Abstract
Aim: To analyze clinical characteristics associated with the occurrence of diabetic ketoacidosis (DKA) at the onset of type 1 diabetes (T1D) in children aged <5 years in order to identify early signs or symptoms useful to prevent DKA appearance. Methods: Data of patients with newly diagnosed TID aged <5 years (Group 1) and 6-10 years old (Group 2) coming from the province of Parma were collected in the period 2012-2016. Results: Mild/moderate ketoacidosis at diabetes diagnosis occurred more frequently in Group 1 than in Group 2 patients (p<0.0015). Severe DKA incidence was higher in children below 5 (21.8%) than in those over 5 years of age (3.75%; p=0.021). Latent period before overt T1D diagnosis was longer in Group 1 than in Group 2 patients (p=0.0081). During this latent period similar indicators were recorded among parents of children <3 years old: frequent use of disposable baby diapers (87%), wet baby diapers because of a large amount of urine (86%), body weight loss (79%). In children aged 3-4 years reported symptoms consisted of polyuria (89%), polydipsia (79%), fatigue (72%). In Group 2 patients predominant signs concern unusual episodes of enuresis. Conclusion s : We believe that it is time to launch a DKA prevention campaign tailored for children under 5 years old and focused just on the above-mentioned three warning signs. Information program must involves pediatricians, pediatric nurses, new moms and nursery school teachers. (www.actabiomedica.it)
Keywords: diabetic ketoacidosis, type 1 diabetes, young children, DKA prevention, baby diapers, pediatrics, polyuria, polydipsia
Introduction
Diabetic ketoacidosis (DKA) is serious complication of diabetes at diagnosis more frequently in children aged <5 years than in older subjects (1, 2). This unacceptable high prevalence conflicts with the expectation of a lowering in the occurrence of DKA, given awareness campaigns everywhere promoted to recognize the earliest symptoms of T1D at onset in childhood (3-6).
In the present paper we analyzed clinical characteristics of DKA occurring at the time of diabetes onset in children aged under 5 years in an area where a capillary awareness campaign produced an important and persistent decrease in rate of DKA in newly diagnosed diabetic children over 5 years old (3, 7, 7). The purpose of this study was to verify whether it was possible to detect in the preclinical history of younger children with newly diagnosed diabetes signs or symptoms useful to prevent the appearance of DKA.
Patients and methods
Data investigation was performed among children with newly diagnosed TID aged < 5 years (Group 1) and 6-10 years old (Group 2) coming from the same province of Parma, admitted to Children Hospital “Pietro Barilla”, University Hospital of Parma, Italy, from 1st January 2012 to 31st December 2016. The province of Parma is located in Northern Italy where a campaign for DKA prevention has been launched since the Nineties according the procedures published elsewhere (3, 7, 7).
Data for this study were collected from medical files of each patient and included: age, gender, blood glucose, capillary pH, 3-beta-hydroxybutyrate (3HB) and glycated hemoglobin (HbA1c) levels, information about symptoms reported by the parents during the days preceding the overt T1D diagnosis. ISPAD criteria were used to define DKA: absent (pH ≥7.30), mild (7.2≤ pH <7.30), moderate (7.1≤ pH <7.20) and severe (pH <7.1) (9). As the clinical implications for mild and moderate DKA are similar, they are considered together in this paper. 3HB serum levels were tested on a fingerstick blood specimen by a hand-held device (Medisens Optium Xceed, Abbott Laboratories, Bedford, MA, USA). 3HB serum levels <0.5 mmol/dl were defined as normal; levels exceeding 1 mmol/dl were retained as hyperketonemia or ketosis; and levels in excess of 3.0 mmol/dl were classified as ketoacidosis (10). HbA1c levels were measured by Bayer DCA 2000 method (upper limit of normal value: 6.0 %).
The study was performed according to the criteria of the Helsinki II Declaration. Tutors or parents of all patients admitted to our department are accustomed to sign an informed consent document for the use of clinical data regarding their children for scientific purposes only. No conflict of interest exists in relation to the subject matter of the present paper.
Statistics
Data were summarized as numbers (n) and frequencies (%) if they were categorical and as mean and standard deviation (SD) if quantitative. If the data were normally distributed a two-tailed unpaired T-test or otherwise a non-parametric Mann-Whitney U-Test was applied to compare results between groups. Chi-square test (ξ2) or Fisher exact test was used to compare frequencies between groups. P-values less than 0.05 were considered as statistically significant.
Results
From 1st January 2012 to 31st December 2016, 135 children aged 1 to 18 years were admitted to the University Children Hospital of Parma, Italy, with newly recognized T1D. Sixty of these children (44.4%) were aged <10 years and came from the province of Parma: 32 patients <5 years old were recruited in the Group 1 and 28 (aged 6-10 years) in the Group 2
Characteristics of Groups patients at T1D diagnosis
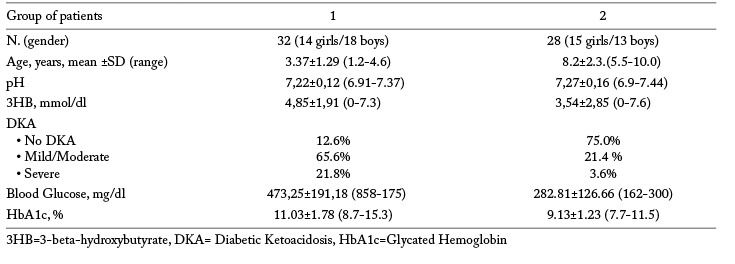
Group 1 patients – Patients gathered in this Group had a mean age of 3.37±1.29 (range:1.2-4.6) years; 18 were males (56.2%) (Table 1). Twenty-one patients (65.6%) had a mild- moderate DKA and 7 patients (21.8%) a severe DKA. The remaining 4 patients did not have DKA (12.6%). Patients without DKA were 4.1-4.6 years old; those with a mild/moderate form of DKA were aged 1.9-2.8 years; and the seven patients with severe DKA had 2.2 and 2.9 years. Blood glucose average was 473,25±191,18 (858-175) mg/dl. Mean HbA1c levels were 11.03±1.78 (8.7-15.3) %. 3HB mean levels was 4,85±1,91 (0-7.3) mmol/dl. Only 7 patients (21.8%) had 3HB levels <1 mmol/dl. Analyzing the frequency of DKA over a span of 5 years, no statistically significant time trend was observed (p<0.243).
Table 1.
Characteristics of study participants at T1D diagnosis
The duration of hyperglycemia-related symptoms before T1D diagnosis was quantified in 12.0±8.0 days according to what the parents reported at hospital admittance. During this latent period parents reported among children <3 years old: frequent use of disposable baby diapers (87%), wet baby diapers because of a large amount of urine (86%), body weight loss (79%), increased thirst and polyuria (76 %). In children aged 3-4 years reported symptoms consisted of polyuria (89%), polydipsia (79%), fatigue (72%).
None parent of Group 1 patients was aware of the existence of a campaign for early diagnosis of T1D and DKA prevention. Parents consulted family pediatrician only at weight loss detection, and did not inform the pediatrician about other symptoms.
Group 2 patients – Patients collected in this Group (13 males, 46.4%) had a mean age of 8.2±2.3.(range: 5.5-10.0) years (Table 1). Twenty-one (75.0%) patients did not have DKA, in 6 patients (21.4%) DKA was mild/moderate, in 1 patient DKA was severe (3.6%). Patients without DKA were 6.6-9.6 years old; patients with a mild/moderate form of DKA were aged 7.9-10 years. Mean blood glucose and HbA1c levels were 282.81±126.66 (162-300) mg/dl and 9.13±1.23 (7.7-11.5) %, respectively. 3HB mean levels were 3,54±1,85 (0-6.6) mmol/dl. Twelve patients (42.8%) had 3HB values <1 mmol/dl.
All parents of children recruited in this Group reported to know that a campaign for early T1D diagnosis was launched in their province. The duration of symptoms before overt T1D diagnosis was estimated in 6.4±1.5 days among children without DKA. Seventy-four percent of parents of these children reported that recurrent episodes of enuresis appeared a few days before, associated with frequent thirst at night (78%), polyuria (75%) and polydipsia (64%). In 18 of 28 patients (64.2%) showing unusual enuresis, the diagnosis of T1D was performed by the family pediatrician directly in his private office by capillary measurement of blood glucose. In the remaining 10 patients (35.7%) the pediatrician underestimated reported symptoms. Remembering the messages about early symptoms of T1D listed in the posters displayed in town pharmacies or at school, the parents of these children consulted spontaneously the Pediatric Emergency Department at Children Hospital of Parma where the diagnosis of T1D was quickly performed.
Comparison between two Groups of patients
During the study period, mild/moderate ketoacidosis at diabetes diagnosis occurred more frequently in Group 1 than in Group 2 patients (p<0.0015). Severe DKA incidence was higher in children below 5 (21.8%) than in those over 5 years of age (3.75%; p=0.021). Children without DKA were more numerous in Group 2 than in Group 1 (p=0.0001). Blood glucose and HbA1c levels were higher in patients of Group 1 than in those of Group 2 (t=2.54; p= 0.016 and t= 3.54; p=0.0012 respectively). Group 2 patients showed lower values of 3HB than Group 1 patients (t= 3.9; p=0.0002). The patients with 3HB levels <1 mmol/l were more numerous in Group 2 than in Group1 but not significantly (t=3.4; p=0.08). Latent period before overt T1D diagnosis was shorter in Group 2 than in Group 1 patients (t= 2.83; p=0.0081).
Discussion
In previous studies we demonstrated that through an educational campaign it is possible to achieve and maintain over time a marked decrease in DKA frequency at diabetes onset in children over 5 years old (3, 7, 7). The findings reported in the present study showed that the goal is still reachable in this children population, but in younger children the same target seems to be still very far to be obtained, even in a province where a DKA information program for teachers, students, parents and pediatricians has been introduced since the Nineties (3).
In analyzing the medical files of children under 5 years of age recruited in this study, we found that DKA frequency occurred with a percentage (65.6%) close to that we reported in older children during the pre-campaign period (3). Among young children severe or mild DKA happened more frequently in those under 3 years old, in agreement with previous reports (11, 12). The same children showed also higher HbA1c and 3HB levels than older patients, features which may explain the long delay in their disease recognition. An optimistic finding was that in 4 patients of the same Group, diabetes has been diagnosed before the appearance of DKA. The diagnosis was performed occasionally because of suspected urinary tract infection facing numerous diapers consumed at home.
Conversely the great majority of patients aged over 5 years had a diabetes uncomplicated by DKA and a short latent period before diagnosis, as proved also by lower HbA1c and 3BH levels. These results may be ascribed to the information campaign promoted for DKA prevention in the area where the study was carried out (3, 7, 7). As expected, unusual enuresis episodes confirmed to be also in this investigation a reliable warning symptoms to promptly diagnose T1D at onset, and to maintain DKA rate low.
It has been reported that greater DKA incidence at D1T onset together with less maturity of metabolic systems combine to predispose younger children to DKA-related complications, e.g. cerebral edema which occurs in about 1% of all episodes of DKA with a high mortality and morbidity rate (13-15). It is not the case of our patients, but given the scenario described in literature, we agree on the opinion of those Authors who sustain that every case of diabetes at onset in a child aged under 5 years has to be regarded as an emergency event (16).
We warmly believe that a new information strategy has to be urgently implemented in order to spread the awareness of early symptoms of a latent hyperglycemia status also in this vulnerable young population. We are aware that early signs or symptoms of an ongoing T1D are not easy to identify in children under 5 years of age (17). We are equally conscious that the appearance of unexpected episodes of enuresis in a child usually “dry” on which we have successfully based the DKA prevention campaign in Nineties (3, 7, 7) cannot involve children whose bladders are still developing.
Among signs observed in younger children, we would like to focus attention on at least three underestimated signs that could be attributable to a latent hyperglycemia status reported in the weeks before overt T1D diagnosis: frequent use of disposable baby diapers (87%), baby diapers abnormally wet due a large amount of urine (86%), body weight loss (79%). All these signs can be linked to a relative insulin deficiency that resulted in hyperglycemia and lipolysis. The combination of hyperglycemia and lipolysis caused osmotic diuresis, dehydration and weight loss. Among these three signs only body weight loss convinced the parents to consult a pediatrician, not reporting the other two more precocious signs. This shows how much moms are sensitive to the weight growth of their children. We believe that this defect in communication has influenced the delay in T1D diagnosis in these children.
We feel it is time to launch a new DKA prevention campaign centered on children under 5 years old and focused just on the above-mentioned three warning signs that over 80% of parents have reported as early signs of the metabolic derangement that was silently happening in their children. This information program must involve pediatricians, pediatric nurses, new moms and nursery school teachers.
During routine checkup visits, it should be good practice that primary care pediatricians and nurses query the moms on how many diapers change daily, and sensitize the same moms on the need to immediately alert the pediatrician if the diapers consumption increases in an unexpected way, and first of all if diapers become abnormally wet due to a large amount of urine. The same recommendations have to be extended to nursery school teachers because they work alongside children for many hours a day, having so the opportunity to perceive an unusual polyuria.
In conclusion, we can debate if the pediatricians have to be equipped with devices for the measurement of capillary blood glucose and glycosuria, supplies for finger pricking, reagents strips and reflectance in order to verify on real time reported signs and symptoms directly in their office. Given the experience made with “Parma campaign” in the Nineties we would be favorable of providing this equipment so useful to shorten the delay in diagnosis and hospitalization, as currently proved also in the present study.
References
- 1.Gale EA. The rise of childhood type 1 diabetes in the 20th century. Diabetes. 2002;51:3353–3361. doi: 10.2337/diabetes.51.12.3353. [DOI] [PubMed] [Google Scholar]
- 2.Bui H. To T. Stein R, et al. Is diabetic ketoacidosis at disease onset a result of missed diagnosis? J Pediatr. 2010;156:472–477. doi: 10.1016/j.jpeds.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 3.M Vanelli. G Chiari. L Ghizzoni, et al. Effectiveness of a prevention program for diabetic ketoacidosis in children. An 8-year study in schools and private practices. Diabetes Care. 1999;22(1):7–9. doi: 10.2337/diacare.22.1.7. [DOI] [PubMed] [Google Scholar]
- 4.King BR. Howard NJ. Verge CF, et al. A diabetes awareness campaign prevents diabetic ketoacidosis in children at their initial presentation with type 1 diabetes. Pediatr Diabetes. 2012;13(8):647–51. doi: 10.1111/j.1399-5448.2012.00896.x. [DOI] [PubMed] [Google Scholar]
- 5.Choleau C. Une campagne nationale lancée en novembre 2010 qui montre ces premiers effets et qui se poursuit pour l’ année 2012. AJD. 2011;58(4):18–19. [Google Scholar]
- 6.Deylami R. Townson J. Mann M. Gregory JW. Systematic review of publicity interventions to increase awareness amongst healthcare professionals and the public to promote earlier diagnosis of type 1 diabetes in children and young people. Pediatric Diabetes. 2017 August;7:1–8. doi: 10.1111/pedi.12565. [DOI] [PubMed] [Google Scholar]
- 7.Vanelli M. Chiari G. Lacava S. Iovane B. Campaign for diabetic ketoacidosis prevention still effective 8 years later. Diabetes Care. 2007 Apr;30(4):e12. doi: 10.2337/dc07-0059. [DOI] [PubMed] [Google Scholar]
- 8.Cangelosi AM. Bonacini I. Serra RP, et al. Spontaneous Dissemination of DKA Prevention Campaign Successfully Launched in Nineties in Parma’s Province. Acta Biomed. 2017 doi: 10.23750/abm.v88i2.6553. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolfsdorf JI. Allgrove J. Craig M, et al. ISPAD clinical practice consensus guidelines 2014. Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes. 2014;15:154–79. doi: 10.1111/pedi.12165. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell GA. Kassovska-Bratinova S. Boukaftane Y, et al. Medical aspects of ketone body metabolism. Clin Invest Med. 1995;18:193–216. [PubMed] [Google Scholar]
- 11.Choleau C. Maitre J. Filipovic Pierucci A, et al. Ketoacidosis at diagnosis of type 1 diabetes in French children and adolescents. Diabetes Metab. 2014;40:137–142. doi: 10.1016/j.diabet.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Szypowska A. Dżygało K. Wysocka-Mincewicz M, et al. High incidence of diabetic ketoacidosis at diagnosis of type 1 diabetes among Polish children aged 10-12 and under 5 years of age: A multicenter study. Pediatr Diabetes. 2017;18:722–728. doi: 10.1111/pedi.12446. [DOI] [PubMed] [Google Scholar]
- 13.Edge JA. Hawkins MM. Winter DL. Dunger DB. The risk and outcome of cerebral oedema developing during diabetic ketoacidosis. Arch Dis Child. 2001;85:16–22. doi: 10.1136/adc.85.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawrence SE. Cummings EA. Gaboury I. Daneman D. Population-based study of incidence and risk factors for cerebral edema in pediatric diabetic ketoacidosis. J Pediatr. 2005;146:688–692. doi: 10.1016/j.jpeds.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 15.Wolfsdorf DJ. Glaser N. Sperling MA. Diabetic Ketoacidosis in Infants, Children, and Adolescents. Diabetes Care. 2006;29:1150–59. doi: 10.2337/diacare.2951150. [DOI] [PubMed] [Google Scholar]
- 16.Neu A. Hofer Se. Karges B, et al. 3 Ketoacidosis at Diabetes Onset Is Still Frequent in Children and Adolescents. Diabetes Care. 2009;32:1647–1648. doi: 10.2337/dc09-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knip M. Natural course of preclinical type 1 diabetes. Horm Res. 2002;57(Suppl 1):6–11. doi: 10.1159/000053305. [DOI] [PubMed] [Google Scholar]


