Abstract
Background/aims: All chronic kidney disease (CKD) can eventually develop into renal fibrosis. We explored the renoprotective effects of a gastric peptide, ghrelin, and investigated whether endoplasmic reticulum stress (ERS) and the NLR family pyrin domain-containing 3 (NLRP3) inflammasome mediate the protective effect of ghrelin in unilateral ureteral obstruction (UUO). Methods: Male C57BL/6J mice were divided into vehicle- or ghrelin-treated sham-operated groups and vehicle- or ghrelin-treated UUO groups. The kidneys were harvested on postoperative day 14. Renal fibrosis was evaluated by periodic acid-Schiff, Masson trichrome, and immunohistochemical (IHC) staining. To assess renal fibrosis, α-smooth muscle actin and type I collagen were detected. NLRP3 inflammasome and ERS activation were also detected via western blotting. The effect of ghrelin on cultured renal cells was further confirmed in HK-2 cells. Results: Compared with the sham mice, UUO mice developed obvious renal fibrosis; pathological and IHC staining showed increased matrix accumulation and elevated ERS, NLRP3 inflammasome was activated both in vivo and in vitro. Ghrelin significantly attenuated collagen fibril accumulation and apoptosis by reducing NLRP3 inflammasome activation and ERS in obstructed kidneys. Conclusions: Ghrelin may attenuate UUO-induced renal fibrosis by inhibiting the NLRP3 inflammasome and ERS in vivo. Therefore, ghrelin might be an effective strategy for preventing CKD.
Keywords: Ghrelin, renal fibrosis, NLRP3 inflammasome, endoplasmic reticulum stress
Introduction
Renal fibrosis is a common pathway of chronic kidney disease (CKD) that ultimately leads to end-stage renal failure. Various primary glomerular diseases such as ureteral obstruction and diabetes can induce inflammatory cell infiltration in the renal interstitium, tubular epithelium cell apoptosis, and myofibroblast accumulation, which can promote pro-fibrotic factors, increasing the production of extracellular matrix (ECM) and reducing its degradation. Subsequently, renal interstitial fibrosis develops and damages renal function [1]. At present, slowing the prognosis by controlling the risk factors that aggravate the deterioration of renal function and renal fibrosis is not satisfactory in the clinic [2]. Accordingly, preventing and curing renal fibrosis effectively presents a major challenge.
Endoplasmic reticulum stress (ERS) is caused by the accumulation of unfolded proteins or false folded protein in the ER, which results in an imbalance of calcium in the cell and ER instability [3]. Accumulating evidence indicates that ERS plays an active role in the development of acute and chronic kidney disease; in particular, it contributes to glomerular and tubular damage in CKD [4]. Furthermore, inhibiting ERS can alleviate renal fibrosis progression [5]. ERS promotes renal fibrosis through multiple signaling pathways, such as epithelial-mesenchymal transition, transforming growth factor-β, and oxidative stress. In rat models, ERS causes podocyte injury and apoptosis, leading to increased proteinuria and the development of renal fibrosis [6]. Therefore, targeted inhibition of ERS may be a promising therapeutic strategy against renal fibrosis.
The inflammasome is a polyprotein complex mainly composed of intracellular pattern recognition receptors, the adaptor ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain), and pro-caspase-1 [7]. The inflammasome is believed to the cellular machinery triggering the maturation of proinflammatory cytokines such as interleukin-1β (IL-1β) to engage the innate immune defenses. Several types of inflammasomes have been identified; among them, the NLR family pyrin domain-containing protein 3 (NLRP3) inflammasome is currently the most fully characterized [8]. Activation of the NLRP3 inflammasome directly causes renal tubular epithelial injury [9]. NLRP3 inflammasome expression is increased in models of unilateral ureteral obstruction (UUO) [10], ischemia-reperfusion (I/R) [11], and lupus nephritis [12]. NLRP3 gene knockout (NLRP3-/-), which blocks NLRP3 inflammasome activation, can significantly attenuate kidney damage.
Ghrelin is a brain peptide found in 1999 by Kojima et al. [13]. Acting on the growth hormone (GH) secretagogue receptor (GHSR), ghrelin can accelerate GH secretion, participate in energy metabolism, improve gastrointestinal function, and protect the cardiovascular system [14]. A recent study demonstrated that the exogenous injection of ghrelin protected against acute kidney injuries [15]. In 2004, Rodriguez et al. [16] found that the serum ghrelin levels of patients with ESRD were significantly increased as compared to the controls. Borges and co-workers have suggested that the increased total ghrelin levels in patients with CKD is mainly due to the impaired renal function of ghrelin removal and degradation, which may be one of the main reasons for the apparent increase in patients with CKD [17].
However, to our knowledge, no direct evidence shows that ghrelin can alleviate renal fibrosis, and the underlying mechanisms of ghrelin alleviation of renal fibrosis remain unexplored. Considering the role of ghrelin in inhibiting the release of inflammatory factors, we assumed that ghrelin and the NLRP3 inflammasome might be linked. To test this hypothesis, we evaluated the effects of ghrelin on UUO-induced renal dysfunction, fibrosis, and inflammation and verified the mechanisms of ghrelin-mediated ERS and NLRP3 inflammasome signaling in renal fibrosis.
Materials and methods
Reagents and antibodies
Ghrelin was purchased from Sigma Chemical (St. Louis, MO, USA) and dissolved in dimethylsulfoxide (DMSO) (Sigma, St. Louis, MO). Antibodies against α-SMA, Collagen-I, fibronectin and GAPDH, were obtained from Abcam (Cambridge, UK). Mouse monoclonal anti-Nlrp3 antibody was purchased from adipoGen (San Diego, CA). Rabbit polyclonal anti-IL-18, IL-1β and anti-caspase-1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against GRP78, Caspase12, IRE1α, CHOP, ATF4, PERK, phospho-PERK, eIF-2α, phospho-eIF-2α, BAX, BCL-2, phospho-Erk, Erk and β-actin were purchased from Cell Signalling Technology.
Animal studies
The study protocols were reviewed and approved by the Institutional Animal Care and Use Committee at Fudan University, China. C57BL/6J mice (male, 20-25 g, 8 weeks old) were housed in a light-, temperature-, and humidity-controlled environment under standard conditions with a 12-h light-dark cycle and free access to water and feed. The mice were divided into four groups (n = 6 per group): Sham (control); UUO; UUO+ghrelin; ghrelin. For the experiments, the mice were anesthetized, an incision was made in the abdominal midline, and the left proximal ureter was exposed and ligated with 6-zero silk sutures. In sham-operated mice, the ureters were manipulated but not ligated. Ghrelin (100 μg/kg/day) was administered by daily subcutaneous injection from the day of surgery until the end of the experiment [18]. The mice were anesthetized, and kidney sections were fixed in 10% formalin and paraffin-embedded. In addition, kidney samples were immediately frozen in liquid nitrogen and stored at -80°C for further analyses.
Cell culture
Human renal proximal tubular cells (HK-2 cells) were cultured in DMEM/F12 medium, supplemented with 10% bovine serum at 37°C and 5% CO2 in a humidified incubator. In vitro experiments, cells were incubated with ghrelin (10 nM) for 1 h, then treated with AngII (10-6 mol/L) for further 24 h [19].
Histopathologic examination
The kidneys were sectioned (4 μm thickness), processed, and stained with periodic acid-Schiff (PAS) and Masson trichrome [20]. After PAS staining, the severity of glomerular injury was evaluated using light microscopy in accordance with the following semi-quantitative grades: grade 0, normal; grade 1, < 25% segmental lesion; grade 2, 25%-50% segmental lesion; grade 3, 50%-75% segmental lesion; grade 4, 75%-100% segmental lesion. At least 20 glomeruli were analyzed per group. Following Masson trichrome staining, the area of tubulointerstitial fibrosis was measured in 10 random fields under ×400 magnification.
Immunohistochemical staining
Kidney tissues were fixed with PBS containing 4% paraformaldehyde and embedded in paraffin. Sections were cut 3-μm. Immunohistochemical staining was performed using primary antibody against type I collagen (1:200) and fibronectin (1:200) overnight at 4°C. The sections were subsequently incubated with the corresponding secondary antibodies at room temperature for 1 h. Diaminobenzidine was added as a color reagent to develop the immune complex; hematoxylin was used as a counterstain [21]. Ten randomly selected fields per section were analyzed manually for type I collagen and fibronectin under microscopy or were automatically quantified using a Nikon inverted Eclipse Ti microscope (×400) with built-in NIS-Elements Advanced Research (AR) software, and measured by a blinded observer using Image-Pro Plus version 6.0.
Western blotting
Harvested renal and cell proteins were boiled at 98°C in Laemmli buffer with 5% β-mercaptoethanol for 5 min, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a polyvinylidene fluoride membrane, blocked with 5% milk in Tris-buffered saline-Tween 20 (10 mM Tris-HCl, pH 8.0; 150 mM NaCl; 0.5% Tween 20) for 1 h [22], and probed with primary antibodies against NLRP3, caspase-1, IL-1β, IL-18, α-SMA, GRP94, CHOP, BAX, Bcl-2, ATF4, PERK, phospho-PERK, EIF2α, GRP78, Caspase12, IRE1α, phospho-Erk, Erk and phospho-EIF2α (all, 1:1000) at 4°C overnight. The membranes were incubated with horseradish peroxidase-labelled secondary antibodies for 1 h at 37°C, then the blots were visualized with the ECL-plus detection system (Merck Millipore, USA), and band density was quantified by ImageJ (http://imagej.nih.gov/ij/).
Statistical analysis
Data are expressed as the mean ± standard error of the mean (SEM). Comparisons between groups were performed with one-way analysis of variance followed by Dunnett’s multiple comparison tests or Student’s t-test. P < 0.05 was considered statistically significant.
Results
Ghrelin attenuated renal injury in UUO mice
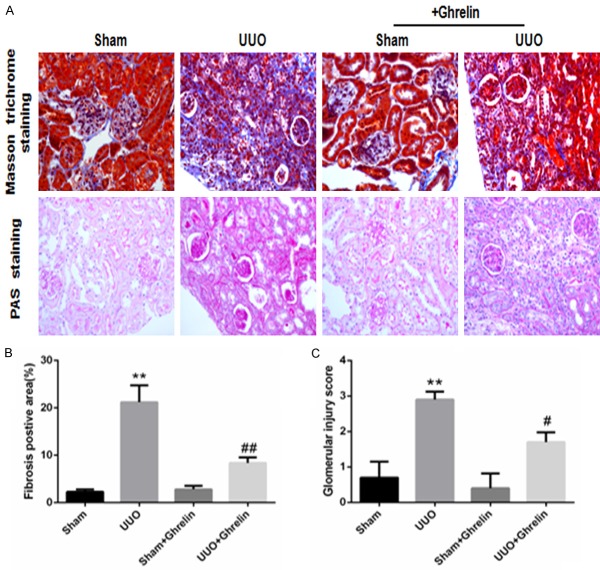
We evaluated renal fibrosis by Masson trichrome staining. The UUO group had increased glomerular and interstitial fibrosis; ghrelin treatment attenuated these changes (Figure 1A). Consistent with this finding, quantitative determination of the tubulointerstitial fibrosis index revealed that UUO induced marked renal fibrosis compared with the Sham group; however, ghrelin treatment inhibited the UUO-induced renal fibrosis (Figure 1A, 1B). Similarly, mesangial matrix expansion was evident in the glomeruli of the UUO group. Compared with the Sham group, mice in the UUO group had significantly larger PAS-positive mesangial matrix areas (Figure 1A). However, treatment with ghrelin significantly attenuated the renal injury and decreased the glomerular injury score (Figure 1C).
Figure 1.
Ghrelin attenuated renal fibrosis and glomerular injury in UUO mice. (A) Representative photomicrographs of PAS-stained kidney sections (×400 magnification) and Masson’s trichrome-stained kidney sections (×400 magnification), (B) tubulointerstitial fibrosis area, and (C) glomerular injury score. All values are means ± SEM (n = 6). **P < 0.01 versus Sham group; ##P < 0.01 versus UUO group.
Ghrelin alleviated the expression of pro-fibrosis factors and renal fibrosis induced by UUO
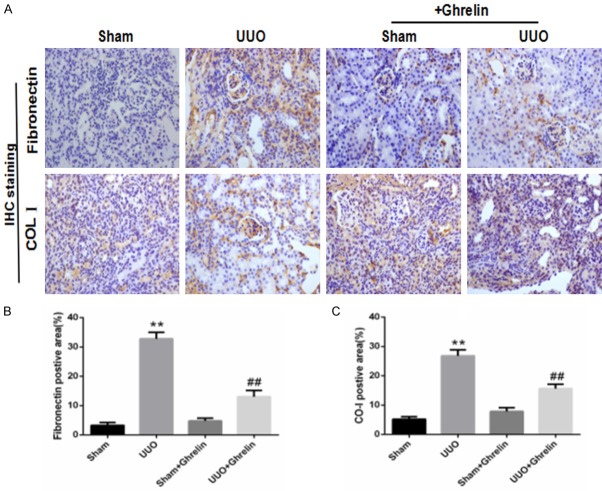
To investigate whether ghrelin regulates UUO-induced renal fibrosis, we quantified the amount of type I collagen, fibronectin, and α-SMA by immunohistochemical staining and western blotting. At 14 days after UUO surgery, there was an obvious increase in collagen deposition in the renal interstitial area in UUO group, which ghrelin treatment significantly reduced renal fibrosis. Consistent with these pathological changes, the kidney cortices of UUO mice had significantly increased expression levels of fibronectin. However, this increase was attenuated in ghrelin-treated UUO mice (Figure 2). UUO induced marked type I collagen and α-SMA protein expression, while ghrelin administration decreased it (Figure 3). Ghrelin inhibited renal fibrosis in uuo mice, may most likely by inhibiting the activation of Erk pathway (Figure 4).
Figure 2.
Ghrelin alleviated the fibronectin and type I collagen expression induced by UUO. (A) Representative immunohistochemistry images show type I collagen (COL 1) and fibronectin expression. (B, C) Bar graphs show the quantification of immunostained areas for fibronectin (B) and type I collagen (CO-I, C). **P < 0.01 versus Sham group; ##P < 0.01 versus UUO group. n = 6.
Figure 3.
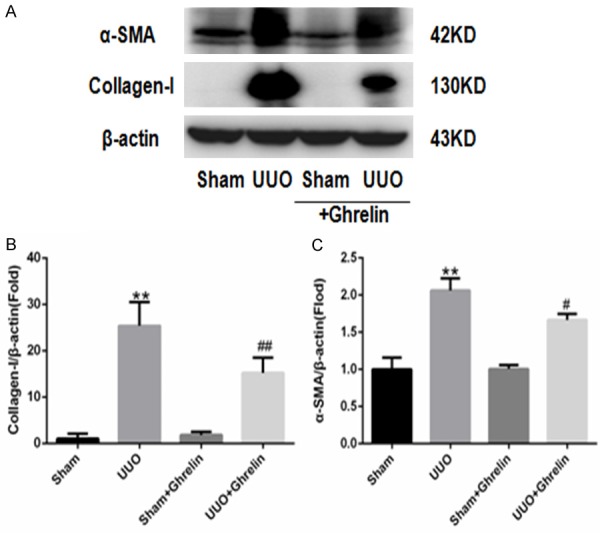
Ghrelin alleviated α-SMA and type I collagen expression in UUO mice. (A) Representative immunoblots of type I collagen and α-SMA. (B, C) Mean quantitative analysis of relative expression of type I collagen (B) and α-SMA (C) in the experimental groups. **P < 0.01 versus Sham group; #P < 0.05 versus UUO group; ##P < 0.01 versus UUO group. n = 6.
Figure 4.
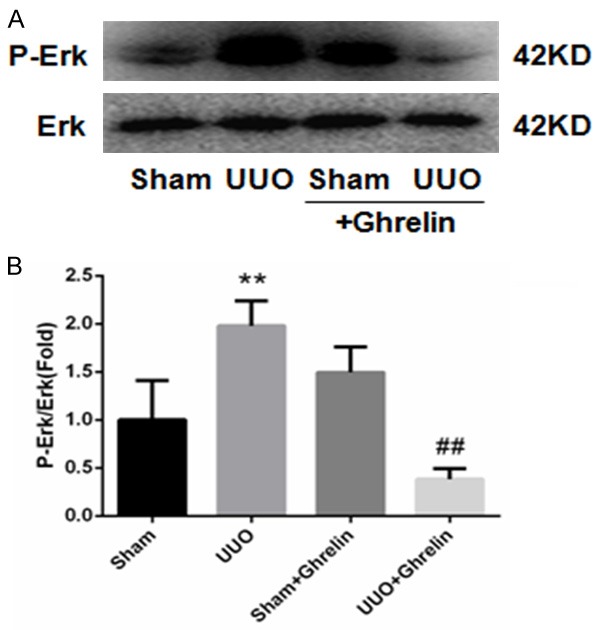
Ghrelin alleviated P-Erk activation in UUO mice. A. Representative immunoblots of P-Erk. B. Mean quantitative analysis of relative expression of P-Erk. **P < 0.01 versus Sham group; ##P < 0.01 versus UUO group. n = 6.
Ghrelin decreased renal cell apoptosis during UUO
As apoptosis is an important detrimental outcome of progressive kidney disease, we detected the effect of ghrelin on renal cell apoptosis and dysfunction in response to UUO by evaluating apoptotic renal cells in a UUO mouse model (Figure 5). As expected, UUO increased renal cell apoptosis, and ghrelin prevented the UUO-mediated Bcl-2 downregulation and Bax upregulation, which subsequently increased the Bcl-2/Bax expression ratio (Figure 5). These results all indicate that ghrelin protected renal function by attenuating apoptosis in UUO mice.
Figure 5.
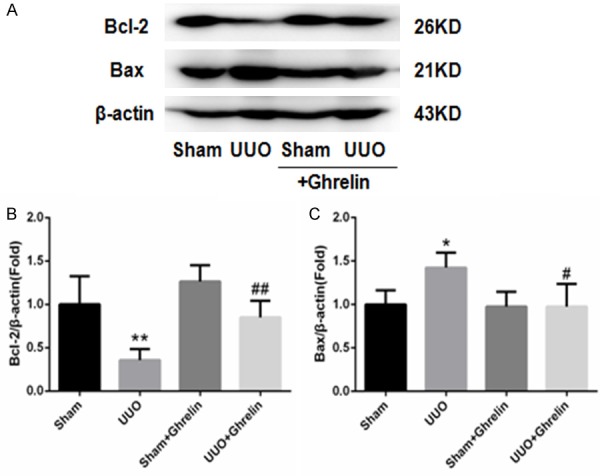
Ghrelin reduced renal cell apoptosis during UUO. (A) Western blot of Bcl-2 and Bax protein. Bcl-2 protein expression was significantly decreased in the UUO group, while Bax expression was significantly increased; ghrelin increased Bcl-2 and decreased Bax. (B, C) Densitometric analysis of Bcl-2 (B) and Bax (C) expression. Results are the mean ± SEM of four experiments (n = 6). **P < 0.01 versus Sham group; #P < 0.05 versus UUO group; ##P < 0.01 versus UUO group.
Ghrelin inhibited NLRP3 inflammasome activation both in vivo and in vitro
To investigate the role of ghrelin on NLRP3 inflammasome activation in UUO-induced renal injury in vivo, we detected NLRP3, caspase-1, IL-1β, and IL-18 levels using western blotting. The UUO group had significantly upregulated renal NLRP3 and caspase-1 protein levels, while treatment with ghrelin markedly inhibited NLRP3 inflammasome activation (Figure 6). Similarly, renal IL-1β and IL-18 protein levels were increased in the UUO group. However, the administration of ghrelin decreased the levels of these inflammatory cytokines (Figure 6). To confirm the protective role of ghrelin in UUO, we detected whether it could inhibit Ang II-induced NLRP3 inflammasome activation in HK-2 cells. As shown in Figure 6C, 6D, treatment with ghrelin significantly prevented NLRP3 inflammasome activation in Ang II-induced HK-2 injury.
Figure 6.
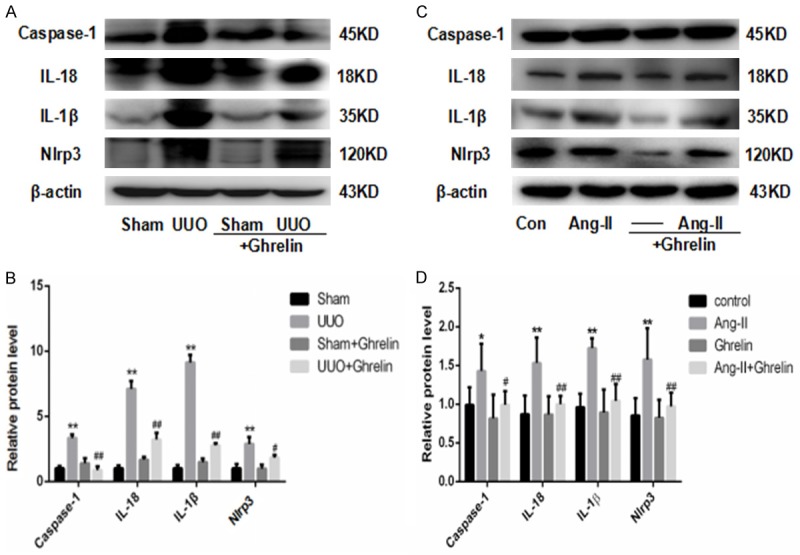
Ghrelin attenuated activation of the NLRP3 inflammasome in vivo and in vitro. (A, B) Expression of renal NLRP3 protein via western blot (A) and (B) relative protein levels normalized against β-actin in mice. **P < 0.01 versus Sham group; #P < 0.05 versus UUO group; ##P < 0.01 versus UUO group. (C, D) Expression of NLRP3 protein via western blot (C) and (D) relative protein levels normalized against β-actin in HK-2 cells. All values are means ± SEM (n = 6). *P < 0.01 versus Con (control) group; **P < 0.01 versus control group; #P < 0.05 versus Ang-II group; ##P < 0.01 versus Ang-II group.
Ghrelin suppressed ERS activation in obstructed kidneys
Growing evidence suggests that ERS is involved in renal injury [18,19]. Therefore, based on the findings discussed above, we hypothesized that ghrelin may be involved in the protective effects against renal injury by inhibiting ERS in UUO mice. Compared with the Sham group, mice in the UUO group had increased expression of the ERS-related protein (Figures 7, 8), and ghrelin treatment decreased ERS marker levels significantly (Figures 7, 8). The ERS-mediated apoptosis pathway was also involved; in particular, the UUO group had markedly increased CHOP and caspase 12 protein levels, and treatment with ghrelin attenuated these changes (Figures 8, 9).
Figure 7.
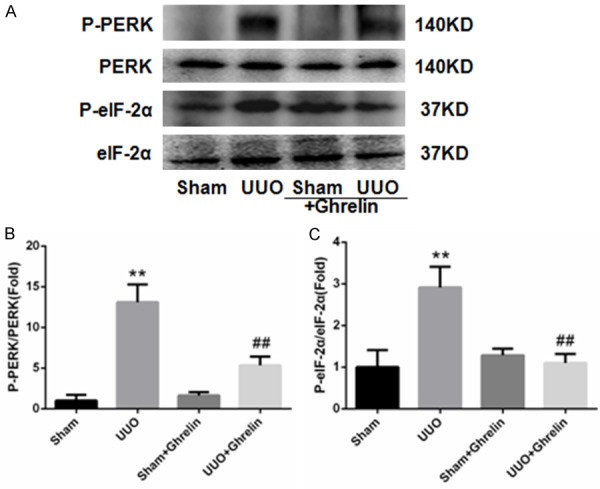
Ghrelin decreases ERS in UUO mice. (A) Western blot showed obvious inhibition of ERS activation by ghrelin. (B, C) Western blot analysis of the ratios of phospho-PERK and PERK (B) and phospho-EIF2α and EIF2α (C). Ghrelin abrogated the UUO-induced increased ERS-relative proteins in the mice. All data are the means ± SEM of four independent experiments (n = 6). **P < 0.01 versus Sham group; ##P < 0.01 versus UUO group.
Figure 8.
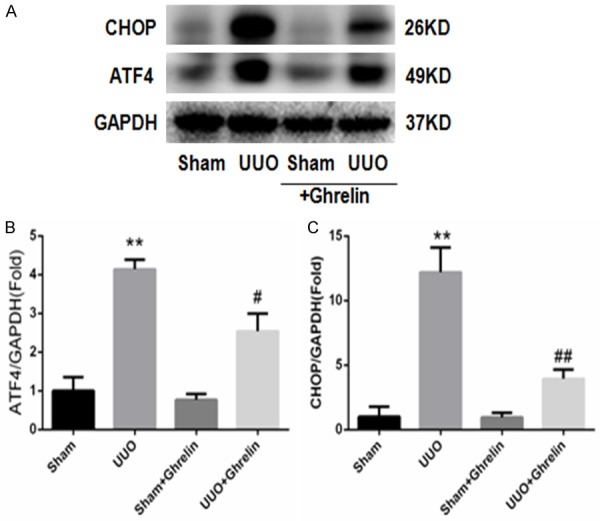
Ghrelin suppressed UUO-induced ERS. (A) Whole renal protein samples were immunoblotted with antibodies against CHOP, ATF4, and GAPDH. (B, C) Graphs indicate the relative abundance of ATF4 (B) and CHOP (C) after normalization with GAPDH. Results are the mean ± SEM of three experiments (n = 6). **P < 0.01 versus Sham group; #P < 0.05 versus UUO group; ##P < 0.01 versus UUO group.
Figure 9.
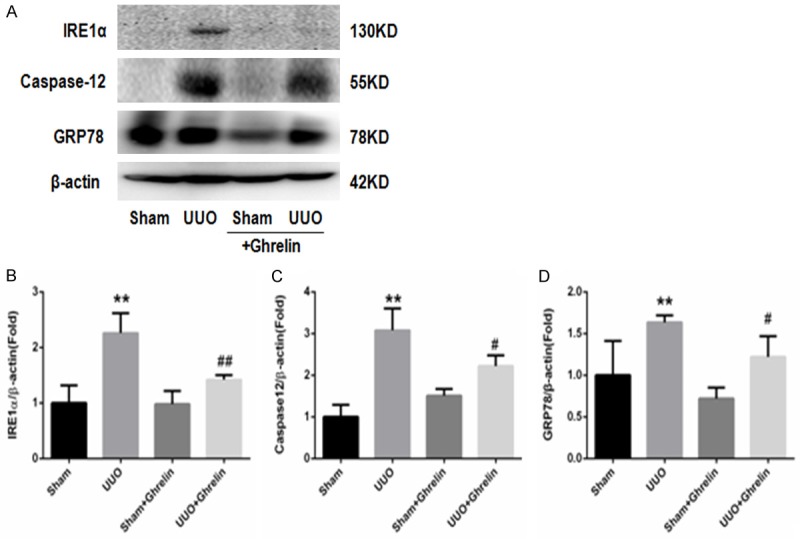
Ghrelin suppressed UUO-induced ERS. (A) Whole renal protein samples were immunoblotted with antibodies against IRE1α, Caspase12, and GRP78. (B-D) Graphs indicate the relative abundance of IRE1α (B), Caspase12 (C) and GRP78 (D) after normalization with GAPDH. Results are the mean ± SEM of three experiments (n = 6). **P < 0.01 versus Sham group; #P < 0.05 versus UUO group; ##P < 0.01 versus UUO group.
Discussion
This study provides evidence of the overall effects and underlying mechanisms of the ghrelin in the pathogenesis of obstructive nephropathy. First, we showed that UUO induced significant renal damage in mice, as characterized by the increased glomerular injury score, tubulointerstitial fibrosis index, collagen, and ECM deposition in the mouse kidneys. Moreover, mice in the UUO group had increased renal cell apoptosis, NLRP3 inflammasome activation, and increased ERS activation. We found that treatment with ghrelin had protective effects against renal fibrosis, renal cell apoptosis, NLRP3 inflammasome activation, and ERS. Therefore, ghrelin may be a potential therapeutic target for reducing UUO-induced kidney damage and renal fibrosis.
Inflammasomes are cytoplasmic multiprotein complexes that were first proposed by the Tschopp research group in 2002 [23]. Inflammasomes are responsible for caspase-1 activation, the subsequent production of the cytokines IL-1β and IL-18, and the initiation of the inflammatory cell death program termed pyroptosis [24]. Four main types of inflammasomes have been found, including NLRP1, NLRP3, NLRC4, and they are absent in melanoma [25]. So far, the NLRP3 inflammasome has been the most extensively studied. The NLRP3 inflammasome is typically composed of a NLRP3 inflammasome sensor, ASC, and the precursor pro-caspase-1 [26]. Considering the inflammatory response is involved in cellular dysfunction during CKD, and an increasing amount of evidence has demonstrated that NLRP3 inflammasome activation is an important pathogenic factor in multiple kidney diseases [27], inflammation appears to be an important destructive process that mediates injury in the kidneys. In addition, NLRP3 inflammasome activation is involved in UUO-induced renal inflammation and leads to renal fibrosis. A recent study also demonstrated that an NLRP3-specific inflammasome inhibitor improved crystal-induced renal inflammation and fibrosis significantly [28]. Consistent with previous studies, the present study shows that the NLRP3 inflammasome activation was increased both in vivo and vitro. Moreover, its downstream cytokines including mature IL-1β and IL-18 were also increased in UUO mice. Taken together, these results suggest that the NLRP3 inflammasome may contribute to UUO-induced renal injury. Treatment with ghrelin markedly attenuated kidney disease and decreased NLRP3 inflammasome activation both in vivo and vitro.
The ER is an important cell organelle, and is a site responsible for protein synthesis, folding, and maturation; it participates in Ca2+ storage, signal transduction, lipid, and glucose metabolism; and regulates protein synthesis, folding, and congregation after synthesis. When the body is exposed to oxidative stress, ischemia, hypoxia, malnutrition, viral infection, and calcium imbalance, unfolded proteins or false folded proteins accumulate in the ER cavity. This accumulation causes cellular calcium imbalance and ER instability, which results in ERS and the unfolded protein response [29]. Previous studies have indicated that ERS plays a critical role in CKD progression [30]. CHOP, an ERS activation marker, is commonly expressed at low levels and is robustly activated in a wide variety of organs as part of the natural stress response. Here, we show that UUO substantially increases CHOP expression, and demonstrate that ghrelin alleviates the ERS response by markedly reducing the ERS signaling pathway in UUO mice.
Ghrelin is a hormone produced by specific cells in the stomach. It can cause increased pituitary secretion of GH. Research on the role of ghrelin has mainly focused on GH secretion and regulation of the balance between growth and energy, and it can promote gastric acid secretion and increase appetite and body weight. At the same time, the universality of its receptor distribution indicates that ghrelin may have a wider biological effect. Recombinant ghrelin was successfully used to alleviate cachexia, anorexia, and gastrointestinal symptoms in patients with cancer and chronic disease [31-33]. In addition, recent studies have shown that ghrelin could attenuate myocardial I/R injury by inhibiting ERS [34]. Recent research has also suggested that ghrelin can alleviate the renal fibrosis caused by UUO, but the mechanism is unknown [35]. In present study, ghrelin alleviated inflammation-induced injury by downregulating the NLRP3 inflammasome, and inhibiting UUO-driven production of IL-18 and IL-1β, suggesting that redundant inflammatory pathways are involved in UUO-induced CKD. Furthermore, ghrelin can block the ERS pathway. The above results suggest that ERS and the NLRP3 inflammasome both contribute to UUO-induced renal injury. Ghrelin may attenuate UUO-induced renal fibrosis by inhibiting the NLRP3 inflammasome and ERS activation.
Conclusions
Our results indicate that ghrelin plays an important role in attenuating UUO-induced renal injury in vivo, possibly by alleviating collagen deposition and preventing NLRP3 inflammasome and ERS activation. Therefore, ghrelin might be an effective strategy for ameliorating obstruction-induced renal injury and the progression of CKD.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China 81270822 (to Yong Gu), 81570661 (to Yong Gu), 81400714 (to Min Yang), Shanghai Pujiang Program 17PJD021 (to Wei Ding), and the Major State Basic Research Development Program of China 2012CB517700 (to Yong Gu).
Disclosure of conflict of interest
None.
References
- 1.Conn PM, Bowers CY. A new receptor for growth hormone-release peptide. Science. 1996;273:923. doi: 10.1126/science.273.5277.923. [DOI] [PubMed] [Google Scholar]
- 2.Leung KC, Tonelli M, James MT. Chronic kidney disease following acute kidney injury-risk and outcomes. Nat Rev Nephrol. 2013;9:77–85. doi: 10.1038/nrneph.2012.280. [DOI] [PubMed] [Google Scholar]
- 3.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunard R. Endoplasmic reticulum stress in the diabetic kidney, the good, the bad and the ugly. J Clin Med. 2015;4:715–740. doi: 10.3390/jcm4040715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu QF, Ye JM, Deng ZY, Yu LX, Sun Q, Li SS. Ameliorating effect of Klotho on endoplasmic reticulum stress and renal fibrosis induced by unilateral ureteral obstruction. Iran J Kidney Dis. 2015;9:291–297. [PubMed] [Google Scholar]
- 6.Ke B, Zhu N, Luo F, Xu Y, Fang X. Targeted inhibition of endoplasmic reticulum stress: New hope for renal fibrosis (Review) Mol Med Rep. 2017;16:1014–1020. doi: 10.3892/mmr.2017.6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 8.Shen J, Wang L, Jiang N, Mou S, Zhang M, Gu L, Shao X, Wang Q, Qi C, Li S, Wang W, Che X, Ni Z. NLRP3 inflammasome mediates contrast media-induced acute kidney injury by regulating cell apoptosis. Sci Rep. 2016;6:34682. doi: 10.1038/srep34682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhuang Y, Ding G, Zhao M, Bai M, Yang L, Ni J, Wang R, Jia Z, Huang S, Zhang A. NLRP3 inflammasome mediates albumin-induced renal tubular injury through impaired mitochondrial function. J Biol Chem. 2014;289:25101–25111. doi: 10.1074/jbc.M114.578260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vilaysane A, Chun J, Seamone ME, Wang W, Chin R, Hirota S, Li Y, Clark SA, Tschopp J, Trpkov K, Hemmelgarn BR, Beck PL, Muruve DA. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J Am Soc Nephrol. 2010;21:1732–1744. doi: 10.1681/ASN.2010020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shigeoka AA, Mueller JL, Kambo A, Mathison JC, King AJ, Hall WF, Correia JS, Ulevitch RJ, Hoffman HM, McKay DB. An inflammasome-independent role for epithelial-expressed Nlrp3 in renal ischemia-reperfusion injury. J Immunol. 2010;185:6277–6285. doi: 10.4049/jimmunol.1002330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao J, Wang H, Dai C, Wang H, Zhang H, Huang Y, Wang S, Gaskin F, Yang N, Fu SM. P2X7 blockade attenuates murine lupus nephritis by inhibiting activation of the NLRP3/ASC/caspase 1 pathway. Arthritis Rheum. 2013;65:3176–3185. doi: 10.1002/art.38174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 14.Delporte C. Structure and physiological actions of ghrelin. Scientifica (Cairo) 2013;2013:518909. doi: 10.1155/2013/518909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeda R, Nishimatsu H, Suzuki E, Satonaka H, Nagata D, Oba S, Sata M, Takahashi M, Yamamoto Y, Terauchi Y, Kadowaki T, Kangawa K, Kitamura T, Nagai R, Hirata Y. Ghrelin improves renal function in mice with ischemic acute renal failure. J Am Soc Nephrol. 2006;17:113–121. doi: 10.1681/ASN.2004080626. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez AE, Pecoits-Filho R, Heimburger O, Lindholm B, Nordfors L, Stenvinkel P. Associations between plasma ghrelin levels and body composition in end-stage renal disease: a longitudinal study. Nephrol Dial Transplant. 2004;19:421–426. doi: 10.1093/ndt/gfg559. [DOI] [PubMed] [Google Scholar]
- 17.Borges N, Moraes C, Barros AF, Carraro-Eduardo JC, Fouque D, Mafra D. Acyl-ghrelin and obestatin plasma levels in different stages of chronic kidney disease. J Ren Nutr. 2014;24:100–104. doi: 10.1053/j.jrn.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Chevalier RL, Forbes MS, Thornhill BA. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 2009;75:1145–1152. doi: 10.1038/ki.2009.86. [DOI] [PubMed] [Google Scholar]
- 19.Fujimura K, Wakino S, Minakuchi H, Hasegawa K, Hosoya K, Komatsu M, Kaneko Y, Shinozuka K, Washida N, Kanda T, Tokuyama H, Hayashi K, Itoh H. Ghrelin protects against renal damages induced by angiotensin-II via an antioxidative stress mechanism in mice. PLoS One. 2014;9:e94373. doi: 10.1371/journal.pone.0094373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding W, Xu C, Wang B, Zhang M. Rotenone attenuates renal injury in aldosterone-infused rats by inhibiting oxidative stress, mitochondrial dysfunction, and inflammasome activation. Med Sci Monit. 2015;21:3136–3143. doi: 10.12659/MSM.895945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang B, Ding W, Zhang M, Li H, Gu Y. Rapamycin attenuates aldosterone-induced tubulointerstitial inflammation and fibrosis. Cell Physiol Biochem. 2015;35:116–125. doi: 10.1159/000369680. [DOI] [PubMed] [Google Scholar]
- 22.Xu C, Ding W, Yang L, Yang M, Zhang M, Gu Y. Contributions of endoplasmic reticulum stress and reactive oxygen species to renal injury in aldosterone/salt-induced rats. Nephron Exp Nephrol. 2014;126:25–32. doi: 10.1159/000357777. [DOI] [PubMed] [Google Scholar]
- 23.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 24.Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–114. doi: 10.1016/s0966-842x(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 25.Ozaki E, Campbell M, Doyle SL. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: current perspectives. J Inflamm Res. 2015;8:15–27. doi: 10.2147/JIR.S51250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 27.Cassel SL, Janczy JR, Bing X, Wilson SP, Olivier AK, Otero JE, Iwakura Y, Shayakhmetov DM, Bassuk AG, Abu-Amer Y, Brogden KA, Burns TL, Sutterwala FS, Ferguson PJ. Inflammasome-independent IL-1beta mediates autoinflammatory disease in Pstpip2-deficient mice. Proc Natl Acad Sci U S A. 2014;111:1072–1077. doi: 10.1073/pnas.1318685111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ludwig-Portugall I, Bartok E, Dhana E, Evers BD, Primiano MJ, Hall JP, Franklin BS, Knolle PA, Hornung V, Hartmann G, Boor P, Latz E, Kurts C. An NLRP3-specific inflammasome inhibitor attenuates crystal-induced kidney fibrosis in mice. Kidney Int. 2016;90:525–539. doi: 10.1016/j.kint.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 29.Safiedeen Z, Andriantsitohaina R, Martinez MC. Dialogue between endoplasmic reticulum and mitochondria as a key actor of vascular dysfunction associated to metabolic disorders. Int J Biochem Cell Biol. 2016;77:10–14. doi: 10.1016/j.biocel.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 30.Ding W, Yang L, Zhang M, Gu Y. Reactive oxygen species-mediated endoplasmic reticulum stress contributes to aldosterone-induced apoptosis in tubular epithelial cells. Biochem Biophys Res Commun. 2012;418:451–456. doi: 10.1016/j.bbrc.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 31.Neary NM, Small CJ, Wren AM, Lee JL, Druce MR, Palmieri C, Frost GS, Ghatei MA, Coombes RC, Bloom SR. Ghrelin increases energy intake in cancer patients with impaired appetite: acute, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89:2832–2836. doi: 10.1210/jc.2003-031768. [DOI] [PubMed] [Google Scholar]
- 32.Wynne K, Giannitsopoulou K, Small CJ, Patterson M, Frost G, Ghatei MA, Brown EA, Bloom SR, Choi P. Subcutaneous ghrelin enhances acute food intake in malnourished patients who receive maintenance peritoneal dialysis: a randomized, placebo-controlled trial. J Am Soc Nephrol. 2005;16:2111–2118. doi: 10.1681/ASN.2005010039. [DOI] [PubMed] [Google Scholar]
- 33.Binn M, Albert C, Gougeon A, Maerki H, Coulie B, Lemoyne M, Rabasa LR, Tomasetto C, Poitras P. Ghrelin gastrokinetic action in patients with neurogenic gastroparesis. Peptides. 2006;27:1603–1606. doi: 10.1016/j.peptides.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Zhang GG, Teng X, Liu Y, Cai Y, Zhou YB, Duan XH, Song JQ, Shi Y, Tang CS, Yin XH, Qi YF. Inhibition of endoplasm reticulum stress by ghrelin protects against ischemia/reperfusion injury in rat heart. Peptides. 2009;30:1109–1116. doi: 10.1016/j.peptides.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 35.Sun G, Ding R, Li M, Guo Y, Fan L, Yue L, Li L, Zhao M. Ghrelin attenuates renal fibrosis and inflammation of obstructive nephropathy. J Urol. 2015;193:2107–2115. doi: 10.1016/j.juro.2014.11.098. [DOI] [PubMed] [Google Scholar]