Introduction
Epidermodysplasia verruciformis (EV) that presents in adulthood is most commonly seen in patients with HIV/AIDS or in organ transplant recipients. We present a case of EV in a young adult who is HIV negative and not a transplant recipient but who does have a newly described immunodeficiency that is responsive to rapamycin. This report reviews the first reported case, to our knowledge, of EV in a patient with activated PI3Kδ syndrome (APDS), proposes a unique treatment strategy, describes the role of whole genome sequencing in cases of acquired EV (AEV), and highlights the growing importance of personalized medicine in dermatology.
Case report
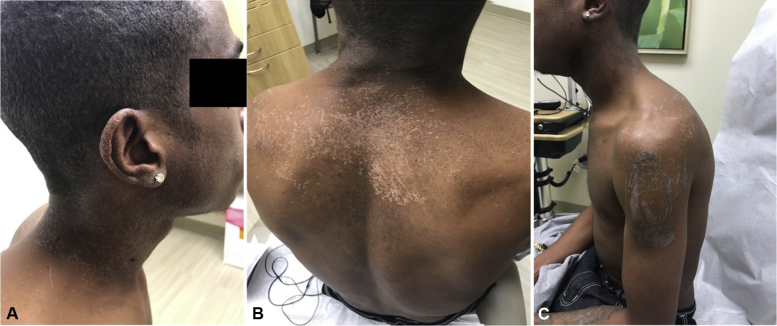
A 25-year-old African-American man presented to dermatology clinic with a 2-month history of a rash that began on the face that slowly progressed to involve his entire body. He experienced occasional pruritus but was otherwise asymptomatic. He had used hydrocortisone 1% cream for 2 weeks with no perceived improvement. The patient has a history of APDS, a recently described immunodeficiency, for which he receives intravenous immunoglobulin quarterly. Recent laboratory findings were normal with the exception of leukopenia (white blood cell count, 3.7 × 109 cells/L) and thrombocytopenia (platelets, 76 × 109/L). An HIV test was negative 2 months prior. The examination was notable for innumerous flat-topped, hypopigmented to gray, pinpoint papules that coalesced into larger plaques on the scalp, face, trunk and bilateral upper and lower extremities (Fig 1). Koebnerization was noted on the back and upper arms. Oral, ocular, anogenital and lymph node findings were normal. A punch biopsy of the right hip yielded a diagnosis of epidermodysplasia verruciformis with classic histologic features (Fig 2). The patient was administered the human papillomavirus 9-valent vaccine, recombinant and started on acitretin, 25 mg daily.
Fig 1.
EV presenting in a patient with activated PI3Kδ syndrome. A to C, Physical examination notable for innumerous flat-topped, hypopigmented to gray, pinpoint papules that coalesce into larger plaques on the scalp, face, trunk, and bilateral upper and lower extremities. Koebnerization noted at some sites.
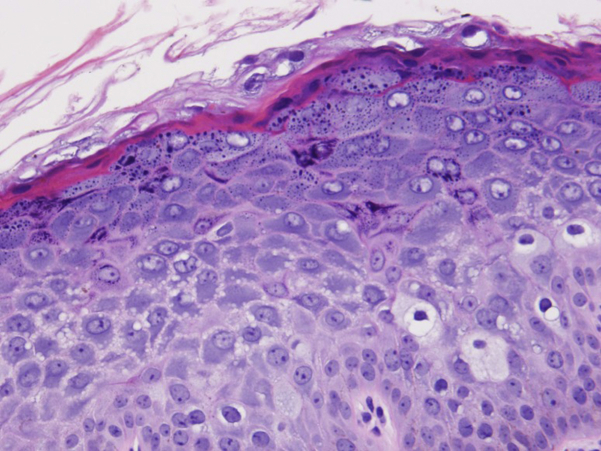
Fig 2.
Histologic analysis of right hip punch biopsy shows flat wart architecture, clumping of keratohyaline granules, and the characteristic steel blue/gray cytoplasm of affected keratinocytes. (Hematoxylin-eosin stain.)
Discussion
EV is classically a rare autosomal recessive genodermatosis that is the result of genetic susceptibility and co-infection by human papillomavirus (HPV), usually manifesting early in life. The most common HPV types implicated in EV are HPV-5 and HPV-8, coined EV-HPV or β-HPV.1 When presenting during adulthood, it is usually the consequence of impaired cell-mediated immunity and is most commonly seen in HIV/AIDS and organ transplant recipients. Case reports of EV are also described in those with lupus, leprosy and Hodgkin lymphoma. Patients with late-onset, or acquired EV (AEV), do not routinely undergo whole genome sequencing. Traditional thought has attributed their cutaneous disease to their existing immunodeficiency rather than to mutations in EVER1 (TMC6) or EVER2 (TMC8) genes, as in the inherited form.2 Our patient with AEV is not only uniquely HIV negative and not a transplant recipient, but also has an immunodeficiency in which EV has never been reported. Furthermore, his immunodeficiency responds to rapamycin, thus opening the door to novel treatment options not available to all patients with EV.
APDS is caused by gain-of-function mutations in the phosphoinositide 3-kinase (PI3K) genes PIK3CD and PIK3R1. These mutations lead to altered maturation of both B and T lymphocytes, which subsequently increases the incidence of both bacterial and viral infections as well as lymphoma.3 mTOR is a downstream effector of the PI3K/AKT pathway, which is important for cell growth, proliferation, and survival. This pathway has been found to be hyperactive in T lymphocytes of APDS patients and is also the mammalian target of rapamycin. It is thought to be the driving force behind this lymphoproliferative immunodeficiency by skewing T lymphocytes to the senescent effector phenotype. Rapamycin is found to normalize the senescent T lymphocyte proliferation in APDS, thus restoring the balance of naïve, effector and memory CD8+ T-lymphocyte population.4 To our knowledge, there is no report of EV occurring in a patient with APDS.
Because EV is notoriously difficult to treat, there is a call for more effective therapy. Treatment aims to not only improve cosmesis but also to reduce progression to malignancy. It is reported that 60% of EV patients will go on to have cutaneous squamous cell carcinoma at an early age.2, 5 Systemic retinoids have been used with varying success, although recurrence is high when treatment is discontinued.6, 7 One report documents clearance with the Gardasil vaccine in a renal transplant patient.8 Interestingly in HIV patients with EV who start antiretroviral therapy, the cutaneous eruption often does not improve despite improvement of CD4 counts.9 Although our patient has improved slightly on acitretin, and his topographical changes are not as prominent, his skin has not normalized. However, unlike most patients with AEV, this patient has undergone whole genome sequencing, and, as a result, a molecular target for therapy has been identified. Rapamycin has improved T-lymphocyte populations in those with APDS. Further studies are needed to determine patients' cutaneous responses to therapy via improved T-cell suppression of HPV. Therefore, we propose investigation into the use of rapamycin in APDS patients with comorbid EV.
Conclusion
This case describes the first report of AEV in a patient with APDS. Furthermore, our patient is HIV negative and not a transplant recipient; thus, his diffuse cutaneous HPV infection may be attributed to comorbid APDS. This diagnosis may have important treatment implications given that T-lymphocyte populations normalize, and immunoregulation improves when APDS patients are treated with rapamycin. Thus, we hypothesize his cutaneous disease will improve indirectly with this course of treatment. This case highlights the importance of a more extensive workup in cases of AEV, including whole genome sequencing, to assess for genetic mutations and possible molecular therapeutic targets in a disease that is disfiguring and resistant to currently available treatment. Finally, this case underscores the importance of interdisciplinary care and illustrates the growing importance of personalized medicine in dermatology.
Footnotes
Funding sources: None.
Conflicts of interest: Dr Atkinson receives research funding from Shire (Baxalta) Pharmaceuticals as a principal investigator. Ms Donaldson and Drs Purnell, Pavlidakey, and Kissel have no conflicts of interest to disclose. This case was previously presented at the 2018 American Academy of Dermatology Annual Meeting, San Diego, California, February 16-20, 2018.
References
- 1.Cardoso J.C., Calonje E. Cutaneous manifestations of human papillomaviruses: a review. Acta Dermatovenerol Alp Pannonica Adriat. 2011;20(3):145–154. [PubMed] [Google Scholar]
- 2.Przybyszewska J., Zlotogorski A., Ramot Y. Re-evaluation of epidermodysplasia verruciformis: Reconciling more than 90 years of debate. J Am Acad Dermatol. 2017;76(6):1161–1175. doi: 10.1016/j.jaad.2016.12.035. [DOI] [PubMed] [Google Scholar]
- 3.Lucas C.L., Chandra A., Nejentsev S., Condliffe A.M., Okkenhaug K. PI3Kdelta and primary immunodeficiencies. Nat Rev Immunol. 2016;16(11):702–714. doi: 10.1038/nri.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lucas C.L., Kuehn H.S., Zhao F. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110delta result in T cell senescence and human immunodeficiency. Nat Immunol. 2014;15(1):88–97. doi: 10.1038/ni.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitsuishi T., Ohara K., Suzuki T., Mochizuki T., Kaneko T., Kawana S. Epidermodysplasia verruciformis with keratoacanthoma, Bowen's disease and squamous cell carcinoma: isolation of high-risk types of HPV 5 and unknown type of human papillomavirus. Eur Acad Dermatol Venereol. 2008:2. doi: 10.1111/j.1468-3083.2007.02526.x. 1468-3083 (Electronic) [DOI] [PubMed] [Google Scholar]
- 6.Rallis E., Papatheodorou G., Bimpakis E., Butanska D., Menounos P., Papadakis P. Systemic low-dose isotretinoin maintains remission status in epidermodysplasia verruciformis. J Eur Acad Dermatol Venereol. 2008;22(4):523–525. doi: 10.1111/j.1468-3083.2007.02394.x. [DOI] [PubMed] [Google Scholar]
- 7.Vohra S., Sharma N.L., Shanker V., Mahajan V.K., Jindal N. Autosomal dominant epidermodysplasia verruciformis: a clinicotherapeutic experience in two cases. Indian J Dermatol Venereol Leprol. 2010;76(5):557–561. doi: 10.4103/0378-6323.69092. [DOI] [PubMed] [Google Scholar]
- 8.Maor D., Brennand S., Goh M.S., Fahey V., Tabrizi S.N., Chong A.H. A case of acquired epidermodysplasia verruciformis in a renal transplant recipient clearing with multimodal treatment including HPV (Gardasil) vaccination. Australas J Dermatol. 2018;59(2):147–148. doi: 10.1111/ajd.12684. [DOI] [PubMed] [Google Scholar]
- 9.Lowe S.M., Katsidzira L., Meys R. Acquired epidermodysplasia verruciformis due to multiple and unusual HPV infection among vertically-infected, HIV-positive adolescents in Zimbabwe. Clin Infect Dis. 2012;54(10):e119–e123. doi: 10.1093/cid/cis118. [DOI] [PMC free article] [PubMed] [Google Scholar]