Highlights
-
•
Biopsies of a large mass are prone to sampling errors and may lead to an incorrect diagnosis.
-
•
MRI imaging of vulvar tumors can aid in surgical planning.
-
•
Large sarcomas of the vulva require a multi-disciplinary approach.
Keywords: Vulvar sarcoma, Vulvar reconstruction
1. Introduction
Primary malignancy of the vulva is rare with approximately 4500 cases reported in the US each year. Non-squamous cell tumors of the vulva are an even more rare entity. Included in this would be primary sarcomas of the vulva, which make up around 1–2% of all vulvar malignancies(Newman and Fletcher, 1991; DiSaia et al., 1971). Vulvar sarcomas may present as large intra-dermal masses that are not as visible as their squamous counterparts. Generally, sarcomas of the vulva are treated with radical surgery for adequate margins; with additional radiation or systemic therapy warranted if advanced disease is found.
Dermatofibrosarcoma protuberans (DFSP) is a slow-growing, superficial tumor that develops mainly on the trunk or extremities. DFSP are often locally aggressive with a high risk of local recurrence, but a lower risk of distant metastatic spread. Fewer than 50 of these tumors presenting on the vulva have been described previously in the literature(Edelweiss and Malpica, 2010). We describe a unique situation with a patient presenting with a massive vulvar mass requiring multi-specialist care.
2. Case
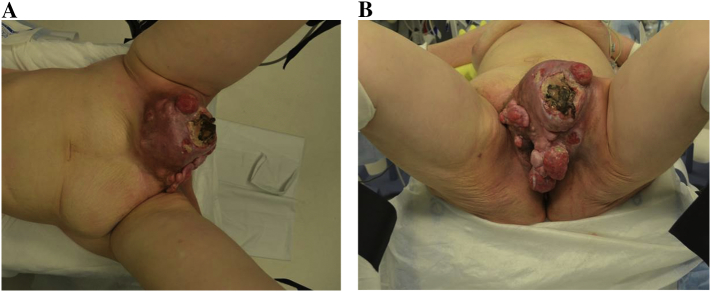
A 57 year old G4P4, visually impaired woman, presented to the emergency department (ED) with a large, bleeding vulvar mass. Patient stated that mass had been present for at least one year, and had grown slowly with more recent bothersome growth. Further questioning revealed she had been seen a couple years prior to current presentation for a new “lump”, but had felt dismissed and ashamed by the provider, prompting no further follow up. The patient's initial admission included an exam under anesthesia (EUA) which revealed a large, fungating mobile left groin mass measuring 20 × 15 cm involving the mons pubis and distorting the left labia majora. A photo of the mass is presented in Fig. 1. Extensive biopsies of the mass revealed a superficial angiomyxoma, prompting surgical planning and initiation of an aromatase inhibitor (AI). Patient remained on an AI for approximately 6 weeks prior to surgery.
Fig. 1.
A/B: Preoperative photo of large vulvar tumor encompassing entire mons pubis and upper labia majora.
Preoperative MRI pelvis revealed a multi-lobulated vulva/left groin mass measuring 13.2 × 16.4 × 19.6 cm. Minimal fat planes were seen between the mass and inguino-femoral neuro-vascular bundle. A slightly enlarged left inguinal nodule measuring 1.3 × 1.9 cm was also present, but no other lymphadenopathy or evidence of metastatic disease was noted. Fig. 2 shows a representative image from MRI. Additional preoperative imaging with CT chest and abdomen did not show evidence of metastatic disease. Plastic surgery was consulted for assistance with closure of the anticipated large defect.
Fig. 2.
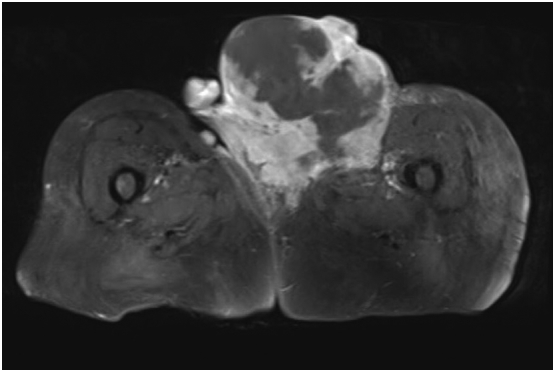
Representative T1 axial MRI pelvis image of the primary tumor.
Following informed consent, the patient underwent a radical vulvectomy with complex wound closure using a vertical rectus abdominis myocutaneous (VRAM) flap. Intraoperatively it was noted that new satellite lesions were present, crossing the midline to the right vulva requiring a large bilateral resection. Although the tumor extended to the deep inguinal-femoral spaces, the tumor compressed, but did not invade into neurovascular structures.
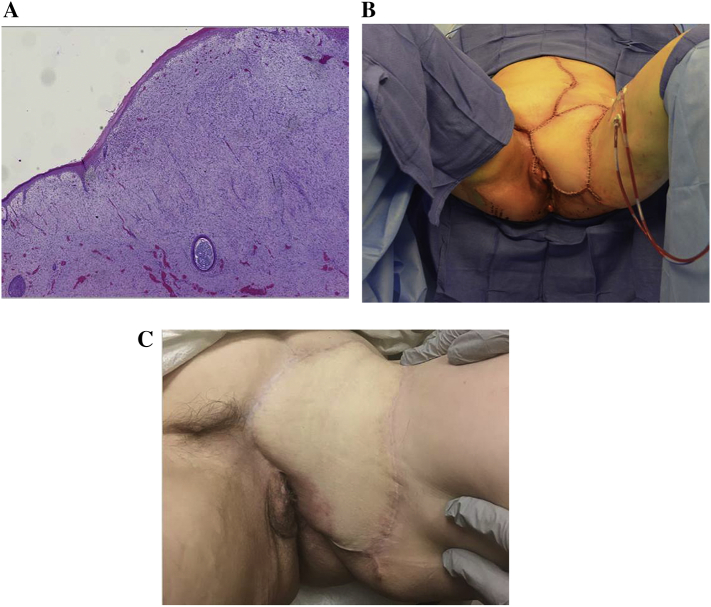
Post-operatively, the patient had an uncomplicated course. Her final pathology was also reviewed by an outside expert, which revealed a fibrosarcomatous variant of dermatofibrosarcoma protuberans (DFSP) [confirmed by 17,22 translocation on FISH] with negative margins and negative surrounding lymph nodes (removed en bloc) (Fig. 3). Histologic staining assisted in the diagnosis, showing positive staining for CD34 and negative for S-100, SOX10, and GFAP (Haycox et al., 1997). Following surgery, the patient was referred to a medical oncologist with extensive expertise in sarcomas given the rarity of this disease. Adjuvant treatment was planned for one year with imatinib 400 mg orally daily. Representative photos of immediate post op and six-month follow up are provided in Fig. 3B/C.
Fig. 3.
A) Representative H&E slide of the primary dermatofibrosarcoma of fibrosarcomatous variant. Picture represents subcutaneous vulva lesion composed of spindle cells arranged into fascicles with mild cellular pleomorphism and brisk mitotic activity (25–30 mitotic figures per 10HPF) with foci of degeneration and necrosis. B) Immediate post-operative photo following vertical rectus-abdominal myocutaneous flap (VRAM) reconstruction. C) Six-month follow up image of vulvar reconstruction.
3. Discussion
Dermatofibrosarcoma protuberans of the vulva is an extremely rare diagnosis. Incidence of this tumor is approximately 4.2 per 1 million in the United States, with primary vulva cases making up a striking minority(Kreicher et al., 2016). While many histologic variants for DFSP exist, one underlying genetic aberration is common. Translocation of chromosomes 17 and 22 [t(17:22)(q22:q13)] is seen in over 90% of DFSP(Noujaim et al., 2015). Translocation of these chromosomes causes alterations in platelet derived growth factor beta (PDGFB1) and collagen type I alpha 1 (COL1A1 resulting in aberrant growth(Greco et al., 1998). Presenting patients will often note a slow growing process, confined to dermal or superficial subcutaneous tissue. If large sized tumors are encountered, it can often reveal a more malignant process with fibrosarcomatous overgrowth on histology. Tumors with this feature have approximately double the chance of distant metastatic spread as well as a lower overall survival(Liang et al., 2014). Importantly to our case was the delay in which our patient sought care, which has been well described in the literature as also being a detriment on outcomes(Jones and Joura, 1999).
The case we present has many features that would point to an aggressive subtype of DFSP. The large size change she had noted over the previous year was indicative of the rapid growth phase seen in these tumors. In addition, pre-operative MRI imaging had shown evidence of deep invasion and possible vascular involvement. Given the difficulty of accurate biopsy on a large mass, it is not surprising that the original diagnosis was revised from angiomyxoma. Similarly, angiomyxoma is a locally aggressive tumor of the vulva without metastatic potential. A distinction of angiomyxoma, compared with DFSP, is their response to anti-hormonal therapy (Giles et al., 2008). Our patient briefly used anti-hormonal therapy while bridging time to surgery. In the setting of a very large tumor, it is important to remember the limitations of single site biopsy and the possibility of incorrect diagnosis based on sampling with biopsies only. If surgical resection is feasible, this is preferred to establish the correct diagnosis and initiate appropriate management.
Management hallmarks for vulvar sarcoma include radical local resection with negative margins. DFSP does not have a standard staging system. AJCC recommendations for soft tissue sarcomas are often used. This system takes into account size of tumor, grading, and nodal/distant metastatic disease. Likewise, treatment of DFSP requires surgery. Mohs' microscopic surgery has been reported for smaller lesions, while large lesions require a 2–3 cm margin of normal tissue (Paradisi et al., 2008; Fields et al., 2011). Routine lymph node assessment has not been described for DFSP, however positive lymph nodes do have prognostic implications.
Vulvar reconstruction following large resection may require myocutaneous flap coverage. An exhaustive review of rotational flaps for vulvar defects is in not in the scope of this article. Previous descriptions include transposition flap such as a V-Y advancement flap, and myocutaneous flaps such as the vertical rectus abdominis (Morrow, 2012). In our case, given the substantial size of the defect, plastic surgery was consulted for assistance in reconstruction. A VRAM flap was chosen which allowed for excellent coverage. Monitoring flap viability by assessing adequate blood flow and preventing infection with good hygiene are the most crucial aspects of the immediate post-operative care for these patients.
Adjuvant therapy for DFSP is based on the risk of recurrence. Final pathologic staging for our patient was a T2N0G3 (stage III) per AJCC guidelines for soft tissue sarcoma. Given a risk of recurrence over 20%, she was counseled on adjuvant treatment. Radiation treatment has not been shown to provide improvement in recurrence risk for this sarcoma subtype, especially in cases of full resection. Two studies showed activity for imatinib in phase II trials (Rutkowski et al., 2010; Kerob et al., 2010) with excellent response rates described. Patients on trial with a durable response were treated for at least 48 weeks, providing the rationale for our patient's planned treatment course. These studies lead to an FDA approval label for DFSP. Patients are monitored for potential toxicities including cardiovascular, hepatic, and dermatologic are monitored for during the year long course of daily oral therapy. Our patient has now been maintained on imatinib without evidence of recurrence for over twelve months and she continues regular surveillance with six-month interval examinations.
Acknowledgments
Acknowledgements
The authors would like to thank Dr. Iouri Ivanov and Dr. Jesus Chavez for their assistance in creation of the pathology micrograph photo.
Conflict of interest statement
The authors of this paper have no relevant conflicts of interest relevant to the manuscript presented.
Author contributions
Robert Neff- Collection of case materials, manuscript preparation, figure design, editing final draft.
Robert Collins- manuscript preparation, editing final draft.
Floor Backes- manuscript preparation, editing final draft.
References
- DiSaia P.J., Rutledge F., Smith J.P. Sarcoma of the vulva. Report of 12 patients. Obstet. Gynecol. 1971;38(2):180–184. [PubMed] [Google Scholar]
- Edelweiss M., Malpica A. Dermatofibrosarcoma protuberans of the vulva: a clinicopathologic and immunohistochemical study of 13 cases. Am. J. Surg. Pathol. 2010;34(3):393–400. doi: 10.1097/PAS.0b013e3181cf7fc1. [DOI] [PubMed] [Google Scholar]
- Fields R.C., Hameed M., Qin L.X., Moraco N., Jia X., Maki R.G. Dermatofibrosarcoma protuberans (DFSP): predictors of recurrence and the use of systemic therapy. Ann. Surg. Oncol. 2011;18(2):328–336. doi: 10.1245/s10434-010-1316-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles D.L., Liu P.T., Lidner T.K., Magtibay P.M. Treatment of aggressive angiomyxoma with aromatase inhibitor prior to surgical resection. Int. J. Gynecol. Cancer. 2008;18(2):375–379. doi: 10.1111/j.1525-1438.2007.01005.x. [DOI] [PubMed] [Google Scholar]
- Greco A., Fusetti L., Villa R., Sozzi G., Minoletti F., Mauri P. Transforming activity of the chimeric sequence formed by the fusion of collagen gene COL1A1 and the platelet derived growth factor b-chain gene in dermatofibrosarcoma protuberans. Oncogene. 1998;17(10):1313–1319. doi: 10.1038/sj.onc.1202051. [DOI] [PubMed] [Google Scholar]
- Haycox C.L., Odland P.B., Olbricht S.M., Piepkorn M. Immunohistochemical characterization of dermatofibrosarcoma protuberans with practical applications for diagnosis and treatment. J. Am. Acad. Dermatol. 1997;37(3 Pt 1):438–444. doi: 10.1016/s0190-9622(97)70146-4. [DOI] [PubMed] [Google Scholar]
- Jones R.W., Joura E.A. Analyzing prior clinical events at presentation in 102 women with vulvar carcinoma. Evidence of diagnostic delays. J. Reprod. Med. 1999;44(9):766–768. [PubMed] [Google Scholar]
- Kerob D., Porcher R., Verola O., Dalle S., Maubec E., Aubin F. Imatinib mesylate as a preoperative therapy in dermatofibrosarcoma: results of a multicenter phase II study on 25 patients. Clin. Cancer Res. 2010;16(12):3288–3295. doi: 10.1158/1078-0432.CCR-09-3401. [DOI] [PubMed] [Google Scholar]
- Kreicher K.L., Kurlander D.E., Gittleman H.R., Barnholtz-Sloan J.S., Bordeaux J.S. Incidence and Survival of primary Dermatofibrosarcoma Protuberans in the United States. Dermatol. Surg. 2016;42(Suppl. 1):S24–S31. doi: 10.1097/DSS.0000000000000300. [DOI] [PubMed] [Google Scholar]
- Liang C.A., Jambusaria-Pahlajani A., Karia P.S., Elenitsas R., Zhang P.D., Schmults C.D. A systematic review of outcome data for dermatofibrosarcoma protuberans with and without fibrosarcomatous change. J. Am. Acad. Dermatol. 2014;71(4):781–786. doi: 10.1016/j.jaad.2014.03.018. [DOI] [PubMed] [Google Scholar]
- Morrow C.P. 2 ed. South Coast Medical Publishing; 2012. Morrow's Gyneocologic Cancer Surgery. [Google Scholar]
- Newman P.L., Fletcher C.D. Smooth muscle tumours of the external genitalia: clinicopathological analysis of a series. Histopathology. 1991;18(6):523–529. doi: 10.1111/j.1365-2559.1991.tb01479.x. [DOI] [PubMed] [Google Scholar]
- Noujaim J., Thway K., Fisher C., Jones R.L. Dermatofibrosarcoma protuberans: from translocation to targeted therapy. Cancer Biol. Med. 2015;12(4):375–384. doi: 10.7497/j.issn.2095-3941.2015.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradisi A., Abeni D., Rusciani A., Cigna E., Wolter M., Scuderi N. Dermatofibrosarcoma protuberans: wide local excision vs. Mohs micrographic surgery. Cancer Treat. Rev. 2008;34(8):728–736. doi: 10.1016/j.ctrv.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Rutkowski P., Van Glabbeke M., Rankin C.J., Ruka W., Rubin B.P., Debiec-Rychter M. Imatinib mesylate in advanced dermatofibrosarcoma protuberans: pooled analysis of two phase II clinical trials. J. Clin. Oncol. 2010;28(10):1772–1779. doi: 10.1200/JCO.2009.25.7899. [DOI] [PMC free article] [PubMed] [Google Scholar]