Abstract
Reporter genes are widely applied in biotechnology and biomedical research owning to their easy observation and lack of toxicity. Taking advantage of the reporter genes in conjunction with imaging technologies, a large number of reporter mouse models have been generated. Reporter mouse models provide systems that enable the studies of live cell imaging, cell lineage tracing, immunological research and cancers etc. in vivo. In this review, we describe the types of different reporter genes and reporter mouse models including, random reporter strains, Cre reporter strains and ROSA26 reporter strains. Collectively, these reporter mouse models have broadened scientific inquires and provided potential strategies for generation of novel reporter animal models with enhanced capabilities.
Keywords: Cre reporter strains, random reporter strains, reporter genes, ROSA26 reporter strains
1. INTRODUCTION
Reporter genes refer to certain genes that encode proteins that can be easily distinguished from a background of endogenous proteins.1 Generally, reporter genes are chosen based on the sensitivity, dynamic range, convenience, and reliability of their assay.2, 3, 4 Reporter proteins can be classified into two categories: nonfluorescent proteins and fluorescent proteins (ie GFP [green fluorescent proteins], RFP [red fluorescent proteins]). Employing the reporter genes, a large number of reporter animal models have also been generated and used in a wide range of research studies. In general, two experimental strategies have been adopted to introduce exogenous genes into animal genomes. Animal genomes can be altered either by random transgenesis or by targeted transgenesis, which relies on direct gene targeting or use of gene editing tools (ie TALENs, CRISPR/Cas9).5, 6, 7 In addition, the conditional reporter animal strains were also developed. In particular, the Cre/loxp system is one of the most commonly use system for generation of conditional reporter animal strains. In the conditional Cre/loxp reporter system, the first reporter gene is flanked by two loxp sites facing the same direction, followed by the second reporter gene.8 In this system, the first reporter gene can be expressed before Cre‐mediated excision, while the second reporter gene can only be expressed after Cre‐mediated excision. Here, we summarized the two categories of reporter genes, mouse random reporter strains, mouse Cre reporter strains and ROSA26 reporter strains.
2. REPORTER GENES
2.1. Non‐fluorescent reporter genes
Chloramphenicol acetyltransferase (CAT) and lacZ gene are commonly employed as nonfluorescent reporter genes. CAT is a bacterial enzyme and the first reporter gene which was used to monitor transcriptional activity in cells.3 Chloramphenicol, an inhibitor of prokaryotic protein synthesis, can be detoxified by CAT through catalyzing the transfer of acetyl groups from acetyl CoA to the 3ʹ‐hydroxyl‐position of chloramphenicol. The advantage of CAT is its stability and lack of endogeneous expression in mammalian cells.9, 10 An automated ELISA can facilitate CAT application; however, the sensitivity of this assay is still not as high as for other reporters.3, 11 The lacZ gene, which encodes a well‐characterized bacterial β‐galactosidase has been the most commonly used reporter gene in molecular biology studies.12 β‐Galactosidase catalyzes the hydrolysis of X‐Gal converting it to a blue product, which can be easily visualized. Therefore, it has the advantage over CAT because the assays tend to be simple.
2.2. Fluorescent reporter genes
Fluorescent reporter genes are used as a tool for biological imaging. The frequently‐used fluorescent reporter genes are green fluorescent protein (GFP) and red fluorescent protein (RFP). The GFP from the jellyfish, Aequorea victoria, discovered in 1962 by Shimomura,13 is a protein composed of 238 amino acid residues (26.9 kDa) that exhibits bright green fluorescence when exposed to light in the blue to ultraviolet range.14, 15 Its discovery triggered intense research interest in the structure, biochemistry, and biophysics of GFP‐like fluorescent proteins, which resulted in an avalanche of scientific reports about fluorescent proteins and their applications to solve a series of basic issues in molecular and cell biology.16 GFP and its variants, such as enhanced yellow (EYFP) and enhanced cyan (ECFP), have been developed and are nowadays used in a wide range of areas.4, 5 GFP has become well established as a marker of gene expression in cell and molecular biology.17 In 1997, Okabe et al.18 generated the first “green mouse,”which expressed enhanced green fluorescent protein (EGFP) driven by a CAG promoter (chicken beta‐actin promoter combined with the cytomegalovirus enhancer element). The successful generation of such ‘green mice’ suggested that EGFP expression is nontoxic in mouse. Variants of green fluorescent protein (EYFP and ECFP) were also rapidly used in mice for living imaging.19, 20 Later GFP and its variants were also applied in other species such as pig.21, 22
The emission spectra of GFP variants (YFP and CFP) are very close and it is difficult to visually differentiate between them with readily available imaging systems.23 In addition, a double reporter system is often required to establish reporter strains; therefore, easily identifiable, spectrally distinct colors, such as red, had to be developed. Over the past few years, a number of RFPs that emit orange, red and far‐red fluorescence have been discovered from anthozoans (corals), and are available for a wide range of biological applications.16, 24, 25 The first RFP isolated from Discocoma sp. was DsRed1.26 Hadjantonakis et al27 tried to generate a DsRed1 transgenic mouse but failed to establish this line, which indicated that DsRed1 was not developmentally neutral or that constitutive transgene expression may not be sustained. Because DsRed1 has slow maturation times and poor solubility, improvements were made for DsRed1 to generate the mutant DsRed S197Y.28 DsRed S197Y is brighter and essentially free from the secondary fluorescence peak, which makes it an ideal reporter for double labeling with GFP. A further improved DsRed variant, DsRed.T3, was produced through random mutagenesis.29 Vintersten et al30 generated an Z/RED ES cell line and the corresponding transgenic reporter mouse, which expresses β‐geo before Cre recombination and DsRed.T3 after Cre excision. These transgenic reporter mice developed normally and DsRed.T3 expression was inherited by their offspring at expected Mendelian ratios. As DsRed.T3 can form multimers, a series of monomeric RFPs were generated subsequently. Campbell et al31 generated the first actual monomeric RFP, monomeric RFP 1 (mRFP1), which was later used for examining the expression of native mRFP1 in ES cells and its germline transmission.32 They found that mRFP1 expression in a wide range of tissues is compatible with normal development and fertility in mRFP1 transgenic mice. Now, many monomeric RFPs improved from DsRed or other fluorescent proteins are available and are also widely applied in biology16 and transgenic reporter strains. Two examples are monomeric cherry (mCherry) and tandem dimer Tomato (tdTomato). mCherry, which is brighter, matures faster, and has higher photostability than mRFP1, has been already used to generate ubiquitous mCherry transgenic reporter lines.33, 34, 35, 36, 37 tdTomato exhibits a short maturation time, greater brightness and folds equivalenty to a monomer, which may minimize toxicity when used in transgenic reporter strains.38 Latterly, Auldridge et al39 reported a versatile novel yellow fluorescent protein (LucY), which may also be used in transgenic reporter mouse models generation.
3. RANDOM REPORTER STRAINS
A series of reporter mice have been generated by random transgenesis. Exogenous DNA with a promoter‐cDNA cassette is either introduced into mouse ES cells via transfection or micro‐injected directly into zygotes.27, 40 Choosing an appropriate promoter is one of the crucial factors for the successful random transgenesis. The most commonly used promoter for ubiquitous expression of a transgene is the CAG promoter.41 However, some studies showed that the CAG promoter might cause non‐ubiquitous or sometimes even silencing effects on expression of transgenes.42, 43 Other promoters, such as the human ubiquitin C (UBC) promoter34, 44 and the ROSA26 promoter,45 are also used for inducing widespread expression of transgenes. Since both UBC and the ROSA26 promoter are derived from endogenous genes, their expression efficiency is lower than the CAG promoter.46 Nevertheless, the recent reports demonstrate that the UBC promoter and ROSA26 promoters with genomic insulators show a more ubiquitous expression of the transgene than the CAG promoter.43, 47
4. MOUSE Cre REPORTER STRAINS
Widely applied in experimental genetics, the Cre/loxP system used alone or in combination with transgenesis technologies allowed generation of conditional genome alterations that are spatially and temporally restricted or activated.48, 49 For example, a double reporter system was developed based on the Cre/loxP system. In the double reporter system, a first reporter gene flanked by two loxP sites, facing the same direction, can be expressed prior to Cre recombination, while the second reporter gene can only be expressed after the Cre recombination takes place. Based on Cre/loxP system, He et al50 further reported a novel dual recombinases system. Regarding this system, the Dre‐rox system allows rigorous control of Cre/loxP recombination, thus enhancing the precision of lineage tracing mediated by Cre/loxP system. By using the Cre/loxP system, a series of double reporter mice expressing a combination of reporter genes including CAG‐CAT‐Z (chloramphenicol acetyltransferase/lacZ),51 Z/EG (lacZ/EGFP),52 Z/AP (lacZ/human alkaline phosphatase)53 have been generated. These Cre random reporter mice are capable of monitoring Cre activity in diverse tissues and cell types. However, when used in the random integration method, those reporter strains showed some drawbacks. Firstly, the expression patterns of reporter lines vary due to different copy numbers and positional effects of the integration sites.54 Moreover, the inserted gene can also be subject to gene‐silencing effects in later offspring.55 Secondly, it is not easy to choose a suitable reporter mouse line owning to differences between laboratories in settings and reporter mice assessment standards.56 Furthermore, reporter mice that show high expression of the fluorescent reporter are often infertile or not viable.57 In order to overcome these issues, the ubiquitously expressed ROSA26 locus was used to generate genetically modified reporter strains.58
5. ROSA26 REPORTER ANIMAL MODELS
5.1. ROSA26 locus
Friedrich et al59 introduced several promoter trap constructs containing fusion lacZ‐neo gene (β‐geo) into mouse ES cells by electroporation or retroviral infection. Embryos from the gene‐trap line ROSAβ‐geo26 (reverse orientation splice acceptor β‐geo 26) showed ubiquitous β‐galactosidase (β‐gal) expression during embryonic development. Zambrowicz et al58 later reported that the gene‐trap vector was integrated into a mouse gene and this gene was subsequently named ROSA26. In mouse, the Rosa26 gene is located on chromosome 6 between THUMPD3 and SETD5 genes and has 3 noncoding transcripts (NR_027008.1, NR_027009.1 and NR_027010.1). ROSA26 transcripts 1 and 2 both contain 2 exons and 1 intron, while transcripts 3 is tail‐to‐tail overlapping (3ʹ to 3ʹ) with the THUMPD3 gene exon 3 (Figure 1). The mouse ROSA26 locus shows ubiquitous transcriptional activity but loss of this gene is not lethal.58 The ubiquitous transcriptional activity of this locus indicates that the genomic region is not affected by chromatin configurations which may cause transcriptional repression of exogenous transgenes. Therefore, this locus is widely used as a permissive site for targeted placement of transgenes in mice,60, 61 with no effect on animal viability or fertility. In mice, transgenes have been introduced into the XbaI site in the first intron of the ROSA26 forward transcript where the presence of a splice acceptor allows the transgene expression to be driven by the ubiquitously expressed endogenous promoter.46 Irion62 and Kobayashi63 demonstrated ubiquitous expression of red‐fluorescent protein cDNA, integrated into the human and rat homolog of the mouse ROSA26 locus through homologous recombination. This indicates that the human and rat ROSA26 locus conserve properties of its orthologs in mouse.
Figure 1.
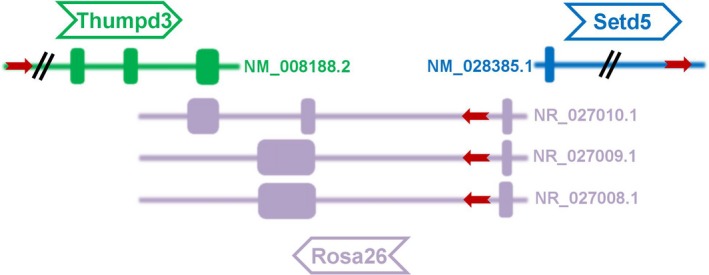
Mouse Rosa26 genomic locus and its adjacent genes (Thumpd3 and Setd5) on chromosome 6. The red arrowheads indicate orientation of transcription of Rosa26, Thumpd3 and Setd5 and genes are shown with exons and introns. Mouse Rosa26 has 3 transcripts (Accession number: NR_027008.1, NR_027009.1 and NR_027010.1) and the transcript NR_027010.1 contains 3 exons and 2 introns. The 3rd exon of NR_027010.1 is tail‐to‐tail overlapping (3ʹ to 3ʹ) with Thumpd3 gene
5.2. ROSA26 reporter strains
Through homologous recombination in ES cells, a series of reporter genes have been inserted into the Rosa26 locus to generate reporter mouse lines with precisely designed genome modifications (Figure 2). Soriano60 constructed the Rosa26 targeting vector which comprises a splice acceptor sequence (SA), a PGK promoter, a neo expression cassette flanked by two loxP sites with the same direction, followed by a triple polyadenylation sequence to prevent neo cassette transcriptional read‐through, a lacZ gene and a polyadenylation sequence. This Rosa26 reporter construct was then linearized and inserted into a unique XbaI site at approximately 300 bp 5ʹ‐upstream of the original gene‐trap integration site in intron 1 of the mouse Rosa26 locus.60 Thus, a reporter mouse line for monitoring Cre recombinase activity at the Rosa26 locus at desired time points was successfully established. However, the endogenous ROSA26 promoter is weaker than exogenous artificial promoters such as CAG promoter,46, 61 resulting in hardly detectable reporter signals in tissues and cells. Therefore, the CAG promoter is often used in knock‐in reporter lines in order to enhance expression activity at the ROSA26 locus (Figure 1).64, 65, 66 The CAG promoter was shown to yield approximately 8‐ to 10‐fold higher expression levels compared to the endogenous ROSA26 promoter.46 A series of reporter genes driven by the CAG promoter were targeted into the mouse Rosa26 locus to generate ROSA26 reporter lines, such as a multifunctional teal‐fluorescent Rosa26 reporter mouse line,67 which strongly expresses mTFP1 (bright teal fluorescent protein) after Cre and Flp mediated recombination. Another example includes, a global double‐fluorescent Cre reporter mouse,38 which expresses membrane‐targeted tandem dimer Tomato (mT) before Cre‐mediated excision or membrane‐targeted green fluorescent protein (mG) after Cre recombination. All of those ROSA26 reporter strains can be used in live cell imaging, lineage tracing, monitoring Cre activity, and analysis of cell morphology and so on.
Figure 2.
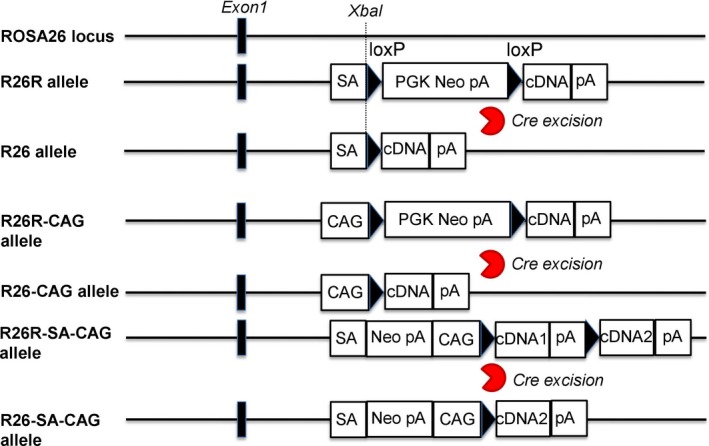
Strategies of targeting reporter genes into the ROSA26 locus. From top to bottom: the wild type ROSA26 locus with the indicated targeting site; the structure of the targeted R26R allele before and after Cre excision of the loxP flanked selection marker with stop cassete; the structure of the targeted R26R‐CAG allele before and after Cre excision of the loxP flanked selection marker with stop cassete, where the CAG promoter is inserted in front of the loxP‐flanked selection marker with stop cassette; the structure of R26R‐SA‐CAG allele before and after Cre excision of the loxP flanked cDNA with stop cassette. loxP sequences are indicated by arrowheads and the ROSA26 exon 1 is shown as black rectangles
In addition, combining the ROSA26 locus with Cre/loxP system, Aya et al68 generated multi‐color fluorescent reporter mice which can be applied for lineage tracing. In these multi‐color fluorescent reporter mice, 4 fluorescent reporter genes (GFP, YFP, RFP and CFP) can be expressed in a random manner after Cre‐mediated DNA excisions and inversions. Szyska et al69 generated dual‐luciferase reporter mouse model expressing an NFAT‐dependent click‐beetle luciferase and a renilla luciferase. This reporter mouse model supports longitudinal and functional monitoring of T cells in vivo. Park et al70 reported estrogen receptor alpha‐iCre mouse line which express codon‐improved Cre (iCre) driven by the Esr1 promoter. They further crossed ROSA26‐LacZ reporter mouse strain with Esr1‐iCre mouse line to characterize the function of lineage‐tracing Esr1‐expressing cells. Plummer et al71 described a new ROSA26 mouse strain for cell ablation by DTA (diphtheria toxin subunit A) which can be switched on by Cre‐dependent flip‐excision (FLEx). Boutet et al72 targeted the Wilms’ tumor gene on the X chromosome (WTX) fused to GFP into the mouse ROSA26 locus and generated a novel ROSA26 mouse strain. This ROSA26 mouse model can conditionally express the WTX in different tissues by crossing with several Cre transgenic mice. Dong et al73 targeted exogenous genes into the ROSA26 locus and generated a ratiometric tdTomato‐GCaMP6f reporter mouse which can be applied in visualizing T‐cell calcium dynamics.
The applications of reporter mouse models are various. For example, reporter animal lines labeled with fluorescent proteins fused to different subcellular localization signals allow for the observation of real‐time states of cells and molecules in specific organelles of living organisms. Lineage tracing is now widely used in stem cell research since it provides information about the cell behavior in the context of intact tissue or organ. It is also a powerful method for understanding tissue development, signals regulating cell‐fate decisions and diseases. The immune system plays a vital role in organisms and has the capacity to recognize and destroy malignant cells and pathogens.74 Reporter animal lines, such as cytokine reporter strains, and immune cell population‐labeled reporter strains are quickly emerging in this field to facilitate immunological studies. In the immune system, cytokines are soluble messenger molecules having important regulatory function.75 For example, IL‐4, which is the hallmark cytokine for Th2 cells, plays an important role in immunity against extracellular pathogens.76 Cytokine reporter strains have been established by placing reporter genes under the control of elements from cytokine genes, thus enabling easy identification of their cellular sources. Based on progress in the development of reporter genes and existing reporter animal models, we believe that abundant novel animal models will be generated in the near future and applied to diverse research fields.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
All listed authors meet the requirements for authorship. SL conceived and wrote the manuscript. XHZ corrected the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (NSFC, No. 31270217 and No. 81471397 No. 31601908); Shanghai Municipal Fund for Science and Technology Development (No. 15140904000); Shanghai Municipal Health and Family Planning Commission Scientific Research Project (No. 20154Y0075) and Fund from Shanghai Public Health Clinical Center (KY‐GW‐2018‐48 and KY‐GW‐2017‐04).
Li S, Chen LX, Peng XH, et al. Overview of the reporter genes and reporter mouse models. Anim Models Exp Med. 2018;1:29–35. 10.1002/ame2.12008
REFERENCES
- 1. Jurgielewicz P, Harmsen S, Wei E, Bachmann MH, Ting R, Aras O. New imaging probes to track cell fate: reporter genes in stem cell research. Cell Mol Life Sci. 2017;74:4455–4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alam J, Cook JL. Reporter genes: application to the study of mammalian gene transcription. Anal Biochem. 1990;188:245–254. [DOI] [PubMed] [Google Scholar]
- 3. Bronstein I, Fortin J, Stanley PE, Stewart GS, Kricka LJ. Chemiluminescent and bioluminescent reporter gene assays. Anal Biochem. 1994;219:169–181. [DOI] [PubMed] [Google Scholar]
- 4. Wood KV. Marker proteins for gene expression. Curr Opin Biotechnol. 1995;6:50–58. [DOI] [PubMed] [Google Scholar]
- 5. Bi H, Yang B. Gene editing with TALEN and CRISPR/Cas in Rice. Prog Mol Biol Transl Sci. 2017;149:81–98. [DOI] [PubMed] [Google Scholar]
- 6. Biagioni A, Chilla A, Andreucci E, et al. Type II CRISPR/Cas9 approach in the oncological therapy. J Exp Clin Cancer Res. 2017;36:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kesavan G, Chekuru A, Machate A, Brand M. CRISPR/Cas9‐mediated zebrafish knock‐in as a novel strategy to study midbrain‐hindbrain boundary development. Front Neuroanat. 2017;11:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li S, Flisikowska T, Kurome M, et al. Dual fluorescent reporter pig for Cre recombination: transgene placement at the ROSA26 locus. PLoS ONE. 2014;9:e102455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schwartz O, Virelizier JL, Montagnier L, Hazan U. A microtransfection method using the luciferase‐encoding reporter gene for the assay of human immunodeficiency virus LTR promoter activity. Gene. 1990;88:197–205. [DOI] [PubMed] [Google Scholar]
- 10. Williams TM, Burlein JE, Ogden S, Kricka LJ, Kant JA. Advantages of firefly luciferase as a reporter gene: application to the interleukin‐2 gene promoter. Anal Biochem. 1989;176:28–32. [DOI] [PubMed] [Google Scholar]
- 11. Pazzagli M, Devine JH, Peterson DO, Baldwin TO. Use of bacterial and firefly luciferases as reporter genes in DEAE‐dextran‐mediated transfection of mammalian cells. Anal Biochem. 1992;204:315–323. [DOI] [PubMed] [Google Scholar]
- 12. Guarente L, Ptashne M. Fusion of Escherichia coli lacZ to the cytochrome c gene of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981;78:2199–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shimomura O, Johnson FH, Saiga Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan. Aequorea. J Cell Comp Physiol. 1962;59:223–239. [DOI] [PubMed] [Google Scholar]
- 14. Prendergast FG, Mann KG. Chemical and physical properties of aequorin and the green fluorescent protein isolated from Aequorea forskalea. Biochemistry. 1978;17:3448–3453. [DOI] [PubMed] [Google Scholar]
- 15. Tsien RY. The green fluorescent protein. Annu Rev Biochem. 1998;67:509–544. [DOI] [PubMed] [Google Scholar]
- 16. Chudakov DM, Matz MV, Lukyanov S, Lukyanov KA. Fluorescent proteins and their applications in imaging living cells and tissues. Physiol Rev. 2010;90:1103–1163. [DOI] [PubMed] [Google Scholar]
- 17. Phillips GJ. Green fluorescent protein‐a bright idea for the study of bacterial protein localization. FEMS Microbiol Lett. 2001;204:9–18. [DOI] [PubMed] [Google Scholar]
- 18. Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. [DOI] [PubMed] [Google Scholar]
- 19. Hadjantonakis AK, Nagy A. FACS for the isolation of individual cells from transgenic mice harboring a fluorescent protein reporter. Genesis. 2000;27:95–98. [DOI] [PubMed] [Google Scholar]
- 20. Srinivas S, Watanabe T, Lin CS, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li L, Pang D, Wang T, et al. Production of a reporter transgenic pig for monitoring Cre recombinase activity. Biochem Biophys Res Commun. 2009;382:232–235. [DOI] [PubMed] [Google Scholar]
- 22. Deng W, Yang D, Zhao B, et al. Use of the 2A peptide for generation of multi‐transgenic pigs through a single round of nuclear transfer. PLoS ONE. 2011;6:e19986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Feng G, Mellor RH, Bernstein M, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. [DOI] [PubMed] [Google Scholar]
- 24. Stepanenko OV, Stepanenko OV, Shcherbakova DM, Kuznetsova IM, Turoverov KK, Verkhusha VV. Modern fluorescent proteins: from chromophore formation to novel intracellular applications. Biotechniques. 2011;51:313–314passim. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Subach FV, Verkhusha VV. Chromophore transformations in red fluorescent proteins. Chem Rev. 2012;112:4308–4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baird GS, Zacharias DA, Tsien RY. Biochemistry, mutagenesis, and oligomerization of DsRed, a red fluorescent protein from coral. Proc Natl Acad Sci U S A. 2000;97:11984–11989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hadjantonakis AK, Macmaster S, Nagy A. Embryonic stem cells and mice expressing different GFP variants for multiple non‐invasive reporter usage within a single animal. BMC Biotechnol. 2002;2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Verkhusha VV, Otsuna H, Awasaki T, Oda H, Tsukita S, Ito K. An enhanced mutant of red fluorescent protein DsRed for double labeling and developmental timer of neural fiber bundle formation. J Biol Chem. 2001;276:29621–29624. [DOI] [PubMed] [Google Scholar]
- 29. Bevis BJ, Glick BS. Rapidly maturing variants of the Discosoma red fluorescent protein (DsRed). Nat Biotechnol. 2002;20:83–87. [DOI] [PubMed] [Google Scholar]
- 30. Vintersten K, Monetti C, Gertsenstein M, et al. Mouse in red: red fluorescent protein expression in mouse ES cells, embryos, and adult animals. Genesis. 2004;40:241–246. [DOI] [PubMed] [Google Scholar]
- 31. Campbell RE, Tour O, Palmer AE, et al. A monomeric red fluorescent protein. Proc Natl Acad Sci U S A. 2002;99:7877–7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Long JZ, Lackan CS, Hadjantonakis AK. Genetic and spectrally distinct in vivo imaging: embryonic stem cells and mice with widespread expression of a monomeric red fluorescent protein. BMC Biotechnol. 2005;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Armstrong JJ, Larina IV, Dickinson ME, Zimmer WE, Hirschi KK. Characterization of bacterial artificial chromosome transgenic mice expressing mCherry fluorescent protein substituted for the murine smooth muscle alpha‐actin gene. Genesis. 2010;48:457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fink D, Wohrer S, Pfeffer M, Tombe T, Ong CJ, Sorensen PH. Ubiquitous expression of the monomeric red fluorescent protein mCherry in transgenic mice. Genesis. 2010;48:723–729. [DOI] [PubMed] [Google Scholar]
- 35. Larina IV, Shen W, Kelly OG, Hadjantonakis AK, Baron MH, Dickinson ME. A membrane associated mCherry fluorescent reporter line for studying vascular remodeling and cardiac function during murine embryonic development. Anat Rec (Hoboken). 2009;292:333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Poche RA, Larina IV, Scott ML, Saik JE, West JL, Dickinson ME. The Flk1‐myr:mCherry mouse as a useful reporter to characterize multiple aspects of ocular blood vessel development and disease. Dev Dyn. 2009;238:2318–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sunmonu NA, Chen L, Li JY. Misexpression of Gbx2 throughout the mesencephalon by a conditional gain‐of‐function transgene leads to deletion of the midbrain and cerebellum in mice. Genesis. 2009;47:667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double‐fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. [DOI] [PubMed] [Google Scholar]
- 39. Auldridge ME, Cao H, Sen S, et al. LucY: a versatile new fluorescent reporter protein. PLoS ONE. 2015;10:e0124272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gordon JW, Scangos GA, Plotkin DJ, Barbosa JA, Ruddle FH. Genetic transformation of mouse embryos by microinjection of purified DNA. Proc Natl Acad Sci U S A. 1980;77:7380–7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Niwa H, Yamamura K, Miyazaki J. Efficient selection for high‐expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. [DOI] [PubMed] [Google Scholar]
- 42. Rhee JM, Pirity MK, Lackan CS, et al. In vivo imaging and differential localization of lipid‐modified GFP‐variant fusions in embryonic stem cells and mice. Genesis. 2006;44:202–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Griswold SL, Sajja KC, Jang CW, Behringer RR. Generation and characterization of iUBC‐KikGR photoconvertible transgenic mice for live time‐lapse imaging during development. Genesis. 2011;49:591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schorpp M, Jager R, Schellander K, et al. The human ubiquitin C promoter directs high ubiquitous expression of transgenes in mice. Nucleic Acids Res. 1996;24:1787–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kisseberth WC, Brettingen NT, Lohse JK, Sandgren EP. Ubiquitous expression of marker transgenes in mice and rats. Dev Biol. 1999;214:128–138. [DOI] [PubMed] [Google Scholar]
- 46. Chen CM, Krohn J, Bhattacharya S, Davies B. A comparison of exogenous promoter activity at the ROSA26 locus using a PhiiC31 integrase mediated cassette exchange approach in mouse ES cells. PLoS ONE. 2011;6:e23376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Abe T, Sakaue‐Sawano A, Kiyonari H, et al. Visualization of cell cycle in mouse embryos with Fucci2 reporter directed by Rosa26 promoter. Development. 2013;140:237–246. [DOI] [PubMed] [Google Scholar]
- 48. Sternberg N, Hamilton D. Bacteriophage P1 site‐specific recombination. I. Recombination between loxP sites. J Mol Biol. 1981;150:467–486. [DOI] [PubMed] [Google Scholar]
- 49. Lakso M, Sauer B, Mosinger B Jr, et al. Targeted oncogene activation by site‐specific recombination in transgenic mice. Proc Natl Acad Sci U S A. 1992;89:6232–6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. He L, Li Y, Li Y, et al. Enhancing the precision of genetic lineage tracing using dual recombinases. Nat Med. 2017;23:1488–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Araki K, Araki M, Miyazaki J, Vassalli P. Site‐specific recombination of a transgene in fertilized eggs by transient expression of Cre recombinase. Proc Natl Acad Sci U S A. 1995;92:160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre‐mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- 53. Lobe CG, Koop KE, Kreppner W, Lomeli H, Gertsenstein M, Nagy A. Z/AP, a double reporter for cre‐mediated recombination. Dev Biol. 1999;208:281–292. [DOI] [PubMed] [Google Scholar]
- 54. Trichas G, Begbie J, Srinivas S. Use of the viral 2A peptide for bicistronic expression in transgenic mice. BMC Biol. 2008;6:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ten Muller U. years of gene targeting: targeted mouse mutants, from vector design to phenotype analysis. Mech Dev. 1999;82:3–21. [DOI] [PubMed] [Google Scholar]
- 56. Abe T, Kiyonari H, Shioi G, et al. Establishment of conditional reporter mouse lines at ROSA26 locus for live cell imaging. Genesis. 2011;49:579–590. [DOI] [PubMed] [Google Scholar]
- 57. Stewart MD, Jang CW, Hong NW, Austin AP, Behringer RR. Dual fluorescent protein reporters for studying cell behaviors in vivo. Genesis. 2009;47:708–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zambrowicz BP, Imamoto A, Fiering S, Herzenberg LA, Kerr WG, Soriano P. Disruption of overlapping transcripts in the ROSA beta geo 26 gene trap strain leads to widespread expression of beta‐galactosidase in mouse embryos and hematopoietic cells. Proc Natl Acad Sci U S A. 1997;94:3789–3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Friedrich G, Soriano P. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 1991;5:1513–1523. [DOI] [PubMed] [Google Scholar]
- 60. Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. [DOI] [PubMed] [Google Scholar]
- 61. Nyabi O, Naessens M, Haigh K, et al. Efficient mouse transgenesis using Gateway‐compatible ROSA26 locus targeting vectors and F1 hybrid ES cells. Nucleic Acids Res. 2009;37:e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Irion S, Luche H, Gadue P, Fehling HJ, Kennedy M, Keller G. Identification and targeting of the ROSA26 locus in human embryonic stem cells. Nat Biotechnol. 2007;25:1477–1482. [DOI] [PubMed] [Google Scholar]
- 63. Kobayashi T, Kato‐Itoh M, Yamaguchi T, et al. Identification of rat Rosa26 locus enables generation of knock‐in rat lines ubiquitously expressing tdTomato. Stem Cells Dev. 2012;21:2981–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Madisen L, Zwingman TA, Sunkin SM, et al. A robust and high‐throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Snippert HJ, van der Flier LG, Sato T, et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–144. [DOI] [PubMed] [Google Scholar]
- 66. Tchorz JS, Suply T, Ksiazek I, et al. A modified RMCE‐compatible Rosa26 locus for the expression of transgenes from exogenous promoters. PLoS ONE. 2012;7:e30011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Imayoshi I, Hirano K, Sakamoto M, et al. A multifunctional teal‐fluorescent Rosa26 reporter mouse line for Cre‐ and Flp‐mediated recombination. Neurosci Res. 2012;73:85–91. [DOI] [PubMed] [Google Scholar]
- 68. Amitai‐Lange A, Berkowitz E, Altshuler A, et al. A method for lineage tracing of corneal cells using multi‐color fluorescent reporter mice. J Vis Exp. 2015;106:e53370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Szyska M, Herda S, Althoff S, et al. A transgenic dual‐luciferase reporter mouse for longitudinal and functional monitoring of T cells in vivo. Cancer Immunol Res. 2018;6:110–120. [DOI] [PubMed] [Google Scholar]
- 70. Park CJ, Chen G, Koo Y, et al. Generation and characterization of an estrogen receptor alpha‐iCre knock‐in mouse. Genesis. 2017;55(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Plummer NW, Ungewitter EK, Smith KG, Yao HH, Jensen P. A new mouse line for cell ablation by diphtheria toxin subunit A controlled by a Cre‐dependent FLEx switch. Genesis. 2017;55(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Boutet A, Comai G, Charlet A, et al. A knock‐in mouse line conditionally expressing the tumor suppressor WTX/AMER1. Genesis. 2017;55(11). [DOI] [PubMed] [Google Scholar]
- 73. Dong TX, Othy S, Jairaman A, et al. T‐cell calcium dynamics visualized in a ratiometric tdTomato‐GCaMP6f transgenic reporter mouse. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dubey P. Reporter gene imaging of immune responses to cancer: progress and challenges. Theranostics. 2012;2:355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Croxford AL, Buch T. Cytokine reporter mice in immunological research: perspectives and lessons learned. Immunology. 2011;132:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol. 2007;19:652–657. [DOI] [PMC free article] [PubMed] [Google Scholar]


