Abstract
Current paradigms of CD8+ T cell-mediated protection in HIV infection center almost exclusively on studies of peripheral blood, which is thought to provide a window into immune activity at the predominant sites of viral replication in lymphoid tissues (LTs). Through extensive comparison of blood, thoracic duct lymph (TDL) and LTs in different species, we show that many LT memory CD8+ T cells bear phenotypic, transcriptional and epigenetic signatures of resident memory T cells (TRMs). Unlike their circulating counterparts in blood or TDL, the majority of total and follicular HIV-specific CD8+ T cells in LTs also resemble TRMs. Moreover, high frequencies of HIV-specific CD8+ TRMs with skewed clonotypic profiles relative to matched blood samples are present in LTs of individuals who spontaneously control HIV replication in the absence of antiretroviral therapy (elite controllers). Single-cell RNA-seq analysis confirmed that HIV-specific TRMs are enriched for effector-related immune genes and signatures compared to HIV-specific non-TRMs in elite controllers. Together, these data indicate that previous studies in blood have largely failed to capture the major component of HIV-specific CD8+ T cell responses resident within LTs.
INTRODUCTION
It is well established that CD8+ T cells are required for effective immune control of HIV. The contemporary literature is also replete with studies that describe the qualitative characteristics of successful HIV-specific CD8+ T cell responses. For example, allotype restriction, antigen sensitivity and specificity, clonotype distribution, cytolytic activity, polyfunctionality, proliferative reserve, and response magnitude have all been associated with differential rates of disease progression (1). However, most of these correlates are based on data acquired from peripheral blood samples, despite the fact that HIV replicates primarily in lymphoid tissues (LTs).
HIV seeds, replicates, and persists in LTs (2). It is therefore important to develop an anatomically consistent view of immunosurveillance, especially in light of the fact that follicular helper T cells (TFHs) facilitate viral replication and harbor much of the viral reservoir (3–5). The efficacy of live-attenuated SIV vaccines can be predicted from the magnitude of virus-specific T cell responses in LTs (6). In addition, CD8+ T cell depletion is associated with a rapid redistribution of productive SIV infection to non-TFHs in elite controller monkeys, suggesting active viral suppression by CD8+ T cells, and/or NK cells, in LTs (7). However, very little is known about the antiviral properties of HIV/SIV-specific CD8+ T cells that reside in LTs.
Through the early work of Gowans and colleagues (8), we know that lymphocytes recirculate between tissues and blood through lymph via the thoracic and lymphatic ducts. More recent studies of human blood have categorized memory CD8+ T cells broadly into two circulating subsets based on their tissue homing properties. Central memory T cells (TCMs) express lymphoid homing receptors (CCR7 and CD62L) and recirculate between blood, LTs, and lymph, while effector memory T cells (TEMs) lack CCR7 and CD62L and recirculate between blood, non-lymphoid tissues (NLTs), and lymph to survey visceral organs and body surfaces (9). Recently, a new subset known as resident memory T cells (TRMs), that reside within NLTs, has been identified (10–13). Most TRMs constitutively express CD69 (14), which downregulates the sphingosine-1-phosphate receptor 1 (S1PR1) (15), thereby preventing tissue egress. NLTs are seeded with TRMs soon after immune priming (11), establishing a front-line tissue defense that can eliminate pathogens largely without the involvement of circulating T cells (16). Importantly, TRMs have distinct functional, phenotypic, and transcriptomic profiles attuned to their compartmentalized state (17).
TRMs have been identified in murine LTs through parabiosis experiments (18), where they are relatively sparse compared with circulating T cells (19). CD8+ TRMs have also been observed in human LTs (20–22). The notion that TRMs may exist in LTs could transform our understanding of CD8+ T cell-mediated control of HIV infection. In line with this possibility, the frequency of HIV-specific CD8+ T cells is generally higher in lymph nodes (LNs) compared with matched peripheral blood samples (23, 24), despite the fact that most circulating HIV-specific CD8+ T cells lack classical lymphoid homing characteristics (25, 26). Moreover, CD8+ T cells accumulate in HIV-infected LTs (27) and respond poorly to S1PR1-mediated signals (28). However, it remains unclear if HIV-specific CD8+ T cells with a TRM phenotype exist in HIV-infected LTs. To resolve this issue, we conducted a detailed assessment of CD8+ T cells in the peripheral blood and LTs of healthy and HIV+ subjects. Our data show that TRMs most often dominate the HIV-specific CD8+ T cell response in LTs, and identify a link between increased magnitudes of HIV-specific CD8+ TRMs and effective immune control of HIV.
RESULTS
CD69+CCR7− CD8+ T cells are expanded in HIV-infected lymph nodes
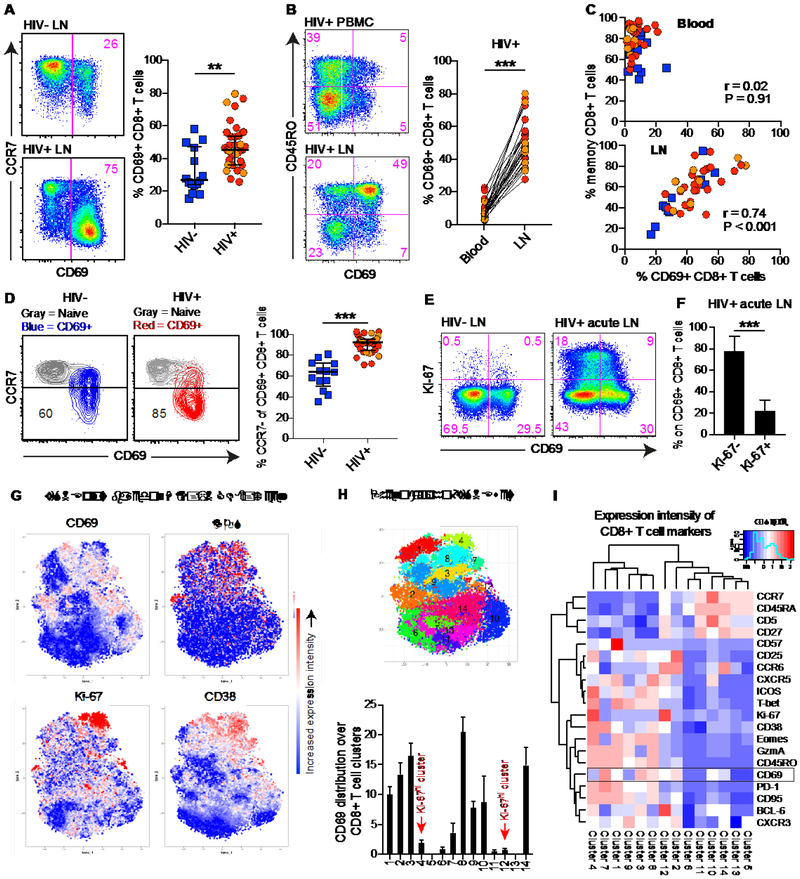
CD69 has often been considered as a marker of early T cell activation (29). We examined CD69 expression on LN CD8+ T cells from HIV+ and HIV− subjects (Table S1). Irrespective of adherence to antiretroviral therapy (ART), higher frequencies of LN CD69+ CD8+ T cells and CCR7− (TEM) cells were present in HIV+ compared with HIV− subjects (Fig. 1A and S1A). In HIV+ subjects, higher frequencies of CD8+ T cells expressed CD69 in LNs compared with blood (Fig. 1B). A strong association was also detected between the frequency of memory CD8+ T cells and the frequency of CD69+ CD8+ T cells in LNs (Fig. 1C and S1B). Moreover, LN CD69+ CD8+ T cells from HIV+ subjects were biased towards a TEM phenotype (Fig. 1D and S1B).
Fig. 1. CD69 is not coupled to an early immune activation signature in HIV-infected LNs.
(A) Frequency of CD69 expression on total LN CD8+ T cells from HIV+ and HIV− subjects. Red indicates ART− and orange indicates ART+ in the HIV+ scatter plot group. (B) Frequency of CD69 expression on total blood (top) and LN (bottom) CD8+ T cells. Matched samples from one subject are shown in the representative flow cytometry plots. Lines connect matched samples across all subjects in the graph. (C) Correlation between the frequency of memory (non-CCR7hi non-CD45RO−) CD8+ T cells and CD69 expression on total blood (top) and LN (bottom) CD8+ T cells. Blue: HIV− subjects: red: HIV+ ART− subjects; orange HIV+ ART+ subjects. (D) Frequency of CCR7− CD69+ memory CD8+ T cells in HIV− (blue) and HIV+ (red: ART−; orange: ART+) subjects. (E) Co-expression pattern of Ki-67 and CD69 in/on total LN CD8+ T cells from an HIV− subject (left) and an HIV+ ART− subject (right) with acute infection (Fiebig V). (F) Frequency of Ki-67–/+ CD69+ CD8+ T cells in LNs from HIV+ subjects with acute infection (n = 7). (G) Expression intensity of CD69, ICOS, Ki-67, and CD38 in multidimensional viSNE space. viSNE plots were derived using 27576 cells (n = 17 subjects) and the markers shown in Fig. 1I. (H) The same viSNE display with sub-populations colored using the PhenoGraph implementation of Cytofkit (top). Distribution of CD69 expression over all 14 sub-populations derived using PhenoGraph (bottom). (I) Hierarchical clustering of expression intensity (z-score) for all assessed markers within the different sub-populations derived using PhenoGraph. Median and IQR are shown for all scatter/bar plots. **P < 0.01; ***P < 0.001. Conventional (fluorescence) flow cytometry was used for all stains in (A-F) and mass cytometry (CYTOF) was used in (G-I).
To determine if LN CD8+ T cells express CD69 as a consequence of activation, we obtained samples from HIV+ subjects in the acute/early stages of infection (Fiebig IV-VI), a time when early cycling (Ki-67+) CD8+ T cells are prevalent in blood (30). Substantially higher frequencies of LN CD8+ T cells expressed Ki-67 in acutely infected HIV+ compared with HIV− subjects (Fig. 1E). These LN Ki-67+ CD8+ T cells also showed evidence of HIV specificity (Fig. S1C), as demonstrated previously in blood (30). However, only a minority of LN CD69+ CD8+ T cells expressed Ki-67 (Fig. 1F and S1D). In addition, we conducted mass cytometry to assess the expression of multiple activation markers on LN CD8+ T cells in HIV+ subjects with chronic disease (Fig. 1G). Similar to our conventional flow cytometry data, mass cytometry also demonstrated higher CD69 levels in LNs compared to blood (Fig. S1E). We next identified fourteen CD8+ T cell clusters algorithmically in t-distributed stochastic neighbor embedding (tSNE) space, where one cluster (#4) displayed high co-expression of several activation markers (CD38, Ki-67, ICOS), but not CD69 (Fig. 1H). In contrast, CD69 expression intensity was high on unique clusters (#7, #8) with elevated levels of CD38, but also dissociated with CD38 expression in other dominant CD69 clusters (#2, #3) (Fig. 1H). Furthermore, some naïve-like clusters (#10, #14) demonstrated low CD69 intensity (Fig. S1F), but these clusters also expressed low to high intensity of BCL-6, CD95 and CXCR5 (#10) as well as CXCR3 and CCR6 (#14), suggestive of memory stem T cell (TSCM)-like features (31). Quantitative assessments demonstrated that several activation markers could be expressed on CD69+ CD8+ T cells (Fig. S1G); however, hierarchical clustering confirmed that CD69 expression was more closely related to PD-1, CD95, and CXCR3 expression than CD38, Ki-67, and ICOS expression (Fig. 1I). Collectively, these data indicate that high levels of CD69 expression on memory CD8+ T cells in HIV-infected LTs cannot be explained by an increased early/cycling (Ki-67+) immune activation profile.
CD69+ CD8+ T cells display enriched traits of residency in lymphoid tissues
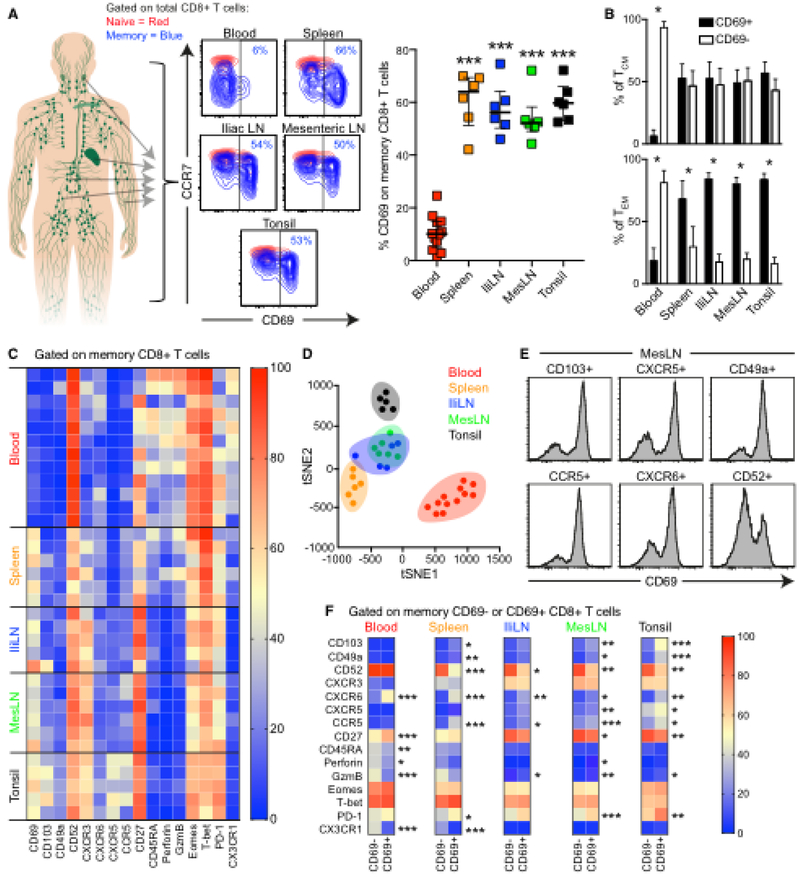
Although CD69 is classically associated with activation, it also marks non-circulating TRMs in NLTs (32). Recent studies in humans have also identified high levels of CD69+ CD8+ T cells in multiple LTs (20–22). However, it remains unclear if these findings reflect early T cell activation or proliferation in inflamed tissues and organs isolated from brain-dead subjects, as previously described in murine models (33). We therefore collected spleen, iliac LNs, mesenteric LNs and tonsils from HIV− subjects without known major inflammatory disease. Using conventional (fluorescence) flow cytometry (Fig. S2A), we found that the majority of LT memory CD8+ T cells expressed CD69, irrespective of tissue origin (Fig. 2A). Most LT TEM cells also expressed CD69 (Fig. 2B), suggestive of a TRM phenotype (32). Similar to our previous analysis on HIV-infected subjects, we also here found some expression of CD69 on naïve-like (CCR7+CD45RA+) cells (Fig. S2B). However, CD69 expression levels on naïve-like cells were not significantly different between blood and LTs and these cells possessed lower CD69 MFI than on paired memory cells (Fig. S2B). Moreover, CD69+CCR7+CD45RA+ were biased towards higher CD95 MFI levels, again indicative of a TSCM phenotype (Fig. S2C). Of note, CD69+ CD8+ T cells expressed lower levels of multiple activation markers compared to in vitro stimulated T cells (Fig. S2D). CD69+ CD8+ T cells did not entirely overlap with the set of activation markers using tSNE analysis (Fig. S2E) and Boolean gating analysis confirmed that the minority of CD69+ CD8+ T cells (median = 33.7 %) expressed any of the assessed activation markers, suggesting that CD69 expression on these cells is not driven exclusively by activation.
Fig. 2. CD69+ CD8+ T cells display TRM characteristics in LTs.
(A) Frequency of CD69 expression on memory CD8+ T cells (blue) in blood, spleen, iliac (Ili) LNs, mesenteric (Mes) LNs, and tonsils from HIV− subjects. Asterisks denote statistical comparisons versus blood. (B) Frequency of CD69–/+ cells among CCR7+ (TCM) and CCR7− (TEM) CD8+ T cells. Bar plots are based on all subjects in (A). (C) Heat-map displaying expression frequency (blue = 0%; red = 100%) for the indicated markers among blood, spleen, IliLN, MesLN, and tonsil memory CD8+ T cells. (D) tSNE clustering on blood (red), spleen (orange), IliLN (blue), MesLN (green), and tonsil (black) memory CD8+ T cells. Values for the tSNE analysis are derived from all markers in (C). (E) CD69 expression on CD103+, CXCR5+, CD49a+, CCR5+, CXCR6+, and CD52+ memory CD8+ T cells from MesLNs. (F) Average expression frequency (blue = 0%; red = 100%) for the indicated markers among blood, spleen, IliLN, MesLN, and tonsil CD69− and CD69+ memory CD8+ T cells. Colors denote average frequencies, derived from all subjects in (C). Median and IQR are shown for all scatter/bar plots. *P < 0.05; **P < 0.01; ***P < 0.001. Conventional flow cytometry was used for all stains in this figure.
In more detailed analyses (Fig. S3A), we found that LT memory CD8+ T cells expressed higher levels of integrins (CD103 and CD49a) and chemokine receptors (CXCR5, CXCR3, CCR5) compared with blood memory CD8+ T cells (Fig. 2C and S3B). In contrast, blood memory CD8+ T cells expressed higher levels of circulation markers (CD52) (34), effector (T-bet, Eomes, perforin, Granzyme B) proteins as well as the fractalkine receptor CX3CR1 (Fig. 2C and S3B). tSNE analysis further showed that LT memory CD8+ T cells formed distinct clusters compared with blood memory CD8+ T cells (Fig. 2D). Importantly, different clusters were observed in spleen and tonsils compared with LNs. We also examined LT CD103+, CXCR5+, CD49a+, CCR5+, and CXCR6+ CD8+ T cells, and found that these populations were more likely to express CD69 (Fig. 2E and S4). Likewise, LT CD69+ CD8+ T cells expressed higher levels of CD103, CXCR5, CD49a, CCR5, CXCR6, PD-1, and Granzyme B compared with CD69− CD8+ T cells, which expressed higher levels of CD52 (Fig. 2F).
LT CD69+ CD8+ T cells have transcriptional, epigenetic, and functional TRM signatures
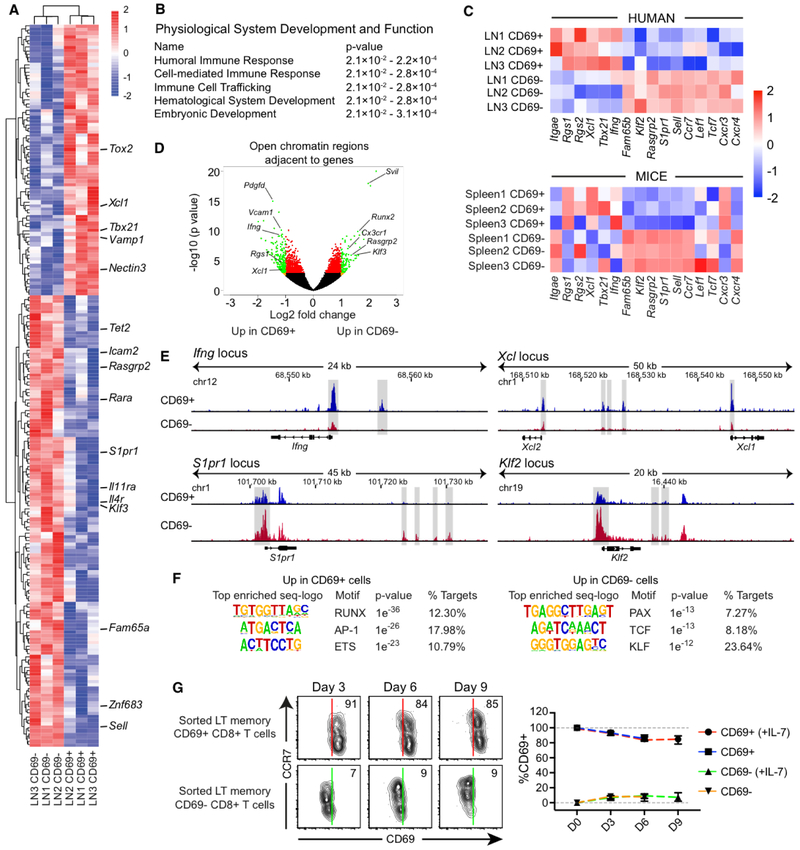
To determine if human LT CD69+ CD8+ T cells have a transcriptional signature resembling TRMs, we sorted mesenteric LN (MesLN) memory CD69− and CD69+ CD8+ T cells (Fig. S5A) and performed RNA sequencing (RNA-seq) analysis. We identified a core signature of genes that were either upregulated or downregulated in LN memory CD69− and CD69+ CD8+ T cells (fold change > 2; P < 0.05), where particular genes that are downregulated in NLT TRMs, such as S1pr1, Klf3, and Sell (35), were also poorly expressed in LN memory CD69+ CD8+ T cells (Fig. 3A and Table S2). Of note, Znf683 (Hobit), previously associated with murine TRM formation (36), was downregulated in LN memory CD69+ CD8+ T cells. Gene-set enrichment analysis (GSEA) revealed that genes linked with human lung CD69+ CD103+ CD8+ T cells (37) were also enriched in LN CD69+ CD8+ T cells (Fig. S5B). Ingenuity pathway analysis further demonstrated that the major differences between CD69− and CD69+ CD8+ T cells included cell-mediated immune responses and cell trafficking (Fig. 3B). In addition, many genes typically associated with TRMs and TCMs/TEMs (17, 38) were upregulated (Itgae, Rgs1, Rgs2, Xcl1, Tbx21, Ifng) or downregulated (Fam65b, Klf2, Rasgrp2, S1pr1, Sell, Ccr7, Lef1, Tcf7, Cxcr3, Cxcr4) in CD69+ CD8+ T cells (Fig. 3C). Many of these genes followed the patterns observed in splenic CD69+ CD8+ T cells and TCMs (CD69− CD62L+) isolated from P14 T cell receptor (TCR)-transgenic mice (Fig. 3C).
Fig. 3. CD69+ CD8+ T cells display functional and transcriptional TRM signatures in LTs.
(A) RNA-seq heat-map showing differentially expressed genes (fold change > 2; P < 0.05) between human MesLN memory CD69− and CD69+ CD8+ T cells. (B) Ingenuity pathway analysis based on all differentially expressed genes (P < 0.05) between CD69− and CD69+ CD8+ T cells in the RNA-seq dataset. The top pathways involved in the “physiological system development and function” arm are shown in the table. (C) Differential expression patterns of TRM-related and TCM/EM-related genes between human LN memory CD69− and CD69+ CD8+ T cells (top) and between splenic CD69− and CD69+ CD8+ T cells from P14 TCR-transgenic mice (bottom). (D) ATAC-seq volcano plot showing open chromatin regions adjacent to specific genes. Green marks ATAC-seq peaks that are differentially enriched (fold change > 2; P < 0.05) in human hepatic LN memory CD69− and CD69+ CD8+ T cells. (E) ATAC-seq tracks from human LN memory CD69− (red) and CD69+ (blue) CD8+ T cells. (F) Enriched ATAC-seq de novo motifs for CD69+ (left) and CD69− (right) CD8+ T cells (G) Human memory CD69− and CD69+ CD8+ T cells from spleen, tonsil, and hepatic LNs (n = 2 each) were assessed over time for CD69 expression in the presence or absence of rhIL-7. Lines represent mean ± SD. Conventional flow cytometry was used for all stains in this figure.
We further conducted Assay for Transposase Accessible Chromatin with high-throughput sequencing (ATAC-seq) to map potential differences in open chromatin regions (OCRs) between LN memory CD69− and CD69+ CD8+ T cells. OCRs adjacent to genes known to be involved in the establishment of residency and immune responses displayed marked differences between these two subsets of LN memory CD8+ T cells (Fig. 3D and Table S3). For example, several OCRs were present next to Ifng, Xcl1, and Xcl2 primarily in CD69+ CD8+ T cells (Fig. 3E), suggesting a readiness to upregulate these effector functions (17, 37). Prdm1 (encoding Blimp-1), recently associated with TRM formation (36), were also enriched for OCRs in CD69+ CD8+ T cells (Fig. S5C). In contrast, CD69− CD8+ T cells were more likely to have multiple OCRs adjacent to genes associated with trafficking, including Klf2 and S1pr1 (Fig. 3F), and the Znf683 locus (Fig. S5C). We further inferred transcription factor occupancy in regulatory elements by motif analysis and found that RUNX, AP-1 and ETS families belonged to the most enriched motifs in CD69+ CD8+ T cells, while CD69− CD8+ T cells showed enriched motifs belonging to the PAX, TCF and KLF families, demonstrating that unique transcription factor families are likely cooperatively responsible for regulating resident and recirculating T cell states (Fig. 3G).
Next, we sorted human LT memory CD69− and CD69+ CD8+ T cells, and found that CD69 was stably expressed on the surface of TRMs for up to 9 days (Fig. 3G). Classical TRMs express low levels of CCR7 and CD62L (32). We found no evidence that supplementation with CCR7 ligands (CCL19 or CCL21) influenced CD69 downregulation on LT memory CD69+ CD8+ T cells (Fig. S5D). However, CD69 was downregulated significantly on CCR7+ CD69+ CD8+ T cells after 9 days in culture (Fig. S5E), and markers associated with residency and effector functions were predominantly expressed by CCR7− CD69+ CD8+ T cells ex vivo (Fig. S5F). Altogether, these data show that LT CD69+ CD8+ T cells show enriched phenotypic, transcriptional, epigenetic and functional traits of tissue residency.
Lack of CD69+ CD8+ T cells in thoracic duct lymph
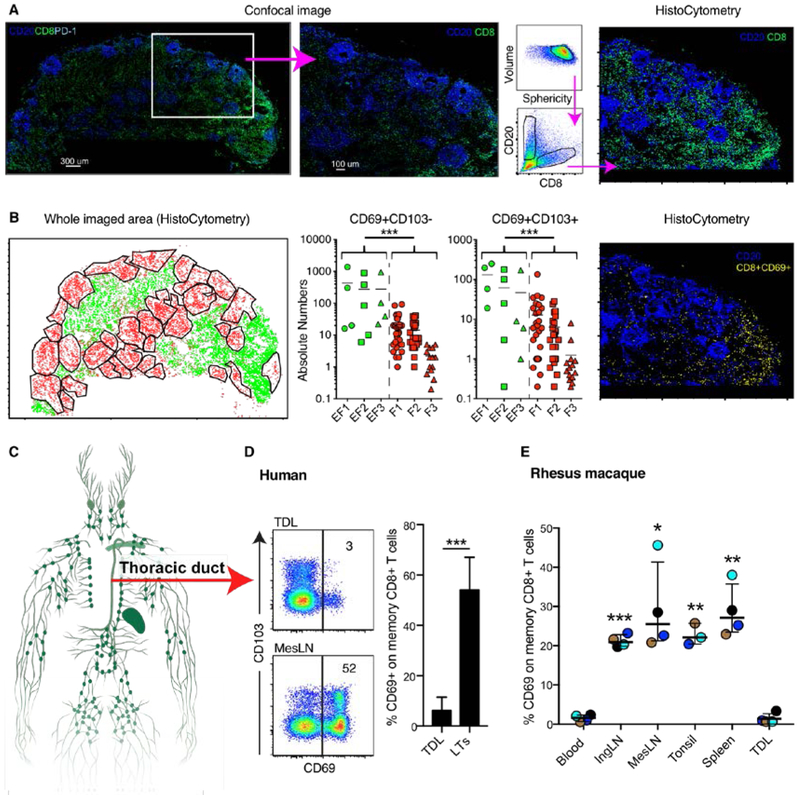
We next applied multiplexed confocal imaging and histo-cytometry to quantify and position where cells with a TRM phenotype were localized in human LNs (Fig. S6A) (39). CD20 was used to distinguish B cell follicles, and did not overlap to a great extent with the anatomical location of CD8+ cells as expected (Fig. 4A and S6B). Imaging and quantitative analysis demonstrated that most CD8+ CD69+CD103− and CD69+CD103+ cells were present in extra-follicular areas (Fig. 4B and S6B). Further analysis revealed that CD69+CD103+/− cells expressed PD-1 to a higher degree than CD69− cells. The CD69+CD103+PD-1+ CD8+ population were localized to an equal degree in B cell follicles and extra-follicular areas (Fig. S6C).
Fig. 4. Anatomical distribution of CD69+ CD8+ T cells in LNs and thoracic duct lymph.
(A) Whole imaged confocal microscopy of mesLN from human (left) and zoomed in area (middle). Histocytometry gating strategy to identify localization of CD8+ T and B cells in the LNs (right). (B) Identification of B cell follicles (red) and extra-follicular areas (green) in the whole imaged area using histocytometry (left). Middle graphs, shows absolute numbers of CD69+CD103− and CD69+CD103+ within all imaged extra-follicular (EF) and B cell follicular (F) areas for three imaged LNs. Example of histocytometry imaging showing the distribution of CD8+CD69+ cells within the zoomed in LN area. (C) Localization in the human body of the thoracic duct. (D) Flow plots of CD69 expression on human thoracic duct lymph (TDL) and mesenteric LN (MesLN) memory CD8+ T cells from two unmatched subjects. Frequency of CD69+ CD8+ T cells in TDL (n = 7 subjects) and LTs from all subjects (n = 25) in Fig. 2A. (E) Frequency of CD69 expression on blood, inguinal (Ing) LN, MesLN, tonsil, spleen, and TDL memory (non-CD28+ non-CD95−) CD8+ T cells from sample-matched rhesus macaques. Each color indicates fluid/tissue from one specific rhesus macaque. Asterisks denote statistical comparisons versus TDL. *P < 0.05; **P < 0.01; ***P < 0.001. Conventional flow cytometry was used for all stains in this figure.
To determine if CD8+ T cells maintain CD69 expression after egress from LNs, we collected efferent lymph from HIV− individuals via cannulation of the human thoracic duct (Fig. 4C). Thoracic duct lymph (TDL) contained very low frequencies of CD69+ CD8+ T cells compared with those detected in LTs (Fig. 4D). Interestingly, both TDL and LT contained a significant proportion of single CD69−CD103+CCR7+ CD8+ T cells, indicating that CD103 expression alone is insufficient to maintain residency in LTs (Fig. 4D, S6D). The overall frequency of CD103+ CD8+ T cells was higher in LTs compared to TDL though, due to the large population of CD69+CD103+CCR7− CD8+ T cells present in LTs. In addition, we collected TDL, blood, and LTs from healthy rhesus macaques. Again, very few blood and TDL CD8+ T cells expressed CD69 compared with donor-matched LT CD8+ T cells (Fig. 4C). Collectively, these data suggest that CD69+ CD8+ T cells likely do not leave LTs.
HIV-specific CD8+ T cells display a TRM phenotype in lymph nodes
The observation that memory CCR7−CD69+ CD8+ T cells are expanded in HIV-infected LNs (Fig. 1) led us to postulate that HIV-specific cells are TRMs. In contrast to matched blood and LN bulk memory CD8+ T cells, most LN HIV-specific CD8+ T cells expressed CD69 (Fig. 5A and S7A). To confirm that HIV-specific CD69+ CD8+ T cells do not leave LTs, we collected TDL from HIV+ subjects. Akin to our previous findings, we detected only very low levels of CD69 on bulk and HIV-specific CD8+ T cells in TDL (Fig. 5A). Of note, CD69 expression on HIV-specific CD8+ T cells was unaffected by ART status (Fig. 5A), indicating that antigen load is not necessarily a determinant of CD69 expression, in contrast to classical immune activation markers in blood (40, 41).
Fig. 5. Majority of HIV-specific CD8+ T cells display a TRM phenotype in LNs.
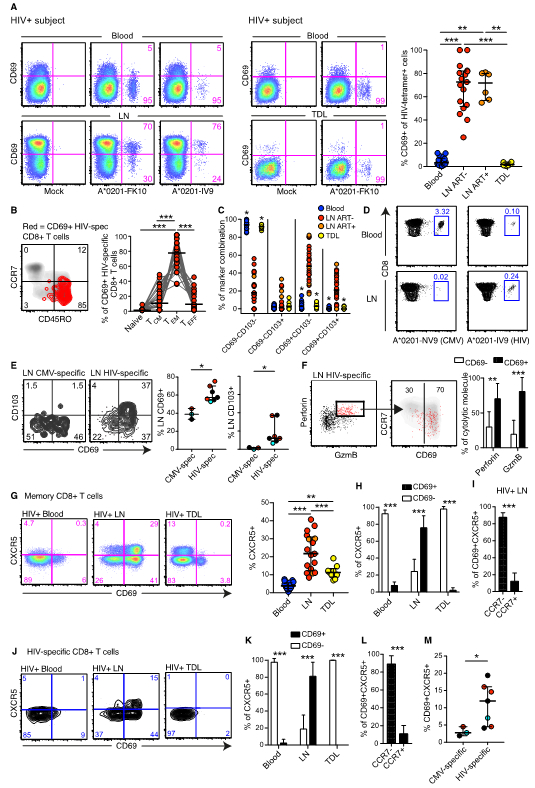
(A) Flow cytometry plots of on memory CD8+ T cells showing CD69 expression on matched HIV-tetramer+ cells in blood and LNs (left), and matched HIV-tetramer+ cells in blood and TDL (middle). Frequency of CD69 expression on HIV-tetramer+ cells in blood (n = 13 subjects), ART− LNs (n = 4 subjects), ART+ LNs (n = 4 subjects), and TDL (n = 7 subjects) (right). (B) Distribution of LN HIV-tetramer+ (spec) CD69+ CD8+ T cells among the naïve (CD45RO− CCR7+), TCM (CD45RO+ CCR7+), TEM (CD45RO+ CCR7−), and effector (TEFF; CD45RO− CCR7−) compartments. (C) CD69 and CD103 expression profiles for blood (blue), LN (red: ART−; orange: ART+), and TDL (yellow) HIV-specific CD8+ T cells. (D) Representative flow cytometry plots showing memory CMV-tetramer+ (left) and HIV-tetramer+ (right) CD8+ T cell frequencies in blood (top) and LNs (bottom) from the same subject. (E) Frequency of CD69 and CD103 expression on LN CMV-NV9-specific and HIV-FK10-specific CD8+ T cells in the same subjects with detectable tetramer+ responses to both viruses. Each color represents a matched subject and a matched tetramer+ response. (F) Representative flow cytometry plots showing CD69 expression on Perforin+ Granzyme B (GzmB)+ HIV-specific CD8+ T cells in LNs (left). Frequency of CD69–/+ cells among Perforin+ (n = 20 responses) and GzmB+ (n = 16 responses) HIV-specific CD8+ T cells in ART+ and ART− LNs (right). (G) Flow plots showing CXCR5 and CD69 co-expression on blood, LN, and TDL memory CD8+ T cells in HIV+ subjects (matched for blood and LNs, but not for TDL). Frequency of CXCR5 expression on memory CD8+ T cells in blood, LNs (red: ART−; orange: ART+), and TDL. (H) Frequency of CD69 expression on blood, LN, and TDL CXCR5+ memory CD8+ T cells in HIV+ subjects. (I) Frequency of CCR7 expression on LN CD69+ CXCR5+ memory CD8+ T cells in HIV+ subjects. Bar plots are based on all subjects in Fig. 5A. (J) Representative flow cytometry plots showing CXCR5 and CD69 co-expression on blood, LN, and TDL HIV-tetramer+ CD8+ T cells (matched for blood and LNs, but not for TDL). (K) Frequency of CD69 expression on blood (n = 18 responses), LN (n = 21 responses), and TDL (n = 10 responses) HIV-specific CXCR5+ CD8+ T cells. (L) Frequency of CCR7 expression on LN (n = 21 responses) HIV-specific CD69+ CXCR5+ CD8+ T cells. (M) Frequency of CD69+ CXCR5+ cells among LN CMV-specific and HIV-specific CD8+ T cells in subjects with detectable tetramer+ responses to both viruses. Each dot represents a single tetramer+ response in all scatter plots. Each color represents a matched subject and a matched tetramer+ response. *P < 0.05, **P < 0.01; ***P < 0.001. Conventional flow cytometry was used for all stains in this figure.
In line with our previous findings, most HIV-specific CD69+ CD8+ T cells (median = 84.1%) did not express CCR7 (Fig. 5B), similar to bulk memory CD8+ T cells (Fig. S7A). Moreover, HIV-specific CD8+ T cells in LNs demonstrated a predominant CD69+ CD103+ or CD69+ CD103− profile, in contrast to HIV-specific CD8+ T cells in blood and TDL (Fig. 5C). We further examined cytomegalovirus (CMV)-specific CD8+ T cells for markers of residency, because CMV does not replicate primarily in LTs (42, 43). In subjects with detectable CMV-specific and HIV-specific CD8+ T cell responses, we consistently found that HIV-specific CD8+ T cells were present in blood and LNs, while CMV-specific CD8+ T cells were always more prevalent in blood compared with LNs (Fig. 5D) (44). HIV-specific CD8+ T cells also expressed higher levels of CD69 and CD103 than CMV-specific CD8+ T cells in LNs (Fig. 5E). In addition, HIV-specific CD69+ CD8+ T cells were more likely to express cytolytic proteins (perforin and/or Granzyme B) than HIV-specific CD69− CD8+ T cells (Fig. 5F). The distribution of cytolytic proteins was similar on CD69+ bulk memory and HIV-specific CD8+ T cells (Fig. S7A), indicating that many characteristics of CD69+ bulk and HIV-specific CD8+ T cells are indistinguishable.
Virus-specific CXCR5+ CD8+ T cells have recently been shown to play a key role in immune protection against chronic viral infections (45–48). However, it is unclear if human CXCR5+ CD8+ T cells exhibit TRM properties akin to TFHs (49). In HIV+ subjects, we observed higher frequencies of CXCR5+ CD8+ T cells in LNs compared with blood and TDL (Fig. 5G). The majority of CXCR5+ CD8+ T cells in LNs co-expressed CD69 and lacked CCR7 (Fig. 5H and5I). Similarly, we found very low levels of HIV-specific CXCR5+ CD8+ T cells in peripheral blood and TDL compared with LTs (Fig. 5J), likely because the majority of LT HIV-specific CXCR5+ CD8+ T cells displayed a residency phenotype, including high levels of CD69 (Fig. 5K) and low levels of CCR7 (Fig. 5L). In addition, we found that LT HIV-specific CD8+ T cells more commonly expressed a CD69+ CXCR5+ phenotype compared with LT CMV-specific CD8+ T cells (Fig. 5M). These data suggest that HIV-specific CXCR5+ are TRMs and induced due to preferential localization of HIV in B cell follicles.
CD69 expression increase on SIV-specific CD8+ T cells over time
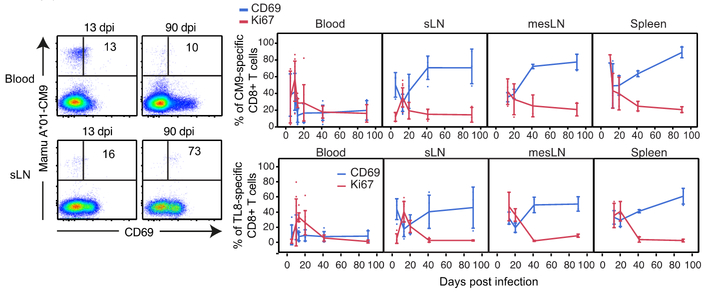
To assess the kinetics of TRM formation, we infected Mamu A*01+ rhesus macaques with SIVmac251, and collected blood and LTs over time. Peripheral blood SIV-specific CD8+ T cells displayed a pronounced transient burst of Ki67 and CD69 expression at d10–14 post-infection (Fig. 6 and S7B). In contrast, LT SIV-specific CD8+ T cells showed an inverse association between Ki67 and CD69 over the same time period, indicating that the majority of early cycling cells were not retained in LTs. After resolution of the acute phase, cycling decreased and CD69 expression steadily increased on LT SIV-specific CD8+ T cells (Fig. 6), suggesting that residency is established late and that CD69 expression is most likely only downregulated after extensive cycling in response to antigen (50).
Fig. 6. SIV-specific CD8+ T cells develop TRM characteristics over time.
Representative flow cytometry plots showing CD69 expression on blood (top) and superficial LN (sLN; bottom) SIV-specific (Mamu A*01-Gag-CM9) CD8+ T cells at 13 and 90 days post-infection (dpi; left). Longitudinal dynamics of CD69 (blue) and Ki-67 (red) expression on/in blood, sLN, MesLN, and spleen SIV-CM9 or TL9-specific CD8+ T cells (13 dpi: n = 12; 90 dpi: n = 3). The longitudinal SIV-CM9 or TL9-specific CD8+ T cell response is plotted at each time point as mean ± SD. *P < 0.05, **P < 0.01; ***P < 0.001. Conventional flow cytometry was used for all stains in this figure.
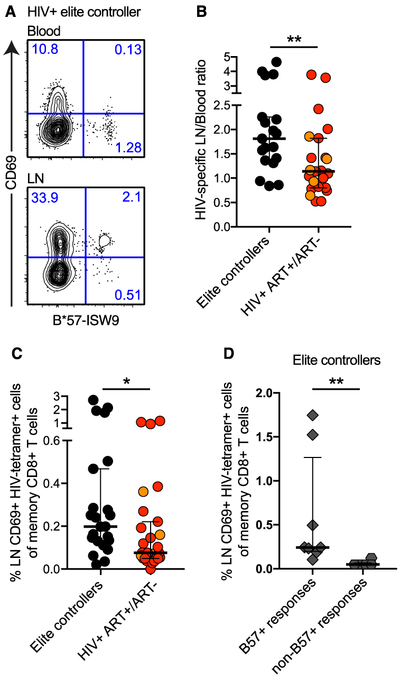
Elite controllers demonstrate high magnitudes of LN HIV-specific TRMs
Recent work has shown that LT CD8+ T cells suppress viral replication in SIV elite controller rhesus macaques (7). In human elite controllers, we found that the majority of LN HIV-specific CD8+ T cells displayed a TRM phenotype (Fig. 7A). Human elite controllers also displayed higher LN/blood ratios of HIV-specific CD8+ T cells (Fig. 7B), and higher frequencies of HIV-tetramer+ CD69+ CD8+ T cells (Fig. 7C), compared with other HIV+ subjects (Fig. S7C). In HLA-B*5701+ elite controllers, we found that TRMs selectively targeted immunodominant HLA-B*5701-restricted epitopes, rather than immunodominant non-HLA-B*5701-restricted epitopes (Fig. 7D).
Fig. 7. Elite controllers exhibit high magnitudes of CD69+ HIV-specific CD8+ T cells in LNs.
(A) Representative flow cytometry plots of memory CD8+ T cells showing CD69 expression on HIV-tetramer+ cells in matched blood and LN samples from an HIV elite controller (EC). (B) Relative distribution of HIV-specific CD8+ T cells in blood and LNs from elite controllers (black) and other HIV+ subjects (red: ART−; orange: ART+). (C) Magnitude of LN HIV-tetramer+ CD69+ CD8+ T cell responses in elite controllers and other HIV+ subjects (red: ART−; orange: ART+). (D) Magnitude of LN HIV-tetramer+ CD69+ CD8+ T cell responses specific for immunodominant HLA-B*5701-restricted and non-HLA-B*5701-restricted epitopes in elite controllers (n = 2). Data points were only included where both HLA-B*5701+ and HLA-B*5701− tetramers were tested to avoid analysis biases. Each dot represents a single tetramer+ response in all scatter plots. *P < 0.05; **P < 0.01. Conventional flow cytometry was used for all stains in this figure.
HIV-specific TRMs demonstrate enriched effector-related immune genes and signatures compared to HIV-specific non-TRMs in LNs
We next index sorted HLA-B*2705-restricted and/or HLA-B*5701-restricted Gag-specific CD8+ T cells from LNs and blood of two elite controllers and conducted single-cell RNA-seq (Fig. S8A). HIV-specific CD8+ T cells from LN and blood clustered differentially in the single-cell space (Fig. S8B and Table S4). Immune-related pathways, including IFN-signatures, were higher in blood, whereas pathways related to maintenance of cell location were higher in LNs, suggesting that both immunological and trafficking properties distinguish the LN response from blood (Fig. S8C). We next compared blood versus LN CD69− HIV-specific CD8+ T cells and found that specific cytolytic genes and pathways involved in lymphocyte degranulation were higher in blood (Fig. S8D). Cytolytic differences at the transcript level were not seen between blood and LN CD69+ HIV-specific CD8+ T cells (Fig. S8F). Finally, to assess if residency was associated with any specific functional gene signatures, we compared CD69− versus CD69+ HIV-specific CD8+ T cells in LNs (Fig. 8A). LN CD69+ HIV-specific CD8+ T cells were enriched for cytolytic and effector-differentiated genes (Fig. 8A) and gene set signatures (Fig. S9A), while LN CD69− HIV-specific CD8+ T cells demonstrated a less-differentiated phenotype instead (Fig. 8A and S9A). Together these findings support the concept that HIV-specific TRMs represent the frontline effector defense in LTs.
Fig. 8. Distribution of HIV-specific clonotypes between LN and blood.
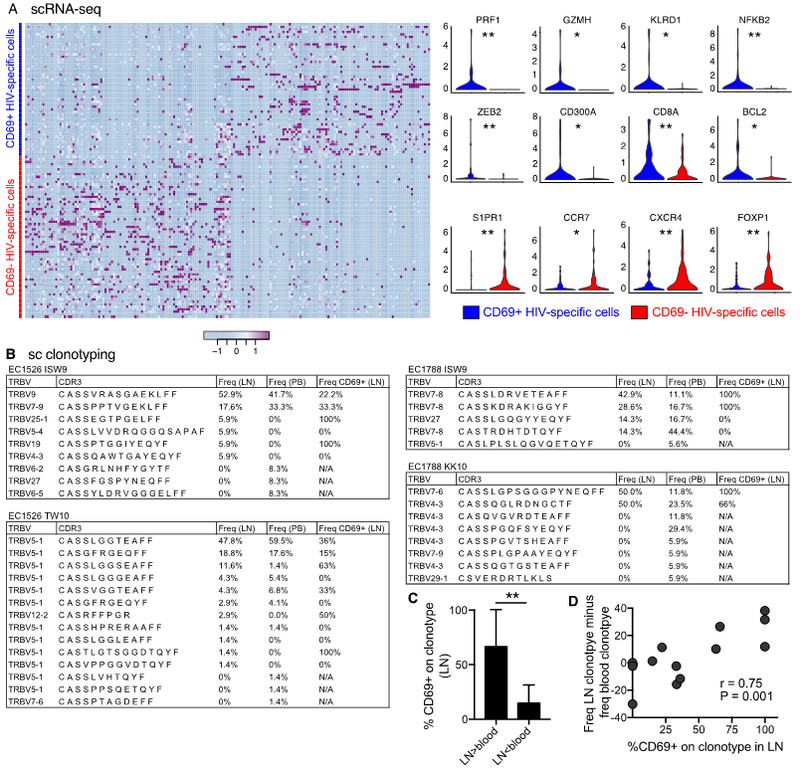
(A) Single-cell (sc) RNA-seq was conducted on CD69+ and CD69− index sorted HLA-B*2705-restricted and/or HLA-B*5701-restricted Gag-specific CD8+ T cells from LNs of two elite controllers. The heat-map (left) illustrates the single-cell gene expression variability of differentially expressed genes (P < 0.01) and violin plots (right) immune-related genes differentially expressed between CD69+ and CD69− single HIV-specific CD8+ T cells. (B) CDR3 amino acid sequence, and percent frequency are shown for each HLA-B*2705-restricted and HLA-B*5701-restricted clonotype. The frequency of CD69 expression for each clonotype was obtained from single-cell index data (right). (C) CD69 expression on LN clonotypes where higher frequencies of specific clonotypes are present in LNs versus blood (LN > blood; n = 7) or vice versa (LN < blood; n = 7). Bars show mean ± SD. (D) Correlation between CD69 expression on LN clonotypes and the distribution of clonotypes between LNs and blood (frequency of a given clonotype in LNs minus the corresponding frequency in blood). Data points were only included where at least one clonotype was present in both compartments. *P < 0.05; **P < 0.01. Conventional flow cytometry was used for all stains in this figure.
CD69 expression on HIV-specific clonotypes is associated with higher clonotype distribution in LN
Based upon the enriched effector signature of CD69+ HIV-specific TRMs in LT, we finally examined whether these cells exhibited differential clonotypic segregation between blood and LT. Subject EC1526 expressed lower levels of CD69 on LN HIV-specific CD8+ T cells (Fig. S9B), and displayed a mixed clonotype distribution between blood and LNs (Fig. 8B). In contrast, subject EC1788 expressed higher levels of CD69 on LN HIV-specific CD8+ T cells (Fig. S9B), and displayed marked repertoire skewing between blood and LNs. Clonotypes present at high frequencies in LNs relative to blood expressed high levels of CD69 in LNs (Fig. 8C). Moreover, the frequency of CD69 expression on clonotypes in LNs was associated with the relative frequency of clonotypes in LNs (Fig. 8D). Together these data show that HIV-specific CD69− CD8+ T cell clonotypes present in blood can also be found in LNs, while HIV-specific CD69+ CD8+ TRM clonotypes with effector-like functional signatures preferentially localize to LNs.
DISCUSSION
TRMs provide constant immunosurveillance and early protection against secondary challenge in NLTs. Here, we show that CD8+ T cells with a TRM phenotype also accumulate in LTs and dominate the LT CD8+ T cell response against HIV. Our key findings were: (i) CD69+ CD8+ T cells are highly expanded in HIV-infected LNs; (ii) LT CD69+ CD8+ T cells are largely not recently activated, based on Ki-67 expression, and instead bear transcriptional and epigenetic signatures of TRM cells distinct from non-resident T cells in LTs; (iii) LT CD69+ TRMs do not recirculate to the blood via the thoracic duct; and (iv) LT CD69+ TRMs most often dominate the HIV-specific CD8+ T cell response in LNs. Moreover, we found high magnitudes of LT HIV-specific TRMs in elite controllers and demonstrate that CD69 expression is closely linked to the distribution of HIV-specific clonotypes between LN and blood. Collectively, these data inform our current understanding of immunosurveillance in LTs and define that ongoing CD8+ T cell-mediated immunity against HIV in LTs is in part mediated by TRMs.
Previous studies have identified bona fide LT CD8+ TRMs in mice (18), but generally at low levels (19), especially compared with the frequencies of human LT CD69+ CD8+ T cells recently described by Farber and colleagues (20, 21). These human studies have generated some controversy, because CD69 expression may occur as a consequence of brain death-induced immune activation (33). Our data show that high frequencies of non-cycling CD69+ CD8+ T cells are indeed generally present in multiple human LTs, and further show that LN CD69+ CD8+ T cells share many of the core transcriptomic signatures of NLT TRMs (17, 35, 37). A recent study confirmed that human LT and NLT CD69+ T cells share several prototypical genes of our LN TRM signature, such as lower levels of S1pr1, Klf3, and Sell, and pathways that are involved in immune defense and lymphocyte migration (51). However, the core signature from Kumar et al, was extracted from both CD4+ and CD8+ T cells and a blood-contaminated lymphoid organ (spleen), whereas we solely focused on LN CD8+ T cells from live subjects. As such, specific genes that were higher on CD69− (e.g. Cx3cr1) or CD69+ (e.g. Pdcd1, Il10, Il2) T cells were not seen in our data set (51). Notably, similar to this (51) and other studies in the human TRM field, we do not find an association between residency in human LTs and the murine TRM-associated transcription factor Hobit (37, 52). We also report the epigenetic structure of human LT CD8+ TRMs; clear epigenetic differences were apparent between the proximal regulatory regions of many genes in CD8+ TRMs versus non-TRMs, including genes associated with cellular motility, migration, adhesion, and function. These data provide evidence that TRMs have a unique epigenetic imprinting for genes that are involved in residency and also aids to explain that certain genes are higher expressed in TRMs. These results strongly support the notion that LT CD69+ cells are enriched for TRMs.
Our data indicate that CD69 can be expressed on CCR7+ cells, including naïve-like cells, whereas CD69+CCR7− cells usually represent bona fide TRMs in both NLTs and LTs in mice. We cannot formally exclude the possibility that CCR7+CD69+ could modulate CD69 to exit LTs. Likewise, it remains unclear whether also CD103+CD69−CCR7+ CD8+ T cells originate from CD8+ TRMs in LTs that modulate expression of CD69 and CCR7, or if these cells represent a recirculating CD8+ T cell memory population transiently observed both in LT and efferent lymph. Indeed, previous studies in KAEDE mice have identified CD103+CD69−CCR7+ migrating CD4+ T cells in both draining lymph nodes and blood originating from the skin after photo-activation that share the same phenotype we observed in both LT and TDL CD8+ T cells (53). One possible mechanism by which T cells can downregulate CD69 is through extensive cycling, leading to increased KLF2 and S1PR1 expression (50). This concept aligns with our acute SIV infection data, which show that CD69+ CD8+ T cell frequencies decline dramatically in LTs when antigen-specific T cells reach a cycling plateau, however then accumulate progressively as LT SIV-specific CD8+ T cells no longer cycle. It remains unclear whether these kinetic changes reflect homeostatic expansion of the original population or the conversion of recirculating cells into TRMs. However, if all LT CD8+ TRMs were free to transiently modulate CD69 and equally recirculate, it would be difficult to explain the observed clonotypic disequilibrium between LNs and blood in subjects with prominent HIV-specific CD8+ CD69+ responses. Importantly, clonotypic disequilibrium has also been observed in LTs with high levels of CD4+ TRMs, such as in Peyer’s patches (54). Coupled with the lack of CD69+ cells in the thoracic duct and extensive expansion of CD69+CCR7− cells in HIV infection, these data suggest that HIV-specific CD8+ T cells, which mostly bear the CD69+CCR7− phenotype, become resident as a consequence of local antigen exposure in LTs.
In light of the fact that CD8+ TRMs are abundant in all human LTs, it seems plausible that these cells serve as regional sentinels that scan all antigen-presenting cells originating from areas that drain to their respective LNs. CD8+ TRMs may therefore acquire unique population-level specificities as a function of pathogen exposure history within different LNs. There are estimated to be at least 600 distinct LNs/LTs in the human body (55), without necessarily including ectopic/tertiary lymphoid structures at mucosal sites (56) that can number in the thousands in the large intestine alone (57). HIV replicates actively in all of these tissues. Accordingly, HIV-specific CD8+ T cell responses must be deployed to all LT sites at all times to maintain overall control of viremia in HIV+ subjects. It should be noted in this context that we only compared the clonal distribution of HIV-specific CD8+ T cells between peripheral blood and a single LT site. In previous studies of peripheral blood, we showed that individual CD8+ T cell clonotypes specific for the same viral antigen can have distinct functional and phenotypic properties (58). The observation that residency status directly influences the anatomical distribution of HIV-specific CD8+ T cell clonotypes therefore has far-reaching implications for our understanding of protective immune responses against HIV. First, our data indicate that HIV-specific CD8+ T cells in blood do not necessarily parallel HIV-specific CD8+ T cells in LTs. Second, there may be distinct HIV-specific CD8+ TRM populations and clonotypes between different LTs, which could impart differential immune pressure on viral replication at each site. Third, it becomes clear that in future studies we need to further explore the functional abilities and potential deficiencies of LT HIV-specific TRMs, because these cells exist in continuous proximity to HIV-infected CD4+ T cells and likely spearhead the cellular immune response against HIV. This view is supported by the observation that elite controllers maintain high levels of HIV-specific TRMs with enhanced effector capabilities, despite low levels of viral replication in LTs.
In summary, our data indicate that CD8+ TRMs likely play a central role in immunosurveillance against HIV. Further studies are now warranted to examine the precise mechanisms through which LT CD8+ TRMs control HIV replication, potentially informing new approaches to the development of vaccines or immunotherapeutic strategies designed to eliminate the viral reservoir in established HIV infection.
MATERIAL AND METHODS
Study design
The present study was an exploratory study aimed at understanding if TRMs are present in HIV-infected LNs and part of HIV-specific immunosurveillance mechanisms. Recruitment of human samples occurred at four sites: INER-CIENI, Case Western Reserve University, University of Pennsylvania and University of California, San Francisco. C57BL/6J (B6) mice were housed in specific pathogen-free conditions at the University of Minnesota. Rhesus macaque samples were obtained from primates housed at the University of Pennsylvania and Yerkes National Primate Research Center. Human samples were collected in accordance to the Declaration of Helsinki and study protocols were approved by the regional review boards. Animals were housed in accordance to regional ethical guidelines and protocols were approved by the regional review boards. Sample sizes were based on the availability of biological samples rather than a pre-specified effect size. Investigators were not blinded to group identity during the course of experimentation.
Supplementary Material
Supplementary Materials and Methods.
Fig. S1. Maturation of and cycling of LN CD8+ T cells
Fig. S2. CD69 expression on naïve CD8+ T cells and immune activation profile of CD69– and CD69+ CD8+ T cells in LTs
Fig. S3. Phenotypic characteristics of memory CD8+ T cells in blood and LTs
Fig. S4. Residency characteristics of CD69– and CD69+ CD8+ T cells
Fig. S5. RNA-seq, ATAC-seq, and CCR7 analysis of LN TRMs
Fig. S6. Imaging analysis and CD103 expression in TDL
Fig S8. Index sorting strategy and scRNA-seq analysis
Fig S9. scRNA-seq analysis of LN HIV-specific CD8+ T cells
Table S1. Cohort characteristics
Table S2. List of significant genes up-/downregulated from RNA-seq
Table S3. List of significant ATAC-seq tracks
Table S4. List of differentially expressed genes between blood and LN HIV-specific CD8+ T cells from scRNA-seq
Acknowledgments:
We express our gratitude to all study participants.
Funding: This research was supported by R01/R56 grants from the National Institutes of Health (AI076066, AI118694, and AI106481 to M.R.B.), the Martin Delaney Collaboratory: Towards an HIV-1 Cure (BEAT: AI126620; DARE: AI096109, A127966), the Penn CFAR (AI045008), the amfAR Institute for HIV Cure Research (amfAR 109301 to S.G.D.), the UCSF/Gladstone Institute of Virology & Immunology CFAR to the SCOPE cohort (P30 AI027763 to S.G.D.), and the Intramural Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (D.C.D). M.B. is funded through the Swedish Research Council (Dnr 537–2014-6829), Karolinska Institutet, Magnus Bergvall and Lars Hiertas Stiftelse. D.A.P. is a Welcome Trust Senior Investigator. D.D.A. and L.F.S. were supported by a Medical Research Grant from the W. W. Smith Charitable Trust Foundation and the Penn CFAR Grant. R.S.H. by K08 grant (AI114852). G.R.T. is funded by the Mexican Government (Comisión de Equidad y Género de las legislaturas LX-LXI, y Comisión de Igualdad de Género de la Legislatura LXII de la H. Cámara de Diputados de la República Mexicana).
Footnotes
Competing interests: The authors declare that they have no competing financial interests, patents, patent applications, or material transfer agreements associated with this study.
Data and materials availability: The sequence data sets reported in this paper have been deposited in the Gene Expression Omnibus with accession no. GSE110684.
REFERENCES
- 1.Migueles SA, Connors M, Success and failure of the cellular immune response against HIV-1. Nat Immunol 16, 563–570 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Estes JD, Pathobiology of HIV/SIV-associated changes in secondary lymphoid tissues. Immunol Rev 254, 65–77 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banga R, Procopio FA, Noto A, Pollakis G, Cavassini M, Ohmiti K, Corpataux JM, de Leval L, Pantaleo G, Perreau M, PD-1(+) and follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. Nat Med 22, 754–761 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Folkvord JM, Armon C, Connick E, Lymphoid follicles are sites of heightened human immunodeficiency virus type 1 (HIV-1) replication and reduced antiretroviral effector mechanisms. AIDS Res Hum Retroviruses 21, 363–370 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Perreau M, Savoye AL, De Crignis E, Corpataux JM, Cubas R, Haddad EK, De Leval L, Graziosi C, Pantaleo G, Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med 210, 143–156 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukazawa Y, Park H, Cameron MJ, Lefebvre F, Lum R, Coombes N, Mahyari E, Hagen SI, Bae JY, Reyes MD 3rd, Swanson T, Legasse AW, Sylwester A, Hansen SG, Smith AT, Stafova P, Shoemaker R, Li Y, Oswald K, Axthelm MK, McDermott A, Ferrari G, Montefiori DC, Edlefsen PT, Piatak M Jr., Lifson JD, Sekaly RP, Picker LJ, Lymph node T cell responses predict the efficacy of live attenuated SIV vaccines. Nat Med 18, 1673–1681 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukazawa Y, Lum R, Okoye AA, Park H, Matsuda K, Bae JY, Hagen SI, Shoemaker R, Deleage C, Lucero C, Morcock D, Swanson T, Legasse AW, Axthelm MK, Hesselgesser J, Geleziunas R, Hirsch VM, Edlefsen PT, Piatak M Jr., Estes JD, Lifson JD, Picker LJ, B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med 21, 132–139 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gowans JL, The recirculation of lymphocytes from blood to lymph in the rat. J Physiol 146, 54–69 (1959). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A, Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999). [DOI] [PubMed] [Google Scholar]
- 10.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR, Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol 10, 524–530 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, Wang J, Casey KA, Barber DL, Kawamura KS, Fraser KA, Webby RJ, Brinkmann V, Butcher EC, Newell KA, Ahmed R, Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med 207, 553–564 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masopust D, Vezys V, Marzo AL, Lefrancois L, Preferential localization of effector memory cells in nonlymphoid tissue. Science 291, 2413–2417 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR, Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science 319, 198–202 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R, Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J Immunol 176, 2079–2083 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M, CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 440, 540–544 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Mackay LK, Wynne-Jones E, Freestone D, Pellicci DG, Mielke LA, Newman DM, Braun A, Masson F, Kallies A, Belz GT, Carbone FR, T-box Transcription Factors Combine with the Cytokines TGF-beta and IL-15 to Control Tissue-Resident Memory T Cell Fate. Immunity 43, 1101–1111 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, Tscharke DC, Heath WR, Inouye M, Carbone FR, Gebhardt T, The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol 14, 1294–1301 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Schenkel JM, Fraser KA, Masopust D, Cutting edge: resident memory CD8 T cells occupy frontline niches in secondary lymphoid organs. J Immunol 192, 2961–2964 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinert EM, Schenkel JM, Fraser KA, Beura LK, Manlove LS, Igyarto BZ, Southern PJ, Masopust D, Quantifying Memory CD8 T Cells Reveals Regionalization of Immunosurveillance. Cell 161, 737–749 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJ, Bickham KL, Lerner H, Goldstein M, Sykes M, Kato T, Farber DL, Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity 38, 187–197 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thome JJ, Yudanin N, Ohmura Y, Kubota M, Grinshpun B, Sathaliyawala T, Kato T, Lerner H, Shen Y, Farber DL, Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell 159, 814–828 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woon HG, Braun A, Li J, Smith C, Edwards J, Sierro F, Feng CG, Khanna R, Elliot M, Bell A, Hislop AD, Tangye SG, Rickinson AB, Gebhardt T, Britton WJ, Palendira U, Compartmentalization of Total and Virus-Specific Tissue-Resident Memory CD8+ T Cells in Human Lymphoid Organs. PLoS Pathog 12, e1005799 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altfeld M, van Lunzen J, Frahm N, Yu XG, Schneider C, Eldridge RL, Feeney ME, Meyer-Olson D, Stellbrink HJ, Walker BD, Expansion of pre-existing, lymph node-localized CD8+ T cells during supervised treatment interruptions in chronic HIV-1 infection. J Clin Invest 109, 837–843 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Connick E, Mattila T, Folkvord JM, Schlichtemeier R, Meditz AL, Ray MG, McCarter MD, Mawhinney S, Hage A, White C, Skinner PJ, CTL fail to accumulate at sites of HIV-1 replication in lymphoid tissue. J Immunol 178, 6975–6983 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Buggert M, Tauriainen J, Yamamoto T, Frederiksen J, Ivarsson MA, Michaelsson J, Lund O, Hejdeman B, Jansson M, Sonnerborg A, Koup RA, Betts MR, Karlsson AC, T-bet and Eomes are differentially linked to the exhausted phenotype of CD8+ T cells in HIV infection. PLoS Pathog 10, e1004251 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Champagne P, Ogg GS, King AS, Knabenhans C, Ellefsen K, Nobile M, Appay V, Rizzardi GP, Fleury S, Lipp M, Forster R, Rowland-Jones S, Sekaly RP, McMichael AJ, Pantaleo G, Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature 410, 106–111 (2001). [DOI] [PubMed] [Google Scholar]
- 27.Lederman MM, Margolis L, The lymph node in HIV pathogenesis. Semin Immunol 20, 187–195 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mudd JC, Murphy P, Manion M, Debernardo R, Hardacre J, Ammori J, Hardy GA, Harding CV, Mahabaleshwar GH, Jain MK, Jacobson JM, Brooks AD, Lewis S, Schacker TW, Anderson J, Haddad EK, Cubas RA, Rodriguez B, Sieg SF, Lederman MM, Impaired T-cell responses to sphingosine-1-phosphate in HIV-1 infected lymph nodes. Blood 121, 2914–2922 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chattopadhyay PK, Roederer M, Good cell, bad cell: flow cytometry reveals T-cell subsets important in HIV disease. Cytometry A 77, 614–622 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ndhlovu ZM, Kamya P, Mewalal N, Kloverpris HN, Nkosi T, Pretorius K, Laher F, Ogunshola F, Chopera D, Shekhar K, Ghebremichael M, Ismail N, Moodley A, Malik A, Leslie A, Goulder PJ, Buus S, Chakraborty A, Dong K, Ndung’u T, Walker BD, Magnitude and Kinetics of CD8+ T Cell Activation during Hyperacute HIV Infection Impact Viral Set Point. Immunity 43, 591–604 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, Wang E, Douek DC, Price DA, June CH, Marincola FM, Roederer M, Restifo NP, A human memory T cell subset with stem cell-like properties. Nat Med 17, 1290–1297 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schenkel JM, Masopust D, Tissue-resident memory T cells. Immunity 41, 886–897 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Floerchinger B, Yuan X, Jurisch A, Timsit MO, Ge X, Lee YL, Schmid C, Tullius SG, Inflammatory immune responses in a reproducible mouse brain death model. Transpl Immunol 27, 25–29 (2012). [DOI] [PubMed] [Google Scholar]
- 34.Clark RA, Watanabe R, Teague JE, Schlapbach C, Tawa MC, Adams N, Dorosario AA, Chaney KS, Cutler CS, Leboeuf NR, Carter JB, Fisher DC, Kupper TS, Skin effector memory T cells do not recirculate and provide immune protection in alemtuzumab-treated CTCL patients. Sci Transl Med 4, 117ra117 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan Y, Tian T, Park CO, Lofftus SY, Mei S, Liu X, Luo C, O’Malley JT, Gehad A, Teague JE, Divito SJ, Fuhlbrigge R, Puigserver P, Krueger JG, Hotamisligil GS, Clark RA, Kupper TS, Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature 543, 252–256 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mackay LK, Minnich M, Kragten NA, Liao Y, Nota B, Seillet C, Zaid A, Man K, Preston S, Freestone D, Braun A, Wynne-Jones E, Behr FM, Stark R, Pellicci DG, Godfrey DI, Belz GT, Pellegrini M, Gebhardt T, Busslinger M, Shi W, Carbone FR, van Lier RA, Kallies A, van Gisbergen KP, Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 352, 459–463 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Hombrink P, Helbig C, Backer RA, Piet B, Oja AE, Stark R, Brasser G, Jongejan A, Jonkers RE, Nota B, Basak O, Clevers HC, Moerland PD, Amsen D, van Lier RA, Programs for the persistence, vigilance and control of human CD8+ lung-resident memory T cells. Nat Immunol 17, 1467–1478 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Appay V, Bosio A, Lokan S, Wiencek Y, Biervert C, Kusters D, Devevre E, Speiser D, Romero P, Rufer N, Leyvraz S, Sensitive gene expression profiling of human T cell subsets reveals parallel post-thymic differentiation for CD4+ and CD8+ lineages. J Immunol 179, 7406–7414 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Gerner MY, Kastenmuller W, Ifrim I, Kabat J, Germain RN, Histo-cytometry: a method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity 37, 364–376 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buggert M, Frederiksen J, Noyan K, Svard J, Barqasho B, Sonnerborg A, Lund O, Nowak P, Karlsson AC, Multiparametric bioinformatics distinguish the CD4/CD8 ratio as a suitable laboratory predictor of combined T cell pathogenesis in HIV infection. J Immunol 192, 2099–2108 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Evans TG, Bonnez W, Soucier HR, Fitzgerald T, Gibbons DC, Reichman RC, Highly active antiretroviral therapy results in a decrease in CD8+ T cell activation and preferential reconstitution of the peripheral CD4+ T cell population with memory rather than naive cells. Antiviral Res 39, 163–173 (1998). [DOI] [PubMed] [Google Scholar]
- 42.Jarvis MA, Nelson JA, Human cytomegalovirus tropism for endothelial cells: not all endothelial cells are created equal. J Virol 81, 2095–2101 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Myerson D, Hackman RC, Nelson JA, Ward DC, McDougall JK, Widespread presence of histologically occult cytomegalovirus. Hum Pathol 15, 430–439 (1984). [DOI] [PubMed] [Google Scholar]
- 44.Ellefsen K, Harari A, Champagne P, Bart PA, Sekaly RP, Pantaleo G, Distribution and functional analysis of memory antiviral CD8 T cell responses in HIV-1 and cytomegalovirus infections. Eur J Immunol 32, 3756–3764 (2002). [DOI] [PubMed] [Google Scholar]
- 45.Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, Sharpe AH, Freeman GJ, Germain RN, Nakaya HI, Xue HH, Ahmed R, Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417–421 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li S, Folkvord JM, Rakasz EG, Abdelaal HM, Wagstaff RK, Kovacs KJ, Kim HO, Sawahata R, MaWhinney S, Masopust D, Connick E, Skinner PJ, Simian Immunodeficiency Virus-Producing Cells in Follicles Are Partially Suppressed by CD8+ Cells In Vivo. J Virol 90, 11168–11180 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mylvaganam GH, Rios D, Abdelaal HM, Iyer S, Tharp G, Mavinger M, Hicks S, Chahroudi A, Ahmed R, Bosinger SE, Williams IR, Skinner PJ, Velu V, Amara RR, Dynamics of SIV-specific CXCR5+ CD8 T cells during chronic SIV infection. Proc Natl Acad Sci U S A 114, 1976–1981 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reuter MA, Del Rio Estrada PM, Buggert M, Petrovas C, Ferrando-Martinez S, Nguyen S, Sada Japp A, Ablanedo-Terrazas Y, Rivero-Arrieta A, Kuri-Cervantes L, Gunzelman HM, Gostick E, Price DA, Koup RA, Naji A, Canaday DH, Reyes-Teran G, Betts MR, HIV-Specific CD8(+) T Cells Exhibit Reduced and Differentially Regulated Cytolytic Activity in Lymphoid Tissue. Cell Rep 21, 3458–3470 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee JY, Skon CN, Lee YJ, Oh S, Taylor JJ, Malhotra D, Jenkins MK, Rosenfeld MG, Hogquist KA, Jameson SC, The transcription factor KLF2 restrains CD4(+) T follicular helper cell differentiation. Immunity 42, 252–264 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rivera J, Proia RL, Olivera A, The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol 8, 753–763 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar BV, Ma W, Miron M, Granot T, Guyer RS, Carpenter DJ, Senda T, Sun X, Ho SH, Lerner H, Friedman AL, Shen Y, Farber DL, Human Tissue-Resident Memory T Cells Are Defined by Core Transcriptional and Functional Signatures in Lymphoid and Mucosal Sites. Cell Rep 20, 2921–2934 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pallett LJ, Davies J, Colbeck EJ, Robertson F, Hansi N, Easom NJW, Burton AR, Stegmann KA, Schurich A, Swadling L, Gill US, Male V, Luong T, Gander A, Davidson BR, Kennedy PTF, Maini MK, IL-2high tissue-resident T cells in the human liver: Sentinels for hepatotropic infection. J Exp Med 214, 1567–1580 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bromley SK, Yan S, Tomura M, Kanagawa O, Luster AD, Recirculating memory T cells are a unique subset of CD4+ T cells with a distinct phenotype and migratory pattern. J Immunol 190, 970–976 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ugur M, Schulz O, Menon MB, Krueger A, Pabst O, Resident CD4+ T cells accumulate in lymphoid organs after prolonged antigen exposure. Nat Commun 5, 4821 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Goroll AH, May LA, Mulley AG, Primary care medicine : office evaluation and management of the adult patient. (Lippincott, Philadelphia, ed. 2nd, 1987), pp. xxi, 1001 p. [Google Scholar]
- 56.Ruddle NH, High Endothelial Venules and Lymphatic Vessels in Tertiary Lymphoid Organs: Characteristics, Functions, and Regulation. Front Immunol 7, 491 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Langman JM, Rowland R, The number and distribution of lymphoid follicles in the human large intestine. J Anat 149, 189–194 (1986). [PMC free article] [PubMed] [Google Scholar]
- 58.Price DA, Brenchley JM, Ruff LE, Betts MR, Hill BJ, Roederer M, Koup RA, Migueles SA, Gostick E, Wooldridge L, Sewell AK, Connors M, Douek DC, Avidity for antigen shapes clonal dominance in CD8+ T cell populations specific for persistent DNA viruses. J Exp Med 202, 1349–1361 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials and Methods.
Fig. S1. Maturation of and cycling of LN CD8+ T cells
Fig. S2. CD69 expression on naïve CD8+ T cells and immune activation profile of CD69– and CD69+ CD8+ T cells in LTs
Fig. S3. Phenotypic characteristics of memory CD8+ T cells in blood and LTs
Fig. S4. Residency characteristics of CD69– and CD69+ CD8+ T cells
Fig. S5. RNA-seq, ATAC-seq, and CCR7 analysis of LN TRMs
Fig. S6. Imaging analysis and CD103 expression in TDL
Fig S8. Index sorting strategy and scRNA-seq analysis
Fig S9. scRNA-seq analysis of LN HIV-specific CD8+ T cells
Table S1. Cohort characteristics
Table S2. List of significant genes up-/downregulated from RNA-seq
Table S3. List of significant ATAC-seq tracks
Table S4. List of differentially expressed genes between blood and LN HIV-specific CD8+ T cells from scRNA-seq