Abstract
Background
Fish meat and its products are usually accepted as good source of biological high value food components and especially for polyunsaturated fatty acids. The quality of fish meat products is considered to be decreased by the lipid peroxidation which leads to reduction in nutritional quality, financial loss and severe health problems. Many tactics are present to reserve their quality and safety. In the present investigation, the extraction and supplementation of optimal total polyphenol extracts (TPC) from vegetable and fruit by−products was explored for lipids oxidative stability and sensoric acceptability of functional fish product samples.
Methods
Vegetable and fruit by−products (cabbage leaves and banana peels) were collected from local fruits and vegetables processing industries. A 3−level five factor Box−Behnken design was used to study the effect of extraction/sonication temperature (°C), amplitude level, water/meal ratio, extraction/sonication time (minutes) and pH conditions for maximum yield of TPC from dried vegetable and fruit samples. The TPC samples were analyzed for chemical composition (total polyphenols, cyanogenic contents and tannins). Natural TPC extracts were supplemented at different concentration (0.5, 1 and 1.5%) to fish meat for preparation of different meat ball samples. The fish meat product samples without supplementation of TPC extract were kept as control. The partial/parfrying of the products was carried out to determine the lipid stability (peroxide value and free fatty acids) stored at refrigerator (for 9 days) and at − 18 °C in a freezer for a storage period of 60−days. The sensoric analysis (color, flavor and overall acceptability) was performed at different storage intervals for experimental treatments.
Results
The percent values of TPC yield from cabbage leave and banana peel samples ranged from a from minimum value of 9.8 ± 0.12% to a maximum value of 19.8 ± 0.15% for cabbage leaves and minimum value of 15.55 ± 0.13% to a maximum value of 24.4 ± 0.17% for banana peels, respectively. The results revealed that extraction conditions significantly affect the TPC yield from cabbage leaves and banana peels. The cabbage leaves and banana peels contain up to 4.8% total phenolics, cyanogenic compounds (1.44 − 1.47 ± 0.14) and tannins (6.55–7.90 ± 0.22). Peroxide values (meqO2 /kg) of meat balls treated with TPC extracts at 4 °C were in the range of 1.31 ± 0.12 to 3.10 ± 0.20 while at − 18 °C ranged was found 1.31 ± 0.12 to 1.55 ± 0.17, respectively. Peroxide values of all the treatments increased at the end of second interval then decreased at the end of last storage interval. Peroxide values of all treatments were higher and significantly different at the beginning and the end of the storage period (p < 0.05). In a similar way, free fatty acids and moisture content values trend was recorded for all experimental treatments. Sensory scores of fish product samples for color, flavor and overall acceptability showed a significant difference in sensory scores at refrigeration temperatures where sensory scores of fish product samples decreased significantly (p < 0.05) throughout refrigeration storage. Whereas, the sensory scores at the − 18 °C shows the good sensory characteristics, relatively.
Conclusions
Phenolic extracts containing antioxidant status can interact with free lipidperoxy or lipidoxy free radicals (formed in result of lipid oxidation) and hence stopping their further self−breakdown. Plant−based phenolic extracts can be used to decrease oxidation process and increase the shelf life of fish meat products. Additional studies should be undertaken to determine the maximal shelf life of food products supplemented with different plant−based polyphenol extracts and treatment of nutritional disorders through their absorption, metabolism and distribution pattern into biological tissues.
Keywords: TPC, Ultrasound optimization, Supplementation, Fish meat, Functional foods, Shelf life
Background
Meat is an important constituent of a well−balanced and healthy diet due to its nutritional status. Some epidemiological data has shown a link between its consumption and increased risk of cardiovascular, metabolic diseases and several forms of cancers [1]. On the other hand, fish meat is considered as abundant source of long chain polyunsaturated fatty acids and valuable nutritional components [2]. However, color and lipid oxidation are main reasons of quality deterioration in the meat products throughout storage which has near about estimate price of the industry loss over $700 million annually. The most instances oxidation is lipid oxidation and it is a free radical chain reaction which can be termed as initiation, propagation, and termination processes [3]. It is a primary mechanism which deteriorates the quality such as development of off−flavor, rancidity, degradation in texture, changing of color in meat and its products during storage, which renders them and made meat unfit for human consumption. These reactions take meat towards loss of nutritive value and eventually declining consumer confidence in the product. Intake of such food products which contain lipid constituents which are oxidized can modify proteins, DNA, tumor initiation and membrane structure in biological system [4, 5].
Improved oxidative stability of raw product is well thought−out valuable for both the processing industry and the end users. In the vegetable and fruit industry, the procedures of preparation and processing may lead to 1/3rd of the product being rejected. The disposal of by−products signifies a rising difficult since the material of plant is typically prone to microbial decay which therefore restrict further exploitation. The residues of fruits and vegetables could be cheap and freely accessible resources of compounds which are bioactive and use in the pharmaceutical and food industries. Diverse antioxidant potency is present in different types of fruit residues and the deviation is very huge [6]. In previous works, the antioxidant strength and amounts of phenolic compounds were noticed to be high in some vegetable and fruit peels which indicates that plant deposits have the ability to be consumed as a source of bioactive compounds e.g. as natural antioxidants [7, 8]. By−products of vegetables and fruits are also good supply of micro−nutrients which are organic in nature; such as poly−phenolics, carotenoids, vitamin C, tocopherols and other bioactive compounds and these compounds can be utilized as food fortification and dietary supplements purposes [9–11].
A sharp responsiveness of customers nowadays is on a relationship between health and diet. The prevention of disease has nurtured growth in the enlargement of ‘functional’ food products. The supplementation of antioxidants in functional diet can protect human body from adverse events and dysfunction of metabolic syndrome because of the beneficial effects of these phytochemicals [12]. Epidemiological work has pointed out that intake of functional foods imparts in health benefits, for example, it reduced the risk of stroke, coronary heart disease as well as certain cancer types [13]. However, available locally and internationally scientific literature does not provide information that consumption of functional meat products supplemented with vegetable and fruit phytochemical extracts are beneficial for health or not. Additionally, research on interactions and stability of phytochemical extracts with meat elements throughout processing process and storage need to be initiated. Therefore, the supplementation study of vegetable and fruit phytochemical extracts in meat products has been targeted in the present investigation which may provide information regarding phytochemicals action to prevent the formation of potential oxidized free fatty acid and peroxide compounds in meat product. In particular, the focus was on the amounts of vegetable and fruit phytochemicals that will not cause any major problems for sensoric acceptability of functional fish meat product.
Methods
Extraction of total polyphenols contents (TPC)
The by−products (cabbage leaves and banana peels) were produced from fresh vegetable and fruit samples. The peel samples were thoroughly washed under tap water to remove dirt, dust, microflora and pesticide residue on the surface. After that, the peel samples were further cut into small pieces using a stainless steel knife and oven dried at 50 °C for 48 h in hot air oven until moisture content fell below 10%. Dried peels were ground to fine powder through the sample mill with sieve size 0.5 mm. The peel powder was weight (100 ± 0.1 g) using the electronic weighing balance (Model Kern 440− 35 N) for each treatment. A 3−level five factor Box−Behnken design was used to study the effect of extraction/sonication temperature (°C), amplitude level, water/meal ratio, extraction/sonication time (minutes) and pH conditions for maximum yield of total polyphenols from dried vegetable and fruit samples.
The percent yield of peel polyphenol extract was assessed by dividing the weight of the extract with the sample weight and multiplying by 100. The coded design of real experiments is given in Table 1. The pH of the solution was monitored continuously and was adjusted by 0.2 mol/L NaOH and HCl, respectively while the temperature of the aqueous system was controlled within ±1.5 °C. The extracted TPC solutions were separated and then dried in a hot air oven at 50 °C. Yield of TPC was calculated as percentage of dried polyphenol extract.
Table 1.
Coded and actual levels of independent variables for optimization of total polyphenol extraction yield as determined by Box−Behnken design
| Independent Variables | Coded Levels | ||
|---|---|---|---|
| –1 | 0 | + 1 | |
| Extraction Temperature (°C) | 40 | 50 | 60 |
| Amplitude Level (%) | 30 | 60 | 90 |
| Water/meal ratio | 20 | 30 | 40 |
| Extraction Time (Minutes) | 20 | 40 | 60 |
| Extraction pH | 4 | 6 | 8 |
The following empirical “black box” modeling presents the relationships among process and response variables
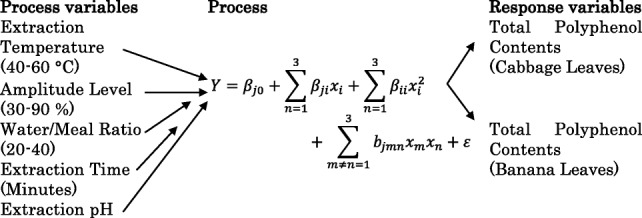
The expression inside the “black box” represents the total polyphenol contents in cabbage and banana leaves when the value of j is changed from 1 to 5. The βjo; βji; βjii; and βjmn represent the constant and coefficients of linear, quadratic and interactive effects, respectively. The xi; and xmxn represent the linear, quadratic and interactive effects of the independent variables, respectively, and ε is the random error primarily to account for the inability to determine the true model.
Analysis of TPC extracts
The phytochemical extracts were analyzed for chemical composition. Total polyphenols were measured by using Folin−Ciocalteu method following the protocol of Singleton et al. [14]. The cyanogenic contents in extract samples were estimated by alkaline titration according to the method outlined in AOAC [15] Method No. 26.115. For tannins determination, at 700 nm through spectrophotometer, the concentrations of each sample were used [16].
Functional meat product development and analysis
Fresh fish rohu (Labeo rohita) was obtained from fish supermarket, Punjab, Pakistan. Natural vegetable and fruit phytochemical extracts were supplemented at different concentration (0.5, 1 and 1.5%) to fish meat for preparation of different meat balls. The fish meat product samples without supplementation of TPC extract were kept as control. The partial/parfrying of the products was carried out to determine the lipid stability. Fried meat product samples were vacuum sealed in plastic bags and then were stored at refrigerator (for 9 days) and at − 18 °C in a freezer for a storage period of 60−days. The moisture content and also the oxidative stability of oils extracted from fish meat products was assessed by measuring peroxide value (AOCS Method No. Cd 8–53) and free fatty acid value (AOCS Method No. Ca 5a–40), respectively [17].
Sensoric evaluation
Experienced and untrained assessors carried out the sensory analysis of meat product samples according to the instructions given by Meilgaard et al. [18]. Each judge gave the written informed consent after explanation of risks and benefits of participation prior to the study. The panelists were provided informative instructions and brief definitions of attributes such as color, flavor and overall acceptability. Samples were presented to participants in sensory booths under white lighting. The order of presentation was balanced to avoid carry−over effects. Each panelist received the samples assigned with random three–digit code numbers. Each panelist was asked to list their preference on a 9–cm comparison line (1 = dislike extremely to 9 = like extremely). The sensoric analysis was performed at different storage intervals for experimental treatments.
Statistical analysis
The behavior of the Box−Behnken Model was explained by the following the quadratic equation. The data of TPC yield obtained for each treatment was subjected to statistical analysis to determine the level of significance by using the software package MATLAB according to the method described by Montgomery [19]. The average of the three runs was reported as the measured value with standard deviation. The sample analysis for storage stability and consumer acceptability was carried out in triplicate and the significant differences were calculated among means at a probability level of 5%.
Results and discussion
Optimal extraction of TPC
A 3−level five factors Box−Behnken design was used to study the effect of extraction temperature (°C), amplitude power level, water/meal ratio, extraction time (minutes) and extraction pH conditions for maximum yield of total polyphenols from cabbage leaves and banana peels. The conditions of response surface experiment were determined by setting the extraction temperature (°C), amplitude level (%), water/meal ratio, extraction time (minutes) and pH conditions on standardizing levels from − 1 to + 1 for each single factor. The total number of experimental runs for each cabbage leaves and banana peels was 46 as determined by the Box−Behnken design. The experimental response regarding TPC yield in cabbage leave and banana peel samples as a result of different extraction treatments have been depicted in Table 2. The percent values of TPC yield from cabbage leave and banana peel samples ranged from a from minimum value of 9.8 ± 0.12% to a maximum value of 19.8 ± 0.15% for cabbage leaves and minimum value of 15.55 ± 0.13% to a maximum value of 24.4 ± 0.17% for banana peels, respectively. The results revealed that extraction conditions significantly affect the TPC yield from cabbage leaves and banana peels. There is very limited published data that provides an information or support to the TPC yield from cabbage leaves and banana peels. The extraction run point 25 (extraction temperature, 60 °C; amplitude/sonication level, 90; extraction pH, 6; water/meal ratio, 30 and extraction time, 40 min) showed maximum TPC yield for cabbage leaves (19.8 ± 0.15%) and banana peel (24.4 ± 0.17%), respectively and the lowest TPC yield 15.55 ± 0.13% and 9.8 ± 0.12% was for extraction run 4 (extraction temperature, 50 °C; extraction pH, 4; water/meal ratio, 30; amplitude level 30 and extraction time, 40 min) of banana peels and extraction run 5 (extraction temperature, 40 °C; extraction pH, 6; water/meal ratio, 30; amplitude level 30 and extraction time, 40 min) of cabbage leaves, respectively.
Table 2.
Percent values of total polyphenol extract yield as determined by the Box−Behnken design
| Extraction run | Independent Variables | Total Polyphenols Extract Yield (%) | |||||
|---|---|---|---|---|---|---|---|
| Extraction Temperature (°C) | Amplitude Level (%) | Water/meal ratio | Extraction Time (Minutes) | Extraction pH | Cabbage Leaves (R1) | Banana Peels (R2) | |
| 1 | 50 (0) | 60 (0) | 40 (+ 1) | 40 (0) | 8(+ 1) | 18.55 | 23.05 |
| 2 | 60 (+ 1) | 60 (0) | 30 (0) | 40 (0) | 8 (+ 1) | 19.82 | 24.34 |
| 3 | 50 (0) | 60 (0) | 20 (−1) | 40 (0) | 4(− 1) | 12.95 | 17.45 |
| 4 | 50 (0) | 30 (− 1) | 30 (0) | 40 (0) | 4 (−1) | 11.05 | 15.55 |
| 5 | 40 (−1) | 30 (− 1) | 30 (0) | 40 (− 1) | 6 (0) | 9.80 | 14.31 |
| 6 | 50 (0) | 30 (−1) | 30 (0) | 60 (+ 1) | 6 (0) | 13.62 | 18.15 |
| 7 | 50 (0) | 60 (0) | 30 (0) | 20 (−1) | 4 (−1) | 12.85 | 17.35 |
| 8 | 50 (0) | 30 (−1) | 20 (− 1) | 40 (0) | 6 (0) | 11.70 | 16.22 |
| 9 | 40 (−1) | 60 (0) | 30 (0) | 40 (0) | 8 (+ 1) | 14.82 | 19.34 |
| 10(C1) | 50 (0) | 60 (0) | 30 (0) | 40 (0) | 6 (0) | 14.85 | 19.33 |
| 11 | 40 (−1) | 60 (0) | 30 (0) | 60 (+ 1) | 6 (0) | 13.60 | 18.11 |
| 12 | 50 (0) | 60 (0) | 40 (+ 1) | 60 (+ 1) | 6 (0) | 17.34 | 21.80 |
| 13 | 60 (+ 1) | 30 (−1) | 30 (0) | 40 (0) | 6 (0) | 14.85 | 19.34 |
| 14 | 60 (+ 1) | 60 (0) | 40 (+ 1) | 40 (0) | 6 (0) | 18.50 | 23.02 |
| 15 | 50 (0) | 30 (−1) | 30 (0) | 40 (0) | 8 (+ 1) | 14.84 | 19.36 |
| 16 | 60 (+ 1) | 60 (0) | 30 (0) | 20 (−1) | 6 (0) | 16.60 | 21.12 |
| 17 | 50 (0) | 60 (0) | 40 (+ 1) | 20 (−1) | 6 (0) | 15.30 | 19.80 |
| 18 | 50 (0) | 90 (+ 1) | 30 (0) | 60 (+ 1) | 6 (0) | 18.62 | 23.13 |
| 19 | 50 (0) | 60 (0) | 30 (0) | 60 (+ 1) | 4 (−1) | 14.85 | 19.35 |
| 20 | 50 (0) | 60 (0) | 20 (−1) | 40 (0) | 8 (+ 1) | 16.70 | 21.20 |
| 21 | 40 (−1) | 60 (0) | 40 (+ 1) | 40 (0) | 6 (0) | 13.51 | 18.04 |
| 22(C2) | 50 (0) | 60 (0) | 30 (0) | 40 (0) | 6 (0) | 14.80 | 19.37 |
| 23 | 50 (0) | 30 (−1) | 30 (0) | 20 (−1) | 6 (0) | 11.60 | 16.10 |
| 24 | 50 (0) | 60 (0) | 20 (−1) | 20 (− 1) | 6 (0) | 13.50 | 18.02 |
| 25 | 60 (+ 1) | 90 (+ 1) | 30 (0) | 40 (0) | 6 (0) | 19.82 | 24.34 |
| 26 | 50 (0) | 60 (0) | 30 (0) | 60 (+ 1) | 8 (−1) | 18.60 | 23.12 |
| 27 | 50 (0) | 30 (−1) | 40 (+ 1) | 40 (0) | 6 (0) | 13.50 | 18.04 |
| 28 | 50 (0) | 90 (+ 1) | 30 (0) | 20 (−1) | 6 (0) | 16.65 | 21.16 |
| 29 | 50 (0) | 90 (+ 1) | 30 (0) | 40 (0) | 6 (0) | 14.83 | 19.34 |
| 30 | 50 (0) | 60 (0) | 20 (−1) | 40 (0) | 6 (0) | 16.70 | 21.22 |
| 31 | 50 (0) | 90 (+ 1) | 20 (−1) | 60 (+ 1) | 6 (0) | 15.50 | 20.04 |
| 32(C3) | 50 (0) | 60 (0) | 30 (0) | 40 (0) | 6 (0) | 14.81 | 19.31 |
| 33 | 40 (−1) | 60 (0) | 20 (− 1) | 40 (0) | 6 (0) | 11.72 | 16.22 |
| 34 | 60 (+ 1) | 60 (0) | 30 (0) | 60 (+ 1) | 6 (0) | 18.60 | 23.10 |
| 35 | 60 (+ 1) | 60 (0) | 30 (0) | 40 (0) | 4 (−1) | 16.05 | 20.55 |
| 36 | 40 (−1) | 90 (+ 1) | 30 (0) | 40 (0) | 6 (0) | 14.80 | 19.30 |
| 37 | 40 (−1) | 60 (0) | 30 (0) | 20 (−1) | 6 (0) | 12.04 | 17.05 |
| 38(C4) | 50 (0) | 60 (0) | 30 (0) | 40 (0) | 6 (0) | 14.82 | 19.34 |
| 39 | 50 (0) | 90 (+ 1) | 30 (0) | 40 (0) | 8 (+ 1) | 19.85 | 24.36 |
| 40 | 40 (−1) | 60 (0) | 30 (0) | 40 (0) | 4 (−1) | 11.05 | 15.55 |
| 41 | 50 (0) | 60 (0) | 40 (+ 1) | 40 (0) | 4 (−1) | 14.75 | 19.25 |
| 42(C5) | 50 (0) | 60 (0) | 30 (0) | 40 (0) | 6 (0) | 14.83 | 19.39 |
| 43 | 60 (+ 1) | 60 (0) | 20 (−1) | 40 (0) | 6 (0) | 16.72 | 21.20 |
| 44 | 50 (0) | 90 (+ 1) | 40 (+ 1) | 40 (0) | 6 (0) | 10.50 | 23.04 |
| 45 | 50 (0) | 60 (0) | 30 (0) | 20 (−1) | 8 (+ 1) | 16.60 | 21.10 |
| 46 | 50 (0) | 90 (+ 1) | 30 (0) | 40 (0) | 4 (−1) | 16.05 | 20.55 |
C1,C2,C3,C4,C5represent extraction process at center points
R1 and R2 represent response factors
The TPC yield obtained from cabbage leaves (15.55 ± 0.13%) and banana peels (24.4 ± 0.17%) was similar to findings of earlier reported studies in literature. Although extraction yields obtained were similar or higher to those described in the literature and former methods of extraction were more aggressive than the proposed in the present work. The results indicated that TPC yield was significantly affected by the model effects (Table 3) of cabbage leaves and banana peels, respectively. The linear and quadratic effects model effects were observed more significant as compared to interaction (p ≤ 0.01). The order of model effects observed during TPC yield from dried cabbage leaves and banana peels was linear>quadratic>interaction. The results further substantiated that the linear coefficients (extraction temperature, extraction pH, amplitude level and extraction time) (p ≤ 0.01) and quadratic term coefficient (extraction pH × extraction pH) (water/meal ratio × water/meal ratio) (p ≤ 0.01) were most significant.
Table 3.
ANOVAs of the predicted second−order polynomial model for total polyphenol extract yield of cabbage leaves and banana peels
| Source of Variation | df | Cabbage Leaves | Banana Peels | |||
|---|---|---|---|---|---|---|
| F value | p value | F value | p value | |||
| Linear | Intercept | 20 | 4482.57*** | < 0.0001 | 442.02*** | <0.0001 |
| A:Extraction Temperature | 1 | 30,628.13*** | < 0.0001 | 33,065.09*** | <0.0001 | |
| B:Amplitude Level | 1 | 31,250.00*** | < 0.0001 | 3095.98*** | <0.0001 | |
| C:Water/meal ratio | 1 | 4050.00*** | < 0.0001 | 401.24*** | <0.0001 | |
| D:Extraction Time | 1 | 4753.13*** | < 0.0001 | 401.24*** | <0.0001 | |
| E:Extraction pH | 1 | 17,578.13*** | < 0.0001 | 1741.49*** | <0.0001 | |
| Interaction | AB | 1 | 0.000 | 1.0000 | 0.000 | 1.0000 |
| AC | 1 | 0.000 | 1.0000 | 0.000 | 1.0000 | |
| AD | 1 | 12.50 | 0.0016 | 0.31 | 1.5829 | |
| AE | 1 | 0.000 | 1.0000 | 0.000 | 1.0000 | |
| BC | 1 | 0.000 | 1.0000 | 0.000 | 1.0000 | |
| BD | 1 | 0.000 | 1.0000 | 0.000 | 1.0000 | |
| BE | 1 | 0.000 | 1.0000 | 0.000 | 1.0000 | |
| CD | 1 | 0.000 | 1.0000 | 0.000 | 1.0000 | |
| CE | 1 | 0.000 | 1.0000 | 0.000 | 1.0000 | |
| DE | 1 | 0.000 | 1.0000 | 0.000 | 1.0000 | |
| Quadratic | A2 | 1 | 1.70 | 0.2036 | 2.70 | < 0.1127 |
| B2 | 1 | 0.19 | 0.6672 | 0.30 | < 0.5886 | |
| C2 | 1 | 232.01 | < 0.0001 | 19.21 | < 0.0002 | |
| D2 | 1 | 288.07 | < 0.0001 | 43.23 | < 0.0001 | |
| E2 | 1 | 1037.12 | < 0.0001 | 94.59 | < 0.0001 | |
| Residual | 25 | – | – | – | – | |
| Lack of Fit | 20 | – | – | – | – | |
| Pure Error | 5 | – | – | – | – | |
| Cor. Total | 45 | – | – | – | – | |
***Significant at 0.05 level
NS Non−Significant
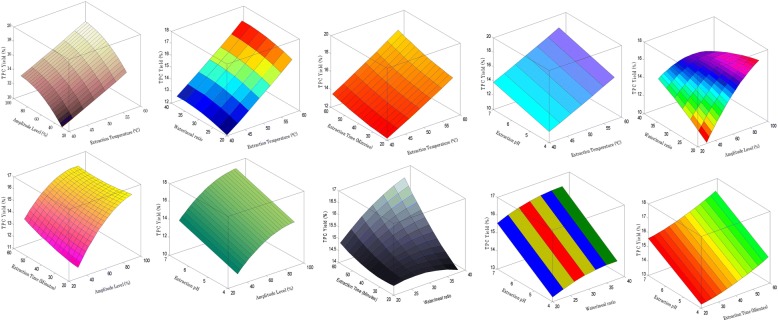
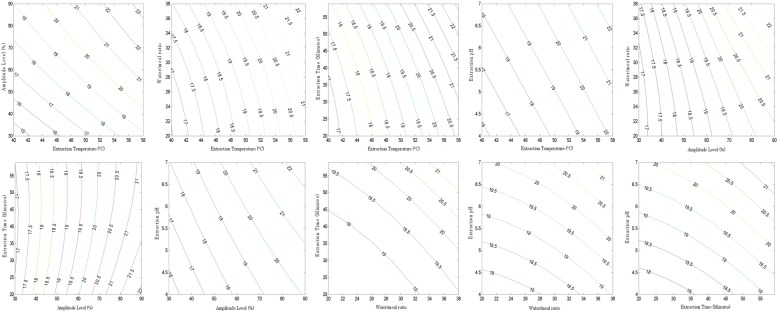
The ultimate objective of employing response surface methodology in this study was to find out the significant effects of parameters viz., extraction temperature, amplitude level, water/meal ratio, pH and extraction/sonication time to find out the optimum conditions for TPC yields. The conditions and mutual interaction terms which determine the rate of maximum TPC yields are difficult to optimize and cannot be manipulated directly from the response model. The validated optimum extraction conditions for the maximum TPC yields were obtained by varying two independent parameters and fixing the other three variables at the coded zero level (Fig. 1 and Fig. 2). The results showed the temperature was major effective factor. It has also been cited that increase in TPC yield is due to the strong effect of extraction time–temperature on the mass transfer rate of the water−soluble polyphenols in the cell wall. However, when extraction temperature and time were kept constant, increase in water:meal ratio was the reason for an exponential increase in the yield. This is due to the availability of more liquid which increases the driving force of polyphenols out of the meal. The researchers showed that the use of the higher temperature is unpractical, because it effects on decreasing resultant ingredient yield of the extract. It was also unpractical to use the meal to water ratio more than optimum, because under these conditions the yield of solids increases not much, but dilution of the system occurs, that reduces its functional properties during further application in food products [20].
Fig. 1.
Response surfaces for the mutual interaction effect of ultrasound−assisted extraction conditions on the yield of total polyphenol extracts in cabbage leaves
Fig. 2.
Contour plots for the mutual interaction effect of extraction conditions on the yield of total polyphenol extracts in banana peels
Chemical characterization of TPC extracts
The cabbage leaves and banana peels contained up to 4.8% total phenolics which were very close to the findings of Wadhwa and Bakshi [21]. The cyanogenic compounds (1.44–1.47 ± 0.14) and tannins (6.55–7.90 ± 0.22) mostly present in the by−product extracts were found responsible for the astringent taste [22].
Peroxide values of functional fish meat product
Table 4 presents the mean values of peroxides in different TPC supplemented fish meat product samples stored at temperatures 4 °C and − 18 °C, respectively. Peroxide values (meqO2 /kg) of meat balls treated with TPC extracts at 4 °C were in the range of 1.31 ± 0.12 to 3.10 ± 0.20 while at − 18 °C ranged was found 1.31 ± 0.12 to 1.55 ± 0.17, respectively. Peroxide values (PV) of all the treatments increased at the end of second interval then decreased at the end of last storage interval. Peroxide values of all treatments were higher and significantly different at the beginning and the end of the storage period (p < 0.05). The decrease of the PV at the end of the storage may occur owing to decomposition of hydro peroxides into secondary oxidation products [23]. A similar trend was also observed by the Yerlikaya et al. [24] during the refrigerated studies of fish patties. The values of peroxide value, free fatty acid, thiobarbutric acid and total volatile base nitrogen in fish burgers at the end of storage increased significantly determined as 4.98 (±0.22) meqO2/kg of fat, 0.94 (±0.01) % of oleic acid, 0.58 (± 0.02) mg MA/kg of sample and 4.78 (±0.02) mg/100 g of sample, respectively [23]. Several lipid oxidation indices were assessed to follow up the development of oxidation in frozen state. Peroxide value and thiobarbituric acid reactive substances showed primary and secondary oxidation, respectively [25]. When the peroxide value exceeded 10 meq oxygen/ kg fat of meat, the meat is then considered unfit for human consumption or refused [26].
Table 4.
Peroxide value (meqO2 /kg) of meat balls treated with total polyphenol extracts
| Treatment | Cabbage Leaves Extracts | Banana Peel Extracts | Cabbage Leaves Extracts | Banana Peel Extracts | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 °C | 4 °C | −18 °C | − 18 °C | |||||||||||||
| 0 day | 3 days | 6 days | 9 days | 0 day | 3 days | 6 days | 9 days | 0 day | 15 days | 30 days | 60 days | 0 day | 15 days | 30 days | 60 days | |
| Control | 1.35 ± 0.14g | 2.02 ± 0.18c | 3.10 ± 0.20a | 2.07 ± 0.19b | 1.34 ± 0.14g | 2.10 ± 0.18c | 2.90 ± 0.20a | 2.60 ± 0.19b | 1.35 ± 0.14g | 1.44 ± 0.16e | 1.55 ± 0.17d | 1.39 ± 0.15f | 1.34 ± 0.14g | 1.43 ± 0.16e | 1.54 ± 0.17d | 1.38 ± 0.15f |
| 0.5% | 1.34 ± 0.15f | 1.85 ± 0.20a | 1.62 ± 0.19b | 1.50 ± 0.18c | 1.33 ± 0.15f | 1.84 ± 0.20a | 1.61 ± 0.19b | 1.49 ± 0.18c | 1.34 ± 0.15f | 1.38 ± 0.16e | 1.41 ± 0.17d | 1.41 ± 0.17d | 1.33 ± 0.15f | 1.37 ± 0.16e | 1.40 ± 0.17d | 1.40 ± 0.17d |
| 1% | 1.33 ± 0.12e | 1.37 ± 0.14c | 1.57 ± 0.15a | 1.48 ± 0.14b | 1.32 ± 0.17d | 1.36 ± 0.18c | 1.56 ± 0.20a | 1.47 ± 0.19b | 1.33 ± 0.12e | 1.35 ± 0.13d | 1.37 ± 0.14c | 1.36 ± 0.14c | 1.32 ± 0.17d | 1.34 ± 0.18c | 1.36 ± 0.18c | 1.35 ± 0.18c |
| 1.5% | 1.32 ± 0.12d | 1.35 ± 0.13c | 1.43 ± 0.15a | 1.39 ± 0.14b | 1.31 ± 0.12d | 1.34 ± 0.13c | 1.42 ± 0.15a | 1.38 ± 0.14b | 1.32 ± 0.12d | 1.33 ± 0.12d | 1.35 ± 0.13c | 1.35 ± 0.13c | 1.31 ± 0.12d | 1.32 ± 0.12d | 1.34 ± 0.13c | 1.34 ± 0.13c |
a-gMeans with different superscripts within an extract category differ significantly (p ≤ 0.05)
Decrease of PV could be attributed to different rates of lipid oxidation [27]. Lipids in cooked meat could be more easily oxidized than those in raw meat [28]. The chemical spoilage associated with fish during storage is mainly due to fish lipid degradation (auto−oxidation). In general, fish have high degree of unsaturated lipids than other food commodities. Fish lipids are subjected to two main changes, lipolysis and auto−oxidation. The main reactants in these processes involves atmospheric oxygen and fish unsaturated lipids, leading to the formation of hydroperoxides, associated with tasteless, flavor and accompanied by brown yellow discoloration of the fish tissue. Upon further degradation of hydroperoxides is the formation of strong rancid flavors e.g. aldehydes and ketones, usually associated with spoilt fatty fish species [26]. The increase of lipid content than the lipid oxidations resulting from action of lipolytic enzymes (lipases and phospholipases) that fish phospholipids undergo degradation to produce hydroperoxides, aldehydes and ketones which are responsible for the development of oxidative rancidity [26]. Oxidation of highly unsaturated lipids is other factor which highly related to the production of off−flavors and odors and also as influencing, protein denaturation and texture changes [29]. The most common cause of oil deterioration is rancidity and the most common cause of rancidity in oils and fats is oxidation. Oxidation of the oil, in oily fish, gives rise to rancid odors and flavors; these can limit the storage life of such species more quickly than the protein changes that govern the extractable protein value. An important stage in the oxidation is the reaction of oxygen with the unsaturated fatty acid molecules to form hydroperoxides and the amount of these can be used as a measure of the extent of oxidation in the early stages. The peroxide test is a measure of the formation of hydroperoxides. An increase in the PV is most useful as an index of the earlier stages of oxidation which on oxidation proceeds and peroxides decrease at final stages and the PV can start to fall [24]. Throughout processing and storage of meat, there are three mechanisms for spoilage of meat and its products as A) autolytic enzymatic spoilage, (B) microbial spoilage and (C) lipid oxidation [30]. A number of hypotheses are associated with the disease caused by the consumption of such rancid meat and a meat product includes cardiovascular diseases, cancers of several types and diabetes [31]. Prolong refrigeration of meat and its product can lead to poor quality due to lipid peroxidation. Antioxidants are being used to decrease oxidation process and increase shelf life [32]. Researchers have shown that lipid oxidation can be delayed or inhibit by synthetic or natural food additives e.g. anti−oxidants [33]. Phenolic antioxidants interact with free lipidperoxy or lipidoxy free radicals (formed in result of lipid oxidation), hence stopping their further self−breakdown. Lipid auto−oxidation inhibition is essential not only in foodstuffs during their heating or storage, but also to reduce lipids oxidation after ingestion and absorption via the intestinal wall [34].
Free fatty acids of functional fish meat product
Table 5 presents the mean values of free fatty acids (FFA) in different TPC supplemented fish product samples stored at temperatures 4 °C and − 18 °C. Free fatty acids of fish products at different storage days was found in parallel to the studies of Vanitha et al. [23]. A similar trend was also observed by the Yerlikaya et al. [24] during the refrigerated studies of fish patties. Genuine FFA contents of lipids from product samples increased in a similar fashion during storage at − 18 °C, while concomitantly their P levels decreased [35]. When fish−based products are frozen and cold stored, unfavorable changes take place in the texture and appearance. Simultaneously, free fatty acids are formed from the lipids. These changes have been attributed to enzymic reactions which take place at a rate governed by the temperature of frozen storage. The FFA generated during storage originated from the phospholipids, the neutral lipids or both. This is in contrast to the mince lipids where the contributions from both phospholipids and neutral lipids to FFA formation could be calculated [35]. Increase in FFA results from the enzymatic hydrolysis of esterified lipids [36]. Free fatty acids content has been used to establish the grade of deterioration. Lipid (glycerol−fatty acids esters) present in the fish muscle undergoes hydrolysis, resulting in the release of fatty acids. Due to lipid hydrolysis, FFA accumulates in the tissue during frozen storage, especially at high temperatures around − 10 °C. Slow freezing rates or fluctuating storage temperatures may result in the lysis of lysosomes and thereby increased activity of some endogenous lipases resulting in increased rates of FFA accumulation [26].
Table 5.
Free fatty acids (% of oleic acid) of meat balls treated with total polyphenol extracts
| Treatment | Cabbage Leaves Extracts | Banana Peel Extracts | Cabbage Leaves Extracts | Banana Peel Extracts | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 °C | 4 °C | −18 °C | −18 °C | |||||||||||||
| 0 day | 3 days | 6 days | 9 days | 0 day | 3 days | 6 days | 9 days | 0 day | 15 days | 30 days | 60 days | 0 day | 15 days | 30 days | 60 days | |
| Control | 0.21 ± 0.15f | 0.31 ± 0.17d | 0.54 ± 0.19b | 0.63 ± 0.20a | 0.20 ± 0.15f | 0.30 ± 0.17d | 0.53 ± 0.19b | 0.62 ± 0.20a | 0.21 ± 0.15f | 0.27 ± 0.16e | 0.33 ± 0.17d | 0.35 ± 0.18c | 0.20 ± 0.15f | 0.26 ± 0.16e | 0.32 ± 0.17d | 0.34 ± 0.18c |
| 0.5% | 0.20 ± 0.15f | 0.30 ± 0.17d | 0.50 ± 0.19b | 0.57 ± 0.20a | 0.19 ± 0.15f | 0.29 ± 0.17d | 0.49 ± 0.19b | 0.56 ± 0.20a | 0.20 ± 0.15f | 0.26 ± 0.16e | 0.28 ± 0.17d | 0.31 ± 0.18c | 0.19 ± 0.15f | 0.25 ± 0.16e | 0.27 ± 0.17d | 0.30 ± 0.18c |
| 1% | 0.22 ± 0.15f | 0.29 ± 0.17d | 0.47 ± 0.19b | 0.51 ± 0.20a | 0.21 ± 0.15f | 0.28 ± 0.17d | 0.46 ± 0.19b | 0.50 ± 0.20a | 0.20 ± 0.15f | 0.25 ± 0.16e | 0.27 ± 0.17d | 0.29 ± 0.18c | 0.19 ± 0.15f | 0.24 ± 0.16e | 0.26 ± 0.17d | 0.28 ± 0.18c |
| 1.5% | 0.19 ± 0.15f | 0.27 ± 0.17d | 0.41 ± 0.19b | 0.49 ± 0.20a | 0.18 ± 0.15f | 0.26 ± 0.17d | 0.40 ± 0.19b | 0.48 ± 0.20a | 0.19 ± 0.15f | 0.24 ± 0.16e | 0.26 ± 0.17d | 0.28 ± 0.18c | 0.18 ± 0.15f | 0.23 ± 0.16e | 0.25 ± 0.17d | 0.27 ± 0.18c |
a-fMeans with different superscripts within an extract category differ significantly (p ≤ 0.05)
Moisture content of functional fish meat product
Table 6 presents the mean values of moisture in different TPC supplemented fish products stored at temperatures 4 °C and − 18 °C. Moisture content of fish products at refrigerated temperature was found in parallel to the studies of Vanitha et al. [23]. While the moisture content of fish products at deep storage was found in parallel to the studies of Mahmoudzadeh et al. [37]. Moisture content increased in all treatments at the end of research trial [37] which is in close to the present findings.
Table 6.
Moisture content (%) of meat balls treated with total polyphenol extracts
| Treatment | Cabbage Leaves Extracts | Banana Peel Extracts | Cabbage Leaves Extracts | Banana Peel Extracts | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 °C | 4 °C | −18 °C | −18 °C | |||||||||||||
| 0 day | 3 days | 6 days | 9 days | 0 day | 3 days | 6 days | 9 days | 0 day | 15 days | 30 days | 60 days | 0 day | 15 days | 30 days | 60 days | |
| Control | 66.54 ± 0.19b | 65.59 ± 0.18c | 65.16 ± 0.17d | 64.23 ± 0.16e | 66.53 ± 0.19b | 65.58 ± 0.18c | 65.15 ± 0.17d | 64.22 ± 0.16e | 66.54 ± 0.19b | 66.53 ± 0.19b | 66.66 ± 0.20a | 66.67 ± 0.20a | 66.53 ± 0.19b | 66.52 ± 0.19b | 66.65 ± 0.20a | 66.66 ± 0.20a |
| 0.5% | 66.17 ± 0.19b | 65.21 ± 0.18c | 65.09 ± 0.17d | 64.15 ± 0.16e | 66.16 ± 0.19b | 65.20 ± 0.18c | 65.08 ± 0.17d | 64.14 ± 0.16e | 66.17 ± 0.19b | 66.16 ± 0.19b | 66.27 ± 0.20a | 66.28 ± 0.20a | 66.16 ± 0.19b | 66.15 ± 0.19b | 66.26 ± 0.20a | 66.27 ± 0.20a |
| 1% | 66.10 ± 0.19b | 65.14 ± 0.18c | 65.07 ± 0.17d | 64.10 ± 0.16e | 66.09 ± 0.19b | 65.13 ± 0.18c | 65.06 ± 0.17d | 64.09 ± 0.16e | 66.10 ± 0.19b | 66.09 ± 0.19b | 66.19 ± 0.20a | 66.20 ± 0.20a | 66.09 ± 0.19b | 66.08 ± 0.19b | 66.18 ± 0.20a | 66.19 ± 0.20a |
| 1.5% | 66.05 ± 0.19b | 65.09 ± 0.18c | 65.01 ± 0.17d | 64.05 ± 0.16e | 66.04 ± 0.19b | 65.08 ± 0.18c | 65.00 ± 0.17d | 64.04 ± 0.16e | 66.05 ± 0.19b | 66.04 ± 0.19b | 66.14 ± 0.20a | 66.15 ± 0.20a | 66.04 ± 0.19b | 66.03 ± 0.19b | 66.13 ± 0.20a | 66.14 ± 0.20a |
a-eMeans with different superscripts within an extract category differ significantly (p ≤ 0.05)
Sensoric acceptability of functional fish meat product
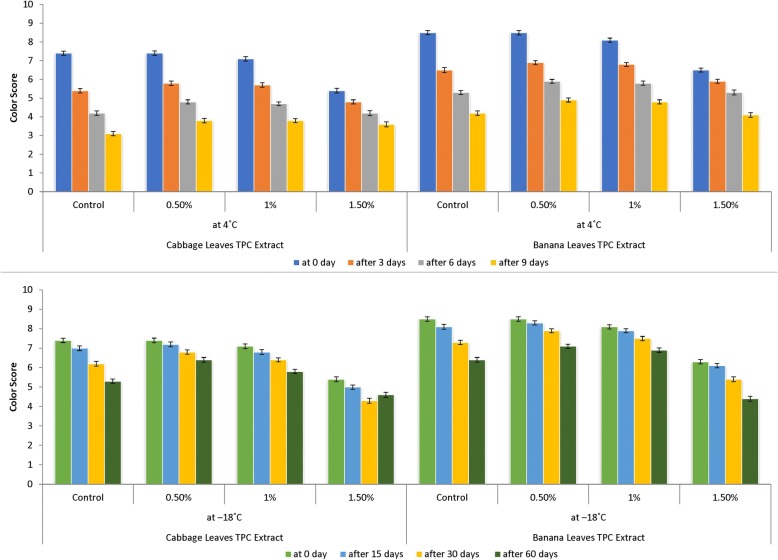
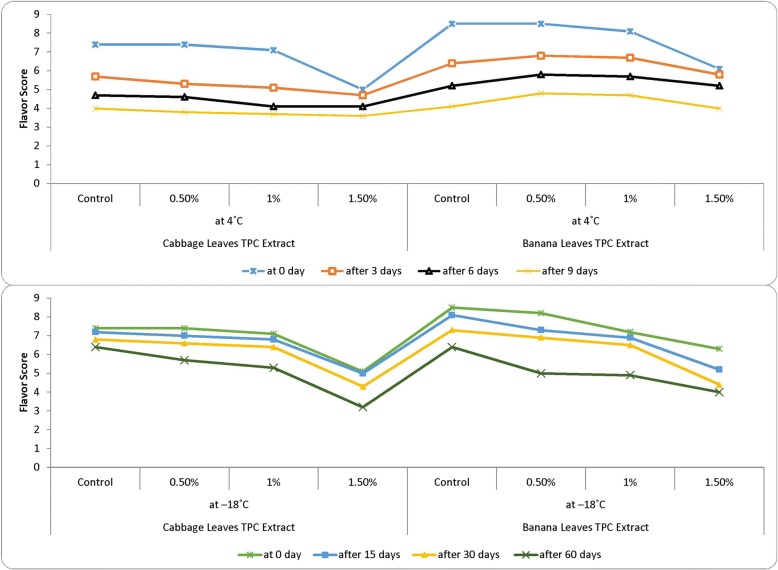
Sensory evaluation is an important tool in a product development. Acceptance of a food product depends upon the consumer’s perception of the color, taste, texture, flavor and overall acceptability into overall impression of quality. Although, chemical, physical and microbiological tests are employed to check the quality of a food product, but these tests cannot provide such kind of information whether consumer will accept it or not. The meatballs prepared, deep fried and then subjected to sensory evaluation by panelist from parent department for different attributes viz., color, flavor and overall acceptability and scores were recorded using a nine−point hedonic scale to assess the liking and disliking of the panelists. Sensory scores of fish product samples for color (Fig. 3), flavor (Fig. 4) and overall acceptability (Fig. 5) show a significant difference in sensory scores at refrigeration temperatures where sensory scores of fish product samples decreased significantly (p < 0.05) throughout refrigeration storage. Whereas the sensory scored at the − 18 °C shows the good sensory characteristics, relatively. Acceptable at end of 2nd month with treatment 0.5% results were in parallel to findings of Mahmoudzadeh et al. [37]. When fish-based products are frozen and cold stored, unfavorable changes take place in the texture and appearance. Both FFA and peroxides have been implicated as the cause of decrease in sensory scores [35]. The appearance, texture, odor and flavor of the fish burger started decreasing at a significant level of 1% level [23]. Turhan et al. [38] determined the shelf life of refrigerated raw anchovy (E. encrasicholus) patties for 6 days at 4 °C. The above−mentioned studies showed the very similar results with the present study. According to Orak and Kayisoglu [29], the decrease in the values of sensory analyses was faster than chemical changes during frozen storage. Appearance of off−flavor may be attributed to WOF (Warm Over Flavor). WOF is usually associated with reheated meats and includes odors and flavors commonly described as stale or rancid also interaction of ketones, aldehydes, alcohols, hydrocarbons, acids, and epoxides with proteins may be produced off−colors [39]. Sensory scores decreased during the storage of anchovy patties at the storage temperature of 4 °C and Anchovy patties were consumable up to 6 days [24]. Fish and meat products undergo rapid post−mortem changes, mostly proteolysis and lipolysis, which takes product towards sensory deterioration and the risks of food−borne illnesses becomes high [40]. Overall, protein and lipid oxidation can account for the toughened texture, off-flavor and unappealing odor of frozen stored sea foods [39].
Fig. 3.
Color evaluation of fish meat balls treated with polyphenol extracts at 4 °C and − 18 °C
Fig. 4.
Flavor scores of fish meat balls treated with polyphenol extracts at different storage temperatures and intervals
Fig. 5.
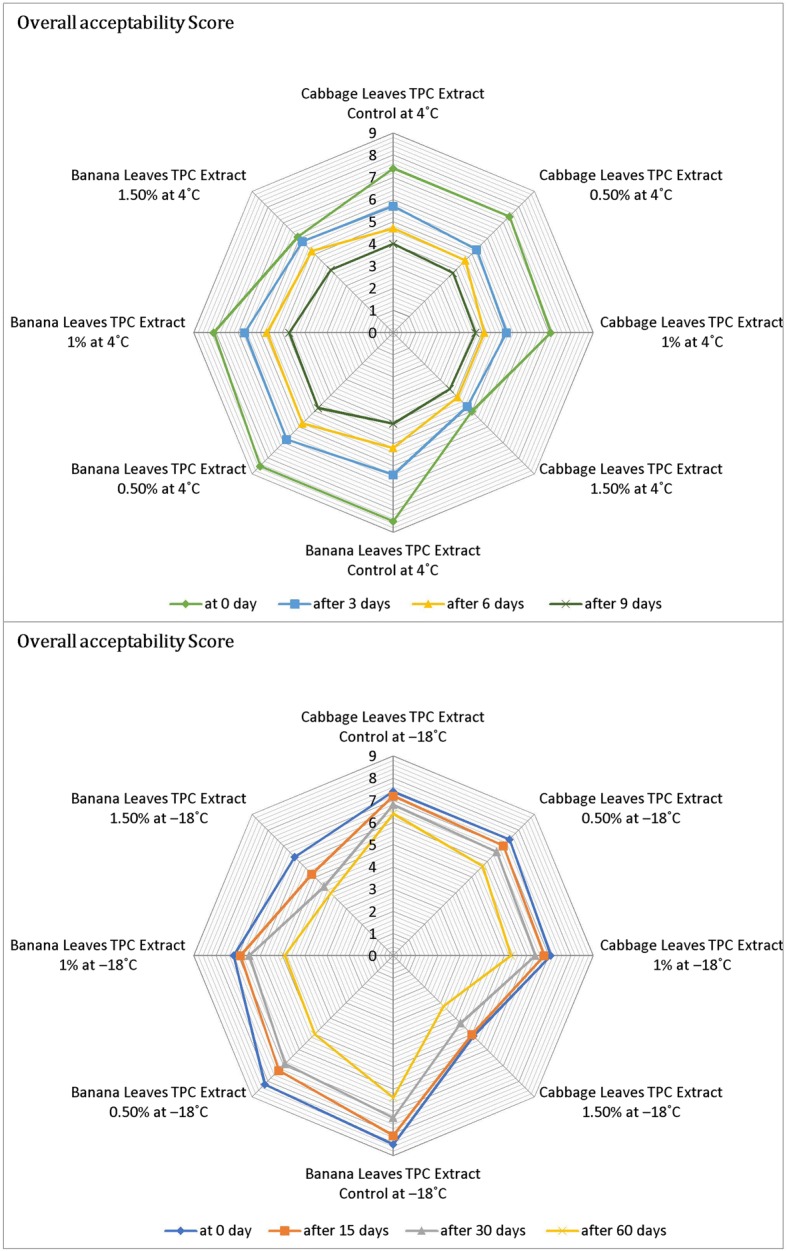
Overall acceptability of fish meat balls treated with polyphenol extracts at 4 °C and − 18 °C during different storage intervals
Conclusions
Various techniques of food preservation are available either for conventional or modern technology preservation methods. In this regard, the preservation methods used to preserve food should be suitable for the food conditions because not all of these methods are able to maintain the freshness and organoleptic properties of the food products. Natural preservatives have gained interest due to the awareness on the harmful effect of consuming artificial preservatives. It can be concluded that the several fruit and vegetable byproducts from food processing industries meet the criteria of antioxidant definition. They are certain to be an excellent source of natural antioxidants if used as high−quality ingredients in functional foods or dietary supplements. The applications of plant food byproducts will definitely bring about added value to both, the industry and the consumer. The industry can benefit from economic incomes and the consumer from the excellent nutritional value of these materials with potential health claims.
Acknowledgements
The authors are highly obliged to the Library Department, Government College University Faisalabad (GCUF), and IT Department, Higher Education Commission (HEC, Islamabad) for access to journals, books and valuable database.
Availability of data materials
The dataset supporting the conclusions of this article is included within the article.
Funding
The research was completed after utilizing the available resources in the Institute and University chemical support (GCUF-RSP 90-F.SCI-1).
Authors’ contributions
MA has done the major research work including the bench work, MI was the research supervisor, MN provided guidance for the lipid oxidative stability, MKK has given technical guidance in bench work especially ultrasound−assisted optimal extraction of polyphenol extracts, MS helped out for analysis of meat samples, HARS and RB helped for drafting the manuscript. “It’s also confirmed that all the authors read and approved the final manuscript”.
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Muhammad Ali, Email: alimalik.ft@gmail.com.
Muhammad Imran, Phone: +92.335.2020050, Email: imran@gcuf.edu.pk.
Muhammad Nadeem, Email: muhammad.nadeem@uvas.edu.pk.
Muhammad Kamran Khan, Email: mk.khan@gcuf.edu.pk.
Muhammad Sohaib, Email: muhammad.sohaib@uvas.edu.pk.
Hafiz Ansar Rasul Suleria, Email: hafiz.suleria@uq.net.au.
Reeja Bashir, Email: reejahashmi@gmail.com.
References
- 1.Pereira PM, Vicente AF. Meat nutritional composition and nutritive role in the human diet. Meat Sci. 2013;93(3):586–592. doi: 10.1016/j.meatsci.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 2.Coppes Petricorena Z. Chemical composition of fish and fishery products. In: Cheung P, Mehta B, editors. Handbook of food chemistry. Berlin, Heidelberg: Springer; 2015. pp. 403–435. [Google Scholar]
- 3.Yogesh K, Ahmad T, Manpreet G, Mangesh K, Das P. Characteristics of chicken nuggets as affected by added fat and variable salt contents. J Food Sci Technol. 2013;50(1):191–196. doi: 10.1007/s13197-012-0617-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porter NA, Caldwell SE, Mills KA. Mechanisms of free radical oxidation of unsaturated lipids. Lipids. 1995;30(4):277–290. doi: 10.1007/BF02536034. [DOI] [PubMed] [Google Scholar]
- 5.Muik B, Lendl B, Molina-Díaz A, Ayora-Cañada MJ. Direct monitoring of lipid oxidation in edible oils by Fourier transform Raman spectroscopy. Chem Phys Lipids. 2005;134(2):173–182. doi: 10.1016/j.chemphyslip.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Deng GF, Shen C, Xu XR, Kuang RD, Guo YJ, Zeng LS, et al. Potential of fruit wastes as natural resources of bioactive compounds. Int J Mol Sci. 2012;13(7):8308–8323. doi: 10.3390/ijms13078308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ajila CM, Naidu KA, Bhat SG, Rao UP. Bioactive compounds and antioxidant potential of mango peel extract. Food Chem. 2007;105(3):982–988. doi: 10.1016/j.foodchem.2007.04.052. [DOI] [Google Scholar]
- 8.Okonogi S, Duangrat C, Anuchpreeda S, Tachakittirungrod S, Chowwanapoonpohn S. Comparison of antioxidant capacities and cytotoxicities of certain fruit peels. Food Chem. 2007;103(3):839–846. doi: 10.1016/j.foodchem.2006.09.034. [DOI] [Google Scholar]
- 9.Schieber A, Stintzing FC, Carle R. By−products of plant food processing as a source of functional compounds—recent developments. Trends Food Sci Technol. 2001;12(11):401–413. doi: 10.1016/S0924-2244(02)00012-2. [DOI] [Google Scholar]
- 10.O'Shea N, Arendt EK, Gallagher E. Dietary fibre and phytochemical characteristics of fruit and vegetable by−products and their recent applications as novel ingredients in food products. Innov Food Sci Emerg Technol. 2012;16:1–10. doi: 10.1016/j.ifset.2012.06.002. [DOI] [Google Scholar]
- 11.Jansen RJ, Robinson DP, Stolzenberg-Solomon RZ, Bamlet WR, de Andrade M, Oberg AL, Rabe KG, Anderson KE, Olson JE, Sinha R, Petersen GM. Nutrients from fruit and vegetable consumption reduce the risk of pancreatic cancer. J Gastrointest Cancer. 2013;44(2):152–161. doi: 10.1007/s12029-012-9441-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scicchitano P, Cameli M, Maiello M, Modesti PA, Muiesan ML, Novo S, di Studio IG. Nutraceuticals and dyslipidaemia: beyond the common therapeutics. J Funct Foods. 2014;6:11–32. doi: 10.1016/j.jff.2013.12.006. [DOI] [Google Scholar]
- 13.Williamson C. Functional foods: what are the benefits? Br J Community Nurs. 2009;14(6):230–236. doi: 10.12968/bjcn.2009.14.6.42588. [DOI] [PubMed] [Google Scholar]
- 14.Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- 15.AOAC . Official methods of analysis, 18th edition. Arlington, VA: Association of Official Analytical Chemists, AOAC Press; 2006. [Google Scholar]
- 16.Schanderi SH. Methods in food analysis. New York: Academic; 1970. p. 709. [Google Scholar]
- 17.AOCS . Official methods and recommended practices of AOCS. 5. Champaign, IL: American Oil Chemists Society; 1998. [Google Scholar]
- 18.Meilgaard MC, Civille GV, Carr BT. Sensory Evaluation Techniques. 4. Boca Raton: CRC Press; 2007. p. 464. [Google Scholar]
- 19.Montgomery DC. Design and Analysis of Experiments. USA: John Wiley & Sons, Inc.; 2008. Response Surface Methods and Designs; p. 440. [Google Scholar]
- 20.Liu YJ, Mo XL, Tang XZ, Li JH, Hu MB, Yan D, Peng W, Wu CJ. Extraction optimization, characterization, and bioactivities of polysaccharides from Pinelliae Rhizoma Praeparatum Cum Alumine employing ultrasound-assisted extraction. Molecules. 2017;22(6):1–19. doi: 10.3390/molecules22060965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wadhwa M, Bakshi MPS. Utilization of fruit and vegetable wastes as livestock feed and as a substrate for generation of other value added products. Ed Harinder P.S. Makkar. Food and Agriculture Organization of United Nations. Bangkok: RAP Publication; 2013. p. 56.
- 22.Emaga TH, Andrianaivo RH, Wathelet B, Tchango JT, Paquot M. Effects of the stage of maturation and varieties on the chemical composition of banana and plantain peels. Food Chem. 2007;103(2):590–600. doi: 10.1016/j.foodchem.2006.09.006. [DOI] [Google Scholar]
- 23.Vanitha M, Dhanapal K, Reddy GVS. Quality changes in fish burger from Catla (Catla Catla) during refrigerated storage. J Food Sci Technol. 2015;52(3):1766–1771. doi: 10.1007/s13197-013-1161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yerlikaya P, Gokoglu N, Uran H. Quality changes of fish patties produced from anchovy during refrigerated storage. Eur Food Res Technol. 2005;220(3–4):287–291. doi: 10.1007/s00217-004-1035-x. [DOI] [Google Scholar]
- 25.Losada V, Barros-Velázquez J, Aubourg SP. Rancidity development in frozen pelagic fish: influence of slurry ice as preliminary chilling treatment. LWT Food Sci Technol. 2007;40(6):991–999. doi: 10.1016/j.lwt.2006.05.011. [DOI] [Google Scholar]
- 26.Khidhir ZK, Murad HOM, Arif ED. Qualitative assessment of imported frozen fish fillets in Sulaimani markets. Iraqi J Vet Sci. 2013;27(1):49–55. [Google Scholar]
- 27.Arvanitoyannis IS, Van Houwelingen-Koukaliaroglou M. Functional foods: a survey of health claims, pros and cons, and current legislation. Crit Rev Food Sci Nutr. 2005;45(5):385–404. doi: 10.1080/10408390590967667. [DOI] [PubMed] [Google Scholar]
- 28.Widayaka K, Setyawardani T, Sumarmono J. The effect of storage and cooking on lipid oxidation of raw and cooked beef and goat meat. Asia Pac J Clin Nutr. 2001;10(4):548. [Google Scholar]
- 29.Orak HH, Kayisoglu S. Quality changes in whole, gutted and filleted three fish species (Gadus euxinus, Mugil cephalus, Engraulis encrasicholus) at frozen storage period (−26 C) Acta Sci Pol Technol Aliment. 2008;7(3):15–28. [Google Scholar]
- 30.Miller RK. Factors affecting the quality of raw meat. In: Joseph K, John K, David L, editors. Meat processing: improving quality. UK.: Woodhead Publishing; 2002. pp. 27–63. [Google Scholar]
- 31.Ruiz-Núñez B, Pruimboom L, Dijck-Brouwer DA, Muskiet FA. Lifestyle and nutritional imbalances associated with Western diseases: causes and consequences of chronic systemic low-grade inflammation in an evolutionary context. J Nutr Biochem. 2013;24(7):1183–1201. doi: 10.1016/j.jnutbio.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Lauridsen C, Buckley DJ, Morrissey PA. Influence of dietary fat and vitamin E supplementation on α−tocopherol levels and fatty acid profiles in chicken muscle membranal fractions and on susceptibility to lipid peroxidation. Meat Sci. 1997;46(1):9–22. doi: 10.1016/S0309-1740(97)00010-7. [DOI] [PubMed] [Google Scholar]
- 33.Sallam KI, Ishioroshi M, Samejima K. Antioxidant and antimicrobial effects of garlic in chicken sausage. LWT Food Sci Technol. 2004;37(8):849–855. doi: 10.1016/j.lwt.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frankel EN. Antioxidants in lipid foods and their impact on food quality. Food Chem. 1996;57(1):51–55. doi: 10.1016/0308-8146(96)00067-2. [DOI] [Google Scholar]
- 35.de Koning AJ, Mol TH. Quantitative quality tests for frozen fish: soluble protein and free fatty acid content as quality criteria for hake (Merluccius capensis) stored at −18C. J Sci Food Agric. 1991;54(3):449–458. doi: 10.1002/jsfa.2740540316. [DOI] [Google Scholar]
- 36.Hwang KT, Regenstein JM. 1993. Characteristics of mackerel mince lipid hydrolysis. J Food Sci. 1993;58(1):79–83. doi: 10.1111/j.1365-2621.1993.tb03216.x. [DOI] [Google Scholar]
- 37.Mahmoudzadeh M, Motallebi AA, Hosseini H, Haratian P, Ahmadi H, Mohammadi M, Khaksar R. Quality assessment of fish burgers from deep flounder (Pseudorhombus elevatus) and brushtooth lizardfish (Saurida undosquamis) during storage at −18°C. Iran J Fish Sci. 2010;9(1):111–126. [Google Scholar]
- 38.Turhan S, Evren M, Yazici F. Shelf−life of refrigerated raw anchovy (Engraulis encrasicholus) patties. EU J Fish Aqua Sci. 2001;18(3–4):391–398. [Google Scholar]
- 39.Thanonkaew A, Benjakul S, Visessanguan W, Decker EA. The effect of metal ions on lipid oxidation, colour and physicochemical properties of cuttlefish (Sepia pharaonis) subjected to multiple freeze–thaw cycles. Food Chem. 2006;95(4):591–599. doi: 10.1016/j.foodchem.2005.01.040. [DOI] [Google Scholar]
- 40.Casaburi A, Nasi A, Ferrocino I, Di Monaco R, Mauriello G, Villani F, Ercolini D. Spoilage−related activity of Carnobacterium maltaromaticum strains in air−stored and vacuum−packed meat. Appl Environ Microbiol. 2011;77(20):7382–7393. doi: 10.1128/AEM.05304-11. [DOI] [PMC free article] [PubMed] [Google Scholar]