Abstract
Background and aims
Autism spectrum disorder (ASD) is currently estimated to affect more than 1% of the world population. For people with ASD, gastrointestinal (GI) distress is a commonly reported but a poorly understood co-occurring symptom. Here, we investigate the physiological basis for GI distress in ASD by studying gut function in a zebrafish model of Phelan-McDermid syndrome (PMS), a condition caused by mutations in the SHANK3 gene.
Methods
To generate a zebrafish model of PMS, we used CRISPR/Cas9 to introduce clinically related C-terminal frameshift mutations in shank3a and shank3b zebrafish paralogues (shank3abΔC). Because PMS is caused by SHANK3 haploinsufficiency, we assessed the digestive tract (DT) structure and function in zebrafish shank3abΔC+/− heterozygotes. Human SHANK3 mRNA was then used to rescue DT phenotypes in larval zebrafish.
Results
Significantly slower rates of DT peristaltic contractions (p < 0.001) with correspondingly prolonged passage time (p < 0.004) occurred in shank3abΔC+/− mutants. Rescue injections of mRNA encoding the longest human SHANK3 isoform into shank3abΔC+/− mutants produced larvae with intestinal bulb emptying similar to wild type (WT), but still deficits in posterior intestinal motility. Serotonin-positive enteroendocrine cells (EECs) were significantly reduced in both shank3abΔC+/− and shank3abΔC−/− mutants (p < 0.05) while enteric neuron counts and overall structure of the DT epithelium, including goblet cell number, were unaffected in shank3abΔC+/− larvae.
Conclusions
Our data and rescue experiments support mutations in SHANK3 as causal for GI transit and motility abnormalities. Reductions in serotonin-positive EECs and serotonin-filled ENS boutons suggest an endocrine/neural component to this dysmotility. This is the first study to date demonstrating DT dysmotility in a zebrafish single gene mutant model of ASD.
Electronic supplementary material
The online version of this article (10.1186/s13229-018-0250-4) contains supplementary material, which is available to authorized users.
Keywords: Digestive transit, Peristaltic rate, Enteroendocrine, Phelan-McDermid syndrome
Background
Autism spectrum disorder (ASD) is estimated to impact more than 1% of the population and is etiologically and clinically heterogeneous [1, 2]. While ASD diagnoses are based upon deficits in social communication and the presence of repetitive behaviors and/or restrictive interests, co-occurring symptoms (comorbidities) are also common. Here, we focus on gastrointestinal (GI) distress, one of the more frequent comorbidities experienced by individuals with ASD [3, 4]. Despite the negative impacts of GI distress on daily life, ASD-associated GI symptoms are poorly understood [3, 5]. Consistent with prospective findings, clinical reports from monogenic causes of ASD regularly document GI distress [3, 6–8]. Our work focuses on a monogenic form of ASD, Phelan-McDermid syndrome, that is caused by mutations that disrupt one copy of the SHANK3 gene resulting in SHANK3 haploinsufficiency [9, 10]. In individuals with PMS, GI distress is characterized by reflux, cyclical vomiting, diarrhea, and/or constipation [11, 12]. To investigate the biological mechanisms underlying GI distress in PMS and ASD, we have generated a zebrafish shank3 mutant model.
The majority of SHANK3 loss-of-function animal models are mammalian and have provided great insight into neural mechanisms related to social and motor behaviors characteristic of ASD [13]. SHANK3 is known to act as a synaptic scaffolding protein in the central nervous system (CNS) where it helps to regulate synaptic development, glutamatergic receptor signaling, actin polymerization, and dendritic spine formation [14–19]. In addition, SHANK3 is also expressed at early developmental stages prior to synapse formation [20, 21], as well as in enterocytes and nitrergic neurons of the enteric nervous system (ENS) [22–24], and has been shown to have important interactions with the Wnt signaling pathway [25]. Studies suggest that SHANK3 may also play important GI-related roles in host/symbiont interactions and Zn metabolism [22, 26–28] and intestinal barrier function [29]. Studies to explore roles for SHANK3 in relation to GI dysfunction, however, are limited.
To understand the etiology of ASD symptoms, zebrafish is a powerful model system [30–32]. Genetically and physiologically similar to humans and mammalian models, zebrafish provide a complementary model system with accessible developmental stages that are transparent, allowing physiological assessment in vivo [31, 33–35]. Additionally, zebrafish and human digestive tracts are largely conserved, with similar hormonal regulation, morphology, cell types, and physiology, albeit simplified in zebrafish [8, 36–40]. For example, in both zebrafish and mammals, digestion rate adapts to the size of the meal [39]; also in both, serotonin, acetylcholine, motilin, and ghrelin increase DT motility [39, 41, 42] while vasoactive intestinal peptide, pituitary adenylate cyclase-activating peptide, and nitric oxide decrease DT motility [41, 43]. Like mammals, the zebrafish DT tract can be divided into sections distinguished by differences in cell type and function: digestive secretions are enriched anteriorly where both nutrient absorption and tissue folding are the greatest, while posterior regions are largely devoid of folding and cell types are largely specialized for water absorption [44, 45]. Additionally, the ENS and key DT regulatory brain regions such as the hypothalamus, secondary gustatory nuclei, vagal motor nucleus, sensory nodose ganglia, and spinal dorsal root ganglia are conserved in zebrafish [32, 40, 46, 47]. Experiments that have used zebrafish to investigate GI dysfunction in Hirschsprung’s and chronic intestinal pseudo-obstruction diseases have found that disrupting conserved zebrafish genes linked to these diseases disrupts both GI motility and ENS development [48, 49], mirroring human symptoms and supporting translatability of the zebrafish model system.
Here, we generate a zebrafish shank3 mutant model to investigate a causal link between shank3 loss-of-function mutations and DT dysfunction. As a result of a genome duplication approximately 300 mya [50], the shank3 gene, along with many other synaptic genes, is duplicated in teleost fishes [51, 52], yielding shank3a and shank3b gene paralogues. Therefore, to model human SHANK3 mutations linked to PMS [53], we used CRISPR/Cas9 to generate frame-shift mutations in the C terminal, proline-rich domains of both zebrafish shank3a (chromosome 18) and shank3b (chromosome 4) (referred to as shank3abΔC).
Methods
Additional materials and methods on histology, statistics, and the Igor Pro code can be found in Additional file 1
Fish maintenance and husbandry
Zebrafish were housed in the University of Miami zebrafish core facility. Both adult and larval zebrafish were maintained at 28 °C in system water and exposed to a 14:10 h circadian light:dark cycle. Zebrafish lines used in this study include AB wild type (WT) and transgenic lines la118Tg:Tg(aldoca:gap43-Venus) [54] and Tg(vglut2a:DsRed) [55]. To generate shank3abΔC+/− animals, WT females were crossed with shank3abΔC−/− males. ZFIN zebrafish gene and protein nomenclature conventions were followed.
Single-stranded guide RNA (sgRNAs) design
Site-specific CRISPR-Cas9 sgRNAs were generated using the online software CHOPCHOP [56]. CHOPCHOP-generated guides were chosen based on location within the gene and minimal predicted off-target sites. Guides were designed to target the C-terminal proline-rich domain of shank3a and shank3b (Additional file 1: Table S1). To produce sgRNA templates for in vitro transcription, phage T7 promoter-adapted sgRNA oligonucleotides were annealed to a universal tracRNA oligonucleotide with complementary overhangs [57]. Each sgRNA template reaction contained: 1× transcription buffer, 1 μM site-specific oligo, 1 μM tracRNA oligo, 500 nM dNTPs, 0.5 U Phusion high-fidelity DNA polymerase (New England Biolabs, NEB; Ipswich, MA), and nuclease-free H2O to 10 μL. Reaction mixtures were heated to 95 °C for 1 min, cooled to 52 °C (0.1 °C/sec), and extended at 72 °C for 10 min. Templates were then used to transcribe single-stranded sgRNAs with a T7 MEGAscript in vitro transcription kit (Ambion; Foster City, CA) following the manufacturer’s protocol. Transcribed sgRNAs were then cleaned using ammonium acetate/ethanol precipitation and quantified by comparison to an RNA sample of known concentration on an agarose gel.
CRISPR-Cas 9 mutagenesis and allele screening
In vitro transcribed sgRNA and Cas 9 protein (PNA Bio, Newbury Park, CA) were mixed and incubated at 37 °C for 5 min. A micro-injector was then used to inject 400:100 pg of sgRNA:Cas 9 into the cell of one-cell stage zebrafish embryos. Injected embryos were allowed to develop for 24 h, and genomic DNA (gDNA) was extracted to screen for mutations. Genes were targeted singly for either shank3aΔC or shank3bΔC then F1 mutants were out-crossed to obtain F2 shank3abΔC heterozygotes. These F2 mutants were then outcrossed to la118Tg:Tg(aldoca:gap43-Venus) and Tg(vglut2a:DsRed) and finally backcrossed to generate F4 and F5 shank3abΔC homozygous mutants. To assay mutations, polymerase chain reaction (PCR) primers were designed to flank the sgRNA target site (Additional file 1: Table S2). Each PCR reaction mixture contained: 1× GOTaq (Promega, Madison, WI), 50 nM forward and reverse primer, 1 μL gDNA, and nuclease-free H2O to 10 μL. PCR products were then sequenced on an AB3130 Sanger sequencer with each sequence PCR mixture containing: 1× BigDye Buffer, 50 nM forward primer, 0.3× 3.1 BigDye mixture, and 1 μL purified PCR product. Identified mutations were then analyzed for unique restriction digest sites that could be used for genotyping (Additional file 1: Figure S1). Post-experimental analysis genotyping using PCR and restriction enzyme digests (Additional file 1: Figure S1) allowed us to conduct experiments blind to genotype.
Western
One hundred milligrams of whole fish brains dissected and pooled from adult shank3ab +/+ (n = 8) and shank3abΔC −/− mutants (n = 8) were used to prepare the postsynaptic density (PSD) fractions following the previously published method [16]. Mouse cortices from Shank3+/+ or Shank3−/−, lacking all Shank3 isoforms, (unpublished) were processed in parallel. Briefly, brains were homogenized in HEPES buffer containing 0.32 M sucrose and protease inhibitors followed by two rounds of low-speed centrifugation (700 g) to remove nuclei. Supernatants were centrifuged at 18,000g to precipitate crude synaptosomal fraction, which were further homogenized in hypotonic HEPES buffer to eliminate synaptic vesicles. Finally, PSD fractions were obtained by four rounds of washing with HEPES buffer containing 0.5% Triton X-100 and high-speed centrifugation (180,000g) and dissolved in HEPES buffer containing 1.8% SDS and 0.85 M urea. PSD lysates were run on SDS-PAGE gel and transferred to PVDF membrane for immunoblot analysis using antibodies against Shank3 (1:100; sc-30,193, Santa Cruz Biotechnology, CA, raised against amino acids 1431–1590 mapping near the C-terminus of isoform 2 of human SHANK3), Tubulin (1:500, ab18207, Abcam), and Actin (1:500, A2066, Sigma).
Cryosectioning and immunohistochemistry
Cryosectioning and antibody staining were performed using previously published methods [58]. Anti-PSD-95 (1:500; Abcam; Cambridge, UK; ab-18,258) and anti-SHANK3 (1:200; sc-30193, Santa Cruz Biotechnology, CA) were used as primary antibodies with secondary antibodies conjugated to Alexa Fluor 568 (Abcam, ab175472) and Alexa Fluor 488 (Thermo Fisher Scientific, R37118), respectively. Images were collected on a SP5 confocal microscope (Leica; Wetzlar, DE) using 40× and 63× oil immersion lenses.
Peristalsis videography and analysis
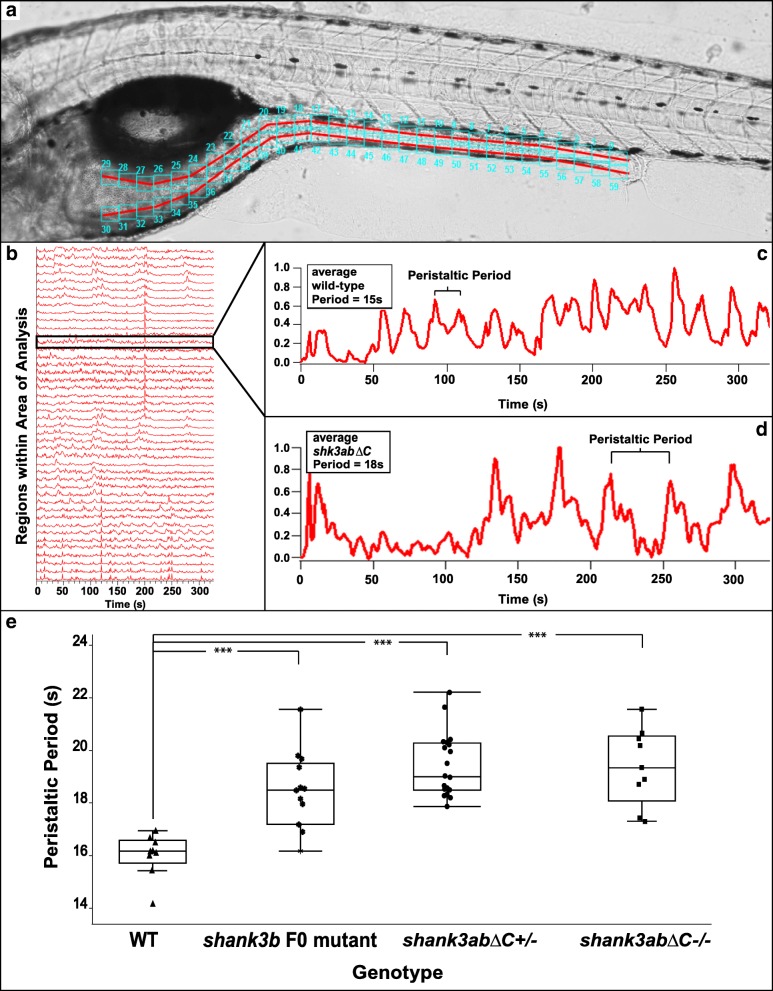
To quantify DT motility, we captured videos of gut peristalsis in intact transparent 7-day-old zebrafish larvae after feeding with a chicken egg yolk emulsion. The yolk emulsion promoted more uniform feeding across the population, making screening easier. To create the emulsion, 1 ml of chicken egg yolk was rapidly and repeatedly pipetted into 1 ml of system water; several drops of the emulsion were then added to a 10 cm petri dish housing 50–100 larva. Both WT controls and shank3abΔC mutant larvae were exposed to the yolk mixture for the same time period (60–90 min). To avoid requiring anesthetic, videos were acquired by embedding single larvae oriented on the sagittal plane in low melt point agarose (LMA; Thermo Fisher, BP165-25) on a glass-bottom 35 mm petri dish. Videos were recorded at 1 frame/second for a total of 10 min on a Canon EOS 5D Mark III; larvae were then processed for genotyping. Videos were compiled blind to genotype, and run through a custom script in Igor Pro. This script allows a user to demarcate a specific area of analysis, or AOA (Fig. 3a, red lines), and determine the number of regions to evaluate along the area of analysis (Fig. 3a, cyan boxes) and the amount of raster averaging for each AOA. Post-analysis, the program produces individual peristaltic periodicity per point of evaluation and overall averages of peristaltic periodicity (see Additional file 1). These outputs were manually annotated to check for artifacts due to muscle twitches that were easy to distinguish from DT peristalsis due to the much faster timescale.
Fig. 3.
shank3abΔC−/− and shank3abΔC+/− larvae have reduced peristaltic frequencies and DT motility compared to WT. a Representative image of a zebrafish embedded for analysis, with the area of analysis (AOA) denoted by the red lines and the regions of evaluation denoted by the cyan boxes (1–60). The AOA was manually drawn in, and the program automatically fills a set number of recording regions along the AOA. For our work, this number was set at 30 dorsal and 30 ventral, but the number and size of the boxes can be adjusted if necessary. b Representative WT data output, showing output from all regions of evaluation within the AOA; as the peristaltic contraction moves down the DT tract, the Igor Pro program registers the change in pixel intensity. c Representative image of an individual analysis readout for a WT larva, with the change in pixel intensity (y-axis) measured as time progresses (x-axis). Igor Pro can separate individual regions of evaluation within the AOA and give data on peristaltic periodicity in a specific region, allowing users to get overall averages throughout multiple regions within the AOA. d Representative image of an individual analysis readout for a shank3abΔC +/− larvae, note the larger space between peaks, indicating reduced peristaltic frequencies. e WT larvae are shown to have peristaltic rates significantly (p < 0.0001) more frequent than that of shank3abΔC mutants. F0 shank3b larvae are significantly slower than WT, while F0, shank3abΔC +/− and ΔC −/− show no significant difference between each other. Each point represents an average frequency from a 7-day-old larva
DT transit
Seven dpf shank3abΔC +/− mutant and WT larvae were fed a mixture of 6 μm fluorescent beads (Polysciences 17156, fluoresbrite beads packaged as 2.5% aqueous suspension) and chicken egg yolk emulsion for 1.5 h. Approximately 10 μl of fluorescent bead solution in 2 ml of egg yolk emulsion was added to the 10 cm petri dish containing the larvae in approximately 20 ml system water. After screening for individuals who had filled their intestinal bulb with the yolk-bead suspension, larvae were embedded in LMA on a 35-mm, glass-bottom petri dish, and a series of pictures of each larva were taken with a Zeiss Axiocam on a stereoscope V20 at 3, 6, 12, and 24 h. Regions of analysis were the pharynx, intestinal bulb, upper intestinal tract, lower intestinal tract, and expelled. This procedure was adapted from [59]. Calculations were made blind to genotype. Using FIJI, the total area encompassed by the fluorescent beads was determined, and percentages of this total were calculated per DT region. Averages for 3, 6, 12, and 24 h time periods were compiled for both WT and shank3abΔC +/− larvae. Supplementary movie figures (shank3abΔC +/−, Additional file 2: Movie S1; and WT, Additional file 3: Movie S2) were processed as described above. Individuals were screened for high quantities of fluorescent intestinal content and were embedded as described. Images were acquired using a Zeiss Axio Imager.Z2 paired with a Zeiss AxioCam MRm Rev3 camera running Zeiss Zen Blue software. Images were acquired every 10 s for 6 h. Image acquisition began late morning and was concluded before 5 pm; as such, the room lights were left on during the duration of the recording process to prevent any confounding effects with shifting circadian rhythm.
Additional file 2: Movie S1. Playback at 5 frames per second, each frame represents 1 z-stack capture per minute. This movie shows the abnormal mutant peristaltic process in a 7 dpf shank3abΔC+/- zebrafish. The fluorescent microspheres can be seen near the intestinal bulb-upper intestine junction; this was a common catch point and the mutant model seems to have the greatest difficultly moving food particles out of the intestinal bulb. Post processing was done to reduce size, change orientation of images, and speed playback up. (MP4 16987 kb)
Additional file 3: Movie S2. Playback at 5 frames per second, each frame represents 1 z-stack capture per minute. This movie shows “normal” wild type peristaltic movements of a 7 dpf zebrafish, highlighting the intestinal region starting at the intestinal bulb-upper intestine junction. Fluorescent microspheres can be seen moving posteriorly down the digestive tract. Post processing was done to reduce size, change orientation of images, and speed playback up. (MP4 23290 kb)
Rescue experiments
Fertilized shank3abΔC +/− embryos were injected with a full-length (designated 5t) human SHANK3 mRNA, or a short isoform (designated 32t, Additional file 1: Table S9) [20]. These fish were allowed to grow to 7 dpf before being analyzed with the microsphere transit assay listed above. Additionally, control injections of loading dye and non-injected WT and shank3abΔC +/− fish were analyzed.
Quantification of gut ENS and enteroendocrine cells
Seven dpf WT and mutant larvae were incubated on ice for 30 min before being fixed in 4% Formaldehyde (PierceTM, Thermo Fisher Scientific, 28906)-1× phosphate buffer solution/0.25% Triton X-100 (1× PBSTx) for 1 h at room temperature. Whole-mount antibody staining and gut dissections were carried out according to previously published methods [60]. Anti-HuC/HuD (1:1000; Invitrogen; A21271) and anti-5-HT (1:1000; serotonin; Immunostar, New Richmond, WI; 20,080) were used as primary antibodies with secondary antibodies conjugated to Alexa Fluor 568 (1:1000; Abcam, ab175472) and Alexa Fluor 633 (1:1000; Thermo Fisher Scientific, A-31575). To sample the anterior and posterior intestinal tract, a 200 × 200-μm field was captured 200 μm from the intestinal bulb (anterior) and 200 μm from the anus (posterior) as in [61]. Images were captured using a 20× dry objective and analyzed using the cell counter application in Fiji.
Results
Generating zebrafish shank3abΔC loss-of-function mutant models of Phelan-McDermid syndrome
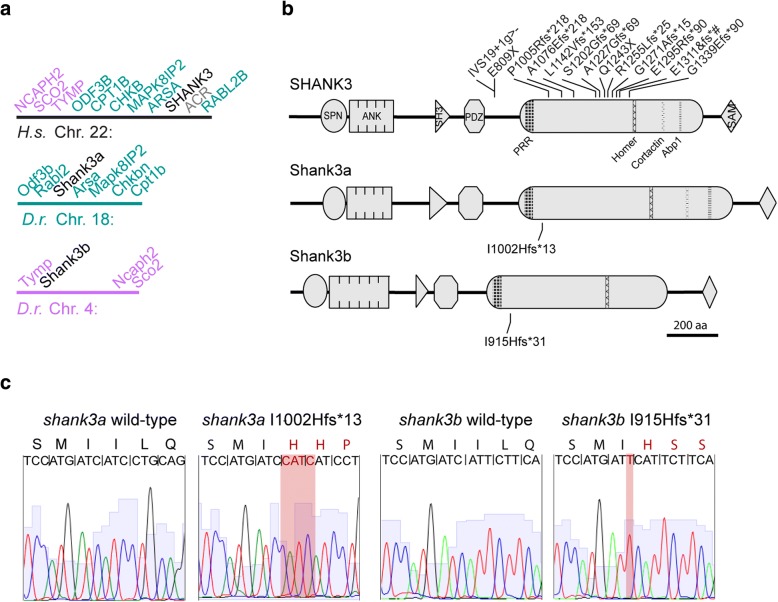
Zebrafish shank3a on chromosome 18 shares human SHANK3 synteny, surrounded by six of the seven genes that neighbor human SHANK3, while shank3b has reduced synteny, flanked by three of the genes that are more distant from human SHANK3 (Fig. 1a). Moreover, while zebrafish Shank3a retains all known protein binding domains, Shank3b has an additional ankyrin repeat and lacks cortactin and Abp1 interaction domains (Fig. 1b). CRISPR-Cas9/sgRNA injections produced frameshift mutations in shank3a and shank3b. Both sgRNAs targeted the largest exon in shank3 that encodes a large C-terminal region containing several protein interaction domains. Similar human frameshift mutations in the SHANK3 C-terminal domain have been associated with ASD [53, 62, 63]. Frameshift mutations were induced in both shank3a and shank3b genes, and alleles were selected to minimize the number of amino acids between frameshift and stop codon (Fig. 1c). In shank3a, a four-base pair insertion converted an isoleucine into a histidine and introduced thirteen amino acids before the stop codon. In shank3b, a single-base pair insertion converted an isoleucine into a histidine and introduced thirty-one amino acids before the stop codon.
Fig. 1.
SHANK3 orthologues are conserved in zebrafish and CRISPR/Cas9-induced frameshift mutations are similar to those found in people with Phelan-McDermid syndrome. a shank3a on chromosome 18 (teal) and shank3b on chromosome 4 (purple) retain synteny with different genes surrounding SHANK3 on chromosome 22 in humans. b Shank3a and Shank3b retain protein binding domains found in humans including Shank/ProSAP N-terminal (SPN), ankyrin repeats, SRC Homology 3 (SH3), postsynaptic density protein/disc large tumor suppressor/zonula occludens-1 protein (PDZ), proline-rich region (PRR), Homer-interaction, cortactin-interaction, actin-binding protein 1 (Abp1), and sterile alpha motif (SAM). Individuals with SHANK3 mutations show a C-terminal bias affecting the largest coding exon preceding the SAM-domain encoding exon (gray cylinder). All indicated mutations show frameshift mutations from people with Phelan-McDermid syndrome above and the two CRISPR generated mutations in zebrafish below. c Raw sequence traces show insertion mutations highlighted in red with corresponding changes in downstream amino acids. Mutations were named according to changes in amino acids (e.g., shank3b isoleucine 915 changed to histidine with 31 frame-shifted amino acids terminating in a stop codon)
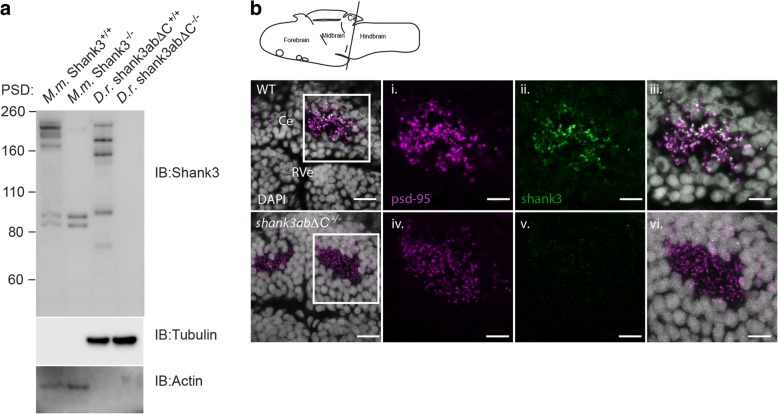
A western blot of protein samples enriched for membrane fractions including post-synaptic densities (PSDs) was then used to determine whether the mouse Shank3 antibody was cross-reactive in zebrafish and to characterize the isoform complexity of zebrafish Shank3ab. Zebrafish protein extracts from adult brain showed multiple isoforms as indicated by the five bands of ranging from 75 to 250 kD in WT. For comparison, PSDs isolated from WT and Shank3−/− mouse brains showed similar Shank3 isoform complexity (Fig. 2a). By contrast, PSD fractions from zebrafish mutant for both shank3a and shank3b (shank3abΔC −/−) showed no visible bands confirming the specificity of the mouse Shank3 antibody for zebrafish Shank3ab.
Fig. 2.
Zebrafish Shank3ab protein shares complex isoform expression with mammals and shows expression in the cerebellum. a Shank3 western blots of postsynaptic densities (PSD) isolated from mouse (M.m) and zebrafish (D.r.) show similar complex isoform expression. Molecular marker weight is expressed in kilodaltons. Tubulin and actin expression were used as loading controls for zebrafish and mouse immunoblots, respectively. Loss of Shank3ab staining in IHC and the western blot supports the immunoreactive specificity of Shank3 antibody. b Shank3ab protein is expressed as distinct overlapping puncta with PSD-95 in the cerebellum from larvae 6 days post-fertilization. Transverse cerebellar sections (see diagram) with enlarged insets show neuropil area dorsal to the rhombencephalic ventricle (RVe). Scale bar represents 10 μm for the first column and 5 μm for the insets (i–vi)
To test for localization of Shank3ab protein in 6 days post-fertilization (dpf) zebrafish brain tissue, we stained fresh frozen sections from both shank3abΔC −/− and WT larvae. Sectioned tissue was co-labeled with PSD-95 antibody because Shank3 is known to co-localize with PSD-95 at mammalian glutamatergic post-synapses [64]. As in mammals, we found that PSD-95 and Shank3 expression co-localized in post-synaptic puncta of WT larvae (Fig. 2b). These puncta were found in the cerebellum that most likely label glutamatergic granule cell synapses. By contrast, Shank3ab expression was absent in shank3abΔC −/− mutants despite robust PSD-95 staining.
DT peristalsis frequency is reduced in shank3abΔC larvae
To test whether shank3abΔC mutations impact DT function, we compared peristaltic rates in WT and different combinations of shank3abΔC mutant alleles in 7 dpf larvae. After feeding, larvae were filmed for a period of 10 min to capture the muscular contractions moving along the DT tract. To determine peristalsis frequency, a custom script in Igor Pro quantifies pixel intensity changes to produce individual plots for regions along the DT (Fig. 3a, cyan boxes). These data show that shank3abΔC+/− larvae have significantly less frequent peristaltic contractions compared to WT larvae (Fig. 3d, WT n = 9, shank3abΔC+/−n = 29, p < 0.001). Peristaltic contractions are shown for the intestinal bulb, as this region is the most responsive to a feeding event. It should be noted, however, that this pattern of significantly reduced motility was also seen in upper and lower intestines (Additional file 1: Table S7). In addition to shank3abΔC+/− larvae, this dysmotility phenotype was also seen in both CRISPR/Cas9/shank3b sgRNA injected embryos (F0 generation; n = 13; p = 0.0001) and shank3abΔC−/− larvae (n = 9, p < 0.0001) compared to WT (similar results were found in the CRISPR/Cas9/shank3a sgRNA injected embryos, but were not recorded with sufficient n to include in the data). Differences between heterozygous and homozygous shank3abΔC mutants were not found to be significant (p = 0.978), suggesting a maximum level of DT effect in shank3abΔC +/− genotypes. Our subsequent analyses therefore focus on this shank3abΔC +/− phenotype that best models human SHANK3 haploinsufficiency.
DT transit time is prolonged in shank3abΔC+/− larvae
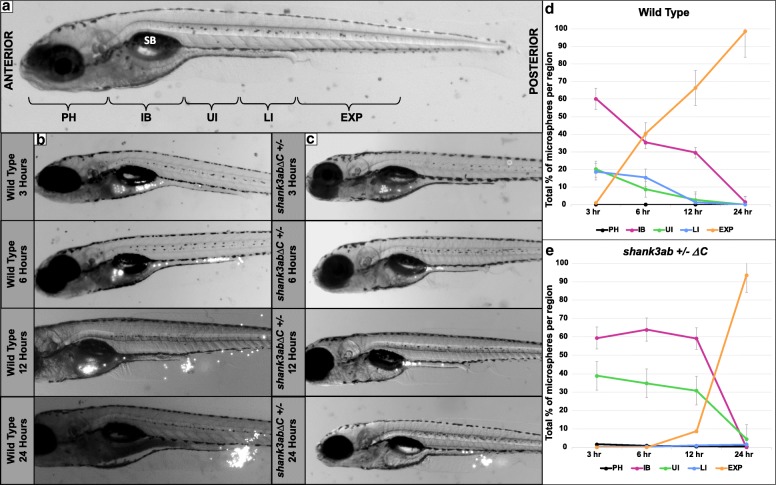
Having established a DT motility phenotype associated with shank3abΔC +/− mutants, we next tested whether the reduced peristaltic frequencies translated into prolonged DT transit times by feeding larvae fluorescent 6 μm microspheres in an emulsion of egg yolk and timing microsphere transit through the DT. Both WT and shank3abΔC+/− larvae were embedded in 1% LMA, immersed in system water, and photographed at 3, 6, 12, and 24 h. Transit differed between WT and shank3abΔC +/− mutants, both in terms of overall rate and the amount of time spent in the upper DT (Fig. 4; Additional file 1: Tables S3–S6). To quantify these differences, we compared the proportions of fluorescent microspheres in DT regions across time and genotypes. After permutation (Additional file 1: Table S8), a repeated measures ANOVA found significant interactions in the percentage of microspheres between genotype (shank3abΔC+/−, n = 15 and WT, n = 13) and time (p < 0.004). When analyzing the rate of bead expulsion specifically (analogous to completed DT transit), significant differences were found between WT and shank3abΔC +/− larvae at 6 h and 12 h transit times (p < 0.006), but not the 24 h time point (p = 0.330). This is indicative of a severely delayed but not entirely obstructed transit. Most WT larvae had passed a large portion of the consumed microspheres by 6 h and had passed the remainder between 12 and 24 h post consumption (Fig. 4b). Comparatively, shank3abΔC +/− larvae took longer than 12 h to begin passing the microspheres and some individuals had not passed the remainder even after 24 h post consumption (Fig. 4c). Of particular interest is the amount of “sloshing” that occurred in shank3abΔC +/− larvae, where the microspheres would repeatedly move anteriorly and posteriorly between the intestinal bulb and upper-intestine. In shank3abΔC +/− larvae, the passage of particles seemed to get delayed in two key areas: the intestinal bulb to upper-intestine transition and near the anus (Additional file 2, shank3ab mutant). This intestinal bulb to upper-intestine transition marks a key region where both anterograde and retrograde peristaltic propulsion transitions to just retrograde propulsion [36]. WT larvae displayed no sloshing and passed the microspheres in a clear anterior to posterior direction; WT similarly lacked the delay in the intestinal bulb to upper-intestine transition that was seen in shank3abΔC +/− larvae.
Fig. 4.
DT transit time of fluorescent beads is prolonged in shank3abΔC+/− mutants compared to WT larvae. a Regions of the DT analyzed for fluorescent bead occupancy are indicated on a 7-day-old zebrafish larva. Beads were tracked as they passed through five key regions: PH (pharynx), IB (intestinal bulb), UI (upper intestine), LI (lower intestine), and EX (expelled). The swim bladder (SB) is labeled for reference. b Images of a representative WT fish at 3, 6, 12, and 24 h post-feed. Beads appear white and can be seen throughout the DT and collecting near the anus post-expulsion. c Images of a representative shank3abΔC +/− larva at 3, 6, 12, and 24 h post-feed. d Graph of bead distribution per time point for WT fish. Each point represents the average +/− standard error of the percentage of total beads held by each region (PH, IB, UI, LI, or EXP) over time (n = 13). e Graph of shank3abΔC +/− mutant average +/− standard error bead distribution over time (n = 15)
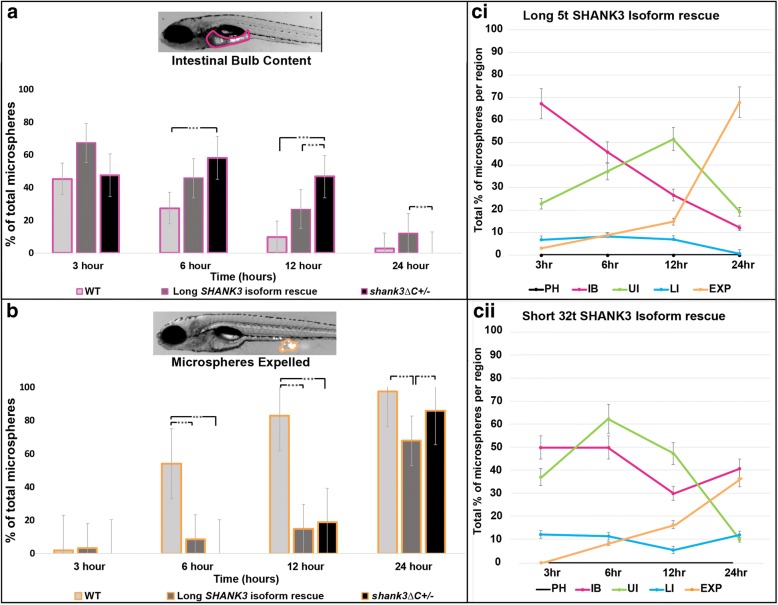
Partial rescue of shank3abΔC+/− transit time with human SHANK3
To test whether providing human SHANK3 mRNA to shank3abΔC +/− zebrafish embryos is sufficient to rescue the DT dysmotility phenotype, we injected mRNA encoding either the longest human SHANK3 isoform that includes all SHANK3 protein domains (5t, n = 19) or a shorter human SHANK3 isoform that includes only the C-terminal proline-rich and SAM domains (32t, n = 6) into fertilized eggs from shank3abΔC +/− mutants. After injection, larvae were reared to 7 dpf and were put through the fluorescent microsphere digestive transit assay. While injecting shank3abΔC +/− larvae with the short 32t SHANK3 isoform further slowed mutant transit times (Fig. 5cii), injecting the long 5t SHANK3 isoform partially rescued mutant transit times (Fig. 5ci); microspheres moved in a clear anterior-posterior direction without any sloshing or pause at the intestinal bulb to upper-intestine transition. The rescue did not return the WT phenotype entirely; while the progression of microspheres was more consistent, the transit rates in rescued shank3abΔC +/− mutants resembled a mid-point between unrescued shank3abΔC +/− and WT larvae (Fig. 5a). By contrast to the partial rescue in the upper intestine, transit rates in the lower intestine were not rescued (Fig. 5b). Therefore, it is likely that RNA rescue was associated with overexpression phenotypes in addition to the partial functional rescue seen with injection of the longest SHANK3 isoform.
Fig. 5.
Full-length human SHANK3 mRNA partially rescues transit time in shank3abΔC +/− larvae. a When measuring the total percentage of fluorescent beads within the intestinal bulb (highlighted region of analysis for in magenta), total content of the long isoform (5t) rescue (dark gray) decreases at a rate quicker than the unrescued shank3abΔC +/− (black) but not as quickly as the WT (light gray). Significant differences of intestinal bulb emptying (total intestinal bulb content) were found between the long isoform rescued and unrescued shank3abΔC +/− larvae at 12 h (p < 0.006) and 24 h (p < 0.03), and between WT and unrescued shank3abΔC +/− at 6 h (p < 0.02) and 12 h (p < 0.01). b Fluorescent microbead expulsion (analogous to completed digestion; highlighted region of analysis in orange) was not rescued, however, and the long isoform rescued larvae showed expulsion rates closer to shank3abΔC +/− than that of WT. Significant differences were found at 6 h between WT, the long isoform rescue, and unrescued shank3abΔC +/− (p < 0.006), at 12 h between WT and unrescued shank3abΔC +/− (p < 0.001), WT and the long isoform rescue (p < 0.001), and at 24 h between unrescued shank3abΔC +/− and the long isoform rescue larvae (p < 0.01), and WT and the long isoform rescue larvae (p < 0.005). ci Graph of bead distribution per time period for the long isoform (5t) rescue larvae (n = 19) and cii short isoform (32t) rescue larvae (n = 6). Each point represents the average +/− standard error percentage of total beads held by each region
Larval DT morphology
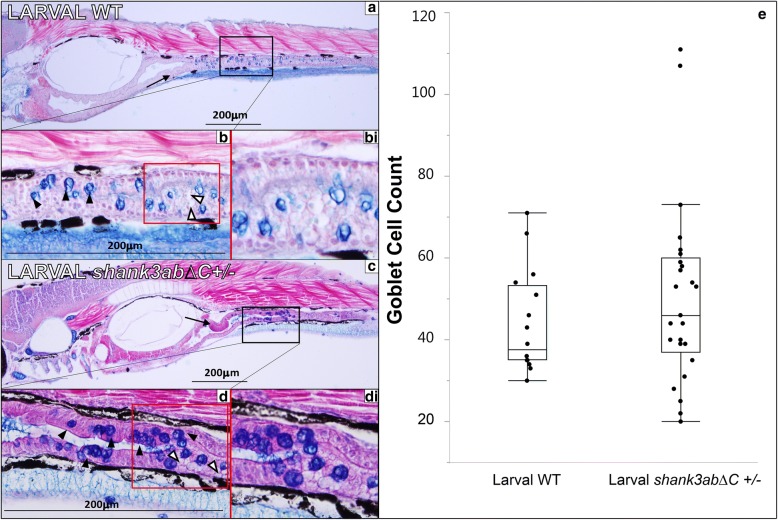
In order to assess the integrity of both WT and shank3abΔC+/− mutant gut tissues, we processed 7 dpf larvae for paraffin sectioning. Larvae were sectioned along the sagittal plane at a thickness of 5 μm and stained with alcian blue and Eosin B as a counterstain, with a left-right anterior to posterior orientation (Fig. 6). Typical features of gut morphology at this stage of development are captured in WT sections (Fig. 6a, b). The intestine is comprised of polarized epithelium and is well-defined with dense cytoplasm. Folding of the epithelium (plicae) can be seen readily in the intestinal bulb extending into the upper-intestine (arrows), but these plicae do not extend any significant distance into the luminal space. Goblet cells and mucin production can be seen throughout the upper-intestine (black arrowheads). In shank3abΔC +/− larval sections (Fig. 6c, d), like that seen in WT, folding can be seen in the intestinal bulb, and extending into the upper-intestine; goblet cells and heavy mucin production can be seen throughout the upper-intestine (black arrowheads); and enterocytes have similar large supranuclear vesicles (white arrowheads) [65]. Statistically, shank3abΔC +/− larvae did not show a significant increase in goblet cells (p = 0.750, n = 25 for shank3abΔC +/− fish, n = 15 for WT).
Fig. 6.
Histology of larval WT and shank3abΔC+/− mutants (n = 25 for shank3abΔC +/−n = 15 for WT). Black boxes in a and c indicate regions shown at 40× magnification, while red boxes in b and d indicate regions of magnified insets in bi and di. a 10× magnification of longitudinal sections through 7 dpf WT fish (anterior left to posterior right); sections were stained with alcian blue and Eosin B. Sections show a well-defined polarized epithelium, with folding beginning in the intestinal bulb and extending into the upper intestine (black arrow). b The goblet cells are stained dark blue (black arrowheads) and intestines (stained purple) are clearly visible, while mucous production can be seen in the luminal space, stained light blue. bi This magnified inset from b shows enterocytes with large supranuclear vesicles (white arrowheads). c 10× magnification of 5-μm sections through 7 dpf shank3abΔC +/− mutants. Mucous production can be seen in luminal space shown in 40× magnification of d, along with goblet cells (black arrowheads) and intestinal lumen. di Similar to what is seen in WT fish, shank3abΔC+/− mutants display enterocytes with large supranuclear vesicles (white arrowheads). e Comparison of WT and shank3abΔC +/− mutant goblet cell count; no significant difference was found at 7 dpf
We also quantified goblet cells in adult DTs from adult (> 1 year old) WT, shank3abΔ+/−, and shank3abΔC−/− animals that had been sacrificed to acquire adequate CNS tissue for western analysis. Paraffin sections were collected near the IB and UI transition zone and stained similar to 7 dpf larvae. In contrast to larvae, goblet cell counts from adult gut tissue were significantly increased in shank3abΔC +/− and shank3abΔC −/− mutant as compared to WT (Additional file 1: Figure S2; p < 0.001, n = 6 for WT, n = 6 for shank3abΔC +/−, and n = 6 for shank3abΔC −/−).
Larval shank3abΔC +/− and shank3abΔC −/− DTs contain fewer serotonin (5-HT+) cells
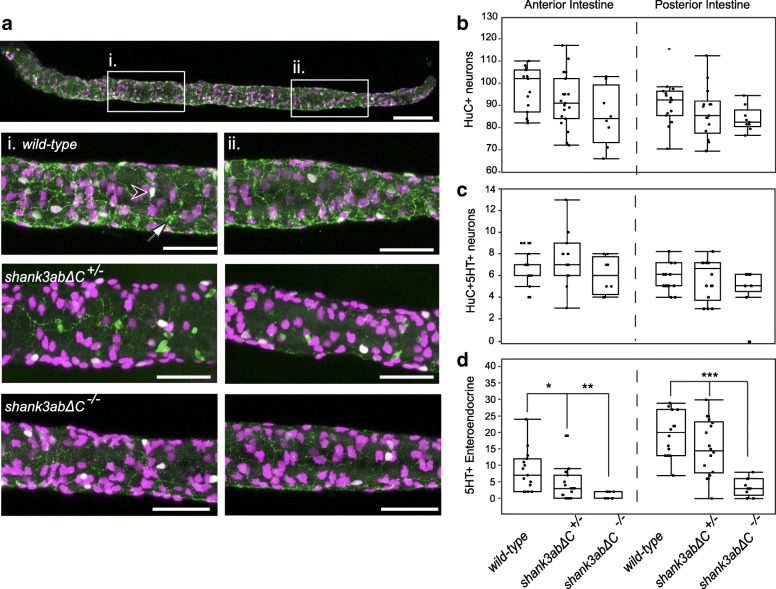
To determine whether impaired gut motility was due to deficits in the ENS or enteroendocrine cells (EECs), shank3abΔC mutants and WT larvae were immunostained for the pan-neuronal marker HuC and 5-HT (Fig. 7, WT, n = 15; shank3abΔC +/−, n = 19; shank3abΔC −/−, n = 9). Enteric neurons (HuC+), serotonin enteric neurons (HuC+/5-HT+), and enteroendocrine (5-HT+) cell bodies from representative anterior and posterior regions (Fig. 7a, (i and ii)) were counted using ImageJ and confocal Z-stacks. There was no significant decrease in enteric neurons along the intestinal tract of shank3abΔC mutants compared to WT larvae. However, there was a significant decrease in serotonin-positive EECs in the intestinal tract of shank3abΔC+/− (anterior, p = 0.0128; posterior, p = 0.0003) and shank3abΔC−/− (anterior, p = 0.0013; posterior p < 0.0001) larvae compared to WT. EECs are mechanosensitive cells that release serotonin in response to mechanical and/or chemical stimulation, which in turn causes the intestine to increase mucus secretion and peristalsis, both of which facilitate transit [66].
Fig. 7.
Shank3abΔC mutants have a reduction in enteroendocrine cells. a Anterior (i.) and posterior (ii.) regions were sampled to quantify the number of enteric neurons (HuC, magenta), serotonin enteric neurons (HuC+/5-HT+, white, black arrowhead), and enteroendocrine cells (5-HT+, green, white arrow). Shank3abΔC mutants did not show a significant decrease in either b enteric neurons or c serotonin positive enteric neurons. d Serotonin positive enteroendocrine cells were significantly decreased in shank3abΔC mutants for both anterior and posterior regions. WT, n = 15;shank3abΔC +/−, n = 19; shank3abΔC −/−, n = 9. Scale bars represent 100 μm (a) and 50 μm (i, ii)
Discussion
Zebrafish as a model system for GI distress in ASD
Our studies are the first to establish DT dysmotility as a robust phenotype in any SHANK3 mutant animal model of ASD. The developmental accessibility and transparency of the zebrafish model system allows highly quantitative assays to be carried out in an intact animal [40, 46]. This type of systems-level analysis is important because GI motility is coordinately regulated by diverse neuronal tissues [67]. For example, the central nervous system (CNS) and parasympathetic ganglia increase GI motility in anticipation of the arrival of food; the vagus nerve releases acetylcholine to promote stomach emptying [53–56]; and coordinated regulation by the enteric nervous system [36, 68–70], enterochromaffin cells [71, 72], interstitial cells of Cajal [73], and microbiome [74–76] all help to promote motility and digestion. In the zebrafish larva, it is possible to quantify this coordinated regulation as food passes through the DT tract. Our analyses of DT function in WT and shank3abΔC zebrafish reveal hypomotility as a phenotype with likely parallels to GI distress described in individuals with PMS. Based on our findings, our working hypothesis is that mutations in shank3ab disrupt EEC regulation of DT motility.
Zebrafish Shank3ab proteins show similar isoform complexity and post-synaptic density enrichment to that seen in mammals
Zebrafish and mouse both express multiple Shank3 isoforms in the brain. Previous studies of the mouse Shank3 gene have identified ten to twelve Shank3 isoforms arising from five transcriptional start sites as well as alternative splicing [62, 77]. While zebrafish shank3ab genes have also been shown to encode at least six isoforms [21], these likely represent an underestimate based on our western data. Despite using an antibody targeting the C-terminus of Shank3 that would only recognize three of the six previously characterized splice-variants, our western identifies five abundant and additional, less abundant Shank3 isoforms that range in size from 75 to 250 kD. These bands are absent in brain-specific PSD extracts from shank3abΔC −/− animals, showing that these bands represent specific Shank3ab protein products. This antibody recognizes residues downstream of the induced mutations; therefore, it is possible that there is residual Shank3 protein in our zebrafish shank3ΔC −/− mutants. Future experiments are needed to assess the full complement of protein products produced by shank3ab genes as they relate to different phenotypes associated with the shank3abΔC mutant genotype.
Immunohistochemistry of larval cerebellar tissue shows that zebrafish Shank3ab proteins co-localize with PSD-95 at excitatory post-synaptic densities, as has been reported previously in mammalian cultured hippocampal neurons [78]. The lack of Shank3ab puncta in shank3abΔC mutants shows that the mammalian Shank3 antibody also specifically recognizes zebrafish Shank3ab in tissue. Taken together, these findings support that there is functional conservation between zebrafish and mammalian Shank3 proteins.
Mutations in shank3ab result in DT hypomotility
Functional comparisons of WT and shank3abΔC +/− mutant larvae show significantly reduced DT peristaltic frequencies with correspondingly prolonged DT transit times. It is important to note that our assay did not test appetite or potential differences in jaw shape that might impact their ability to forage. Comparisons of overall size between WT and shank3abΔC mutant larvae found no significant differences (data not shown), but further study of aspects such as jaw size/shape and potential differences in appetite should be investigated in future research. Although we saw no signs of regurgitation in larval shank3abΔC mutants, fluorescent microspheres remained in the intestinal bulb for prolonged periods of time, and in a small number of cases, fluorescent microspheres moved back into the pharynx in shank3abΔC +/− individuals, a behavior not seen in the WT larvae. Such prolonged transit times and longer dwell times in anterior DT in shank3abΔC +/− larvae are consistent with reflux and vomiting in individuals with PMS [11, 12] and phenotypes reported in a zebrafish chd7 mutant model of CHARGE syndrome [79].
Hypomotility in shank3abΔC+/− larvae can be partially rescued by human SHANK3 mRNA
Prolonged dwell times in the intestinal bulb of 7-day-old shank3abΔC +/− larvae could be partially rescued by injecting human SHANK3 mRNA at the one cell stage. Human SHANK3 RNA specifically rescued transit from the intestinal bulb (analogous to the mammalian stomach) to the upper intestine but not overall intestinal motility. Unlike endogenous transcripts, RNAs injected at fertilization are expressed transiently, for approximately 48 h, throughout the embryo [80]. Therefore, such a manipulation would likely rescue early shank3ab-dependent processes like hindbrain morphogenesis [20], but perhaps not later shank3ab-dependent processes like ENS migration, EEC differentiation, growth and function, and general epithelial maintenance.
Structurally intact DT tissue in shank3ab mutant larvae contains fewer serotonin-expressing enteroendocrine cells
Histological analysis of shank3abΔC +/− and WT larval DTs reveals normal tissue structure in mutants. Despite the fact that inflammation and leaky gut have been implicated in ASD-linked GI distress [81, 82], at least at larval stages, shank3abΔC +/− zebrafish exhibited an intact columnar epithelium and similar numbers of goblet cells to their WT counterparts and to the literature. Likewise, analysis of the larval ENS showed similar numbers of enteric neurons in WT and shank3abΔC mutants.
By staining for serotonin in the DT, we showed that EECs are significantly reduced in both shank3abΔC +/− and shank3abΔC −/− larvae. EECs in the gut synthesize 95% of the body’s serotonin and, during digestion, serotonin release helps to coordinate secretion and motility as well as satiety and pain sensation [83, 84]. RNA-seq analysis from cells dissociated and sorted from 6 dpf WT larvae show shank3a and shank3b are detectable in EECs as well other intestinal epithelial cells, with shank3a expressed at higher levels than shank3b (personal communication from Lihua Ye, Roger A. Liddle, and John F. Rawls, Duke University). Therefore, Shank3 dosage-dependent reductions in DT EECs could help to explain dysmotility phenotypes. Future experiments are required to establish a causal relationship between EEC reductions and DT dysmotility phenotypes. Additionally, future studies will need to explore other cell types, such as interstitial cells of Cajal and smooth muscle cells, as defects in these may also explain the observed motility phenotype.
Unlike larval shank3abΔC +/−, DTs from both shank3abΔC +/− and shank3abΔC −/− adults show significantly more goblet cells. Such a goblet cell expansion could result from disrupting Delta-Notch signaling and/or increasing inflammation. As in mammals, zebrafish goblet cell differentiation (and DT epithelial maintenance in general) is reliant on Delta-Notch signaling [85, 86]; therefore, it is possible that Shank3ab normally interacts with Delta-Notch signaling in a way that is pivotal to GI differentiation and maintenance. Recent research reports such interactions between ascl1a (a transcription factor important for neuronal commitment and differentiation) and Delta-Notch have drastic impacts on both motility and secretory cell expression [87]. An alternate possibility is that reduced motility feeds back on microbial communities to cause inflammation [88]. Zebrafish serve as an excellent model system for studying inflammation, and it has been observed that such inflammation can cause a dramatic expansion of goblet cells [89]. Importantly, inflammation is reported to occur in ASD, and the potential impact it might have on patients is not yet known [90]. Distinguishing between these possibilities and expanding on their potential impact is an area of interest for future studies.
Conclusions
The shank3abΔC mutant zebrafish generated herein mimics clinically relevant mutations shown previously to cause Phelan-McDermid syndrome. Westerns and immunohistochemistry show zebrafish Shank3 to be functionally conserved with mammalian Shank3 in terms of isoform complexity and enrichment at post-synaptic sites in the cerebellum, validating the zebrafish model system. shank3abΔC mutant zebrafish show reduced DT peristalsis, increased digestive transit time, and reductions in EECs consistent with reduced GI functionality and symptoms of GI distress reported in people with Phelan-McDermid syndrome. Prolonged passage time in shank3abΔC mutants can be rescued by human SHANK3 mRNA establishing reduced Shank3 in the zebrafish model system as causal for GI dysmotility. This zebrafish model system provides a basis for studying the functional relationships between the brain, gut, and microbiome as these relate to GI distress common in Phelan-McDermid syndrome.
Additional files
Figure S1. Insertion mutations in zebrafish shank3 orthologues produce unique restriction maps for genotyping. Figure S2. a Transverse 5 μm section of adult WT upper intestinal tissue stained with alcian blue and Eosin B (n = 6 for WT, n = 6 for shank3abΔC +/− and n = 6 for shank3abΔC −/−). b 40x magnification shows dense plicae that extend to the point of nearly occluding the luminal space. c, d In shank3abΔC +/− upper intestinal tissue, increased counts of goblet cells (black arrowheads) suggest inflammation. e, f Homozygous shank3abΔC −/− adults also show increased goblet cell count. Table S1. Oligonucleotides used for sgRNA synthesis. Table S2. Primers used for PCR and sanger sequencing. Table S3. % bead occupancy in WT and shank3abΔC+/− larvae at 3 h post-feed. Table S4. % bead occupancy in WT and shank3abΔC+/− larvae at 6 h post-feed. Table S5. % bead occupancy in WT and shank3abΔC+/− larvae at 12 h post-feed. Table S6. % bead occupancy in WT and shank3abΔC+/− larvae at 24 h post-feed. Table S7. Upper and lower GI rate of peristaltic period (seconds between contractions). Table S8. R script for Permutation. Table S9. shank3abΔC+/− injected with either Human SHANK3 mRNA short (32T) or long (5 T) isoform, 3–24 h post feed. (DOCX 4255 kb)
Acknowledgements
We would like to thank the Phelan McDermid Syndrome Foundation for including us in their International Family Conference, where we first learned of GI distress in kids with Phelan McDermid Syndrome. We would also like to thank Nick Talley, Elizabeth Davidson, Kevin Collins, James Baker, and Mary Dallman for discussion and feedback on writing, James D. Baker for giving us time on his microscope and camera for motility and for helping with confocal, Bill Browne for helping with movies of bead transit, Chris Searcy, Stephanie Diaz, and David C. Howell for assistance with statistical analysis and R-programming, the Biology department for core histology equipment, and Ricardo Cepeda for excellent care of the zebrafish.
Funding
This work was supported by the Seaver Foundation and NIH grant MH101584 to JDB, Bridge Funds from the College of Arts and Sciences at the University of Miami and NIH grants NIMH R03MH103857 and NICHD R21HD093021 to JED and an IMSD graduate fellowship from NIH parent grant R25GM076419, HHMI teaching fellowship, and McKnight Dissertation Fellowship to DMJ.
Availability of data and materials
The datasets used and/or analyzed during the current study as well as the animal models are available from the corresponding author on reasonable request.
Abbreviations
- 5-HT
Serotonin
- ASD
Autism spectrum disorder
- CNS
Central nervous system
- DT
Digestive tract
- ENS
Enteric nervous system
- GI
Gastrointestinal
- PMS
Phelan-McDermid syndrome
- WT
Wild type
Authors’ contributions
DMJ, RAK, and JED shared in the writing of the manuscript, with extensive editing by JED. JED and RAK designed and RAK implemented CRISPR/Cas9 mutagenesis of shank3a and shank3b loci in zebrafish. JED and DMJ designed and DMJ implemented assays of DT function. MK wrote Igor Pro program for motility analyses and ALW analyzed DT motility blind to genotype. DMJ carried out paraffin sections with ECS doing cell counts blind to genotype, and RAK carried out ENS and EEC analyses. BM advised on transit assays, provided clinical perspective, and helped with writing. YK and JDB helped with post-synaptic density purification from zebrafish brains and western blotting and writing of the “Methods” and “Results” sections relating to those techniques. All authors read and approved the final manuscript.
Ethics approval
All procedures with animal are described in protocols 15–191 and 18–128 and were approved by the Institutional Animal Care and Use Committee of University of Miami.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Christensen DL, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years--autism and developmental disabilities monitoring network, 11 sites, United States, 2012. MMWR Surveill Summ. 2016;65(3):1–23. doi: 10.15585/mmwr.ss6503a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.IAAC. In: I.A.C.C. (IACC), editor. 2016–2017 Interagency Autism Coordinating Committee Strategic Plan For Autism Spectrum Disorder; 2017. Retrieved from the U.S. Department of Health and Human Services Interagency Autism Coordinating Committee website: https://iacc.hhs.gov/publications/strategic-plan/2017/. Accessed 23 Dec 2018.
- 3.Hsiao EY. Gastrointestinal issues in autism spectrum disorder. Harv Rev Psychiatry. 2014;22(2):104–111. doi: 10.1097/HRP.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 4.Chaidez V, Hansen RL, Hertz-Picciotto I. Gastrointestinal problems in children with autism, developmental delays or typical development. J Autism Dev Disord. 2014;44(5):1117–1127. doi: 10.1007/s10803-013-1973-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuhlthau KA, et al. Associations of quality of life with health-related characteristics among children with autism. Autism. 2018;22(7):804-813. [DOI] [PubMed]
- 6.Bresnahan M, et al. Association of maternal report of infant and toddler gastrointestinal symptoms with autism: evidence from a prospective birth cohort. JAMA Psychiatry. 2015;72(5):466–474. doi: 10.1001/jamapsychiatry.2014.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grubisic V, et al. Pitt-Hopkins mouse model has altered particular gastrointestinal transits in vivo. Autism Res. 2015;8(5):629–633. doi: 10.1002/aur.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernier R, et al. Disruptive CHD8 mutations define a subtype of autism early in development. Cell. 2014;158(2):263–276. doi: 10.1016/j.cell.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Betancur C, Buxbaum JD. SHANK3 haploinsufficiency: a "common" but underdiagnosed highly penetrant monogenic cause of autism spectrum disorders. Mol Autism. 2013;4(1):17. doi: 10.1186/2040-2392-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phelan K, McDermid HE. The 22q13.3 deletion syndrome (Phelan-McDermid syndrome) Mol Syndromol. 2012;2(3–5):186–201. doi: 10.1159/000334260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soorya L, et al. Prospective investigation of autism and genotype-phenotype correlations in 22q13 deletion syndrome and SHANK3 deficiency. Mol Autism. 2013;4(1):18. doi: 10.1186/2040-2392-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarasua SM, et al. Clinical and genomic evaluation of 201 patients with Phelan-McDermid syndrome. Hum Genet. 2014;133(7):847–859. doi: 10.1007/s00439-014-1423-7. [DOI] [PubMed] [Google Scholar]
- 13.Jiang YH, Ehlers MD. Modeling autism by SHANK gene mutations in mice. Neuron. 2013;78(1):8–27. doi: 10.1016/j.neuron.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mei Y, et al. Adult restoration of Shank3 expression rescues selective autistic-like phenotypes. Nature. 2016;530(7591):481–484. doi: 10.1038/nature16971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peixoto RT, et al. Early hyperactivity and precocious maturation of corticostriatal circuits in Shank3B(−/−) mice. Nat Neurosci. 2016;19(5):716–724. doi: 10.1038/nn.4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bozdagi O, et al. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol Autism. 2010;1(1):15. doi: 10.1186/2040-2392-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roussignol G, et al. Shank expression is sufficient to induce functional dendritic spine synapses in aspiny neurons. J Neurosci. 2005;25(14):3560–3570. doi: 10.1523/JNEUROSCI.4354-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peca J, et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472(7344):437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bockers TM, et al. Synaptic scaffolding proteins in rat brain. Ankyrin repeats of the multidomain Shank protein family interact with the cytoskeletal protein alpha-fodrin. J Biol Chem. 2001;276(43):40104–12. doi: 10.1074/jbc.M102454200. [DOI] [PubMed] [Google Scholar]
- 20.Kozol RA, et al. Two knockdown models of the autism genes SYNGAP1 and SHANK3 in zebrafish produce similar behavioral phenotypes associated with embryonic disruptions of brain morphogenesis. Hum Mol Genet. 2015;24(14):4006–4023. doi: 10.1093/hmg/ddv138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu CX, et al. Developmental profiling of ASD-related shank3 transcripts and their differential regulation by valproic acid in zebrafish. Dev Genes Evol. 2016;226(6):389–400. doi: 10.1007/s00427-016-0561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huett A, et al. The cytoskeletal scaffold Shank3 is recruited to pathogen-induced actin rearrangements. Exp Cell Res. 2009;315(12):2001–2011. doi: 10.1016/j.yexcr.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaudhury A. Molecular handoffs in nitrergic neurotransmission. Front Med (Lausanne) 2014;1:8. doi: 10.3389/fmed.2014.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raab M, Boeckers TM, Neuhuber WL. Proline-rich synapse-associated protein-1 and 2 (ProSAP1/Shank2 and ProSAP2/Shank3)-scaffolding proteins are also present in postsynaptic specializations of the peripheral nervous system. Neuroscience. 2010;171(2):421–433. doi: 10.1016/j.neuroscience.2010.08.041. [DOI] [PubMed] [Google Scholar]
- 25.Qin L, et al. Social deficits in Shank3-deficient mouse models of autism are rescued by histone deacetylase (HDAC) inhibition. Nat Neurosci. 2018;21(4):564–575. doi: 10.1038/s41593-018-0110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grabrucker AM. A role for synaptic zinc in ProSAP/Shank PSD scaffold malformation in autism spectrum disorders. Dev Neurobiol. 2014;74(2):136–146. doi: 10.1002/dneu.22089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grabrucker S, et al. Zinc deficiency dysregulates the synaptic ProSAP/Shank scaffold and might contribute to autism spectrum disorders. Brain. 2014;137(Pt 1):137–152. doi: 10.1093/brain/awt303. [DOI] [PubMed] [Google Scholar]
- 28.Pfaender S, et al. Zinc deficiency and low enterocyte zinc transporter expression in human patients with autism related mutations in SHANK3. Sci Rep. 2017;7:45190. doi: 10.1038/srep45190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei SC, et al. SHANK3 regulates intestinal barrier function through modulating ZO-1 expression through the PKCepsilon-dependent pathway. Inflamm Bowel Dis. 2017;23(10):1730–1740. doi: 10.1097/MIB.0000000000001250. [DOI] [PubMed] [Google Scholar]
- 30.Abrams AJ, et al. Mutations in SLC25A46, encoding a UGO1-like protein, cause an optic atrophy spectrum disorder. Nat Genet. 2015;47(8):926–932. doi: 10.1038/ng.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ijaz S, Hoffman EJ. Zebrafish: a translational model system for studying neuropsychiatric disorders. J Am Acad Child Adolesc Psychiatry. 2016;55(9):746–748. doi: 10.1016/j.jaac.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kozol RA, et al. Function over form: modeling groups of inherited neurological conditions in zebrafish. Front Mol Neurosci. 2016;9:55. doi: 10.3389/fnmol.2016.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baraban SC, Dinday MT, Hortopan GA. Drug screening in Scn1a zebrafish mutant identifies clemizole as a potential Dravet syndrome treatment. Nat Commun. 2013;4:2410. doi: 10.1038/ncomms3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoffman EJ, et al. Estrogens suppress a behavioral phenotype in zebrafish mutants of the autism risk gene, CNTNAP2. Neuron. 2016;89(4):725–733. doi: 10.1016/j.neuron.2015.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White R, Rose K, Zon L. Zebrafish cancer: the state of the art and the path forward. Nat Rev Cancer. 2013;13(9):624–636. doi: 10.1038/nrc3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shepherd I, Eisen J. Development of the zebrafish enteric nervous system. Methods Cell Biol. 2011;101:143–160. doi: 10.1016/B978-0-12-387036-0.00006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen YC, et al. Zebrafish Agr2 is required for terminal differentiation of intestinal goblet cells. PLoS One. 2012;7(4):e34408. doi: 10.1371/journal.pone.0034408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rich A, et al. Kit signaling is required for development of coordinated motility patterns in zebrafish gastrointestinal tract. Zebrafish. 2013;10(2):154–160. doi: 10.1089/zeb.2012.0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jordi J, et al. A high-throughput assay for quantifying appetite and digestive dynamics. Am J Phys Regul Integr Comp Phys. 2015;309(4):R345–R357. doi: 10.1152/ajpregu.00225.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao X, Pack M. Modeling intestinal disorders using zebrafish. Methods Cell Biol. 2017;138:241–270. doi: 10.1016/bs.mcb.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 41.Holmberg A, et al. Ontogeny of the gut motility control system in zebrafish Danio rerio embryos and larvae. J Exp Biol. 2004;207(Pt 23):4085–4094. doi: 10.1242/jeb.01260. [DOI] [PubMed] [Google Scholar]
- 42.Olsson C, Holmberg A, Holmgren S. Development of enteric and vagal innervation of the zebrafish (Danio rerio) gut. J Comp Neurol. 2008;508(5):756–770. doi: 10.1002/cne.21705. [DOI] [PubMed] [Google Scholar]
- 43.Holmberg A, Olsson C, Holmgren S. The effects of endogenous and exogenous nitric oxide on gut motility in zebrafish Danio rerio embryos and larvae. J Exp Biol. 2006;209(Pt 13):2472–2479. doi: 10.1242/jeb.02272. [DOI] [PubMed] [Google Scholar]
- 44.Wallace KN, et al. Intestinal growth and differentiation in zebrafish. Mech Dev. 2005;122(2):157–173. doi: 10.1016/j.mod.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Wallace KN, Pack M. Unique and conserved aspects of gut development in zebrafish. Dev Biol. 2003;255(1):12–29. doi: 10.1016/S0012-1606(02)00034-9. [DOI] [PubMed] [Google Scholar]
- 46.Ganz J, Melancon E, Eisen JS. Zebrafish as a model for understanding enteric nervous system interactions in the developing intestinal tract. Methods Cell Biol. 2016;134:139–164. doi: 10.1016/bs.mcb.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 47.Yanez J, et al. Gustatory and general visceral centers and their connections in the brain of adult zebrafish: a carbocyanine dye tract-tracing study. J Comp Neurol. 2017;525(2):333–362. doi: 10.1002/cne.24068. [DOI] [PubMed] [Google Scholar]
- 48.Jiang Q, et al. Functional loss of semaphorin 3C and/or semaphorin 3D and their epistatic interaction with ret are critical to Hirschsprung disease liability. Am J Hum Genet. 2015;96(4):581–596. doi: 10.1016/j.ajhg.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bonora E, et al. Mutations in RAD21 disrupt regulation of APOB in patients with chronic intestinal pseudo-obstruction. Gastroenterology. 2015;148(4):771–782.e11. doi: 10.1053/j.gastro.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glasauer SM, Neuhauss SC. Whole-genome duplication in teleost fishes and its evolutionary consequences. Mol Gen Genomics. 2014;289(6):1045–1060. doi: 10.1007/s00438-014-0889-2. [DOI] [PubMed] [Google Scholar]
- 51.Bayes A, et al. Evolution of complexity in the zebrafish synapse proteome. Nat Commun. 2017;8:14613. doi: 10.1038/ncomms14613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hurley IA, et al. A new time-scale for ray-finned fish evolution. Proc Biol Sci. 2007;274(1609):489–498. doi: 10.1098/rspb.2006.3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leblond CS, et al. Meta-analysis of SHANK mutations in autism spectrum disorders: a gradient of severity in cognitive impairments. PLoS Genet. 2014;10(9):e1004580. doi: 10.1371/journal.pgen.1004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hsieh JY, et al. Rapid development of Purkinje cell excitability, functional cerebellar circuit, and afferent sensory input to cerebellum in zebrafish. Front Neural Circuits. 2014;8:147. doi: 10.3389/fncir.2014.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Satou C, Kimura Y, Higashijima S. Generation of multiple classes of V0 neurons in zebrafish spinal cord: progenitor heterogeneity and temporal control of neuronal diversity. J Neurosci. 2012;32(5):1771–1783. doi: 10.1523/JNEUROSCI.5500-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Montague TG, et al. CHOPCHOP: a CRISPR/Cas9 and TALEN web tool for genome editing. Nucleic Acids Res. 2014;42(Web Server issue):W401–W407. doi: 10.1093/nar/gku410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hwang WY, et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol. 2013;31(3):227–229. doi: 10.1038/nbt.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ganser LR, et al. Distinct phenotypes in zebrafish models of human startle disease. Neurobiol Dis. 2013;60:139–151. doi: 10.1016/j.nbd.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Field HA, et al. Analysis of gastrointestinal physiology using a novel intestinal transit assay in zebrafish. Neurogastroenterol Motil. 2009;21(3):304–312. doi: 10.1111/j.1365-2982.2008.01234.x. [DOI] [PubMed] [Google Scholar]
- 60.Uyttebroek L, et al. Neurochemical coding of enteric neurons in adult and embryonic zebrafish (Danio rerio) J Comp Neurol. 2010;518(21):4419–4438. doi: 10.1002/cne.22464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simonson LW, et al. Characterization of enteric neurons in wild-type and mutant zebrafish using semi-automated cell counting and co-expression analysis. Zebrafish. 2013;10(2):147–153. doi: 10.1089/zeb.2012.0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang X, et al. Transcriptional and functional complexity of Shank3 provides a molecular framework to understand the phenotypic heterogeneity of SHANK3 causing autism and Shank3 mutant mice. Mol Autism. 2014;5:30. doi: 10.1186/2040-2392-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Rubeis S, Siper PM, Durkin A, Weissman J, Muratet F, Halpern D, MdP T, Frank Y, Lozano R, Wang AT, Holder JL Jr, Betancur C, Buxbaum JD, Kolevzon A. Delineation of the genetic and clinical spectrum of Phelan-McDermid syndrome caused by SHANK3 point mutations. Mol Autism. 2018;9:31-51. [DOI] [PMC free article] [PubMed]
- 64.Tu JC, et al. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23(3):583–592. doi: 10.1016/S0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- 65.Ng AN, et al. Formation of the digestive system in zebrafish: III. Intestinal epithelium morphogenesis. Dev Biol. 2005;286(1):114–35. doi: 10.1016/j.ydbio.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 66.Linan-Rico A, et al. Mechanosensory signaling in enterochromaffin cells and 5-HT release: potential implications for gut inflammation. Front Neurosci. 2016;10:564. doi: 10.3389/fnins.2016.00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Holtmann G, Talley NJ. The stomach-brain axis. Best Pract Res Clin Gastroenterol. 2014;28(6):967–979. doi: 10.1016/j.bpg.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 68.Groneberg D, Voussen B, Friebe A. Integrative control of gastrointestinal motility by nitric oxide. Curr Med Chem. 2016;23(24):2715–2735. doi: 10.2174/0929867323666160812150907. [DOI] [PubMed] [Google Scholar]
- 69.Grundy D, et al. Fundamentals of neurogastroenterology: basic science. Gastroenterology. 2006;130(5):1391–1411. doi: 10.1053/j.gastro.2005.11.060. [DOI] [PubMed] [Google Scholar]
- 70.Holmberg A, Olsson C, Hennig GW. TTX-sensitive and TTX-insensitive control of spontaneous gut motility in the developing zebrafish (Danio rerio) larvae. J Exp Biol. 2007;210(Pt 6):1084–1091. doi: 10.1242/jeb.000935. [DOI] [PubMed] [Google Scholar]
- 71.Browning KN. Role of central vagal 5-HT3 receptors in gastrointestinal physiology and pathophysiology. Front Neurosci. 2015;9:413. doi: 10.3389/fnins.2015.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bellono NW, et al. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell. 2017;170(1):185–198.e16. doi: 10.1016/j.cell.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Uyttebroek L, et al. The zebrafish mutant lessen: an experimental model for congenital enteric neuropathies. Neurogastroenterol Motil. 2016;28(3):345–357. doi: 10.1111/nmo.12732. [DOI] [PubMed] [Google Scholar]
- 74.Wiles TJ, et al. Host gut motility promotes competitive exclusion within a model intestinal microbiota. PLoS Biol. 2016;14(7):e1002517. doi: 10.1371/journal.pbio.1002517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Parracho HM, et al. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol. 2005;54(Pt 10):987–991. doi: 10.1099/jmm.0.46101-0. [DOI] [PubMed] [Google Scholar]
- 76.Yano JM, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Waga C, et al. Identification of two novel Shank3 transcripts in the developing mouse neocortex. J Neurochem. 2014;128(2):280–293. doi: 10.1111/jnc.12505. [DOI] [PubMed] [Google Scholar]
- 78.Qualmann B, et al. Linkage of the actin cytoskeleton to the postsynaptic density via direct interactions of Abp1 with the ProSAP/Shank family. J Neurosci. 2004;24(10):2481–2495. doi: 10.1523/JNEUROSCI.5479-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cloney K, et al. Etiology and functional validation of gastrointestinal motility dysfunction in a zebrafish model of CHARGE syndrome. FEBS J. 2018;285(11):2125-2140. [DOI] [PubMed]
- 80.Hyatt TM, Ekker SC. Vectors and techniques for ectopic gene expression in zebrafish. Methods Cell Biol. 1999;59:117–126. doi: 10.1016/S0091-679X(08)61823-3. [DOI] [PubMed] [Google Scholar]
- 81.Samsam M, Ahangari R, Naser SA. Pathophysiology of autism spectrum disorders: revisiting gastrointestinal involvement and immune imbalance. World J Gastroenterol. 2014;20(29):9942–9951. doi: 10.3748/wjg.v20.i29.9942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Magistris L, et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J Pediatr Gastroenterol Nutr. 2010;51(4):418–424. doi: 10.1097/MPG.0b013e3181dcc4a5. [DOI] [PubMed] [Google Scholar]
- 83.Gershon MD, Drakontides AB, Ross LL. Serotonin: synthesis and release from the myenteric plexus of the mouse intestine. Science. 1965;149(3680):197–199. doi: 10.1126/science.149.3680.197. [DOI] [PubMed] [Google Scholar]
- 84.Sikander A, Rana SV, Prasad KK. Role of serotonin in gastrointestinal motility and irritable bowel syndrome. Clin Chim Acta. 2009;403(1–2):47–55. doi: 10.1016/j.cca.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 85.Crosnier C, et al. Delta-Notch signalling controls commitment to a secretory fate in the zebrafish intestine. Development. 2005;132(5):1093–1104. doi: 10.1242/dev.01644. [DOI] [PubMed] [Google Scholar]
- 86.Troll JV, et al. Microbiota promote secretory cell determination in the intestinal epithelium by modulating host Notch signaling. Development. 2018;145(4). 10.1242/dev.155317. [DOI] [PMC free article] [PubMed]
- 87.Roach G, et al. Loss of ascl1a prevents secretory cell differentiation within the zebrafish intestinal epithelium resulting in a loss of distal intestinal motility. Dev Biol. 2013;376(2):171–186. doi: 10.1016/j.ydbio.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rolig AS, et al. The enteric nervous system promotes intestinal health by constraining microbiota composition. PLoS Biol. 2017;15(2):e2000689. doi: 10.1371/journal.pbio.2000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brugman S. The zebrafish as a model to study intestinal inflammation. Dev Comp Immunol. 2016;64:82–92. doi: 10.1016/j.dci.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 90.Ashwood P, et al. Intestinal lymphocyte populations in children with regressive autism: evidence for extensive mucosal immunopathology. J Clin Immunol. 2003;23(6):504–517. doi: 10.1023/B:JOCI.0000010427.05143.bb. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Insertion mutations in zebrafish shank3 orthologues produce unique restriction maps for genotyping. Figure S2. a Transverse 5 μm section of adult WT upper intestinal tissue stained with alcian blue and Eosin B (n = 6 for WT, n = 6 for shank3abΔC +/− and n = 6 for shank3abΔC −/−). b 40x magnification shows dense plicae that extend to the point of nearly occluding the luminal space. c, d In shank3abΔC +/− upper intestinal tissue, increased counts of goblet cells (black arrowheads) suggest inflammation. e, f Homozygous shank3abΔC −/− adults also show increased goblet cell count. Table S1. Oligonucleotides used for sgRNA synthesis. Table S2. Primers used for PCR and sanger sequencing. Table S3. % bead occupancy in WT and shank3abΔC+/− larvae at 3 h post-feed. Table S4. % bead occupancy in WT and shank3abΔC+/− larvae at 6 h post-feed. Table S5. % bead occupancy in WT and shank3abΔC+/− larvae at 12 h post-feed. Table S6. % bead occupancy in WT and shank3abΔC+/− larvae at 24 h post-feed. Table S7. Upper and lower GI rate of peristaltic period (seconds between contractions). Table S8. R script for Permutation. Table S9. shank3abΔC+/− injected with either Human SHANK3 mRNA short (32T) or long (5 T) isoform, 3–24 h post feed. (DOCX 4255 kb)
Data Availability Statement
The datasets used and/or analyzed during the current study as well as the animal models are available from the corresponding author on reasonable request.