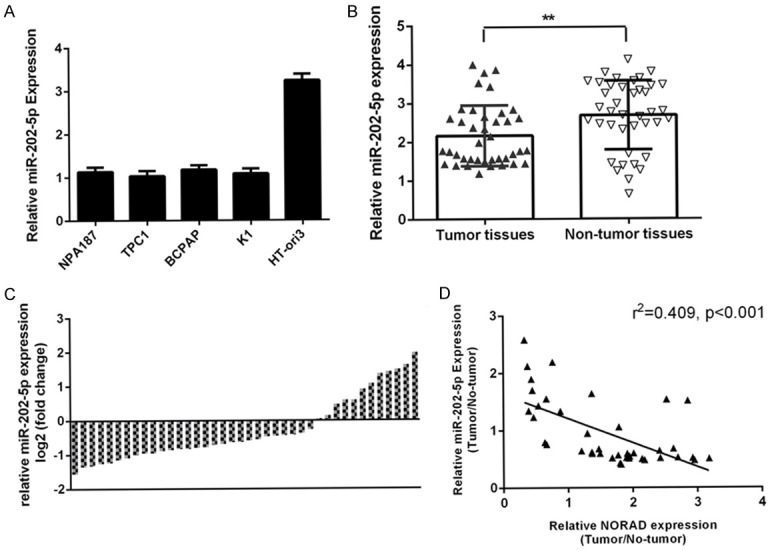
miR-202-5p was decreased in PTC cell lines and samples. A. The expression of miR-202-5p in four PTC cell lines (NPA187, TPC1, BCPAP and K1) and a normal thyroid cell line (HT-ori3) was determined by qRT-PCR analysis. B. The expression of miR-202-5p in PTC tissues was measured by qRT-PCR. C. Of 40 PTC samples, 28 (70%) showed downregulation of NORAD expression compared to adjacent tissues. Data are presented as the log2 fold change in PTC tissues relative to nontumor tissues. D. The expression of miR-202-5p was negatively correlated with NORAD expression in PTC samples. **P < 0.01.
NORAD regulated miR-202-5p expression in PTC cells
To study the downstream genes regulated by NORAD, we searched for its associated miRNAs. There was a potential binding site for miR-202-5p on NORAD (Figure 3A). The expression of miR-202-5p and NORAD was upregulated in PTC cells transduced with the miR-202-5p mimic or pcDNA-NORAD, respectively (Figure 3B). We further performed luciferase reporter assays and found that ectopic expression of miR-202-5p decreased luciferase activity in PTC cells transduced with the WT NORAD reporter vector (Figure 3C). Furthermore, we showed that overexpression of NORAD suppressed miR-202-5p expression in PTC cells (Figure 3D).
Figure 3.
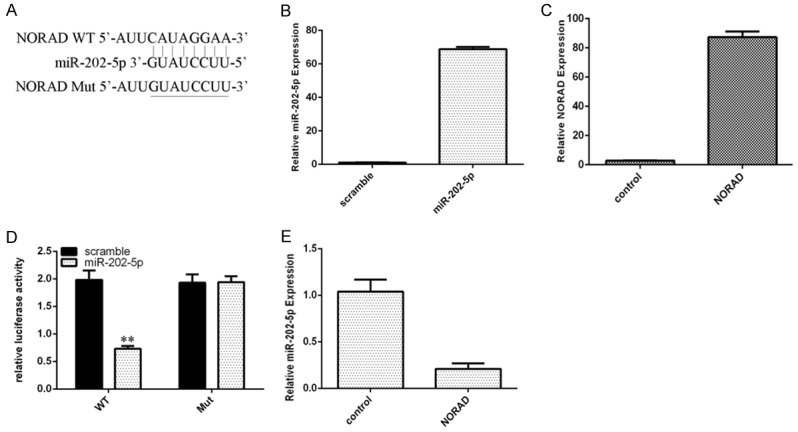
NORAD regulated miR-202-5p expression in PTC cells. A. There is a potential binding site for miR-202-5p in NORAD. B. The expression of miR-202-5p in TPC1 cells was determined by qRT-PCR. C. The expression of NORAD in TPC1 cells was determined by qRT-PCR. D. Ectopic expression of miR-202-5p decreased luciferase activity in PTC cells transduced with the WT NORAD-reporter vector. E. Overexpression of NORAD suppressed miR-202-5p expression in TPC1 cells. **P < 0.01.
Ectopic expression of NORAD promoted PTC cell growth, invasion and migration
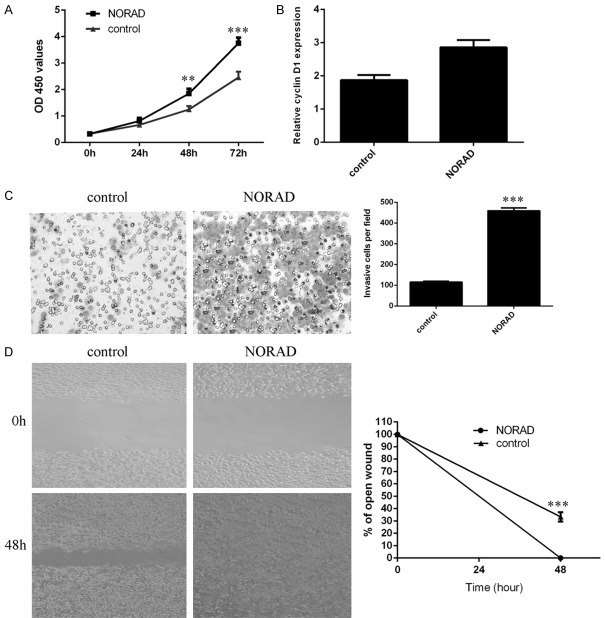
By using the CCK-8 assay, we showed that the ectopic expression of NORAD increased PTC cell proliferation (Figure 4A). We also found that overexpression of NORAD promoted cyclin D1 expression in PTC cells (Figure 4B). Furthermore, we showed that ectopic expression of NORAD promoted PTC cell invasion, and the relative cell invasion is shown (Figure 4C). To study the effect of NORAD on cell migration, wound scratch assays were performed. It was found that elevated expression of NORAD promoted PTC cell migration (Figure 4D).
Figure 4.
Ectopic expression of NORAD promoted PTC cell growth, invasion and migration. A. Ectopic expression of NORAD increased PTC cell proliferation, as shown by CCK-8 assays. B. Overexpression of NORAD promoted cyclin D1 expression in TPC1 cells. C. Ectopic expression of NORAD promoted PTC cell invasion, and relative cell invasion is shown on the right. D. Elevated expression of NORAD promoted PTC cell migration; relative wound closure is shown on the right. **P < 0.01 and ***P < 0.001.
Overexpression of NORAD promotes epithelial-to-mesenchymal transition (EMT) progression in the PTC cell
Furthermore, we showed that ectopic expression of NORAD induced the expression of ZEB1 expression in the PTC cell (Figure 5A). In addition, overexpression of NORAD promoted the expression of Vimentin (Figure 5B) and N-cadherin (Figure 5C) in the PTC cell. Elevated expression of NORAD decreased the E-cadherin expression in the PTC cell. In line with this, we demonstrated that overexpression of NORAD promoted the expression of ZEB1, Vimentin and N-cadherin and suppressed the expression of N-cadherin in the PTC cell (Figures 5D, S1). These data suggested that Overexpression of NORAD promotes EMT progression in the PTC cell.
Figure 5.
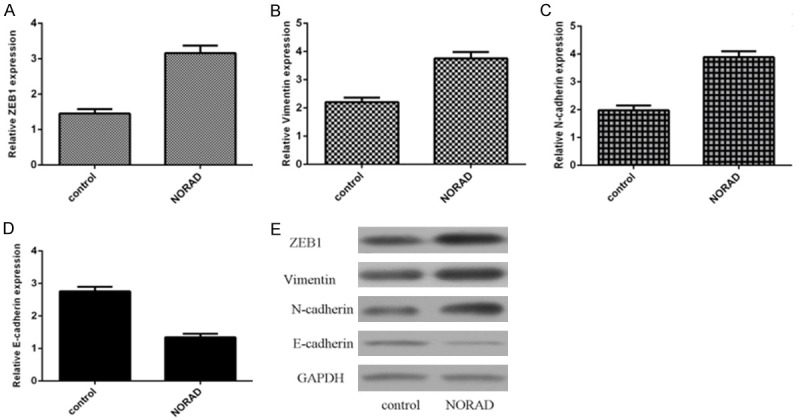
Overexpression of NORAD promotes epithelial-to-mesenchymal transition (EMT) progression in the PTC cell. A. The expression of ZEB1 was detected by using qRT-PCR analysis. B. Ectopic expression of NORAD promoted the vimentin expression in the PTC cell. C. The expression of N-cadherin was measured by using qRT-PCR analysis. D. Ectopic expression of NORAD suppressed the E-cadherin expression in the PTC cell. E. The protein expression of ZEB1, vimentin, N-cadherin and E-cadherin was measured by western blotting. GAPDH was used as the internal control.
Overexpression of miR-202-5p suppressed PTC cell growth, invasion and migration
By CCK-8 assay, we found that ectopic expression of miR-202-5p decreased PTC cell proliferation (Figure 6A). We also showed that overexpression of miR-202-5p suppressed cyclin D1 expression in PTC cells (Figure 6B). Furthermore, we showed that ectopic expression of miR-202-5p suppressed PTC cell invasion, and the relative cell invasion is shown (Figure 6C). To study the effect of miR-202-5p on cell migration, wound scratch assays were performed. The data indicated that elevated expression of miR-202-5p inhibited PTC cell migration (Figure 6D).
Figure 6.
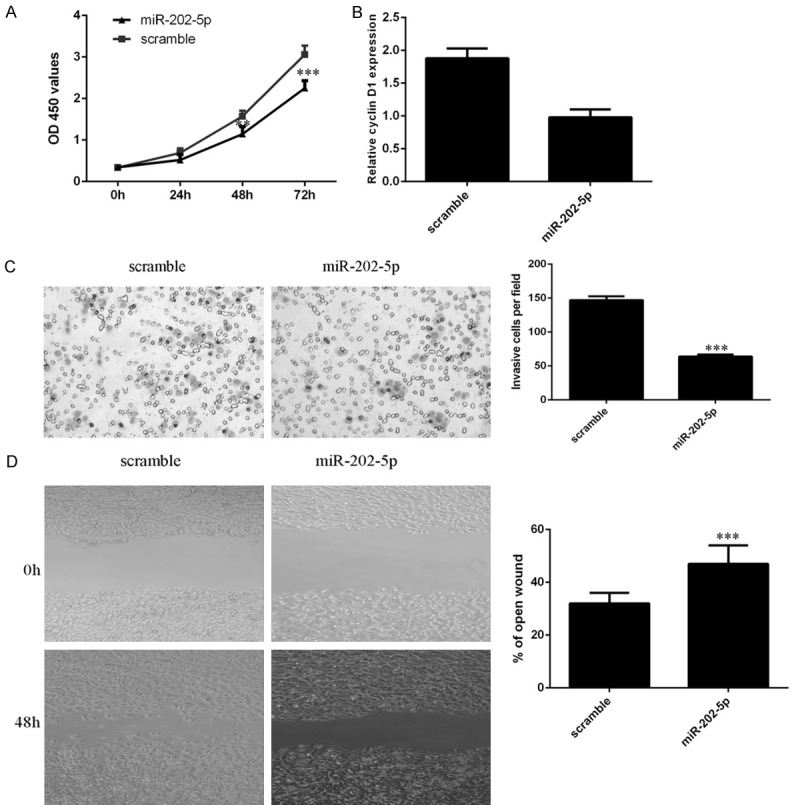
Overexpression of miR-202-5p suppressed PTC cell growth, invasion and migration. A. Ectopic expression of miR-202-5p decreased PTC cell proliferation, as shown by CCK-8 assays. B. Overexpression of miR-202-5p suppressed cyclin D1 expression in TPC1 cells. C. Ectopic expression of miR-202-5p inhibited PTC cell invasion, and relative cell invasion is shown on the right. D. Elevated expression of miR-202-5p decreased PTC cell migration; relative wound closure is shown on the right. **P < 0.01 and ***P < 0.001.
Elevated expression of NORAD promoted PTC cell growth, invasion and migration by regulating miR-202-5p
To further explore the molecular mechanisms by which NORAD influences cell function, we conducted rescue experiments. We demonstrated that ectopic expression of miR-202-5p decreased PTC cell proliferation induced by NORAD overexpression (Figure 7A). We also showed that overexpression of miR-202-5p inhibited the cyclin D1 expression induced by NORAD (Figure 7B). Furthermore, the data indicated that ectopic expression of miR-202-5p decreased the PTC cell invasion induced by NORAD (Figure 7C). Via the wound scratch assay, we found that miR-202-5p overexpression suppressed NORAD-induced PTC cell migration (Figure 7D).
Figure 7.
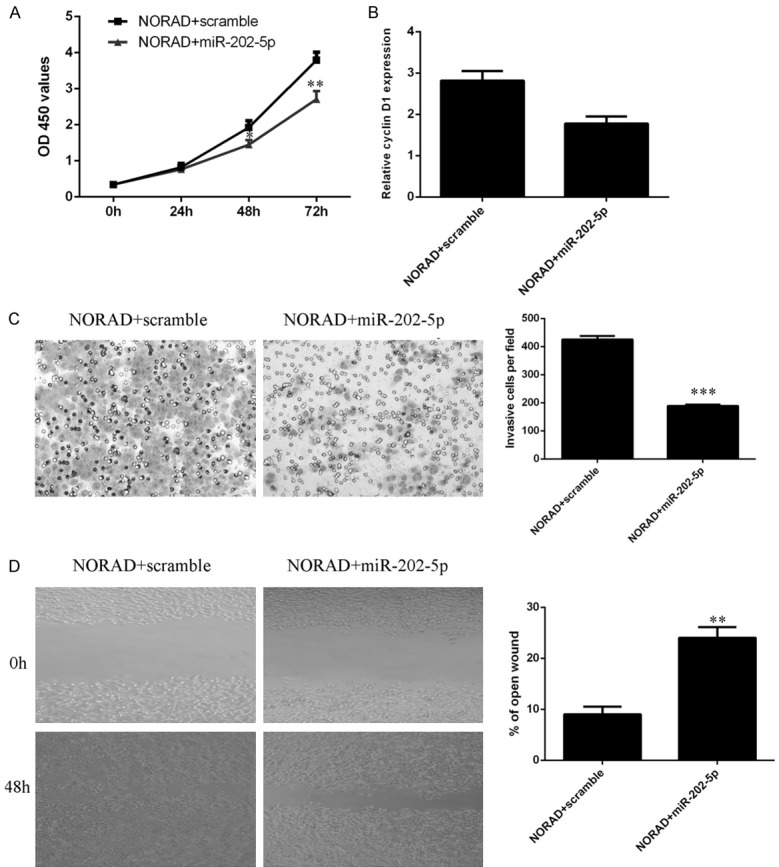
Elevated expression of NORAD promoted PTC cell growth, invasion and migration by regulating miR-202-5p. A. Cell growth was determined by using CCK-8 assays. B. The mRNA expression of cyclin D1 was analyzed by qRT-PCR analysis. C. Ectopic expression of miR-202-5p decreased PTC cell invasion induced by NORAD; relative cell invasion is shown on the right. D. By wound scratch assay, we found that miR-202-5p overexpression suppressed PTC cell migration induced by NORAD. The relative wound closure is shown on the right. *P < 0.05, **P < 0.01 and ***P < 0.001.
xidentified. Wu et al. [26] showed that the expression of NORAD was upregulated in esophageal squamous cell carcinoma (ESCC) tissues compared to adjacent normal samples. In addition, high NORAD expression was associated with T stage and larger tumor size. ESCC patients with high expression of NORAD have poor disease-free survival. Li et al. [27] showed that the expression of NORAD was upregulated in pancreatic cancer samples, and higher expression of NORAD was correlated with shorter OS (overall survival). Ectopic expression of NORAD increases pancreatic carcinoma cell invasion and migration. Furthermore, Zhang et al. [28] indicated that NORAD expression was upregulated in colorectal cancer (CRC) samples, and high NORAD expression was correlated with poor prognosis and CRC metastasis. Knockdown of NORAD suppresses CRC cell invasion, migration and proliferation by regulating miR-202-5p expression. However, the expression and function of NORAD are largely unknown in PTC. In our study, we first determined the expression of NORAD in PTC cells and tissues. We demonstrated that NORAD expression was upregulated in PTC cell lines and samples. Ectopic expression of NORAD promoted PTC cell growth, invasion and migration.
LncRNAs can act as sponges by binding to miRNAs and modulating their function [37-39]. Wang et al. [40] showed that the lncRNA MEG3 was downregulated in cervical cancer tissues and suppressed cell growth by modulating miR-21 expression. Sun et al. [41] demonstrated that the expression of the lncRNA MALAT1 was upregulated in uveal melanoma tissues and that overexpression of MALAT1 increased uveal melanoma cell proliferation and invasion by silencing miR-140 expression. Moreover, Zhang et al. [28] showed that knockdown of NORAD inhibited CRC cell invasion, migration and proliferation by regulating miR-202-5p expression. We also performed luciferase reporter assays and found that overexpression of NORAD suppressed miR-202-5p expression in PTC cells. We then showed that the expression of miR-202-5p was downregulated in PTC cell lines and samples and that miR-202-5p expression was negatively correlated with NORAD expression in PTC tissues. Overexpression of miR-202-5p suppressed PTC cell growth, invasion and migration. In addition, we demonstrated that elevated expression of NORAD promoted PTC cell growth, invasion and migration by inhibiting miR-202-5p expression.
In summary, we demonstrated that the expression of NORAD was upregulated in PTC cell lines and tissues. Ectopic expression of NORAD increased PTC cell proliferation, invasion and migration, partly by regulating miR-202-5p expression. These results suggest that the lncRNA NORAD acts as an oncogene in PTC progression, partly by regulating miR-202-5p expression.
Acknowledgements
The work was supported by the China Postdoctoral Science Foundation Project (2018M641840) and the Heilongjiang Provincial Chinese Medicine Research Project (ZHY12-Z176) and the Haiyan Scientific Research Fund in the Affiliated Tumor Hospital of Harbin Medical University (No. JJMS2014-02).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Ma Y, Qin H, Cui Y. MiR-34a targets GAS1 to promote cell proliferation and inhibit apoptosis in papillary thyroid carcinoma via PI3K/Akt/Bad pathway. Biochem Biophys Res Commun. 2013;441:958–963. doi: 10.1016/j.bbrc.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Zhao M, Wang KJ, Tan Z, Zheng CM, Liang Z, Zhao JQ. Identification of potential therapeutic targets for papillary thyroid carcinoma by bioinformatics analysis. Oncol Lett. 2016;11:51–58. doi: 10.3892/ol.2015.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lima CR, Geraldo MV, Fuziwara CS, Kimura ET, Santos MF. MiRNA-146b-5p upregulates migration and invasion of different Papillary Thyroid Carcinoma cells. BMC Cancer. 2016;16 doi: 10.1186/s12885-016-2146-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu C, Chen T, Zeng W, Wang S, Xiong Y, Liu Z, Huang T. Reevaluating the prognostic significance of male gender for papillary thyroid carcinoma and microcarcinoma: a SEER database analysis. Sci Rep. 2017;7:11412. doi: 10.1038/s41598-017-11788-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Lu X, Geng Z, Yang G, Shi Y. LncRNA PTCSC3/miR-574-5p governs cell proliferation and migration of papillary thyroid carcinoma via Wnt/β-catenin signaling. J Cell Biochem. 2017;118:4745–4752. doi: 10.1002/jcb.26142. [DOI] [PubMed] [Google Scholar]
- 6.Liu C, Zhao Q, Zeng W, Chen C, Ming J, Wang S, Xiong Y, Zhang C, Chen T, Liu Z, Huang T. Do patients with oxyphilic cell papillary thyroid carcinoma have a poor prognosis? Analysis of the surveillance, epidemiology, and end results database 2004-2013 with propensity score matching. Oncotarget. 2017;8:77075–77085. doi: 10.18632/oncotarget.20355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lv T, Zhu C, Di Z. Risk factors stratifying malignancy of nodules in contralateral thyroid lobe in patients with pre-operative ultrasound indicated unilateral papillary thyroid carcinoma: a retrospective analysis from single centre. Clin Endocrinol (Oxf) 2018;88:279–284. doi: 10.1111/cen.13506. [DOI] [PubMed] [Google Scholar]
- 8.Mansour J, Sagiv D, Alon E, Talmi Y. Prognostic value of lymph node ratio in metastatic papillary thyroid carcinoma. J Laryngol Otol. 2018;132:8–13. doi: 10.1017/S0022215117002250. [DOI] [PubMed] [Google Scholar]
- 9.Viola D, Materazzi G, Valerio L, Molinaro E, Agate L, Faviana P, Seccia V, Sensi E, Romei C, Piaggi P, Torregrossa L, Sellari-Franceschini S, Basolo F, Vitti P, Elisei R, Miccoli P. Prophylactic central compartment lymph node dissection in papillary thyroid carcinoma: clinical implications derived from the first prospective randomized controlled single institution study. J Clin Endocrinol Metab. 2015;100:1316–1324. doi: 10.1210/jc.2014-3825. [DOI] [PubMed] [Google Scholar]
- 10.Li G, Lei J, Peng Q, Jiang K, Chen W, Zhao W, Li Z, Gong R, Wei T, Zhu J. Lymph node metastasis characteristics of papillary thyroid carcinoma located in the isthmus: a single-center analysis. Medicine (Baltimore) 2017;96:e7143. doi: 10.1097/MD.0000000000007143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Londero S, Krogdahl A, Bastholt L, Overgaard J, Pedersen H, Hahn C, Bentzen J, Schytte S, Christiansen P, Gerke O, Godballe C. Papillary thyroid carcinoma in Denmark, 1996-2008: outcome and evaluation of established prognostic scoring systems in a prospective national cohort. Thyroid. 2015;25:78–84. doi: 10.1089/thy.2014.0294. [DOI] [PubMed] [Google Scholar]
- 12.Yang C, Wu K, Wang S, Wei G. Long non-coding RNA XIST promotes osteosarcoma progression by targeting YAP via miR-195-5p. J Cell Biochem. 2018;119:5646–5656. doi: 10.1002/jcb.26743. [DOI] [PubMed] [Google Scholar]
- 13.Alipoor F, Asadi M, Torkzadeh-Mahani M. Miat lncRNA is overexpressed in breast cancer and its inhibition triggers senescence and G1 arrest in MCF7 cell line. J Cell Biochem. 2018;119:6470–6481. doi: 10.1002/jcb.26678. [DOI] [PubMed] [Google Scholar]
- 14.Zhao J, Gao Z, Zhang C, Wu H, Gu R, Jiang R. Long non-coding RNA ASBEL promotes osteosarcoma cell proliferation, migration and invasion by regulating microRNA-21. J Cell Biochem. 2018;119:6461–6469. doi: 10.1002/jcb.26671. [DOI] [PubMed] [Google Scholar]
- 15.Wang ZW, Jin YY, Ren HT, Ma XL, Wang BF, Wang YL. Downregulation of the long non-coding RNA TUSC7 promotes NSCLC cell proliferation and correlates with poor prognosis. Am J Transl Res. 2016;8:680–7. [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Lian Y, Yan C, Cai Z, Ding J, Ma Z, Peng P, Wang K. Long non-coding RNA FOXP4-AS1 is an unfavourable prognostic factor and regulates proliferation and apoptosis in colorectal cancer. Cell Prolif. 2017;50 doi: 10.1111/cpr.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xin Y, Li Z, Shen J, Chan M, Wu W. CCAT1: a pivotal oncogenic long non-coding RNA in human cancers. Cell Prolif. 2016;49:255–260. doi: 10.1111/cpr.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z, Shen J, Chan M, Wu W. TUG1: a pivotal oncogenic long non-coding RNA of human cancers. Cell Prolif. 2016;49:471–475. doi: 10.1111/cpr.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang YH, Fu J, Zhang ZJ, Ge CC, Yi Y. LncRNA-LINC00152 down-regulated by miR-376c-3p restricts viability and promotes apoptosis of colorectal cancer cells. Am J Transl Res. 2016;8:5286–5297. [PMC free article] [PubMed] [Google Scholar]
- 20.Huang CS, Liu SG, Wang HJ, Zhang ZC, Yang Q, Gao F. LncRNA PVT1 overexpression is a poor prognostic biomarker and regulates migration and invasion in small cell lung cancer. Am J Transl Res. 2016;8:5025–5034. [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang YY, Yang R, Lian JC, Xu HY. LncRNA Sox2ot overexpression serves as a poor prognostic biomarker in gastric cancer. Am J Transl Res. 2016;8:5035–5043. [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang XY, Zhang LX, Tian CJ, Tang XY, Zhao LM, Guo YL, Cheng DJ, Chen XL, Ma LJ, Chen ZC. LncRNAs BCYRN1 promoted the proliferation and migration of rat airway smooth muscle cells in asthma via upregulating the expression of transient receptor potential 1. Am J Transl Res. 2016;8:3409–3418. [PMC free article] [PubMed] [Google Scholar]
- 23.Dou J, Ni YY, He XF, Wu D, Li M, Wu SY, Zhang R, Guo M, Zhao FS. Decreasing lncRNA HOTAIR expression inhibits human colorectal cancer stem cells. Am J Transl Res. 2016;8:98–108. [PMC free article] [PubMed] [Google Scholar]
- 24.Zou ZW, Ma C, Medoro L, Chen LL, Wang B, Gupta R, Liu T, Yang XZ, Chen TT, Wang RZ, Zhang WJ, Li PD. LncRNA ANRIL is up-regulated in nasopharyngeal carcinoma and promotes the cancer progression via increasing proliferation, reprograming cell glucose metabolism and inducing side-population stem-like cancer cells. Oncotarget. 2016;7:61741–61754. doi: 10.18632/oncotarget.11437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee S, Kopp F, Chang T, Sataluri A, Chen B, Sivakumar S, Yu H, Xie Y, Mendell J. Noncoding RNA NORAD regulates genomic stability by sequestering PUMILIO proteins. Cell. 2016;164:69–80. doi: 10.1016/j.cell.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu X, Lim Z, Li Z, Gu L, Ma W, Zhou Q, Su H, Wang X, Yang X, Zhang Z. NORAD expression is associated with adverse prognosis in esophageal squamous cell carcinoma. Oncol Res Treat. 2017;40:370–374. doi: 10.1159/000464465. [DOI] [PubMed] [Google Scholar]
- 27.Li H, Wang X, Wen C, Huo Z, Wang W, Zhan Q, Cheng D, Chen H, Deng X, Peng C, Shen B. Long noncoding RNA NORAD, a novel competing endogenous RNA, enhances the hypoxia-induced epithelial-mesenchymal transition to promote metastasis in pancreatic cancer. Mol Cancer. 2017;16:169. doi: 10.1186/s12943-017-0738-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Li X, Hu P, Ding Y. LncRNA NORAD contributes to colorectal cancer progression by inhibition of miR-202-5p. Oncol Res. 2018 doi: 10.3727/096504018X15190844870055. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Li Z, Zheng W, Li X, Wang Z, Cui Y, Jiang X. LncRNA-ATB: an indispensable cancer-related long noncoding RNA. Cell Prolif. 2017;50 doi: 10.1111/cpr.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiong GY, Yang LH, Chen Y, Fan ZN. Linc-POU3F3 promotes cell proliferation in gastric cancer via increasing T-reg distribution. Am J Transl Res. 2015;7:2262–2269. [PMC free article] [PubMed] [Google Scholar]
- 31.Peng W, Si S, Zhang Q, Li C, Zhao F, Wang F, Yu J, Ma R. Long non-coding RNA MEG3 functions as a competing endogenous RNA to regulate gastric cancer progression. J Exp Clin Cancer Res. 2015;34:79. doi: 10.1186/s13046-015-0197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cai L, Lv J, Zhang Y, Li J, Wang Y, Yang H. The lncRNA HNF1A-AS1 is a negative prognostic factor and promotes tumorigenesis in osteosarcoma. J Cell Mol Med. 2017;21:2654–2662. doi: 10.1111/jcmm.12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo Q, Qian Z, Yan D, Li L, Huang L. LncRNA-MEG3 inhibits cell proliferation of endometrial carcinoma by repressing notch signaling. Biomed Pharmacother. 2016;82:589–594. doi: 10.1016/j.biopha.2016.02.049. [DOI] [PubMed] [Google Scholar]
- 34.Modali SD, Parekh VI, Kebebew E, Agarwal SK. Epigenetic regulation of the lncRNA MEG3 and its target c-MET in pancreatic neuroendocrine tumors. Mol Endocrinol. 2015;29:224–237. doi: 10.1210/me.2014-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang F, Xue X, Bi J, Zheng L, Zhi K, Gu Y, Fang G. Long noncoding RNA CCAT1, which could be activated by c-Myc, promotes the progression of gastric carcinoma. J Cancer Res Clin Oncol. 2013;139:437–445. doi: 10.1007/s00432-012-1324-x. [DOI] [PubMed] [Google Scholar]
- 36.Zhu H, Zhou X, Chang H, Li H, Liu F, Ma C, Lu J. CCAT1 promotes hepatocellular carcinoma cell proliferation and invasion. Int J Clin Exp Pathol. 2015;8:5427–5434. [PMC free article] [PubMed] [Google Scholar]
- 37.Ma MZ, Chu BF, Zhang Y, Weng MZ, Qin YY, Gong W, Quan ZW. Long non-coding RNA CCAT1 promotes gallbladder cancer development via negative modulation of miRNA-218-5p. Cell Death Dis. 2015;6:e1583. doi: 10.1038/cddis.2014.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng L, Yang SB, Xu FF, Zhang JH. Long noncoding RNA CCAT1 promotes hepatocellular carcinoma progression by functioning as let-7 sponge. J Exp Clin Cancer Res. 2015;34:18. doi: 10.1186/s13046-015-0136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang S, Zhang W, Wu X, Weng M, Zhang M, Cai Q, Zhou D, Wang J, Quan Z. The lncRNA MALAT1 functions as a competing endogenous RNA to regulate MCL-1 expression by sponging miR-363-3p in gallbladder cancer. J Cell Mol Med. 2016;20:2299–2308. doi: 10.1111/jcmm.12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang J, Yao T, Wang Y, Yu J, Liu Y, Lin Z. Long noncoding RNA MEG3 is downregulated in cervical cancer and affects cell proliferation and apoptosis by regulating miR-21. Cancer Biol Ther. 2016;17:104–113. doi: 10.1080/15384047.2015.1108496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun L, Sun P, Zhou Q, Gao X, Han Q. Long noncoding RNA MALAT1 promotes uveal melanoma cell growth and invasion by silencing of miR-140. Am J Transl Res. 2016;8:3939–3946. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.