Abstract
Background
Kerala, the southern state of India, has experienced sudden rise in the prevalence estimates of diabetes. A cohort study on the incidence of type 2 diabetes mellitus (T2DM) in Kerala state thus aptly bridges the lacuna of incidence estimate of T2DM from a population at risk.
Methods
A 10-year prospective cohort study was carried out in two urban wards of central Kerala. The individuals who participated in the baseline survey in 2007 were again invited for a follow-up study in 2017. The data was analyzed using IBM SPSS Statistics for windows (version 21.0). Logistic regression analysis was used to estimate odds ratios and 95% confidence intervals. Findings are based on the 10-year follow-up data from 869 participants from the cohort.
Results
The overall follow-up and response rate of the study was 68.9 and 86.9% respectively. During the follow-up period, 190 people (21.9%) developed T2DM. The incidence rate of T2DM and impaired fasting glucose (IFG) were 24.5 per 1000 person years and 45.01 per 1000 person years respectively. Nearly 60% of participants with baseline IFG were converted to T2DM group in the follow-up period. Age > 45 years, family history of T2DM, BMI ≥ 25 kg/m2 and presence of central obesity emerged as important risk factors for incident T2DM.
Conclusion
High incidence of prediabetes over diabetes observed in this study shows an epidemic trend of T2DM in Kerala, India. It requires an immediate public health action.
Keywords: Type 2 diabetes, Prediabetes, Asian Indians, Incidence, Cohort
Background
Globally, around 450 million people are suffering from diabetes mellitus. The age-standardized global prevalence of diabetes mellitus among adult population has nearly doubled since year 1980, rising from 4.7 to 8.5% [1]. The greatest increase in the prevalence of diabetes mellitus is reported from low and middle-income countries [1, 2]. Asia, being the epicenter for the epidemics of diabetes, is responsible for more than 60% of the global burden of diabetes mellitus [3, 4]. Type 2 diabetes mellitus (T2DM) is the most common form of diabetes. One of the reasons cited for this increasing trend in the prevalence of T2DM in low and middle income countries is Asia paradox [5]. Asia paradox refers to the rapid socio-economic and demographic changes in the Asian population towards that of a developed economy. This can be evident in its economic development, urbanization, and nutritional transition [6]. India, with 69.2 million people with T2DM, is the country with 2nd highest number of people living with diabetes mellitus worldwide next to China [7]. The federal state of Kerala in India is unique in that the health indicators of Kerala are on par with that of developed countries [8–10]. However, T2DM is now highly visible across all sections of society within Kerala and that implies the existence of Asia paradox in Kerala State. Majority of the data on burden of T2DM in India is derived from the prevalence estimates [11]. Though incidence studies could provide more valid estimates of disease trend, such studies on T2DM are limited. A cohort study on incidence of T2DM in Kerala State thus aptly bridges the lacuna of incidence estimate of T2DM from a population at risk.
Methods
Setting
India, representing low and middle income country, is constituted by various geographic/administrative federal units called States. In each State there are geographic/administrative subunits such as District, Corporation/Municipality/Panchayat and Wards. Wards are the smallest unit of administration. Kerala State is one among the southern States in India. The study was conducted in two adjacent Wards of Venmony Panchayat of Alappuzha District in Kerala State that are semi-urban.
Study design and study participants
Study of Life Style Diseases in Central Kerala (SLICK) is an ongoing epidemiological project conducted under the auspices of Medical Trust Hospital and Diabetes Care Centre, Pathanamthitta District, Kerala State, India. Institutional Ethics committee, constituted as per the norms of Indian Council of Medical Research [12], of Medical Trust Hospital and Diabetes Care Centre had approved the study protocol. Study was conducted after obtaining informed written consent from the study participants. The project was initiated in the year 2007, which involved baseline cross-sectional survey and cohort follow-up. The detailed description and findings of the baseline study was published [13]. In brief, the sample frame was constituted based on electoral roll of year 2007: all citizens aged ≥18 years in two adjacent Wards of Venmony panchayat. Of the 1990 adults, 1645 (Male: 624 (37.9%), Female: 1021(62.1%) residents participated in the baseline door-to-door cross- sectional survey (response rate: 82.7%). The baseline assessments included anthropometric measurements (weight, height, waist circumference and hip circumference), assessment of blood pressure and biochemical parameters such as fasting plasma glucose and fasting total cholesterol. The overall crude prevalence of T2DM was 14.6% (241/1645) and Impaired Fasting Glucose (IFG) was 5.1% (84/1645) [13].
The cohort of residents who participated in the baseline survey was re-assessed after a mean period of 10 years. Flow diagram of this cohort is shown in Fig. 1. Accordingly, we intended to follow 1404 participants after excluding 241 participants who were identified as having diabetes in baseline study. In those 1404 eligible participants, 143 (10.2%) participants died before the follow-up study and therefore, 1261 participants became eligible for the follow-up study. While conducting the follow-up study only 1000 participants were accessible as the remaining 261 (18.6%) participants either migrated or were not available. Among the 1000 eligible participants for the follow-up study 118 participants were not willing to participate in the study and 13 participants were sick. The remaining 869 residents (Male: 261 (30.0%), Female: 608 (70.0%)) participated in the study. Thus, the response rate of the follow-up study was 86.9% (869 × 100/1000) and the follow-up rate was 68.9% (869 × 100/1261).
Fig. 1.
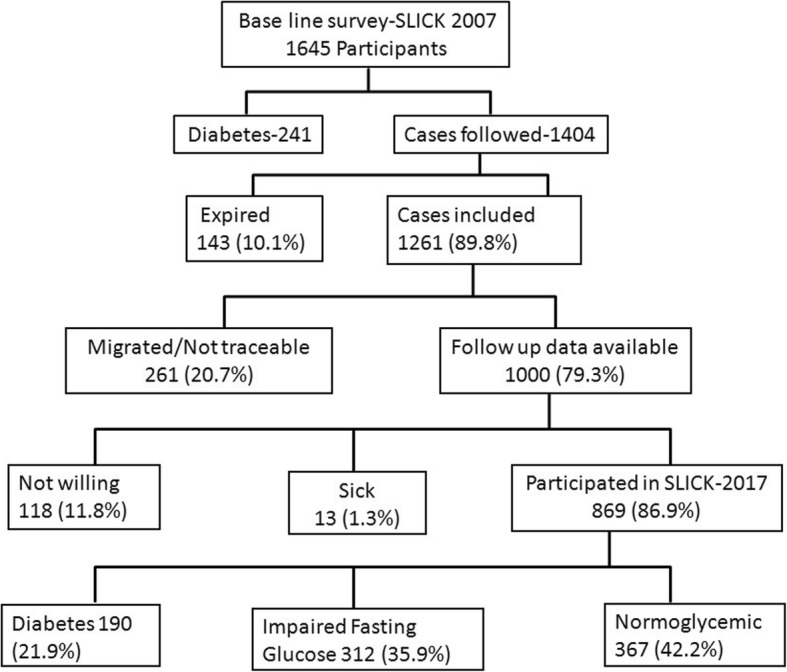
Flow chart on the study participants. The baseline, Study of Life Style Diseases in Central Kerala (SLICK) was conducted in 2007 having 1645 participants. Out of the 1404 participants, who did not have T2DM in 2007, 1000 participants were available for the current study on incidence of T2DM and 869 people were able to participate showing a response rate of 86.9%
Study enrolment and data collection
Inclusion criteria for the 10-year follow-up were: those who participated in the baseline survey; those who had normal fasting glucose or impaired fasting glucose in baseline; those who provided informed written consent. Those who were identified as having T2DM during baseline survey were excluded. The data collection period extended from 13th February 2017 to 3rd April 2017. Study enrolment and data collection involved door-to-door visit to eligible participants. In addition to socio-demographic details, data on non-communicable diseases, anthropometry and biochemical parameters were collected. These are described in detail below.
Assessment of non-communicable disease (NCD) risk factors
NCD risk factors were assessed using a structured interview schedule adapted from World Health Organization STEP instrument for NCD risk factor surveillance [14]. The interview schedule captured behavioral risk factors such as consumption of tobacco and alcohol, physical activity and diet as well as biological risk factors such as personal history of NCDs and family history of NCDs.
Assessment of anthropometric measurements
By following WHO standard protocol [14], we measured height in meter (using SECA 213 standalone stadiometer), weight in kilogram (using SECA 813 Electronic flat weighing scale) and waist circumference in centimeter (using waist measuring inch tape). Blood pressure (BP) was recorded using Digital Omron apparatus (OMRON-4, Omron Corporation, Kyoto, Japan) as an average of three consecutive readings.
Assessment of biochemical parameters
Fasting plasma was used to estimate parameters such as Fasting Plasma Glucose (FPG) and Fasting Total Cholesterol (FTC). FPG and FTC were measured respectively by Hexokinase/Glucose-6-phosphate dehydrogenase method and cholesterol-peroxidase method using Beckman Coulter AU480 instrument (Beckman Coulter Inc., Ireland).
Outcome variables
One of the following defines presence of T2DM: Current use of hypoglycemic medication and/or FPG ≥126 mg/dL [15]. Impaired Fasting Glucose (IFG) is defined as FPG in the range 100–125 mg/dL without being on hypoglycemic medication [15]. One of the following defines Hypercholesterolemia: Current use of lipid lowering medication and/or FTC > 200 mg/dL [16]. One of the following satisfies Hypertension: Current use of hypertensive medicine or Systolic blood pressure ≥ 140 mmHg and/or Diastolic blood pressure ≥ 90 mmHg [17]. Central obesity was defined as per Asian Indian cut off for waist circumference (males ≥85 cm and females ≥80 cm) [18]. Body mass index (BMI) (kg/m2) was calculated with patient’s weight (kg) divided by height squared (m2). BMI was categorized into three groups such as Underweight (BMI < 18.5), Normal (BMI: 18.5–24.9) and Overweight/Obese (BMI ≥ 25) [19].
Statistical analysis
Data analyses were done using IBM SPSS Statistics for windows v21.0. The descriptive characteristics of participants were described in frequency and percentages. Paired ‘t’ test was used to compare the continuous variables and Pearson chi-square test was used for the categorical variables between baseline year and follow-up year. One way ANOVA was used for comparing present glycemic status of the participants with their baseline characteristics. Person time denominator for assessing incidence rate estimated based on the assumption that T2DM (event) occurred at the mid-point of the interval (5 years). Unadjusted relative risks were estimated from bivariate analysis. Logistic regression models, using all variables in bivariate analysis, were used to estimate the odds ratio with 95% confidence interval. Stepwise forward likelihood ratio method was used for developing the model. Adjusted relative risks were estimated from odds ratio using Zhang-YU method using the equation: RR = OR/ (1 - P0) + (P0 X OR), where OR refers to Odds ratio derived from logistic regression model and P0refers to incidence of outcome of interest (T2DM) in the non-exposed group [20]. We estimated population attributable risk (PAR) for modifiable risk factors using Miettinen’s formula [21]. Population attributable risk, PAR = Pd X (RR-1/RR), where Pd is proportion of cases exposed to a risk factor and RR is adjusted relative risk.
Results
Results are based on the 10-year follow-up data obtained from the 869 participants of the cohort. Mean age of the participants was 54.50 ± 14.47 years. Table 1 shows demographic and behavioral characteristics of the study participants. Among current users of tobacco, mean ages for initiating smoking and smokeless tobacco are 21.83 ± 10.57 years and 37.52 ± 17 years respectively. The average duration of alcohol consumption among current alcohol users is 22.04 ± 11.5 years.
Table 1.
Demographic and behavioral characteristics of study participants (n = 869) on follow-up survey
| Characteristics | Frequency | (%) |
|---|---|---|
| Age | ||
| Age Up to 44 years | 245 | 28.2 |
| Age 45 to 59 years | 295 | 33.9 |
| Age 60 and above | 329 | 37.9 |
| Sex | ||
| Male | 261 | 30.0 |
| Female | 608 | 70.0 |
| Education | ||
| Up to 10 years of school education | 623 | 71.7 |
| Greater than 10 years of school education | 246 | 28.3 |
| Diet | ||
| Vegetarian | 55 | 6.3 |
| Non vegetarian | 814 | 93.7 |
| Smoking & Alcohol | ||
| Ever smoker | 152 | 17.5 |
| Current smoker | 61 | 7.0 |
| Ever use of smokeless tobacco | 120 | 13.8 |
| Current use of smokeless tobacco | 61 | 7.0 |
| Ever use of alcohol | 105 | 19.0 |
| Current use of alcohol (within 30 days) | 82 | 9.4 |
| Physical activity | ||
| Low (< 600 MET# minutes/week) | 315 | 36.2 |
| Moderate (600–2999 MET minutes/week) | 254 | 29.2 |
| High (≥3000 MET minutes/week) | 300 | 34.5 |
Current smokers = Smoked beedi, cigarettes or others within the past 30 days. Current users of smokeless tobacco = Used smokeless tobacco products such as chewing tobacco and betel leaves, snuff, kaini or others within the past 30 days
MET Metabolic Equivalent Task
Physical and biochemical characteristics of the participants
Significant changes (p < 0.001) were observed in BMI, Waist circumference, Systolic blood pressure, Diastolic blood pressure, FPG and FTC from baseline to the 10-year follow-up period (Table 2). Increase in age can be one of the factors for these differences in the physical and biochemical characteristics.
Table 2.
Comparison of participants’ physical and biochemical characteristics between baseline survey (2007) and follow-up survey (2017)
| Variables (N = 869) | Baseline (2007) Mean (Standard deviation) |
Follow-up (2017) Mean (Standard deviation) |
p-value |
|---|---|---|---|
| Body Mass Index | 23.69 (4.20) | 24.74 (4.54) | < 0.0001 |
| Waist circumference | 80.55 (10.50) | 88.65 (12.59) | < 0.0001 |
| Systolic blood pressure | 127.79 (19.24) | 141.69 (25.11) | < 0.0001 |
| Diastolic blood pressure | 78.48 (10.76) | 86.17 (13.11) | < 0.0001 |
| Fasting plasma glucose | 77.19 (12.69) | 113·40 (40·43) | < 0.0001 |
| Fasting total cholesterol | 187.44 (32.98) | 213·54 (42·95) | < 0.0001 |
p-value is calculated using paired t test
Incidence of type 2 diabetes mellitus and impaired fasting glucose
During the 10-year mean follow-up period, 190 people developed T2DM, yielding an incidence rate of 24.5 per 1000 person years (190/7740X1000, 95% confidence interval, 21.2–28.2). The cumulative incidence of T2DM was 21.9% (at 95% confidence intervals, 19.1–24.3). The annual incidence of T2DM was 2.9%. Thirty five participants (60%) who had IFG at baseline (n = 58) developed T2DM, yielding an incidence rate of T2DM among participants with IFG, 86.41 per 1000 person year (35/405X1000, 95% confidence interval, 61.1–118.9). Incidence rate of T2DM among participants with baseline normal glycemic status is 21.13 per 1000 person year (155/7335X1000, 95% confidence interval, 18.0–24.6). Regarding IFG, 298 (cumulative incidence 36.7%; 95% confidence interval, 33.4–40.2) participants had IFG, yielding an incidence rate of 45.01 per 1000 person years (298/6620X1000; 95% confidence interval, 45.0–50.0).
Risk factors for developing type 2 diabetes mellitus
Comparison of baseline age, family history of T2DM, anthropometric measurements and biochemical measurements of those who remained normoglycemic, those who converted to T2DM and those who converted to IFG showed that there were significant differences between the groups for all the parameters (Table 3). With respect to the risk factors; age greater than 45 years, central obesity, overweight/obesity (BMI ≥ 25), family history of T2DM and hypertension showed strong association (p < 0.001) with the incidence of T2DM (Table 4). Whereas, parameters such as, sex of the participant, physical activity and hypercholesterolemia did not show any relation with the incidence of T2DM (Table 4).
Table 3.
Comparison of baseline characteristics of the participants (n = 811) of normalglycemic status with their glycemic status at follow-up period
| Variables | Remained as normal glycemica (n = 358) | Converted to IFG (n = 298) | Converted to DM (n = 155) | p Value |
|---|---|---|---|---|
| Age | 41.82 ± 14·6 | 44.9 ± 14.0 | 47.3 ± 13.5 | < 0.001 |
| BMI | 22.3 ± 3.8 | 24.0 ± 3.9 | 25.9 ± 4.6 | < 0.001 |
| Waist circumference | 77.4 ± 9.9 | 81.1 ± 10.4 | 85.7 ± 9·9 | < 0.001 |
| Systolic blood pressure | 123.5 ± 16.2 | 129.90 ± 21.8 | 132.05 ± 18.7 | < 0.001 |
| Diastolic blood pressure | 76.5 ± 9.6 | 79·3 ± 11·2 | 80.7 ± 10.6 | < 0.001 |
| Fasting plasma glucose | 73.56 ± 8.9 | 75.19 ± 10.4 | 77.9 ± 10.3 | < 0.001 |
| Fasting total cholesterol | 183.0 ± 30.9 | 189.5 ± 32.9 | 191.48 ± 35.9 | < 0.008 |
| Family history of diabetesb | 110 (30.8%) | 94 (31.6%) | 65 (41.9%) | 0.038 |
acomparison group. Statistical analyses is done using One way ANOVA
bAnalyses is done by chi-square test
Table 4.
Incidence of diabetes mellitus and its associated risk factors at baseline
| Risk factors | Incidence (N) | Percentage (%) | Un adjusted RR (95% CI) | Adjusted RR (95%CI) | p value |
|---|---|---|---|---|---|
| i) Non-modifiable risk factors | |||||
| Age | |||||
| 18–45 years | 86 | 17.6 | 1 | 1 | |
| > 45 years | 104 | 27.3 | 1.55 (1.20–1.99) | 1.76 (1.21–2.56) | 0.001 |
| Sex | |||||
| Female | 130 | 21.4 | 1 | 1 | |
| Male | 60 | 23 | 0.93 (0.71–1.21) | 1.32 (0.90–1.92) | 0.599 |
| Family history of diabetes | |||||
| Absent | 107 | 18.7 | 1 | 1 | |
| Present | 83 | 28.2 | 1.51 (1.17–1.94) | 1.63 (1.15–2.32) | 0.001 |
| ii) Modifiable risk factors | |||||
| Central obesity | |||||
| Absent | 62 | 13.6 | 1 | 1 | |
| Present | 128 | 30.9 | 2.26 (.0.72–2.98) | 1.59 (1.03–2.43) | 0.001 |
| Obesity | |||||
| Absent (BMI < 25.0) | 81 | 14.6 | 1 | 1 | |
| Present (BMI ≥25.0) | 109 | 34.7 | 2.37 (1.84–3.05) | 2.28 (1.49–3.47) | 0.001 |
| Physical activity | |||||
| Physical activity ≥600MET/week | 169 | 21.8 | 1 | 1 | |
| Physical activity < 600 MET/week | 20 | 23 | 1.05 (0.70–1.58) | 1.17 (0.66–2.06) | 0.796 |
| Hypertension | |||||
| Absent | 116 | 19.1 | 1 | 1 | |
| Present | 74 | 28.1 | 1.47 (1.14–1.89) | 1.08 (0.73–1.59) | 0.003 |
| Hypercholesterolemia | |||||
| Absent | 111 | 19.9 | 1 | 1 | |
| Present | 79 | 25.5 | 1·28 (0.99–1.65) | 1.04 (0.73–1.4) | 0.055 |
Statistical analyses is done using Chi Square test
T2DM type 2 diabetes mellitus, MET metabolic equalent task, BMI Body mass index
Participants with higher baseline BMI had higher incidence of T2DM (Fig. 2). The population attributable risks for overweight/obesity and central obesity were 27.2 and 21.8% respectively (Table 5). Since cholesterol and blood pressure can be intermediary risk factors for obesity, we estimated relative risk and population attributable risk for overweight/obesity adjusted and unadjusted for cholesterol and blood pressure. The relative risk of BMI ≥ 25 adjusted for Cholesterol and Blood pressure was 1·90 (at 95% confidence interval, 1·38–2·50) and the corresponding population attributable risk was 27.2% (PAR = 109/190X (1.90–1/1.90) = 27.2%). Whereas, the relative risk for BMI ≥ 25 unadjusted for Cholesterol and Blood pressure was 1.88 (at 95% confidence interval, 1·38–2·48) and the corresponding population attributable risk was 26.9% (PAR = 109/190 X (1. 88–1/1.88) = 26.9%).
Fig. 2.
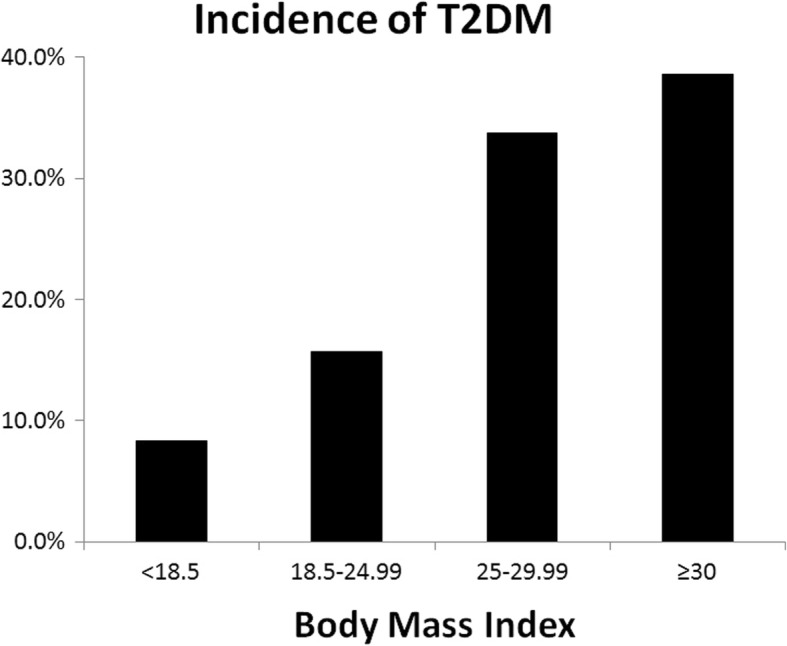
Relationship between baseline BMI and the incidence of T2DM. Study participants were categorized into different BMI groups based on their baseline BMI in 2007 (X-Axis). Y-axis shows the % of people who contracted T2DM in the follow-up period during the current study
Table 5.
Results of multivariate analysis: baseline risk factors for incidence of diabetes mellitus
| Risk factors | Adjusted RR (95% CI) | PAR (%) |
|---|---|---|
| Age group | ||
| 18–45 years | 1 | |
| > 45 years | 1·59 (1·23–2·01) | – |
| Family H/O DM | ||
| Absent | 1 | |
| Present | 1·45 (1·36–1·85) | – |
| BMI ≥ 25 | ||
| Absent | 1 | |
| Present | 1·90 (1·38–2·50) | 27.2 |
| Central Obesity | ||
| Absent | 1 | |
| Present | 1·48 (1·04–2·05) | 21.8 |
Variables adjusted in the model are sex, physical activity, dyslipidemia and hypertension
Comparison of responders versus non-responders
We observed significant difference in age, sex, BMI, FBS, cholesterol and religion between responders and non-responders (Table 6). It was noted that mean age of non-responders is comparatively less than the responders group. This can be the reason for the significant difference in other baseline variables such as BMI, FBS and Cholesterol level. Less follow-up for younger participants is mainly due to the huge migration of younger participants for job or marriage. The observed differences in baseline characteristics between responders and non-responders will not make much difference in the overall study result as we have a good 68.9% follow-up rate.
Table 6.
Comparison between the baseline characteristics of responders and non-responders of the follow-up study
| Baseline characteristics | Responders 869 |
Non responders 392 |
P-Value |
|---|---|---|---|
| Age in years | 44.3 (14.3) | 39.0 (17.7) | < 0.001 |
| BMI | 23.7 (4.2) | 22.9 (4.1) | 0.003 |
| Average sys B.P | 127.7 (19.2) | 126.0 (17.9) | 0.138 |
| FBS | 77.2 (12.6) | 75.2 (11.0) | 0.006 |
| Total Cholesterol | 187.4 (32.9) | 180.4 (32.9) | < 0.000 |
| Sex | |||
| Female | 608 (74.6) | 207 (25.4) | < 0.001 |
| Male | 261 (58.5) | 185 (41.5) | |
| Family history of DM | |||
| Present | 573 (69.8) | 248 (30.2) | 0.3 |
| Absent | 294 (67.1) | 144 (32.9) | |
| Literacy | |||
| Literate | 821 (69.4) | 362 (30.6) | 0.146 |
| Illiterate | 48 (61.5) | 30 (38.5) | |
| Family type | |||
| Nuclear | 377 (69.7) | 164 (30.3) | 0.608 |
| Joint | 492 (68.3) | 228 (31.7) | |
| Religion | |||
| Hindu | 546 (72.1) | 248 (30.2) | 0.001 |
| Christian | 294 (67.1) | 144 (32.9) | |
| Muslim | 120 (59.1) | 83 (40.9) | |
Student’s t-test done for continuous variable and data presented as Mean and standard deviation
Chi-square test was done for categorical variables and data showed in frequency and percentage
Discussion
Our study describes the incidence of T2DM and IFG during 10 years of follow-up. Our finding that crude incidence of T2DM is 21.9% or 24.5 per 1000 person years re-emphasizes the high burden of T2DM in India. Similar to this finding, high incidence of T2DM was reported in south India by Mohan et al. in 2008 [22], Ghorpade et al. in 2013 [23] and Anjana et al. in 2015 [24]. The incidence of T2DM from those studies were 20.2, 21.5, and 22.2 per 1000 person years respectively. Our cohort attracts special attention in that inspite of being a semi-urban cohort, the incidence is still high in comparison to the two studies from urban south India [22, 24] and the one from rural south India [23]. It is to be noted that high incidence of T2DM is reported from other low and middle income countries too. Latifi et al. in 2016 reported a T2DM incidence rate of 21.9 per 1000 person year from Iran [25]. These observations confirm that the epidemic trends of T2DM is widespread across India as well as across nations.
With the additional information that nearly 60% of participants with baseline IFG converted to T2DM in 10-year follow-up, it is justifiable to conclude that we are facing an epidemic trend of T2DM. Again, this conversion rate (60% or 86.41 per 1000 person years) is much more than the conversion rates from other studies in India. Mohan et al. reported that 40.5% of baseline prediabetes participants converted to T2DM within 8 years of follow-up [22]. Another recent study from South India reported incidence rate of 58.9% or 78.9 per 1000 person-years [24]. Matching these findings, studies from high income country also reported very high incidence of diabetes among participants with baseline IFG [26, 27]. The high incidence rate of prediabetes as compared to T2DM and the high conversion rate of prediabetes to T2DM signal uncontrolled progressive increase in prevalence of T2DM.
Another important finding from our study is the high crude incidence of IFG (36.7%). The high incidence of IFG as compared to incidence of T2DM (crude incidence of 36.7% vs 21.9%) indicates high incidence of prediabetes in the community. In India, a recent multi centric cross- sectional study done in 15 states documented high prevalence of prediabetes as compared to T2DM (24.7% vs. 7.3%) [28]. Similar findings on high incidence of prediabetes were reported from other Asian countries. A study conducted by Latifi et al. in Iran reported the incidence rate of prediabetes as 40.8 per 1000 person years [25]. A study conducted by Vaidya et al. in China also reported high incidence rate of prediabetes (62.6 per 1000 person year) as compared to incidence rate of T2DM, which is 10.0 per 1000 person years [29]. Another 9-year follow-up study from Iran also reported high incidence of prediabetes, 46.1 per 1000 person-years in men and 36.8 per 1000 person-years in women [30]. Thus prediabetes too is at an epidemic trend in our cohort as elsewhere.
Among all the factors studied, obesity emerged as the single-most modifiable risk factor for T2DM. Prevalence of overweight or obesity is quite high (46%) in this cohort. 10% increase in the prevalence of overweight or obesity within 10 year was observed in this cohort. Also, nearly 70% of participants have central obesity based on Asian Indian cutoff. High prevalence of overweight or obesity (25%) was documented in a study conducted on non-communicable risk factors among adults in Kerala in 2009 [31]. In our study, we found that BMI ≥ 25 and central obesity were the significant risk factors for the incidence of T2DM. A person with BMI ≥25 has nearly 1.9 times higher risk than those whose BMI is below 25 (Table 5). The population attributable risk for BMI ≥ 25 is nearly 27% which shows that reduction in overweight or obesity can significantly reduce the incidence of T2DM. Stratified analysis also showed that BMI is independently associated with T2DM as significant risk factor for both men and women. In contrast to our finding, a study done in Iranian population reported BMI as an independent risk factor only in men [32]. Several cohort study as well as cross-sectional study from all around the world reported overweight or obesity and central obesity as important risk factors for T2DM [22, 23, 25, 28, 29, 33–43]. The drastic increase in the prevalence of central obesity (47.6% vs 70.2%) and overweight or obesity (36.2% vs. 46.1%) in this cohort over 10 years amplifies the grave situation. We believe that reduction in obesity or over weight and abdominal obesity can produce substantial reduction in incidence of T2DM. Our study thus emphasizes the importance of addressing obesity in the primary prevention of T2DM. In our study, we measured only total cholesterol among lipid profile. Since we did not measure triglyceride and HDL-cholesterol, burden of metabolic vascular syndrome [44] was not estimated in our cohort.
We observed high crude incidence of T2DM among those aged 60 and above (27.1%). This is consistent with the study done in Canada by Lipscombe et al. in 2007 where they observed high incidence of T2DM over the age group 50 years [45]. Similar association of age as significant risk factor was documented in several studies [25, 28, 35, 36, 42, 46].
In our study, participants with age higher than 45 years has nearly 1.6 times higher risk of having T2DM. The proportion of those aged 60 and above is 37.9% and that could partly explain the high incidence of T2DM in our study population. Age-adjusted incidence of T2DM in our study came to be 14.56 per 1000 person years. But this would not minimize the relevance of our finding since global demographic trend in modern era is towards increasing life expectancy and ageing of population. Hence the burden of T2DM will ever be continually increasing unless preventive measures are adopted.
Another important non-modifiable risk factors observed in this study is family history of T2DM. Nearly 28% of participants with T2DM have a family history of T2DM and this association is significant. This is consistent to several other studies finding that family history of T2DM is an important risk factor for T2DM [25, 27, 28, 34, 45]. High incidence of T2DM in the present cohort ultimately gets reflected as high rates of family history of T2DM in successive generations, thereby spiraling the T2DM burden.
In the current study we didn’t find genderwise difference in the incidence of T2DM which is in contrast to some studies in Asia that reported high incidence of T2DM among males [23, 25, 29]. Whereas, similar to our study, absence of genderwise difference in the incidence of diabetes was noted in a study done in Iran [32]. Over 10 years, prevalence of hypertension (30.3% vs 59.8%) and hypercholesterolemia (35.7% vs. 70.2%) have doubled. Though we had observed high incidence of T2DM among those with hypertension and hypercholesterolemia, the association is not significant.
The high rate of conversion from IFG at baseline into T2DM at 10-year follow-up identifies people with IFG as the target population for preventive measures. From our study, it is also evident that obese individuals are the single most important target population while envisaging preventive strategies against incident T2DM. Moreover, in resource deficit countries, interventions targeting at-risk population are considered resource efficient. Hence, for states/countries with demographics matched to Kerala, public health preventive interventions targeting people with IFG and/or obesity will be the most effective and resource efficient step in preventing T2DM.
Among Indian states, the state of Kerala is known for its demographic indicators and health indicators progressing towards those of developed countries [10]. Thus the state of Kerala represents a transitional zone between developing countries and developed countries. It is likely that any state/country in transition from developing economy to developed economy might face similar trends in non-communicable diseases. This provides an opportunity for such states/countries to anticipate health outcomes and thereby take precautionary measures. A major strength of our study is the good follow-up rate and response rate. We could follow 68.9% of participants from the baseline survey, which is much higher than any other Indian studies.
Conclusion
The current prospective study estimates that the cumulative incidence of T2DM in Kerala is 21.9% and the incidence of prediabetes is 36.7%. Nearly 60% of the participants who had impaired plasma glucose at baseline converted to T2DM at present, shows an epidemic trend for T2DM in Kerala. Overweight/obesity and central obesity emerged as the most important modifiable risk factors for developing T2DM in Kerala. The findings of this study provide conclusive evidence that incidence of T2DM and prediabetes is increasing rapidly in developing countries.
Acknowledgments
We greatly acknowledge the all the study participants, community volunteers and staffs of Medical Trust Hospital and Diabetes Care Centre Authors’ information (optional).
Funding
This work was supported by the intramural grant of Rajiv Gandhi Centre for Biotechnology, Ministry of Science and Technology, Government of India and by the P Gadadharan Namboodiri Memorial Charitable Trust, Pandalam, Kerala. The funders of this study had no role in the study design, data collection, data analysis, interpretation or writing of the report.
Availability of data and materials
The datasets generated and analyzed during the current study are not publicly available, however, will be made available on reasonable request.
Authors’ contributions
GV and AJ designed the study, provided the resources, take responsibility for the integrity of the data, accuracy of data analysis and contributed in writing. SM, RV, LS and LMS contributed in data collection, analyses and writing. AV, GKS, AR and VRK involved in the data analyses and in critically revising the manuscript. All the authors read and approved the manuscript.
Ethics approval and consent to participate
Institutional Ethics committee, constituted as per the norms of Indian Council of Medical Research [12], of Medical Trust Hospital and Diabetes Care Centre had approved the study protocol. Study was conducted after obtaining informed written consent from the study participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gadadharan Vijayakumar, Email: drgvijayakumar@gmail.com.
Sreeja Manghat, Email: sreejamnair05@gmail.com.
Revathi Vijayakumar, Email: revathipathani@gmail.com.
Leena Simon, Email: leenathop@gmail.com.
Liss Maria Scaria, Email: lissmariascaria@gmail.com.
Aswathi Vijayakumar, Email: anjuvv@gmail.com.
Ganapathy K. Sreehari, Email: sreeharigk@gmail.com
V. Raman Kutty, Email: rkutty@sctimst.ac.in.
Arun Rachana, Email: arunrdr@gmail.com.
Abdul Jaleel, Email: jaleel@rgcb.res.in.
References
- 1.World Health Organization. Global Report on Diabetes. 2016. http://apps.who.int/iris/bitstream/handle/10665/204871/9789241565257_eng.pdf;jsessionid=1C87D795D989E8C7FD4703DED26C9157?sequence=1. Accessed 5 Nov 2017.
- 2.Bhutani J, Bhutani S. Worldwide burden of diabetes. Indian J Endocrinol Metab. 2014;18(6):868–870. doi: 10.4103/2230-8210.141388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Diabetes Federation. Diabetes Atlas. 8th ed; 2017. http://www.diabetesatlas.org/resources/2017-atlas.html. Accessed 9 Nov 2017.
- 4.Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care. 2011;34(6):1249–1257. doi: 10.2337/dc11-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zimmet PZ, Magliano DJ, Herman WH, Shaw JE. Diabetes: a 21st century challenge. Lancet Diabetes Endocrinol. 2014;2(1):56–64. doi: 10.1016/S2213-8587(13)70112-8. [DOI] [PubMed] [Google Scholar]
- 6.Bishwajit G. Nutrition transition in South Asia: the emergence of non-communicable chronic diseases. F1000Res. 2015;4:8. doi: 10.12688/f1000research.5732.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sen A. Health: perception versus observation. BMJ. 2002;324(7342):860–861. doi: 10.1136/bmj.324.7342.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parayil G. The “Kerala model” of development: development and sustainability in the Third World. Third World Q. 1996;17(5):941–957. doi: 10.1080/01436599615191. [DOI] [PubMed] [Google Scholar]
- 9.Kannan KP, Thankappan KR, Ramankutty V, Aravindan KP. Kerala: a unique model of development. Health Millions. 1991;17(5):30–33. [PubMed] [Google Scholar]
- 10.Brown A. Growth and success in Kerala. Yale Rev Int Stud. 2013;4(1):85–95. [Google Scholar]
- 11.Joshi SR. Incidence data on diabetes from India. J Assoc Physicians India. 2008;56:149–151. [PubMed] [Google Scholar]
- 12.Indian Council of Medical Research (ICMR). National Ethical Guidelines for Biomedical and Health Research Involving Human Participants. https://icmr.nic.in/sites/default/files/guidelines/ICMR_Ethical_Guidelines_2017.pdf. Accessed 16 May 2016.
- 13.Vijayakumar G, Arun R, Kutty VR. High prevalence of type 2 diabetes mellitus and other metabolic disorders in rural Central Kerala. J Assoc Physicians India. 2009;57:563–567. [PubMed] [Google Scholar]
- 14.World Health Organization. Noncommunicable diseases and their risk factors. STEPwise approach to surveillance (STEPS). http://www.who.int/ncds/surveillance/steps/en/. Accessed 19 Nov 2017.
- 15.American Diabetes Association Classification and diagnosis of diabetes. Diabetes Care. 2015;38:s8–s16. doi: 10.2337/dc15-S005. [DOI] [PubMed] [Google Scholar]
- 16.Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adults treatment panel III). Executive summary of the third report of the National Cholesterol Education Program (NCEP). JAMA. 2001;285:2486–97. [DOI] [PubMed]
- 17.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 18.Snehalatha C, Viswanathan V, Ramachandran A. Cutoff values for normal anthropometric variables in asian Indian adults. Diabetes Care. 2003;26(5):1380–1384. doi: 10.2337/diacare.26.5.1380. [DOI] [PubMed] [Google Scholar]
- 19.World health Organization. Helath Topics. Obesity. http://www.who.int/topics/obesity/en/. Accessed 25 Nov 2017.
- 20.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 21.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88(1):15–19. doi: 10.2105/AJPH.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohan V, Deepa M, Anjana RM, Lanthorn H, Deepa R. Incidence of diabetes and pre-diabetes in a selected urban south Indian population (CUPS-19) J Assoc Physicians India. 2008;56:152–157. [PubMed] [Google Scholar]
- 23.Ghorpade AG, Majgi SM, Sarkar S, Kar SS, Roy G, Ananthanarayanan PH, Das AK. Diabetes in rural Pondicherry, India: a population-based studyof the incidence and risk factors. WHO South East Asia J Public Health. 2013;2(3):149–155. doi: 10.4103/2224-3151.206761. [DOI] [PubMed] [Google Scholar]
- 24.Anjana RM, Shanthi Rani CS, Deepa M, Pradeepa R, Sudha V, Divya Nair H, Lakshmipriya N, Subhashini S, Binu VS, Unnikrishnan R, et al. Incidence of diabetes and prediabetes and predictors of progression among Asian Indians: 10-year follow-up of the Chennai urban rural epidemiology study (CURES) Diabetes Care. 2015;38(8):1441–1448. doi: 10.2337/dc14-2814. [DOI] [PubMed] [Google Scholar]
- 25.Latifi SM, Karandish M. Incidence of prediabetes and type 2 diabetes among people aged over 20 years in ahvaz: a 5-year perspective study (2009–2014). Journal of Diabetes Research. 2016: Article ID 4908647. [DOI] [PMC free article] [PubMed]
- 26.Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Meigs JB, Bonadonna RC, Muggeo M. Population-based incidence rates and risk factors for type 2 diabetes in white individuals: the Bruneck study. Diabetes. 2004;53(7):1782–1789. doi: 10.2337/diabetes.53.7.1782. [DOI] [PubMed] [Google Scholar]
- 27.Valdes S, Botas P, Delgado E, Alvarez F, Cadorniga FD. Population-based incidence of type 2 diabetes in northern Spain: the Asturias study. Diabetes Care. 2007;30(9):2258–2263. doi: 10.2337/dc06-2461. [DOI] [PubMed] [Google Scholar]
- 28.Anjana RM, Deepa M, Pradeepa R, Mahanta J, Narain K, Das HK, Adhikari P, Rao PV, Saboo B, Kumar A, et al. Prevalence of diabetes and prediabetes in 15 states of India: results from the ICMR-INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol. 2017;5(8):585–596. doi: 10.1016/S2213-8587(17)30174-2. [DOI] [PubMed] [Google Scholar]
- 29.Vaidya A, Cui L, Sun L, Lu B, Chen S, Liu X, Zhou Y, Xie X, Hu FB, Wu S, et al. A prospective study of impaired fasting glucose and type 2 diabetes in China: the Kailuan study. Medicine (Baltimore) 2016;95(46):e5350. doi: 10.1097/MD.0000000000005350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hadaegh F, Derakhshan A, Zafari N, Khalili D, Mirbolouk M, Saadat N, Azizi F. Pre-diabetes tsunami: incidence rates and risk factors of pre-diabetes and its different phenotypes over 9 years of follow-up. Diabet Med. 2017;34(1):69–78. doi: 10.1111/dme.13034. [DOI] [PubMed] [Google Scholar]
- 31.Thankappan KR, Shah B, Mathur P, Sarma PS, Srinivas G, Mini GK, Daivadanam M, Soman B, Vasan RS. Risk factor profile for chronic non-communicable diseases: results of a community-based study in Kerala, India. Indian J Med Res. 2010;131:53–63. [PubMed] [Google Scholar]
- 32.Derakhshan A, Sardarinia M, Khalili D, Momenan AA, Azizi F, Hadaegh F. Sex specific incidence rates of type 2 diabetes and its risk factors over 9 years of follow-up: Tehran lipid and glucose study. PLoS One. 2014;9(7):e102563. doi: 10.1371/journal.pone.0102563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world--a growing challenge. N Engl J Med. 2007;356(3):213–215. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 34.Ramachandran A, Mary S, Yamuna A, Murugesan N, Snehalatha C. High prevalence of diabetes and cardiovascular risk factors associated with urbanization in India. Diabetes Care. 2008;31(5):893–898. doi: 10.2337/dc07-1207. [DOI] [PubMed] [Google Scholar]
- 35.Satman I, Omer B, Tutuncu Y, Kalaca S, Gedik S, Dinccag N, Karsidag K, Genc S, Telci A, Canbaz B, et al. Twelve-year trends in the prevalence and risk factors of diabetes and prediabetes in Turkish adults. Eur J Epidemiol. 2013;28(2):169–180. doi: 10.1007/s10654-013-9771-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keen H, Jarrett RJ, McCartney P. The ten-year follow-up of the Bedford survey (1962-1972): glucose tolerance and diabetes. Diabetologia. 1982;22(2):73–78. doi: 10.1007/BF00254832. [DOI] [PubMed] [Google Scholar]
- 37.Motala AA, Pirie FJ, Gouws E, Amod A, Omar MA. High incidence of type 2 diabetes mellitus in South African Indians: a 10-year follow-up study. Diabet Med. 2003;20(1):23–30. doi: 10.1046/j.1464-5491.2003.00782.x. [DOI] [PubMed] [Google Scholar]
- 38.Dedov I, Shestakova M, Benedetti MM, Simon D, Pakhomov I, Galstyan G. Prevalence of type 2 diabetes mellitus (T2DM) in the adult Russian population (NATION study) Diabetes Res Clin Pract. 2016;115:90–95. doi: 10.1016/j.diabres.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 39.Corbin LJ, Richmond RC, Wade KH, Burgess S, Bowden J, Smith GD, Timpson NJ. BMI as a modifiable risk factor for type 2 diabetes: refining and understanding causal estimates using Mendelian randomization. Diabetes. 2016;65(10):3002–3007. doi: 10.2337/db16-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zou D, Ye Y, Zou N, Yu J. Analysis of risk factors and their interactions in type 2 diabetes mellitus: a cross-sectional survey in Guilin, China. J Diabetes Investig. 2017;8(2):188–194. doi: 10.1111/jdi.12549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pinto G, Beltran-Sanchez H. Prospective study of the link between overweight/obesity and diabetes incidence among Mexican older adults: 2001-2012. Salud Publica Mex. 2015;57(Suppl 1):S15–S21. doi: 10.21149/spm.v57s1.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gyawali B, Sharma R, Neupane D, Mishra SR, van Teijlingen E, Kallestrup P. Prevalence of type 2 diabetes in Nepal: a systematic review and meta-analysis from 2000 to 2014. Glob Health Action. 2015;8:29088. doi: 10.3402/gha.v8.29088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen GY, Li L, Dai F, Li XJ, Xu XX, Fan JG. Prevalence of and risk factors for type 2 diabetes mellitus in hyperlipidemia in China. Med Sci Monit. 2015;21:2476–2484. doi: 10.12659/MSM.894246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scholz GH, Hanefeld M. Metabolic vascular syndrome: new insights into a multidimensional network of risk factors and diseases. Visc Med. 2016;32(5):319–326. doi: 10.1159/000450866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lipscombe LL, Hux JE. Trends in diabetes prevalence, incidence, and mortality in Ontario, Canada 1995-2005: a population-based study. Lancet. 2007;369(9563):750–756. doi: 10.1016/S0140-6736(07)60361-4. [DOI] [PubMed] [Google Scholar]
- 46.Ramachandran A, Snehalatha C, Vijay V. Temporal changes in prevalence of type 2 diabetes and impaired glucose tolerance in urban southern India. Diabetes Res Clin Pract. 2002;58(1):55–60. doi: 10.1016/S0168-8227(02)00125-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available, however, will be made available on reasonable request.


