Abstract
Background & Aims
The small intestine regulates plasma triglyceride (TG) concentration. Within enterocytes, dietary TGs are packaged into chylomicrons (CMs) for secretion or stored temporarily in cytoplasmic lipid droplets (CLDs) until further mobilization. We and others have shown that oral and intravenous glucose enhances CM particle secretion in human beings, however, the mechanisms through which this occurs are incompletely understood.
Methods
Two separate cohorts of participants ingested a high-fat liquid meal and, 5 hours later, were assigned randomly to ingest either a glucose solution or an equivalent volume of water. In 1 group (N = 6), plasma and lipoprotein TG responses were assessed in a randomized cross-over study. In a separate group (N = 24), duodenal biopsy specimens were obtained 1 hour after ingestion of glucose or water. Ultrastructural and proteomic analyses were performed on duodenal biopsy specimens.
Results
Compared with water, glucose ingestion increased circulating TGs within 30 minutes, mainly in the CM fraction. It decreased the total number of CLDs and the proportion of large-sized CLDs within enterocytes. We identified 2919 proteins in human duodenal tissue, 270 of which are related to lipid metabolism and 134 of which were differentially present in response to glucose compared with water ingestion.
Conclusions
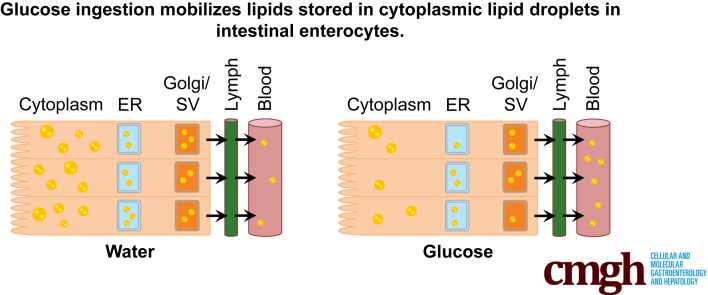
Oral glucose mobilizes TGs stored within enterocyte CLDs to provide substrate for CM synthesis and secretion. Future studies elucidating the underlying signaling pathways may provide mechanistic insights that lead to the development of novel therapeutics for the treatment of hypertriglyceridemia.
Keywords: Intestine, Glucose, Triglycerides, Cytoplasmic Lipid Droplets
Abbreviations used in this paper: CLD, cytoplasmic lipid droplet; CM, chylomicron; ER, endoplasmic reticulum; FA, fatty acid; GLP-2, glucagon-like peptide-2; GO, Gene Ontology; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor; TG, triglyceride; TRL, triglyceride-rich lipoprotein; VLDL, very-low-density lipoprotein
Graphical abstract
See editorial on page 291.
Summary.
Triglycerides are retained in the human gut long after ingestion of dietary fat. Oral glucose subsequently mobilizes triglyceride stores from the gut by recruiting cytoplasmic lipid droplets for chylomicron synthesis and secretion.
Hypertriglyceridemia, resulting from accumulation of circulating triglyceride (TG)-rich lipoprotein (TRL) particles in both fasting and postprandial states, is a highly prevalent condition and a significant risk factor for cardiovascular disease.1 TGs, the main form of dietary fat, are hydrolyzed into fatty acids (FAs), glycerol, and monoglycerides by digestive enzymes in the intestinal lumen. These digestive products of dietary TGs are taken up by absorptive cells of the small intestine (enterocytes), where the majority of re-esterified TG is packaged into chylomicrons (CMs) and secreted into the circulation via the lymphatic system.2 There is increasing evidence that, beyond the dominant regulation by lipid substrate availability, the intestine actively participates in the regulation of whole-body lipid metabolism via nutrient, hormonal, metabolic, and neural regulatory pathways.3
Aside from rapid TG incorporation into CMs, the intestine can store a considerable quantity of fat for several hours after the absorptive phase.4 Studies in human beings suggest that dietary lipids originating from an earlier high-fat meal may contribute to CM TG after prolonged storage in the gut.5, 6, 7, 8 In addition, abundant lipid droplets are detected in human enterocytes 6 hours after ingestion of a high-fat liquid meal,9 and in mice up to 12 hours after an oral fat gavage.10 The exact site(s) of retained intestinal lipid stores and the quantity stored in each location have not been well characterized. Lipid droplets have been visualized in the cytoplasm of jejunal enterocytes in human beings9 and mice,10 and CMs have been observed in intracellular secretory pathways, in the lamina propria, and lacteals of the mesenteric lymphatics in human beings9 and rodents.10, 11, 12 Cytoplasmic lipid droplets (CLDs) are the best studied of these various lipid pools with respect to lipid storage and mobilization. CLDs consist of a neutral lipid core surrounded by a phospholipid monolayer. Numerous CLD-associated proteins have been identified and several have been shown to regulate CLD storage and metabolism.13, 14 The exact role of CLDs in the process of dietary fat absorption and their contribution to CM assembly and secretion is unknown, but studies in mice have indicated that CLD stores undergo dynamic changes in response to a dietary fat challenge.15 Therefore, it is thought that CLDs may function as a temporary storage pool of neutral lipids for incorporation in CMs at later time points.16, 17
Various dietary and hormonal factors play a role in mobilizing TGs stored within enterocytes from a previous meal. Several stimuli, including mixed meals,18 glucose ingestion,9 the gut hormone glucagon-like peptide-2 (GLP-2),19 and sham fat feeding,8 may trigger the mobilization of intestinal lipid stores. Ingestion of a mixed meal after a previous high-fat meal has been shown to elicit a peak in plasma TGs before the absorption of lipid from the current meal.20 Glucose ingestion 5 hours after a high-fat meal decreases lipid stores in human enterocytes.9 In healthy men, under the conditions of constant intraduodenal feeding and a pancreatic clamp, subcutaneous injection of GLP-2 caused a rapid and transient increase in plasma TGs and TRL particles.19 In the latter study we showed that GLP-2 mobilized lipid that was ingested 7 hours earlier, which likely was retained in 1 or more of the earlier-mentioned intestinal lipid pools.19 Furthermore, sham fat feeding was shown to stimulate CM secretion, suggesting the involvement of a neural regulatory pathway in intestinal lipid mobilization.8 Collectively, mounting evidence supports the existence of TG stores in the human intestine that are subject to release in response to certain stimuli. However, the specific mechanism(s) by which mobilization of intestinal TG stores occurs remain unclear.
The goal of this study was to investigate the mechanism by which oral glucose mobilizes TGs stored within enterocytes in human beings and to identify the specific lipid pools that are mobilized. In each experiment, participants ingested a high-fat liquid meal and, 5 hours later, ingested glucose or water. In aim 1, in vivo circulating lipid responses to oral glucose were examined. In aim 2, duodenal biopsy specimens were obtained and ultrastructural and molecular responses were characterized.
Results
Oral Glucose Ingested 5 Hours After a High-Fat Liquid Meal Acutely Increases Plasma TG Concentration
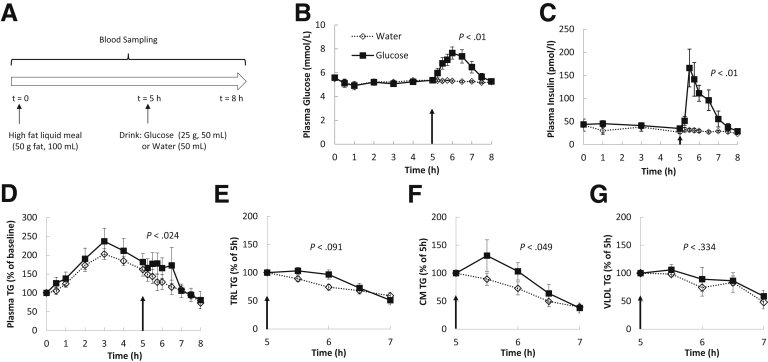
Lipid responses to oral glucose and water were measured in 6 healthy participants (Table 1) in a study design illustrated in Figure 1A. As anticipated in aim 1, plasma glucose levels increased in response to the ingestion of glucose, but not water (Figure 1B). After glucose ingestion, plasma insulin levels also increased from a basal level of approximately 30 pmol/L to peak at approximately 150 pmol/L at 30 minutes, followed by a gradual decline to basal level 2 hours later (Figure 1C). In both groups plasma TGs increased to a postprandial peak at approximately 3 hours after fat ingestion before decreasing toward baseline (Figure 1D). With water ingestion, the decrease in plasma TGs continued unabated and approached basal levels at approximately 7 hours. However, after glucose ingestion, plasma TGs plateaued during the following 2 hours (P = .024 glucose vs water).
Table 1.
Demographics and Biochemical Characteristics of Aim 1 Participants
| Subject | Age, y | Weight, kg | Height, cm | BMI, kg/m2 | Waist, cm | Fasting glucose level, mmol/L | Fasting TG level, mmol/L | Fasting insulin level, pmol/L |
|---|---|---|---|---|---|---|---|---|
| 1 | 58 | 72 | 178 | 23 | 95.5 | 5.6 | 0.76 | 35 |
| 2 | 46 | 77 | 166 | 27 | 101 | 4.9 | 0.73 | 48 |
| 3 | 46 | 87 | 182 | 26 | 100 | 4.5 | 0.97 | 37 |
| 4 | 53 | 76 | 172 | 25.7 | 87 | 4.9 | 1.99 | 94 |
| 5 | 29 | 87 | 182 | 26.4 | 94 | 4.4 | 0.68 | 36.5 |
| 6 | 57 | 84 | 179 | 26 | 88 | 4.5 | 0.59 | 29 |
| Means | 48.2 | 80.5 | 176.5 | 25.7 | 94.3 | 4.8 | 1.0 | 46.6 |
| SE | 4.4 | 2.6 | 2.6 | 0.6 | 2.4 | 0.2 | 0.2 | 9.8 |
BMI, body mass index.
Figure 1.
Lipid responses to oral glucose ingestion. (A) Study design. After an overnight fast, subjects ingested a high-fat liquid meal and 5 hours later ingested a glucose solution or equivalent volume of water in 2 randomized visits. (B) Blood glucose and (C) insulin concentrations during the study period. (D) TG concentrations in plasma during the study period, expressed as a percentage of baseline. (E–G) TG concentrations in total TRL, CM-sized TRL, and VLDL-sized TRL 2 hours after glucose or water ingestion, expressed as the percentage of levels at t = 5 hours. Arrows indicate time of glucose or water ingestion. All P values were with repeated-measures analysis of variance between 5 and 7 hours.
Oral Glucose Ingested 5 Hours After a High-Fat Liquid Meal Increased TGs in Total and CM-Sized, but Not in Smaller Very-Low-Density Lipoprotein–Sized, TRL Particles in the Circulation
Circulating total TRL TG tended to be higher after glucose vs water ingestion (P = .091) (Figure 1E). To identify whether large or small TRLs were most responsible for the increase in plasma and TRL TGs after glucose ingestion, TRLs were separated further by ultracentrifugation into larger CM-sized particles (Svedberg flotation > 400, predominantly comprising CMs) and smaller very-low-density lipoprotein (VLDL)-sized particles (Svedberg flotation 20–400, likely comprising both hepatically derived VLDL particles and smaller, intestinally derived CMs). An increase in TGs in the larger CM-sized TRL particles was observed with glucose ingestion (P = .049, analysis of variance) (Figure 1F). Despite interindividual variations, as is the usual case for most human mechanistic studies, the response was statistically significant because each subject showed a response to glucose, either a reversal of the decrease or an attenuated decrease. Changes in the smaller VLDL-sized TRL particles were similar with both glucose and water ingestion (P = .340) (Figure 1G). These results suggest that the increase in plasma TGs in response to glucose ingestion was owing exclusively to an increase in CM-sized TRL particles.
Presence of Lipid Pools Within the Intestinal Mucosa
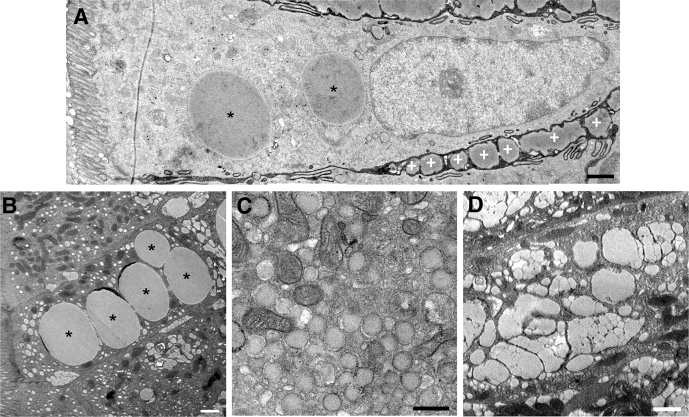
Duodenal biopsy specimens were obtained 1 hour after glucose or water ingestion from 24 participants (Table 2). Enterocytes within biopsy specimens were subjected to ultrastructural analysis using transmission electron microscopy. Consistent with previous observations, the duodenal samples obtained 6 hours after fat ingestion contained considerable quantities of lipids both intracellularly and extracellularly (Figure 2A). Within enterocytes, lipids were observed in large CLDs (Figure 2B), in smaller lipid droplets within the endoplasmic reticulum (ER) (Figure 2C), and within the Golgi (Figure 2D). In addition, secreted CMs were present in the intercellular spaces between enterocytes. Overall, the enterocyte ultrastructure and lipid pools observed in human duodenal enterocytes appeared similar to what has been observed previously in mice.12
Table 2.
Demographics of Aim 2 Participants
| Glucose | Placebo | |
|---|---|---|
| N | 12 | 12 |
| BMI, kg/m2 | 25.3 ± 0.9 | 25.5 ± 1.5 |
| Age, y | 34.6 ± 2.9 | 34.7 ± 3.1 |
| Sex | 4 M/8 F | 2 M/10 F |
NOTE. Data are means ± SE for BMI and age.
BMI, body mass index; F, female; M, male.
Figure 2.
Lipid pools within the intestinal mucosa. (A) A transmission electron microscopy image of an enterocyte from a duodenal biopsy specimen obtained 6 hours after a high-fat liquid meal and 1 hour after glucose ingestion. Lipid present within CLDs is shown (asterisk), as well as in secreted CMs in the intercellular space (white plus symbol). (B) An enterocyte containing lipid within several large CLDs (asterisk). (C) An enterocyte containing lipid within smaller lipid droplets in the ER, which are surrounded by a bilayer membrane and usually are observed at the apical side of the cell. (D) Lipid present within the Golgi of an enterocyte, which normally was observed above the nucleus. Scale bars: 1 μm (A, B, and D), and 0.5 μm (C).
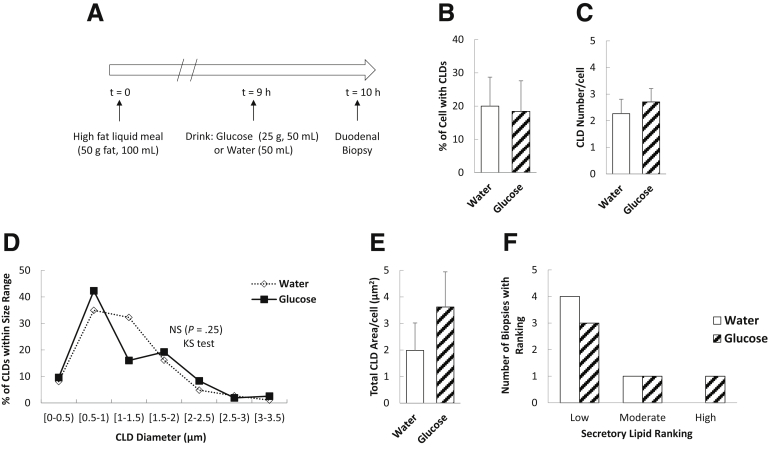
Oral Glucose Mobilizes Enterocyte CLD Stores
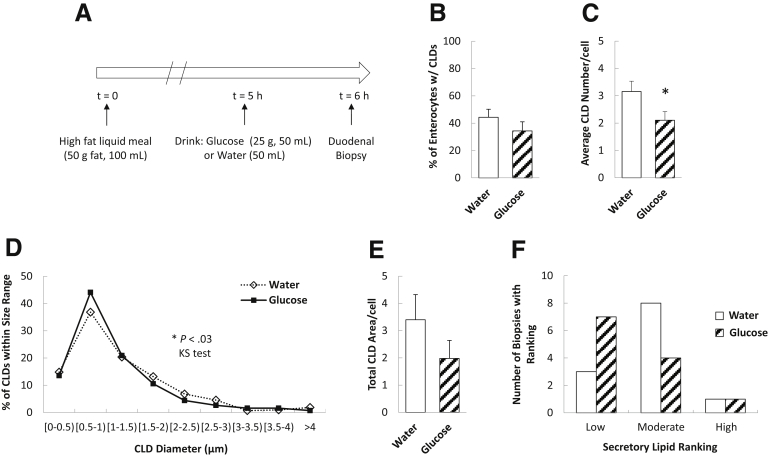
We performed quantitative analyses of enterocyte CLDs in duodenal biopsy specimens obtained in a design similar to that of aim 1 (Figure 3A). After glucose and water ingestion, 34% and 44% of enterocytes per biopsy sample contained CLDs, respectively (Figure 3B) (P = .14). In the samples containing CLDs, there were fewer CLDs per cell in response to glucose compared with water (Figure 3C) (P = .02). Although the average diameters (P = .18) and areas (P = .17) of individual CLDs were not significantly different between treatments (data not shown), there were differences in the CLD diameter distributions, with more CLDs falling into the smaller size ranges and fewer into larger size ranges after glucose compared with water ingestion (Figure 3D) (P = .03). However, the difference between treatments in total CLD area per enterocyte did not reach statistical significance (Figure 3E) (P = .11). We also assessed the amount of lipids within the secretory pathway in enterocytes, which included lipids in the ER, Golgi, and Golgi-derived secretory vesicles. There were no significant differences in the proportion of biopsy specimens containing low, moderate, and high amounts of secretory lipids in response to glucose compared with water ingestion (Figure 3F) (P = .29, Fisher exact test). Taken together, glucose ingestion resulted in fewer CLDs in enterocytes and a shift toward smaller-sized CLDs.
Figure 3.
Oral glucose mobilizes enterocyte CLD stores. (A) Study design. Enterocyte CLD and secretory lipid stores were analyzed 6 hours after a high-fat liquid meal and 1 hour after glucose or water ingestion (N = 12 patients per group). (B) Percentage of enterocytes containing CLDs (P = .14, t test). (C) Average CLD number per cell (P = .022, t test). (D) CLD diameter distribution (P = .03, Kolmogorov–Smirnov test) and (E) average total CLD area per cell (P = .11, t test). (F) Amount of lipid within the secretory pathway (includes lipid in ER, Golgi, and secretory vesicles) (P = .29 Fisher exact test).
Oral Glucose Does Not Mobilize Lipids Within Enterocytes After Delayed Fasting
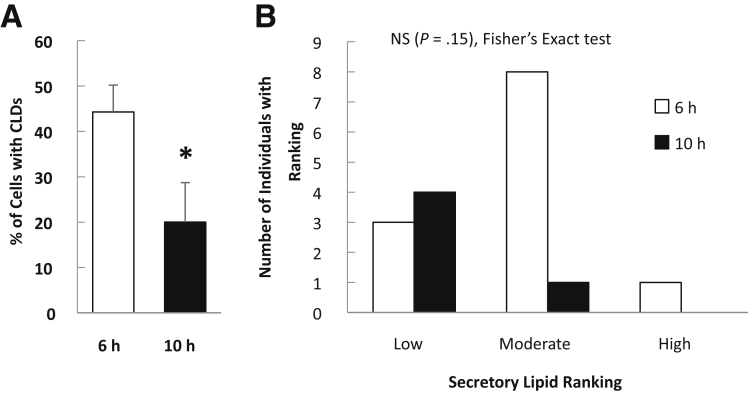
To investigate whether glucose mobilization of intestinal lipid stores persists after more prolonged fasting, a separate group of subjects (Table 3) ingested glucose or water 9 hours after the high-fat liquid meal. Duodenal biopsy specimens were collected 1 hour later (10 hours after ingesting the high-fat liquid meal). Under these conditions, no stimulatory effect of glucose on lipid mobilization was observed. There were no significant differences in the percentage of cells with CLDs, CLD number or size, or in the amount of lipids within the secretory pathway in enterocytes in response to glucose compared with water ingestion (Figure 4). This likely was owing to a lower proportion of enterocytes containing CLDs after prolonged fasting compared with the 6-hour fast (Figure 5). Thus, mobilization of enterocyte CLDs by oral glucose appears to depend on the presence of a sufficient pool of intestinal lipid stores retained in the enterocyte after fat ingestion.
Table 3.
Demographics of Additional Participants Participating in Aim 2 With Delayed Fasting
| Glucose | Placebo | |
|---|---|---|
| N | 5 | 5 |
| BMI, kg/m2 | 23.8 ± 1.2 | 22.2 ± 1.4 |
| Age, y | 33.0 ± 3.8 | 33.6 ± 3.2 |
| Sex | 1 M/4 F | 1 M/4 F |
NOTE. Data are means ± SE for BMI and age.
BMI, body mass index; F, female; M, male.
Figure 4.
Analysis of enterocyte lipid stores in response to glucose or water ingestion after a delayed fast. (A) Study design. Duodenal biopsy specimens were obtained 10 hours after ingestion of a high-fat liquid meal and 1 hour after ingestion of glucose or water. (B) Percentage of enterocytes containing CLDs (P = .45). (C) Average CLD number per cell (P = .28). (D) CLD diameter distribution and (E) average total CLD area per cell (P = .18). (F) Amount of lipid within the secretory pathway (includes lipid in ER, Golgi, and secretory vesicles) (P = 1, Fisher exact test). Average CLD number and total CLD area per cell were compared with a t test.
Figure 5.
Comparison of enterocyte lipid stores after different fasting times. (A) Percentage of cells containing CLDs (P = .025) and (B) amount of lipid within the secretory pathway (P = .15, Fisher exact test) in individuals at 6 hours compared with 10 hours after the high-fat liquid meal (and 1 h after water consumption). *P < .05, t test.
Differential Expression of Proteins in Duodenal Biopsy Specimens From Subjects Administered Glucose or Water After a High-Fat Liquid Meal
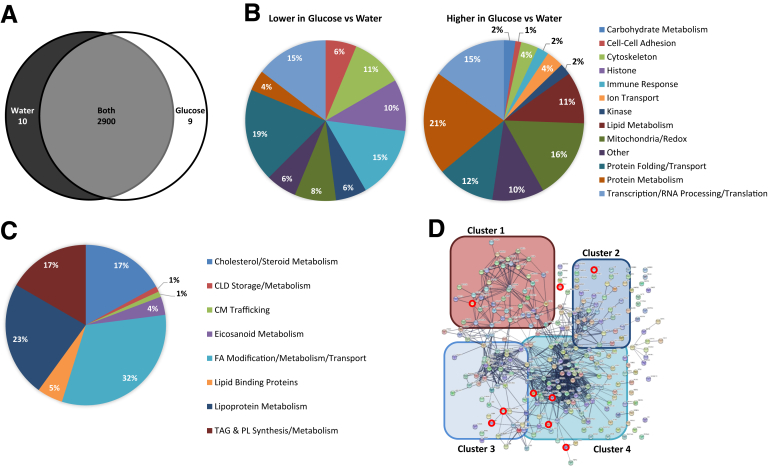
Untargeted proteomic analysis of duodenal biopsy specimens identified 2919 proteins, with 2900 present in both water and glucose ingestion conditions, only 9 were identified in response to glucose and only 10 were identified in response to water ingestion (Figure 6A). A total of 48 of these proteins were present at relatively lower levels and 86 were present at relatively higher levels in response to glucose compared with water (Table 4). After correction for multiple statistical tests, the relative levels of 7 proteins remained significantly different between treatments.
Figure 6.
Proteins present in duodenal biopsy specimens from subjects administered glucose or water after a high-fat liquid meal. Duodenal biopsy specimens were collected 6 hours after lipid and 1 hour after glucose or water ingestion from patients undergoing a diagnostic endoscopy (N = 12 patients per group). (A) Venn diagram of proteins identified in response to glucose or water ingestion. Proteins present in at least 3 samples in 1 group and 0 samples in the other group were considered present in only 1 group. Proteins identified in at least 3 samples in 1 group and at least 1 sample in the other group were considered present in both groups. (B) Percentage of proteins within broad functional groups that were present at either relatively lower (48 total proteins) or relatively higher (86 total proteins) levels in response to glucose compared with water ingestion, as classified based on their biological/molecular functions. Only proteins that were identified in at least 3 samples in both groups and present at relatively different levels (P < .05, t test), or at least 3 samples in 1 group and 0 samples in the other group, were included in this classification. A Database for Annotation, Visualization, and Integrated Discovery search of the 2919 identified proteins resulted in the identification of 270 proteins with GO terms related to lipid metabolism. (C) Percentage of the 270 lipid metabolism-related proteins involved in more specific lipid-related functions. (D) String analysis of the 270 lipid metabolism-related proteins. The thickness of the line represents the strength of evidence of a structural/functional relationship between 2 proteins. Cluster 1 is enriched in proteins involved in TG and phospholipid (PL) synthesis and metabolism, cluster 2 is enriched in proteins involved in lipoprotein metabolism, cluster 3 is enriched in proteins involved in cholesterol/steroid metabolism, and cluster 4 is enriched in proteins involved in FA modification/metabolism/transport. Proteins that were present at relatively different levels in response to glucose compared with water consumption (P < .05, t test) are circled in red. TAG, triacylglycerol.
Table 4.
Proteins Present at Relatively Different Levels in Duodenal Biopsy Specimens From Subjects Administered Glucose or Water After a High-Fat Liquid Meal
| Uniprot accession | Protein name | Gene name | Fold change | t test P value | Function |
|---|---|---|---|---|---|
| P05997 | Collagen α-2(V) chaina | COL5A2 | -7.4957 | .0030 | Other (extracellular matrix protein) |
| Q5T5C0 | Syntaxin-binding protein 5a | STXBP5 | -6.8628 | 4.72E-22 | Protein folding/transport |
| Q8N2S1 | Latent-transforming growth factor β-binding protein 4a | LTBP4 | -6.7854 | .0002 | Protein folding/transport |
| O14672 | Disintegrin and metalloproteinase domain-containing protein 10a | ADAM10 | -6.7264 | .0010 | Protein metabolism |
| P17480 | Nucleolar transcription factor 1a | UBTF | -6.2100 | .0063 | Transcription/RNA processing/translation |
| Q8N8S7 | Protein enabled homologa | ENAH | -5.7620 | .0007 | Cytoskeleton |
| Q7Z6K5 | Arpina | ARPIN | -5.7573 | 2.02E-19 | Cytoskeleton |
| Q9UQ35 | Serine/arginine repetitive matrix protein 2a | SRRM2 | -5.5687 | .0002 | Transcription/RNA processing/translation |
| P49790 | Nuclear pore complex protein Nup153a | NUP153 | -5.1794 | .0079 | Transcription/RNA processing/translation |
| Q9NRG7 | Epimerase family protein SDR39U1a | SDR39U1 | -4.1142 | 3.26E-28 | Mitochondria/redox |
| P16403 | Histone H1.2 | HIST1H1C | -1.0513 | .0213 | Histone |
| P01860 | Immunoglobulin heavy constant γ 3 | IGHG3 | -1.0063 | .0007 | Immune response |
| P13284 | γ-interferon–inducible lysosomal thiol reductase | IFI30 | -1.0048 | .0116 | Mitochondria/redox |
| P35580 | Myosin-10 | MYH10 | -0.9176 | .0449 | Cytoskeleton |
| P08590 | Myosin light chain 3 | MYL3 | -0.8947 | .0464 | Other (regulation of muscle contraction) |
| Q71UI9 | Histone H2A.V | H2AFV | -0.8933 | .0072 | Histone |
| Q71DI3 | Histone H3.2 | -0.8572 | .0338 | Histone | |
| A0A0B4J1X5 | Immunoglobulin heavy variable 3–74 | IGHV3-74 | -0.8112 | .0076 | Immune response |
| Q99829 | Copine-1 | CPNE1 | -0.7877 | .0101 | Transcription/RNA processing/translation |
| P01780 | Immunoglobulin heavy variable 3–7 | IGHV3-7 | -0.7127 | .0154 | Immune response |
| P42167 | Lamina-associated polypeptide 2; isoforms β/γ | TMPO | -0.6933 | .0453 | Cell–cell adhesion |
| P0CG06 | Immunoglobulin λ constant 2 | IGLC2 | -0.6852 | .0260 | Immune response |
| Q96KA5 | Cleft lip and palate transmembrane protein 1–like protein | CLPTM1L | -0.6539 | .0031 | Other (apoptosis) |
| P01859 | Immunoglobulin heavy constant γ 2 | IGHG2 | -0.6532 | .0234 | Immune response |
| P84243 | Histone H3.3 | H3F3A | -0.6487 | .0236 | Histone |
| Q8IUX7 | Adipocyte enhancer-binding protein 1 | AEBP1 | -0.637 | .0226 | Transcription/RNA processing/translation |
| Q09666 | Neuroblast differentiation-associated protein AHNAK | AHNAK | -0.635 | .0397 | Cell–cell adhesion |
| Q9BY50 | Signal peptidase complex catalytic subunit SEC11C | SEC11C | -0.6182 | .0288 | Protein metabolism |
| P07305 | Histone H1.0 | H1F0 | -0.5824 | .0446 | Histone |
| P01857 | Immunoglobulin heavy constant γ 1 | IGHG1 | -0.5542 | .0206 | Immune response |
| Q9UEW8 | STE20/SPS1-related proline-alanine–rich protein kinase | STK39 SPAK | -0.5436 | .0033 | Kinase |
| P30405 | Peptidyl-prolyl cis-trans isomerase F; mitochondrial | PPIF | -0.5252 | .0168 | Protein folding/transport |
| P56378 | 6.8-kilodalton mitochondrial proteolipid | MP68 | -0.4738 | .0418 | Mitochondria/redox |
| P61758 | Prefoldin subunit 3 | VBP1 | -0.4401 | .0427 | Protein folding/transport |
| Q96L92 | Sorting nexin-27 | SNX27 | -0.427 | .0158 | Protein folding/transport |
| O75323 | Protein NipSnap homolog 2 | GBAS | -0.4059 | .0315 | Mitochondria/redox |
| O75190 | DnaJ homolog subfamily B member 6 | DNAJB6 | -0.3578 | .0384 | Protein folding/transport |
| Q86UP2 | Kinectin | KTN1 | -0.3174 | .0401 | Cell–cell adhesion |
| Q15629 | Translocating chain-associated membrane protein 1 | TRAM1 | -0.3048 | .0452 | Protein folding/transport |
| Q9BWS9 | Chitinase domain-containing protein 1 | CHID1 | -0.2768 | .0223 | Immune response |
| O00186 | Syntaxin-binding protein 3 | STXBP3 | -0.2611 | .0332 | Protein folding/transport |
| Q02543 | 60S ribosomal protein L18a | RPL18A | -0.2238 | .0089 | Transcription/RNA processing/translation |
| P13861 | cAMP-dependent protein kinase type II-α regulatory subunit | PRKAR2A | -0.217 | .0098 | Kinase |
| P84085 | ADP-ribosylation factor 5 | ARF5 | -0.1788 | .0354 | Protein folding/transport |
| P28482 | Mitogen-activated protein kinase 1 | MAPK1 | -0.1528 | .0078 | Kinase |
| P59998 | Actin-related protein 2/3 complex subunit 4 | ARPC4 | -0.1477 | .0141 | Cytoskeleton |
| O15145 | Actin-related protein 2/3 complex subunit 3 | ARPC3 | -0.145 | .0464 | Cytoskeleton |
| Q5VTE0 | Putative elongation factor 1-α–like 3 | EEF1A1P5 | -0.134 | .0151 | Transcription/RNA processing/translation |
| Q8IZ83 | Aldehyde dehydrogenase family 16 member A1 | ALDH16A1 | 0.1539 | .0433 | Mitochondria/redox |
| Q96A33 | Coiled-coil domain-containing protein 47 | CCDC47 | 0.1676 | .0403 | Other (calcium ion homeostasis, ERAD) |
| Q9NPA0 | ER membrane protein complex subunit 7 | EMC7 | 0.1744 | .0474 | Other (carbohydrate binding) |
| Q15417 | Calponin-3 | CNN3 | 0.1993 | .0488 | Cytoskeleton |
| P21281 | V-type proton ATPase subunit B; brain isoform | ATP6V1B2 | 0.2023 | .0460 | Ion transport |
| P48556 | 26S proteasome non-ATPase regulatory subunit 8 | PSMD8 | 0.209 | .0347 | Protein metabolism |
| O14734 | Acyl-coenzyme A thioesterase 8 | ACOT8 | 0.2101 | .0177 | Lipid metabolism |
| Q9NS69 | Mitochondrial import receptor subunit TOM22 homolog | TOMM22 | 0.2105 | .0281 | Mitochondria/redox |
| P11940 | Polyadenylate-binding protein 1 | PABPC1 | 0.2111 | .0441 | Transcription/RNA processing/translation |
| P78344 | Eukaryotic translation initiation factor 4 γ 2 | EIF4G2 | 0.2114 | .0490 | Transcription/RNA processing/translation |
| Q14974 | Importin subunit β-1 | KPNB1 | 0.2177 | .0417 | Protein folding/transport |
| Q13200 | 26S proteasome non-ATPase regulatory subunit 2 | PSMD2 | 0.2182 | .0108 | Protein metabolism |
| O95782 | AP-2 complex subunit α-1 | AP2A1 | 0.219 | .0059 | Protein folding/transport |
| Q93034 | Cullin-5 | CUL5 | 0.2198 | .0058 | Protein metabolism |
| Q9UNZ2 | NSFL1 cofactor p47 | NSFL1C | 0.2214 | .0150 | Protein metabolism |
| Q9BTM9 | Ubiquitin-related modifier 1 | URM1 | 0.2221 | .0047 | Transcription/RNA processing/translation |
| O75436 | Vacuolar protein sorting-associated protein 26A | VPS26A | 0.2305 | .0230 | Protein folding/transport |
| P25788 | Proteasome subunit α type-3 | PSMA3 | 0.2323 | .0358 | Protein metabolism |
| Q9Y2Z0 | Protein SGT1 homolog | SUGT1 | 0.2356 | .0128 | Protein metabolism |
| Q9P2J5 | Leucine-tRNA ligase; cytoplasmic | LARS | 0.2405 | .0207 | Transcription/RNA processing/translation |
| P38606 | V-type proton ATPase catalytic subunit A | ATP6V1A | 0.2418 | .0123 | Ion transport |
| Q93008 | Probable ubiquitin carboxyl-terminal hydrolase FAF-X | USP9X | 0.2423 | .0467 | Protein metabolism |
| P11142 | Heat shock cognate 71-kilodalton protein | HSPA8 | 0.253 | .0051 | Protein folding/transport |
| P55060 | Exportin-2 | CSE1L | 0.2541 | .0385 | Protein folding/transport |
| O75146 | Huntingtin-interacting protein 1–related protein | HIP1R | 0.259 | .0285 | Cytoskeleton |
| O96008 | Mitochondrial import receptor subunit TOM40 homolog | TOMM40 | 0.2593 | .0076 | Mitochondria/redox |
| P15531 | Nucleoside diphosphate kinase A | NME1 | 0.271 | .0213 | Kinase |
| P46734 | Dual-specificity mitogen-activated protein kinase kinase 3 | MAP2K3 | 0.2752 | .0373 | Kinase |
| P28070 | Proteasome subunit β type-4 | PSMB4 | 0.2764 | .0158 | Protein Metabolism |
| O75381 | Peroxisomal membrane protein PEX14 | PEX14 | 0.2803 | .0238 | Protein folding/transport |
| Q9NUQ8 | ATP-binding cassette subfamily F member 3 | ABCF3 | 0.2846 | .0347 | Cell–cell adhesion |
| Q9Y697 | Cysteine desulfurase; mitochondrial | NFS1 | 0.2919 | .0167 | Protein metabolism |
| Q02790 | Peptidyl-prolyl cis-trans isomerase FKBP4 | FKBP4 | 0.2953 | .0477 | Protein folding/transport |
| Q15020 | Squamous cell carcinoma antigen recognized by T cell 3 | SART3 | 0.3007 | .0457 | Transcription/RNA processing/translation |
| Q01813 | ATP-dependent 6-phosphofructokinase; platelet type | PFKP | 0.3087 | .0367 | Carbohydrate metabolism |
| Q5H9R7 | Serine/threonine-protein phosphatase 6 regulatory subunit 3 | PPP6R3 | 0.3154 | .0231 | Protein metabolism |
| O95433 | Activator of 90-kilodalton heat shock protein ATPase homolog 1 | AHSA1 | 0.3177 | .0091 | Protein folding/transport |
| O00231 | 26S proteasome non-ATPase regulatory subunit 11 | PSMD11 | 0.3221 | .0158 | Protein metabolism |
| P31689 | DnaJ homolog subfamily A member 1 | DNAJA1 | 0.3234 | .0479 | Protein folding/transport |
| O75915 | PRA1 family protein 3 | ARL6IP5 | 0.3236 | .0439 | Cytoskeleton |
| Q9ULA0 | Aspartyl aminopeptidase | DNPEP | 0.3283 | .0271 | Protein metabolism |
| Q99757 | Thioredoxin; mitochondrial | TXN2 | 0.3371 | .0343 | Mitochondria/redox |
| Q9NTX5 | Ethylmalonyl-CoA decarboxylase | ECHDC1 | 0.341 | .0450 | Lipid metabolism |
| Q96GK7 | Fumarylacetoacetate hydrolase domain-containing protein 2A | FAHD2A | 0.35 | .0195 | Other (potential hydrolase) |
| Q9Y3D9 | 28S ribosomal protein S23; mitochondrial | MRPS23 | 0.3593 | .0114 | Transcription/RNA processing/translation |
| P23526 | Adenosylhomocysteinase | AHCY | 0.3976 | .0216 | Other (regulation of methylation) |
| P18827 | Syndecan-1 | SDC1 | 0.4174 | .0314 | Other (cell migration) |
| P08621 | U1 small nuclear ribonucleoprotein 70 kilodaltons | SNRNP70 | 0.4216 | .0144 | Transcription/RNA processing/translation |
| P28838 | Cytosol aminopeptidase | LAP3 | 0.4431 | .0436 | Protein metabolism |
| Q9NR19 | Acetyl-coenzyme A synthetase; cytoplasmic | ACSS2 | 0.4512 | .0470 | Lipid metabolism |
| Q8N5G0 | Small integral membrane protein 20 | SMIM20 | 0.4532 | .0296 | Mitochondria/redox |
| P49247 | Ribose-5-phosphate isomerase | RPIA | 0.4698 | .0326 | Carbohydrate metabolism |
| Q9Y333 | U6 snRNA-associated Sm-like protein LSm2 | LSM2 | 0.4741 | .0355 | Transcription/RNA processing/translation |
| Q9H490 | Phosphatidylinositol glycan anchor biosynthesis class U protein | PIGU | 0.4799 | .0487 | Lipid metabolism |
| O75382 | Tripartite motif-containing protein 3 | TRIM3 | 0.485 | .0174 | Immune response |
| Q15125 | 3-β-hydroxysteroid-Δ(8); Δ(7)-isomerase | EBP | 0.4859 | .0413 | Lipid metabolism |
| Q16881 | Thioredoxin reductase 1; cytoplasmic | TXNRD1 | 0.4905 | .0148 | Mitochondria/redox |
| P07108 | Acyl-CoA binding protein | DBI | 0.4917 | .0382 | Lipid metabolism |
| P48637 | Glutathione synthetase | GSS | 0.4944 | .0440 | Other (glutathione synthesis) |
| O76003 | Glutaredoxin-3 | GLRX3 | 0.4953 | .0403 | Mitochondria/redox |
| Q12882 | Dihydropyrimidine dehydrogenase [NADP(+)] | DPYD | 0.5076 | .0165 | Mitochondria/redox |
| Q9NWU5 | 39S ribosomal protein L22; mitochondrial | MRPL22 | 0.526 | .0101 | Transcription/RNA processing/translation |
| Q9NVS9 | Pyridoxine-5'-phosphate oxidase | PNPO | 0.5395 | .0337 | Mitochondria/redox |
| Q9UHY7 | Enolase-phosphatase E1 | ENOPH1 | 0.5544 | .0307 | Protein metabolism |
| P16930 | Fumarylacetoacetase | FAH | 0.5694 | .0236 | Protein metabolism |
| P48506 | Glutamate-cysteine ligase catalytic subunit | GCLC | 0.5745 | .0241 | Mitochondria/redox |
| Q8N983 | 39S ribosomal protein L43; mitochondrial | MRPL43 | 0.5932 | .0266 | Transcription/RNA processing/translation |
| Q9UBM7 | 7-dehydrocholesterol reductase | DHCR7 | 0.6045 | .0283 | Lipid metabolism |
| P48147 | Prolyl endopeptidase | PREP | 0.6099 | .0244 | Protein metabolism |
| P82673 | 28S ribosomal protein S35; mitochondrial | MRPS35 | 0.6118 | .0363 | Transcription/RNA processing/translation |
| Q8WVX9 | Fatty acyl-CoA reductase 1 | FAR1 | 0.6231 | .0223 | Lipid metabolism |
| Q9Y679 | Ancient ubiquitous protein 1 | AUP1 | 0.6292 | .0093 | Protein metabolism |
| P37840 | α-synuclein | SNCA | 0.6332 | .0063 | Mitochondria/redox |
| Q9BRF8 | Serine/threonine-protein phosphatase CPPED1 | CPPED1 | 0.6702 | .0313 | Protein metabolism |
| Q6UX53 | Methyltransferase-like protein 7B | METTL7B | 0.7067 | .0210 | Other (probable methyltransferase) |
| P02792 | Ferritin light chain | FTL | 0.8169 | .0124 | Ion transport |
| P02794 | Ferritin heavy chain | FTH1 | 0.8959 | .0144 | Mitochondria/redox |
| Q9BVL4 | Selenoprotein Ob | SELENOO | 5.0987 | .0002 | Mitochondria/redox |
| Q9HB07 | UPF0160 protein MYG1; mitochondrialb | C12orf10 | 5.9843 | 5.8E-16 | Mitochondria/redox |
| P22090 | 40S ribosomal protein S4b | RPS4Y1 | 6.7537 | .0018 | Transcription/RNA processing/translation |
| Q9P003 | Protein cornichon homolog 4b | CNIH4 | 7.1618 | 1.74E-15 | Protein folding/transport |
| O60938 | Keratocanb | KERA | 7.4582 | .0050 | Other (keratan sulfate metabolism/cornea development) |
| Q9C0D9 | Ethanolaminephosphotransferase 1b | SELENOI | 7.4638 | .0005 | Lipid metabolism |
| Q96HV5 | Transmembrane protein 41Ab | TMEM41A | 7.5997 | .0023 | Other (transmembrane protein) |
| P62306 | Small nuclear ribonucleoprotein Fb | SNRPF | 7.7847 | 3.21E-17 | Transcription/RNA processing/translation |
| Q8NDA2 | Hemicentin-2b | HMCN2 | 11.5204 | 3.81E-16 | Immune response |
NOTE. Proteins identified in at least 3 samples in both groups, or at least 3 samples in 1 group and 0 samples in the other group, were compared. Proteins present at significantly different levels within the 2 treatment groups (P < .05, t test) are shown. Average fold change of proteins in response to glucose relative to water consumption are presented. Numbers in the “Fold change” column represent how much higher (or lower if negative) the protein levels were in the glucose group compared with the water group. Proteins are listed in descending order according to relative fold change, with negative fold change values indicating relative down-regulation by glucose (listed at the top of the table) followed by those up-regulated by glucose indicated by a positive fold change (with greatest positive fold change listed at the bottom of table).
ADP, adenosine diphosphate; ATP, adenosine triphosphate; ATPase, adenosine triphosphatase; cAMP, cyclic adenosine monophosphate; ERAD, endoplasmic-reticulum-associated protein degradation; NADP, nicotinamide adenine dinucleotide phosphate; redox, reduction-oxidation; tRNA, transfer ribonucleic acid.
Only identified in response to water.
Only identified in response to glucose.
The differentially expressed proteins (defined as P < .05 between treatment groups) were classified into broad groups based on gene ontology (GO) terms for biological processes and molecular functions (Table 5, Figure 6B). Among the 48 proteins present at relatively lower levels in response to glucose, protein folding/transport (19%), immune response (15%), and transcription/RNA processing/translation (15%) were the most abundant functions. Of the 86 proteins present at relatively higher levels in response to glucose, those involved in protein metabolism (21%), mitochondria/redox (16%), and transcription/RNA processing/translation (15%) were the most abundant. Interestingly, in response to glucose compared with water ingestion, histone proteins were present at relatively lower levels, while those involved in carbohydrate metabolism, ion transport, and lipid metabolism all were present at relatively higher levels.
Table 5.
GO Terms Associated With Lipid Metabolism-Related Proteins Present in Duodenal Biopsy Specimens 6 Hours After a High-Fat Liquid Meal
| Cholesterol/steroid metabolism | |
| UP_KEYWORDS | Cholesterol biosynthesis |
| GOTERM_BP_DIRECT | Cholesterol biosynthetic process |
| UP_KEYWORDS | Cholesterol metabolism |
| UP_KEYWORDS | Steroid biosynthesis |
| KEGG_PATHWAY | Steroid hormone biosynthesis |
| UP_KEYWORDS | Steroid metabolism |
| UP_KEYWORDS | Sterol biosynthesis |
| GOTERM_MF_DIRECT | Sterol esterase activity |
| UP_KEYWORDS | Sterol metabolism |
| CLD storage/metabolism | |
| GOTERM_CC_DIRECT | Lipid droplet |
| GPTERM_BP_DIRECT | Lipid storage |
| CM trafficking | |
| GOTERM_CC_DIRECT | COPII vesicle coat |
| GOTERM_CC_DIRECT | ER to Golgi transport vesicle membrane |
| GOTERM_MF_DIRECT | SNARE binding |
| GOTERM_CC_DIRECT | SNARE complex |
| GOTERM_BP_DIRECT | Vesicle fusion |
| Eicosanoid metabolism | |
| UP_KEYWORDS | Leukotriene biosynthesis |
| GOTERM_BP_DIRECT | Leukotriene biosynthetic process |
| GOTERM_BP_DIRECT | Leukotriene metabolic process |
| GOTERM_BP_DIRECT | Prostaglandin biosynthetic process |
| FA modification/metabolism/transport | |
| GOTERM_BP_DIRECT | Fatty acid biosynthetic process |
| GOTERM_MF_DIRECT | 3-hydroxyacyl-CoA dehydrogenase activity |
| INTERPRO | 3-hydroxyacyl-CoA dehydrogenase, conserved site |
| INTERPRO | 3-hydroxyacyl-CoA dehydrogenase, C-terminal |
| INTERPRO | 3-hydroxyacyl-CoA dehydrogenase, NAD binding |
| GOTERM_MF_DIRECT | Acyl-CoA dehydrogenase activity |
| INTERPRO | Acyl-CoA dehydrogenase, conserved site |
| INTERPRO | Acyl-CoA dehydrogenase/oxidase |
| INTERPRO | Acyl-CoA dehydrogenase/oxidase C-terminal |
| INTERPRO | Acyl-CoA dehydrogenase/oxidase, N-terminal |
| GOTERM_MF_DIRECT | Acyl-CoA hydrolase activity |
| GOTERM_BP_DIRECT | Acyl-CoA metabolic process |
| INTERPRO | Acyl-CoA oxidase |
| PIR_SUPERFAMILY | Acyl-CoA oxidase |
| INTERPRO | Acyl-CoA oxidase, C-terminal |
| INTERPRO | Acyl-CoA oxidase/dehydrogenase, central domain |
| INTERPRO | AMP binding, conserved site |
| INTERPRO | AMP-dependent synthetase/ligase |
| GOTERM_MF_DIRECT | Decanoate-CoA ligase activity |
| INTERPRO | Domain of unknown function DUF4009 |
| GOTERM_BP_DIRECT | Fatty acid β-oxidation |
| GOTERM_BP_DIRECT | Fatty acid β-oxidation using acyl-CoA dehydrogenase |
| GOTERM_BP_DIRECT | Fatty acid β-oxidation using acyl-CoA oxidase |
| KEGG_PATHWAY | Fatty acid biosynthesis |
| KEGG_PATHWAY | Fatty acid degradation |
| GOTERM_BP_DIRECT | Fatty acid elongation |
| GOTERM_BP_DIRECT | Fatty acid metabolic process |
| KEGG_PATHWAY | Fatty acid metabolism |
| GOTERM_BP_DIRECT | Fatty acid transport |
| GOTERM_MF_DIRECT | Fatty-acyl-CoA binding |
| GOTERM_BP_DIRECT | Fatty-acyl-CoA biosynthetic process |
| GOTERM_BP_DIRECT | Lipid homeostasis |
| GOTERM_BP_DIRECT | Long-chain fatty acid import |
| GOTERM_BP_DIRECT | Long-chain fatty acid metabolic process |
| GOTERM_MF_DIRECT | Long-chain fatty acid-CoA ligase activity |
| GOTERM_BP_DIRECT | Long-chain fatty-acyl-CoA biosynthetic process |
| GOTERM_BP_DIRECT | Long-chain fatty-acyl-CoA metabolic process |
| GOTERM_MF_DIRECT | Very long-chain fatty acid-CoA ligase activity |
| Lipid binding proteins | |
| INTERPRO | Acyl-CoA-binding protein, ACBP |
| INTERPRO | Acyl-CoA-binding protein, ACBP, conserved site |
| INTERPRO | Cytosolic fatty-acid binding |
| UP_SEQ_FEATURE | Domain: ACB |
| INTERPRO | Lipocalin/cytosolic fatty-acid binding protein domain |
| INTERPRO | Lipocalin/cytosolic fatty-acid binding protein domain |
| GOTERM_MF_DIRECT | Retinal binding |
| GOTERM_MF_DIRECT | Retinoic acid binding |
| GOTERM_MF_DIRECT | Retinoid binding |
| GOTERM_MF_DIRECT | Retinol binding |
| UP_KEYWORDS | Retinol binding |
| UP_KEYWORDS | Retinol binding |
| UP_KEYWORDS | Vitamin A |
| Lipoprotein metabolism | |
| INTERPRO | Apolipoprotein A1/A4/E |
| GOTERM_MF_DIRECT | Cholesterol binding |
| GOTERM_BP_DIRECT | Cholesterol efflux |
| GOTERM_BP_DIRECT | Cholesterol homeostasis |
| GOTERM_BP_DIRECT | Cholesterol metabolic process |
| GOTERM_MF_DIRECT | Cholesterol transporter activity |
| GOTERM_CC_DIRECT | Chylomicron |
| UP_KEYWORDS | Chylomicron |
| GOTERM_BP_DIRECT | Chylomicron remnant clearance |
| UP_KEYWORDS | HDL |
| GOTERM_CC_DIRECT | High-density lipoprotein particle |
| GOTERM_BP_DIRECT | High-density lipoprotein particle assembly |
| GOTERM_BP_DIRECT | High-density lipoprotein particle clearance |
| GOTERM_MF_DIRECT | High-density lipoprotein particle receptor binding |
| GOTERM_BP_DIRECT | High-density lipoprotein particle remodeling |
| GOTERM_CC_DIRECT | Intermediate-density lipoprotein particle |
| UP_KEYWORDS | LDL |
| SMART | LDLa |
| GOTERM_MF_DIRECT | Lipase inhibitor activity |
| UP_KEYWORDS | Lipid transport |
| GOTERM_BP_DIRECT | Lipid transport |
| GOTERM_MF_DIRECT | Lipid transporter activity |
| GOTERM_BP_DIRECT | Lipoprotein biosynthetic process |
| GOTERM_BP_DIRECT | Lipoprotein metabolic process |
| INTERPRO | LDL-receptor class A repeat |
| INTERPRO | LDL-receptor class A, conserved site |
| GOTERM_CC_DIRECT | LDL particle |
| GOTERM_BP_DIRECT | LDL particle remodeling |
| GOTERM_BP_DIRECT | Negative regulation of cholesterol transport |
| GOTERM_BP_DIRECT | Negative regulation of lipid catabolic process |
| GOTERM_BP_DIRECT | Negative regulation of lipid metabolic process |
| GOTERM_BP_DIRECT | Negative regulation of receptor-mediated endocytosis |
| GOTERM_BP_DIRECT | Negative regulation of VLDL particle clearance |
| GOTERM_BP_DIRECT | Negative regulation of VLDL particle remodeling |
| GOTERM_BP_DIRECT | Neuron projection regeneration |
| GOTERM_MF_DIRECT | Phosphatidylcholine binding |
| GOTERM_MF_DIRECT | Phosphatidylcholine-sterol O-acyltransferase activator activity |
| GOTERM_BP_DIRECT | Phospholipid efflux |
| GOTERM_BP_DIRECT | Positive regulation of cholesterol esterification |
| GOTERM_BP_DIRECT | Positive regulation of fatty acid biosynthetic process |
| GOTERM_BP_DIRECT | Positive regulation of lipoprotein lipase activity |
| GOTERM_BP_DIRECT | Positive regulation of triglyceride catabolic process |
| GOTERM_BP_DIRECT | Regulation of Cdc42 protein signal transduction |
| GOTERM_BP_DIRECT | Regulation of intestinal cholesterol absorption |
| GOTERM_BP_DIRECT | Reverse cholesterol transport |
| GOTERM_CC_DIRECT | Spherical HDL particle |
| GOTERM_BP_DIRECT | Triglyceride homeostasis |
| GOTERM_CC_DIRECT | VLDL particle |
| GOTERM_BP_DIRECT | VLDL particle remodeling |
| UP_KEYWORDS | VLDL |
| TAG and PL synthesis/metabolism | |
| GOTERM_MF_DIRECT | 1-acylglycerol-3-phosphate O-acyltransferase activity |
| GOTERM_BP_DIRECT | Acylglycerol catabolic process |
| GOTERM_MF_DIRECT | Acylglycerol lipase activity |
| GOTERM_BP_DIRECT | CDP-diacylglycerol biosynthetic process |
| GOTERM_BP_DIRECT | Ether lipid biosynthetic process |
| GOTERM_BP_DIRECT | Glycerolipid metabolic process |
| GOTERM_BP_DIRECT | Glycerophospholipid biosynthetic process |
| GOTERM_BP_DIRECT | Glycerophospholipid catabolic process |
| KEGG_PATHWAY | Glycerophospholipid metabolism |
| GOTERM_BP_DIRECT | GPI anchor biosynthetic process |
| GOTERM_MF_DIRECT | Lysophospholipase activity |
| GOTERM_BP_DIRECT | Phosphatidic acid biosynthetic process |
| UP_KEYWORDS | Phospholipid biosynthesis |
| GOTERM_BP_DIRECT | Phospholipid biosynthetic process |
| GOTERM_BP_DIRECT | Phospholipid catabolic process |
| GOTERM_BP_DIRECT | Phospholipid metabolic process |
| UP_KEYWORDS | Phospholipid metabolism |
| GOTERM_BP_DIRECT | Phospholipid transport |
| INTERPRO | Phospholipid/glycerol acyltransferase |
| SMART | PlsC |
| UP_SEQ_FEATURE | Short sequence motif: HXXXXD motif |
| GOTERM_BP_DIRECT | Triglyceride biosynthetic process |
| GOTERM_MF_DIRECT | Triglyceride lipase activity |
ABCP, Acyl-CoA-binding protein; ACB, acyl-CoA-binding; AMP, Adenosine monophosphate; CDP, Cytidine Diphosphate; COPII, cytoplasmic coat protein complex II; GPI, glycosylphosphatidylinositol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LDLa, low-density lipoprotein receptor domain class A; NAD, Nicotinamide adenine dinucleotide.
Because our goal was to identify mechanisms by which intestinal lipid stores are mobilized in response to glucose ingestion, we then specifically examined lipid metabolism-related proteins. Of the 2919 proteins identified, 270 (9%) are known to be involved in lipid/lipoprotein metabolism and transport. The majority of these proteins are involved in FA modification, metabolism, and transport (32%, cluster 4) and lipoprotein metabolism (23%, cluster 2); however, proteins involved in cholesterol/steroid metabolism (cluster 3), TG/phospholipid metabolism (cluster 1), lipid binding, eicosanoid metabolism, CLD storage/metabolism, and CM trafficking also were identified (Figure 6C and D). In response to glucose compared with water ingestion, 9 of these lipid-related proteins were present at relatively higher levels (P < .05) (Table 6). Of note, ethanolaminephosphotransferase 1 was identified only in response to glucose ingestion.
Table 6.
Lipid Metabolism Proteins Present at Relatively Different Levels in Duodenal Biopsy Specimens From Subjects Administered Glucose or Water After a High-Fat Liquid Meal
| Uniprot accession | Protein name | Gene name | Fold change | t test P value | Lipid metabolism-related function |
|---|---|---|---|---|---|
| O14734 | Acyl-coenzyme A thioesterase 8 | ACOT8 | 0.2101 | .0177 | FA modification/ metabolism/transport |
| Q9NTX5 | Ethylmalonyl-CoA decarboxylase | ECHDC1 | 0.341 | .0450 | FA modification/ metabolism/transport |
| Q9NR19 | Acetyl-coenzyme A synthetase; cytoplasmic | ACSS2 | 0.4512 | .0470 | FA modification/ metabolism/transport |
| Q9H490 | Phosphatidylinositol glycan anchor biosynthesis class U protein | PIGU | 0.4799 | .0487 | TAG and PL synthesis/metabolism |
| Q15125 | 3-β-hydroxysteroid-Δ(8); Δ(7)-isomerase | EBP | 0.4859 | .0413 | Cholesterol/steroid metabolism |
| P07108 | Acyl-CoA-binding protein | DBI | 0.4917 | .0382 | Lipid binding protein |
| Q9UBM7 | 7-dehydrocholesterol reductase | DHCR7 | 0.6045 | .0283 | Cholesterol/steroid metabolism |
| Q8WVX9 | Fatty acyl-CoA reductase 1 | FAR1 | 0.6231 | .0223 | FA modification/metabolism/transport |
| Q9C0D9 | Ethanolaminephosphotransferase 1a | SELENOI | 7.4638 | .0005 | TAG and PL synthesis/metabolism |
NOTE. Proteins known to play a role in lipid metabolism were identified based on GO terms. Relative levels of proteins identified in at least 3 duodenal biopsy samples per group, or identified in at least 3 samples in 1 group and 0 samples in the other group, were compared. Proteins present at significantly different levels within the 2 treatment groups (P < .05, t test) are shown. Average fold changes of proteins in response to glucose relative to water consumption are presented. Numbers in the “Fold change” column represent how much higher (or lower if negative) the protein levels were in the glucose group compared with the water group. All of these lipid metabolism proteins were up-regulated by glucose relative to water consumption and are listed in ascending order of magnitude of fold change.
PL, phospholipid; TAG, triacylglycerol.
Only identified in response to glucose.
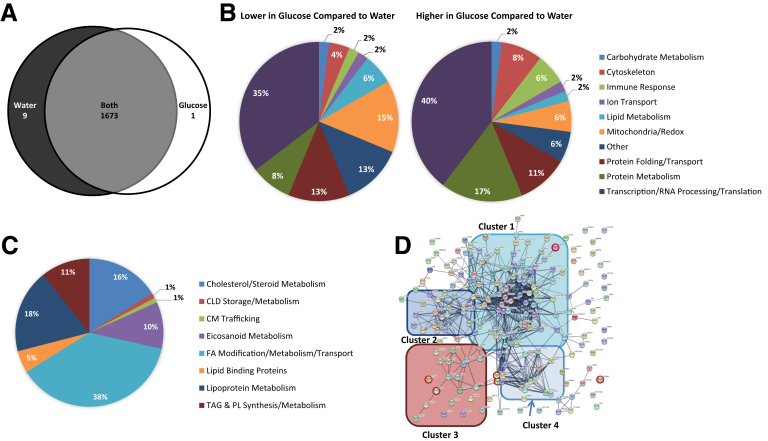
A similar comparative proteomic analysis was performed in response to glucose or water ingestion after a longer, overnight fast after the ingestion of a high-fat meal (samples collected 10 hours after a high-fat meal and 1 hour after glucose/water ingestion), in which there were no observed differences in enterocyte CLD stores. This analysis identified 1683 proteins, with 1673 common to both groups, 9 identified only in response to water, and 1 identified only in response to glucose ingestion (Figure 7A). The 96 proteins that were differentially present in this analysis are involved in a variety of cellular processes, with a greater proportion of proteins associated with transcription and translation (GO terms) compared with the initial study (Tables 7 and 8, Figure 7B). The 186 lipid metabolism-related proteins identified in this analysis are involved in similar processes as the initial study (Figure 7C and D); however, none of the 6 lipid metabolism proteins that were differentially present in response to glucose compared with water ingestion were the same as those identified at 6 hours after ingesting a high-fat meal (Table 9).
Figure 7.
Proteins present in intestinal biopsy specimens from subjects administered glucose or water after an overnight fast after the consumption of a high-fat liquid meal. Duodenal biopsy specimens were collected 10 hours after lipid and 1 hour after glucose or water ingestion from patients undergoing a diagnostic endoscopy (n = 5 patients per group). (A) Venn diagram of proteins identified in response to glucose or water ingestion. Proteins present in at least 3 samples in 1 group and 0 samples in the other group were considered present in only 1 group. Proteins identified in at least 3 samples in 1 group and at least 1 sample in the other group were considered present in both groups. (B) Percentage of proteins within broad functional groups that were present at either relatively lower (48 total proteins) or relatively higher (48 total proteins) levels in response to glucose compared with water ingestion, as classified based on their biological/molecular functions. Only proteins that were identified in at least 3 samples in both groups and present at relatively different levels (P < .05, t test), or at least 3 samples in 1 group and 0 samples in the other group, were included in this classification. A Database for Annotation, Visualization, and Integrated Discovery search of the 1683 identified proteins resulted in the identification of 186 proteins with GO terms related to lipid metabolism. (C) Percentage of the 186 lipid metabolism-related proteins involved in more specific lipid-related functions. (D) String analysis of the 186 lipid metabolism-related proteins. The thickness of the line represents the strength of evidence of a structural/functional relationship between 2 proteins. Cluster 1 is enriched in proteins involved in FA modification/metabolism/transport, cluster 2 is enriched in proteins involved in lipoprotein metabolism, cluster 3 is enriched in proteins involved in TG and phospholipid (PL) synthesis and metabolism, and cluster 4 is enriched in proteins involved in cholesterol/steroid metabolism. Proteins that were present at relatively different levels (P < .05, t test) in response to glucose compared with water ingestion are circled in red.
Table 7.
Proteins Present at Relatively Different Levels in Duodenal Biopsy Specimens From Subjects Administered Glucose or Water After an Overnight Fast After the Consumption of a High-Fat Liquid Meal
| Uniprot accession | Protein name | Gene name | Fold change | t test P value | Function |
|---|---|---|---|---|---|
| P02760 | Protein AMBPa | AMBP | -10.9586 | 2.33E-05 | Protein metabolism |
| P35613 | Basigina | BSG | -7.4781 | 1.28E-05 | Other (extracellular matrix organization) |
| P42025 | β-centractina | ACTR1B | -6.6073 | .0003 | Cytoskeleton |
| Q96GA7 | Serine dehydratase-likea | SDSL | -6.4238 | 4.13E-05 | Protein metabolism |
| Q9NP81 | Serine-tRNA ligase; mitochondriala | SARS2 | -6.4093 | .0001 | Transcription/RNA processing/translation |
| Q07837 | Neutral and basic amino acid transport protein rBATa | SLC3A1 | -5.9686 | .0001 | Protein folding/transport |
| Q9NW15 | Anoctamin-10a | ANO10 | -5.8317 | 6.88E-13 | Ion transport |
| P33897 | ATP-binding cassette subfamily D member 1a | ABCD1 | -5.296 | .0011 | Lipid metabolism |
| Q9Y320 | Thioredoxin-related transmembrane protein 2a | TMX2 | -4.7109 | .0055 | Mitochondria/redox |
| P62899 | 60S ribosomal protein L31 | RPL31 | -1.4463 | .0016 | Transcription/RNA processing/translation |
| P26583 | High-mobility group protein B2 | HMGB2 | -1.3698 | .0205 | Transcription/RNA processing/translation |
| P62841 | 40S ribosomal protein S15 | RPS15 | -1.339 | .0217 | Transcription/RNA processing/translation |
| O43895 | Xaa-Pro aminopeptidase 2 | XPNPEP2 | -1.2009 | .0161 | Protein metabolism |
| Q00688 | Peptidyl-prolyl cis-trans isomerase FKBP3 | FKBP3 | -1.1226 | .0486 | Protein folding/transport |
| P62424 | 60S ribosomal protein L7a | RPL7A | -1.0994 | .0165 | Transcription/RNA processing/translation |
| P99999 | Cytochrome c | CYCS | -1.0634 | .0237 | Mitochondria/redox |
| P14927 | Cytochrome b-c1 complex subunit 7 | UQCRB | -0.9939 | .0352 | Mitochondria/redox |
| O43181 | NADH dehydrogenase (ubiquinone) iron-sulfur protein 4; mitochondrial | NDUFS4 | -0.9391 | .0220 | Mitochondria/redox |
| P46783 | 40S ribosomal protein S10 | RPS10 | -0.8004 | .0412 | Transcription/RNA processing/translation |
| Q9UNX3 | 60S ribosomal protein L26-like 1 | RPL26L1 | -0.795 | .0441 | Transcription/RNA processing/translation |
| P62241 | 40S ribosomal protein S8 | RPS8 | -0.7606 | .0362 | Transcription/RNA processing/translation |
| Q00013 | 55-kilodalton erythrocyte membrane protein | MPP1 | -0.6858 | .0323 | Immune response |
| Q9Y3U8 | 60S ribosomal protein L36 | RPL36 | -0.6824 | .0149 | Transcription/RNA processing/translation |
| P04792 | Heat shock protein β-1 | HSPB1 | -0.6317 | .0415 | Protein folding/transport |
| P20674 | Cytochrome c oxidase subunit 5A; mitochondrial | COX5A | -0.6209 | .0430 | Mitochondria/redox |
| Q9BXW7 | Haloacid dehalogenase-like hydrolase domain-containing 5 | HDHD5 | -0.5832 | .0196 | Lipid metabolism |
| P62081 | 40S ribosomal protein S7 | RPS7 | -0.5799 | .0368 | Transcription/RNA processing/translation |
| Q92520 | Protein FAM3C | FAM3C | -0.5691 | .0467 | Other (cytokine activity) |
| Q86VU5 | Catechol O-methyltransferase domain-containing protein 1 | COMTD1 | -0.5488 | .0242 | Other (putative O-methyltransferase) |
| Q15233 | Non-POU domain-containing octamer-binding protein | NONO | -0.5382 | .0131 | Transcription/RNA processing/translation |
| P08708 | 40S ribosomal protein S17 | RPS17 | -0.5285 | .0429 | Transcription/RNA processing/translation |
| Q5SSJ5 | Heterochromatin protein 1-binding protein 3 | HP1BP3 | -0.5232 | .0430 | Transcription/RNA processing/translation |
| P26232 | Catenin α-2 | CTNNA2 | -0.5041 | .0231 | Cytoskeleton |
| O60825 | 6-phosphofructo-2-kinase/fructose-2;6-bisphosphatase 2 | PFKFB2 | -0.4706 | .0010 | Carbohydrate metabolism |
| Q9BPW8 | Protein NipSnap homolog 1 | NIPSNAP1 | -0.4247 | .0273 | Mitochondria/redox |
| Q9BUJ2 | Heterogeneous nuclear ribonucleoprotein U-like protein 1 | HNRNPUL1 | -0.4204 | .0322 | Transcription/RNA processing/translation |
| P51148 | Ras-related protein Rab-5C | RAB5C | -0.4166 | .0228 | Protein folding/transport |
| A0AV96 | RNA-binding protein 47 | RBM47 | -0.4047 | .0211 | Other (RNA binding) |
| P26373 | 60S ribosomal protein L13 | RPL13 | -0.3879 | .0418 | Transcription/RNA processing/translation |
| Q9Y6N9 | Harmonin | USH1C | -0.3863 | .0445 | Other (brush-border assembly, regulation of microvillus length) |
| Q5IFJ7 | 60S ribosomal protein L9 | RPL9 | -0.3797 | .0444 | Transcription/RNA processing/translation |
| P36543 | V-type proton ATPase subunit E 1 | ATP6V1E1 | -0.3609 | .0298 | Mitochondria/redox |
| P15880 | 40S ribosomal protein S2 | RPS2 | -0.313 | .0103 | Transcription/RNA processing/translation |
| Q00169 | Phosphatidylinositol transfer protein α isoform | PITPNA | -0.2974 | .0315 | Lipid metabolism |
| Q9Y4W6 | AFG3-like protein 2 | AFG3L2 | -0.2882 | .0427 | Protein metabolism |
| Q9UBQ0 | Vacuolar protein sorting-associated protein 29 | VPS29 | -0.283 | .0334 | Protein folding/transport |
| Q13232 | Nucleoside diphosphate kinase 3 | NME3 | -0.2655 | .0405 | Other (nucleotide triphosphate synthesis) |
| P61106 | Ras-related protein Rab-14 | RAB14 | -0.2269 | .0176 | Protein folding/transport |
| Q8NEV1 | Casein kinase II subunit α 3 | CSNK2A3 | 0.1846 | .0033 | Protein metabolism |
| Q9BPX5 | Actin-related protein 2/3 complex subunit 5-like protein | ARPC5L | 0.2192 | .0094 | Transcription/RNA processing/translation |
| Q7L5N1 | COP9 signalosome complex subunit 6 | COPS6 | 0.2227 | .0237 | Protein metabolism |
| O60313 | Dynamin-like 120-kilodalton protein; mitochondrial | OPA1 | 0.2682 | .0189 | Mitochondria/redox |
| Q15029 | 116-kilodalton U5 small nuclear ribonucleoprotein component | EFTUD2 | 0.2702 | .0022 | Transcription/RNA processing/translation |
| Q92841 | Probable ATP-dependent RNA helicase DDX17 | DDX17 | 0.2774 | .0277 | Transcription/RNA processing/translation |
| Q9Y265 | RuvB-like 1 | RUVBL1 | 0.2863 | .0143 | Transcription/RNA processing/translation |
| Q08211 | ATP-dependent RNA helicase A | DHX9 | 0.2969 | .0021 | Transcription/RNA processing/translation |
| P56192 | Methionine-tRNA ligase; cytoplasmic | MARS | 0.2985 | .0005 | Transcription/RNA processing/translation |
| O00303 | Eukaryotic translation initiation factor 3 subunit F | EIF3F | 0.3017 | .0358 | Transcription/RNA processing/translation |
| P50990 | T-complex protein 1 subunit theta | CCT8 | 0.3103 | .0462 | Protein folding/transport |
| Q13363 | C-terminal-binding protein 1 | CTBP1 | 0.3117 | .0193 | Transcription/RNA processing/translation |
| O76094 | Signal recognition particle subunit SRP72 | SRP72 | 0.317 | .0088 | Transcription/RNA processing/translation |
| P50851 | Lipopolysaccharide-responsive and beige-like anchor protein | LRBA | 0.3239 | .0462 | Immune response |
| Q13409 | Cytoplasmic dynein 1 intermediate chain 2 | DYNC1I2 | 0.3304 | .0080 | Cytoskeleton |
| Q6P2Q9 | Pre–messenger RNA-processing-splicing factor 8 | PRPF8 | 0.3428 | .0263 | Transcription/RNA processing/translation |
| P46940 | Ras GTPase-activating-like protein IQGAP1 | IQGAP1 | 0.3464 | .0427 | Other (cellular response to calcium and growth factor stimuli) |
| O95782 | AP-2 complex subunit α-1 | AP2A1 | 0.3545 | .0240 | Protein folding/transport |
| P17987 | T-complex protein 1 subunit α | TCP1 | 0.3631 | .0078 | Protein folding/transport |
| Q14152 | Eukaryotic translation initiation factor 3 subunit A | EIF3A | 0.3778 | .0231 | Transcription/RNA processing/translation |
| O95394 | Phosphoacetylglucosamine mutase | PGM3 | 0.3795 | .0164 | Carbohydrate metabolism |
| O43143 | Pre–messenger RNA-splicing factor ATP-dependent RNA helicase DHX15 | DHX15 | 0.3864 | .0448 | Transcription/RNA processing/translation |
| Q9P2J5 | Leucine-tRNA ligase; cytoplasmic | LARS | 0.3966 | .0183 | Transcription/RNA processing/translation |
| P13010 | X-ray repair cross-complementing protein 5 | XRCC5 | 0.4032 | .0284 | Transcription/RNA processing/translation |
| O75643 | U5 small nuclear ribonucleoprotein 200-kilodalton helicase | SNRNP200 | 0.404 | .0136 | Transcription/RNA processing/translation |
| Q53EL6 | Programmed cell death protein 4 | PDCD4 | 0.4079 | .0175 | Transcription/RNA processing/translation |
| Q8N163 | Cell cycle and apoptosis regulator protein 2 | CCAR2 | 0.4108 | .0094 | Transcription/RNA processing/translation |
| Q15008 | 26S proteasome non-ATPase regulatory subunit 6 | PSMD6 | 0.4158 | .0475 | Protein metabolism |
| P00325 | Alcohol dehydrogenase 1B | ADH1B | 0.427 | .0394 | Mitochondria/redox |
| P07478 | Trypsin-2 | PRSS2 | 0.4359 | .0328 | Protein metabolism |
| Q9Y262 | Eukaryotic translation initiation factor 3 subunit L | EIF3L | 0.4529 | .0493 | Transcription/RNA processing/translation |
| Q93009 | Ubiquitin carboxyl-terminal hydrolase 7 | USP7 | 0.455 | .0294 | Protein metabolism |
| Q86VP6 | Cullin-associated NEDD8-dissociated protein 1 | CAND1 | 0.4561 | .0490 | Protein metabolism |
| O00410 | Importin-5 | IPO5 | 0.4754 | .0232 | Protein folding/transport |
| Q15393 | Splicing factor 3B subunit 3 | SF3B3 | 0.4946 | .0349 | Transcription/RNA processing/translation |
| P07437 | Tubulin β chain | TUBB | 0.5145 | .0031 | Cytoskeleton |
| P55011 | Solute carrier family 12 member 2 | SLC12A2 | 0.5237 | .0405 | Ion transport |
| Q14974 | Importin subunit β-1 | KPNB1 | 0.5474 | .0326 | Protein folding/transport |
| P0DOX7 | Immunoglobulin κ light chain | 0.5546 | .0356 | Immune response | |
| P68363 | Tubulin α-1B chain | TUBA1B | 0.5982 | .0022 | Cytoskeleton |
| P55786 | Puromycin-sensitive aminopeptidase | NPEPPS | 0.5983 | .0495 | Protein metabolism |
| P11766 | Alcohol dehydrogenase class-3 | ADH5 | 0.5992 | .0360 | Mitochondria/redox |
| P05451 | Lithostathine-1-α | REG1A | 0.6105 | .0107 | Other (positive regulator of cell proliferation, carbohydrate binding) |
| Q9BUF5 | Tubulin β-6 chain | TUBB6 | 0.6265 | .0332 | Cytoskeleton |
| P01619 | Immunoglobulin κ variable 3–20 | IGKV3-20 | 1.0274 | .0314 | Immune response |
| O00534 | von Willebrand factor A domain-containing protein 5A | VWA5A | 1.1874 | .0346 | Other (may act as tumor suppressor) |
| P08311 | Cathepsin G | CTSG | 1.8843 | .0438 | Protein metabolism |
| Q8IV08 | Phospholipase D3b | PLD3 | 5.5249 | 1.31E-08 | Lipid metabolism |
NOTE. Duodenal biopsy samples were collected 10 hours after lipid and 1 hour after glucose or water ingestion from patients undergoing a diagnostic endoscopy (n = 5 patients per group). Proteins that were identified in at least 3 samples in both groups and present at relatively different levels (P < .05, t test), or at least 3 samples in 1 group and 0 samples in the other group, are shown. Average fold changes of proteins in response to glucose relative to water consumption are presented. Numbers in the “Fold change” column represent how much higher (or lower if negative) the protein levels were in the glucose group compared with the water group.
AMBP, alpha-1-microglobulin/bikunin precursor; AP-2, adaptor protein complex 2; ATP, adenosine triphosphate; ATPase, adenosine triphosphatase; GTPase, guanosine triphosphatase; rBAT, neutral and basic amino acid transport protein; redox, reduction-oxidation; tRNA, transfer ribonucleic acid.
Only identified in response to water.
Only identified in response to glucose.
Table 8.
GO Terms Associated With Lipid Metabolism-Related Proteins Present in Duodenal Biopsy Specimens 10 Hours After a High-Fat Liquid Meal
| Cholesterol/steroid metabolism | |
| UP_KEYWORDS | Cholesterol biosynthesis |
| GOTERM_BP_DIRECT | Cholesterol biosynthetic process |
| UP_KEYWORDS | Cholesterol metabolism |
| GOTERM_BP_DIRECT | Isoprenoid biosynthetic process |
| UP_KEYWORDS | Steroid biosynthesis |
| KEGG_PATHWAY | Steroid hormone biosynthesis |
| GOTERM_BP_DIRECT | Steroid metabolic process |
| UP_KEYWORDS | Steroid metabolism |
| UP_KEYWORDS | Sterol biosynthesis |
| GOTERM_MF_DIRECT | Sterol esterase activity |
| UP_KEYWORDS | Sterol metabolism |
| CLD storage/metabolism | |
| GOTERM_CC_DIRECT | Lipid droplet |
| GOTERM_BP_DIRECT | Lipid storage |
| CM trafficking | |
| GOTERM_CC_DIRECT | ER to Golgi transport vesicle membrane |
| Eicosanoid metabolism | |
| GOTERM_MF_DIRECT | Arachidonic acid epoxygenase activity |
| KEGG_PATHWAY | Arachidonic acid metabolism |
| GOTERM_BP_DIRECT | Cyclooxygenase pathway |
| GOTERM_BP_DIRECT | Epoxygenase P450 pathway |
| GOTERM_BP_DIRECT | Leukotriene metabolic process |
| UP_KEYWORDS | Prostaglandin biosynthesis |
| GOTERM_BP_DIRECT | Prostaglandin biosynthetic process |
| UP_KEYWORDS | Prostaglandin metabolism |
| GOTERM_MF_DIRECT | Steroid hydroxylase activity |
| FA modification/metabolism/transport | |
| GOTERM_MF_DIRECT | Acyl-CoA dehydrogenase activity |
| INTERPRO | Acyl-CoA dehydrogenase, conserved site |
| INTERPRO | Acyl-CoA dehydrogenase/oxidase |
| INTERPRO | Acyl-CoA dehydrogenase/oxidase, C-terminal |
| INTERPRO | Acyl-CoA dehydrogenase/oxidase, N-terminal |
| GOTERM_MF_DIRECT | Acyl-CoA hydrolase activity |
| GOTERM_BP_DIRECT | Acyl-CoA metabolic process |
| INTERPRO | Acyl-CoA oxidase/dehydrogenase, central domain |
| INTERPRO | Acyltransferase ChoActase/COT/CPT |
| INTERPRO | AMP binding, conserved site |
| INTERPRO | AMP-dependent synthetase/ligase |
| UP_SEQ_FEATURE | Binding site: carnitine |
| GOTERM_MF_DIRECT | Decanoate-CoA ligase activity |
| INTERPRO | Domain of unknown function DUF4009 |
| GOTERM_BP_DIRECT | Fatty acid α-oxidation |
| GOTERM_BP_DIRECT | Fatty acid β-oxidation |
| GOTERM_BP_DIRECT | Fatty acid β-oxidation using acyl-CoA dehydrogenase |
| UP_KEYWORDS | Fatty acid biosynthesis |
| KEGG_PATHWAY | Fatty acid biosynthesis |
| KEGG_PATHWAY | Fatty acid degradation |
| UP_KEYWORDS | Fatty acid metabolism |
| KEGG_PATHWAY | Fatty acid metabolism |
| GOTERM_MF_DIRECT | Flavin adenine dinucleotide binding |
| GOTERM_MF_DIRECT | Hydroxyacyl-CoA dehydrogenase activity |
| GOTERM_BP_DIRECT | Lipid homeostasis |
| GOTERM_BP_DIRECT | Long-chain fatty acid import |
| GOTERM_BP_DIRECT | Long-chain fatty acid metabolic process |
| GOTERM_MF_DIRECT | Long-chain fatty acid–CoA ligase activity |
| GOTERM_BP_DIRECT | Negative regulation of fatty acid metabolic process |
| GOTERM_MF_DIRECT | Oxidoreductase activity, acting on the CH-CH group of donors |
| GOTERM_MF_DIRECT | Oxidoreductase activity, acting on the CH-CH group of donors, with a flavin as acceptor |
| GOTERM_MF_DIRECT | Palmitoyl-CoA hydrolase activity |
| UP_SEQ_FEATURE | Region of interest: coenzyme A binding |
| GOTERM_MF_DIRECT | Transferase activity, transferring acyl groups |
| GOTERM_MF_DIRECT | Very-long-chain fatty acid–CoA ligase activity |
| Lipid binding proteins | |
| INTERPRO | Acyl-CoA binding protein, ACBP |
| INTERPRO | Acyl-CoA binding protein, ACBP, conserved site |
| INTERPRO | Cytosolic fatty acid binding |
| UP_SEQ_FEATURE | Domain: ACB |
| GOTERM_MF_DIRECT | Fatty-acyl-CoA binding |
| INTERPRO | Lipocalin/cytosolic fatty acid binding protein domain |
| GOTERM_MF_DIRECT | Retinoic acid binding |
| Lipoprotein metabolism | |
| INTERPRO | Apolipoprotein A1/A4/E |
| GOTERM_MF_DIRECT | Cholesterol binding |
| GOTERM_BP_DIRECT | Cholesterol efflux |
| GOTERM_BP_DIRECT | Cholesterol homeostasis |
| GOTERM_BP_DIRECT | Cholesterol metabolic process |
| GOTERM_MF_DIRECT | Cholesterol transporter activity |
| GOTERM_CC_DIRECT | Chylomicron |
| UP_KEYWORDS | Chylomicron |
| GOTERM_BP_DIRECT | Chylomicron remnant clearance |
| UP_KEYWORDS | High-density lipoprotein |
| GOTERM_CC_DIRECT | High-density lipoprotein particle |
| GOTERM_BP_DIRECT | High-density lipoprotein particle assembly |
| GOTERM_BP_DIRECT | High-density lipoprotein particle clearance |
| GOTERM_MF_DIRECT | High-density lipoprotein particle receptor binding |
| GOTERM_BP_DIRECT | High-density lipoprotein particle remodeling |
| GOTERM_CC_DIRECT | Intermediate-density lipoprotein particle |
| GOTERM_MF_DIRECT | Lipase inhibitor activity |
| UP_KEYWORDS | Lipid transport |
| GOTERM_BP_DIRECT | Lipid transport |
| GOTERM_MF_DIRECT | Lipid transporter activity |
| GOTERM_BP_DIRECT | Lipoprotein biosynthetic process |
| GOTERM_BP_DIRECT | Lipoprotein metabolic process |
| GOTERM_CC_DIRECT | Low-density lipoprotein particle |
| GOTERM_BP_DIRECT | Low-density lipoprotein particle remodeling |
| GOTERM_BP_DIRECT | Negative regulation of cholesterol transport |
| GOTERM_BP_DIRECT | Negative regulation of lipid catabolic process |
| GOTERM_BP_DIRECT | Negative regulation of lipid metabolic process |
| GOTERM_BP_DIRECT | Negative regulation of receptor-mediated endocytosis |
| GOTERM_BP_DIRECT | Negative regulation of VLDL particle clearance |
| GOTERM_BP_DIRECT | Negative regulation of VLDL particle remodeling |
| GOTERM_MF_DIRECT | Phosphatidylcholine binding |
| GOTERM_MF_DIRECT | Phosphatidylcholine-sterol O-acyltransferase activator activity |
| GOTERM_BP_DIRECT | Phospholipid efflux |
| GOTERM_BP_DIRECT | Positive regulation of cholesterol esterification |
| GOTERM_BP_DIRECT | Positive regulation of fatty acid biosynthetic process |
| GOTERM_BP_DIRECT | Positive regulation of lipoprotein lipase activity |
| GOTERM_BP_DIRECT | Positive regulation of triglyceride catabolic process |
| GOTERM_BP_DIRECT | Regulation of Cdc42 protein signal transduction |
| GOTERM_BP_DIRECT | Regulation of intestinal cholesterol absorption |
| GOTERM_BP_DIRECT | Reverse cholesterol transport |
| GOTERM_CC_DIRECT | Spherical high-density lipoprotein particle |
| GOTERM_BP_DIRECT | Triglyceride catabolic process |
| GOTERM_BP_DIRECT | Triglyceride homeostasis |
| GOTERM_CC_DIRECT | VLDL particle |
| GOTERM_BP_DIRECT | VLDL particle remodeling |
| UP_KEYWORDS | VLDL |
| TAG and PL synthesis/metabolism | |
| GOTERM_MF_DIRECT | 1-Acylglycerol-3-phosphate O-acyltransferase activity |
| GOTERM_MF_DIRECT | 1-Acylglycerol-3-phosphate O-acyltransferase activity |
| GOTERM_BP_DIRECT | Acylglycerol catabolic process |
| GOTERM_BP_DIRECT | CDP-diacylglycerol biosynthetic process |
| GOTERM_BP_DIRECT | CDP-diacylglycerol biosynthetic process |
| GOTERM_BP_DIRECT | Glycerophospholipid biosynthetic process |
| Glycerophospholipid biosynthetic process | |
| GOTERM_BP_DIRECT | Glycerophospholipid catabolic process |
| KEGG_PATHWAY | Glycerophospholipid metabolism |
| GOTERM_BP_DIRECT | Phosphatidic acid biosynthetic process |
| UP_KEYWORDS | Phospholipid biosynthesis |
| GOTERM_BP_DIRECT | Phospholipid biosynthetic process |
| UP_KEYWORDS | Phospholipid metabolism |
| INTERPRO | Phospholipid/glycerol acyltransferase |
| GOTERM_BP_DIRECT | Triglyceride biosynthetic process |
| GOTERM_BP_DIRECT | Triglyceride lipase activity |
ACBP, acyl-CoA-binding protein; AMP, adenosine monophosphate; CDP, cytidine diphosphate; COT/CPT, carnitine octanoyltransferase/carnitine palmitoyltransferase.
Table 9.
Lipid Metabolism Proteins Present at Relatively Different Levels in Duodenal Biopsy Specimens in Response to Glucose or Water Consumption After an Overnight Fast After the Consumption of a High-Fat Liquid Meal
| Uniprot accession | Protein name | Gene name | Fold change | t test P value | Lipid metabolism-related function |
|---|---|---|---|---|---|
| P33897 | ATP binding cassette subfamily D member 1a | ABCD1 | -5.296 | .00108 | FA modification/ metabolism/transport |
| Q9BXW7 | Haloacid dehalogenase-like hydrolase domain-containing 5 | HDHD5 | -0.5832 | .0196 | TAG and PL synthesis/metabolism |
| Q00169 | Phosphatidylinositol transfer protein α isoform | PITPNA | -0.2974 | .0315 | Lipoprotein metabolism |
| P00325 | Alcohol dehydrogenase 1B | ADH1B | 0.427 | .0394 | FA modification/ metabolism/transport |
| P11766 | Alcohol dehydrogenase class-3 | ADH5 | 0.5992 | .0360 | FA modification/ metabolism/transport |
| Q8IV08 | Phospholipase D3b | PLD3 | 5.5249 | 1.31E-08 | TAG and PL synthesis/ metabolism |
NOTE. Duodenal biopsy samples were collected 10 hours after lipid and 1 hour after glucose or water ingestion from patients undergoing a diagnostic endoscopy (n = 5 patients per group). Lipid metabolism-related proteins were identified based on GO terms, and relative levels of proteins identified in at least 3 duodenal biopsy samples per group, or identified in at least 3 samples in 1 group and 0 samples in the other group, were compared. Numbers in the “Fold change” column represent how much higher (or lower if negative) the protein levels were in the glucose group compared with the water group. Proteins present at significantly different levels within the 2 treatment groups (P < .05, t test) are shown.
ATP, adenosine triphosphate; PL, phospholipid, TAG, triacylglycerol.
Only identified in response to water.
Only identified in response to glucose.
Discussion
In the current study we investigated the effect of oral glucose ingestion on lipid stored in the intestine from a previous meal. We not only confirmed the ability of oral glucose to mobilize intestinal lipid stores and increase plasma CM TGs, but also expanded this observation with high-quality visualization of subcellular CLDs and lipids within the secretory pathway, as well as an examination of the intestinal proteome, to explore cellular mechanisms. Through detailed quantitative analysis of subcellular lipid depots, we showed that glucose ingestion reduced both the number and size of CLDs within enterocytes. Furthermore, our proteomic analysis of duodenal biopsy specimens showed marked differential presence of intestinal proteins in response to oral glucose compared with water, some of which may be involved in regulating the mobilization of intestinal lipid stores.
The results of the current study provide further evidence that lipid can be retained within the small intestine for many hours after fat ingestion and subsequently mobilized by a stimulus, as reviewed in the introduction. Although visualization of lipid depots in jejunal biopsy specimens was reported in a previous study,9 our study added to the literature with examination of duodenal biopsy specimens and provided visualization of the subcellular localization of lipid droplets in the cell and in the secretory pathway. We detected the presence of abundant lipid depots, especially CLDs, within the duodenal enterocytes of subjects who ingested a high-fat meal 6 hours before the biopsy and water 1 hour before the intestinal biopsy (ie, the control study). This lipid retention in duodenal enterocytes was seen at a time that plasma TGs had almost returned to baseline, clearly showing that lipids are being retained in the intestine. Glucose ingestion acutely (within 1 hour) reduced the total amount of lipid retained in enterocytes, providing evidence of glucose-stimulated lipid mobilization. This corresponded to a spike in total plasma TGs, which was mainly owing to an increase in CM TGs. Because there was no other food intake during the study period, the high-fat liquid meal likely was the source of this TG spike. Together, these results suggest that considerable dietary lipid is retained in intestinal CLDs well into the late postprandial period, which subsequently can be mobilized and secreted within CMs. Although the results of the current study show an intracellular mechanism of CLD mobilization, lymph flow and mobilization of extracellular (eg, in lamina propria) CMs also could contribute to the overall mobilization of intestinal lipid stores. Glucose in the luminal fluid increases sympathetic activity, leading to vasodilation of the submucosal arterioles, and enhances intestinal blood flow in rodent models. Changes in vasodilation and blood flow and the potential in mediating the total response of lipid mobilization to glucose ingestion were not assessed in the current study. Increased insulin secretion after glucose ingestion also may lead to vasodilation in muscle. This may help mobilize total TG stores, but the effects of insulin on CLD mobilization are unknown. These aspects warrant further study using animal models.
This study identifies CLDs as dynamic and regulated lipid storage depots that mediate intestinal lipid handling and CM secretion. The ability to store TGs in CLDs and mobilize this lipid pool at later times likely contributes to the efficiency of dietary fat absorption and helps prevent toxicity both locally within enterocytes as well as systemically.4, 16, 17 In the enterocyte, CLDs provide a buffering depot for lipids that cannot be rapidly incorporated into CMs for secretion. Systemically, this process also may attenuate an otherwise rapid increase in postprandial lipids that could overwhelm the lipid storage and buffering capacity of adipose tissues, resulting in fatty acid spillover and lipotoxicity.21 Furthermore, early postprandial CLD mobilization may serve as a priming function for the enterocyte’s CM assembly and secretion pathway, which needs to rapidly and efficiently cope with the large influx of ingested dietary lipids during food ingestion. Quantifying lipid depots in subcellular compartments and organelles, such as detailed assessment of size distribution of chylomicrons within Golgi, may yield important information on the underlying mechanism, which was not possible with EM visualization. Together with the increase in plasma and CM TG concentrations, the results of the current study support that oral glucose functioned as a stimulus to mobilize enterocyte lipid stores for use in CM synthesis and secretion. Based on the known and well-described biology of CM assembly and secretion,2 it is hypothesized that glucose ingestion would have initiated a sequence of events, including hydrolysis of CLD TGs, TG resynthesis in the ER membrane, CM assembly and secretion from the enterocytes, and CM transport through the lymphatics into circulation. It remains to be determined if other stimuli for intestinal lipid mobilization (eg, sham fat feeding8 and GLP-219) mobilize intestinal lipids by a similar mechanism, and whether their effects are quantitatively similar to that of oral glucose. Although glucose ingestion may stimulate the secretion of GLP-2, which mobilizes CM release, the observed effects of glucose ingestion on CLDs was unlikely owing to GLP-2 secretion. GLP-2 concentrations after 25-gram glucose ingestion is unknown and was not measured in this study because of a lack of blood samples with dipeptidyl peptidase-4 inhibitor. In healthy subjects, a standard oral glucose tolerance test (75 g D-glucose) increased plasma glucose concentrations from approximately 5 to approximately 9 mmol/L, and plasma GLP-2 concentrations from approximately 15 to 49 pmol/L.22 Mixed meal ingestion increased plasma GLP-2 (intact, 16 ± 3 to 73 ± 10 pmol/L at 90 min), and subcutaneous injection of 400 ug GLP-2 increased intact GLP-2 to maximally 1493 ± 250 pmol/L at 45 minutes in healthy volunteers.23 In our previous study in which GLP-2 promoted release of preformed chylomicrons, a more than 3-fold higher dose of GLP-2 was used (1500 ug),19 which is expected to increase circulating GLP-2 even more significantly. In addition, because GLP-1 and GLP-2 are co-secreted, glucose ingestion–stimulated secretion of GLP-2 is accompanied by secretion of GLP-1, which is known to suppress CM secretion.24 Interestingly, we found that the effects of glucose ingestion are dependent on sufficient lipid stores within the enterocyte. After prolonged fasting (10 hours after fat ingestion), which resulted in a significant reduction in intestinal CLD stores, glucose had no effect on intestinal lipid stores.
To gain further insight into proteins potentially regulating the observed glucose-stimulated lipid mobilization within the small intestine, we performed a comparative proteomic analysis of the duodenal biopsy specimens in response to glucose compared with water ingestion. Previous studies have used both untargeted and targeted approaches to identify duodenal proteins in insulin-resistant compared with insulin-sensitive individuals, but these duodenal tissue samples were collected in the fasted state.25, 26 Although validation of the presence of the identified proteins within the small intestine by additional methods is needed, the present study provides us with candidate proteins that are present in the duodenum in response to dietary fat and glucose ingestion. This study also identified potential glucose-regulated proteins within the duodenum, some of which also may be involved in regulating glucose-stimulated mobilization of lipids from the small intestine. In the current study we initially used a P value less than .05 as the cut-off value to identify proteins differentially present between treatment groups. After Bonferroni correction for multiple comparisons, a few proteins were still present at significantly different levels between the groups. It is important to note that the Bonferroni correction is a stringent correction factor that minimizes false-positive results, but it also increases false-negative results. Therefore, although this correction factor helps prioritize candidate proteins for further investigation, the current proteomic analysis is a hypothesis-generating experiment, and it is therefore also important to not completely disregard the proteins that were no longer present at significantly different levels after this correction.
To identify proteins potentially involved in regulating glucose-stimulated lipid mobilization from the small intestine, we performed a targeted search of our proteomics data to identify proteins with known roles in intestinal lipid/lipoprotein metabolism and transport. The results suggest differential regulation of proteins involved in fatty acid metabolism, cholesterol synthesis, and lipid binding. Little is known about the particular roles of these proteins within the small intestine specifically. However, acyl-coenzyme A binding protein previously was shown to be present at high levels in mouse intestinal epithelium and to co-localize with fatty acid binding protein 2 (intestinal fatty-acid binding protein).27 Fatty acid binding protein 2 also was identified in the current study but was present at similar levels in both treatments. Interestingly, we found that ethanolaminephosphotransferase 1 was relatively up-regulated by glucose ingestion. This protein is involved in the synthesis of phosphatidylethanolamine.28 Mutations in several enzymes involved in phospholipid synthesis are associated with diseases including fatty liver, lipodystrophy, and obesity.29 In addition, altering the phospholipid composition of CLDs, CMs, and the ER all have the potential to impact lipid storage and secretion, such as in phospholipid remodeling protein lysophosphatidylcholine acyltransferase-3 deficiency.30 Therefore, it is possible that higher levels of ethanolaminephosphotransferase 1 in the intestine in response to glucose has an impact on membrane composition of the ER and/or CMs that ultimately promotes CM secretion. Validation of the presence and localization of these proteins within the small intestine, which was not possible owing to a lack of suitable samples in this study, is required in future studies, but their initial identification and differential presence in the 2 treatment groups suggests there may be a general increase in intestinal lipid metabolism in response to glucose consumption. Furthermore, the differentially present lipid metabolism-related proteins identified in biopsy specimens after prolonged (10 hours) fasting were not the same as those identified in the shorter (6 hour) fasting study, but they were involved in similar processes. This suggests that glucose may exert different regulatory effects depending on dietary status (ie, when the last meal was consumed).
Interestingly, we did not see differences in the levels of several proteins with established roles in CM synthesis and secretion or CLD metabolism between treatments. We identified both perilipin 2 and perilipin 3, which play roles in regulating CLD storage,31 but these proteins were present at similar levels in both treatment groups. In addition, we only identified 1 of the 4 enzymes involved in cytoplasmic TG lipolysis, monoacylglycerol lipase, in the current study, and it was not differentially present in response to glucose compared with water ingestion. This is consistent, however, with the lack of identification of any cytoplasmic lipases other than monoacylglycerol lipase within duodenal tissues collected from fasted insulin-sensitive or insulin-resistant individuals.26 It is possible that these proteins are present at levels below the mass spectrometry detection limit, and/or that other enzymes or pathways play more of a role in TG mobilization from enterocytes under the conditions of the current study. In fact, we identified lysosomal acid lipase, which hydrolyzes TGs within the lysosome during lipophagy,32 at similar levels in both treatment groups, along with several other lipases. We also identified key proteins involved in CM synthesis and trafficking (apolipoprotein B, microsomal triglyceride transfer protein, secretion associated ras related GTPase 1B), but again did not see differences in their protein levels in response to glucose compared with water ingestion. However, it still is possible that glucose ingestion alters the activities (eg, through phosphorylation) or subcellular localization of proteins involved in CM synthesis and CLD metabolism, which requires further investigation in future studies.
To examine whether proteins without known roles in lipid metabolism are involved in the observed glucose-stimulated mobilization from the small intestine, we also examined non–lipid-related proteins. This analysis showed that glucose down-regulated syntaxin-binding protein 5. This protein has been shown to be a negative regulator of soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) protein assembly required for insulin exocytosis in β cells. In addition, glucose has been shown to inhibit syntaxin-binding protein 5 and induce its degradation in these cells to promote insulin secretion.33 Furthermore, it regulates vesicle exocytosis in other cell types, including platelets and endothelial cells.34 In enterocytes, SNARE complex is required for prechylomicron transport vesicle intracellular transport during chylomicron synthesis and secretion. After budding from the ER, prechylomicron transport vesicles are directed by vesicle SNARE toward the Golgi. Vesicle associated membrane protein 7 of the vesicle SNARE joins with syntaxin-5, rbet1, and vti1a of the target SNARE to form the SNARE complex, which facilitates docking and fusion of prechylomicron transport vesicle with the Golgi membrane.35 The roles of syntaxin-binding protein 5 in enterocytes are not elucidated, but our data suggest that glucose negatively regulates this protein in enterocytes, and that this regulation may have an impact on intestinal lipid mobilization. Another protein that draws attention is epimerase family protein SDR39U1, which was up-regulated by glucose. This protein is expressed in small intestine, including the duodenum.36 It belongs to a family of enzymes involved in the metabolism of a large variety of compounds, including steroid hormones, prostaglandins, retinoids, lipids, and xenobiotics.37 Genetic defects in SDR genes underlie several inherited metabolic diseases.38 Further investigation into the intestine-specific functions of these proteins would be beneficial because of their possible involvement in glucose-stimulated lipid mobilization from the intestine.
Conclusions
Here, we present evidence from both in vivo lipid responses and intestinal biopsy specimens that support a role of glucose ingestion in mobilizing lipid stores from the human intestine. Glucose ingestion mobilizes enterocyte CLDs to provide substrate for CM synthesis and secretion, likely involving multiple molecular players. Although the precise mechanisms by which intestinal lipid stores are mobilized remain unknown, the current study has highlighted candidate proteins and pathways that may regulate this process and can inform future studies investigating the regulation of this process. An increased understanding of the regulation of intestinal lipid storage and mobilization may help provide novel dietary guidance for lowering blood TG levels and identify novel therapeutic targets for treatment of hypertriglyceridemia to reduce cardiovascular disease risk.
Materials and Methods
Aim 1
Subjects
Six healthy men were recruited through advertisement in a local newspaper. Subjects were in good health, with no known medical conditions, and were not taking any medication. The study protocol was approved by the Research Ethics Board at the University Health Network, University of Toronto. All participants provided written informed consent.
Study protocol
Each subject was studied on 2 occasions, 4–6 weeks apart, in random order. On each occasion, subjects were admitted to the Metabolic Test Centre at the Toronto General Hospital after an overnight fast. An indwelling catheter was inserted into a superficial arm vein for blood sampling. At 7 AM (referred to as t = 0), subjects ingested a 100-mL high-fat liquid meal (Calogen; Nutricia Advanced Medical Nutrition, Wiltshire, UK). Each 100 mL of the liquid meal contains 450 kcal energy, 50 g fat (5.3 g saturated fat, 30.4 g monounsaturated fat, 14.3 g polyunsaturated fat), 0 g protein, and 0.1 g carbohydrate. Five hours later (t = 5 h), subjects ingested a glucose solution (50%, 50 mL) in 1 arm of the study and 50 mL water in the other arm, 4–6 weeks apart. Blood samples were drawn at baseline and at regular intervals until the end of the study (t = 8 h).
Laboratory analysis
TRL was first isolated as previously described.24 CM and VLDL-sized particles were isolated further from TRL, according to a previously described method with modifications.19, 39 TRL fractions (1 mL) were transferred to a 6-mL centrifuge tube on ice, carefully overlaid with 1.006 g/mL density NaBr solution, and centrifuged at 13,500 rpm for 30 minutes at 12°C using a Ti50.4 rotor. Clear separation was visible between the top and bottom fractions. The top 0.5 mL was collected as CM by tube slicing, whereas the bottom fraction was collected as VLDL-sized lipoproteins. Plasma glucose was measured at the bedside with a glucose analyzer (Analox Instruments, Stourbridge, UK). TG in plasma and lipoprotein fractions were measured with a commercial kit (Roche Diagnostics, Indianapolis, IN). Plasma insulin was measured with a human insulin radioimmunoassay kit (Millipore, Burlington, MA).
Aim 2
Subjects
Twenty-four individuals undergoing diagnostic gastroduodenoscopy for gastrointestinal symptoms were recruited after obtaining informed consent. Subjects were referred for endoscopy after complaints of heartburn, dyspepsia, bloating, abdominal pain, nausea without vomiting, reflux, gas, and regurgitation. Participants had no known duodenal pathology and were observed to have normal duodenal mucosa by visual inspection during the endoscopy. The study protocol was approved by the Human Research Ethics Board of the University Health Network, University of Toronto. All participants provided written informed consent.
Study protocol
After providing informed consent, participants were block-randomized to receive either oral glucose or water treatment. At 7 AM after an overnight fast (referred to as t = 0), subjects ingested a 100-mL high-fat liquid meal containing 50 g of fat (Calogen; Nutricia Advanced Medical Nutrition). Five hours later (t = 5 h), subjects ingested either a glucose solution (50%, 50 mL) or 50 mL water. One hour after ingesting either glucose or water (t = 6 h), duodenal biopsy samples were obtained during an endoscopy. Although quantitatively jejunum is responsible for the majority of lipid absorption, active absorption also occurs in duodenum40 and obtaining biopsy specimens from the duodenum as compared with jejunum is more technically feasible and was acceptable to our human ethics review committee. Samples were snap-frozen in dry ice and stored at -80°C for later proteomic analysis or preserved in 2.5% glutaraldehyde in 0.1 mol/L sodium cacodylate (pH 7.4) and stored at 4°C for electron microscopy.
Delayed fasting
To further examine the time course of fat retention in the intestine and its subsequent mobilization by oral glucose, duodenal biopsy specimens were taken from an additional 10 individuals. These individuals were randomly assigned to ingest glucose or water 9 hours after ingestion of the high-fat liquid meal, which was administered at 10 PM the night before undergoing the gastroduodenoscopy. The study protocol was otherwise identical to that described earlier for aim 2.
Transmission electron microscopy
Duodenal biopsy samples were immediately fixed using 2.5% glutaraldehyde in 0.1 mol/L sodium cacodylate (pH 7.4) and stored at 4°C until processed. The tissues then were fixed with a secondary fixative, 1% osmium tetroxide in 0.1 mol/L sodium cacodylate (pH 7.4) for 1 hour at room temperature, washed repeatedly in distilled deionized water, dehydrated with a graded series of ethanol, and embedded in Embed 812 resin (Electron Microscopy Sciences, Hatfield, PA). Thick sections (0.5 μm) were stained with 1% toluidine blue and examined by light microscopy to confirm tissue orientation. Thin sections (80 nm) were cut on a Leica (Leica Microsystems Inc, Buffalo Grove, IL) UC6 ultramicrotome and stained with 2% uranyl acetate and lead citrate. Images were either acquired on a Tecnai T20 transmission electron microscope (FEI, Hillsboro, OR) equipped with an LaB6 source and operating at 100 kV, or a CM-100 transmission electron microscope (FEI/Philips, Hillsboro, OR) operating at 80 kV. Intact enterocytes were examined for the presence of CLDs (40–63 enterocytes/sample, 5 or 12 samples/group). Quantitative analyses were performed on enterocytes containing CLDs. The number of CLDs per enterocyte was counted and the diameters of individual CLDs were measured using ImageJ software (NIH, Bethesda, MD). Measured diameters were used to estimate the area of individual CLDs (area = π (diameter/2)2), and the total CLD area per enterocyte was estimated by multiplying the average CLD area by the average CLD number. Qualitative assessments of lipids within the secretory pathway (ER, Golgi, and secretory vesicles) were made with a ranking system. Because it was too difficult to determine quantitatively the area of lipids within the secretory pathway, an in-house ranking system was used to arbitrarily classify each enterocyte as containing high, moderate, or low lipid content. Individual enterocytes were classified as containing low, moderate, or high amounts of secretory lipid, and then this information was used to assign each biopsy sample an overall ranking of low, moderate, or high. Previous electron microscopic analyses of intestinal lipid stores were used as a reference for the identification of intestinal lipid storage pools in the current study.41, 42, 43, 44, 45
Sample preparation for liquid chromatography–mass spectrometry/mass spectrometry analysis
Biopsy samples were washed once with 100 μL purified water followed by 100 μL washes using 100 mmol/L ammonium bicarbonate until the supernatant was clear, to remove the presence of blood in some samples. Tissue samples then were placed into 2-mL reinforced tubes containing 2.8-mm ceramic (zirconium oxide) beads (Cayman Chemical, Ann Arbor, MI). A total of 200 μL of 100 mmol/L ammonium bicarbonate was added to each sample, and the tubes were loaded into a Precellys 24 homogenizer (Berlin Instruments, Rockville, MD). The tissue was homogenized at 6500 rpm using 3 cycles of 20 seconds each. Protein concentrations were determined for each of the tissue solutions using a Pierce BCA assay kit (Thermo Scientific, Waltham, MA). An aliquot containing 100 μg protein was taken for processing. Before the digestion, the protein was precipitated and concentrated from solution using acetone. After drying the precipitated pellets, the protein samples were reduced using 10 mmol/L 1,4-dithiothreitol followed by alkylation using iodoethanol. Sequence grade Lys-C/Trypsin (Promega, Madison, WI) was used to enzymatically digest the protein samples in the Barocycler NEP2320 (Pressure Biosciences, Inc, Easton, MA) at 50°C under 20,000 psi for 1 hour. Digested samples were cleaned using C18 spin columns (Nest Group, Southborough, MA) and dried. Resulting pellets were resuspended in 97% purified water/3% acetonitrile/0.1% formic acid before liquid chromatography/mass spectrometry analysis.
Liquid chromatography–mass spectrometry/mass spectrometry
Digested samples were analyzed using the Dionex UltiMate 3000 RSLC Nano System coupled to a Q Exactive HF Hybrid Quadrupole-Orbitrap Mass Spectrometer (Thermo Fisher Scientific). Peptides generated during the digestion were loaded onto a 300 μm inner diameter × 5 mm C18 PepMap 100 (Thermo Fisher Scientific) trap column and washed with 98% purified water/2% acetonitrile/0.01% formic acid using a flow rate of 5 μL/min. The trap column was switched in-line with the analytical column after 5 minutes, and peptides were separated over a 75 μm × 150 mm reverse-phase Acclaim PepMap RSLC C18 analytical column using a 120-minute method at a flow rate of 300 nL/min. Mobile phase A contained 0.01% formic acid in water and mobile phase B consisted of 0.01% formic acid in 80% acetonitrile. The linear gradient began at 5% B and reached 30% B in 80 minutes, 45% B in 91 minutes, and 100% B in 93 minutes. The column was held at 100% B for the next 5 minutes before returning to 5% B, where it was equilibrated for 20 minutes. Samples were injected into the QE HF through the Nanospray Flex Ion Source fitted with a stainless-steel emission tip (Thermo Scientific). Data acquisition was performed by monitoring the top 20 precursors at 120,000 resolution with an injection time of 100 ms.
Proteomic data analysis
The results from the mass spectrometer were processed using the MaxQuant (Max-Planck Institute for Biochemistry, Martinsried, Germany) computational proteomics platform.46 The peak list generated was searched against the Homo sapiens sequences from UniProt and a common contaminants database. The following settings were used for the MaxQuant run: trypsin and Lys-C digestion enzymes with 2 missed cleavages allowed, ethanolyl addition to cysteine as a fixed modification, N-terminal acetylation and oxidation of methionine as variable modifications, with 3 modifications allowed for each peptide, default Orbitrap parameters, minimum peptide length of 7 amino acids, label-free quantification was selected, match between runs was selected and the interval was set to 1 minute, and the protein false-discovery rate was set to 1%. An in-house script was used to perform the following on the MaxQuant results: remove all of the contaminant proteins, log transform (log2[x]) the label-free quantification intensity values, and input missing values using half of the highest intensity when all the values for a given protein were missing in 1 group and present in at least 3 samples of the other group. Only proteins that were identified in at least 3 samples in 1 treatment group were considered present in the duodenal biopsy samples. Only the relative label-free quantification values of proteins that were identified in at least 3 samples in both treatment groups, or identified in at least 3 samples in 1 treatment group and 0 samples in the other treatment group (imputed values used) were compared statistically. The statistical analyses were performed in the R environment (www.cran.r-project.org). A t test was performed on the label-free quantification intensities, with a P value < .05 considered a statistically significant difference between the groups. The differentially present proteins were classified into broad groups based on GO terms for biological process or molecular function using the Database for Annotation, Visualization, and Integrated Discovery version 6.7 and the UniProt database. Proteins with GO terms related to lipid (TG, phospholipid, cholesterol, and fatty acid) metabolism, lipoprotein metabolism and transport, and CLD storage and metabolism were identified and classified using the Database for Annotation, Visualization, and Integrated Discovery functional annotation clustering and functional annotation tables as well as the UniProt database. Protein–protein interactions were visualized with Search Tool for the Retrieval of Interacting Genes/Proteins version 10.5 using the confidence view (high confidence, score 0.700).
Statistical analysis
Data are presented as means ± SE. Plasma glucose concentrations, plasma TG concentration vs time curves, and lipoprotein fractions (TRL, CM, and VLDL) were compared using repeated-measures analysis of variance with post hoc analysis using a paired t test. Mean CLD numbers, diameters, and areas were compared using a t test. CLD diameter distribution was compared with the Kolmogorov–Smirnov test. Secretory lipids were assessed using a Fisher exact test.
Footnotes
Author contributions Changting Xiao, Satya Dash, and Gary F. Lewis were responsible for the study concept and design; Changting Xiao, Priska Stahel, Alicia L. Carreiro, Yu-Han Hung, and Ian Bookman performed data acquisition; Changting Xiao, Priska Stahel, Alicia L. Carreiro, Satya Dash, Kimberly K. Buhman, and Gary F. Lewis analyzed and interpreted data; Changting Xiao and Gary F. Lewis wrote the manuscript; Changting Xiao, Priska Stahel, Alicia L. Carreiro, Satya Dash, Kimberly K. Buhman, and Gary F. Lewis revised the manuscript and were responsible for important intellectual content; and Gary F. Lewis obtained funding and performed study supervision.
Conflicts of interest The authors disclose no conflicts.
Funding This work was supported by an operating grant from the Canadian Institutes of Health Research; the Drucker Family Chair in Diabetes Research and the Sun Life Financial Chair in Diabetes (G.F.L.); a Banting and Best Diabetes Centre Hugh Sellers Postdoctoral Fellowship and a Diabetes Action Canada Postdoctoral Fellowship (P.S.); a Purdue Research Foundation Fellowship and a Purdue Bilsland Dissertation Fellowship (A.L.C.); and a Diabetes Canada New Investigator grant and the Reuben and Helene Dennis Scholarship from the Banting and Best Diabetes Centre (S.D.).
References
- 1.Lewis G.F., Xiao C., Hegele R.A. Hypertriglyceridemia in the genomic era: a new paradigm. Endocr Rev. 2015;36:131147. doi: 10.1210/er.2014-1062. [DOI] [PubMed] [Google Scholar]
- 2.Mansbach C.M., Siddiqi S.A. The biogenesis of chylomicrons. Annu Rev Physiol. 2010;72:315–333. doi: 10.1146/annurev-physiol-021909-135801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dash S., Xiao C., Morgantini C., Lewis G.F. New insights into the regulation of chylomicron production. Annu Rev Nutr. 2015;35:265–294. doi: 10.1146/annurev-nutr-071714-034338. [DOI] [PubMed] [Google Scholar]
- 4.Xiao C., Stahel P., Carreiro A.L., Buhman K.K., Lewis G.F. Recent advances in triacylglycerol mobilization by the gut. Trends Endocrinol Metab. 2018;29:151–163. doi: 10.1016/j.tem.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Cohn J.S., McNamara J.R., Krasinski S.D., Russell R.M., Schaefer E.J. Role of triglyceride-rich lipoproteins from the liver and intestine in the etiology of postprandial peaks in plasma triglyceride concentration. Metabolism. 1989;38:484–490. doi: 10.1016/0026-0495(89)90203-5. [DOI] [PubMed] [Google Scholar]
- 6.Fielding B.A., Callow J., Owen R.M., Samra J.S., Matthews D.R., Frayn K.N. Postprandial lipemia: the origin of an early peak studied by specific dietary fatty acid intake during sequential meals. Am J Clin Nutr. 1996;63:36–41. doi: 10.1093/ajcn/63.1.36. [DOI] [PubMed] [Google Scholar]
- 7.Mattes R.D. Brief oral stimulation, but especially oral fat exposure, elevates serum triglycerides in humans. Am J Physiol Gastrointest Liver Physiol. 2009;296:G365–G371. doi: 10.1152/ajpgi.90591.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chavez-Jauregui R.N., Mattes R.D., Parks E.J. Dynamics of fat absorption and effect of sham feeding on postprandial lipema. Gastroenterology. 2010;139:1538–1548. doi: 10.1053/j.gastro.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robertson M.D., Parkes M., Warren B.F., Ferguson D.J.P., Jackson K.G., Jewell D.P., Frayn K.N. Mobilisation of enterocyte fat stores by oral glucose in humans. Gut. 2003;52:834–839. doi: 10.1136/gut.52.6.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu J., Lee B., Buhman K.K., Cheng J.-X. A dynamic, cytoplasmic triacylglycerol pool in enterocytes revealed by ex vivo and in vivo coherent anti-Stokes Raman scattering imaging. J Lipid Res. 2009;50:1080–1089. doi: 10.1194/jlr.M800555-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahara E.-I., Mantani Y., Udayanga K.G.S., Qi W.-M., Tanida T., Takeuchi T., Yokoyama T., Hoshi N., Kitagawa H. Ultrastructural demonstration of the absorption and transportation of minute chylomicrons by subepithelial blood capillaries in rat jejunal villi. J Vet Med Sci. 2013;75:1563–1569. doi: 10.1292/jvms.13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hung Y.-H., Carreiro A.L., Buhman K.K. Dgat1 and Dgat2 regulate enterocyte triacylglycerol distribution and alter proteins associated with cytoplasmic lipid droplets in response to dietary fat. Biochim Biophys Acta. 2017;1862:600–614. doi: 10.1016/j.bbalip.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beilstein F., Bouchoux J., Rousset M., Demignot S. Proteomic analysis of lipid droplets from Caco-2/TC7 enterocytes identifies novel modulators of lipid secretion. PLoS One. 2013;8:e53017. doi: 10.1371/journal.pone.0053017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Aquila T., Sirohi D., Grabowski J.M., Hedrick V.E., Paul L.N., Greenberg A.S., Kuhn R.J., Buhman K.K. Characterization of the proteome of cytoplasmic lipid droplets in mouse enterocytes after a dietary fat challenge. PLoS One. 2015;10:e0126823. doi: 10.1371/journal.pone.0126823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uchida A., Whitsitt M.C., Eustaquio T., Slipchenko M.N., Leary J.F., Cheng J.-X., Buhman K.K. Reduced triglyceride secretion in response to an acute dietary fat challenge in obese compared to lean mice. Front Physiol. 2012;3:26. doi: 10.3389/fphys.2012.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beilstein F., Carriere V., Leturque A., Demignot S. Characteristics and functions of lipid droplets and associated proteins in enterocytes. Exp Cell Res. 2015;340:172–179. doi: 10.1016/j.yexcr.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 17.D’Aquila T., Hung Y.-H., Carreiro A., Buhman K.K. Recent discoveries on absorption of dietary fat: presence, synthesis, and metabolism of cytoplasmic lipid droplets within enterocytes. Biochim Biophys Acta. 2016;1861:730–747. doi: 10.1016/j.bbalip.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fielding B.A., Reid G., Grady M., Humphreys S.M., Evans K., Frayn K.N. Ethanol with a mixed meal increases postprandial triacylglycerol but decreases postprandial non-esterified fatty acid concentrations. Br J Nutr. 2000;83:597–604. doi: 10.1017/s0007114500000763. [DOI] [PubMed] [Google Scholar]
- 19.Dash S., Xiao C., Morgantini C., Connelly P.W., Patterson B.W., Lewis G.F. Glucagon-like peptide-2 regulates release of chylomicrons from the intestine. Gastroenterology. 2014;147:1275–1284. doi: 10.1053/j.gastro.2014.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans K., Kuusela P.J., Cruz M.L., Wilhelmova I., Fielding B.A., Frayn K.N. Rapid chylomicron appearance following sequential meals: effects of second meal composition. Br J Nutr. 1998;79:425–429. doi: 10.1079/bjn19980072. [DOI] [PubMed] [Google Scholar]
- 21.Lewis G.F., Carpentier A., Adeli K., Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002;23:201–229. doi: 10.1210/edrv.23.2.0461. [DOI] [PubMed] [Google Scholar]
- 22.Westberg-Rasmussen S., Starup-Linde J., Hermansen K., Holst J.J., Hartmann B., Vestergaard P., Gregersen S. Differential impact of glucose administered intravenously or orally on bone turnover markers in healthy male subjects. Bone. 2017;97:261–266. doi: 10.1016/j.bone.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 23.Hartmann B., Harr M.B., Jeppesen P.B., Wojdemann M., Deacon C.F., Mortensen P.B., Holst J.J. In vivo and in vitro degradation of glucagon-like peptide-2 in humans. J Clin Endocrinol Metab. 2000;85:2884–2888. doi: 10.1210/jcem.85.8.6717. [DOI] [PubMed] [Google Scholar]
- 24.Xiao C., Bandsma R.H., Dash S., Szeto L., Lewis G.F. Exenatide, a glucagon-like peptide-1 receptor agonist, acutely inhibits intestinal lipoprotein production in healthy humans. Arterioscler Thromb Vasc Biol. 2012;32:1513–1519. doi: 10.1161/ATVBAHA.112.246207. [DOI] [PubMed] [Google Scholar]
- 25.Couture P., Tremblay A.J., Kelly I., Lemelin V., Droit A., Lamarche B. Key intestinal genes involved in lipoprotein metabolism are downregulated in dyslipidemic men with insulin resistance. J Lipid Res. 2014;55:128–137. doi: 10.1194/jlr.M040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bourassa S., Fournier F., Nehmé B., Kelly I., Tremblay A., Lemelin V., Lamarche B., Couture P., Droit A. Evaluation of iTRAQ and SWATH-MS for the quantification of proteins associated with insulin resistance in human duodenal biopsy samples. PLoS One. 2015;10:e0125934. doi: 10.1371/journal.pone.0125934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yanase H., Shimizu H., Kanda T., Fujii H., Iwanaga T. Cellular localization of the diazepam binding inhibitor (DBI) in the gastrointestinal tract of mice and its coexistence with the fatty acid binding protein (FABP) Arch Histol Cytol. 2001;64:449–460. doi: 10.1679/aohc.64.449. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed M.Y., Al-Khayat A., Al-Murshedi F., Al-Futaisi A., Chioza B.A., Pedro Fernandez-Murray J., Self J.E., Salter C.G., Harlalka G.V., Rawlins L.E., Al-Zuhaibi S., Al-Azri F., Al-Rashdi F., Cazenave-Gassiot A., Wenk M.R., Al-Salmi F., Patton M.A., Silver D.L., Baple E.L., McMaster C.R., Crosby A.H. A mutation of EPT1 (SELENOI) underlies a new disorder of Kennedy pathway phospholipid biosynthesis. Brain J Neurol. 2017;140:547–554. doi: 10.1093/brain/aww318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Payne F., Lim K., Girousse A., Brown R.J., Kory N., Robbins A., Xue Y., Sleigh A., Cochran E., Adams C., Dev Borman A., Russel-Jones D., Gorden P., Semple R.K., Saudek V., O’Rahilly S., Walther T.C., Barroso I., Savage D.B. Mutations disrupting the Kennedy phosphatidylcholine pathway in humans with congenital lipodystrophy and fatty liver disease. Proc Natl Acad Sci U S A. 2014;111:8901–8906. doi: 10.1073/pnas.1408523111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Veen J.N., Kennelly J.P., Wan S., Vance J.E., Vance D.E., Jacobs R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim Biophys Acta. 2017;1859:1558–1572. doi: 10.1016/j.bbamem.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Itabe H., Yamaguchi T., Nimura S., Sasabe N. Perilipins: a diversity of intracellular lipid droplet proteins. Lipids Health Dis. 2017;16:83. doi: 10.1186/s12944-017-0473-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaishy B., Abel E.D. Lipids, lysosomes, and autophagy. J Lipid Res. 2016;57:1619–1635. doi: 10.1194/jlr.R067520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang W., Lilja L., Mandic S.A., Gromada J., Smidt K., Janson J., Takai Y., Bark C., Berggren P.-O., Meister B. Tomosyn is expressed in beta-cells and negatively regulates insulin exocytosis. Diabetes. 2006;55:574–581. doi: 10.2337/diabetes.55.03.06.db05-0015. [DOI] [PubMed] [Google Scholar]
- 34.Zhu Q., Yamakuchi M., Ture S., de la Luz Garcia-Hernandez M., Ko K.A., Modjeski K.L., LoMonaco M.B., Johnson A.D., O’Donnell C.J., Takai Y., Morrell C.N., Lowenstein C.J. Syntaxin-binding protein STXBP5 inhibits endothelial exocytosis and promotes platelet secretion. J Clin Invest. 2014;124:4503–4516. doi: 10.1172/JCI71245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mansbach C.M., Siddiqi S. Control of chylomicron export from the intestine. Am J Physiol Gastrointest Liver Physiol. 2016;310:G659–G668. doi: 10.1152/ajpgi.00228.2015. [DOI] [PubMed] [Google Scholar]
- 36.Fagerberg L., Hallström B.M., Oksvold P., Kampf C., Djureinovic D., Odeberg J., Habuka M., Tahmasebpoor S., Danielsson A., Edlund K., Asplund A., Sjöstedt E., Lundberg E., Szigyarto C.A.-K., Skogs M., Takanen J.O., Berling H., Tegel H., Mulder J., Nilsson P., Schwenk J.M., Lindskog C., Danielsson F., Mardinoglu A., Sivertsson A., von Feilitzen K., Forsberg M., Zwahlen M., Olsson I., Navani S., Huss M., Nielsen J., Ponten F., Uhlén M. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics. 2014;13:397–406. doi: 10.1074/mcp.M113.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Persson B., Kallberg Y., Bray J.E., Bruford E., Dellaporta S.L., Favia A.D., Duarte R.G., Jörnvall H., Kavanagh K.L., Kedishvili N., Kisiela M., Maser E., Mindnich R., Orchard S., Penning T.M., Thornton J.M., Adamski J., Oppermann U. The SDR (short-chain dehydrogenase/reductase and related enzymes) nomenclature initiative. Chem Biol Interact. 2009;178:94–98. doi: 10.1016/j.cbi.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oppermann U.C., Filling C., Jörnvall H. Forms and functions of human SDR enzymes. Chem Biol Interact. 2001;130–132:699–705. doi: 10.1016/s0009-2797(00)00301-x. [DOI] [PubMed] [Google Scholar]
- 39.Lemieux S., Fontani R., Uffelman K.D., Lewis G.F., Steiner G. Apolipoprotein B-48 and retinyl palmitate are not equivalent markers of postprandial intestinal lipoproteins. J Lipid Res. 1998;39:1964–1971. [PubMed] [Google Scholar]
- 40.Booth C.C., Read A.E., Jones E. Studies on the site of fat absorption: 1. The sites of absorption of increasing doses of I-labelled triolein in the rat. Gut. 1961;2:23–31. doi: 10.1136/gut.2.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cardell R.R., Badenhausen S., Porter K.R. Intestinal triglyceride absorption in the rat. An electron microscopical study. J Cell Biol. 1967;34:123–155. doi: 10.1083/jcb.34.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friedman H.I., Cardell R.R. Effects of puromycin on the structure of rat intestinal epithelial cells during fat absorption. J Cell Biol. 1972;52:15–40. doi: 10.1083/jcb.52.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sabesin S.M., Frase S. Electron microscopic studies of the assembly, intracellular transport, and secretion of chylomicrons by rat intestine. J Lipid Res. 1977;18:496–511. [PubMed] [Google Scholar]
- 44.Young S.G., Cham C.M., Pitas R.E., Burri B.J., Connolly A., Flynn L., Pappu A.S., Wong J.S., Hamilton R.L., Farese R.V. A genetic model for absent chylomicron formation: mice producing apolipoprotein B in the liver, but not in the intestine. J Clin Invest. 1995;96:2932–2946. doi: 10.1172/JCI118365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jersild R.A. A time sequence study of fat absorption in the rat jejunum. Am J Anat. 1966;118:135–162. doi: 10.1002/aja.1001180108. [DOI] [PubMed] [Google Scholar]
- 46.Cox J., Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]