Abstract
Background: Rheumatic diseases (RDs) are the most common cause of severe long-term pain and physical disability, affecting hundreds of millions of people around the world. Smartphones technology have the potential to become an important tool that rheumatologist can employ in the clinical care management of RD. Methods: Research of the published literature on the principle electronic databases available as Ovid MEDLINE, Health Technology Assessment Database, Embase, and PsycINFO was conducted, and the studies evaluated eligible were reviewed. Results: Our search produced 120 results from which 47 eligible articles were identified reporting studies of smartphone apps for patients with RD. All examined feasibility and five assessed the efficacy of a smartphone intervention for clinical care management. Conclusions: It has been demonstrated a strong evidence for the feasibility of using smartphone to enhance care of patients with RD. Based on the available literature and our personal experiences, we consider useful the development of some mobile phone apps, to simplify and assist the rheumatologist during his clinical practice. Still remains limited data on the efficacy of such interventions. (www.actabiomedica.it)
Keywords: Rheumatic disease, mHealth, smartphone, patient-reported outcomes, clinical care management
Introduction
Rheumatic diseases (RDs) are the most common cause of severe long-term pain and physical disability, and they affect hundreds of millions of people around the world (1-4). The reported disease prevalence of RD complaints ranged broadly from 9.8% to 33.2% (5-8). Almost one third of people aged over 75 has a significant musculoskeletal problem, and the prevalence of locomotor disability rises from 3.1% in those aged less than 60 to almost 50% in those aged more than 75 (9, 10). In a survey carried in Italy, the point prevalence of chronic pain caused by a RD is estimated at 27% of the general adult population (11), the prevalence is higher among women and increases markedly with age.
In the 2010 World Health Organization (WHO) Global Burden of Disease Study, RD were reported to be the second leading cause of disability worldwide, as measured by years lived with disability (12). Results for specific diseases and impairments have been extensively reported (13-17): not only rheumatic disorders are progressive debilitating diseases, but they also have a devastating impact on health-related quality of life (HRQoL) (18, 19). HRQoL has become an important measure when studying health status and health outcome infact surveys from the industrialized world revealed a high prevalence of RDs and its negative effect on the perceived HRQoL, as compared with other common chronic conditions (20-25). Traditional methods of evaluation, with a focus on the locomotor system and measures of impairment, may fail to describe the extensive multi-dimensional issues associated with RDs. Patient-reported outcomes (PROs) are an attractive option in a busy medical practice, as the time burden is transferred from the clinician to the patient. PROs include physical function or HRQoL, pain, general health status, side effects, medical costs and other factors, and instruments for measuring PROs are easier to administer and less expensive than physician-observed disease activity and process measures. Although, in Italy, the use of the instruments is still quite limited, the validity and usefulness of PRO data in evaluating and monitoring patients with RDs have been well documented (26-29). Electronic data collection improves data quality by providing software safeguard against entry omission and inconsistent response sets, and by eliminating data entry errors made by researcher’s (30-32). The development and spread of smartphones offers several advantages, it is a device that combine the characteristics of a typical mobile phone with the capabilities of a personal computer, due to the presence of a full and autonomous operating system. The fields of application of mobile health are various and heterogeneous, without a special training automated health tracking and timely interventions are possible. In this paper, we aim to review the published studies of smartphone apps applied for the care of patients with rheumatic disorders and explore the current evidence base for their potential impact on clinical care.
Eligibility criteria and study selection
Only original English research articles were included in the review. We included any study that reported on any quantitative outcomes of a smartphone-based intervention among patients with RD. An electronic database search of Ovid MEDLINE, Health Technology Assessment Database, Embase and PsycINFO was conducted on March 20, 2017, using the following keyword search algorithm: (“smartphone*” or “mobile phone*” or “cell phone” or “iPhone” or “mobile app*” or “phone app*”) AND (“rheumatic diseases”, rheumatoid arthritis”, “osteoarthritis”, “fibromyalgia”, “spondyloarthritis” and “chronic pain sindrome”). All eligible studies were systematically reviewed, and proportional meta-analyses were applied to pooled data on recruitment, retention, and adherence to examine the overall feasibility of smartphone interventions for RDs.
Search Findings
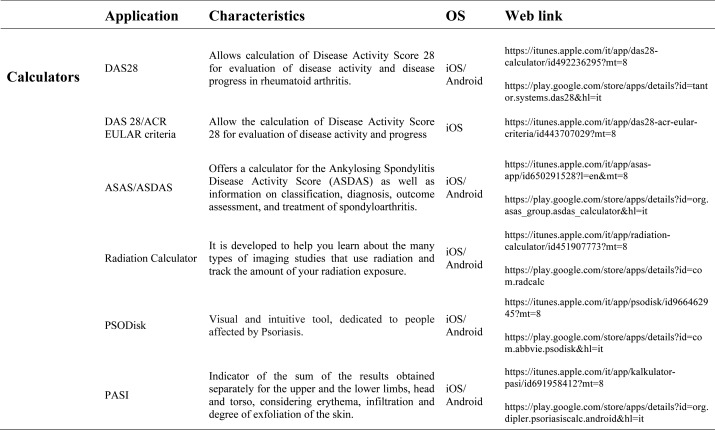
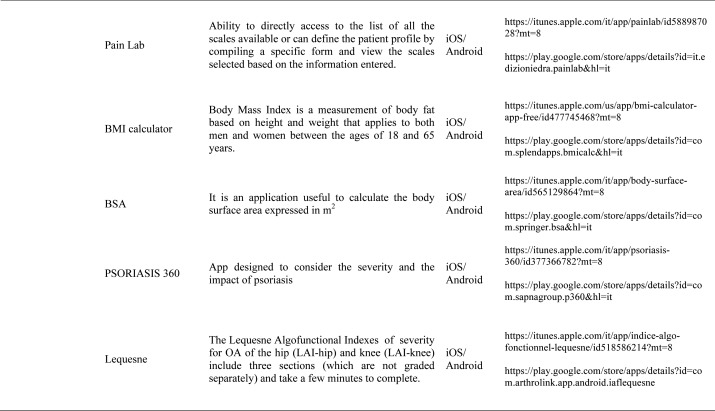
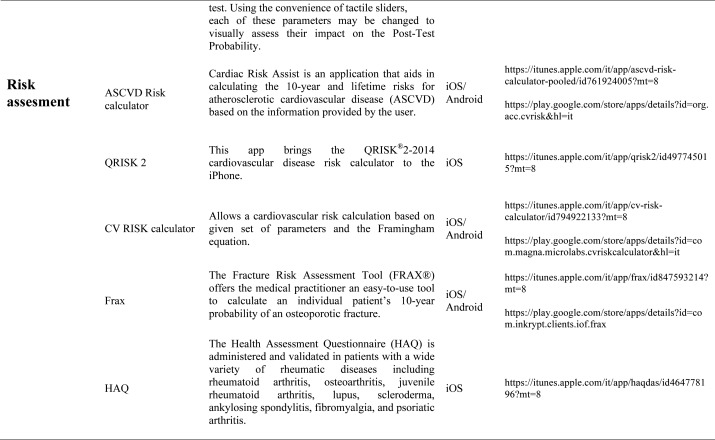
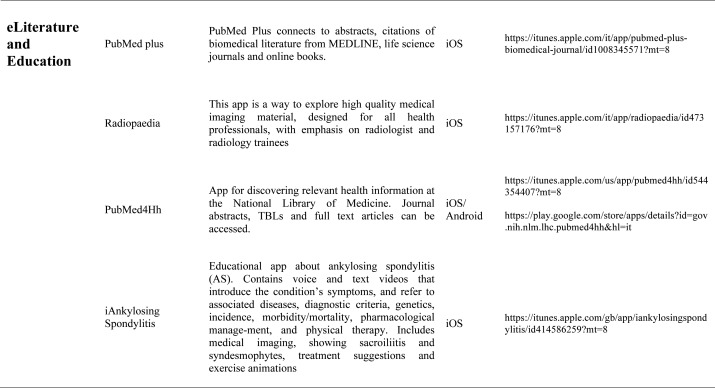
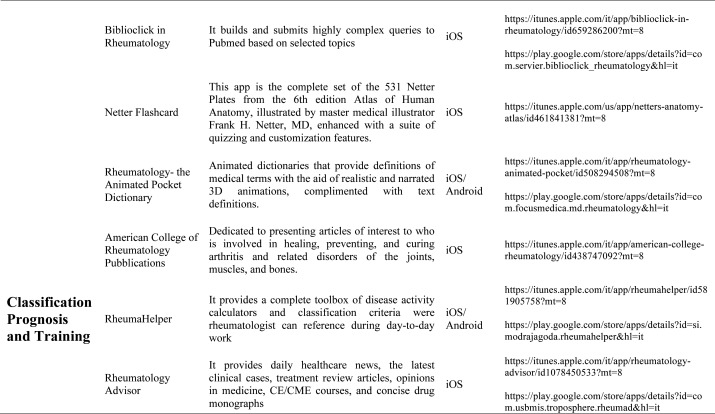
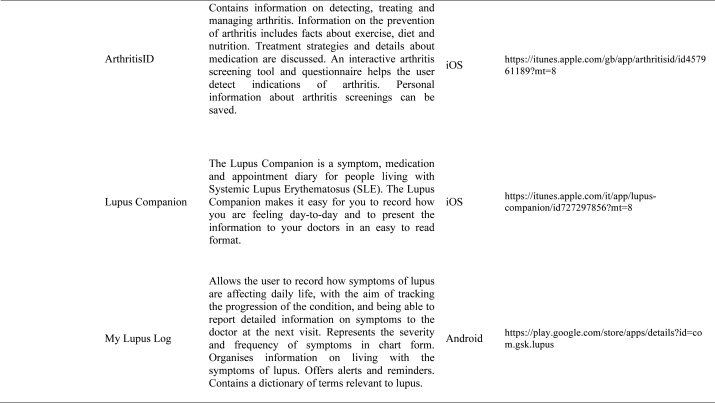
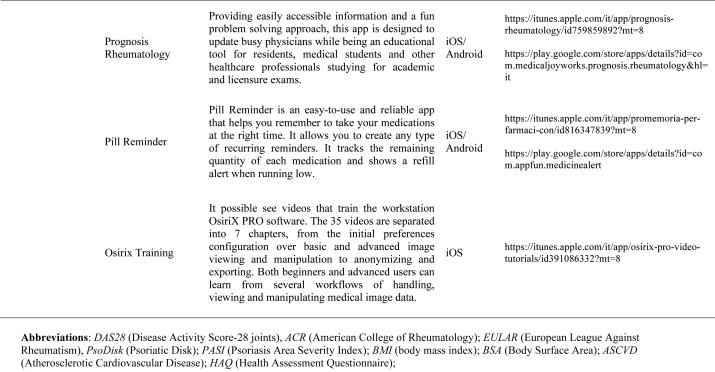
Our search produced 120 results from which 47 eligible articles were identified reporting studies of smartphone apps for patients with RDs. All examined feasibility and five assessed the efficacy of a smartphone intervention for clinical care management. However, there was substantial heterogeneity across studies, due to the fact that each app was unique. Table 1 provides summary information from apps presented in the context of different individual study, selected based on their content quality, user-friendliness, availability and time optimization.
Table 1.
Medical applications for the rheumatologist
The emergence and enhancement of electronic medical applications and web-internet based tools have been driven by private and public sector initiatives with the goals of improving individual patient care, reducing medical errors and health care costs, increasing physician access to information for medical decision making, and facilitating communication between providers (33-34). Medical apps are being used with increasing frequency in rheumatological practices to make relevant patient data more readily available at the time of the patient encounter. They were subdivided into 5 categories: medical calculators, risk assessment tools, eLiterature, classification prognosis & training and applications for medical and nursing students.
Medical calculators: A medical calculator or clinical calculator is a software program for calculating various clinical scores and indices such as Disease Activity Score - 28 joints (DAS-28), Clinical Disease Activity Index (CDAI), Simple Disease Activity Index (SDAI), for rheumatoid arthritis, Lequesne index for knee and hip osteoarthritis or Ankylosing Spondylitis Disease Activity Score (ASDAS) for ankylosing spondylitis. Usually, calculation of composite clinical scores or indices involves complex formulas using several input parameters. Medical calculators typically provide a user interface to enter parameters and calculate scores using a standard formula. Physicians and/or patients do not need to use or even know the actual formula for calculating a clinical score or index. For example, body mass index (BMI) is the most commonly used measure of obesity in all over the world. Other applications available are, Corticonverter, that is a quickly and useful application to perform corticosteroids unit converter, DocNomo, a graphical tool to enhance the bedside interpretation of a diagnostic test result, a digital adaptation of Two-Step Fagan Nomogram which is the updated version of the original Fagan’s nomogram developed by Fagan in 1975 (35).
Risk Assessment calculators: More and more frequently the rheumatologist is requested to assess the cardiovascular risk of each single patient suffering from an inflammatory disease: in this sense are available different tools. The QRISK®2-2014 is a cardiovascular disease risk calculator for the iPhone, it uses traditional risk factors (age, systolic blood pressure, smoking status and ratio of total serum cholesterol to high-density lipoprotein cholesterol) together with body mass index, ethnicity, measures of deprivation, family history, chronic kidney disease, rheumatoid arthritis, atrial fibrillation, diabetes mellitus, and antihypertensive treatment. The QRISK®2 algorithm has been developed by doctors and academics working in the UK National Health Service and is based on routinely collected data from many thousands of GPs across the country who have freely contributed data for medical research. It is updated annually refitted to the latest data to remain as accurate as possible. The ASCVD Risk Estimator is published jointly by the American College of Cardiology (ACC) and the American Heart Association (AHA) to help health care providers and patients estimate 10-year and lifetime risks for atherosclerotic cardiovascular disease (ASCVD) using the Pooled Cohort Equations and lifetime risk prediction tools. This app is intended as a companion tool to the 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk and the 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults. The ASCVD Risk Estimator provides easy access to recommendations specific to calculate risk estimates. Additionally, the app includes readily accessible guideline reference information for both providers and patients related to therapy, monitoring, and lifestyle. Osteoporosis is a disease characterized by low bone mass and structural deterioration of bone tissue, with a consequent increase in bone fragility and susceptibility to fracture. Many risk assessment tools are available to predict fracture incidence over a period, and these may be used to aid decision making. These tools are limited in that they may not include all risk factors, or may lack details of some risk factors. The most used tool is the Fracture Risk Assessment Tool (FRAX), it was developed using baseline and follow up data from nine prospective population-based cohorts (including Europe, Australia, Canada and Japan) and validated in 11 prospective population-based cohorts (> 1 million patient years). The FRAX’s app offers to the medical practitioner an easy-to-use tool to calculate an individual patient’s 10-year probability of an osteoporotic fracture.
Literature search applications: Literature search applications for healthcare professionals facilitate searching biomedical literature databases to find and display medical reference information, thus providing a usefull resource both for physicians and students. Some apps that we consider are PubMed plus, that connects to abstracts, citations of literature from Medline, Netter flashcard, a complete set of all Netter Plates from the 6thedition Atlas of Human Anatomy. Other literature apps for rheumatologists are American College of Rheumatology Publications and the Animated Pocket Dictionary-Rheumatology, the first one is dedicated to presenting articles of interest to researchers, physicians and health professional regarding arthritis and related disorders of the joints, muscles and bones, the second one is animated dictionary that provides definitions of medical terms with the aid of realistic and narrated 3D animations with text definitions. There is also the possibility to analize various imaging material usind Radiopedia, exploring a collection of high quality medical imaging, for all health professionals. The app useful to learn about spine condition is iAnkylosing-spondylitis in fact it contains voice and text videos introducing the symptoms, associated diseases, incidence, morbidity/mortality, genetics, diagnostic criteria, physical therapy and pharmacological management of the ankylosing spondylitis.
Medical classification prognosis & training applications: Smartphones are also used for medical training and continuing medical education (CME). CME provides training in the most current evidence-based medical practice. OsiriX HD (Di Pixmeo SARL) is a full DICOM image viewer for iOS (DICOM Files & DICOM Network protocol support) so that you can access to medical images, download and manipulate them using iPhone or iPad. It works with all imaging modalities: ultrasound, CT scanner, MRI, PET, etc. in their native standard DICOM format used by the medical/scientific industry. It’s designed to work seamlessly with any DICOM compatible software, including PACS, medical workstations, acquisition modalities. It also supports communications through the iOS built-in VPN for secure and encrypted connections. RheumaHelper is a mobile rheumatology assistant. It provides a complete toolbox of disease classification criteria the informed rheumatologist can reference during day-to-day work. Easy to use and always with you on mobile phone, all the included classifications and disease activity calculators are based on referenced equations. My lupus log allows the user to record how symptoms of lupus are affecting daily life, tracking the progression of the condition, and report detailed information on symptoms to the physician. Prognosis Rheumatology it is an easy and reliable app to access information with a fun problem solving approach. It explores 16 varied clinical cases based on actual patients and update knowledge on the latest therapeutic guidelines. Providing easily accessible information and a fun problem solving approach, this app is designed to update busy physicians while being an educational tool for residents, medical students and other healthcare professionals studying for academic and licensure exams.
Applications for medical and nursing students: There are many smartphone-based applications containing primarily as educational material for medical or nursing students. They are Netter’ s Atlas of Human Anatomy, Netter’ s Anatomy Flash Cards, Rheumatology Advisor, PubMed4Hh, Oxford Handbook of Clinical Specialties, Medicine Toolkit and Radiation Calculator.
Our smartphone applications for rheumatic disease management
The health care of patients was improved associating the use of smartphone on the curative strategies, allowing more rapid decisions, a better quality on the managing of data, and a more effective way to reach outcomes. Based on the available literature and our personal experiences, we considered useful the development of some mobile phone apps, to simplify and assist the rheumatologist during his clinical practice. In the following section are described some examples of these Apps.
a) Simple Psoriatic Arthritis Screening (SiPAS)
Psoriatic arthritis (PsA) has an estimated prevalence in Italy of 0.5% in the general population (11) and the prevalence of PsA among patients with psoriasis is reported from 6% to 44% (36). To date, several screening tools have been realized to identify psoriasis patients with musculoskeletal manifestations of PsA. In this respect, recent guidelines for managing psoriatic recommend the usage of questionnaires to screen for the presence of PsA (37). Most of these screening tools have been validated in a variety of independent populations and in several clinical settings. However, the sensitivity and specificity of these instruments is well under 50% when the polyarticular forms of arthritis are excluded (38) and no Italian versions of these tools have yet been developed and validated in the dermatology and rheumatology settings. The Simple Psoriatic Arthritis Screening (SiPAS) questionnaire, is a valid and efficient, self-administered, user-friendly PsA screening tool, to screen psoriasis patients for signs and symptom of PsA, starting from the questions coming from the already existing questionnaires (39-40). The development of the SiPAS followed multiple major steps: identification of a specific patient population, item pool development, item reduction, internal consistency, pre-testing of the prototype instrumenta validation study (Figure 1A).
Figure 1.
(A) Simple Psoriatic Arthritis Screening (SiPAS) and (B) Detection of arthritis in Inflammatory bowel diseases (DETAIL) calculators
b) Detection of arthritis in inflammatory bowel diseases (DETAIL) calculator
The presence of an inflammatory arthropathy is the commonest extra-intestinal manifestation in patient suffering from an inflammatory bowel disease (IBD), involving from the 4% to the 23% of the subjects, classified in the context of SpA (41-44). We developed a new self-administered screening tool, called DETection of Arthritis in Inflammatory boweL diseases (DETAIL) tool, in patients suffering from IBD not previously diagnosed as having a SpA (Figure 1B). One-hundred and twenty-eight patients were tested with the DETAIL questionnaire in the gastroenterology setting. After the rheumatologic assessment, in 21 (16.4%) subjects was diagnosed a SpA according to the ASAS classification criteria. Of the six items of the DETAIL questionnaire, the best positive likelihood ratio (LR+) has been found in item 2 (LR+ 3.82), exploring dactylitis, and in item 6 (LR+ 3.82) and item 5 (LR+ 3.40), two questions exploring inflammatory low back pain. Enthesitis (item 3 – LR+ 2.87) and peripheral synovitis (item 1 – LR+ 2.81) gave similar results, while item 4, exploring the duration of low back pain, resulted in the worst performance (LR+ 1.99). Three of the six items answered in affirmative way gave a post-test probability ≥75%.
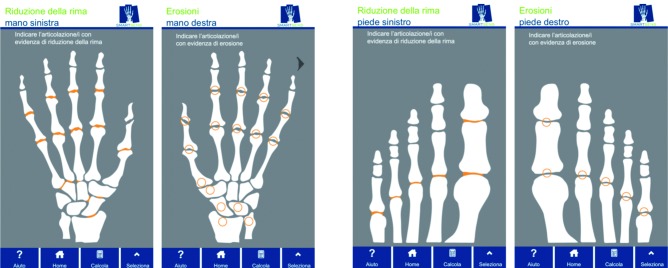
c) Simplified Erosion and Narrowing Score (SENS)
Rheumatoid Arthritis (RA) is a chronic systemic disease of unknown origin that, predominantly, involves synovial tissue. RA affects 0.5% of the global population, with a clear predilection for women. Conventional radiography (plain radiographs or X-rays) is the most widely used imaging technique for diagnosing and monitoring the progression of RA (45). Advanced imaging techniques (e.g. MRI, computed tomography, ultrasound, and nuclear scintigraphy), that are better suited for detecting soft-tissue inflammation are available, but they are costlier and some of them may expose the patient to higher doses of radiation. Plain film radiographs are inexpensive, easy to generate, can be compared with baseline and prospective films, and provide a permanent, reproducible record. The plain radiographs of the hands and feet can detect the features that are specific to RA such as joint space narrowing or erosions, and serial radiography can be used as a objective marker for monitoring treatment response in clinical trials, since assessing abnormalities radiologically is one of the most powerful means available to the clinical investigator for determining the effects of RA. Progression of structural damage to joints is commonly used as an outcome measure in RA and in observational studies (46, 47). Radiographic scores, such as the Sharp scoresand their modifications, are the standard semiquantitative methods for determining joint damage and its progression. The scoring time is one drawback of both Sharp method and Sharp/van der Heijde method, related to their detailed evaluation. In order to overcome these limitations, it has been developed the Simplified Erosion and Narrowing Score (called SENS), that is entirely based on the van der Heijde modification of the Sharp score (48). It exploits the same joints of hands and feet, but as opposed to applying a semiquantitative scale of 0-4 for joint space narrowing and 0-5 for erosions, the SENS simply dichotomizes (bimodal answer modality) whether an erosion is absent (score of 0) or present (score of 1), and whether joint space narrowing is absent (score of 0) or present (score of 1). The hand score per joint can, therefore, range from 0 to 2. Joint erosions are scored in 32 joints in the hands and wrists and 12 joints in the feet. JSN is scored in 30 joints in the hands and wrists and in 12 joints in the feet. Consequently, the maximum total erosion score is 44, the maximum total JSN score is 42 and the maximum total score is 86. The SENS showed a good intra- and inter-reader reliability, and is sensitive to change. Its decisive advantage is its feasibility in clinical practice (49) (Figure 2).
Figure 2.
Simplified Erosion and Narrowing Score (SENS)
d) Italian DElphi in psoriatic Arthritis (IDEA)
To create a protocol for PsA diagnosis and global assessment of patients with an algorithm based on anamnestic, clinical, laboratory and imaging procedures, we established a Delphi study on a national scale, the Italian DElphi in Psoriatic Arthritis (IDEA). After a literature search, a Delphi pool, involving 52 rheumatologists, was performed. Based on the literature search 202 potential items were identified, the steering committee planned at least two Delphi rounds. A total of 43 recommended diagnosis and assessment procedures, recognized as items, were derived by combination of the Delphi survey and two National Expert Meetings, and grouped in different areas including medical (familial and personal) history, physical evaluation, imaging tool, second level laboratory tests, disease activity measurement and extrarticular manifestations. In the context of any area, a rank was assigned for each item, by Expert Committee members in order to create the logical sequence the algorithm. The final list of recommended diagnosis and assessment procedures, by the Delphi survey and the two National Expert Meetings, was reported also as algorithm. The IDEA algorithm might lead to a multidimensional approach and could represent a useful and practical tool for addressing the diagnosis and for assessing appropriately the disease (50) (Fig. 3).
Figure 3.
Italian Delphi in psoriatic Arthritis (IDEA) tool
e) The PsAID-12 questionnaire
The overall assessment of PsA is challenging and include may domains. The European League Against Rheumatism (EULAR) developed two PsA Impact of Disease questionnaires (PsAID) including both physical and psychological domains: one for clinical practice (12 domains of health) and one for clinical trials (nine domains). ThePsAID score is developed, translated and validated across several countries; it is free of charge and fast, making it feasible and widely applicable (35). The longer questionnaire, developed for clinical practice contains components to assess 12 domains, each with 0–10 NRS that are perceived by patients to be particularly important for their health. Each domain has different weight. The final score has a range from 0 to 10 (10 worst health). The PsAID scores had satisfactory psychometric properties in an international validation study (51, 52). The touch-screen mode of administration of PsAID-12 can be a feasible and suit-able alternative to the paper-and-pencil mode for the assessment of patients with PsA (32, 52) (Figure 4). In our study 159 patients with PsA, as a part of clinical treatment, on the waiting room perfomed both the paper-and-pencil and touch screen version of PsAID-12 showing a high concordance between them (32).
Figure 4.
The PsA Impact of Disease questionnaires (PsaID)-12 questionnaire
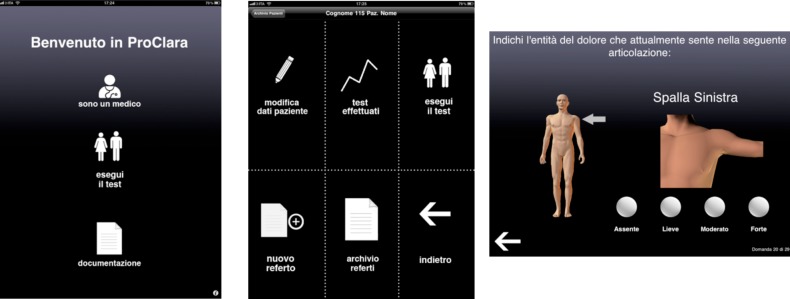
f) Patient-Reported Outcome –Clinical Arthritis Activity (PRO-CLARA) index
RA PRO-CLARA is a validated, short and easy to complete self-administered index to evaluate RA diseases activity, without formal joint counts, combining three items on patient’s physical function (as measured by Recent-Onset Arthritis Disability – ROAD – questionnaire), self-administered tender joint count (TJC) and patient global assessment (PtGA) into a single measure of disease activity (26) (Figure 5). The total score is a sum of scores of the three individual measures divided by three, and ranges from 0 to 10. The ROAD questionnaire is a reliable, valid and responsive tool for measuring physical functioning in patients with RA, and it is suitable for use in clinical trials and daily clinical practice (53-55). The self-administered TJC is evaluated according to joint list of the Rheumatoid Arthritis Disease Activity Index (RADAI). The PtGA, is scored with the following question: “Considering all the ways in which illness and health conditions may affect you now, please make a mark below to show how you are doing” on a 0-10 numerical rating scale (NRS) with very well (0) and very poorly (10) as anchors. The three 0–10 scores are added together for a raw score of 0–30, and divided by 3 to give an adjusted 0–10 score.
Figure 5.
The Patient-Reported Outcome –Clinical Arthritis Activity (PRO-CLARA) index
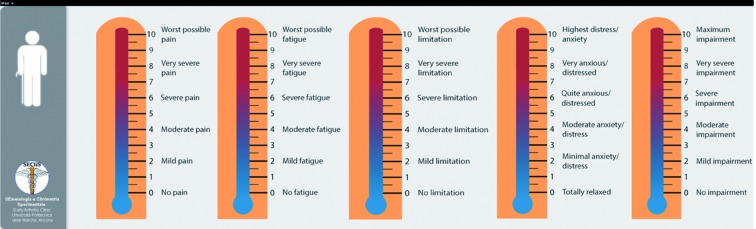
g) PROs Thermomer – 5 items scale: a brief assessment tool for rapid evaluation of rheumatic diseases in research and clinical practice
Patients with RDs conditions have been shown to suffer deficits in HRQoL along several physical functioning and mental health dimensions (5, 11, 19, 56-58). A comprehensive assessment of the multiple symptoms domains associated with RDs and their impact on multidimensional aspects of HRQoL should be a routine part of the care of patients. Clinical trials and long term clinical registries have used disparate outcome measures (59-60). We developed a PROs Thermomer – 5 items (5T-PROs) scales that measure overall health status in patients with a five-items domain (pain, fatigue, physical functioning, anxiety/depression, general health status). These five-items measures in which participants mark their subjective status on a graphic thermometer scale, afford simple and rapid administration, and increased comprehension and completion rates (Figure 6).
Figure 6.
The PROs Thermomer – 5 item scale (5T-PROs)
Conclusions
Over the past decade, smartphones have radically changed many aspects of our everyday lives, from banking to shopping to entertainment. With innovative digital technologies, cloud computing and machine learning, the medicalized smartphone is going to upend every aspect of health care. Although the current literature on the role of smartphones in RD is small, results suggest high feasibility and acceptability. However, there is currently limited data on the efficacy of smartphone apps. With further research and clinical innovation, smartphone may provide an effective means to improve access to rheumatology care. This would allow for both direct patient care by rheumatologists and support of primary care providers, who can be educated, mentored and given diagnostic and management advice.
Acknowledgements
We wish to thank Appycom s.r.l. (www.appycom.it) for the realization of the touch-screen and smartphone Apps and the technical assistance provided dur-ing the studies.
Disclosure statement: FS has attended advisory board meetings and has received speaking fees from Bristol-Myers Squibb, Abbvie, Roche, Pfizer and Janssen. SF and MDC declare that they have no conflict of interest.
References
- 1.Centers for Disease Control and Prevention: Health-related quality of life among adults with arthritis-Behavioral Risk Factor Surveillance System. 11 states, 1996-1998. Morb Mortality Wkly Rep. 2000;49:366-91–3. [PubMed] [Google Scholar]
- 2.Reginster J-Y. The prevalence and burden of arthritis. Rheumatology. 2002;41(suppl. 1):3–6. [PubMed] [Google Scholar]
- 3.March L. Smith EU. Hoy DG, et al. Burden of disability due to musculoskeletal (MSK) disorders. Best Pract Res Clin Rheumatol. 2014;28(3):353–66. doi: 10.1016/j.berh.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Al Maini M. Adelowo F. Al Saleh J, et al. The global challenges and opportunities in the practice of rheumatology: white paper by the World Forum on Rheumatic and Musculoskeletal Diseases. Clin Rheumatol. 2015;34(5):819–29. doi: 10.1007/s10067-014-2841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Picavet HSJ. Hazes JMW. Prevalence of self-reported musculoskeletal diseases is high. Ann Rheum Dis. 2003;62:644–504. doi: 10.1136/ard.62.7.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrianakos A. Trontzas P. Christoyannis F, et al. Prevalence of rheumatic diseases in Greece: a cross-sectional population based epidemiological study. The ESORDIG study. J Rheumatol. 2003;30:1589–601. [PubMed] [Google Scholar]
- 7.Carmona L. Ballina J. Gabriel R. Laffon A on behalf of the EPISER Study Group. The burden of musculoskeletal diseases in the general population of Spain: results from a national survey. Ann Rheum Dis. 2001;60:1040–5. doi: 10.1136/ard.60.11.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laine V. Rheumatic complaints in an urban population in Finland. Acta Rheumatol Scand. 1962;8:81–8. doi: 10.3109/rhe1.1962.8.issue-1-4.09. [DOI] [PubMed] [Google Scholar]
- 9.Hagen KB. Bjorndal A. Uhlig T. Kvien TK. A population study of factors associated with general pratictioner consultation for non-inflammatory musculoskeletal pain. Ann Rheum Dis. 2000;59:788–93. doi: 10.1136/ard.59.10.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urwin M. Symmons D. Allison T, et al. Estimating the burden of musculoskeletal disorders in the community: the comparative prevalence of symptoms at different anatomical sites, and the relation to social deprivation. Ann Rheum Dis. 1998;57:649–55. doi: 10.1136/ard.57.11.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salaffi F. De Angelis R. Grassi W. MArche Pain Prevalence; INvestigation Group (MAPPING) study. Prevalence of musculoskeletal conditions in an Italian population sample: results of a regional community-based study. I. The MAPPING study. Clin Exp Rheumatol. 2005;23(6):819–28. [PubMed] [Google Scholar]
- 12.Vos T. Flaxman AD. Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2013;380(9859):2163–6. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baxter AJ. Brugha TS. Erskine HE. Scheurer RW. Vos T. Scott JG. The epidemiology and global burden of autism spectrum disorders. Psychol Med. 2014;45:1–13. doi: 10.1017/S003329171400172X. [DOI] [PubMed] [Google Scholar]
- 14.Cross M. Smith E. Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323–30. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 15.Driscoll T. Jacklyn G. Orchard J, et al. The global burden of occupationally related low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73:975–81. doi: 10.1136/annrheumdis-2013-204631. [DOI] [PubMed] [Google Scholar]
- 16.Hoy DG. Smith E. Cross M, et al. The global burden of musculoskeletal conditions for 2010: an overview of methods. Ann Rheum Dis. 2014;73:982–89. doi: 10.1136/annrheumdis-2013-204344. [DOI] [PubMed] [Google Scholar]
- 17.Whiteford HA. Degenhardt L. Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–86. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- 18.Woolf AD. Pfleger B. Burden of major musculoskeletal conditions. Bulletin World Health Organization. 2003;81:646–56. [PMC free article] [PubMed] [Google Scholar]
- 19.Salaffi F. De Angelis R. Stancati A. Grassi W. Marche Pain. Prevalence Investigation Group (MAPPING) study. Health-related quality of life in multiple musculoskeletal conditions: a cross-sectional population based epidemiological study. II. The MAPPING study. Clin Exp Rheumatol. 2005;23(6):829–39. [PubMed] [Google Scholar]
- 20.Parker L. Moran GM. Roberts LM. Calvert M. McCahon D. The burden of common chronic disease on health-related quality of life in an elderly community-dwelling population in the UK. Fam Pract. 2014;31(5):557–63. doi: 10.1093/fampra/cmu035. [DOI] [PubMed] [Google Scholar]
- 21.Brettschneider C. Leicht H. Bickel H, et al. MultiCare Study Group. Relative impact of multimorbid chronic conditions on health-related quality of life - results from the MultiCare Cohort Study. PloSOne. 2013;8(6):e66742. doi: 10.1371/journal.pone.0066742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoels M. Wong J. Scott DL, et al. Economic aspects of treatment options in rheumatoid arthritis: a systematic literature review informing the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2010;69:995–1003. doi: 10.1136/ard.2009.126714. [DOI] [PubMed] [Google Scholar]
- 23.Hallinen TA. Soini E. Eklund K. Puolakka K. Cost–utility of different treatment strategies after the failure of tumour necrosis factor inhibitor in rheumatoid arthritis in the Finnish setting. Rheumatology. 2010;49:767–77. doi: 10.1093/rheumatology/kep425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sprangers MA. de Regt EB. Andries F, et al. Which chronic conditions are associated with better or poorer quality of life? J Clin Epidemiol. 2000;53:895–907. doi: 10.1016/s0895-4356(00)00204-3. [DOI] [PubMed] [Google Scholar]
- 25.Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. >Lancet. 2015, 22;386(9995):743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salaffi F. Migliore A. Scarpellini M, et al. Psychometric properties of an index of three patient reported outcome (PRO) measures, termed the CLinical ARthritis Activity (PRO-CLARA) in patients with rheumatoid arthritis. The NEW INDICES study. Clin Exp Rheumatol. 2010;28:186–200. [PubMed] [Google Scholar]
- 27.Linde L. Sørensen J. Ostergaard M. Hørslev-Petersen K. Hetland Ml. Health-related quality of life: validity, reliability, and responsiveness of SF-36, 15D, EQ-5D, RAQoL, and HAQ in patients with rheumatoid arthritis. J Rheumatol. 2008;35:1528–37. [PubMed] [Google Scholar]
- 28.Gossec L. Dougados M. Rincheval N, et al. Elaboration of the preliminary Rheumatoid Arthritis Impact of Disease (RAID) score: a EULAR initiative. Ann Rheum Dis. 2009;68:1680–5. doi: 10.1136/ard.2008.100271. [DOI] [PubMed] [Google Scholar]
- 29.Kalyoncu U. Dougados M. Daurès Jp. Gossec L. Reporting of patient-reported outcomes in recent trials in rheumatoid arthritis: a systematic literature review. Ann Rheum Dis. 2009;68:183–90. doi: 10.1136/ard.2007.084848. [DOI] [PubMed] [Google Scholar]
- 30.Salaffi F. Gasparini S. Grassi W. The use of computer touch-screen technology for the collection of patient-reported outcome data in rheumatoid arthritis: comparison with standardized paper questionnaires. Clin Exp Rheumatol. 2009;27(3):459–68. [PubMed] [Google Scholar]
- 31.Salaffi F. Gasparini S. Ciapetti A. Gutierrez M. Grassi W. Usability of an innovative and interactive electronic system for collection of patient-reported data in axial spondyloarthritis: comparison with the traditional paper-administered format. Rheumatology (Oxford) 2013;52(11):2062–70. doi: 10.1093/rheumatology/ket276. [DOI] [PubMed] [Google Scholar]
- 32.Salaffi F. Di Carlo M. Carotti M. Farah S. Gutierrez M. The Psoriatic Arthritis Impact of Disease 12-item questionnaire: equivalence, reliability, validity, and feasibility of the touch-screen administration versus the paper-and-pencil version. Ther Clin Risk Manag. 2016;12:631–42. doi: 10.2147/TCRM.S101619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salaffi F. Carotti M. Ciapetti A, et al. Effectiveness of a telemonitoring intensive strategy in early rheumatoid arthritis: comparison with the conventional management approach. BMC Musculoskelet Disord. 2016;2(17):146. doi: 10.1186/s12891-016-1002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salaffi F. Ciapetti A. Gasparini S. Atzeni F. Sarzi-Puttini P. Baroni M. Web/Internet-based telemonitoring of a randomized controlled trial evaluating the time-integrated effects of a 24-week multicomponent intervention on key health outcomes in patients with fibromyalgia. Clin Exp Rheumatol. 2015;33(1 Suppl 88):S93–101. [PubMed] [Google Scholar]
- 35.Fagan TJ. Nomogram for Bayes’s theorem. N Engl J Med. 1975;293(5):257. doi: 10.1056/NEJM197507312930513. [DOI] [PubMed] [Google Scholar]
- 36.Salvarani C. Lo Scocco G. Macchioni P, et al. Prevalence of psoriatic arthritis in Italian psoriatic patients. J Rheumatol. 1995;22:1499–1503. [PubMed] [Google Scholar]
- 37.Canete JD. Dauden E. Queiro R, et al. Recommendations for the coordinated management of psoriatic arthritis by rheumatologists and dermatologists: a Delphi study. Actas Dermosifiliogr. 2014;105:216–32. doi: 10.1016/j.adengl.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 38.Walsh JA. Callis DK. Krueger GG. Clegg DO. Limitations in screening instruments for psoriatic arthritis: a comparison of instruments in patients with psoriasis. J Rheumatol. 2013;40:287–93. doi: 10.3899/jrheum.120836. [DOI] [PubMed] [Google Scholar]
- 39.Salaffi F. Di Carlo M. Bugatti L. Lato V. Nicolini M. Carotti M. Development and pilot-testing of a new tool to screen psoriasis patients for the presence of psoriatic arthritis: the Simple Psoriatic Arthritis Screening (SiPAS) questionnaire. JEADV. 2016;31(5):e167–9. doi: 10.1111/jdv.13902. [DOI] [PubMed] [Google Scholar]
- 40.Salaffi F. Di Carlo M. Lucchetti M, et al. A new tool to screen psoriasis patients for the presence of psoriatic arthritis:validation of the Simple Psoriatic Arthritis Screening (SiPAS) questionnaire. Clin Exp Rheumatol. 2017;(in press) [PubMed] [Google Scholar]
- 41.D’Incà R. Podswiadek M. Ferronato A. Punzi L. Salvagnini M. Sturniolo GC. Articular manifestations in inflammatory bowel disease patients: a prospective study. Dig Liv Dis. 2009;41:565–9. doi: 10.1016/j.dld.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 42.Rudwaleit M. van der Heijde D. Landewe R, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68:777–83. doi: 10.1136/ard.2009.108233. [DOI] [PubMed] [Google Scholar]
- 43.Feldtkeller E. Khan MA. van der Heijde D. van der Linden S. Braun J. Age at disease onset and diagnosis delay in HLA- B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int. 2003;23:61–6. doi: 10.1007/s00296-002-0237-4. [DOI] [PubMed] [Google Scholar]
- 44.Sieper J. Srinivasan S. Zamani O, et al. Comparison of two referral strategies for diagnosis of axial spondyloarthritis: the Recognising and Diagnosing Ankylosing Spondylitis Reliably (RADAR) study. Ann Rheum Dis. 2013;72:1621–7. doi: 10.1136/annrheumdis-2012-201777. [DOI] [PubMed] [Google Scholar]
- 45.Salaffi F. Carotti M. Di Carlo M.Conventional radiology in rheumatoid arthritis: new scientific insights and practical application. Int J Clin Exp Med. 2016;9(9):17012–27. [Google Scholar]
- 46.Salaffi F. Ferraccioli G. Peroni M. Carotti M. Bartoli E. Cervini C. Progression of erosion and joint space narrowing scores in rheumatoid arthritis assessed by nonlinear models. J Rheumatol. 1994;21(9):1626–30. [PubMed] [Google Scholar]
- 47.Salaffi F. Carotti M. Ciapetti A. Gasparini S. Filippucci E. Grassi W. Relationship between time-integrated disease activity estimated by DAS28-CRP and radiographic progression of anatomical damage in patients with early rheumatoid arthritis. BMC Musculoskelet Disord. 2011;12:120. doi: 10.1186/1471-2474-12-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van der Heijde D. Dankert T. Nieman F. Rau R. Boers M. Reliability and sensitivity to change of a simplification of the Sharp/van der Heijde radiological assessment in rheumatoid arthritis. Rheumatology (Oxford) 1999;38:941–7. doi: 10.1093/rheumatology/38.10.941. [DOI] [PubMed] [Google Scholar]
- 49.Barnabe C. Hazlewood G. Barr S. Martin L. Comparison of radiographic scoring methods in a cohort of RA patients treated with anti-TNF therapy. Rheumatology. 2012;51:878–81. doi: 10.1093/rheumatology/ker418. [DOI] [PubMed] [Google Scholar]
- 50.Lapadula G. Marchesoni A. Salaffi F, et al. Evidence-based algorithm for diagnosis and assessment in psoriatic arthritis: results by Italian DElphi in psoriatic Arthritis (IDEA) Reumatismo. 2016;68(3):126–36. doi: 10.4081/reumatismo.2016.913. [DOI] [PubMed] [Google Scholar]
- 51.Gossec L. de Wit M. Kiltz U, et al. EULAR PsAID Taskforce. A patient-derived and patient-reported outcome measure for assessing psoriatic arthritis: elaboration and preliminary validation of the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire, a 13-country EULAR initiative. Ann Rheum Dis. 2014;73(6):1012–9. doi: 10.1136/annrheumdis-2014-205207. [DOI] [PubMed] [Google Scholar]
- 52.Di Carlo M. Becciolini A. Lato V. Crotti C. Favalli EG. Salaffi F. The 12-item Psoriatic Arthritis Impact of Disease Questionnaire: Construct Validity, Reliability, and Interpretability in a Clinical Setting. J Rheumatol. 2017;44(3):279–85. doi: 10.3899/jrheum.160924. [DOI] [PubMed] [Google Scholar]
- 53.Salaffi F. Carotti M. Gutierrez M. Di Carlo M. De Angelis R. Patient Acceptable Symptom State in Self-Report Questionnaires and Composite Clinical Disease Index for Assessing Rheumatoid Arthritis Activity: Identification of Cut-Off Points for Routine Care. Biomed Res Int. 2015;2015:930756. doi: 10.1155/2015/930756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salaffi F. Bazzichi L. Stancati A, et al. Development of a functional disability measurement tool to assess early arthritis: the Recent-Onset Arthritis Disability (ROAD) questionnaire. Clin Exp Rheumatol. 2005;23(5):628–36. [PubMed] [Google Scholar]
- 55.Salaffi F. Stancati A. Neri R. Grassi W. Bombardieri S. Measuring functional disability in early rheumatoid arthritis: the validity, reliability and responsiveness of the Recent-Onset Arthritis Disability (ROAD) index. Clin Exp Rheumatol. 2005;23(5 Suppl 39):S31–42. [PubMed] [Google Scholar]
- 56.Salaffi F. Cimmino MA. Malavolta N, et al. The burden of prevalent fractures on health-related quality of life in postmenopausal women with osteoporosis: the IMOF study. J Rheumatol. 2007;34(7):1551–60. [PubMed] [Google Scholar]
- 57.Salaffi F. Sarzi-Puttini P. Girolimetti R. Atzeni F. Gasparini S. Grassi W. Health-related quality of life in fibromyalgia patients: a comparison with rheumatoid arthritis patients and the general population using the SF-36 health survey. Clin Exp Rheumatol. 2009;27(5 Suppl 56):67–74. [PubMed] [Google Scholar]
- 58.Van Tuyl LH. Boers M. Patient-reported outcomes in core domain sets for rheumatic diseases. Nat Rev Rheumatol. 2015;11(12):705–12. doi: 10.1038/nrrheum.2015.116. [DOI] [PubMed] [Google Scholar]
- 59.Idzerda L. Rader T. Tugwell P. Boers M. Can we decide which outcomes should be measured in every clinical trial? A scoping review of the existing conceptual frameworks and processes to develop core outcome sets. J Rheumatol. 2014;41(5):986–93. doi: 10.3899/jrheum.131308. [DOI] [PubMed] [Google Scholar]
- 60.Tugwell PS. Petersson IF. Boers M, et al. Domains selection for patient-reported outcomes: current activities and options for future methods. J Rheumatol. 2011;38(8):1702–10. doi: 10.3899/jrheum.110389. [DOI] [PubMed] [Google Scholar]