Abstract
Ewing's sarcoma is a kind of undifferentiated reticulocytic sarcoma, which was first reported in 1921 by James Ewing. It is difficult to differentiate Ewing's sarcoma from osteomyelitis on computed tomography (CT) and X-ray and hence cytological confirmation is needed. Fluorodeoxy glucose being a nonspecific tracer cannot differentiate between malignant and inflammatory lesions. However, it is found that Ewing's sarcoma has increased LAT1 transporter expression at the cell surface. This property has been utilized to specifically target the tumor cells and differentiate them from inflammatory lesions. 18F-fluoroethyl tyrosine (FET) is a radiotracer which shows increased uptake in tumors having LAT1 expression and no uptake in inflammatory lesions. Thus, FET positron emission tomography-computed tomography can serve as a useful tool in diagnosing recurrence or residual Ewing's sarcoma from infective pathology. Besides, it is also helpful in monitoring response to therapy.
Keywords: Ewing's sarcoma, flouro-ethyl tyrosine, osteomyelitis
INTRODUCTION
Ewing's sarcoma is the second-most common bone tumor in children and adolescents. Ewing's sarcoma is a kind of undifferentiated reticulocytic sarcoma, which was first reported in 1921 by James Ewing. The fluorodeoxy glucose (FDG) positron emission tomography-computed tomography (PET-CT) study became a valuable imaging method in the staging, restaging, and evaluation of therapeutic response in patients with Ewing's tumor.[1] However, a wide range of benign processes reveal glycolytic metabolism, as is the case of infectious or inflammatory diseases (tuberculosis, pneumonia, abscess, osteomyelitis, etc.), postsurgical and postradiotherapy status, and utilization of granulocyte-colony-stimulating factors (promote expansion with consequential uptake by the bone marrow), among others. Inflammatory alterations secondary to surgical, traumatic, or radiotherapy procedures may demonstrate glycolytic activity even 3 months after the event; thus, Ewing's sarcoma is difficult to differentiate from osteomyelitis through current imaging modalities and hence cytological confirmation is often required.[1] Moreover, it is difficult to differentiate Ewing's sarcoma from osteomyelitis on CT images as well as both share similar radiographic findings.[2] Here, we report the case of a 22-year-old male with recurrence of Ewing's sarcoma and concurrent osteomyelitis. Both FDG and fluoroethyl tyrosine (FET) PET/CT scans were acquired and we have tried to differentiate between infection and malignancy of the bone through FET PET/CT imaging modality and later confirmed with cyto/histopathological examination.
CASE REPORT
A 22-year-old male, who was a known case of Ewing's sarcoma in the region of left humerus, had been operated upon for the sarcoma and had received radiotherapy. He was referred to our institute for PET-CT for the evaluation of recurrence. At the time of the study, the boy complained of a palpable swelling in the vault of the skull and discharging sinus from the left humerus where he had received radiotherapy and a surgical implant 5 years back.
An 18F-FDG PET-CT study was performed 60 min after intravenous injection of 370 MBq 18F-FDG, after 6-h fasting with a whole-body full-ring PET-CT camera (Discovery STE16-GE, Chicago, illinois, USA) which provided three-dimensional acquisition, processing, and display of CT, PET, and PET-CT images. The CT portion was performed according to a soft-tissue protocol and acquisition on a 16-slice scanner. Finally, the acquisition of PET emission images was performed (2 min per bed position).
The CT data were used for attenuation correction of PET emission images and for fusion with PET data for accurate localization of lesions. Nonattenuated data were reconstructed after scan acquisition had been completed. Reconstruction of attenuation-corrected data was executed concurrently. All digital images were interpreted on a dedicated Xeleris™ workstation (GE, Chicago, Illinois, USA).
There was evidence of a FDG-avid lytic-sclerotic lesion with destructive pattern in the mid-humerus and distal humerus with associated soft-tissue mass [Figures 1 and 2]. Another poorly defined lytic lesion was seen in the high frontoparietal region of the skull vault with an associated soft-tissue mass extending into the scalp [Figure 3]. A large extradural component was noted indenting the underlying brain parenchyma. The lesion showed heterogeneously increased FDG uptake [Figure 3].
Figure 1.
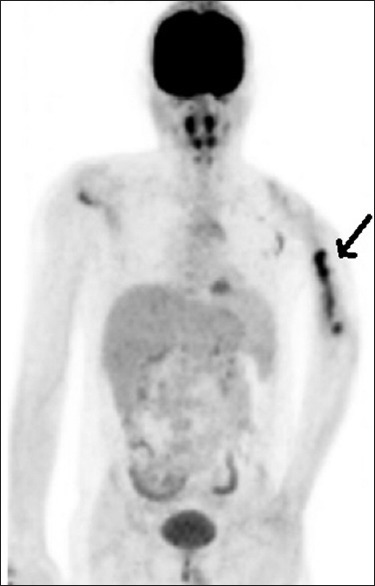
Maximum intensity projection image of fluorodeoxy glucose scan with fluorodeoxy glucose-avid lesion in the left humerus marked by a thick arrow, while the skull lesion cannot be appreciated due to high adjacent normal brain activity
Figure 2.
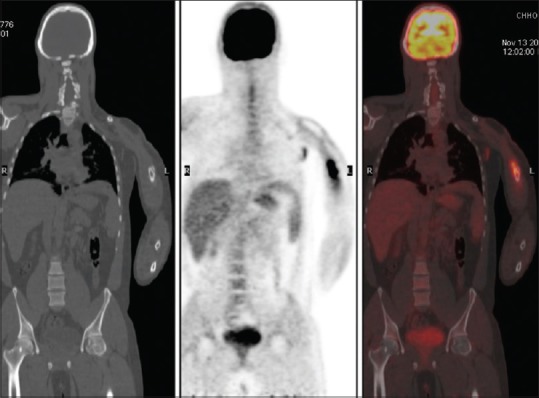
Coronal sections of computed tomography, positron emission tomography, and fused positron emission tomography/computed tomography images, respectively, showing an increased fluorodeoxy glucose uptake in lytic-sclerotic lesion of the left humerus
Figure 3.
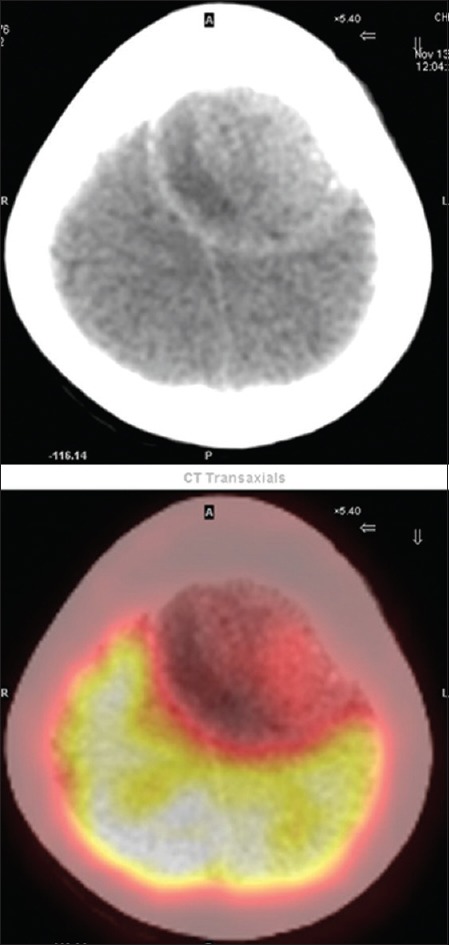
Transaxial computed tomography and fused transaxial fluorodeoxy glucose scan showing fluorodeoxy glucose-avid lytic lesions. Due to increased fluorodeoxy glucose uptake in the normal brain parenchyma, the fluorodeoxy glucose uptake in the skull lesion cannot be well appreciated
The patient was called again after 2 days for FET PET/CT scan. The study was performed 60 min after intravenous injection of 370 MBq 18F-FET, and PET/CT images were acquired and reconstructed using the similar procedure as mentioned before.
FET PET-CT revealed no uptake of tracer in the lytic-sclerotic lesion of the humerus [Figure 4], but an increased uptake was noted in the lytic lesion of the skull [Figure 5]. The patient was followed up with cytopathological reports from both lesions, i.e., from the skull and left humerus. The skull lesion confirmed recurrence of small-round-cell tumor, whereas biopsy from the left humerus reflected only inflammatory cells and chronic infectious osteomyelitis.
Figure 4.
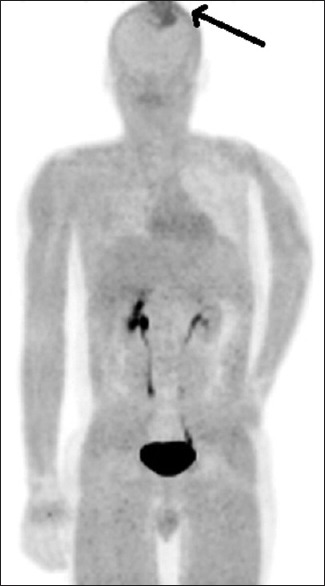
Maximum intensity projection positron emission tomography image of fluoroethyl tyrosine scan showing a single lesion in the skull marked with an arrow having increased fluoroethyl tyrosine uptake; however, no uptake in the left humerus is noted
Figure 5.
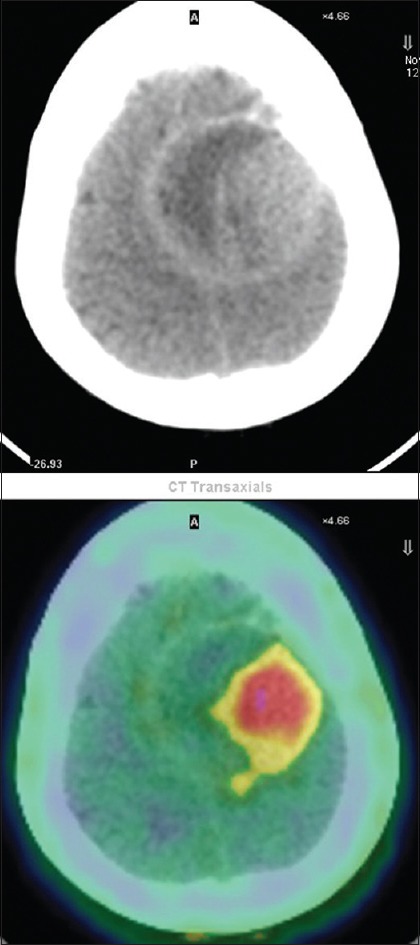
Transaxial computed tomography and fused transaxial fluoroethyl tyrosine positron emission tomography-computed tomography scan showing an increased fluoroethyl tyrosine uptake in lytic lesion in the skull marked by an arrow
DISCUSSION
FDG has already proved its utility in staging, diagnosing, and evaluating response to therapy for Ewing's sarcoma; however, FDG is nonspecific. Hence, many a times, FDG scan findings can be misleading when evaluating response to therapy, especially in the postsurgical cases where chances of infection of the graft are quite high.[1] Moreover, Ewing's sarcoma is one tumor where radiological findings are similar with that of osteomyelitis.[2] Therefore, a situation may often arise with a diagnostic dilemma of residual disease or a postsurgical infection while following such cases. This may also lead to uncertainty in deciding the further course of management. Fine-needle aspiration cytology though conclusive of malignancy or residual tumor can give false-negative results.[3] FDG being a nonspecific tracer is expected to show uptake in infection and inflammation as well as tumor or metastasis.[1] FDG has inferior capability in distinguishing neoplastic from inflammatory or treatment-related lesions as opposed to an aminoacid PET tracer.[4] A need, therefore, arises to differentiate malignant pathology from infective pathology through imaging modalities where detection through invasive cytological procedures is difficult to perform (e.g., lesions involving spinal cord).[5] Kebir et al. reported the role of FET PET/CT for the differential diagnosis of tumefactive multiple sclerosis versus glioma and found FET being specific to tumor, whereas no uptake was noted in inflammatory pathology.[6] Similarly, in a preclinical study, Lee et al. examined FET and FLT along with FDG to differentiate tumor from inflammation. They found that FET selectively localized in tumor tissues but not in inflammation,[7] and similar results were also reported by Tsuji et al.[8] FET depicts aminoacid metabolism and has specific uptake in the cells through LAT1 and LAT2 aminoacid transporters. It has been found that Ewing's sarcoma has increased LAT1 transporter expression at the cell surface.[9] This property has been utilized to specifically target the tumor cells and differentiate them from inflammatory lesions. FDG scan shows an increased uptake in the lesion of skull and left humerus, whereas FET uptake was noted only in the skull lesion. In addition, from the above findings, a biopsy was done from the humerus lesion and skull lesion, of which the skull lesion confirmed the recurrence of small-round-cell tumor, whereas biopsy from the humerus reflected only inflammatory cells and chronic infectious osteomyelitis. Thus, FET PET-CT can serve as a useful tool in diagnosing recurrence or residual Ewing's sarcoma and response to therapy.
CONCLUSION
FET PET/CT can be used to differentiate Ewing's sarcoma from osteomyelitis and can be used as a sole modality for recurrence/residual evaluation and response to treatment. However, further studies are required to validate it as a sole modality.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Guimarães JB, Rigo L, Lewin F, Emerick A. The importance of PET/CT in the evaluation of patients with Ewing tumors. Radiol Bras. 2015;48:175–80. doi: 10.1590/0100-3984.2013.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCarville MB, Chen JY, Coleman JL, Li Y, Li X, Adderson EE, et al. Distinguishing osteomyelitis from Ewing sarcoma on radiography and MRI. AJR Am J Roentgenol. 2015;205:640–50. doi: 10.2214/AJR.15.14341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kilpatrick SE, Geisinger KR. Soft tissue sarcomas: The usefulness and limitations of fine-needle aspiration biopsy. Am J Clin Pathol. 1998;110:50–68. doi: 10.1093/ajcp/110.1.50. [DOI] [PubMed] [Google Scholar]
- 4.Buck D, Förschler A, Lapa C, Schuster T, Vollmar P, Korn T, et al. 18F-FDG PET detects inflammatory infiltrates in spinal cord experimental autoimmune encephalomyelitis lesions. J Nucl Med. 2012;53:1269–76. doi: 10.2967/jnumed.111.102608. [DOI] [PubMed] [Google Scholar]
- 5.Kebir S, Kimmich O, Niehusmann P, Gaertner FC, Essler M, Landsberg J, et al. 18F-fluoroethyl-L-tyrosine positron emission tomography-guided diagnosis of a malignant intramedullary spinal cord tumor. Oncol Lett. 2016;12:4705–7. doi: 10.3892/ol.2016.5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kebir S, Gaertner FC, Mueller M, Nelles M, Simon M, Schäfer N, et al. 18F-fluoroethyl-L-tyrosine positron emission tomography for the differential diagnosis of tumefactive multiple sclerosis versus glioma: A case report. Oncol Lett. 2016;11:2195–8. doi: 10.3892/ol.2016.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee TS, Ahn SH, Moon BS, Chun KS, Kang JH, Cheon GJ, et al. Comparison of 18F-FDG, 18F-FET and 18F-FLT for differentiation between tumor and inflammation in rats. Nucl Med Biol. 2009;36:681–6. doi: 10.1016/j.nucmedbio.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Tsuji AB, Kato K, Sugyo A, Okada M, Sudo H, Yoshida C, et al. Comparison of 2-amino-[3-11C] isobutyric acid and 2-deoxy-2-[18F] fluoro-D-glucose in nude mice with xenografted tumors and acute inflammation. Nucl Med Commun. 2012;33:1058–64. doi: 10.1097/MNM.0b013e328356efb0. [DOI] [PubMed] [Google Scholar]
- 9.Pauleit D, Zimmermann A, Stoffels G, Bauer D, Risse J, Flüss MO, et al. 18F-FET PET compared with 18F-FDG PET and CT in patients with head and neck cancer. J Nucl Med. 2006;47:256–61. [PubMed] [Google Scholar]


