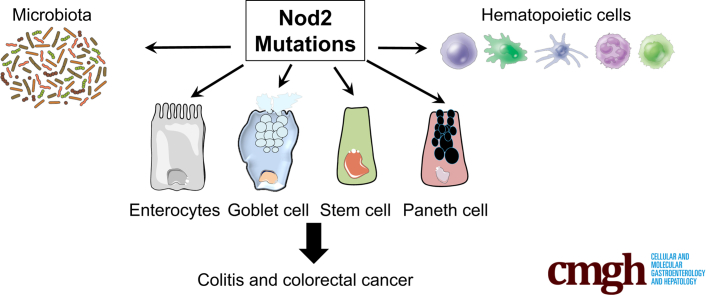
Abstract
Nucleotide-binding oligomerization domain 2 (NOD2) is an intracellular pattern recognition receptor that senses bacterial peptidoglycan-conserved motifs in cytosol and stimulates host immune response including epithelial and immune cells. The association of NOD2 mutations with a number of inflammatory pathologies including Crohn’s disease (CD), graft-versus-host diseases, or Blau syndrome, highlights its pivotal role in inflammatory response and the associated-carcinogenesis development. Since its identification in 2001 and its association with CD, the role of NOD2 in epithelial cells and immune cells has been investigated extensively but the precise mechanism by which NOD2 mutations lead to CD and the associated carcinogenesis development is largely unknown. In this review, we present and discuss recent developments about the role of NOD2 inside epithelial cells on the control of the inflammatory process and its linked carcinogenesis development.
Keywords: Nod2, Cancer, Colitis, Gut, Epithelial cells
Abbreviations used in this paper: AMP, antimicrobial peptide; CARD, caspase activation and recruitment domain; CD, Crohn’s disease; CEC, colonic epithelial cells; CRC, colorectal cancer; HD, human defensin; IFN, interferon; IRF4, interferon regulatory factor 4; KO, knockout; MAPK, mitogen-activated protein kinase; MDP, muramyl dipeptide; MLCK, myosin light-chain kinase; mRNA, messenger RNA; Muc, mucin; NF-κB, nuclear factor-κB; NOD2, nucleotide-binding oligomerization domain 2; PP, Peyer’s patch; TLR, Toll-like receptor; TNBS, trinitrobenzene sulfonic acid; TNF, tumor necrosis factor; WT, wild-type
Graphical abstract
Summary.
Although the association between Crohn’s disease susceptibility and nucleotide-binding oligomerization domain 2 polymorphisms was shown in 2001, the mechanisms involved remain largely unknown. In this review, we report the role of nucleotide-binding oligomerization domain 2 in epithelial cells in the development of colitis and associated carcinogenesis.
The gastrointestinal mucosa constitutes the largest interface of the human body between the external environment and the organism interior milieu. It establishes a dynamic barrier that excludes potentially harmful compounds (microbes, toxic ingested molecules) present in the intestinal lumen, while permitting sampling and absorption of the luminal content.1 To maintain this barrier, it is necessary to renew the gut epithelium continuously.
The surface of the intestine is composed of a columnar epithelial mucosa, within which the crypts are located. At the base of the crypts are the intestinal stem cells, ensuring the renewal of the epithelial lining by proliferating, giving rise to progenitor cells that differentiate into the 7 specialized lineages composing the intestinal epithelium: enterocytes, which represent the large majority of the epithelial cell population and allow absorption of nutrients and water; goblet cells, which secrete mucus as a protective barrier; Paneth cells, which are present only in the small intestine crypts, and secrete antimicrobial peptides (AMPs) and paracrine molecules, which participate in the stem cell niche; enteroendocrine cells, which secrete hormones; the newly identified tuft cells, which are thought to secrete prostaglandin precursors, with immunologic functions and interacting with the gut nervous system2; M cells (microfold cells), which have a pivotal role in antigen presentation from the luminal content to immune cells1; and, finally, cup cells, which are involved in the induction of immune response to luminal bacteria.3 In the intestinal mucosa, these different cell types interact together to form a continuous epithelium, isolating the luminal content from the internal milieu.4 This physical barrier is reinforced by the presence of a mucus wall, including mucins and AMPs, made by a coordinated secretion from the intestinal cells. The last element of the intestinal barrier is achieved by the microbiota, which limits the pathogen invasion.
In healthy people, interactions between the 3 compartments of the digestive mucosa, namely the immune system, the epithelial layer, and the microbiota, are characterized by a homeostatic state. A very large panel of human diseases, including burns, sepsis, inflammatory bowel disease, celiac disease, irritable bowel syndrome, intestinal ischemia, graft-versus-host disease, cirrhosis, graft rejection after small-bowel transplantation, food intolerance, allergy, malnutrition, rheumatoid arthritis, obesity, diabetes, and colorectal cancer, are linked to a loss of gut barrier homeostasis.
The nucleotide-binding oligomerization domain containing 2 (NOD2, also known as caspase activation and recruitment domain [CARD]15 and Nod-like receptor-C2) gene is a member of the evolutionarily conserved Nod-like receptors family, which sense components of the microbial cell wall. In the past decade, numerous studies have reported that Nod2 plays a pivotal role in the regulation of chronic inflammatory conditions.5 NOD2 polymorphisms were found to be associated with an increased risk of Crohn’s disease (CD)6 and colorectal cancer (CRC)7 since 2001 and 2004, respectively. The most commonly studied polymorphisms includes 2 missense mutations and a frameshift mutation, located within coding regions and affecting the function of NOD2 by altering its amino acid sequence. Although an abundant amount of literature since then has mainly confirmed the link between NOD2 polymorphisms and CD susceptibility, its association with different cancers, including gastric, colorectal, endometrial, breast, ovarian, and laryngeal, remains unclear by a lack of consensus between the different studies reported. Nevertheless, a recent meta-analysis has shown that NOD2 rs2066844 C/T, rs2066845 C/G, and rs2066847 (3020insC) polymorphisms are associated with an increased cancer risk, especially in regard to gastrointestinal cancer.8
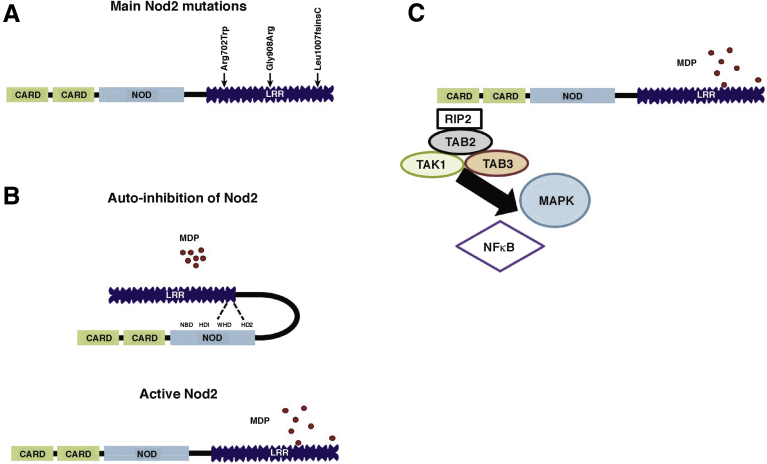
In the intestine, NOD2 is expressed by both hematopoietic9 and nonhematopoietic cells forming the intestinal epithelium.10, 11, 12, 13, 14 NOD2 senses the muramyl dipeptide (MDP), which arises from the partial degradation of a bacterial component (peptidoglycan).15 After stimulation by MDP, NOD2 promotes host defense through the production of cytokines,16, 17 chemokines,16 AMPs,18 mucins,12 and activation of both innate and adaptive immune responses. Under basal conditions, NOD2 protein, which has 3 domains including CARDs, NACHT (or NOD), and leucine-rich repeats (Figure 1), is auto-inhibited through the interaction between its different domains. The chaperone protein heat shock protein 90 is involved in this phenomenon.19 MDP interacts directly with the leucine-rich repeat domain, allowing activation of the NACHT domain and the interaction of the CARD domains with other CARD-containing proteins (Figure 1). As a result, activation of NOD2 by MDP triggers oligomerization of the receptors via their NOD domains and the recruitment of mediators needed to form a signaling complex named nodosome,20 enhancing nuclear factor-κB (NF-κB) and mitogen activated protein kinase (MAPK) pathways.21, 22, 23
Figure 1.
Structure and main intracellular pathway induced by NOD2. (A) NOD2 protein shows 3 domains including CARDs, NOD, and leucine-rich repeats (LRRs). Within the LRR region, ↓ indicates an amino acid change owing to a CD-associated polymorphism. (B) The NOD module contains a nucleotide-binding domain (NBD), a winged helix (WH), and 2 helix domains (HD1 and HD2). The interaction between NBD and WH is important to stabilize Nod2 in an inactive form, and is maintained by adenosine diphosphate-mediated packed conformation. Upon ligand binding, HD2 mediates conformational changes of the NBD, WH, and HD1 to allow adenosine diphosphate–adenosine triphosphate exchange, self-oligomerization, and downstream signaling. The effector CARD domains mediate intracellular signaling after interaction between the LRR domain and MDP. (C) NOD2 oligomerization induces a signaling complex named nodosome. NOD2 attracts receptor-interacting serine/threonine-protein kinase 2 (RIP2) via a CARD–CARD homotypic interaction followed by transforming growth factor β-activated kinase 1 (TAK1) and TAK1 binding protein 2 and 3 (TAB2 or 3). This complex induces the activation of both MAPKs and NF-κB pathways.
Since the discovery of its association with CD and CRC, the role of NOD2 in epithelial cells and immune cells has been studied extensively. In this review, we present and discuss recent developments about the role of NOD2 inside epithelial cells to control the inflammatory process and the linked carcinogenesis development.
Role of Nod2 in Epithelial Intestinal Cells
The integrity of the intestinal epithelium is maintained by the continual renewal of epithelial cells as a result of the accelerated division of crypt cells that migrate upward from the base of the crypts. Today, although NOD2 is known to be expressed by epithelial enterocytes,13, 14 goblet cells,12 Paneth cells,10 and intestinal stem cells,11 no information is available concerning enteroendocrine, tuft, cup, and M cells. The highest expression of NOD2 has been reported in Paneth cells and intestinal stem cells. This probably explains why it initially was described that crypt epithelial cells express approximately 85-fold more NOD2 messenger RNA (mRNA) than villus epithelial cells.24 However, no data are available regarding the level of NOD2 expression in human small vs large intestine.
Enterocytes
Enterocytes are the most numerous cells of the intestinal mucosa (Figure 2A). Although their main roles are to ensure the absorption of nutrients and water, they also have the ability to synthesize AMPs, cytokines, and chemokines to control the stability of the microbiota and to induce the immune response. Finally, by their abilities to form a ring of tight junctions at the apical side of cells, enterocyte cells play an important role in the regulation of the gut paracellular permeability. Since 2003, we have known that enterocyte cells express NOD2 mRNA and protein in human colonic carcinoma epithelial cell lines and primary intestinal epithelial cells.13, 14 After tumor necrosis factor-α (TNF-α) or interferon-γ (IFN-γ) stimulation, NOD2 mRNA and protein levels are increased in human colonic carcinoma.13, 14 Moreover, a synergism between TNF-α and IFN-γ strongly enhances NOD2 expression.14 This NOD2 overexpression is mediated by the NF-κB pathway.14 Two NF-κB binding sites are identified in the promoter of NOD2. The deletion of either site, or the overexpression of a NF-κB dominant negative, leads to reduced levels of TNF-α/IFN-γ.14 Furthermore, this increased expression of NOD2 is associated with a reduced number of viable internalized Salmonella typhimurium in human epithelial cell lines compared with epithelial cells weakly expressing NOD2.13 In the context of Campylobacter jejuni infection, only Nod2knockout (KO) mice show a higher intestinal commensal Escherichia coli load associated with higher levels of IL6, TNF-α, and IL18, and a reduced IL22 level. This excessive immune response is probably owing to a reduced number of proliferating cells involved in the renewal of the intestinal epithelium.25 Because Nod2 expression is inducible upon stimulation with bacterial products such as lipopolysaccharide,26 or after colonization of GF mice,27 it is possible that Nod2 expression might be induced by the simple contact of epithelial intestinal cells with nonpathogenic commensal bacterium as E coli K12. Indeed, in vitro analyses have shown that the simple contact with E coli K12 is sufficient to induce Nod2 mRNA and protein in human intestinal epithelial cells.28 However, this induction is not observed in human intestinal epithelial cells transfected with a plasmid encoding dominant-negative Toll-like receptor (TLR)-5. Furthermore, flagellin-negative E coli mutants failed to induce Nod2.28 Moreover, microbial metabolites such as butyrate allow the up-regulation of the NOD2 expression in the intestinal epithelial cells.29 Its expression in human colonic carcinoma cell line (T84) also is increased when cells are stimulated with a noninvasive E coli plus the hydrogen ionophore dinitrophenol, a disruptor of mitochondrial adenosine triphosphate synthesis.30 Furthermore, an increased internalization of bacteria by epithelia presenting dysfunctional mitochondria (treated with dinitrophenol) is potentiated in NOD2-/- cells.30 This uptake of bacteria is dependent on reactive oxygen species and MAPK, and the increased viable intracellular bacteria in NOD2-/- cells likely reflect a reduced ability to recognize and kill bacteria.30 Collectively, these studies indicate that Nod2 expression in enterocytes is regulated by commensal bacteria or microbial components.
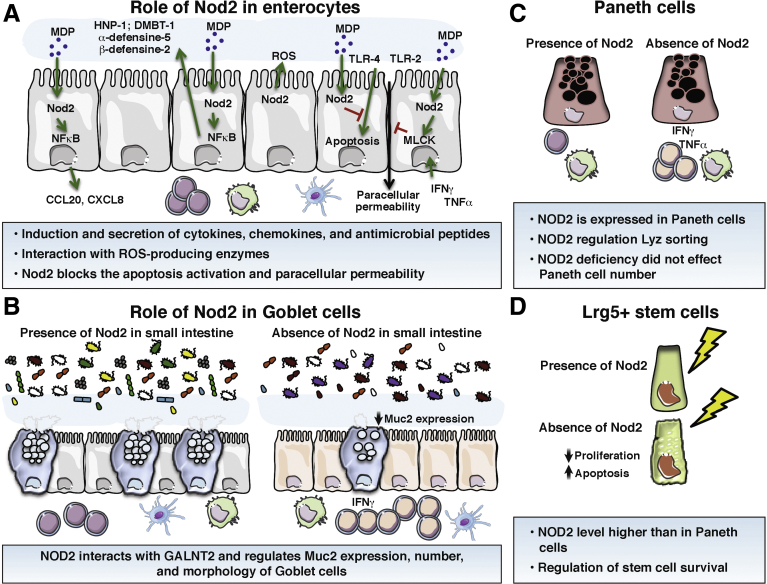
Figure 2.
Role of Nod2 in the homeostasis of the main cell types of the intestinal epithelium. (A) Enterocytes. Intestinal enterocyte cells express NOD2 and its expression is up-regulated by inflammatory cytokines. In response to MDP, intestinal epithelial cells synthesize AMPs, controlling the growth of pathogenic bacteria. This AMP secretion is lost in case of NOD2 mutation or deficiency. MDP stimulation also is able to induce the secretion of CXCL-8 to recruit neutrophil cells. Nod2 stimulation blocks the apoptosis induced by TLR-4 and the increased paracellular permeability induced by TLR-2, TLR-4, or by TNF-α and IFN-γ. (B) Goblet cells. A reduced mucin 2 expression as well as a diminished number of goblet cells have been described in the small intestine of Nod2KO mice. Nod2KO mice also showed fewer mucin granules per goblet cell than in WT mice. (C) Paneth cells. They express NOD2, and NOD2 expression is up-regulated in Paneth cells of CD patients. In these cells, NOD2 activation by MDP enhances the expression of α-human defensin-5 and -6 through induction of the NF-κB pathway. (D) Intestinal stem cells. In the gut, Lgr5+ stem cells strongly expressed Nod2. NOD2 stimulation by MDP promotes colonic epithelial cell growth and protection against apoptosis. NOD2 depletion results in a reduced ability to grow cells and increased levels of apoptosis by conferring cytoprotection against oxidative stress–mediated cell death. CCL20, Chemokine (C-C motif) ligand 20; CXCL-8, C-X-C motif chemokine ligand 8; DMBT-1, deleted in malignant brain tumors 1; GALNT2, Polypeptide N-Acetylgalactosaminyltransferase 2; HNP-1, Human Neutrophil peptide-1; Lyz, lysosome; ROS, reactive oxygen species.
The functional consequences of NOD2 stimulation by MDP are multiple. In response to MDP, intestinal epithelial cells synthesize and release at the apical side of AMPs such as C-C motif chemokine ligand 2028 or human neutrophil peptide,31 controlling the growth and/or survival of E coli and S typhimurium, respectively (Figure 1A). This AMP secretion is lost in NOD2 mutation (F3020insC) condition. MDP stimulation also induces secretion of chemokine (C-X-C motif) ligand-8 by intestinal epithelial cells, allowing the recruitment of neutrophils at the inflammatory site (Figure 1A). In the context of necrotizing enterocolitis, TLR-4 activation causes apoptosis in newborn intestine but not in adult mice.32 TLR-4 expression and activation in intestinal epithelial cells are described to be influenced by NOD2.33 Indeed, NOD2 activation inhibits TLR-4 expression and activation in enterocytes, but not in macrophages, and reverses the effects of TLR-4 on intestinal mucosal injury and repair33 (Figure 2A). Similar observations have been reported in the regulation of paracellular permeability of the intestinal mucosa, which is increased by TLR-2 or TLR-4 stimulations and normalized by Nod2 activation by MDP (Figure 3A).34 In an inflammatory context, excessive levels of TNF-α and IFN-γ are reported to increase the intestinal paracellular permeability involving an enhanced long myosin light-chain kinase (MLCK) expression and activity.35 MLCK overexpression induced by TNF-α and IFN-γ is mediated by an increased expression of the TNF receptor (TNF-R) 2 receptor, triggering an induction of the NF-κB pathway35 (Figure 2A). TNF-α, through the TNF-R1 receptor, have been shown recently to also increase NOD2 expression.35 Then, activation of NOD2 by MDP is able to suppress the overexpression of MLCK (Figure 2A).35 Together these data show a main role for NOD2 in enterocytes cells, to maintain the intestinal homeostasis by modulating secretion of AMPs, chemokines, and the paracellular permeability. This beneficial impact of NOD2 is lost in CD-linked gene mutations, which can impair the enterocyte homeostasis and favor the development and/or relapses of CD.
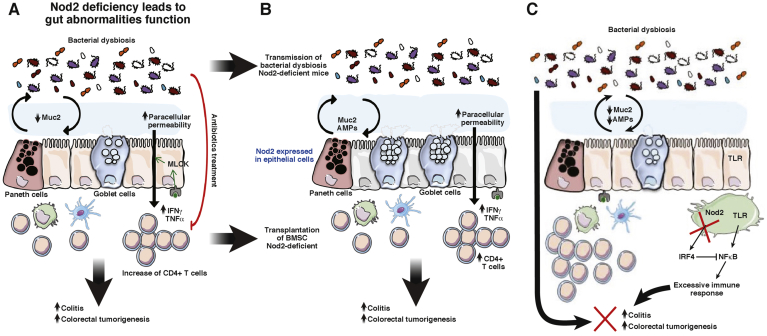
Figure 3.
Mechanisms by which Nod2, in epithelial cells, participates in the development of inflammation and inflammatory-associated carcinogenesis. (A) Nod2 deficiency or mutations have been described to alter gut epithelial permeability, AMP, and mucin expression; to increase CD4 T cells expressing proinflammatory cytokines; and to induce a microbial dysbiosis favoring the development of inflammation and CRC. Antibiotics suppressed the excessive permeability and the alteration of the immune compartment of Nod2KO mice. (B) Transfer of gut microbiota dysbiosis linked to Nod2 deficiency to WT mice alters the expression of both AMPs and mucins secreted by epithelial cells and increases colitis and colorectal cancer susceptibility. Transfer of bone marrow stem cells show that Nod2 expression in the hematopoietic compartment increases CD4 T cells and regulates gut epithelial permeability. However, bone marrow transfer does not change the gut microbiota, showing that Nod2 expression in the immune system plays a pivotal role in the regulation of colitis, independently of microbiota composition. (C) This notion was highlighted recently by the study by Udden et al,77 reporting the development of colonic inflammation and linked colorectal carcinogenesis in Nod2KO mice independently of gut microbiota dysbiosis but resulting from an absence of TLR down-regulation by Nod2.
Goblet Cells
Goblet cells, the second most numerous cells of the intestinal mucosa, are mainly involved in the production of the mucus layer (Figure 2B). This layer is composed of a dense, firmly attached inner layer and a removable outer layer overlying the epithelium. It promotes the elimination of gut contents and provides defense against injuries caused by ingested food, toxic particles, and microbes. Most of the commensal bacteria are trapped in the outer layer and then eliminated by peristaltic movements.36 The major components of the mucus layer are secreted glycoprotein mucins forming a gel at the surface of the epithelial cells.37 In addition to mucins, goblet cells synthesize AMPs such as trefoil factors, resistin-like molecule β, and Fc fragment of immunoglobulin G (IgG) binding protein.38 Synergism between mucins and AMPs is required to maintain the homeostasis of the mucus layer.37, 39 For example, trefoil factor 3 synergizes with secreted mucin 2 to enhance the protective properties of the mucus layer by increasing its viscosity.40 Resistin-like molecule β is known to increase the expression of some mucins.41 Recently, reduced mRNA mucin 2 (Muc2) expression as well as diminished numbers of Muc2-positive cells and goblet cells per villi were described in the small intestine of Nod2KO mice (Figure 2B).12 Furthermore, Nod2KO mice show fewer mucin granules per goblet cell, and many of these granules showed an abnormal fused appearance that was barely detected in wild-type (WT) mice (Figure 2B).12 By using chimeric mice for Nod2 expression, it has been shown that Nod2 deficiency in the hematopoietic compartment is sufficient to reduce the goblet cell number per villi.12 These goblet cell abnormalities are dependent on the expansion of Bacteroides vulgatus, a common member of the gut microbiota, exacerbating the proinflammatory status of the intestinal mucosa of Nod2KO mice.12 However, the reduced mRNA and protein expression of Muc2 as well as the goblet cell numbers in the small intestine of Nod2KO mice were not observed in other studies.42, 43 This could be explained by an absence of B vulgatus.42, 43 Nevertheless, although mRNA expression of Muc2 is similar between WT and Nod2KO mice, a reduced goblet cell number has been observed in colonic mucosa of Nod2KO mice.42 In addition, mRNA and protein expressions of Muc2 are not altered in chimeric mice that do not express Nod2 in their hematopoietic compartments.43 Finally, treatment with either Nod1 or Nod2 agonist did not modify the number of periodic acid–Schiff–stained goblet cells and Muc2-expressing cells at the colonic level in WT mice.44 However, simultaneous treatment of WT mice with Nod1 and Nod2 agonists increase the number of goblet cells.44 Moreover, Nod1 and 2 agonists up-regulate the β1,3-N-acetylglucosaminyltransferase, an important enzyme involved in the synthesis of mucin 2.44
In conclusion, these data support a role for Nod2 in goblet cells to maintain intestinal homeostasis by modulating mucin secretion. This beneficial impact of Nod2 is lost in case of deletion, which could impair the mucus homeostasis and favor the development and/or relapse of CD.
Paneth Cells
Paneth cells are secretory epithelial cells that reside at the base of the small intestinal crypts, in close proximity to intestinal stem cells (Figure 2C). These cells express a collection of antimicrobial substances, stored in cytoplasmic granules, which are released into the crypt lumen,45 and they also secrete molecules for the stem cell environment. Paneth cells, by secreting AMPs, are key players of the innate mucosal immunity to maintain the intestinal homeostasis between a host and its colonizing microbes.46 In human Paneth cells, the most expressed AMPs are as follows: α-defensin 5 and 6 (human defensin [HD]5 and HD6), lysozyme, and the secretory phospholipase A2.45 These AMPs have not only an antibacterial function against gram-positive and gram-negative bacteria, but also show antimicrobial activity against viruses, fungi, and protozoa.46, 47 Given their important involvement within the intestinal crypts, several studies have linked defective Paneth cells to CD pathogenesis. Expression of α-defensins has been shown to be diminished in CD patients, correlating with altered gut microbiota.48 Furthermore, CD patients with ATG16L1 mutations show abnormal Paneth cell morphology characterized by malformed disordered granules.49 Mice carrying this mutation and mice expressing hypomorphic Atg16l1 alleles have similar malformed disordered granules in Paneth cells, which is associated with an increased risk of intestinal inflammation.50 Finally, it recently was shown that endoplasmic reticulum stress exacerbated by autophagy dysfunction, more specifically within Paneth cells, initiates gut inflammation.51
NOD2 gene mutations initially described to affect Paneth cell function are strongly linked to CD.52 Paneth cells actually express NOD2, and this expression is up-regulated in CD patients.24 Moreover, exposure of isolated intestinal crypts to MDP induced a release of granules from Paneth cells into the crypt lumen.53 Likewise, Nod2 stimulation has been reported to play a role in lysozyme secretion by Paneth cells within the crypt via the Receptor-interacting serine/threonine-protein kinase 2 pathway.54, 55 Thus, because CD mutations in the NOD2 gene result in defective sensing of MDP, the Nod2–Paneth cell axis is thought to play a role in CD pathogenesis. Indeed, Stappenbeck’s laboratory has shown that the proportion of abnormal Paneth cells (with disordered, diminished, diffuse, or excluded granule phenotypes) is associated with the number of CD-associated NOD2 risk alleles.56 Thus, the number of disordered and diminished granules containing lysozyme are strongly increased in Paneth cells from CD patients carrying at least 2 NOD2 mutations.56 Moreover, the cumulative number of NOD2 and ATG16L1 risk alleles had an additive effect on the proportion of abnormal Paneth cells.56 However, although mutations in NOD2 are highly correlated with the incidence of CD,6 Simms et al57 reported that the reduced expression of α-defensins in the ileum of CD patients appeared to be related to the inflammatory status of the mucosa, but not to NOD2 polymorphisms. Similarly, although initial studies established a link between Nod2 deficiency and reduced secretion of α-defensins by Paneth cells,18, 52, 58 recent data clearly have shown that small intestines from Nod2KO mice do not present a default in defensin expression in Paneth cells.42, 43, 59, 60, 61 In contrast, a reduced expression of secretory phospholipase A2 from intestinal epithelial cells has been described in the context of Nod2 deficiency in the nonhematopoietic compartment.35 Organoids from small intestine of Nod2KO mice also are not impaired in α-defensin expression or antibacterial activity.61 Likewise, stimulation of murine miniguts with bacterial products, including MDP, does not induce the secretion of granules from Paneth cells.62 In a new in vitro model of Paneth cells, using Caco-2 cells (human colonic carcinoma) treated with fibroblast growth factor 9, Tan et al63 investigated the role of NOD2 in the synthesis of α-defensins. During the differentiation period (treatment with fibroblast growth factor 9), MDP stimulation reduced the expression of HD5 and HD6, while in differentiated Paneth cells, MDP treatment increased the expression of HD5 and HD6 through induction of the NF-κB pathway.63
In conclusion, no report clearly has shown that in Paneth cells either NOD2 mutations or deletions are linked to reduced α-defensin expression of Paneth cells, or that MDP is able to stimulate α-defensin release. Our opinion is that altered expression of α-defensins in CD patients is independent of NOD2 mutations, and that MDP stimulation is unable to trigger the secretion of α-defensins. However, recent studies have shown that Nod2 might control other functions in Paneth cells. For example, on one hand, mRNA expression of IL23 by Paneth cells is up-regulated by MDP stimulation,64 while on the other hand, this increased expression is decreased in Paneth cells from Nod2KO mice.64 Similarly, in mice carrying a Nod2 deletion in Paneth cells, treated with an anti-CD3 antibody to induce small intestinal inflammation, an increased number of apoptotic epithelial cells and higher expression of TNF-α and IL22 were observed.65 Thus, although NOD2 involvement in α-defensin expression in Paneth cells remains debated, its involvement in IL23 secretion by Paneth cells is clearly shown. However, no study has reported a possible role of NOD2 in the stem cell nursing function of the Paneth cells.
Intestinal Stem Cells
Intestinal stem cell progeny undergo lineage differentiation into the different intestinal epithelial cell types (Figure 2D). To achieve this, stem cells must divide to give rise to a daughter stem cell and a committed daughter cell, which will differentiate toward a fully differentiated epithelial cell. Two types of intestinal cells with stem cell–like properties have been identified in the intestinal crypts: the crypt-based columnar cells66 and the +4 label-retaining cells.66 Markers used to identify these stem cells are Lgr5+ for crypt-based columnar cells66 and Bmi1 for +4 label-retaining cells.67 The intestinal crypt is a site of interactions between microbiota products, stem cells, and other cell types found in this niche such as Paneth cells, and thus offers a potential for commensal microbes to influence the host epithelium. In the colonic mucosa, the highest levels of NOD2 expression were detected in proliferating crypt epithelial cells (Figure 2D).68 In vitro, NOD2 stimulation by MDP promotes colonic epithelial cell (CEC) growth, although this growth is impaired in CECs from Nod2KO mice (Figure 2D).68 In vivo, CEC proliferation also is reduced and apoptosis is increased in the intestinal epithelium from Nod2KO mice (Figure 2D).68 Similarly, depletion of NOD2 expression in human colonic carcinoma results in decreased survival owing to an increased level of apoptosis.68 These data were confirmed only partially by a recent study showing that the number of Ki67-positive cells was reduced in the intestine, but not in the colon, of Nod2KO mice compared with WT mice.25 Currently, the high Nod2 expression in Lgr5+ stem cells was confirmed by Nigro et al11 (Figure 2D). Nod2 stimulation by MDP also has been described to promote stem cell survival.11 Indeed, Nod2 stimulation promotes a strong cytoprotection against oxidative stress-mediated cell death (Figure 2D).11 Thus, gut epithelial restitution is Nod2-dependent and triggered by the presence of microbiota-derived molecules. Taken together, these data show that Nod2 affords stem cell protection and links microbes to gut epithelial regeneration. Thus, under NOD2 mutations, the lack of NOD2 stimulation by MDP could play a pivotal role in the altered renewal of the intestinal mucosa.
Involvement of Nod2 Epithelial Expression in Initiation or Chronicity of Intestinal Inflammation and its Associated Carcinogenesis
The constant exposure of the intestinal tissue to gut microorganisms maintains the mucosa in a state of physiological inflammation, which balances tolerogenic and proinflammatory-type responses to maintain homeostasis. Since the discovery of the association between NOD2 polymorphisms and diseases susceptibilities (CD, CRC, and others), studies have investigated the impact of NOD2 deletion or mutation on the homeostasis of the intestinal mucosa, as well as the development of the inflammatory process and the associated carcinogenesis.69, 70, 71, 72 Thus, Nod2 deficiency or mutation in all cell types of mice have been described to alter the major elements forming the intestinal barrier function, including microbiota,5, 73 the mucus layer43 including AMPs,18, 43 and the sealing of the intestinal epithelium74 favoring the development of inflammation,69, 71, 74 and CRC (Figure 3A).69 However, recent studies using bone marrow transplantation or specific deletion of Nod2 in epithelial or immune cells allowed us to understand the specific role of Nod2 in each cell type (Figure 3A and B). Over the past decade, increasing evidence has allowed consideration of different molecular and cellular mechanisms involved in Nod2 control of intestinal homeostasis.
Role of Nod2 in the Hematopoietic Compartment
In 2010, using bone marrow chimeras in an experimental trinitrobenzene sulfonic acid (TNBS) colitis model, Penack et al9 observed that Nod2 deficiency in hematopoietic cells results in increased intestinal inflammation. Furthermore, they showed that Nod2 regulates graft-versus-host disease development through its inhibitory effect on host antigen-presenting cell function.9 Likewise, transfer of bone marrow hematopoietic cells that express Nod2 to Nod2KO mice reduce the paracellular permeability in Peyer’s patches (PPs) and ileum linked to Nod2 deficiency in the epithelial compartment.43 Reciprocally, transfer of bone marrow cells that do not express Nod2 to WT mice increased the paracellular permeability in PPs and ileum expressing Nod2.16, 43 However, transfer of bone marrow cells expressing Nod2 to Nod2KO mice (or inversely Nod2KO to WT mice) did not modify the expression of mucin and antimicrobial peptides, or the composition of gut microbiota.43 Similar conclusions have been obtained in chimera axenic mice reconstituted with normal microbiota, or microbiota from gut dysbiosis linked to Nod2 deficiency.43 Finally, the deficiency of Nod2 in hematopoietic cells is enough to reproduce all the immune alterations observed in PPs of full Nod2KO mice.43 Taken together, these data support that Nod2 in hematopoietic cells controls the homeostasis of the gut-associated lymphoid tissue as well as the permeability of the intestinal epithelium, and the increased susceptibility of the gut mucosa to develop inflammation (Figure 3A and B).
Role of Nod2 in the Nonhematopoietic Compartment
By using bone marrow chimeras in an experimental TNBS colitis model, it was shown that Nod2 deficiency in nonhematopoietic cells does not modify colitis severity.9 Similarly, Nod2 deficiency in nonhematopoietic cells does not alter the homeostasis of the gut-associated lymphoid tissue as well as the paracellular permeability of the ileum and the follicle-associated epithelium.16, 43 In contrast, Nod2 deficiency in nonhematopoietic cells is sufficient to alter the expression of mucins and antimicrobial peptides and the associated gut microbiota.43 Thus, these data support that Nod2 in nonhematopoietic cells controls the homeostasis of mucins and antimicrobial peptide expression and the associated gut microbiota. As discussed earlier, other studies did not support a role of Nod2 in the regulation of mucins and AMP expression in nonchimeric mice models. However, given that Nod2 deletion leads to bacterial dysbiosis, and that this bacterial dysbiosis alters the secretion of mucins and AMP by epithelial cells, it remains important to consider the impact of bacterial dysbiosis and the intrinsic role of Nod2 on epithelial function. Thus, as we have shown recently, gut microbiota dysbiosis linked to Nod2 deficiency is dominant and transmissible into WT mice, altering the epithelial expression of some mucins and AMP.42 In conclusion, Nod2 in nonhematopoietic cells and/or the associated gut microbiota dysbiosis controls the expressions of mucins and antimicrobial peptides from epithelial cells.
Impact of Nod2-Deficiency–Mediated Intestinal Homeostasis
The increase of paracellular permeability in Nod2KO mice is abolished after antibiotic exposure, highlighting the role of microbiota in the regulation of epithelial barrier function (Figure 3A).34 Changes in intestinal microbial composition have been observed in Nod2KO mice. Although this dysbiosis was associated with an alteration of mucins and AMP expression in Nod2 deficiency in nonhematopoietic cells, it was not the case in hematopoietic lineages.43, 69, 73, 75 Furthermore, transmission of bacterial dysbiosis linked to Nod2 deficiency to WT mice alters mucins and AMP expressions without altered permeability and homeostasis of the gut-associated lymphoid follicle (Figure 3A and B).42 Similarly, the susceptibility of colitis and associated epithelial dysplasia induced by DSS in Nod2KO or Rip2KO mice is dependent on the microbiota dysbiosis, which is transmissible through the microbiota to WT mice (Figure 3A and B).69 Given that gut epithelial restitution is Nod2 dependent and triggered by the presence of microbiota-derived molecules, it is plausible that enhanced epithelial dysplasia in Nod2KO mice is linked to survival and regeneration of stem cells.11 An acceptable rationale to explain these findings may involve an imbalance between proinflammatory and anti-inflammatory cytokines, leading to the loss of autophagy and apoptosis stimuli. This eventually could induce an increased risk of infection, chronic inflammation, and cancer. Nevertheless, microbial composition may play an important role in the determination of colitis or colitis-associated cancer susceptibility. Indeed, Amendola et al76 found that Nod2 deficiency was associated with decreased TNBS-induced colitis. This colitis is associated with gut dysbiosis, resulting in the development of a microbiome supporting the development of regulatory cells that suppressed inflammation in these mice. However, it should be noted that this study was the only one showing that Nod2 deficiency does not increase colitis susceptibility in an animal model.76
In contrast with the fact that gut microbiota dysbiosis is linked to Nod2 deficiency and is a key element in the development of colonic inflammation and its linked colorectal carcinogenesis, it recently was shown that Nod2KO mice are highly susceptible to experimental colorectal tumorigenesis independently of gut microbiota dysbiosis (Figure 3C).77 Thus, the relationship of Nod2-deficiency–associated dysbiosis and colitis is more ambiguous and complex. However, this study was performed in full Nod2KO mice, and whether the high susceptibility to develop inflammation and tumorigenesis was mediated by Nod2 deficiency in hematopoietic and/or nonhematopoietic compartments remains unclear. Nevertheless, expression of inflammatory genes and activation of inflammatory pathways, including NF-κB, extracellular signal–regulated kinase, and signal transducer and activator of transcription 3, are up-regulated in colons from Nod2KO mice during colitis and colorectal tumorigenesis.77 Consistent with increased inflammation, a greater proliferation of epithelial cells is found in hyperplastic regions of colon from Nod2KO mice.77 Exploring the role of Nod2 in the regulation of intestinal homeostasis, in vitro studies performed on bone marrow–derived macrophages from WT and Nod2KO mice showed that although NOD2 activates the NF-κB and MAPK pathways in response to MDP, it inhibited TLR-mediated activation of NF-κB and MAPK.77 Moreover, NOD2-mediated down-regulation of NF-κB and MAPK was associated with the induction of interferon regulatory factor 4 (IRF4).71, 77 Furthermore, NOD2 deficiency, leading to increased tumorigenesis, can be attributed to failure of NOD2 immunoregulation via IRF4.77 On the other hand, it is interesting to note that NOD2-induced activation of IRF4 has effects outside of the mucosal immune system because it can regulate insulin resistance and obesity.78
Thus, NOD2 plays a critical role in the suppression of inflammation and tumorigenesis in the colon via down-regulation of the TLR signaling pathways as shown for inflammatory cytokine production,71 intestinal permeability,34 and response to some pathogenic bacteria.17, 79 Indeed, NOD2 is known to mostly regulate TLR-2, TLR-3, and TLR-4 responses.70, 80, 81 Administration of MDP induced a reduced TLR response in mice carrying a normal NOD2 transgene compared with mice carrying a NOD2 transgene with a CD-associated frameshift abnormality.70, 71
Role of Nod2 on Cancer Development
Given the role of Nod2 on mucosal homeostasis and in shaping microbiota composition, recent studies have shown that deficient NOD2 function confers an increased risk of inflammatory bowel disease and CRC. Not surprisingly, many population-based studies have attempted to explore the association of NOD2 mutations with the pathogenesis of CRC (see Branquinho et al82). To date, most studies addressing NOD2 polymorphisms and CRC essentially rely on a specific country or region. However, considering that NOD2 polymorphism incidence shows a significant geographic variability, and that genome-wide association studies often recur to samples from diverse countries, the effect of these polymorphisms in a specific population may go unnoticed.8, 83 In 2004, Kurzawski et al7 reported an association of the Nod2 frameshift mutation - rs2066847 insC (3020insC) with the risk of CRC. This observation later was supported by other clinical studies.84, 85 Similar to a NOD2 frameshift mutation, 2 other missense mutations rs2066845 C/G (G908R) and rs2066844 C/T (R702W) also have been associated with CRC susceptibility.83, 84, 85, 86, 87, 88 Recently, 2 meta-analyses suggested that polymorphisms in NOD2 are linked with CRC.8, 83 Finally, Yazdanyar and Nordestgaard86 showed that only patients carrying 2 NOD2 mutations present a risk of gastrointestinal cancer. However, other studies have failed to identify an increased susceptibility to CRC for these mutations.89, 90, 91, 92, 93, 94 Thus, the findings concerning the association between NOD2 variants and the risk of CRC show a discrepancy among different cohorts (ie, Finnish, German, Greek, Hungarian, New Zealand, and Polish CRC patients).7, 84, 87, 89, 90, 91 Moreover, the frequency of these variants also varies among different populations (ie, between Europeans and Asians).95 Therefore, it is very likely that reports of a modest genetic association may be the result of a bias owing to the human cohorts used in the reports, as found for the majority of candidate gene studies before the genome-wide association study era.8, 83 Thus, for an adequate analysis, one has to keep in mind the geographic variability, the source of control, and that NOD2 polymorphisms present different prevalences depending on the studied populations. These reasons may explain why the routine detection of NOD2 mutations remains weakly used in the management of CRC patients despite the availability of a simple and cost-effective genetic screening of CRC that includes Nod296 and that the 3020insC NOD2 mutation has been associated with a differential adjuvant chemotherapy response in CRC patient treatment.85
Experimental evidence supports the role of NOD2 in CRC pathogenesis. However, the mechanistic understanding of the functional role of NOD2 in carcinogenesis remains unclear. Couturier-Maillard et al69 showed that microbial dysbiosis linked to Nod2 deficiency can increase colitis-associated cancer susceptibility. Indeed, Nod2KO mice showed an increased tumor load in the distal colon compared with WT animals.69 Interestingly, this susceptibility was shown to be transmittable to WT animals by co-housing them with the Nod2KO mice, highlighting a role of bacterial dysbiosis linked to Nod2 deficiency in CRC sucseptibility.69 An acceptable rationale to these findings may involve an imbalance between proinflammatory and anti-inflammatory cytokines and the loss of autophagy and apoptosis stimuli.97, 98 This eventually could lead to an increased risk of infection and/or chronic inflammation, promoting cancer development. However, a study by Udden et al77 reported that inflammation and tumorigenesis can be associated with Nod2 deficiency in the absence of dysbiosis. Finally, another study reported that vitamin D supplementation reduces colitis severity and decreases the number of inflammation-associated colorectal tumors independently of Nod2.99 Thus, given the discrepancy of the literature regarding the involvement, or not, of the microbiota dysbiosis linked to Nod2 deficiency in the development of neoplastic lesions in Nod2KO mice, we cannot clearly state that it is involved in cancer development.
In conclusion, studies presented in this review highlight the pivotal role played by Nod2 in the homeostasis of the main epithelial cells of the intestinal epithelium, controlling the physiological status of the digestive tract mucosa. In case of Nod2 malfunctioning, the homeostasis of the intestinal barrier function is disrupted, favoring the development of gut microbiota dysbiosis, leading to higher susceptibility to inflammation and colorectal cancer development.
Footnotes
Author contributions Ziad Al Nabhani, Audrey Ferrand, and Frédérick Barreau were responsible for the review design and concept; and Audrey Ferrand, Ziad Al Nabhani, Emmanuel Mas, Núria Solà Tapias, Jean-Pierre Hugot, and Frédérick Barreau wrote the manuscript.
Conflicts of interest The authors disclose no conflicts.
References
- 1.Jung C., Hugot J.P., Barreau F. Peyer's patches: the immune sensors of the intestine. Int J Inflam. 2010;2010:823710. doi: 10.4061/2010/823710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerbe F., Legraverend C., Jay P. The intestinal epithelium tuft cells: specification and function. Cell Mol Life Sci. 2012;69:2907–2917. doi: 10.1007/s00018-012-0984-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madara J.L., Carlson S.L. Cup cells: further structural characterization of the brush border and the suggestion that they may serve as an attachment site for an unidentified bacillus in guinea pig ileum. Gastroenterology. 1985;89:1374–1386. doi: 10.1016/0016-5085(85)90658-4. [DOI] [PubMed] [Google Scholar]
- 4.Barreau F., Hugot J. Intestinal barrier dysfunction triggered by invasive bacteria. Curr Opin Microbiol. 2014;17C:91–98. doi: 10.1016/j.mib.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Al Nabhani Z., Dietrich G., Hugot J.P., Barreau F. Nod2: the intestinal gate keeper. PLoS Pathog. 2017;13:e1006177. doi: 10.1371/journal.ppat.1006177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hugot J.P., Chamaillard M., Zouali H., Lesage S., Cezard J.P., Belaiche J., Almer S., Tysk C., O'Morain C.A., Gassull M., Binder V., Finkel Y., Cortot A., Modigliani R., Laurent-Puig P., Gower-Rousseau C., Macry J., Colombel J.F., Sahbatou M., Thomas G. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 7.Kurzawski G., Suchy J., Kladny J., Grabowska E., Mierzejewski M., Jakubowska A., Debniak T., Cybulski C., Kowalska E., Szych Z., Domagala W., Scott R.J., Lubinski J. The NOD2 3020insC mutation and the risk of colorectal cancer. Cancer Res. 2004;64:1604–1606. doi: 10.1158/0008-5472.can-03-3791. [DOI] [PubMed] [Google Scholar]
- 8.Liu J., He C., Xu Q., Xing C., Yuan Y. NOD2 polymorphisms associated with cancer risk: a meta-analysis. PLoS One. 2014;9:e89340. doi: 10.1371/journal.pone.0089340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penack O., Smith O.M., Cunningham-Bussel A., Liu X., Rao U., Yim N., Na I.K., Holland A.M., Ghosh A., Lu S.X., Jenq R.R., Liu C., Murphy G.F., Brandl K., van den Brink M.R. NOD2 regulates hematopoietic cell function during graft-versus-host disease. J Exp Med. 2009;206:2101–2110. doi: 10.1084/jem.20090623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogura Y., Lala S., Xin W., Smith E., Dowds T.A., Chen F.F., Zimmermann E., Tretiakova M., Cho J.H., Hart J., Greenson J.K., Keshav S., Nunez G. Expression of NOD2 in Paneth cells: a possible link to Crohn's ileitis. Gut. 2003;52:1591–1597. doi: 10.1136/gut.52.11.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nigro G., Rossi R., Commere P.H., Jay P., Sansonetti P.J. The cytosolic bacterial peptidoglycan sensor Nod2 affords stem cell protection and links microbes to gut epithelial regeneration. Cell Host Microbe. 2014;15:792–798. doi: 10.1016/j.chom.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Ramanan D., Tang M.S., Bowcutt R., Loke P., Cadwell K. Bacterial sensor Nod2 prevents inflammation of the small intestine by restricting the expansion of the commensal Bacteroides vulgatus. Immunity. 2014;41:311–324. doi: 10.1016/j.immuni.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hisamatsu T., Suzuki M., Reinecker H.C., Nadeau W.J., McCormick B.A., Podolsky D.K. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology. 2003;124:993–1000. doi: 10.1053/gast.2003.50153. [DOI] [PubMed] [Google Scholar]
- 14.Rosenstiel P., Fantini M., Brautigam K., Kuhbacher T., Waetzig G.H., Seegert D., Schreiber S. TNF-alpha and IFN-gamma regulate the expression of the NOD2 (CARD15) gene in human intestinal epithelial cells. Gastroenterology. 2003;124:1001–1009. doi: 10.1053/gast.2003.50157. [DOI] [PubMed] [Google Scholar]
- 15.Girardin S.E., Boneca I.G., Viala J., Chamaillard M., Labigne A., Thomas G., Philpott D.J., Sansonetti P.J. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 16.Alnabhani Z., Montcuquet N., Biaggini K., Dussaillant M., Roy M., Ogier-Denis E., Madi A., Jallane A., Feuilloley M., Hugot J.P., Connil N., Barreau F. Pseudomonas fluorescens alters the intestinal barrier function by modulating IL-1beta expression through hematopoietic NOD2 signaling. Inflamm Bowel Dis. 2015;21:543–555. doi: 10.1097/MIB.0000000000000291. [DOI] [PubMed] [Google Scholar]
- 17.Meinzer U., Barreau F., Esmiol-Welterlin S., Jung C., Villard C., Leger T., Ben-Mkaddem S., Berrebi D., Dussaillant M., Alnabhani Z., Roy M., Bonacorsi S., Wolf-Watz H., Perroy J., Ollendorff V., Hugot J.P. Yersinia pseudotuberculosis effector YopJ subverts the Nod2/RICK/TAK1 pathway and activates caspase-1 to induce intestinal barrier dysfunction. Cell Host Microbe. 2012;11:337–351. doi: 10.1016/j.chom.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi K.S., Chamaillard M., Ogura Y., Henegariu O., Inohara N., Nunez G., Flavell R.A. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 19.Boyle J.P., Parkhouse R., Monie T.P. Insights into the molecular basis of the NOD2 signalling pathway. Open Biol. 2014;4 doi: 10.1098/rsob.140178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tattoli I., Travassos L.H., Carneiro L.A., Magalhaes J.G., Girardin S.E. The Nodosome: Nod1 and Nod2 control bacterial infections and inflammation. Semin Immunopathol. 2007;29:289–301. doi: 10.1007/s00281-007-0083-2. [DOI] [PubMed] [Google Scholar]
- 21.Zhong Y., Kinio A., Saleh M. Functions of NOD-like receptors in human diseases. Front Immunol. 2013;4:333. doi: 10.3389/fimmu.2013.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Opitz B., Puschel A., Schmeck B., Hocke A.C., Rosseau S., Hammerschmidt S., Schumann R.R., Suttorp N., Hippenstiel S. Nucleotide-binding oligomerization domain proteins are innate immune receptors for internalized Streptococcus pneumoniae. J Biol Chem. 2004;279:36426–36432. doi: 10.1074/jbc.M403861200. [DOI] [PubMed] [Google Scholar]
- 23.Theivanthiran B., Batra S., Balamayooran G., Cai S., Kobayashi K., Flavell R.A., Jeyaseelan S. NOD2 signaling contributes to host defense in the lungs against Escherichia coli infection. Infect Immun. 2012;80:2558–2569. doi: 10.1128/IAI.06230-11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Lala S., Ogura Y., Osborne C., Hor S.Y., Bromfield A., Davies S., Ogunbiyi O., Nunez G., Keshav S. Crohn's disease and the NOD2 gene: a role for Paneth cells. Gastroenterology. 2003;125:47–57. doi: 10.1016/s0016-5085(03)00661-9. [DOI] [PubMed] [Google Scholar]
- 25.Bereswill S., Grundmann U., Alutis M.E., Fischer A., Kuhl A.A., Heimesaat M.M. Immune responses upon Campylobacter jejuni infection of secondary abiotic mice lacking nucleotide-oligomerization-domain-2. Gut Pathog. 2017;9:33. doi: 10.1186/s13099-017-0182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gutierrez O., Pipaon C., Inohara N., Fontalba A., Ogura Y., Prosper F., Nunez G., Fernandez-Luna J.L. Induction of Nod2 in myelomonocytic and intestinal epithelial cells via nuclear factor-kappa B activation. J Biol Chem. 2002;277:41701–41705. doi: 10.1074/jbc.M206473200. [DOI] [PubMed] [Google Scholar]
- 27.Petnicki-Ocwieja T., Hrncir T., Liu Y.J., Biswas A., Hudcovic T., Tlaskalova-Hogenova H., Kobayashi K.S. Nod2 is required for the regulation of commensal microbiota in the intestine. Proc Natl Acad Sci U S A. 2009;106:15813–15818. doi: 10.1073/pnas.0907722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Begue B., Dumant C., Bambou J.C., Beaulieu J.F., Chamaillard M., Hugot J.P., Goulet O., Schmitz J., Philpott D.J., Cerf-Bensussan N., Ruemmele F.M. Microbial induction of CARD15 expression in intestinal epithelial cells via toll-like receptor 5 triggers an antibacterial response loop. J Cell Physiol. 2006;209:241–252. doi: 10.1002/jcp.20739. [DOI] [PubMed] [Google Scholar]
- 29.Leung C.H., Lam W., Ma D.L., Gullen E.A., Cheng Y.C. Butyrate mediates nucleotide-binding and oligomerisation domain (NOD) 2-dependent mucosal immune responses against peptidoglycan. Eur J Immunol. 2009;39:3529–3537. doi: 10.1002/eji.200939454. [DOI] [PubMed] [Google Scholar]
- 30.Saxena A., Lopes F., Poon K.K.H., McKay D.M. Absence of the NOD2 protein renders epithelia more susceptible to barrier dysfunction due to mitochondrial dysfunction. Am J Physiol Gastrointest Liver Physiol. 2017;313:G26–G38. doi: 10.1152/ajpgi.00070.2017. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto-Furusho J.K., Barnich N., Hisamatsu T., Podolsky D.K. MDP-NOD2 stimulation induces HNP-1 secretion, which contributes to NOD2 antibacterial function. Inflamm Bowel Dis. 2010;16:736–742. doi: 10.1002/ibd.21144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Mandat Schultz A., Bonnard A., Barreau F., Aigrain Y., Pierre-Louis C., Berrebi D., Peuchmaur M. Expression of TLR-2, TLR-4, NOD2 and pNF-kappaB in a neonatal rat model of necrotizing enterocolitis. PLoS One. 2007;2:e1102. doi: 10.1371/journal.pone.0001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson W.M., Sodhi C.P., Russo A., Siggers R.H., Afrazi A., Gribar S.C., Neal M.D., Dai S., Prindle T., Jr., Branca M., Ma C., Ozolek J., Hackam D.J. Nucleotide-binding oligomerization domain-2 inhibits toll-like receptor-4 signaling in the intestinal epithelium. Gastroenterology. 2010;139:904–917. doi: 10.1053/j.gastro.2010.05.038. 17 e1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barreau F., Madre C., Meinzer U., Berrebi D., Dussaillant M., Merlin F., Eckmann L., Karin M., Sterkers G., Bonacorsi S., Lesuffleur T., Hugot J.P. Nod2 regulates the host response towards microflora by modulating T cell function and epithelial permeability in mouse Peyer's patches. Gut. 2010;59:207–217. doi: 10.1136/gut.2008.171546. [DOI] [PubMed] [Google Scholar]
- 35.Al Nabhani Z., Montcuquet N., Roy M., Dussaillant M., Hugot J.P., Barreau F. Complementary roles of Nod2 in hematopoietic and nonhematopoietic cells in preventing gut barrier dysfunction dependent on MLCK activity. Inflamm Bowel Dis. 2017;23:1109–1119. doi: 10.1097/MIB.0000000000001135. [DOI] [PubMed] [Google Scholar]
- 36.Lievin-Le Moal V., Servin A.L. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: mucins, antimicrobial peptides, and microbiota. Clin Microbiol Rev. 2006;19:315–337. doi: 10.1128/CMR.19.2.315-337.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hollingsworth M.A., Swanson B.J. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 38.Kim Y.S., Ho S.B. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep. 2010;12:319–330. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Makkink M.K., Schwerbrock N.M., Mahler M., Boshuizen J.A., Renes I.B., Cornberg M., Hedrich H.J., Einerhand A.W., Buller H.A., Wagner S., Enss M.L., Dekker J. Fate of goblet cells in experimental colitis. Dig Dis Sci. 2002;47:2286–2297. doi: 10.1023/a:1020147630032. [DOI] [PubMed] [Google Scholar]
- 40.Taupin D., Podolsky D.K. Trefoil factors: initiators of mucosal healing. Nat Rev Mol Cell Biol. 2003;4:721–732. doi: 10.1038/nrm1203. [DOI] [PubMed] [Google Scholar]
- 41.Krimi R.B., Kotelevets L., Dubuquoy L., Plaisancie P., Walker F., Lehy T., Desreumaux P., Van Seuningen I., Chastre E., Forgue-Lafitte M.E., Marie J.C. Resistin-like molecule beta regulates intestinal mucous secretion and curtails TNBS-induced colitis in mice. Inflamm Bowel Dis. 2008;14:931–941. doi: 10.1002/ibd.20420. [DOI] [PubMed] [Google Scholar]
- 42.Al Nabhani Z., Lepage P., Mauny P., Montcuquet N., Roy M., Le Roux K., Dussaillant M., Berrebi D., Hugot J.P., Barreau F. Nod2 deficiency leads to a specific and transmissible mucosa-associated microbial dysbiosis which is independent of the mucosal barrier defect. J Crohns Colitis. 2016;10:1428–1436. doi: 10.1093/ecco-jcc/jjw095. [DOI] [PubMed] [Google Scholar]
- 43.Alnabhani Z., Hugot J.P., Montcuquet N., Le Roux K., Dussaillant M., Roy M., Leclerc M., Cerf-Bensussan N., Lepage P., Barreau F. Respective roles of hematopoietic and nonhematopoietic Nod2 on the gut microbiota and mucosal homeostasis. Inflamm Bowel Dis. 2016;22:763–773. doi: 10.1097/MIB.0000000000000749. [DOI] [PubMed] [Google Scholar]
- 44.Wang H., Kim J.J., Denou E., Gallagher A., Thornton D.J., Shajib M.S., Xia L., Schertzer J.D., Grencis R.K., Philpott D.J., Khan W.I. New role of nod proteins in regulation of intestinal goblet cell response in the context of innate host defense in an enteric parasite infection. Infect Immun. 2015;84:275–285. doi: 10.1128/IAI.01187-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Porter E.M., Bevins C.L., Ghosh D., Ganz T. The multifaceted Paneth cell. Cell Mol Life Sci. 2002;59:156–170. doi: 10.1007/s00018-002-8412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bevins C.L., Salzman N.H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011;9:356–368. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 47.Selsted M.E., Ouellette A.J. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6:551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- 48.Ostaff M.J., Stange E.F., Wehkamp J. Antimicrobial peptides and gut microbiota in homeostasis and pathology. EMBO Mol Med. 2013;5:1465–1483. doi: 10.1002/emmm.201201773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cadwell K., Liu J.Y., Brown S.L., Miyoshi H., Loh J., Lennerz J.K., Kishi C., Kc W., Carrero J.A., Hunt S., Stone C.D., Brunt E.M., Xavier R.J., Sleckman B.P., Li E., Mizushima N., Stappenbeck T.S., Virgin H.W. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456:259–263. doi: 10.1038/nature07416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lassen K.G., Kuballa P., Conway K.L., Patel K.K., Becker C.E., Peloquin J.M., Villablanca E.J., Norman J.M., Liu T.C., Heath R.J., Becker M.L., Fagbami L., Horn H., Mercer J., Yilmaz O.H., Jaffe J.D., Shamji A.F., Bhan A.K., Carr S.A., Daly M.J., Virgin H.W., Schreiber S.L., Stappenbeck T.S., Xavier R.J. Atg16L1 T300A variant decreases selective autophagy resulting in altered cytokine signaling and decreased antibacterial defense. Proc Natl Acad Sci U S A. 2014;111:7741–7746. doi: 10.1073/pnas.1407001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adolph T.E., Tomczak M.F., Niederreiter L., Ko H.J., Bock J., Martinez-Naves E., Glickman J.N., Tschurtschenthaler M., Hartwig J., Hosomi S., Flak M.B., Cusick J.L., Kohno K., Iwawaki T., Billmann-Born S., Raine T., Bharti R., Lucius R., Kweon M.N., Marciniak S.J., Choi A., Hagen S.J., Schreiber S., Rosenstiel P., Kaser A., Blumberg R.S. Paneth cells as a site of origin for intestinal inflammation. Nature. 2013;503:272–276. doi: 10.1038/nature12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wehkamp J., Salzman N.H., Porter E., Nuding S., Weichenthal M., Petras R.E., Shen B., Schaeffeler E., Schwab M., Linzmeier R., Feathers R.W., Chu H., Lima H., Jr., Fellermann K., Ganz T., Stange E.F., Bevins C.L. Reduced Paneth cell alpha-defensins in ileal Crohn's disease. Proc Natl Acad Sci U S A. 2005;102:18129–18134. doi: 10.1073/pnas.0505256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ayabe T., Satchell D.P., Wilson C.L., Parks W.C., Selsted M.E., Ouellette A.J. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000;1:113–118. doi: 10.1038/77783. [DOI] [PubMed] [Google Scholar]
- 54.Rocha J.D., Schlossmacher M.G., Philpott D.J. LRRK2 and Nod2 promote lysozyme sorting in Paneth cells. Nat Immunol. 2015;16:898–900. doi: 10.1038/ni.3255. [DOI] [PubMed] [Google Scholar]
- 55.Wang H., Zhang X., Zuo Z., Zhang Q., Pan Y., Zeng B., Li W., Wei H., Liu Z. Rip2 Is Required for Nod2-mediated lysozyme sorting in Paneth cells. J Immunol. 2017;198:3729–3736. doi: 10.4049/jimmunol.1601583. [DOI] [PubMed] [Google Scholar]
- 56.VanDussen K.L., Liu T.C., Li D., Towfic F., Modiano N., Winter R., Haritunians T., Taylor K.D., Dhall D., Targan S.R., Xavier R.J., McGovern D.P., Stappenbeck T.S. Genetic variants synthesize to produce Paneth cell phenotypes that define subtypes of Crohn's disease. Gastroenterology. 2014;146:200–209. doi: 10.1053/j.gastro.2013.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simms L.A., Doecke J.D., Walsh M.D., Huang N., Fowler E.V., Radford-Smith G.L. Reduced alpha-defensin expression is associated with inflammation and not NOD2 mutation status in ileal Crohn's disease. Gut. 2008;57:903–910. doi: 10.1136/gut.2007.142588. [DOI] [PubMed] [Google Scholar]
- 58.Wehkamp J., Harder J., Weichenthal M., Schwab M., Schaffeler E., Schlee M., Herrlinger K.R., Stallmach A., Noack F., Fritz P., Schroder J.M., Bevins C.L., Fellermann K., Stange E.F. NOD2 (CARD15) mutations in Crohn's disease are associated with diminished mucosal alpha-defensin expression. Gut. 2004;53:1658–1664. doi: 10.1136/gut.2003.032805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robertson S.J., Zhou J.Y., Geddes K., Rubino S.J., Cho J.H., Girardin S.E., Philpott D.J. Nod1 and Nod2 signaling does not alter the composition of intestinal bacterial communities at homeostasis. Gut Microbes. 2013;4:222–231. doi: 10.4161/gmic.24373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shanahan M.T., Carroll I.M., Grossniklaus E., White A., von Furstenberg R.J., Barner R., Fodor A.A., Henning S.J., Sartor R.B., Gulati A.S. Mouse Paneth cell antimicrobial function is independent of Nod2. Gut. 2014;63:903–910. doi: 10.1136/gutjnl-2012-304190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilson S.S., Tocchi A., Holly M.K., Parks W.C., Smith J.G. A small intestinal organoid model of non-invasive enteric pathogen-epithelial cell interactions. Mucosal Immunol. 2015;8:352–361. doi: 10.1038/mi.2014.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Farin H.F., Karthaus W.R., Kujala P., Rakhshandehroo M., Schwank G., Vries R.G., Kalkhoven E., Nieuwenhuis E.E., Clevers H. Paneth cell extrusion and release of antimicrobial products is directly controlled by immune cell-derived IFN-gamma. J Exp Med. 2014;211:1393–1405. doi: 10.1084/jem.20130753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tan G., Zeng B., Zhi F.C. Regulation of human enteric alpha-defensins by NOD2 in the Paneth cell lineage. Eur J Cell Biol. 2015;94:60–66. doi: 10.1016/j.ejcb.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 64.Tan G., Liang E., Liao K., Deng F., Zhang W., Chen Y., Xu J., Zhi F. NOD2 up-regulates TLR2-mediated IL-23p19 expression via NF-kappaB subunit c-Rel in Paneth cell-like cells. Oncotarget. 2016;7:63651–63660. doi: 10.18632/oncotarget.11467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zanello G., Goethel A., Rouquier S., Prescott D., Robertson S.J., Maisonneuve C., Streutker C., Philpott D.J., Croitoru K. The cytosolic microbial receptor Nod2 regulates small intestinal crypt damage and epithelial regeneration following T cell-induced enteropathy. J Immunol. 2016;197:345–355. doi: 10.4049/jimmunol.1600185. [DOI] [PubMed] [Google Scholar]
- 66.Barker N., van Es J.H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P.J., Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 67.Sangiorgi E., Capecchi M.R. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cruickshank S.M., Wakenshaw L., Cardone J., Howdle P.D., Murray P.J., Carding S.R. Evidence for the involvement of NOD2 in regulating colonic epithelial cell growth and survival. World J Gastroenterol. 2008;14:5834–5841. doi: 10.3748/wjg.14.5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Couturier-Maillard A., Secher T., Rehman A., Normand S., De Arcangelis A., Haesler R., Huot L., Grandjean T., Bressenot A., Delanoye-Crespin A., Gaillot O., Schreiber S., Lemoine Y., Ryffel B., Hot D., Nunez G., Chen G., Rosenstiel P., Chamaillard M. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J Clin Invest. 2013;123:700–711. doi: 10.1172/JCI62236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Watanabe T., Kitani A., Murray P.J., Strober W. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat Immunol. 2004;5:800–808. doi: 10.1038/ni1092. [DOI] [PubMed] [Google Scholar]
- 71.Watanabe T., Asano N., Murray P.J., Ozato K., Tailor P., Fuss I.J., Kitani A., Strober W. Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis. J Clin Invest. 2008;118:545–559. doi: 10.1172/JCI33145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maeda S., Hsu L.C., Liu H., Bankston L.A., Iimura M., Kagnoff M.F., Eckmann L., Karin M. Nod2 mutation in Crohn's disease potentiates NF-kappaB activity and IL-1beta processing. Science. 2005;307:734–738. doi: 10.1126/science.1103685. [DOI] [PubMed] [Google Scholar]
- 73.Rehman A., Sina C., Gavrilova O., Hasler R., Ott S., Baines J.F., Schreiber S., Rosenstiel P. Nod2 is essential for temporal development of intestinal microbial communities. Gut. 2011;60:1354–1362. doi: 10.1136/gut.2010.216259. [DOI] [PubMed] [Google Scholar]
- 74.Barreau F., Meinzer U., Chareyre F., Berrebi D., Niwa-Kawakita M., Dussaillant M., Foligne B., Ollendorff V., Heyman M., Bonacorsi S., Lesuffleur T., Sterkers G., Giovannini M., Hugot J.P. CARD15/NOD2 is required for Peyer's patches homeostasis in mice. PLoS One. 2007;2:e523. doi: 10.1371/journal.pone.0000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mondot S., Barreau F., Al Nabhani Z., Dussaillant M., Le Roux K., Dore J., Leclerc M., Hugot J.P., Lepage P. Altered gut microbiota composition in immune-impaired Nod2(-/-) mice. Gut. 2012;61:634–635. doi: 10.1136/gutjnl-2011-300478. [DOI] [PubMed] [Google Scholar]
- 76.Amendola A., Butera A., Sanchez M., Strober W., Boirivant M. Nod2 deficiency is associated with an increased mucosal immunoregulatory response to commensal microorganisms. Mucosal Immunol. 2012;7:391–404. doi: 10.1038/mi.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Udden S.M.N., Peng L., Gan J.L., Shelton J.M., Malter J.S., Hooper L.V., Zaki M.H. NOD2 suppresses colorectal tumorigenesis via downregulation of the TLR pathways. Cell Rep. 2017;19:2756–2770. doi: 10.1016/j.celrep.2017.05.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cavallari J.F., Fullerton M.D., Duggan B.M., Foley K.P., Denou E., Smith B.K., Desjardins E.M., Henriksbo B.D., Kim K.J., Tuinema B.R., Stearns J.C., Prescott D., Rosenstiel P., Coombes B.K., Steinberg G.R., Schertzer J.D. Muramyl dipeptide-based postbiotics mitigate obesity-induced insulin resistance via IRF4. Cell Metab. 2017;25:1063–1074 e3. doi: 10.1016/j.cmet.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 79.Jung C., Meinzer U., Montcuquet N., Thachil E., Chateau D., Thiebaut R., Roy M., Alnabhani Z., Berrebi D., Dussaillant M., Pedruzzi E., Thenet S., Cerf-Bensussan N., Hugot J.P., Barreau F. Yersinia pseudotuberculosis disrupts intestinal barrier integrity through hematopoietic TLR-2 signaling. J Clin Invest. 2012;122:2239–2251. doi: 10.1172/JCI58147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Netea M.G., Kullberg B.J., de Jong D.J., Franke B., Sprong T., Naber T.H., Drenth J.P., Van der Meer J.W. NOD2 mediates anti-inflammatory signals induced by TLR2 ligands: implications for Crohn's disease. Eur J Immunol. 2004;34:2052–2059. doi: 10.1002/eji.200425229. [DOI] [PubMed] [Google Scholar]
- 81.Netea M.G., Ferwerda G., de Jong D.J., Jansen T., Jacobs L., Kramer M., Naber T.H., Drenth J.P., Girardin S.E., Kullberg B.J., Adema G.J., Van der Meer J.W. Nucleotide-binding oligomerization domain-2 modulates specific TLR pathways for the induction of cytokine release. J Immunol. 2005;174:6518–6523. doi: 10.4049/jimmunol.174.10.6518. [DOI] [PubMed] [Google Scholar]
- 82.Branquinho D., Freire P., Sofia C. NOD2 mutations and colorectal cancer - where do we stand? World J Gastrointest Surg. 2016;8:284–293. doi: 10.4240/wjgs.v8.i4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tian Y., Li Y., Hu Z., Wang D., Sun X., Ren C. Differential effects of NOD2 polymorphisms on colorectal cancer risk: a meta-analysis. Int J Colorectal Dis. 2010;25:161–168. doi: 10.1007/s00384-009-0809-9. [DOI] [PubMed] [Google Scholar]
- 84.Papaconstantinou I., Theodoropoulos G., Gazouli M., Panoussopoulos D., Mantzaris G.J., Felekouras E., Bramis J. Association between mutations in the CARD15/NOD2 gene and colorectal cancer in a Greek population. Int J Cancer. 2005;114:433–435. doi: 10.1002/ijc.20747. [DOI] [PubMed] [Google Scholar]
- 85.Omrane I., Mezlini A., Baroudi O., Stambouli N., Bougatef K., Ayari H., Medimegh I., Bouzaienne H., Uhrhammer N., Bignon Y.J., Benammar-Elgaaied A., Marrakchi R. 3020insC NOD2/CARD15 polymorphism associated with treatment of colorectal cancer. Med Oncol. 2014;31:954. doi: 10.1007/s12032-014-0954-z. [DOI] [PubMed] [Google Scholar]
- 86.Yazdanyar S., Nordestgaard B.G. NOD2/CARD15 genotype and common gastrointestinal diseases in 43,600 individuals. J Intern Med. 2010;267:228–236. doi: 10.1111/j.1365-2796.2009.02137.x. [DOI] [PubMed] [Google Scholar]
- 87.Roberts R.L., Gearry R.B., Allington M.D., Morrin H.R., Robinson B.A., Frizelle F.A. Caspase recruitment domain-containing protein 15 mutations in patients with colorectal cancer. Cancer Res. 2006;66:2532–2535. doi: 10.1158/0008-5472.CAN-05-4165. [DOI] [PubMed] [Google Scholar]
- 88.Freire P., Portela F., Donato M.M., Figueiredo P., Ferreira M., Amaro P., Sa A., Andrade P., Gouveia H., Sofia C. CARD15 mutations and colorectal cancer in a South European country. Int J Colorectal Dis. 2010;25:1211–1219. doi: 10.1007/s00384-010-1028-0. [DOI] [PubMed] [Google Scholar]
- 89.Alhopuro P., Ahvenainen T., Mecklin J.P., Juhola M., Jarvinen H.J., Karhu A., Aaltonen L.A. NOD2 3020insC alone is not sufficient for colorectal cancer predisposition. Cancer Res. 2004;64:7245–7247. doi: 10.1158/0008-5472.CAN-04-2364. [DOI] [PubMed] [Google Scholar]
- 90.Lakatos P.L., Hitre E., Szalay F., Zinober K., Fuszek P., Lakatos L., Fischer S., Osztovits J., Gemela O., Veres G., Papp J., Ferenci P. Common NOD2/CARD15 variants are not associated with susceptibility or the clinicopathologic characteristics of sporadic colorectal cancer in Hungarian patients. BMC Cancer. 2007;7:54. doi: 10.1186/1471-2407-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Szeliga J., Sondka Z., Jackowski M., Jarkiewicz-Tretyn J., Tretyn A., Malenczyk M. NOD2/CARD15 polymorphism in patients with rectal cancer. Med Sci Monit. 2008;14:CR480–CR484. [PubMed] [Google Scholar]
- 92.Mockelmann N., von Schonfels W., Buch S., von Kampen O., Sipos B., Egberts J.H., Rosenstiel P., Franke A., Brosch M., Hinz S., Roder C., Kalthoff H., Folsch U.R., Krawczak M., Schreiber S., Broring C.D., Tepel J., Schafmayer C., Hampe J. Investigation of innate immunity genes CARD4, CARD8 and CARD15 as germline susceptibility factors for colorectal cancer. BMC Gastroenterol. 2009;9:79. doi: 10.1186/1471-230X-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tuupanen S., Alhopuro P., Mecklin J.P., Jarvinen H., Aaltonen L.A. No evidence for association of NOD2 R702W and G908R with colorectal cancer. Int J Cancer. 2007;121:76–79. doi: 10.1002/ijc.22651. [DOI] [PubMed] [Google Scholar]
- 94.Lau T.P., Roslani A.C., Lian L.H., Lee P.C., Hilmi I., Goh K.L., Chua K.H. NOD2/CARD15 variants in Malaysian patients with sporadic colorectal cancer. Genet Mol Res. 2014;13:7079–7085. doi: 10.4238/2014.March.19.3. [DOI] [PubMed] [Google Scholar]
- 95.Esters N., Pierik M., van Steen K., Vermeire S., Claessens G., Joossens S., Vlietinck R., Rutgeerts P. Transmission of CARD15 (NOD2) variants within families of patients with inflammatory bowel disease. Am J Gastroenterol. 2004;99:299–305. doi: 10.1111/j.1572-0241.2004.04040.x. [DOI] [PubMed] [Google Scholar]
- 96.Stojcev Z., Banasiewicz T., Kaszuba M., Sikorski A., Szczepkowski M., Bobkiewicz A., Paszkowski J., Krokowicz L., Biczysko M., Szmeja J., Jurkowska M., Majewski P., Mackiewicz A., Lamperska K., Drews M., Wojciechowicz J. Development of a new, simple and cost-effective diagnostic tool for genetic screening of hereditary colorectal cancer--the DNA microarray assay. Acta Biochim Pol. 2013;60:195–198. [PubMed] [Google Scholar]
- 97.Mokarram P., Albokashy M., Zarghooni M., Moosavi M.A., Sepehri Z., Chen Q.M., Hudecki A., Sargazi A., Alizadeh J., Moghadam A.R., Hashemi M., Movassagh H., Klonisch T., Owji A.A., Los M.J., Ghavami S. New frontiers in the treatment of colorectal cancer: autophagy and the unfolded protein response as promising targets. Autophagy. 2017;13:781–819. doi: 10.1080/15548627.2017.1290751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moradi Marjaneh R., Hassanian S.M., Ghobadi N., Ferns G.A., Karimi A., Jazayeri M.H., Nasiri M., Avan A., Khazaei M. Targeting the death receptor signaling pathway as a potential therapeutic target in the treatment of colorectal cancer. J Cell Physiol. 2018;233:6538–6549. doi: 10.1002/jcp.26640. [DOI] [PubMed] [Google Scholar]
- 99.Elimrani I., Koenekoop J., Dionne S., Marcil V., Delvin E., Levy E., Seidman E.G. Vitamin D reduces colitis- and inflammation-associated colorectal cancer in mice independent of NOD2. Nutr Cancer. 2017;69:276–288. doi: 10.1080/01635581.2017.1263346. [DOI] [PubMed] [Google Scholar]