Abstract
Excess iron deposition in patients with beta thalassemia major (BTM) causes excess free radical formation, damages the hypothalamic pituitary testicular axis and production of sperms with DNA defects. As antioxidants were reported to improve fertility in healthy males; their effectiveness to improve sperm DNA defects in adult males with BTM was studied. Twenty fully pubertal BTM patients were included consecutively, all had semen analysis; 10 were found to be azoospermic, so further analysis for sperms and DNA defects was conducted on the remaining 10 participants. Semen was analyzed for antioxidants in seminal plasma and sperms for defects including the DNA fragmentation index, sperm deformity index, teratozospermia index and acrosomal index. Participants were then given L-carnitine and N-acetylcysteine for 6 months. All semen parameters were reassessed after treatment. The sperm deformity index and teratozospermia index increased significantly after treatment from 1.90±0.33 to 2.46±0.61 and from 1.59±0.22 to 1.86±0.28 respectively. So, apparently antioxidants accentuated sperm deformities in men with BTM. Therefore, the results of this study are not in favour with the use of antioxidants in BTM patients for improving potential fertility. Larger studies, however, are needed to confirm these preliminary results. (www.actabiomedica.it)
Keywords: thalassemia major, sperm analysis, antioxidants, fertility
Introduction
The improved care of patients with β-thalassemia major (BTM) poses a challenge for endocrinologists regarding their fertility potential once they reach adulthood. Hypogonadotropic hypogonadism affects 80-90% of BTM patients worldwide (1) which is largely mediated by iron deposition and generation of reactive oxygen species (ROS) (2). Oxidative stress affects the hypothalamic-pituitary-gonadal axis in a dose-dependent manner (3). The detrimental effects of ROS on sperm membrane, its structural components and nucleus are also well documented (2). Sperms are particularly vulnerable to oxidant mediated injury due to their high content of unsaturated fatty acids enriching their lipid membrane. This oxidant mediated injury, once initiated, will self-propagate into a vicious cycle culminating into loss of functionality and sperm death (4).
Functional sperm defects are by far the most common cause of human infertility identified till now (4). Only few studies addressed sperm DNA damage in patients with BTM (2, 5). It was thought to be solely related to iron overload (3) with recent evidence that desferrioxamine may have detrimental effect on spermatogenesis (5).
Antioxidant treatment in healthy males yielded controversial results (6-9) and a recent Cochrane review concluded that this form of treatment may improve the pregnancy rate and the live birth rates; however, the evidence is of low quality (10). L- carnitine increased pregnancy rates in spouses of males with idiopathic oligoasthenozoospermia (11). L-carnitine was lower in the seminal plasma of infertile males compared to that of fertile controls (12) and it improved sperm motility in infertile males (13-15). N-acetyl cysteine is a physiologic antioxidant that depends on reduced glutathione system to reduce the DNA fragmentation index (FI) as well as the sperm decondensation index (9).
The DNA FI is considered an indicator of improved sperm quality that is independent on morphology, count and motility (12). Antioxidants reduced oxidative stress caused by excess iron overload in BTM patients (16); however, data on the use of antioxidants for treatment of sperm DNA defects in pubertal males with BTM are lacking.
This study was an exploratory convenience interventional study that aimed at examination of potential sperm DNA damage in adult male patients with BTM and evaluation of the benefit of using L-carnitine and N-acetyl cysteine for the treatment of the existent DNA defects to fill this knowledge gap. We hypothesized that giving these antioxidants will improve sperm DNA defects in adults with BTM.
Patients
Participant recruitment into the study from the hematology/oncology and endocrinology clinics, Ain Shams University, continued over a period of 10 months from February – November 2015. Inclusion criteria included pubertal patients (Tanner stage 4 or 5) without endocrinopathies that could interfere with sperm production and willingness to participate. The exclusion criteria were: a) BTM patients with renal insufficiency; b) bone marrow transplanted patients; c) patients HIV positive and d) patients with cardiac failure.
Medical records were revised for age at diagnosis, frequency of blood transfusion, mean pre-transfusion hemoglobin level, chelation history and splenectomy. With regards to chelation therapy, 7 were using desferrioxamine while 3 were on deferiprone. Compliance of patients who were on desferrioxamine was defined as taking 30-50 mg/kg 5-7 times/week for at least 8 hours/week (17). Good compliance on oral deferiprone was defined as the patient taking the drug on >85% of days of the month, fair 50- 85% and bad <50% (18). Three patients (30%) had good compliance, four (40%) had fair compliance and three (30%) had bad compliance. The mean pre-transfusion haemoglobin level was 7.4±0.74 gm/dL.
Clinical evaluation included vital data, weight and height with calculation of standard deviation scores (SDS) (19) calculation of body mass index (20), chest, heart, abdominal examination and Tanner pubertal staging (21).
Methods
Metabolic and endocrinal investigation included fasting blood glucose (FBG), fasting insulin, serum calcium, phosphorus, parathyroid hormone (PTH), free thyroxine (fT4) and thyroid stimulating hormone (TSH), basal luteinizing hormone (LH), basal follicle stimulating hormone (FSH) and total testosterone.
Twenty fully pubertal BTM patients were included consecutively, all had semen analysis; 10 were found to be azoospermic, so further analysis for sperms and DNA defects was conducted on the remaining 10 participants, each one was followed up for a period of 6 months. Participants received L-carnitine: 2 g/day and N-acetyl cysteine: 600 mg/day for 6 months with a monthly review to check drug compliance and to provide treatment. All 10 zoospermic participants were compliant to treatment and follow up.
Semen samples were collected by masturbation after at least 3 days of abstinence at the beginning and end of study. Semen samples were examined within 30 minutes of liquefaction according to WHO guidelines (22).
Seminal plasma was obtained after centrifugation at 3500 rpm for 15 minutes, loaded into aliquots and stored at -80°C awaiting antioxidant analysis then used for measurement of superoxide dismutase, glutathione peroxidase and reductase. The deposits were used for sperm chromatin structure assay (SCSA).
Sperm motility was classified into four categories: rapid progressive motility (type a), slow progressive motility (type b), non-progressive motility and immotile spermatozoa, and was assayed at exactly 0.5 and 2h after liquefaction. Total progressive motility was defined as the combination of type a and type b motility. Morphology was measured by recording the percentage of abnormal forms in the sample and types of abnormality (head, mid piece and tail defects).
Teratozoospermia index (TZI) was calculated as the total number of defects/number of sperms with defects. Sperm deformity index (SDI) was calculated as the total number of defects/total number of spermatozoa.
Acrosomal index (AI) was calculated as the total number of sperms-number of sperms with abnormal acrosome (sperms with absent acrosome plus sperms with small head) per 100 examined sperm (23).
Assays
SCSA was done by sperm DNA fragmentation test supplied by Halotech DNA-Bio Madrid. The method is based on the sperm chromatin dispersion test. Intact unfixed spermatozoa were immersed in an inert agarose microgel on a pretreated slide. An initial acid treatment denatured DNA in those sperm cells with fragmented DNA. Following this, the lysing solution removed most of the nuclear proteins, and in the absence of massive DNA breakage produced nucleoids with large halos of spreading DNA loops, emerging from a central core.
However, the nucleoids from spermatozoa with fragmented DNA either did not show a dispersion halo or the halo was minimal. A minimum of 500 spermatozoa per sample were studied, adopting the criteria of Fernández et al. (24). Cells close to the edge of the micro gel were not scored.Spermatozoa without DNA fragmentation were either spermatozoa with big halo; those whose halo width is similar or higher than the minor diameter of the core or spermatozoa with medium sized halo; their halo size is between those with large and very small halo. Spermatozoa with fragmented DNA were either spermatozoa with small halo; the halo width is similar or smaller than 1/3 of the minor diameter of the core, spermatozoa without halo or spermatozoa without halo and degraded; those that show no halo and present a core irregularly or weakly stained. Cell nuclei which do not correspond to spermatozoa were distinguished by the absence of tail.
Antioxidant enzymes were assayed by the commercially available manual kinetics kits supplied by RANDOX. The analysis was done spectrophotometrically on Biosystem BTS 330 supplied by MYCO.
The method used for superoxide dismutase (SOD)employed xanthine and xanthine oxidase to generate superoxide radicals which reacted with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride (INT) to form a red formazan dye. The superoxide dismutase activity was then measured by the degree of inhibition of this reaction. One unit of SOD is that which causes a 50% inhibition of the rate of reduction of INT under the conditions of the assay.
Glutathione peroxidase (GPX) and Glutathione reductase (GSSG) was based on the method of Paglia and Valentine (25).
Hormonal assessments
FBG, calcium and phosphoruswere measured by Cobasintegra 800 autoanalyzer supplied by Roche Diagnostics.Serum FSH, LH, TSH, FT4, total testosterone, PTH, insulin and ferritin were measured by Architect I series autoanalyzer supplied by Abbott Ireland Diagnostics Division.
Ethical approval
All patients were informed about the aim and the methods of the study. All patients signed an informed consent prior to recruitment, and the study protocol was approved by the local ethics committee of Ain Shams University.
Statistical analysis
Data were collected, coded, revised and entered to the Statistical Package for Social Science (IBM SPSS) version 20. The data were presented as number and percentages for the qualitative data, mean, standard deviations and ranges for the quantitative data with parametric distribution. Chi-square test was used in the comparison between two groups with qualitative data and Fisher exact test was used instead of the Chi-square test when the expected count in any cell found less than 5. The comparison between two independent groups with quantitative data and parametric distribution were done by using Independent t-testwhile comparison between two groups with non-parametric data was done by using Mann-Whitney test. The paired groups with quantitative data and parametric distribution were compared by using Paired t-test; while the non-parametric distribution was done by using Wilcoxon-rank test.
Spearman correlation coefficients were used to assess the relation between two quantitative parameters in the same group. The confidence interval was set to 95% and the margin of error accepted was set to 5%.
Results
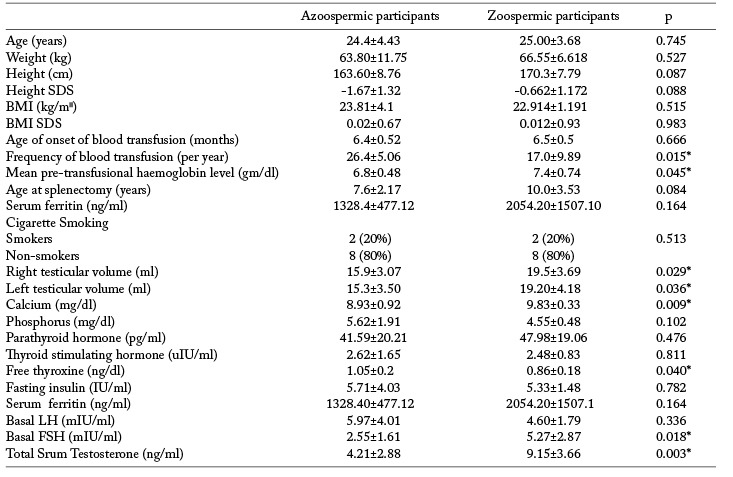
Descriptive data of the included patients with comparison of azoospermic and zoospermic participants are shown in table 1. Both groups of participants differed only regarding the frequency of blood transfusion and the mean pre-transfusional haemoglobin level. Testicular volumes were significantly lower in azoospermic males with BTM as well as FSH and total testosterone.
Table 1.
Comparison between clinical and laboratory data of the included participants
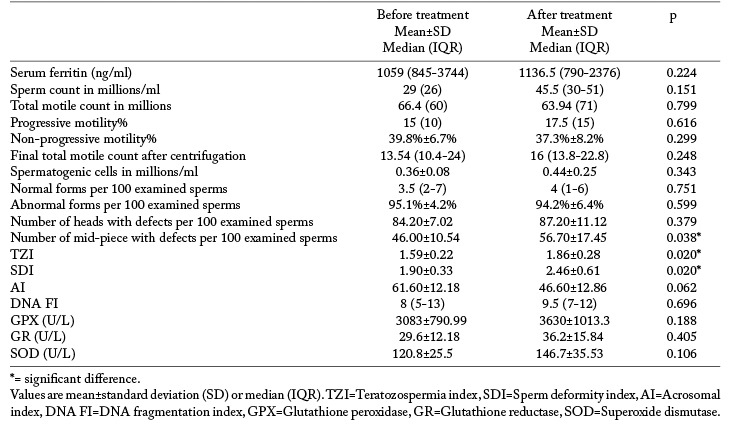
Semen analysis data of the zoospermic participants before and after treatment are listed in table 2 and the statistical comparison is shown in table 3 where it is evident that serum ferritin didn’t show significant difference after treatment and didn’t correlate with any of the semen or seminal plasma parameters. All participants had a DNA FI of less than 30% before treatment. The number of mid-piece with defects, TZI and SDI increased significantly after treatment with L-carnitine and N-acetyl cysteine for 6 months. There were no significant changes in the DNA FI or the AI (table 3). Glutathione peroxidase, glutathione reductase and superoxide dismutase levels in seminal plasma didn’t differ significantly before and after treatment and didn’t correlate with any of the indices of sperm damage (table 3).
Table 2.
Semen analysis parameters before and after 6 months of antioxidant treatment
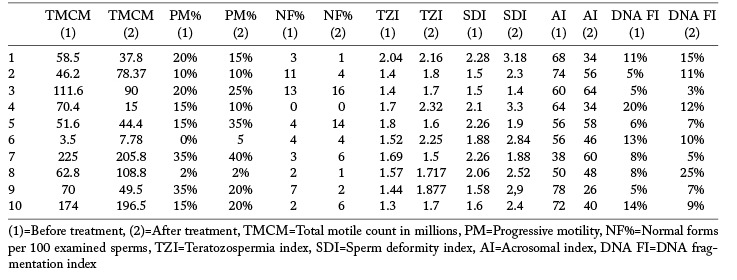
Table 3.
Comparison of serum ferritin and semen analysis parameters before and after 6 months of antioxidant treatment
Discussion
This study showed that zoospermic adult males with BTM had significantly higher mean pre-transfusional hemoglobin and lower frequency of blood transfusions with no other significant differences regarding history or anthropometric measures compared to their azoospermic counterparts. Genetic modifiers which control the rate of hemolysis and the phenotypic severity of thalassemia (26) may thus have a role in the development of late onset male hypogonadism (27) that manifests by aspermia. This link between genetic modifiers and sperm production in males with BTM has not been addressed before and this association needs verification by further studies.
This study shed some light on an unknown aspect regarding spermatogenesis in BTM which is still an evolving subject and very little is known about it as up to two thirds of patients with BTM have pubertal problems ranging from pubertal failure to infertility (28). Less than half of males with BTM who progress to full sexual maturity have adequate sperm count and motility (29). Moreover, chelation therapy poses some risk to normal spermatogenesis (30) which puts an additional burden on achieving normal fertility in these patients.
A previous study on 6 patients with BTM pointed to the presence of sperm DNA damage and the degree of this damage correlated with the degree of iron overload (1). However, in our study serum ferritin didn’t show any correlation with markers of DNA damage and our participants had a lower median for serum ferritin than the study by Perera et al. (1). All of our participants had a DNA FI of <30% i.e. below the critical value above which DNA damage was demonstrated to reduce the pregnancy rate thus having a negative effect on fertility (31). Increased sperm DNA FI was consistently associated with reduced fertility in several studies (32-34). In this study, DNA FI didn’t differ significantly after antioxidant treatment; however, other parameters of sperm damage; number of middle piece with defects, SDI and TZI increased significantly. This was considered a potential side effect of treatment with L-carnitine and N-acetyl cysteine in the studied BTM patients.
Our observations support the findings of Ménézo et al. (12) who reported some untoward effects of using antioxidants in the form of increased sperm DNA decondensation by 25%. They attributed the accentuated decondensation to the deleterious effect of the powerful antioxidant vitamin C on chromatin packaging and the tertiary structure of sperm DNA. Therefore the use of antioxidants in those whose sperm decondensation exceeds 20% is not recommended to avoid reaching the 28% threshold that adversely affect the occurrence of pregnancy due to its negative effect on sperm DNA compaction during the pre-implantation event, a process which needs good paternal gene expression in order to achieve pregnancy and avoid chromosomal anomalies (12). The increased TZI and SDI in our study advocate against the use of antioxidants in men with BTM to improve fertility as the SDI negatively correlates with the fertilization rate in conventional in vitro fertilization techniques and predicts fertilization failure (35).
Our study carries some limitations: Firstly, DNA fragmentation was normal in all men. Therefore, this made evaluation of DNA fragmentation changes challenging. Secondly, the study analysis was possible only in 10 BTM patients. Nevertheless, our study gives further information on spermatogenesis in thalassemia major patients and to our best knowledge, this pilot study is the first to evaluate the effects of antioxidants in patients with BTM.
Conclusion
Zoospermic participants with BTM had no significant sperm DNA damage as evidenced by their DNA FI; however, they showed significant increase in the SDI and TZI with the use of antioxidants. Therefore, the results of this study are not in favour with the use of antioxidants in BTM patients for improving potential fertility. Larger studies, however, are needed to confirm these preliminary results.
Acknowledgement
The authors would like to acknowledge Walaa Salah, Inas Ayman, Rana El-Garhy and Shady Wafa for helping with patient recruitment, laboratory analysis and provision of antioxidant treatment.
References
- 1.Perera D. Pizzey A. Campbell A. Katz M. Porter J. Petrou M. Irvine DS. Chatterjee R. Sperm DNA damage in potentially fertile homozygous beta-thalassaemia patients with iron overload. Hum Reprod. 2002;17:1820–5. doi: 10.1093/humrep/17.7.1820. [DOI] [PubMed] [Google Scholar]
- 2.Aitken RJ. Gordon E. Harkiss D. Twigg JP. Milne P. Jennings Z. Irvine DS. Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol Reprod. 1998;59:1037–46. doi: 10.1095/biolreprod59.5.1037. [DOI] [PubMed] [Google Scholar]
- 3.Chatterjee R. Katz M. Reversible hypogonadotrophic hypogonadism in sexually infantile male thalassaemic patients with transfusional iron overload. Clin Endocrinol (Oxf) 2000;53:33–42. doi: 10.1046/j.1365-2265.2000.00962.x. [DOI] [PubMed] [Google Scholar]
- 4.Aitken RJ. Smith TB. Jobling MS. Baker MA. De Iuliis G. Oxidative stress and male reproductive health. Asian J Androl. 2014;16:31–8. doi: 10.4103/1008-682X.122203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Sanctis V. Perera D. Katz M. Fortini M. Gamberini MR. Spermatozoal DNA damage in patients with B thalassaemia syndromes. Pediatr Endocrinol Rev. 2008;6(Suppl 1):185–9. [PubMed] [Google Scholar]
- 6.Hughes EG. Grantmyre J. Zini A. An integrated approach to male-factor subfertility: bridging the gap between fertility specialists trained in urology and gynaecology. J Obstet Gynaecol Can. 2015;37:258–65. doi: 10.1016/S1701-2163(15)30312-1. [DOI] [PubMed] [Google Scholar]
- 7.Gual-Frau J. Abad C. Amengual MJ. Hannaoui N. Checa MA. Ribas-Maynou J. Lozano I. Nikolaou A. Benet J. García-Peiró A. Prats J. Oral antioxidant treatment partly improves integrity of human sperm DNA in infertile grade I varicocele patients. Hum Fertil (Camb) 2015;18:225–9. doi: 10.3109/14647273.2015.1050462. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal A. Nallella KP. Allamaneni SS. Said TM. Role of antioxidants in treatment of male infertility: an overview of the literature. Reprod Biomed Online. 2004;8:616–27. doi: 10.1016/s1472-6483(10)61641-0. [DOI] [PubMed] [Google Scholar]
- 9.Dattilo M. Cornet D. Amar E. Cohen M. Menezo Y. The importance of the one carbon cycle nutritional support in human male fertility: a preliminary clinical report. Reprod Biol Endocrinol. 2014 Jul 29;12:71. doi: 10.1186/1477-7827-12-71. doi: 10.1186/1477-7827-12-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Showell MG. Brown J. Yazdani A. Stankiewicz MT. Hart RJ. Antioxidants for male subfertility. Cochrane Database Syst Rev. 2014;(12):CD007411. doi: 10.1002/14651858.CD007411.pub3. doi: 10.1002/14651858.CD007411. [DOI] [PubMed] [Google Scholar]
- 11.Shang XJ. Wang LL. Mo DS. Cai HC. Zheng DD. Zhou YZ. Effect and safety of L-carnitine in the treatment of idiopathic oligoasthenozoospermia: a systemic review. Zhonghua Nan Ke Xue. 2015;21:65–73. [PubMed] [Google Scholar]
- 12.Ménézo YJ. Hazout A. Panteix G. Robert F. Rollet J. Cohen-Bacrie P. Chapuis F. Clément P. Benkhalifa M. Antioxidants to reduce sperm DNA fragmentation: an unexpected adverse effect. Reprod Biomed Online. 2007;14:418–21. doi: 10.1016/s1472-6483(10)60887-5. [DOI] [PubMed] [Google Scholar]
- 13.Lenzi A. Sgrò P. Salacone P. Paoli D. Gilio B. Lombardo F. Santulli M. Agarwal A. Gandini L. A placebo-controlled double-blind randomized trial of the use of combined l-carnitine and l-acetyl-carnitine treatment in men with asthenozoospermia. Fertil Steril. 2004;81(6):1578–84. doi: 10.1016/j.fertnstert.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 14.Peivandi S. Karmpour A. Moslemizadeh N. Effects of L-carnitine on Infertile Men’s Spermogram; a Randomized Clinical Trial. J Reprod Infertil. 2010;10 [Google Scholar]
- 15.Zhou X. Liu F. Zhai S. Effect of L-carnitine and/or L-acetyl-carnitine in nutrition treatment for male infertility: a systematic review. Asia Pac J Clin Nutr. 2007;16(Suppl 1):383–90. [PubMed] [Google Scholar]
- 16.Darvishi Khezri H. Salehifar E. Kosaryan M. Aliasgharian A. Jalali H. Hadian Amree A. Potential Effects of Silymarin and Its Flavonolignan Components in Patients with β-Thalassemia Major: A Comprehensive Review in 2015. Adv Pharmacol Sci. 2016;2016:3046373. doi: 10.1155/2016/3046373. doi: 10.1155/2016/304637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rashid M. Karimi M. Compliance of deferoxamine injection in beta-thalassaemia major patients in Iran. Transfus Med. 2012;22:2. doi: 10.1111/j.1365-3148.2011.01130.x. [DOI] [PubMed] [Google Scholar]
- 18.Olivieri NF. Brittenham GM. Matsui D. Berkovitch M. Blendis LM. Cameron RG. McClelland RA. Liu PP. Templeton DM. Koren G. Iron-chelation therapy with oral deferiprone in patients with thalassemia major. N Engl J Med. 1995;332:918–22. doi: 10.1056/NEJM199504063321404. [DOI] [PubMed] [Google Scholar]
- 19.Tanner JM. Whitehouse RH. Takaishi M. Standards from birth to maturity for height, weight, height velocity and weight velocity: British children. 1966;41:613–35. doi: 10.1136/adc.41.220.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cole TJ. A chart to link child centiles of Body Mass Index, weight and height. Eur J Clin Nutr. 2002;56:1194–9. doi: 10.1038/sj.ejcn.1601473. [DOI] [PubMed] [Google Scholar]
- 21.Marshall WA. Tanner JM. Variations in the Pattern of Pubertal Changes in Boys Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organisation. 5th ed. Geneva: World Health Organization; 2010. WHO Laboratory Manual for the Examination and Processing of Human Semen; pp. 7–47. [Google Scholar]
- 23.Menkveld R. Wong WY. Lombard CJ. Wetzels AM. Thomas CM. Merkus HM. Steegers-Theunissen RP. Semen parameters, including WHO and strict criteria morphology, in a fertile and subfertile population: an effort towards standardization of in-vivo thresholds. Hum Reprod. 2001;16:1165–71. doi: 10.1093/humrep/16.6.1165. [DOI] [PubMed] [Google Scholar]
- 24.Fernández JL. Muriel L. Goyanes V. Segrelles E. Gosálvez J. Enciso M. LaFromboise M. De Jonge C. Simple determination of human sperm DNA fragmentation with an improved sperm chromatin dispersion test. Fertil Steril. 2005;84:833–42. doi: 10.1016/j.fertnstert.2004.11.089. [DOI] [PubMed] [Google Scholar]
- 25.Paglia DE. Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–69. [PubMed] [Google Scholar]
- 26.Galanello R. Origa R. Beta-thalassemia. Orphanet J Rare Dis. 2010;5:11. doi: 10.1186/1750-1172-5-11. doi: 10.1186/1750-1172-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Sanctis V. Soliman AT. Elsedfy H. Soliman NA. Elalaily R. Late-onset Male Hypogonadism and Fertility Potential in Thalassemia Major Patients: Two Emerging Issues. Mediterr J Hematol Infect Dis. 2015;7:e2015047. doi: 10.4084/MJHID.2015.047. doi: 10.4084/MJHID.2015.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Sanctis V. Soliman AT. Candini G. Yassin M. Raiola G. Galati MC. Elalaily R. Elsedfy H. Skordis N. Garofalo P. Anastasi S. Campisi S. Karimi M. Kattamis C. Canatan D. Kilinc Y. Sobti P. Fiscina B. El Kholy M. Insulin-like Growth Factor-1 (IGF-1): Demographic, Clinical and Laboratory Data in 120 Consecutive Adult Patients with Thalassaemia Major. Mediterr J Hematol Infect Dis. 2014;6(1):e2014074. doi: 10.4084/MJHID.2014.074. doi: 10.4084/MJHID.2014.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Sanctis V. Borsari G. Brachi S. Govoni M. Carandina G. Spermatogenesis in young adult patients with beta-thalassaemia major long-term treated with desferrioxamine. Georgian Med News. 2008;156:74–7. [PubMed] [Google Scholar]
- 30.Larson KL. DeJonge CJ. Barnes AM. Jost LK. Evenson DP. Sperm chromatin structure assay parameters as predictors of failed pregnancy following assisted reproductive techniques. Hum Reprod. 2000;15:1717–22. doi: 10.1093/humrep/15.8.1717. [DOI] [PubMed] [Google Scholar]
- 31.Kodama H. Yamaguchi R. Fukuda J. Kasai H. Tanaka T. Increased oxidative deoxyribonucleic acid damage in the spermatozoa of infertile male patients. Fertil Steril. 1997;68:519–24. doi: 10.1016/s0015-0282(97)00236-7. [DOI] [PubMed] [Google Scholar]
- 32.Evenson DP. Larson KL. Jost LK. Sperm chromatin structure assay: its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J Androl. 2002;23:25–43. doi: 10.1002/j.1939-4640.2002.tb02599.x. [DOI] [PubMed] [Google Scholar]
- 33.Henkel R. Hajimohammad M. Stalf T. Hoogendijk C. Mehnert C. Menkveld R. Gips H. Schill WB. Kruger TF. Influence of deoxyribonucleic acid damage on fertilization and pregnancy. Fertil Steril. 2004;81:965–72. doi: 10.1016/j.fertnstert.2003.09.044. [DOI] [PubMed] [Google Scholar]
- 34.Hammadeh ME. Radwan M. Al-Hasani S. Micu R. Rosenbaum P. Lorenz M. Schmidt W. Comparison of reactive oxygen species concentration in seminal plasma and semen parameters in partners of pregnant and non-pregnant patients after IVF/ICSI. Reprod Biomed Online. 2006;13:696–706. doi: 10.1016/s1472-6483(10)60661-x. [DOI] [PubMed] [Google Scholar]


