Extinction threatens at least 60% of wild coffee species, including those that are key to the future of the global coffee sector.
Abstract
Wild coffee species are critical for coffee crop development and, thus, for sustainability of global coffee production. Despite this fact, the extinction risk and conservation priority status of the world’s coffee species are poorly known. Applying IUCN Red List of Threatened Species criteria to all (124) wild coffee species, we undertook a gap analysis for germplasm collections and protected areas and devised a crop wild relative (CWR) priority system. We found that at least 60% of all coffee species are threatened with extinction, 45% are not held in any germplasm collection, and 28% are not known to occur in any protected area. Existing conservation measures, including those for key coffee CWRs, are inadequate. We propose that wild coffee species are extinction sensitive, especially in an era of accelerated climatic change.
INTRODUCTION
Coffee as a crop and wild species
Coffee (Coffea L.) is one of the world’s most widely consumed beverages, supporting a multibillion-dollar sector (1) spanning a lengthy value chain from farmer to consumer. As coffee production is largely in the hands of smallholder farmers, the livelihood value is immense, with an estimated 100 million coffee farmers worldwide (2). Global coffee trade relies on two species: Arabica (Coffea arabica) comprising c. 60% of traded coffee, and robusta (Coffea canephora), the remaining 40% (1). Liberica coffee (Coffea liberica), a third beverage species, is cultivated worldwide (and used as a grafting rootstock for Arabica and robusta) but is insignificant in terms of global trade (1). C. arabica, a product of the ancient hybridization of C. canephora and Coffea eugenioides (3, 4), occurs naturally in Ethiopia and South Sudan (5); C. liberica and C. canephora occur wild across much of wet tropical Africa (6).
Arabica coffee has been farmed for at least several hundred years and may have been wild harvested for millennia, first as a food and then later as a beverage (7). Farming of robusta was first recorded in Africa in the early to mid-1800s (8) but probably predates records by hundreds of years. Liberica coffee cultivation was first documented in the early 1870s, but despite great hopes, its cup qualities failed to meet the taste requirements of the consumer, and thus the aspirations of growers and coffee merchants, particularly in Sri Lanka (9). Arabica and robusta are unusual among major crop plants, in that the time period over which domestication occurred is short and their level of domestication (i.e., variance from wild types) is variable and mostly minimal, except in the cases of interspecies hybrids. Wild variants of both species can be harvested and processed to produce coffee with sensory qualities that are similar to or indistinguishable from cultivated and domesticated types.
Robusta gains ground over Arabica
Despite early records of robusta coffee farming, this species was not recognized by science until 1897, based on material from West Africa (Gabon) (10). Cultivation outside Africa developed rapidly from the early 1900s onward (11), in many cases replacing widely grown Arabica and newly planted Liberica coffee. Robusta gained market share against Arabica due to its resistance to coffee leaf rust (CLR; Hemileia vastatrix Berk. & Broome) (8), broader agroecological envelope (6), higher productivity (12), and lower market price (1). Although robusta has some negative sensory qualities (e.g., tasting notes of wood and tobacco), it is favored in some instances for its taste, high caffeine content, and ability to add body to espresso and espresso-based coffees; it is now the species of choice for instant coffee. Robusta coffee has been transformed from a poorly known minor African crop to a major global commodity in just c. 150 years. Today, robusta comprises c. 40% of global coffee trade (1), although its true proportion in the global market is probably higher, given that it is used to adulterate Arabica coffee (13). Moreover, robusta has been vital for breeding CLR-resistant cultivars of Arabica coffee, via backcrossing with Arabica-robusta hybrids (14), the most notable of these being the Timor hybrid (15). Robusta coffee has therefore been responsible for overcoming most of the key issues for coffee sector sustainability, either by direct replacement or through use in breeding new cultivars, rendering the development and use of other coffee species unnecessary. Robusta coffee provides a good example of how a (relatively) newly discovered wild species has transformed a globally important crop.
Wild coffee species as a resource for the sustainability of the coffee sector
Despite the overwhelming agronomic and economic success of Arabica and robusta, a myriad of new threats are now evident for the global coffee sector. These include climate change (16), especially the increasing incidence and duration of drought, the spread and escalating severity of devastating fungal pathogens, most notably CLR for Arabica in Central and northern South America (14), and coffee wilt disease (CWD; Gibberella xylarioides R. Heim & Sacca) for robusta in Africa (17), the emergence and/or spread of other diseases and pests (18), and social, economic, and market-based factors. Meeting these challenges will require clear vision, a broad range of interventions, and good governance. There will also be an increasing demand for germplasm: the raw material of crop development. Wild variants of Arabica and robusta will be of primary importance, but other wild coffee species [crop wild relatives (CWRs)] are likely to be required. Wild coffee species are once again coming into focus (19, 20), reviving the considerable interest that existed during earlier eras of coffee research (12, 21, 22).
Most consumers, and even many coffee sector representatives, are unaware that there are more than two or three coffee species. There are 124 coffee species known to science (6, 23), occurring naturally (wild) in tropical Africa, the Indian Ocean islands (Madagascar, Comoros Islands, and Mascarene Islands), Asia, and Australasia (fig. S1) (24). All Coffea species have the characteristic coffee bean (seed) morphology (25), and several of the noncommercial species are (or have been) used on a local or regional scale as a substitute for Arabica coffee (12, 21, 22, 26, 27). These species have useful traits for coffee development, such as climatic tolerance (6) and especially drought tolerance, pest and disease resistance (21, 28–31), low or zero caffeine content (32), and sensory (taste) amelioration (12, 22).
Given the importance of coffee CWRs for coffee sector sustainability, two critical questions come into focus: What is the extinction risk of wild coffee species? And which species should be prioritized for conservation and crop development? To answer these questions, we report here a global assessment of extinction risk for all known coffee species by rigorously applying International Union for the Conservation of Nature (IUCN) Red List Categories and Criteria (33); a priority system for coffee CWRs, based on phylogenetic data and plant breeding information; and a gap analysis of ex situ conservation in germplasm collections and in situ conservation in protected areas.
RESULTS
At least 60% of coffee species are threatened with extinction
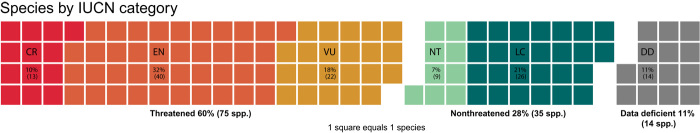
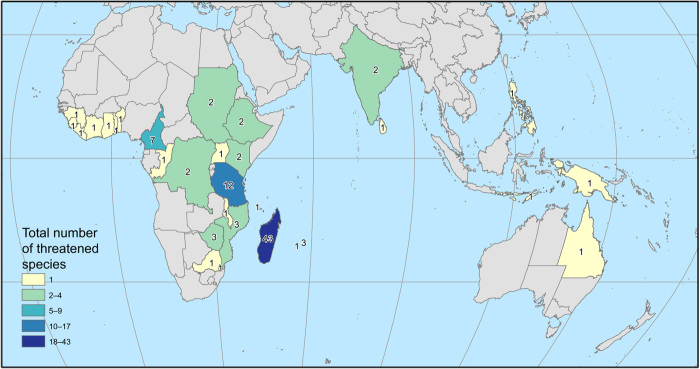
The application of the IUCN Red List Categories and Criteria (33) resulted in 75 coffee species (60%) being assessed as threatened with extinction, including 13 Critically Endangered (CR), 40 Endangered (EN), and 22 Vulnerable (VU) species; 35 species were assessed as not threatened [Near Threatened (NT) or Least Concern (LC)], and 14 species were Data Deficient (DD) (Fig. 1). Information on individual species, including distribution range, habitat and ecology, threats, conservation actions, and assessment information, can be accessed via the IUCN Red List of Threatened Plant Species portal (34); a species summary is provided in table S1, and regional and area summaries in tables S2 and S3. Madagascar has the highest number of threatened species (43 species) (Fig. 2), but proportions of threatened species in Madagascar (72%) were similar to those in Tanzania (12 species; 71%) (table S3).
Fig. 1. IUCN extinction risk categories for coffee species.
Waffle chart, showing the proportion and number of threatened, nonthreatened, and DD coffee species in main blocks, and the proportion and number of coffee species assigned to each IUCN extinction risk category. The total number of species is 124 [CR, 10.5% (13 species); EN, 32.3% (40 species); VU, 17.7% (22 species); NT, 8% (10 species); LC, 21% (26 species); DD, 11.3% (14 species)]. Each square is equal to one species.
Fig. 2. Total number of coffee species threatened with extinction by area.
Map showing threatened coffee species by TDWG level 3 areas (countries or subdivisions of countries; see Materials and Methods for the definition of TDWG level 3). See fig. S1 for number of coffee species by area.
The 14 species (11.3%) assessed as DD were spread across the distribution range of wild coffees (fig. S2). A taxon is assessed as DD when there is inadequate information to make a direct or indirect assessment of its risk of extinction based on its distribution and/or population status (35). Inadequacy of information is attributable to one or more of a range of factors including uncertain provenance, taxonomic uncertainty, old records, and uncertain threats. Thirteen coffee species were categorized as DD because of the paucity of ground point data (occurrence) records; a fourteenth coffee species, Coffea rhamnifolia (northern Kenya and Somalia), lacks recent knowledge of population trends or threats. The lack of recent ground point data arises either from the lack of research observations of these species in the wild over several decades, due to conflict-engendered inaccessibility of a particular region (e.g., in Angola, for Coffea carrisoi, Coffea kapakata, and Coffea melanocarpa), or perhaps from a lack of rigorous, targeted fieldwork (e.g., in Asia, for Coffea fragrans, Coffea horsfieldiana, and Coffea madurensis). Nine of the DD species have not been seen since 1940, and five of these (all Asian) are only known from three or fewer herbarium records made before 1900 (Coffea cochinchinensis, Coffea floresiana, C. fragrans, C. horsfieldiana, and Coffea malabarica). It is likely that some of these species are threatened (36) and that some could be extinct.
The preliminary extinction risk assessments reported in Davis et al. (6) suggest a higher percentage (69%) of threatened species for wild coffee than is reported here, and, for many species, the category applied then (in 2006) differs markedly from that in the complete assessment reported here. However, these earlier assessments were exceedingly provisional, being based primarily on an approximation of geographical range size, without detailed consideration of threats; the IUCN Red List Categories and Criteria (33) were not rigorously applied, and the assessments were not peer reviewed and submitted to (or ratified by) the IUCN Red List (34). Thus, the differences between the assessments of Davis et al. (6) and those reported here should not be interpreted as indicators of trends in the extinction risk of wild coffee species.
Priority coffee CWRs have a high extinction risk
Three CWR priority groups were recognized; assignment of species is given in table S1. CWR priority group I is composed of four species, including farmed and wild variants of each crop species (C. arabica, C. canephora, and C. liberica) and C. eugenioides, a parental species of C. arabica (3, 4). Wild C. arabica is the only threatened (EN) species of group I (37). CWR priority group II is composed of 38 species and includes all African species and African clades (24, 32) except species formerly placed in Psilanthus (see the Supplementary Materials). In group II, 23 species (61%) are threatened (Table 1). CWR priority group III contains 82 species, including all species from Madagascar, Comoros lslands, and Mascarene Islands and their respective clades (4, 24, 32) and all species (African and Asian) formerly placed in Psilanthus (Supplementary Materials). In group III, 51 species (62%) are threatened.
Table 1. Number and percentage of coffee species held in germplasm collections (ex situ) and occurring in protected areas (in situ), organized by CWR priority group and extinction risk category (33).
Numbers in parentheses represent percentage for each CWR priority group or each IUCN category. n/a, not applicable.
| CWR priority group | Number of species |
Total number (%) of threatened species per CWR group |
Number (%) of species in germplasm collections (ex situ) |
Number (%) of species in protected areas (in situ) |
Number (%) of species lacking ex situ or in situ representation |
| Group I | |||||
| EN | 1 | — | 1 (100) | 1 (100) | 0 (0) |
| LC | 3 | — | 3 (100) | 3 (100) | 0 (0) |
| Totals | 4 | 1 (25) | 4 (100) | 4 (100) | 0 (0) |
| Group II | |||||
| CR | 6 | — | 1 (17) | 1 (17) | 4 (67) |
| EN | 9 | — | 4 (44) | 9 (100) | 0 (0) |
| VU | 8 | — | 2 (25) | 8 (100) | 0 (0) |
| NT | 4 | — | 3 (75) | 4 (100) | 0 (0) |
| LC | 8 | — | 5 (63) | 8 (100) | 0 (0) |
| DD | 3 | — | 1 (33) | n/a | n/a |
| Totals | 38 | 23 (61) | 16 (42) | 30 (79) | 4 (11) |
| Group III | |||||
| CR | 7 | — | 2 (29) | 2 (29) | 3 (43) |
| EN | 30 | — | 18 (60) | 20 (67) | 4 (13) |
| VU | 14 | — | 11 (79) | 13 (93) | 1 (7) |
| NT | 5 | — | 4 (80) | 5 (100) | 0 (0) |
| LC | 15 | — | 11 (73) | 15 (100) | 0 (0) |
| DD | 11 | — | 2 (18) | n/a | n/a |
| Totals | 82 | 51 (62) | 48 (59) | 55 (67) | 8 (10) |
| Totals | 124 | 75 (60) | 68 (55) | 89 (72) | 12 (10) |
Threatened species are inadequately represented ex situ and in situ
Our gap analysis shows that just over half of all coffee species (55%) are held within ex situ germplasm collections (Table 1 and table S4). The most highly threatened species are poorly represented, with only 23% of CR species held ex situ; EN and VU species have better coverage, at 58 and 59%, respectively (table S4). Ex situ representation of CWR priority groups is as follows: 4 species (100%) for group I, 16 species (42%) for group II, and 48 species (59%) for group III (Table 1).
Around two-thirds of species (89; 72%) occur within at least one protected area (in situ); 22 species (18%) have no in situ protection, and the protected area coverage of 13 species is unknown (including 11 DD species). In situ representation for each CWR priority group is as follows: 4 species (100%) for group I, 30 species (79%) for group II, and 55 species (67%) for group III (Table 1).
Overall, in situ coverage is greater than ex situ: 72% versus 55%, respectively. This difference is particularly marked in CWR priority group II (79% in situ versus 42% ex situ), while proportions in group III vary less (67% versus 59%). Twelve species (10%) have no ex situ or in situ representation, including seven CR species (four in CWR priority group II) and four EN species.
DISCUSSION
At 60%, the proportion of coffee species threatened with extinction is high compared with a global figure of 22% for all plants (38), and one of the highest levels recorded for a plant group. For the near-endemic Madagascan palm genus Dypsis (Arecaceae), 73.8% of its species are threatened (34, 39), which is comparable to the percentage of threatened Madagascan and Indian Ocean island coffee species (71.4%). Other examples include 66% threatened species for Encephalartos (Zamiaceae), 58% for Parodia (Cactaceae), and 48.7% for Magnolia (Magnoliaceae) (34). To date, complete genus-wide extinction risk assessments for CWRs, including woody representatives, are scarce. A recent, comprehensive assessment of tea relatives (Camellia; Theaceae) reported one species (<1%) Extinct (EX), 45.4% threatened, and 21% DD (40). Other woody CWR examples include hazelnut (Corylus; Betulaceae), with 6.2% species threatened; pistachio (Pistacia; Anacardiacae), with 9% threatened; and mango (Mangifera; Anacardiacae), with 52.4% threatened (34). The proportion of threatened coffee species may prove ultimately to be higher than the values reported here, as it has been shown that DD species with old and/or few records (as is the case for at least five coffee species) tend to have high levels of predicted extinction risk (36).
C. arabica has the most thorough extinction risk assessment of any coffee species (37), due to plentiful high-quality ground point data, rigorous ground truthing, and inclusion of climate change projections (5, 16). Moat et al. (37) show that when climate change projections are incorporated in the extinction risk assessment, C. arabica moves three categories, from LC to EN. While it may be some time before we generate enough data to treat all coffee species in this way, these findings (37) signal considerable additional concern for the fate of coffee species when climate change projections are included in extinction risk assessments.
The percentage of coffee CWRs (priority groups I and II) lacking ex situ collections is 52% (45% for all coffee species), much higher than that reported for other CWRs, e.g., 29.1% for 313 taxa associated with 63 crops (41). In general, coffee species are difficult, expensive, and risk-laden subjects for ex situ conservation (42, 43). Unlike many other CWRs, coffee seeds are recalcitrant, i.e., not storable using conventional methods (at low moisture and low temperatures) (43). Maintaining living collections of coffee is particularly costly (42–44), and the genetic integrity of species is susceptible due to the open pollination environment (45). Cryopreservation of seeds and slow growth in vitro methods provide better options and can be cost-effective, but so far, these methods are largely restricted to the main coffee crop species (44).
Coffee species of CWR priority group I (C. arabica, C. eugenioides, C. canephora, and C. liberica) would seem to be in a more secure position than other CWR priority groups, since each species is included in at least one protected area and is present in germplasm collections. There is, however, an underlying issue of inadequate diversity coverage in both ex situ– and in situ–protected environments, including those of C. arabica and C. canephora (see discussion in the Supplementary Materials). Because of rapid deforestation (37, 46), climate change (5, 37), and genetic erosion (47), options to collect further material of wild C. arabica for ex situ conservation and use (e.g., plant breeding) are diminishing. In Ethiopia, the practices of low-intensity farming within native humid forest and harvesting of wild coffee afford a good measure of in situ protection for wild Arabica populations (7, 16, 48, 49); the income generated by coffee production means that the forest has an immediate and tangible value and is thus preserved. The metrics for CWR priority group II are of more concern: 61% of the species in this group are threatened, 58% are not represented ex situ, and four (11%) CR species have neither ex situ nor in situ representation (Table 1).
At face value, the number and percentage of species (89; 72%) occurring within protected areas (in situ) might seem encouraging, but within-species diversity coverage is a major concern. Looking at the example of C. arabica, only c. 4% (1681 km2) of the potential forest area for this species is contained within the protected areas of Ethiopia and South Sudan (37). Moreover, a large proportion of the protected area is under increasing threat from human pressure (50) and global environmental change (51). It should also be made clear that our definition of in situ conservation is restricted to occurrence within a protected area, such as nature reserve or national park. Many protected areas fail to conserve the diversity encompassed within their borders, and workable management plans would be required to ensure that target species are effectively conserved (52).
The main drivers for coffee species extinction risk are small distribution sizes (table S5) and low number of locations (34), i.e., “a geographically or ecologically distinct area in which a single threatening event will affect all individuals” (33), in conjunction with ongoing threats, particularly habitat loss (fig. S3). For almost all species, there is a continuing decline in the quality, area, and extent of available habitat (34). Habitat loss is mainly due to land use change, especially forest loss, predominantly because of agriculture (general), livestock farming, and settlement and development, mostly associated with farming (fig. S3). Timber collection is also a threat for many coffee species; coffee timber is often straight, hard, and termite resistant and frequently collected for minor construction purposes (fig. S3) and fuelwood. On the basis of the research undertaken for this contribution, alongside other coffee research, and after more than two decades of field research, we propose that coffee species are extinction sensitive. Wild coffee species generally exhibit narrow climatic envelopes with restricted habitat (niche) specificity (5, 6, 16, 37), have low adaptive potential (5), and are mostly forest dwelling (6). As with other members of the coffee family (Rubiaceae), they also require good quality habitat, have limited capacity for regeneration unless conditions are optimal, and do not act as pioneer species (53). There are, no doubt, other reasons for the prevalence of range restriction, including perhaps the presence of near-universal obligate outcrossing, due to strong gametophytic self-incompatibility (54).
At a time when so much focus is on addressing food security and livelihood income shortfalls for farmers, it is of great concern that the raw materials for possible solutions are highly threatened. Coffee CWRs have provided major sustainability solutions for the global coffee sector for the last 400 years and to the present day. It is highly likely that similar resources will be called on again to deal with production issues, particularly those linked to disease, pests, and worsening climatic suitability, especially as the global demand for coffee increases (1). This situation is particularly acute for Arabica coffee, given its climatic inflexibility (5, 16) and susceptibility to CLR (14) and other diseases and pests (17). Robusta coffee is also vulnerable to climatic conditions that fall outside the species’ climatic requirements, as shown by the recent media reports of crop failure and plant death due to drought conditions in Brazil. In addition, despite resistance to CLR, robusta is highly susceptible to specific fungal pathogens, such as CWD (17).
Ultimately, we need to conserve existing wild coffee species in situ to ensure the preservation of remaining genetic diversity. This objective necessitates a major commitment and would require input by multiple stakeholders, from host countries and externally. Large, protected areas under strict control have lower human impact and, accordingly, less biodiversity loss (52) but are not immune to other pressures, such as climate change and natural events. In the case of coffee, however, better solutions might be found where there is the potential for human engagement that benefits both livelihoods and biodiversity, such as the case of wild Arabica forests in Ethiopia (7, 16, 48, 49). For ex situ collections, we need to improve the quantity and quality of coffee germplasm inventories (including plant identification and reduction of genetic redundancy), improve management (including data storage and dissemination), and secure vital long-term funding, especially for coffee CWR priority species and core collections required for breeding purposes. The Global Conservation Strategy for Coffee Genetic Resources, which focuses on the ex situ conservation of the main coffee crop species, elaborates on the requirements of effective germplasm management and governance, including recommendations and specific priority actions (42). It has been argued that the use and conservation of wild coffee species outside their countries of origin are hampered by the lack of rigorous and mutually workable access and benefit sharing mechanisms for germplasm (42), an impediment also reported for other crops and CWRs (55). African countries that both cultivate coffee and are home to wild coffee species in natural environments are well placed to develop and conserve their wild coffee resources. They should be supported to do so by the international development and conservation communities.
MATERIALS AND METHODS
Ground point and field observation data
For the IUCN extinction risk assessments of the 124 coffee species, we used a dataset of 5434 ground point records, including 3798 herbarium specimen records, consulted from over 40 herbaria; BM, BR, BRLU, BZ, C, COI, DSM, EA, ETH, G, K, L, LISC, P, SCA, TAN, TEF, UPS, VNM, WAG, and YA [herbarium codes following standard abbreviations (56, 57)] provided the majority of records. A total of 162 data points were sourced online, via Tropicos (104 records) (58), Global Biodiversity Information Facility (43 records) (59), and the Natural History Museum, Paris (3 records) (60); 12 photographic records were accessed through iNaturalist (61). We also used 1624 field and plot observations for wild Arabica from previous studies (5, 16). Herbarium records are verifiable in space (locality), time (when they were collected), and form (species identification) and are therefore well suited for the purposes of the study. All ground point data were georeferenced (if not already available), manually checked for geolocation accuracy, and corrected if necessary. We used an error radius of 2 km for data points, except in cases where we needed reference point for general mapping purposes and for data-poor species (including DD species). Extensive fieldwork was undertaken in areas of high coffee species diversity, in Africa (Cameroon, Kenya, Tanzania, South Sudan, and Uganda), Madagascar (11 field expeditions), and the Indian Ocean islands (Mauritius). A specific focus was given to the two main crop species, Arabica and robusta coffee, including fieldwork in Ethiopia and South Sudan for the former species, and comprehensive herbarium survey for the latter. Observations made during fieldwork were mostly accompanied by the production of herbarium vouchers, other than the numerous recorded observations of Arabica coffee made in Ethiopia (1624 records) (5, 16). Specimen verifications were undertaken by A.P.D.
Production of extent of occurrence and area of occupancy metrics
Extent of occurrence (EOO) and area of occupancy (AOO) are key measurements for applying the IUCN Red List Categories and Criteria (33). EOO is defined as the area containing all the known, inferred, or projected sites of present occurrence of a taxon (i.e., a species) within the shortest continuous imaginary boundary, which can be drawn to encompass the sites; EOO can often be measured by a minimum convex polygon. For convenience, EOO can be referred to as the geographical range. AOO is defined as the area within its “extent of occurrence,” which is occupied by a taxon (33), excluding cases of vagrancy; the size of the AOO “will be a function of the scale at which it is measured, and should be at a scale appropriate to relevant biological aspects of the taxon, the nature of threats, and the available data” (33). The recommended IUCN standard 2 km by 2 km grid cell was used for calculations of AOO. Calculations for the AOO and EOO of each coffee species were made using GeoCAT (62) and rCAT (63).
AOO was in all but one case calculated from observation data. C. arabica was the exception, as the assessment used predicted niche (37) rather than AOO. We only expect AOO values to be accurate at lower thresholds (e.g., <10 km2); those for wider-ranging species are unlikely to be accurate as the ground data will largely underrepresent the species.
Extinction risk assessments (application of the IUCN Red List Categories and Criteria)
Extinction risk assessments were completed for all 124 coffee species, following the IUCN Red List Categories and Criteria (33). The assessments were documented and managed in the Species Information Service (SIS) of IUCN, via their web application (64), following explicit documentation and consistency standards (33, 65). After the application of the criteria, each coffee species was assigned to one of six categories within the IUCN extinction risk assessment system: (i) threatened: CR, EN, or VU; (ii) nonthreatened: NT or LC; or (iii) DD. No species were placed in the EX, Extinct in the Wild (EW), or Not Evaluated (NE) categories. Species with preexisting IUCN extinction risk assessments that did not require updating were retained for our analyses (only two species: Coffea schliebenii and Coffea ligustroides). Reports for each species were generated via SIS (64), internally reviewed by the authors, and then externally by country/regional/group specialists, in batches, by region (West Africa, East Africa, Madagascar, Mascarene Islands, and Asia/Australasia) or group, as part of the formal IUCN review system. Herbarium specimen label data, field observations (1997–2017), and literature sources (including web-based information) were used as additional evidence-based support for applying the IUCN Red List Categories and Criteria (35), e.g., for understanding threats, population declines, and quality and suitability of habitat. Satellite imagery viewed via Google Earth Pro (66) was used to further gauge habitat status (e.g., deforestation) and threats visible from space (e.g., agricultural development and mining). Historical imagery was accessed in Google Earth by using the historical imagery function and the time slider tool to move between satellite image acquisition dates. For wild coffee species localities, the most common date range was the early 1980s to 2016, although acquisition dates for a single scene can go as far back as the mid-20th century (aerial photography).
Mapping and statistical analysis
The IUCN extinction risk assessment metrics were exported from IUCN’s SIS (64) and used as the basis for mapping and statistical analysis. To map coffee species at the country level, species data from the World Checklist of Selected Plant families (67) were matched to Taxonomic Databases Working Group (TDWG) geographical scheme level 3 geography (68). TDWG level 3 denotes a “botanical country,” where most regions are subdivided into units generally equating to a political country, but large countries may be split or outlying areas omitted. These outputs provided species totals and percentages against extinction risk assessment category (CR, EN, VU, NT, LC, and DD) for each country. Data were displayed in ArcGIS 10.5 (69) using the Winkel I projection orientated around the 45° east meridian (through Madagascar). The map colors for Fig. 1 and figs. S1 and S2 were derived from ColorBrewer version 2 (70) to highlight color representation (i.e., allow for differentiation of classes and reproducibility in print and online).
Construction of CWR priority groups
A widely used definition of CWR is “a wild plant taxon that has an indirect use derived from its relatively close genetic relationship to a crop” and “this relationship is defined in terms of the CWR belonging to Gene Pools 1 or 2, or Taxon Groups 1 to 4 of the crop” (71). Assigning a species to a gene pool relies on the presence of genetic diversity information and/or cross pollination data, while assignment to a taxon group requires reference to a taxonomic classification. The gene pool approach of Harlan and de Wet (72) can be summarized as follows: primary gene pool (GP-1), within which GP-1A are the cultivated forms and GP-1B are the wild or weedy forms of the crop; the secondary gene pool (GP-2) includes the coenospecies (less closely related species) from which gene transfer to the crop is possible but difficult using conventional breeding techniques; and the tertiary gene pool (GP-3) includes the species from which gene transfer to the crop is impossible or, if possible, requires sophisticated techniques, such as embryo rescue, somatic fusion, or genetic engineering. Maxted et al. (71) pointed out the difficulties of applying a gene pool classification (72) and, in the absence of the required data, proposed the taxon group concept, based on taxonomic hierarchy (71): taxon group 1a (the crop), taxon group 1b (the same species as the crop), taxon group 2 (the same series or section as the crop), taxon group 3 (the same subgenus as the crop), taxon group 4 (same genus), and taxon group 5 (the same tribe but different genus to crop). Subgenus, section, and series are hierarchical taxonomic divisions of a genus, in order of diminishing taxonomic inclusivity.
There is ample molecular diversity data for coffee, both at the species level (4, 24, 32, 73) and at the genus level (74, 75). These works challenged the historical taxonomic circumscription of the coffee genus (Coffea) and the divisions within it (6, 25), replacing them with a stable and workable system for dividing the genus into groups of related species (clades), which can be used to assist the construction of CWR priority groups. There are also several studies reporting the results of crossing experiments between coffee species and taxa (27, 76–78). Crossing refers to conventional breeding methods, although we recognize that more sophisticated methods are available (e.g., embryo rescue, somatic fusion, or genetic engineering) and that this is a developing area of research. In general, coffee suffers from low postcrossing fertility (flowers, pollen, and seeds), although pre- and postcrossing chromosome duplication and restoration of fertility via backcrossing can increase and restore fertility, respectively (29, 77).
Using the available data, we constructed a CWR priority group classification system for coffee, based on a combination of the gene pool approach (72), which includes crossing data, and the taxon group method (71), but using molecular systematic data (clades) rather than a formal taxonomy. Our proposed criteria for coffee CWR priority groups are as follows: CWR priority group I (to include the cultivated and wild variants of the main coffee crop species, and hybrid progenitor species), CWR priority group II (to include species closely related to the crop species, from which gene transfer to the crop is proven or assumed, with low to high postcrossing fertility rates), and CWR priority group III [to include species more distantly related to the crop species (within the genus Coffea), including species from which gene transfer to the crop is demonstrated or assumed to be difficult or impossible without laboratory procedures, with low (or unknown) postcrossing fertility rates]. Our combined classification system for CWR priority ranking is similar to that proposed by Wiersema et al. (79), both being based on breeding information and relatedness; their terms primary, secondary, and tertiary overlap with our CWR priority groups I, II, and III, respectively. Our overall approach differs because we use phylogenetic data (clades) rather than taxonomic information (a classification system), and our CWR priority group I diverges from the primary status category (79) by including only the main crop species and their progenitors.
Gap analysis for germplasm collections (ex situ) and protected areas (in situ)
To ascertain whether a coffee species is held within a coffee germplasm collection (ex situ), we surveyed literature sources (32, 42, 43, 73, 80–86) and herbarium collections [which often house herbarium vouchers for germplasm collections (for recording and verification purposes)]. Site visits were made to the living collections at the Kianjavato Coffee Research Station in Madagascar (in 2000, 2006, and 2011) and to the L’Institut de recherche pour le développement (IRD), Montpellier, France (in 2001). For our first-pass assessment of in situ occurrences, we used the coffee data collated within SIS (64). We then compared ground point data for all species, against the World Database on Protected Areas (WDPA), accessed via Protected Planet (87) and GeoCat (62).
Supplementary Material
Acknowledgments
We are grateful to in-country partners in Africa and Madagascar (for assistance with fieldwork), germplasm managers and field staff (for access to collections), and herbarium curators worldwide (for access to herbarium material). We are grateful to the RBG, Kew staff S. Bachman, J. Clarkson, and B. Walker for advice and assistance and intern students H. Batchelor and A. Duarte and volunteers M. Black and L. Murray for work on data gathering and error checking of ground point data. We would also like to thank the many specialists who undertook review of coffee species as part of the IUCN Red List evaluation process and M. Rivers for assistance with various IUCN Red List enquiries. Funding: This work was supported by the IUCN and Toyota Motor Corporation through the project “The IUCN Red List of Threatened Species and Toyota Motor Corporation” and is one of the products of the Global Coffee Assessment, undertaken by the Plant Assessment Unit (RBG, Kew). Fieldwork in Ethiopia was conducted under the project Building a Climate Resilient Coffee Economy for Ethiopia, within the Strategic Climate Institutions Programme (SCIP) Fund, financed by the governments of the United Kingdom (DFID), Denmark, and Norway. Fieldwork in Africa was supported by the Amar-Franses and Foster-Jenkins Trust and the Bentham-Moxon Trust. Author contributions: A.P.D., J.M., S.H., and E.N.L. conceived of the project and designed the research. A.P.D. undertook the field research with assistance from R.O. and J.M. A.P.D. identified all the specimens. H.C., A.P.D., R.O., and J.M. undertook the IUCN Red List assessments. S.H. coordinated species assessment, review, and submission. J.M., E.N.L., and A.P.D. undertook the various analyses. Competing interests: The authors declare that they have no competing interests. Data and materials availability: Extinction risk assessments and supporting data are available via the IUCN Red List of Threatened Species (34). All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/1/eaav3473/DC1
Supplementary Text
Fig. S1. Total number of coffee species by area.
Fig. S2. Total number of DD coffee species by area.
Fig. S3. Summary of threats for coffee species.
Table S1. List of coffee species with main distribution area, IUCN extinction risk category, CWR priority group, occurrence in germplasm collections (ex situ), and occurrence in protected areas (in situ).
Table S2. Main distribution area and number and percentage of species against IUCN extinction risk category.
Table S3. Number and percentage of threatened and DD coffee species arranged by TDWG level 3.
Table S4. Number and percentage of coffee species held in germplasm collections (ex situ) and occurring in protected areas (in situ).
Table S5. Size distribution of species numbers according to EOO in km2.
REFERENCES AND NOTES
- 1.International Coffee Organization (ICO), Trade Statistics (2018); www.ico.org/trade_statistics.asp.
- 2.Vega F. E., Rosenquist E., Collins W., Global project needed to tackle coffee crisis. Nature 425, 343 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Lashermes P., Combes M.-C., Robert J., Trouslot P., D’Hont A., Anthony F., Charrier A., Molecular characterisation and origin of the Coffea arabica L. genome. Mol. Gen. Genet. 261, 259–266 (1999). [DOI] [PubMed] [Google Scholar]
- 4.Maurin O., Davis A. P., Chester M., Mvungi E. F., Jaufeerally-Fakim Y., Fay M. F., Towards a phylogeny for Coffea (Rubiaceae): Identifying well-supported lineages based on nuclear and plastid DNA sequences. Ann. Bot. 100, 1565–1583 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis A. P., Gole T. W., Baena S., Moat J., The impact of climate change on natural populations of Arabica coffee (Coffea arabica): Predicting future trends and identifying priorities. PLOS ONE 7, e47981 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis A. P., Govaerts R., Bridson D. M., Stoffelen P., An annotated taxonomic conspectus of the genus Coffea (Rubiaceae). Bot. J. Linn. Soc. 152, 465–512 (2006). [Google Scholar]
- 7.A. P. Davis, Z. K. Challa, J. Williams, S. Baena, T. W. Gole, J. Moat, Coffee Atlas of Ethiopia (Royal Botanic Gardens, Kew, 2018). [Google Scholar]
- 8.G. Wrigley, Coffee – Tropical Agriculture Series (Longman Scientific & Technical, 1988). [Google Scholar]
- 9.G. A. Cruwell, Liberian Coffee In Ceylon: The History of the Introduction and Progress of the Cultivation up to April 1878 (A.M. & J. Ferguson, 1878). [Google Scholar]
- 10.Froehner A., IV, Übersicht über die Arten der Gattung Coffea. Notizbl. Bot. Gart. Berlin Dahlem 1, 230–238 (1897). [Google Scholar]
- 11.S. McCrook, Coffee: A comprehensive guide to the bean, the beverage, and the industry, in The Ecology of Taste: Robusta Coffee and the Limits of the Specialty Revolution, R. W. Thurston, J. Morris, S. Steiman, Eds. (Rowman & Littlefield, 2013), pp. 248–261. [Google Scholar]
- 12.F. L. Wellman, Coffee: Botany, Cultivation and Utilization (Leonard Hill [Books] Limited/Interscience Publishers Inc, 1961). [Google Scholar]
- 13.Gunning Y., Defernez M., Watson A. D., Beadman N., Colquhoun I. J., le Gall G., Philo M., Garwood H., Williamson D., Davis A. P., Kemsley E. K., 16-O-methylcafestol is present in ground roast Arabica coffees: Implications for authenticity testing. Food Chem. 248, 52–60 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avelino J., Cristancho M., Georgiou S., Imbach P., Aguilar L., Bornemann G., Läderach P., Anzueto F., Hruska A. J., Morales C., The coffee rust crises in Colombia and Central America (2008–2013): Impacts, plausible causes and proposed solutions. Food Secur. 7, 303–321 (2015). [Google Scholar]
- 15.Clarindo W. R., Carvalho C. R., Caixeta E. T., Koehler A. D., Following the track of “Híbrido de Timor” origin by cytogenetic and flow cytometry approaches. Genet. Resour. Crop. Evol. 60, 2253–2259 (2013). [Google Scholar]
- 16.Moat J., Williams J., Baena S., Wilkinson T., Gole T. W., Challa Z. K., Demissew S., Davis A. P., Resilience potential of the Ethiopian coffee sector under climate change. Nat. Plants 3, 17081 (2017). [DOI] [PubMed] [Google Scholar]
- 17.A. L. Gaitán, M. A. Cristancho, B. L. Castro Caicedo, C. A. Rivillas, G. Cadena Gómez, Compendium of Coffee Diseases and Pests (APS Press, 2015). [Google Scholar]
- 18.Jaramillo J., Muchugu E., Vega F. E., Davis A., Borgemeister C., Chabi-Olaye A., Some like it hot: The influence and implications of climate change on coffee berry borer (Hypothenemus hampei) and coffee production in East Africa. PLOS ONE 6, e24528 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brozynska M., Furtado A., Henry R. J., Genomics of crop wild relatives: Expanding the gene pool for crop improvement. Plant Biotechnol. J. 14, 1070–1085 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breitler J.-C., Dechamp E., Campa C., Zebral Rodrigues L. A., Guyot R., Marraccini P., Etienne H., CRISPR/Cas9-mediated efficient targeted mutagenesis has the potential to accelerate the domestication of Coffea canephora. Plant Cell Tissue Organ Cult. 134, 383–394 (2018). [Google Scholar]
- 21.P. J. S. Cramer, A Review of Literature of Coffee Research in Indonesia (Turrialba: SIC Editorial, Inter-American Institute of Agricultural Sciences, 1957). [Google Scholar]
- 22.R. H. Cheney, A Monograph of the Economic Species of the Genus Coffea L (New York Univ. Press, 1925). [Google Scholar]
- 23.Davis A. P., Psilanthus mannii, the type species of Psilanthus, transferred to Coffea. Nord. J. Bot. 29, 471–472 (2011). [Google Scholar]
- 24.Davis A. P., Tosh J., Ruch N., Fay M. F., Growing coffee: Psilanthus (Rubiaceae) subsumed on the basis of plastid and nuclear DNA sequences; implications for the size, morphology, distribution and evolutionary history of Coffea. Bot. J. Linn. Soc. 167, 357–377 (2011). [Google Scholar]
- 25.A. P. Davis, D. M. Bridson, F. Rakotonasolo, A reexamination of Coffea subgenus Baracoffea and comments on the morphology and classification of Coffea and Psilanthus (Rubiaceae-Coffeeae), in Festschrift for William G. DArcy: The legacy of a taxonomist, R. C. Keating, V. C. Hollowell, T. Croat, Eds. (Monograph in Systematic Botany 104, MBG Press, 2005), pp. 398–420. [Google Scholar]
- 26.H. M. Burkill, Useful Plants of West Tropical Africa Volume 4, families M–R (Royal Botanic Gardens, Kew, 1997). [Google Scholar]
- 27.A. Charrier, La structure génétique des caféiers spontanés de la région Malgache (Mascarocoffea), Memoires ORSTOM No. 87 (ORSTOM, 1978).
- 28.Lashermes P., Combes M.-C., Ribas A., Cenci A., Mahé L., Etienne H., Genetic and physical mapping of the SH3 region that confers resistance to leaf rust in coffee tree (Coffea arabica L.). Tree Genet. Genomes 6, 973–980 (2010). [Google Scholar]
- 29.Medina Filho H. P., Carvalho A., Medina D. M., Germoplasma de Coffea racemosa e seu potencial de melhoramento do cafeeiro. Bragantia 36, 43–46 (1977). [Google Scholar]
- 30.Silva M. d. C., Várzea V., Guerra-Guimarães L., Azinheira H. G., Fernandez D., Petitot A.-S., Bertrand B., Lashermes P., Nicole M., Coffee resistance to the main diseases: Leaf rust and coffee berry disease. Braz. J. Plant Physiol. 18, 119–147 (2006). [Google Scholar]
- 31.D. Ganesh, A. S. Ram, N. S. Prakash, D. Padmajyothi, M. K. Mishra, J. Ahmed, M. Jagadeesan, A. G. S. Reddy, C. S. Srinivasan, Central Coffee Research Institute, Evaluation of Coffea liberica × Coffea eugenioides and its progenies for yield, leaf rust tolerance and quality, in Proceedings of the 15th Plantation Crops Symposium Placrosym XV, Mysore, India, 10 to 13 December 2002, pp. 72–77. [Google Scholar]
- 32.Hamon P., Grover C. E., Davis A. P., Rakotomalala J.-J., Raharimalala N. E., Albert V. A., Sreenath H. L., Stoffelen P., Mitchell S. E., Couturon E., Hamon S., de Kochko A., Crouzillat D., Rigoreau M., Sumirat U., Akaffou S., Guyot R., Genotyping-by-sequencing provides the first well-resolved phylogeny for coffee (Coffea) and insights into the evolution of caffeine content in its species: GBS coffee phylogeny and the evolution of caffeine content. Mol. Phylogenet. Evol. 109, 351–361 (2017). [DOI] [PubMed] [Google Scholar]
- 33.IUCN Standards and Petitions Subcommittee, Guidelines for using the IUCN Red List Categories and Criteria. Version 13. Prepared by the Standards and Petitions Subcommittee (2017); www.iucnredlist.org/documents/RedListGuidelines.pdf.
- 34.International Union for Conservation of Nature (IUCN), The IUCN Red List of Threatened Species (2018); www.iucnredlist.org.
- 35.International Union for Conservation of Nature (IUCN), IUCN Red List Categories and Criteria. Version 3.1. Second edition (2012); https://portals.iucn.org/library/sites/library/files/documents/RL-2001-001-2nd.pdf.
- 36.Bland L. M., Bielby J., Kearney S., Orme C. D. L., Watson J. E. M., Collen B., Toward reassessing data-deficient species. Conserv. Biol. 31, 531–539 (2017). [DOI] [PubMed] [Google Scholar]
- 37.J. Moat, T. W. Gole, A. P. Davis, Least Concern to Endangered: Applying climate change projections profoundly influences the extinction risk assessment for wild Arabica coffee, Glob. Chang. Biol. 10.1111/gcb.14341 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brummitt N. A., Bachman S. P., Griffiths-Lee J., Lutz M., Moat J. F., Farjon A., Donaldson J. S., Hilton-Taylor C., Meagher T. R., Albuquerque S., Aletrari E., Andrews A. K., Atchison G., Baloch E., Barlozzini B., Brunazzi A., Carretero J., Celesti M., Chadburn H., Cianfoni E., Cockel C., Coldwell V., Concetti B., Contu S., Crook V., Dyson P., Gardiner L., Ghanim N., Greene H., Groom A., Harker R., Hopkins D., Khela S., Lakeman-Fraser P., Lindon H., Lockwood H., Loftus C., Lombrici D., Lopez-Poveda L., Lyon J., Malcolm-Tompkins P., McGregor K., Moreno L., Murray L., Nazar K., Power E., Quiton Tuijtelaars M., Salter R., Segrott R., Thacker H., Thomas L. J., Tingvoll S., Watkinson G., Wojtaszekova K., Nic Lughadha E. M., Green plants in the red: A baseline global assessment for the IUCN Sampled Red List Index for Plants. PLOS ONE 10, e0135152 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rakotoarinivo M., Dransfield J., Bachman S. P., Moat J., Baker W. J., Comprehensive red list assessment reveals exceptionally high extinction risk to Madagascar palms. PLOS ONE 9, e103684 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.E. Beech, M. Barstow, M. T. Rivers, The Red List of Theaceae [Botanic Gardens Conservation International (BGCI), 2017]; http://globaltrees.org/wp-content/uploads/2018/01/The-Red-List-of-Theaceae.pdf.
- 41.Castañeda-Álvarez N. P., Khoury C. K., Achicanoy H. A., Bernau V., Dempewolf H., Eastwood R. J., Guarino L., Harker R. H., Jarvis A., Maxted N., Müller J. V., Ramirez-Villegas J., Sosa C. C., Struik P. C., Vincent H., Toll J., Global conservation priorities for crop wild relatives. Nat. Plants 2, 16022 (2016). [DOI] [PubMed] [Google Scholar]
- 42.P Brame, S Krishna, D Horna, B Lainoff, C Montagnon, Global Conservation Strategy For Coffee Genetic Resources (Crop Trust & World Coffee Research, 2017); https://worldcoffeeresearch.org/media/documents/Coffee_Strategy_Low_Res.pdf.
- 43.F. Engelmann, M. E. Dulloo, C. Astorga, S. Dussert, F. Anthony, Conserving Coffee Genetic Resources: Complementary Strategies for Ex Situ Conservation of Coffee (Coffea Arabica L.) Genetic Resources. A Case Study in CATIE, Costa Rica (Bioversity International, 2007). [Google Scholar]
- 44.S. Dussert, N. Vasquez, K. Salazar, F. Anthony, F. Engelmann, VI. Cryopreservation of coffee genetic resources, in Conserving Coffee Genetic Resources: Complementary Strategies for Ex Situ Conservation of Coffee (Coffea arabica L.) Genetic Resources. A Case Study in CATIE, Costa Rica, F. Engelmann, E. Dulloo, C. Astorga, S. Dussert, F. Anthony, Eds. (Bioversity International, 2007), pp. 49–58. [Google Scholar]
- 45.Krishnan S., Ranker T. A., Davis A. P., Rakotomalala J. J., An assessment of the genetic integrity of ex situ germplasm collections of three endangered species of Coffea from Madagascar: Implications for the management of field germplasm collections. Genet. Resour. Crop. Evol. 60, 1021–1036 (2013). [Google Scholar]
- 46.Thomas A. S., The wild Arabica coffee on the Boma Plateau. Anglo-Egyptian Sudan. Empire J. Exp. Agric. 10, 207–212 (1942). [Google Scholar]
- 47.Aerts R., Berecha G., Gijbels P., Hundera K., Glabeke S., Vandepitte K., Muys B., Roldán-Ruiz I., Honnay O., Genetic variation and risks of introgression in the wild Coffea arabica gene pool in south-western Ethiopian montane rainforests. Evol. Appl. 6, 243–252 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.J. Moat, J. Williams, S. Baena, T. Wilkinson, S. Demissew, Z. K. Challa, T. W. Gole, A.P. Davis, Coffee Farming and Climate Change in Ethiopia: Impacts, Forecasts, Resilience and Opportunities — Summary Report 2017 (The Strategic Institutions Programme (SCIP). Royal Botanic Gardens, Kew, 2017).
- 49.Hylander K., Nemomissa S., Delrue J., Enkosa W., Effects of coffee management on deforestation rates and forest integrity. Conserv. Biol. 27, 1031–1040 (2013). [DOI] [PubMed] [Google Scholar]
- 50.Jones K. R., Venter O., Fuller R. A., Allan J. R., Maxwell S. L., Negret P. J., Watson J. E. M., One-third of global protected land is under intense human pressure. Science 360, 788–791 (2018). [DOI] [PubMed] [Google Scholar]
- 51.Stork N. E., Re-assessing current extinction rates. Biodivers. Conserv. 19, 357–371 (2010). [Google Scholar]
- 52.Laurance W. F., Carolina Useche D., Rendeiro J., Kalka M., Bradshaw C. J. A., Sloan S. P., Laurance S. G., Campbell M., Abernethy K., Alvarez P., Arroyo-Rodriguez V., Ashton P., Benítez-Malvido J., Blom A., Bobo K. S., Cannon C. H., Cao M., Carroll R., Chapman C., Coates R., Cords M., Danielsen F., de Dijn B., Dinerstein E., Donnelly M. A., Edwards D., Edwards F., Farwig N., Fashing P., Forget P. M., Foster M., Gale G., Harris D., Harrison R., Hart J., Karpanty S., John Kress W., Krishnaswamy J., Logsdon W., Lovett J., Magnusson W., Maisels F., Marshall A. R., McClearn D., Mudappa D., Nielsen M. R., Pearson R., Pitman N., van der Ploeg J., Plumptre A., Poulsen J., Quesada M., Rainey H., Robinson D., Roetgers C., Rovero F., Scatena F., Schulze C., Sheil D., Struhsaker T., Terborgh J., Thomas D., Timm R., Nicolas Urbina-Cardona J., Vasudevan K., Joseph Wright S., Carlos Arias-G. J., Arroyo L., Ashton M., Auzel P., Babaasa D., Babweteera F., Baker P., Banki O., Bass M., Bila-Isia I., Blake S., Brockelman W., Brokaw N., Brühl C. A., Bunyavejchewin S., Chao J. T., Chave J., Chellam R., Clark C. J., Clavijo J., Congdon R., Corlett R., Dattaraja H. S., Dave C., Davies G., de Mello Beisiegel B., de Nazaré Paes da Silva R., di Fiore A., Diesmos A., Dirzo R., Doran-Sheehy D., Eaton M., Emmons L., Estrada A., Ewango C., Fedigan L., Feer F., Fruth B., Giacalone Willis J., Goodale U., Goodman S., Guix J. C., Guthiga P., Haber W., Hamer K., Herbinger I., Hill J., Huang Z., Fang Sun I., Ickes K., Itoh A., Ivanauskas N., Jackes B., Janovec J., Janzen D., Jiangming M., Jin C., Jones T., Justiniano H., Kalko E., Kasangaki A., Killeen T., King H. B., Klop E., Knott C., Koné I., Kudavidanage E., Lahoz da Silva Ribeiro J., Lattke J., Laval R., Lawton R., Leal M., Leighton M., Lentino M., Leonel C., Lindsell J., Ling-Ling L., Eduard Linsenmair K., Losos E., Lugo A., Lwanga J., Mack A. L., Martins M., Scott McGraw W., McNab R., Montag L., Myers Thompson J., Nabe-Nielsen J., Nakagawa M., Nepal S., Norconk M., Novotny V., O'Donnell S., Opiang M., Ouboter P., Parker K., Parthasarathy N., Pisciotta K., Prawiradilaga D., Pringle C., Rajathurai S., Reichard U., Reinartz G., Renton K., Reynolds G., Reynolds V., Riley E., Rödel M. O., Rothman J., Round P., Sakai S., Sanaiotti T., Savini T., Schaab G., Seidensticker J., Siaka A., Silman M. R., Smith T. B., de Almeida S. S., Sodhi N., Stanford C., Stewart K., Stokes E., Stoner K. E., Sukumar R., Surbeck M., Tobler M., Tscharntke T., Turkalo A., Umapathy G., van Weerd M., Vega Rivera J., Venkataraman M., Venn L., Verea C., Volkmer de Castilho C., Waltert M., Wang B., Watts D., Weber W., West P., Whitacre D., Whitney K., Wilkie D., Williams S., Wright D. D., Wright P., Xiankai L., Yonzon P., Zamzani F., Averting biodiversity collapse in tropical forest protected areas. Nature 489, 290–294 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Davis A. P., Govaerts R., Bridson D. M., Ruhsam M., Moat J., Brummitt N. A., A global assessment of distribution, diversity, endemism, and taxonomic effort in the Rubiaceae. Ann. Mo. Bot. Gard. 96, 68–78 (2009). [Google Scholar]
- 54.Nowak M. D., Davis A. P., Anthony F., Yoder A. D., Expression and trans-specific polymorphism of self-incompatibility RNases in Coffea (Rubiaceae). PLOS ONE 6, e21019 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.M. Lightbourne, Food Security, Biological Diversity and Intellectual Property Rights (Rutledge, 2016). [Google Scholar]
- 56.P. K. Holmgren, N. H. Holmgren, L. C. Barnett, Index Herbariorum. Part 1: The Herbaria of the World, 8th edn. Regnum Vegetabile (New York Botanical Garden, 1990). [Google Scholar]
- 57.B. Thiers, Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden's Virtual Herbarium (2018); http://sweetgum.nybg.org/science/ih/.
- 58.Missouri Botanical Garden, Tropicos.org Missouri Botanical Garden (2018); http://www.tropicos.org.
- 59.Global Biodiversity Information Facility (GBIF) (2018); https://www.gbif.org/.
- 60.Natural History Museum Paris (2018); https://science.mnhn.fr/institution/mnhn/search.
- 61.iNaturalist.org (2018); http://www.inaturalist.org.
- 62.Bachman S., Moat J., Hill A., Torre J., Scott B., Supporting Red List threat assessments with GeoCAT: Geospatial conservation assessment tool. ZooKeys 126, 117–126 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.J. Moat, rCAT: conservation assessment tools and functions in R (2017); https://cran.r-project.org/web/packages/rCAT/index.html; https://cran.r-project.org/web/packages/rCAT/rCAT.pdf.
- 64.International Union for Conservation of Nature (IUCN), Species Information Service (SIS) Toolkit, rev. 2.0 (2017); https://sis.iucnsis.org/apps/org.iucn.sis.server/SIS/index.html.
- 65.International Union for Conservation of Nature (IUCN), Documentation standards and consistency checks for IUCN Red List assessments and species accounts. Version 2. Adopted by the IUCN Red List Committee and IUCN SSC Steering Committee (2013); http://cmsdocs.s3.amazonaws.com/keydocuments/RL_Standards_Consistency.pdf.
- 66.Google Earth, Google Earth Pro, Version 7.3.1 (2018); www.google.com/intl/en_uk/earth/desktop.
- 67.WCSPF, World Checklist of Selected Plant Families. Royal Botanic Gardens, Kew (2018); http://apps.kew.org/wcsp/.
- 68.R. K. Brummitt, S. Pando Hollis, N. A. Brummitt, Plant Taxonomic Database Standards No. 2, ed. 2. World Geographical Scheme for Recording Plant Distributions (TDWG/Hunt Institute for Botanical Documentation, 2001). [Google Scholar]
- 69.Environmental Systems Research Institute (ESRI), ArcGIS (R) Desktop Release 10.5. Redlands, CA. (2016).
- 70.C. A. Brewer, ColorBrewer Ver. 2. Geography, Pennsylvania State University (2018); http://www.ColorBrewer.org.
- 71.Maxted N., Ford-Lloyd B. V., Jury S., Kell S., Schoten M., Towards a definition of a crop wild relative. Biodivers. Conserv. 15, 2673–2685 (2006). [Google Scholar]
- 72.Harlan J. R., Wet J. M. J., Towards a rational classification of cultivated plants. Taxon 20, 509–517 (1971). [Google Scholar]
- 73.Razafinarivo N. J., Guyot R., Davis A. P., Couturon E., Hamon S., Crouzillat D., Rigoreau M., Dubreuil-Tranchant C., Poncet V., de Kochko A., Rakotomalala J.-J., Hamon P., Genetic structure and diversity of coffee (Coffea) across Africa and the Indian Ocean islands revealed using microsatellites. Ann. Bot. 111, 229–248 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Davis A. P., Chester M., Maurin O., Fay M. F., Searching for the relatives of Coffea (Rubiaceae, Ixoroideae): The circumscription and phylogeny of Coffeeae based on plastid sequence data and morphology. Am. J. Bot. 94, 313–329 (2007). [DOI] [PubMed] [Google Scholar]
- 75.Tosh J., Davis A. P., Dessein S., de Block P., Huysmans S., Fay M. F., Smets E., Robbrecht E., Phylogeny of Tricalysia (Rubiaceae) and its relationships with allied genera based on plastid DNA data: Resurrection of the genus Empogona. Ann. Mo. Bot. Gard. 96, 194–213 (2009). [Google Scholar]
- 76.Couturon E., Lashermes P., Charrier A., First intergeneric hybrids (Psilanthus ebracteolatus Hiern x Coffea arabica L.) in coffee trees. Can. J. Bot. 76, 542–546 (1998). [Google Scholar]
- 77.Carvalho A., Monaco L. C., Relaciones geneticas de especies seleccionadas de Coffea. Cafe 9, 1–19 (1968). [Google Scholar]
- 78.Medina Filho H. P., Carvalho A., Mônaco L. C., Melhoramento do cafeeiro. XXXVII – Observações sobre a resistência do cafeeiro ao bicho mineiro. Bragantia 36, 131–137 (1977). [Google Scholar]
- 79.Wiersema J. H., León B., Garvey E. J., Identifying wild relatives of subtropical and temperate fruit and nut crops. Acta Hortic. 948, 285–288 (2012). [Google Scholar]
- 80.E. Couturon, N. E. Raharimalala, J.-J. Rakotomalala, S. Hamon, A. de Kochko, R. Guyot, P. Hamon, Wild Coffee-Trees: A Threatened Treasure in the Heart of Tropical Forests! Caféiers Sauvages: Un Trésor en Péril au Cœur des Forêts Tropicales (Private publication, 2016). [Google Scholar]
- 81.A. C. Andrade, in Achieving Sustainable Cultivation of Coffee: Breeding and Quality Traits, P. Lashermes, Ed. (Burleigh Dodds Science Publishing Limited, 2018), pp. 1–12. [Google Scholar]
- 82.Cubry P., De Bellis F., Pot D., Musoli P., Leroy T., Global analysis of Coffea canephora Pierre ex A.Froehner (Rubiaceae) from the Guineo-Congolese region reveals impacts from climatic refuges and migration effects. Genet. Resour. Crop. Evol. 60, 483–501 (2013). [Google Scholar]
- 83.Garavito A., Montagnon C., Guyot R., Bertrand B., Identification by the DArTseq method of the genetic origin of the Coffea canephora cultivated in Vietnam and Mexico. BMC Plant Biol. 16, 242 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gomez C., Dussert S., Hamon P., Hamon S., de Kochko A., Poncet V., Current genetic differentiation of Coffea canephora Pierre ex A.Froehn in the Guineo-Congolian African zone: Cumulative impact of ancient climatic changes and recent human activities. BMC Evol. Biol. 9, 167 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.N’Diaye A., Poncet V., Louran J., Hamon S., Noirot M., Genetic differentiation between Coffea liberica var. liberica and C. liberica var. dewevrei and comparison with C. canephora. Plant Syst. Evol. 253, 95–104 (2005). [Google Scholar]
- 86.F. Anthony, S. Dussert, M. E. Dulloo, II. Coffee genetic resources, in Conserving Coffee Genetic Resources: Complementary Strategies for Ex Situ Conservation Of Coffee (Coffea Arabica L.) GENETIC Resources. A Case Study in CATIE, Costa Rica, F. Engelmann, M. E. Dulloo, C. Astorga, S. Dussert, F. Anthony, Eds. (Bioversity International, 2007), pp. 12–22. [Google Scholar]
- 87.UNEP-WCMC and IUCN, Protected Planet: The World Database on Protected Areas (WDPA)/The Global Database on Protected Areas Management Effectiveness (GD-PAME) Cambridge, UK (2018); https://www.protectedplanet.net. [accessed 30 April 2018].
- 88.Andrianasolo D. N., Davis A. P., Razafinarivo N. J., Hamon S., Rakotomalala J.-J., Sabatier S.-A., Hamon P., High genetic diversity of in situ and ex situ populations of Madagascan coffee species: Further implications for the management of coffee genetic resources. Tree Genet. Genomes 9, 1295–1312 (2013). [Google Scholar]
- 89.Nagai C., Rakotomalala J.-J., Katahira R., Li Y., Yamagata K., Ashihara H., Production of a new low-caffeine hybrid coffee and the biochemical mechanism of low caffeine accumulation. Euphytica 164, 133–142 (2008). [Google Scholar]
- 90.Rakotomalala J.-J. R., Cros E., Clifford M. N., Charrier A., Caffeine and theobromine in green beans from Mascarocoffea. Phytochemistry 31, 1271–1272 (1992). [Google Scholar]
- 91.F. G. Meyer, L. M. Fernie, R. L. Narasimhaswamy, L. C. Monaco, D. J. Greathead, FAO coffee mission to Ethiopia, 1964–1965 (Food and Agriculture Organization of the United Nations, Rome, 1968). [Google Scholar]
- 92.Guillaumet J.-L., Hallé F., Echantillonnage du matérial récolté en Ethiopie. Bull. IFCC 14, 13–18 (1978). [Google Scholar]
- 93.F. Anthony, B. Bertrand, C. Astorga, P. Lashermes, Characterization and assessment of Coffea arabica L. genetic respources conserved in the CATIE field genebank, in Conserving Coffee Genetic Resources: Complementary Strategies for Ex Situ Conservation Of Coffee (Coffea Arabica L.) GENETIC Resources. A Case Study in CATIE, COSTA RICA, F. Engelmann, M. E. Dulloo, C. Astorga, S. Dussert, F. Anthony, Eds. (Bioversity International, 2007), pp. 35–44. [Google Scholar]
- 94.Davis A. P., Six species of Psilanthus transferred to Coffea (Coffeeae, Rubiaceae). Phytotaxa 10, 41–45 (2010). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/1/eaav3473/DC1
Supplementary Text
Fig. S1. Total number of coffee species by area.
Fig. S2. Total number of DD coffee species by area.
Fig. S3. Summary of threats for coffee species.
Table S1. List of coffee species with main distribution area, IUCN extinction risk category, CWR priority group, occurrence in germplasm collections (ex situ), and occurrence in protected areas (in situ).
Table S2. Main distribution area and number and percentage of species against IUCN extinction risk category.
Table S3. Number and percentage of threatened and DD coffee species arranged by TDWG level 3.
Table S4. Number and percentage of coffee species held in germplasm collections (ex situ) and occurring in protected areas (in situ).
Table S5. Size distribution of species numbers according to EOO in km2.