Abstract
Several immune suppressive mechanisms that evade the host immune response have been described in patients with hepatocellular carcinoma (HCC); one of these mechanisms is expansion of myeloid-derived suppressor cells (MDSCs). MDSCs have been shown to inhibit T cell responses in tumor-bearing mice, but little is known about these cells in humans. Here, we have analyzed and characterized the effect of MDSCs on the innate immune system, in particular, their interaction with natural killer (NK) cells in patients with HCC. MDSCs from patients with HCC inhibited autologous NK cell cytotoxicity and cytokine secretion when cultured together in vitro. This suppression was dependent on cell contact, but did not rely on the arginase activity of MDSCs, which is a hallmark function of these cells. However, MDSC-mediated inhibition of NK cell function was dependent mainly on the NKp30 on NK cells. Conclusion: Our study suggests a new role for MDSCs in patients with HCC in disarming the innate immune system and further contributing to the immune suppressor network in these patients. These findings have important implications when designing immunotherapy protocols.
Tumors have evolved different mechanisms to generate a suppressive network and evade the host’s immune response. One such mechanism is an increase in myeloid-derived suppressor cells (MDSCs). MDSCs are a heterogeneous population of myeloid cells including macrophages, granulocytes, and other cells that express both Gr-1 and CD11b in mice and suppress immune responses in vivo and in vitro.1 In humans, MDSCs have not been well characterized owing to the lack of specific markers. Only limited data are available on different myeloid cell populations with suppressor function in patients with head and neck cancer, squamous cell carcinoma, non–small cell lung cancer, and colon and breast cancers. The phenotype of these cells has been shown to be mainly CD34, CD33, CD15, and CD13, CD14−/lin−.2–5
Our earlier studies have demonstrated that hepatocellular carcinoma (HCC), similar to other types of cancer, induces a suppressive network to evade the host immune response. We have shown an increase in regulatory T cells6 as well as defective antigen-presenting cells7 as some of the suppressive mechanisms in patients with HCC. Recently, we have characterized a new population of MDSCs that are CD14+ human leukocyte antigen (HLA)-DR−/low, significantly increased in peripheral blood and tumors of patients with HCC, have arginase activity, and suppress autologous T cell proliferation.8 Interestingly, our study showed that MDSCs in patients with HCC induce CD4+CD25+FoxP3 regulatory T cells, which also suppress antitumor immune responses.8
Natural killer (NK) cells are effector cells of the innate immune system, where they have the ability to kill tumors or virus-infected cells, secrete cytokines, and regulate both innate and adaptive immune responses.9 It has previously been shown that NK cells in patients with cancer are reduced in their cytotoxic function.10,11 In particular, several studies in patients with HCC have shown that NK cells from these patients are defective in their lytic function and cytokine secretion.12,13 However, the cause for the impaired function of NK cells is not known.
We hypothesized that MDSCs can also exert their inhibitory function on the innate arm of the immune system and its effector cells, the NK cells. In order to investigate if MDSCs can regulate NK cell function, we analyzed their interaction with NK cells when isolated directly from peripheral blood of patients with HCC and healthy donors. In this study, we show that NK cells in patients with HCC are defective in their lytic function and cytokine secretion as compared to healthy donors. We also provide evidence for the potential linkage between the suppression of NK cell function and increase in MDSCs seen in patients with HCC. We show that MDSCs inhibit autologous NK cell cytotoxicity and cytokine release in patients with HCC when cocultured in vitro. This inhibition is cell contact–dependent and is mediated through the NKp30. We suggest that MDSCs are able to inhibit and regulate NK cells as effectors of the innate immune system contributing further to immune suppressor mechanisms in patients with HCC. These finding have important implications when designing immunotherapy protocols in patients with HCC.
Patients and Methods
Patients and Healthy Donors.
Blood samples were collected from patients with HCC seen at the Department of Gastroenterology, Hepatology and Endocrinology, Hannover Medical School (Hannover, Germany). HCC was diagnosed according to the guidelines of the European Association for the Study of the Liver. Written consent was obtained from all patients before blood and tumor sampling, and the Ethics Committee of Hannover Medical School approved the study protocol. Table 1 shows the clinical characteristics of all patients with HCC in this study.
Table 1.
Patient Characteristics
| Characteristic | Value |
|---|---|
| Male/Female | 25/5 |
| Average age | 69 years |
| Cause of cirrhosis | |
| HBV | 7 |
| HCV | 6 |
| Ethanol | 9 |
| Other | 3 |
| Unknown | 5 |
| Liver cirrhosis | |
| No cirrhosis | 3 |
| Child-Pugh A | 17 |
| Child-Pugh B | 9 |
| Child-Pugh C | 1 |
| Tumor stage | |
| BCLC A | 6 |
| BCLC B | 7 |
| BCLC C | 16 |
| BCLC D | 1 |
HBV, hepatitis B virus; HCV, hepatitis C virus; BCLC, Barcelona Clinic Liver Cancer.
Cell Isolation and Sorting.
Peripheral blood mononucleocytes (PBMCs) were isolated from freshly obtained blood by Ficoll density gradient centrifugation (Biochrom, Berlin, Germany) as described.8 CD14+ cells were purified using CD14 Microbeads and AutoMACS (magnetic cell sorting) separation unit (Miltenyi Biotech, Bergisch Gladbach, Germany). CD14+HLA-DR−/low and CD14+HLA-DR+ cells were isolated from CD14+ cells using BD FACS (fluorescence-activated cell sorting) Aria cell sorting system (Becton Dickinson, Heidelberg, Germany) as described.8 CD56+CD3− NK cells were purified using the CD14-depleted fraction or PBMCs. The purity of the cells after sorting was >98%.
Isolation of Tumor-Infiltrating Lymphocytes.
Tumor specimens were collected at the time of surgery and processed by cutting into small pieces and digested with 3000 U/mL collagenase (Sigma-Aldrich, St. Louis, MO) and 130 U/mL dispase I (Roche, Mannheim, Germany) for 30 minutes. Resulting cells were washed with phosphate-buffered saline, and lymphocytes were isolated by Ficoll density gradient as described.8
Cell-Mediated Cytotoxicity Assay.
Lytic function of NK cells was determined by a standard 4-hour chromium-release assay. Briefly, FACS-sorted NK cells, NK cells cocultured with CD14+HLA-DR−/low cells, or PBMCs depleted of CD14+ cells were added to 51Cr-labeled K562 target cells in triplicate at different ratios of effector to target cell.
Flow Cytometry Analysis and Blocking Antibodies.
To determine the phenotype of MDSCs and NK cells ex vivo and upon coculturing, FACS analysis was done using the following antibodies: anti-CD16, anti–HLA-DR (ImmunoTools, Friesoythe, Germany); anti-CD14, anti-CD56, anti-69, anti-CD314 (anti-NKG2D), anti-CD336 (anti-NKp44) (Miltenyi Biotech, Bergisch Gladbach, Germany); anti-CD94, anti–HLA-ABC (Becton Dickinson, Heidelberg, Germany); anti-CD86 (Caltag, Hamburg, Germany); anti-CD337 (NKp30) (Beckman Coulter, Fullerton, CA); and anti-NKp80 (R&D Systems, Minneapolis, MN). For intracellular staining, cells were lysed using BD Cytofix/Cytoperm Fixation/Permeabilization Kit and stained with anti–interferon-γ (IFN-γ), (Becton Dickinson, Heidelberg, Germany). For blocking experiments, the following antibodies were used: anti-CD337 (2–20 μg/mL, Clone P3015), anti-CD336 (5 μg/mL, Clone P44–8) (Biolegend, San Diego, CA); anti-CD94 (20 μg/mL, Clone 131412), and anti-CD314 (1 μg/mL, Clone 149810) (R&D Systems, Minneapolis, MN). Flow cytometry was done using Becton Dickinson FACSCalibur. Analysis of FACS data was done with FlowJo software (TreeStar Inc., Ashland, OR). Isotype-matched antibodies were used as indicated.
Suppression Assay.
NK cells, CD14+HLA-DR−/low and CD14+HLA-DR+ cells were purified as described. NK cells were stimulated with 250 IU/mL interleukin-2 (IL-2; Chiron, Amsterdam, Netherlands) and CD14+ cells were added as indicated. IFN-γ secretion was measured in supernatants after 48 hours by enzyme-linked immunosorbent assay (ELISA; ImmunoTools, Friesoythe, Germany). Transwell inserts, blocking antibodies, or inhibitors were added as indicated.
Determination of STAT Expression by Quantitative Polymerase Chain Reaction.
NK cells and CD14+ cells were purified as described. Cells were cocultured for 12 hours, and RNA was isolated using RNeasy Micro Kit (Qiagen, Hilden, Germany). Complementary DNA synthesis was done with iScript Kit (Bio-Rad, München, Germany). Quantitative polymerase chain reaction was performed using the following primers (300 nmol each): signal transducer and activator of transcription 1 (STAT1) forward, 5′-tgc aaa acc ttg cag aac ag-3′; STAT1 reverse, 5′-ggg cat tct ggg taa gtt ca-3′; STAT3 forward, 5′-ctg gcc ttt ggt gtt gaa at-3′; STAT3 reverse, 5′-ctc tgc cca gcc tta ctc ac-3′; STAT4 forward, 5′-agc ctt gcg aag ttt caa ga-3′; STAT4 reverse, 5′-aca ccg cat aca cac ttg ga-3′; STAT5 forward, 5′-aca aag att gtt ggg gca ag-3′; STAT5 reverse, 5′-cat atg tgc aca ccc aga gg-3′; cyclophilin A forward, 5′-atg ctc aac ccc acc gtg t-3′; cyclophilin A reverse, 5′-tct gct gtc ttt ggg acc ttg tc-3′. Reactions were done in triplicate using Sybr Green (Bio-Rad, München, Germany) and normalized to endogenous cyclophilin A messenger RNA level.
Statistical Analysis.
Data are expressed as mean ± standard error of the mean (SEM) for percentages. Statistical analysis was done using Student t test to assess the differences between the study groups. P values <0.05 were considered statistically significant.
Results
NK Cells from Patients with HCC Are Reduced in their Cytotoxicity.
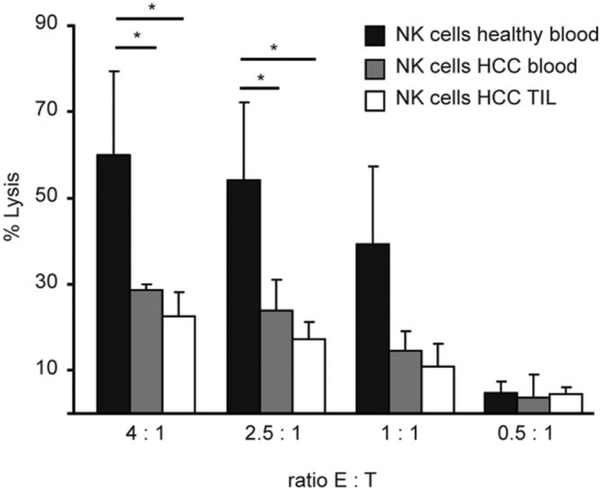
To characterize NK cells from patients with HCC, CD56+CD3− cells were sorted from PBMCs and tumor-infiltrating lymphocytes of patients with HCC. Lytic activity was compared to NK cells from healthy donors in a 51Cr-release assay against K562 target cells at different ratios. Cytotoxicity of NK cells from peripheral blood and tumor of patients with HCC was significantly reduced as compared to healthy donors at all ratios tested (Fig. 1). At the 4:1 ratio, there was more than 50% reduction in lysis by NK cells isolated from PBMCs and tumor of patients with HCC as compared to that from healthy donors.
Fig. 1.
NK cell cytotoxicity is reduced in patients with HCC. NK cells were FACS-sorted from PBMCs of either healthy donors (n = 10) or patients with HCC (n = 10), or from tumor-infiltrating lymphocytes (n = 5) by gating on CD56+CD3− population and used in a 51Cr-release assay against K562 target cells. Figure shown is average of four independent experiments (*P < 0.05).
MDSCs Inhibit NK Cell Cytotoxicity and IFN-γ Release.
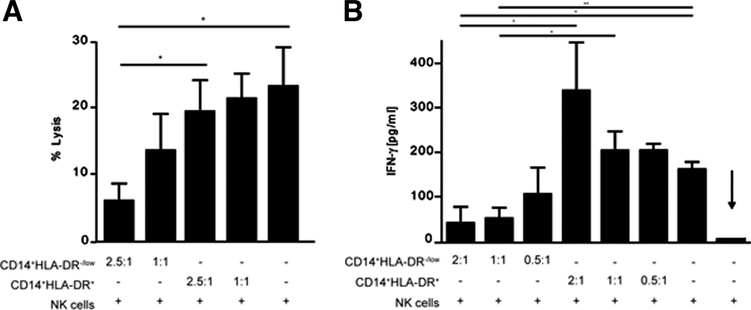
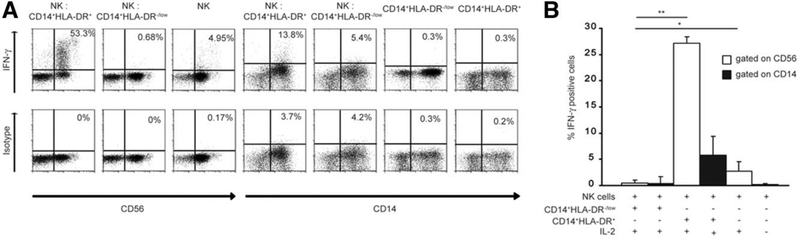
We have previously shown a significant increase in frequency of CD14+HLA-DR−/low MDSCs in peripheral blood and tumor of patients with HCC. In order to see whether MDSCs can impair NK cell cytotoxicity, MDSCs and NK cells were sorted by FACS from peripheral blood of patients with HCC and cultured together. After 12 hours, the NK cells were tested in a standard 51Cr-release assay. CD14+HLA-DR+ monocytes were used as a control. NK cell cytotoxicity was significantly impaired after coculture of NK cells with MDSCs, which was still observed at a 1:1 ratio (Fig. 2A). However, no suppression was observed when NK cells were cocultured with CD14+HLA-DR+ cells. In addition, MDSCs did not lyse K562 cells at any ratio tested, and there was no lysis of MDSCs by the NK cells detected (data not shown). Next, MDSCs or CD14+HLA-DR+ cells from peripheral blood of patients with HCC were cocultured with NK cells, and the supernatant was tested for IFN-γ secretion. As shown in Fig. 2B, IFN-γ release by NK cells was also significantly reduced (more than 80% at 2:1 and more than 60% at 0.5:1 ratio) when cocultured with MDSCs but not with CD14+HLA-DR+ cells. Intracellular FACS analysis was performed in order to investigate the source of IFN-γ. As shown in Fig. 3A, NK cells released IFN-γ (53.3% IFN-γ CD56+ cells) when cocultured with CD14+HLA-DR+ cells, but not with MDSCs (0.7% IFN-γ CD56+ cells). Analysis of MDSCs revealed that CD14+HLA-DR+ cells released more IFN-γ after coincubation with NK cells (13.8% IFN-γ CD14+ cells) than with MDSCs (5.4%) (Fig. 3A,B).
Fig. 2.
CD14+HLA-DR−/low cells suppress NK cell function. (A) Purified NK cells were cultured in the absence or presence of different ratios of CD14+HLA-DR+ or CD14+HLA-DR−/low cells as indicated. After 12 hours, K562 cells were added at a ratio of 2.5:1 (E:T) and lysis was determined by standard 51Cr-release assay (P < 0.05). Shown are cumulative results from four independent experiments. (B) NK cells were stimulated with IL-2 and cultured in the presence or absence of CD14+HLA-DR−/low or CD14+HLA-DR+ cells as indicated. IFN-γ release was determined after 48 hours by ELISA. Shown are cumulative results from five independent experiments (*P < 0.05, **P < 0.001). NK cells cultured without IL-2 were used as background (arrow).
Fig. 3.
Intracellular cytokine analysis of CD14+HLA-DR−/low in the presence or absence of NK cells. (A) NK cells were stimulated with IL-2 and cultured either alone, with CD14+HLA-DR−/low, or with CD14+HLA-DR+ cells. IFN-γ was analyzed after 48 hours by intracellular FACS as shown in representative dot plots. (B) Cumulative results of three independent experiments are shown (*P < 0.05; **P < 0.001). Cytokine secretion was analyzed gating on CD14+ cells (black bars) and CD56+ cells (white bars).
Phenotypic Characterization of MDSCs and NK Cells.
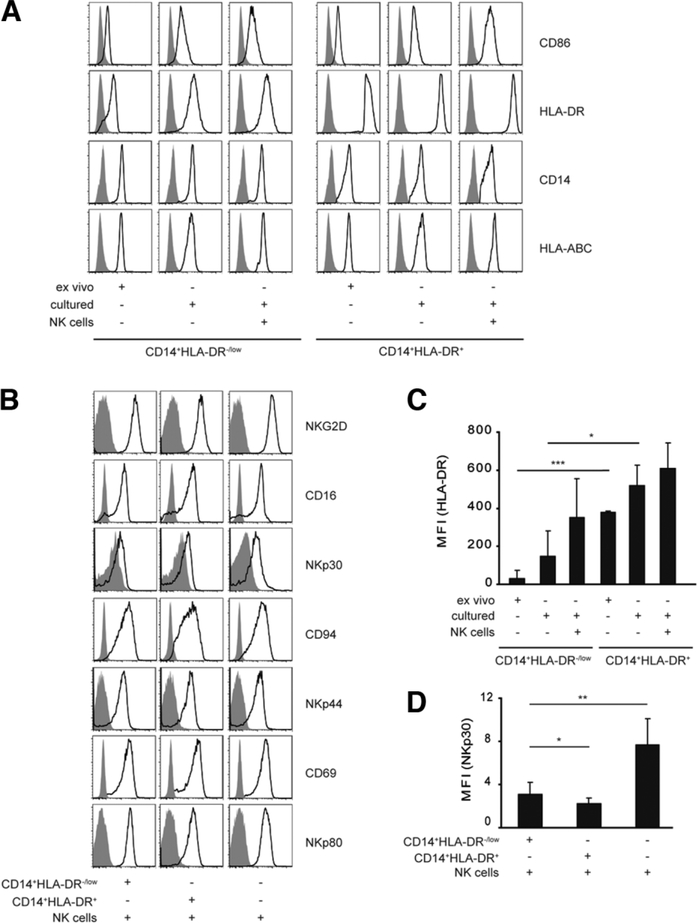
Here, the phenotype of NK cells and MDSCs was analyzed before and after coculture. FACS-sorted MDSC and NK cells were incubated together for 24 hours, and the number and phenotype of both NK cells and MDSCs were analyzed by FACS. The numbers of MDSCs or NK cells did not change significantly upon coculture (data not shown). MDSCs retained the expression of all their surface markers; these markers were similar when the MDSCs were cultured alone or with NK cells (Fig. 4A). There was an up-regulation of HLA-DR expression on MDSCs upon coculture with NK cells (Fig. 4A,C). Similarly, NKG2D, CD16, CD94, NKp44, CD69, and NKp80 expression did not change on NK cells upon coculture with MDSCs (Fig. 4B). In contrast, there was a significant reduction in expression of NKp30 on NK cells upon coculture with MDSCs (Fig. 4B,D). It should be noted that NK cells also had a reduction in expression of NKp30 when cocultured with monocytes.
Fig. 4.
Phenotypic analysis of NK cells and CD14+HLA-DR−/low cells upon coculture. Purified CD14+HLA-DR−/low or CD14+HLA-DR+ cells were cultured alone or with FACS-sorted autologous NK cells as indicated, and the expression of different surface markers (black lines) or isotype control (filled histograms) was analyzed by FACS. Representative histograms for (A) CD14+HLA-DR+, CD14+HLA-DR−/low or (B) NK cells are shown. Cumulative results of seven independent experiments for (C) HLA-DR and (D) NKp30 are shown (*P < 0.05; **P < 0.001, ***P < 0.0001).
Suppression of NK Cells by MDSCs Is Dependent on Cell Contact.
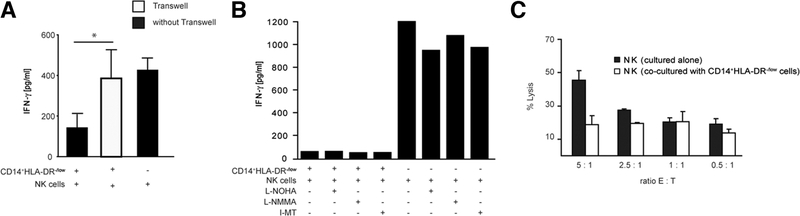
In order to investigate the possible mechanisms as to how MDSCs inhibit NK cell function, MDSCs and NK cells were incubated either together or in separate wells of a transwell, and IFN-γ concentration in cell supernatants was measured after 48 hours. Figure 5A shows that the inhibition of NK cells by MDSCs is mainly dependent on cell contact, because up to 80% of the inhibition was reversed when the cells were separated by use of a transwell.
Fig. 5.
Inhibition of IFN-γ production by MDSC is mainly cell contact–dependent, but independent of arginase and NO. (A) CD14+HLA-DR−/low cells were cocultured with NK cells as described and transwell inserts were used as indicated (white bar). IFN-γ secretion was determined by ELISA. Shown are cumulative results from three independent experiments (*P < 0.05). (B) Purified NK cells and CD14+HLA-DR−/low cells were cocultured in the absence or presence of N-omega-hydroxy-L-arginine (L-NOHA), N(G)-monomethyl-L-arginine (L-NMMA), 1-MT (10 μmol/L each), or media alone as indicated. IFN-γ production was measured by ELISA after 48 hours. Figure shown is a representative of three independent experiments. (C) Purified NK cells and CD14+HLA-DR−/low cells were cocultured for 36 hours. NK cells were reisolated, and a 51Cr-release assay against K562 target cells was performed. Shown is representative data from three independent experiments.
Suppression of NK Cells by MDSCs Is Independent of Arginase or Inducible Nitric Oxide Synthase Function.
Next, we assessed the role of arginase I and inducible NO synthase on MDSC-mediated suppression of NK cells. We have previously shown that MDSCs express high levels of arginase.8 MDSCs were treated with N-omega-hydroxy-L-arginine, N(G)-monomethyl-L-arginine, and 1-Methyl-Tryptophane (I-MT) (specific inhibitor for indoleamine 2,3-deoxygenase [IDO]) and incubated with NK cells. None of these inhibitors affected MDSC mediated inhibition of cytokine secretion by NK cells (Fig. 5B). These results show that MDSCs inhibit NK cells through another pathway independent of arginase I, inducible NO synthase, and IDO.
The Suppressive Effect of MDSCs on NK Cells Is Long-Lasting.
We also incubated NK cells with MDSCs for 24 hours, then re-sorted NK cells from these cocultures and used them in a chromium-release assay against K562 cells. NK cells re-isolated from MDSC cocultures had a reduced lytic function as compared to uncultured NK cells and NK cells cocultured with CD14+HLA-DR+ cells (Fig. 5C). This suggests that the suppressive effect of MDSCs on NK cells is long-lasting and not a transient outcome.
MDSCs Inhibit NK Cell Function Using the NKp30 Receptor.
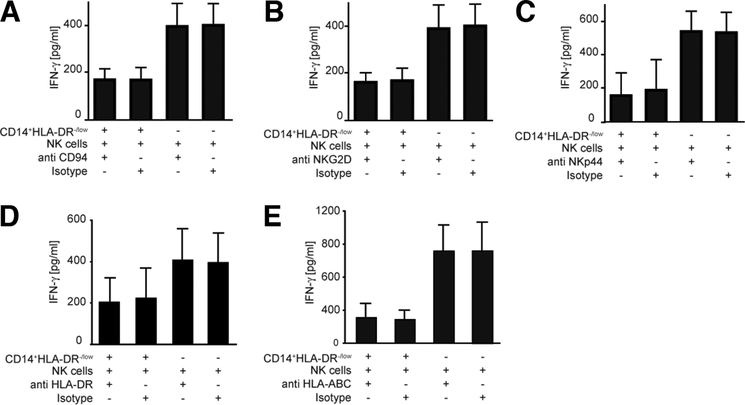
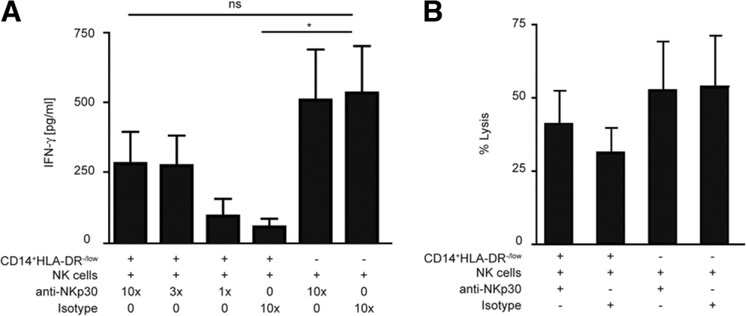
To analyze the molecular basis of NK cell inhibition by MDSCs, we tested to see if blocking of any of the receptors on NK cells could affect the interaction between MDSCs and NK cells and subsequent inhibition. MDSCs and NK cells were coincubated in the presence of anti-NKG2D, anti-CD94, anti-NKp44, anti–major histocompatibility complex I (MHC I), anti-MHC-II, and anti-NKp30. Addition of anti-NKp44, anti-NKG2D, anti-CD94 monoclonal antibody or anti-MHC I or anti-MHC II had no effect on MDSC-mediated inhibition of IFN-γ release by NK cells (Fig. 6). In addition, there was also no effect on NK cell lysis (data not shown). However, blocking NKp30 substantially reduced the inhibitory function of MDSCs on NK cells. Indeed, in the presence of anti-NKp30, both cytolytic activity and IFN-γ release by NK cells were reversed. Similar amounts of IFN-γ were measured in supernatants from NK cells alone and NK cells cocultured with MDSCs when NKp30 blocking antibody was used (Fig. 7A,B). There was no effect of anti-NKp30 antibody on NK cells alone (data not shown). These results suggest that NKp30 engagement is involved in MDSC-mediated inhibition of NK cell function.
Fig. 6.
MDSC-mediated suppressive mechanism is independent of anti-CD94, anti-NKG2D, anti-NKp44, anti-HLA-DR, and anti-MHC class I. Purified NK cells were cultured with IL-2 in the absence or presence of different blocking antibodies as indicated. (A-E) IFN-γ secretion was measured in supernatants after coincubation in the presence of the indicated antibodies by ELISA. Shown are cumulative results from two independent experiments.
Fig. 7.
MDSC-mediated suppressive mechanism is dependent on NKp30. (A) NK cells were cultured with IL-2, CD14+HLA-DR−/low in the absence or presence of anti-NKp30 antibody or isotype control. IFN-γ secretion was measured by ELISA. Cumulative results ± SEM of three independent experiments are shown (*P < 0.05). (B) NK cells were cultured with CD14+HLA-DR−/low in the presence or absence of anti-NKp30 antibody or isotype control. K562 cells were added at a ratio of 2.5:1 (E:T) and lysis was measured. Cumulative results ± SEM of three independent experiments are shown.
Role of STATs in MDSC-Mediated Inhibition of NK Cells.
Because the STAT signaling pathways are known to be important in NK cell function and recently have been shown to be involved in MDSC-mediated inhibition of NK cells in mice,14 we investigated the expression of messenger RNA level of different STATs in our cocultures. Quantitative polymerase chain reaction was performed on NK cells and CD14+HLA-DR−/low cells under different conditions, as indicated. A significant up-regulation of STAT1 expression was observed when NK cells and CD14+HLA-DR−/low cells were cultured together (Supporting Fig. 1A; 0.56 ± 0.03 to 0.08 ± 0.02), but no differences in expression level could be detected for STAT3, STAT4, and STAT5 (Supporting Fig. 1B-D). However, adding the nucleoside analogue fludarabine as an inhibitor for STAT1 phosphorylation15 showed no effect on the IFN-γ secretion of NK cells (Supporting Fig. 1E). Intracellular staining for p-STAT1 showed an activation of STAT1 in CD14+HLA-DR−/low cells (Supporting Fig. 1F, panel I) cocultured with NK cells but also when cultured alone (Supporting Fig. 1F, panel III), whereas NK cells showed no phosphorylation of STAT1 (Supporting Fig. 1F, panels II,IV). These findings suggest that STAT1 is not involved in the suppression of NK cells by CD14+HLA-DR−/low cells in humans.
Discussion
It has become increasingly clear that host-tumor interactions are quite complex, leading to tumor escape mechanisms in patients with cancer.16,17 MDSC expansion is one of the mechanisms that HCC tumors develop to evade the host immune response seen in both mice and humans.8 This study demonstrates a novel role for MDSCs in patients with HCC.
We have previously shown that patients with HCC mount spontaneous tumor-specific immune responses, but still progress with their disease,6 suggesting that immune-suppressor mechanisms might counterbalance antitumor immune responses. Indeed, we have been able to detect different types of immune escape mechanisms such as an increase in CD4+CD25+ regulatory T cells and defective dendritic cell function in patients with HCC.7,18 Recently, we have identified a new population of MDSCs in patients with HCC; these cells are CD14+HLA-DR−/low, are significantly increased in peripheral blood and tumors of HCC patients, and can inhibit autologous T cell proliferation.8 More importantly, we could also demonstrate that these cells induce CD4+CD25+FoxP3 regulatory T cells.8
Here, we show a novel role for MDSCs in patients with HCC, where MDSCs contribute to the immune suppression in these patients by inhibiting NK cells, the effector cells of the innate immune system. NK cells as the effectors of the innate arm of the immune system play an important role in eliminating virus-infected cells as well as in controlling tumor cell growth.19 It has also been shown that human NK cells kill immature autologous dendritic cells.20 Therefore, defective NK cell function in patients with HCC not only impairs clearance of tumor cells with MHC class I down-regulation but also can lead to accumulation of these immature cells, which can be tolerogenic to T cells. Therefore, MDSCs play a pivotal role in influencing anti-tumor immune responses by regulating both adaptive and innate immunity.
Impairment in the function of NK cells in HCC patients has recently been described supporting our observations.12,13 However, until now the mechanism by which NK cells in patients with HCC are impaired has not been clear. We have previously shown that MDSCs induce regulatory T cells in patients with HCC. Interestingly, it has also been shown that regulatory T cells are inhibitors of NK cell function in patients with cancer,21 suggesting a possible additional pathway of MDSC-mediated inhibition of NK cell function in patients with HCC.
It is important to mention that our study does not address how the cross-talk between NK cells and MDSCs contributes to HCC pathogenesis in vivo. It has been shown that NK cells are also impaired in their function in patients with liver cirrhosis.22 We have tested MDSC-NK interactions in patients with liver cirrhosis as well as patients with hepatitis C virus (HCV). In either case, MDSCs were able to suppress NK cell function (Supporting Fig. 2). In addition, there were no differences between HCC patients with or without HCV. We suggest that MDSC-mediated NK cell impairment is a general mechanism regardless of the patient population, whether healthy donors, patients with liver cirrhosis, or patients with hepatitis. However, in our previous study, we have shown that the frequency of MDSCs is only increased in peripheral blood of patients with HCC but not in patients with liver cirrhosis or HCV infection.8 Therefore, we suggest that the increase in frequency of MDSCs in patients with HCC is one mechanism responsible for suppression of NK cell function in these patients.
Several previous murine studies have demonstrated a tumor-dependent increase of MDSCs.1,23 In mice, conflicting results have been described on the MDSC-NK crosstalk in mouse tumor models. In one study, CD11b Gr-1+ cells activated NK cells through a STAT1-mediated mechanism,14 whereas two other studies described an inhibitory role of these cells in mice with tumors.24,25 However, nothing is known about the NK-MDSC crosstalk in humans and in particular in patients with HCC.
The mechanism by which MDSCs inhibit NK cell function in patients with HCC is not clear. Our previous study showed that MDSCs in these patients have high arginase activity, which is a hallmark function of MDSCs.8 However, in this study, the inhibition of NO production as well as the blocking of arginase function did not affect the inhibitory effect of MDSCs. Blocking direct cell contact between MDSCs and NK cells, however, reversed the inhibitory effect of MDSCs on NK cells. Inhibition of NK cell function by MDSCs was mainly mediated through the NK-activating receptor NKp30, one of the three natural cytotoxicity receptors on NK cells. NKp30 has been shown to be primarily responsible for the NK cell–mediated lysis of autologous immature dendritic cells.26 Because inhibition of NK cells by MDSCs was dependent on cell contact, it suggests that MDSCs express one or more ligands for NKp30. However, at this point, the ligand(s) for NKp30 on MDSCs is not known. Nonetheless, it cannot be ruled out that there are additional factors possibly acting through direct cell contact that play a role in MDSC-mediated NK cell suppression. It is also important to note that NKp30 is an activating receptor which here, in interaction with MDSCs, results in inhibition of NK cell function. We believe that the level of stimulation or inhibition has to do with the density of NKp30 ligand expressed on the surface of MDSCs, which remains to be identified.
Further work is required to elucidate the exact mechanism used by MDSCs in this suppression. Additional studies are also needed to see whether the NK cells can also affect the MDSCs in humans and in patients with HCC. The multifaceted role of MDSCs illustrates further the complexity of the immune-suppressive mechanisms in patients with HCC, and as such, the need to design new immune-based therapies targeting these pathways.
In summary, our study has uncovered another new mechanism of immune evasion for HCC tumors whereby MDSCs regulate the innate immunity and its effector cells, the NK cells. Impaired NK cells can affect antitumor immune responses, which contributes further to tumor escape from both innate and adaptive immune responses in patients with HCC.
Supplementary Material
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG) (KFO119), the H.W. & J. Hector Foundation, and by the Initiative and Networking Fund of the Helmholtz Association within the Helmholtz Alliance on Immunotherapy of Cancer.
Abbreviations:
- ELISA
enzyme-linked immunosorbent assay
- FACS
fluorescence-activated cell sorting
- HCC
hepatocellular carcinoma
- IFN
interferon
- MDSC
myeloid-derived suppressor cell
- MHC
major histocompatibility complex
- NK
natural killer cell
- PBMC
peripheral blood mononuclear cells
- STAT
signal transducer and activator of transcription
Footnotes
Potential conflict of interest: Nothing to report
References
- 1.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol 2006;16:53–65. [DOI] [PubMed] [Google Scholar]
- 2.Pak AS, Wright MA, Matthews JP, Collins SL, Petruzzelli GJ, Young MR. Mechanisms of immune suppression in patients with head and neck cancer: presence of CD34(+) cells which suppress immune functions within cancers that secrete granulocyte-macrophage colony-stimulating factor. Clin Cancer Res 1995;1:95–103. [PubMed] [Google Scholar]
- 3.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J,et al. Arginase-producingmyeloidsuppressorcellsinrenalcellcarcinoma patients: a mechanism of tumor evasion. Cancer Res 2005;65:3044–3048. [DOI] [PubMed] [Google Scholar]
- 4.Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, et al. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res 2006;66:9299–9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol 2001;166:678–689. [DOI] [PubMed] [Google Scholar]
- 6.Korangy F, Ormandy LA, Bleck JS, Klempnauer J, Wilkens L, Manns MP,et al. Spontaneous tumor-specific humoral and cellular immune responses to NY-ESO-1 in hepatocellular carcinoma. Clin Cancer Res 2004;10: 4332–4341. [DOI] [PubMed] [Google Scholar]
- 7.Ormandy LA, Farber A, Cantz T, Petrykowska S, Wedemeyer H, Horning M, et al. Direct ex vivo analysis of dendritic cells in patients with hepatocellular carcinoma. World J Gastroenterol 2006;12:3275–3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology 2008;135:234–243. [DOI] [PubMed] [Google Scholar]
- 9.Caligiuri MA. Human natural killer cells. Blood 2008;112:461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrega P, Morandi B, Costa R, Frumento G, Forte G, Altavilla G, et al. Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56 bright CD16(−) cells and display an impaired capability to kill tumor cells. Cancer 2008;112:863–875. [DOI] [PubMed] [Google Scholar]
- 11.Fauriat C, Moretta A, Olive D, Costello RT. Defective killing of dendritic cells by autologous natural killer cells from acute myeloid leukemia patients. Blood 2005;106:2186–2188. [DOI] [PubMed] [Google Scholar]
- 12.Jinushi M, Takehara T, Tatsumi T, Hiramatsu N, Sakamori R, Yamaguchi S, et al. Impairment of natural killer cell and dendritic cell functions by the soluble form of MHC class I-related chain A in advanced human hepatocellular carcinomas. J Hepatol 2005;43:1013–1020. [DOI] [PubMed] [Google Scholar]
- 13.Cai L, Zhang Z, Zhou L, Wang H, Fu J, Zhang S, et al. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol 2008;129:428–437. [DOI] [PubMed] [Google Scholar]
- 14.Liu C, Yu S, Kappes J, Wang J, Grizzle WE, Zinn KR, et al. Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood 2007;109:4336–4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frank DA, Mahajan S, Ritz J. Fludarabine-induced immunosuppression is associated with inhibition of STAT1 signaling. Nat Med 1999;5:444–447. [DOI] [PubMed] [Google Scholar]
- 16.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002;3:991–998. [DOI] [PubMed] [Google Scholar]
- 17.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004;21:137–148. [DOI] [PubMed] [Google Scholar]
- 18.Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res 2005;65:2457–2464. [DOI] [PubMed] [Google Scholar]
- 19.Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol 2007;7:329–339. [DOI] [PubMed] [Google Scholar]
- 20.Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med 2002;195:335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghiringhelli F, Menard C, Terme M, Flament C, Taieb J, Chaput N, et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med 2005; 202:1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chuang WL, Liu HW, Chang WY, Chen SC, Hsieh MY, Wang LY. Natural killer cell activity in patients with liver cirrhosis relative to severity of liver damage. Dig Dis Sci 1991;36:299–302. [DOI] [PubMed] [Google Scholar]
- 23.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev 2008;222:162–179. [DOI] [PubMed] [Google Scholar]
- 24.Nausch N, Galani IE, Schlecker E, Cerwenka A. Mononuclear myeloid-derived “suppressor” cells express RAE-1 and activate NK cells. Blood 2008;112:4080–4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol 2009;182:240–249. [DOI] [PubMed] [Google Scholar]
- 26.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Munz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med 2002;195:343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.