Introduction
Dermatomyositis, one of the subtypes of idiopathic inflammatory myopathies, presents with a prominent skin rash in up to 80% of patients with or without proximal muscle weakness.1 Dermatomyositis treatment can be very challenging. In particular, cutaneous disease is usually refractory and impacts the quality of life in patients with dermatomyositis.2 Systemic steroids are still considered first-line treatment; however, their chronic use should be limited.3 Many steroid-sparing agents have been used with variable outcomes.3 Currently used immunomodulators include antimalarials, azathioprine, methotrexate, mycophenolate mofetil, intravenous immunoglobulin, rituximab, and oral calcineurin inhibitors.4Apremilast is a phosphodiesterase-4 (PDE-4) inhibitor recently used in the treatment of several inflammatory diseases including psoriasis, psoriatic arthritis, and Behçet disease.5, 6 Apremilast was not previously reported in the treatment of patients with dermatomyositis. We report the efficacy of off-label use of apremilast in the treatment of 3 patients with recalcitrant dermatomyositis.
Case report
Patient 1
Patient 1 is a 57-year-old woman with a long-standing history of classic dermatomyositis diagnosed 16 years ago. The patient initially presented with the characteristic rash of dermatomyositis: heliotrope sign, V-sign with poikiloderma over the chest, shawl sign on the back, and proximal nailfold with ragged cuticles and capillaries looping. She also had violaceous patches and plaques over the extensors, scalp pruritus, scale, and proximal muscle weakness. Skin biopsy done over the chest found diffuse mucin in the dermis with mixed perivascular and lichenoid lymphocytic inflammatory infiltrates consistent with dermatomyositis.
Laboratory work up was significant for elevated levels of creatine kinase (CK) and aldolase. No underlying malignancy was found. Systemic steroids were initiated as well as hydroxychloroquine. She developed a pruritic eruption secondary to hydroxychloroquine. This treatment was discontinued, and over the next years she continued to be steroid dependent despite adequate trials of multiple immunosuppressive agents, including mycophenolate mofetil, azathioprine, methotrexate, acitretin, intravenous immunoglobulin, tacrolimus, chlorambucil, infliximab, and rituximab. Chronic steroid use resulted in insulin-dependent diabetes mellitus as well as other steroid-associated side effects. Her disease continued to flare despite these therapies with a cutaneous dermatomyositis activity and severity index (CDASI) score of 43. Severe cutaneous disease is defined by a CDASI score of more than 14.7 Although she was on stable doses of mycophenolate mofetil and prednisone, apremilast given at 30 mg orally twice daily was added to her treatment regimen. Steroids and steroid-sparing agents were kept at a stable dose for the first 3 months. The patient had follow-up 1 month after apremilast initiation and every 3 months thereafter. One month into her treatment, she noticed significant improvement of her skin disease and then clearance after 3 months of apremilast with a CDASI score of 0. Her muscle weakness lagged behind and she noticed improvement after 9 months of being on apremilast with normalization of her aldolase and CK. The patient weaned off all immunosuppressive agents and prednisone and continues on apremilast as monotherapy. The patient experienced mild nausea and diarrhea with apremilast that improved 4 weeks into the treatment. She was able to discontinue insulin and lose weight and she continues to be clear of both skin and muscle symptoms on apremilast for more than 2 years.
Patient 2
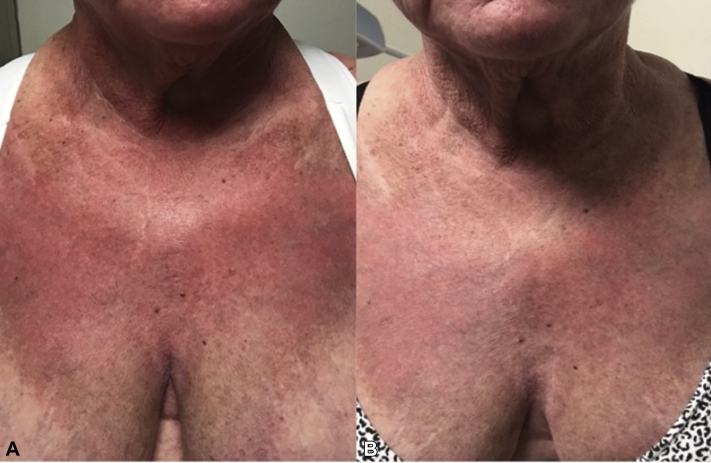
Patient 2 is a 64-year-old woman known to have dermatomyositis for the last 4 years complicated by calcinosis cutis over her extremities. She presented initially with a heliotrope sign; erythematous patches and plaques over the face, back, and extensors; V-sign with poikiloderma; scalp pruritus; and scale. She had severe cutaneous disease with a CDASI score of 41. There was neither muscle involvement nor an underlying malignancy. The patient was treated with systemic steroids, mycophenolate mofetil, hydroxychloroquine, colchicines, and intravenous sodium thiosulfate. Her disease continued to flare despite her treatment. Apremilast, 30 mg orally twice a day, was added to prednisone and mycophenolate mofetil. Steroids and steroid-sparing agents were kept at a stable dose for the first 3 months. She had follow-up 1 month after apremilast initiation and every 3 months thereafter. One month into her treatment, she noticed a decrease in her pruritus and improvement of her rash. After 3 months of apremilast, she had significant improvement of her skin disease with a CDASI score of 7 (Fig 1, A and B). Prednisone and mycophenolate mofetil were tapered off, and the patient remained controlled on apremilast alone. She did not experience any side effects with apremilast.
Fig 1.
Clinical images of the eruption over the chest of patient 2 before and after 3 months of apremilast. A, Violaceous plaque with scale and poikiloderma over the chest. B, Mild erythematous patch over the chest after 3 months of treatment with apremilast.
Patient 3
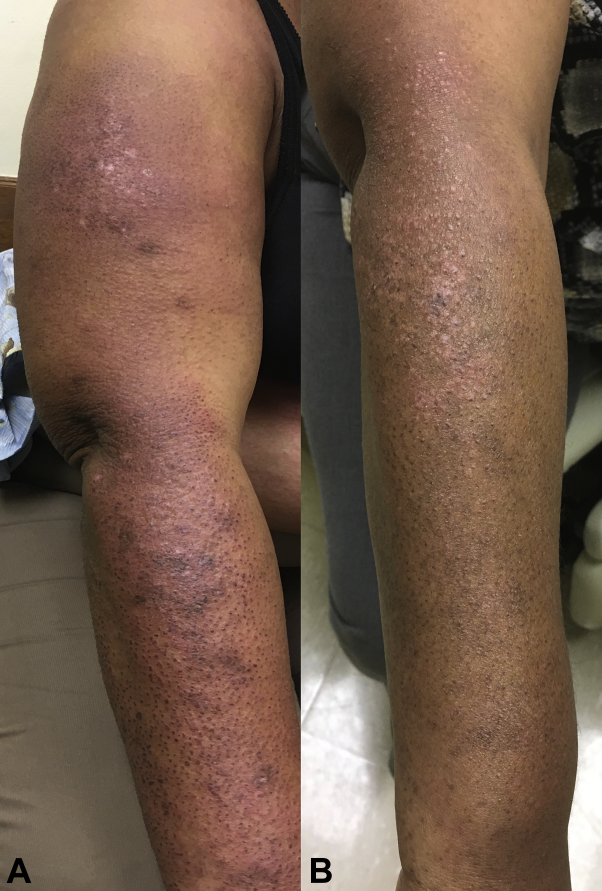
Patient 3 is a 62-year-old woman with classic dermatomyositis diagnosed 2 years ago. She presented with a heliotrope rash and severe periorbital edema together with facial swelling. She had violaceous plaques with crusting, ulceration, and poikiloderma on the chest, back, and the extensors. She also complained of scalp pruritus, alopecia, and proximal muscle weakness. Skin biopsy performed on the chest found lichenoid lymphocytic dermatitis consistent with connective tissue diseases. Laboratory workup was significant for elevated levels of CK. Screening tests for an underlying malignancy were negative. The patient was treated with systemic steroids, hydroxychloroquine, and mycophenolate mofetil. Despite her treatment, she remained with severe cutaneous disease and CDASI score of 62. Apremilast, 30 mg orally twice a day, was added to prednisone, mycophenolate mofetil, and hydroxychloroquine. Steroids and steroid-sparing agents were kept at a stable dose for the first 3 months. The patient had follow-up 1 month after apremilast initiation and every 3 months thereafter. One month into her treatment, she noticed a decrease in her pruritus and mild improvement of her rash. After 3 months of apremilast, she had significant improvement of her skin disease with a CDASI score of 18 (Fig 2, A and B). The patient was stable for 9 months on her treatment regimen before flaring of her skin disease. Since her flare with no identified inciting event, patient 3 has been refractory to all treatments; she received high-dose steroid pulse and is planned to start on intravenous immunoglobulin. She did not experience any side effects with apremilast.
Fig 2.
Clinical images of the eruption over the extensors of patient 3 before and after 3 months of apremilast. A, Violaceous plaques with scale and crusting over the extensors. B, Mild erythematous papules and macules over the extensors thereafter 3 months of treatment with apremilast.
Discussion
This case series discussed 3 patients with refractory cutaneous dermatomyositis treated with apremilast at our dermatology clinic. All 3 patients were women with severe CDASI score of more than 14.7 In all cases, CDASI was calculated by reviewing individual score criteria in our detailed history and physical examination as well as clinical pictures. There were no underlying malignancies. Each patient had been treated with oral steroids and multiple steroid-sparing agents without clearing of their skin for months to years.
Apremilast given at 30 mg orally twice daily was added to their treatment regimens. On average, 85% improvement was seen in CDASI skin score 3 months after the addition of apremilast. Steroids and steroid-sparing agents were tapered off as improvement was documented. Patients 1 and 2 were able to discontinue all other medications and continued on apremilast as a monotherapy. All patients noticed improvement in their muscle disease that lagged behind the skin disease.
There was normalization of their muscle enzymes (creatine kinase and aldolase). Apremilast was well tolerated with transient gastrointestinal symptoms in patient 1.
The pathogenesis of dermatomyositis is multifactorial with environmental, genetic, and immune factor contribution.1 T helper-1 (Th1) and T helper-2 (Th2) immune pathways play a fundamental role in dermatomyositis.8 There is an increase in proinflammatory cytokines including tumor necrosis factor α, interleukin (IL)-1, IL-6, and interferon-α and interferon-γ, all of which shift the immune balance to a Th1 response.1 IL-4 released by lymphocytes infiltrating the skin and muscles in dermatomyositis patients contributes to increase in Th2 response in conjunction with Th1 response.8
Apremilast is a PDE-4 inhibitor currently used for psoriasis and psoriatic arthritis.5 However, its use on patients with dermatomyositis has not been investigated.
By inhibiting PDE-4, apremilast increases the level of cyclic adenosine monophosphate, leading to decreased expression of proinflammatory cytokines including tumor necrosis factor-α and interferon-γ, thus inhibiting Th1 response.6 Apremilast may also block Th2 response by interfering with the level of IL-6 secreted by type 2 macrophages.5 Although the mechanism of action of apremilast in dermatomyositis is unknown, we suggest that apremilast can be a potential treatment for dermatomyositis through interfering with the Th1 and Th2 response.
Apremilast, although not cytotoxic, can cause significant diarrhea and other gastrointestinal effects.5Apremilast is a well-tolerated oral medicine that can be added to other immunomodulating agents to maximize the therapeutic response in recalcitrant dermatomyositis. Our observations are based on a small number of patients, and further studies are needed to better understand the role of apremilast in the treatment of dermatomyositis.
Footnotes
Funding sources: None.
Conflicts of interest: Dr Boh serves on the advisory board for Celgene. Drs Bitar, Stumpf, and Boh received research support from Celgene to conduct an investigator-initiated trial. Drs Maghfour and Ho-Pham have no conflicts of interest disclose.
References
- 1.Thompson C., Piguet V., Choy E. The pathogenesis of dermatomyositis. Br J Dermatol. 2018;179(6):1256–1262. doi: 10.1111/bjd.15607. [DOI] [PubMed] [Google Scholar]
- 2.Wolstencroft P.W., Chung L., Li S., Casciola-Rosen L., Fiorentino D.F. Factors associated with clinical remission of skin disease in dermatomyositis. JAMA Dermatol. 2018;154:44–51. doi: 10.1001/jamadermatol.2017.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandel D.E., Malemud C.J., Askari A.D. Idiopathic inflammatory myopathies: a review of the classification and impact of pathogenesis. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18051084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anyanwu C.O., Chansky P.B., Feng R., Carr K., Okawa J., Werth V.P. The systemic management of cutaneous dermatomyositis: results of a stepwise strategy. Int J Women's Dermatol. 2017;3:189–194. doi: 10.1016/j.ijwd.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maier C., Ramming A., Bergmann C. Inhibition of phosphodiesterase 4 (PDE4) reduces dermal fibrosis by interfering with the release of interleukin-6 from M2 macrophages. Ann Rheum Dis. 2017;76:1133–1141. doi: 10.1136/annrheumdis-2016-210189. [DOI] [PubMed] [Google Scholar]
- 6.Hatemi G., Melikoglu M., Tunc R. Apremilast for Behcet's syndrome—a phase 2, placebo-controlled study. N Engl J Med. 2015;372:1510–1518. doi: 10.1056/NEJMoa1408684. [DOI] [PubMed] [Google Scholar]
- 7.Anyanwu C.O., Fiorentino D.F., Chung L. Validation of the Cutaneous Dermatomyositis Disease Area and Severity Index: characterizing disease severity and assessing responsiveness to clinical change. Br J Dermatol. 2015;173:969–974. doi: 10.1111/bjd.13915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giris M., Durmus H., Yetimler B., Tasli H., Parman Y., Tuzun E. Elevated IL-4 and IFN-gamma levels in muscle tissue of patients with dermatomyositis. In Vivo. 2017;31:657–660. doi: 10.21873/invivo.11108. [DOI] [PMC free article] [PubMed] [Google Scholar]