Abstract
Life satisfaction is increasingly recognized as an important determinant of health; however, prospective population-based studies on this topic are limited. We estimated the risk of chronic disease and death according to life satisfaction among a population-based cohort in Ontario, Canada (n = 73,904). The cohort included 3 pooled cycles of the Canadian Community Health Survey (2003–2008) linked to 6 years of follow-up (to 2015), using population-based health databases and validated disease-specific registries. The databases capture incident and prevalent cases of diabetes, cancer, chronic obstructive pulmonary disease, heart disease, and death. Multivariable Cox proportional hazard models were used to estimate hazards of incident chronic disease and death, and were adjusted for sociodemographic, behavioral, and clinical confounders, including age, sex, comorbidity, mood disorder, smoking, alcohol consumption, physical activity, body mass index, immigrant status, education, and income. In the fully adjusted models, risk of both death and incident chronic disease was highest for those most dissatisfied with life (for mortality, hazard ratio = 1.59, 95% confidence interval: 1.15, 2.19; for chronic disease, hazard ratio = 1.70, 95% confidence interval: 1.16, 2.51). In this population-based cohort, poor life satisfaction was an independent risk factor for incident chronic disease and death, supporting the idea that interventions and programs that improve life satisfaction will affect population health.
Keywords: chronic disease, life satisfaction, mortality
Life satisfaction, a dimension of quality of life encompassing physical, mental, and social well-being, is increasingly being recognized as a meaningful determinant of health (1). Life satisfaction is part of the broader construct of psychological well-being, which also includes domains such as positive emotion, optimism, and purpose (2). Self-reported measures of life satisfaction have been shown to be stable and reliable over time (3, 4). Poor life satisfaction has been linked repeatedly to undesirable health outcomes, including elevated risk of chronic disease (2, 5, 6) and death (7–9). This association is observed less consistently after adjusting for key predictors of incident chronic disease, including lifestyle and comorbidity (10).
These findings suggest that life satisfaction may influence chronic disease and death outcomes independently of traditional risk factors. However, in observational studies to date, the focus either has been on selected populations (e.g., women or older adults) or not explicitly on capturing life satisfaction before the chronic disease incidence or death, and the study designs were varied in their control for confounding variables, particularly variables accounting for health status. Consequently, in these studies, researchers had limited ability to assess a comprehensive range of health outcomes prospectively, independent of behavioral and sociodemographic risk factors. Using a large, representative, population-based cohort offers greater opportunity to appropriately assess the direct impact of life satisfaction on chronic disease and death risk, and can provide important evidence to inform prevention efforts.
The objective for this study was to determine the association between life satisfaction and chronic disease and death in a population-based prospective cohort, using validated disease outcomes for key burdensome chronic diseases (namely, diabetes, coronary heart disease (CHD), chronic obstructive respiratory disease (COPD), and cancer) and population vital statistics for death. In our analyses, we leveraged powerful linkages between survey responses and administrative health data, which allowed us to prospectively follow-up chronic disease and mortality outcomes for up to 6 years after life satisfaction was assessed. Multilinked data were used to establish whether the association persisted after adjustment for age and sex, comorbidity measured using health records, mood disorder, health-relevant behaviors (i.e., smoking, alcohol consumption, and physical activity), and immigrant status.
METHODS
Data sources
Participants were pooled from 3 cycles of the Canadian Community Health Survey (CCHS): cycle 2.1 (2003–2004), cycle 3.1 (2005–2006), and cycle 4.1 (2007–2008). Each participant was linked deterministically (i.e., via exact matching) and individually to population-based health administrative databases for Ontario, Canada. Deterministic linkage refers to an all-or-nothing linkage approach, wherein records are matched on the basis of an exact match of unique identifying information. This approach is superior in contrast to probabilistic linkage, where exact matches may not be found (11). The CCHS is a cross-sectional survey administered by Statistics Canada and is representative of 98% of the Canadian population aged 12 years or older living in private dwellings; response rates are greater than 75%. The CCHS gathers up-to-date, cross-sectional data about the distribution of health determinants and outcomes, as well as health care use, across Canada (11). CCHS data are representative at national and provincial levels; detailed survey methodology is available elsewhere (12).
This study received ethics approval from the University of Toronto Research Ethics Board (protocol reference no. 32666). Of survey respondents, 84% consented to have their responses linked to the data from Ontario’s single-payer health insurance program, the Ontario Health Insurance Plan (OHIP), which contains all the data to capture that are relevant to chronic disease diagnosis. Our study cohort was restricted to CCHS respondents who consented to have their data linked, reported on life satisfaction, were eligible for OHIP throughout the follow-up period, and were aged 18 years or older as of the CCHS interview date. All survey respondents were also linked to Ontario’s population registry, the Registered Persons Database, which captures core demographic (i.e., age, sex) and death information for all OHIP-eligible residents.
The analytic sample included adults who reported on life satisfaction, were eligible for OHIP throughout the study period, and did not have a prevalent chronic disease at baseline. Because OHIP eligibility requires residency in Ontario, Canada, our sample was effectively limited to Ontario CCHS respondents. Overall, 101,719 individuals were successfully linked across the 3 combined survey cycles. After removing duplicate records (n = 532) and excluding people younger than 18 years of age (n = 9,633), those missing life satisfaction data (n = 1,723), and those without complete OHIP eligibility (n = 121), there were 89,710 individuals included, of whom 73,904 had no prevalent chronic disease (i.e., diabetes, CHD, or COPD) at baseline. The creation of our analytic sample is described in Web Figure 1 (available at https://academic.oup.com/aje).
Variable definitions
Exposure
Information about participants’ life satisfaction was captured using the question “How satisfied are you with your life in general?” with response options of “very satisfied,” “satisfied,” “neither satisfied nor dissatisfied,” “dissatisfied” or “very dissatisfied.” Very dissatisfied and dissatisfied were pooled, resulting in a 4-level life satisfaction variable as the main independent variable. Self-report has been shown to be a reliable and valid measure of life satisfaction in population survey data (13, 14). Furthermore, single-item measures of life satisfaction perform highly similarly to equivalent multidimensional measures (15).
Outcome
Information on chronic conditions was captured using validated derived chronic disease case–detection algorithms for diabetes, myocardial infarction, congestive heart failure, and COPD (16–19). Incident myocardial infarction and congestive heart failure cases were grouped together as CHD in the analysis. Cancer data were captured from the Ontario Cancer Registry (20). Information on deaths was captured using the Registered Persons’ Database and Vital Statistics data.
Other covariates
Interview questions were used to capture baseline sociodemographic and behavioral covariates, body mass index, and mood disorder diagnoses. Sociodemographic characteristics captured included age, sex, household income quintile, household educational attainment, and immigrant status. Health-relevant behaviors included smoking, alcohol consumption, and physical activity level. Mood disorder diagnosis was based on participant self-report of a physician-diagnosed mood disorder, such as depression, bipolar disorder, mania, or dysthymia. Comorbidity at baseline was captured using Aggregated Diagnosis Group (ADG) scores (21) based on data from administrative health records. ADG clusters are broad diagnostic categories that can be derived from clinical diagnoses recorded in administrative health records. Examples of ADG groups include Asthma (ADG 6); Chronic Medical: Unstable, such as anemia or cystic fibrosis (ADG 11); and Signs/Symptoms: Minor, such as headache or limb pain (ADG 28) (21, 22). ADG scores, which measure the number of ADGs a patient has a diagnosis in, have been validated for use in Ontario and are reliable for morbidity adjustment (22, 23).
Statistical analyses
Cox proportional hazards models were run to estimate the hazards associated with baseline life satisfaction on risk of separate chronic disease endpoints (i.e., diabetes, cancer, COPD, CHD) developing and death. Time was defined as the interval between interview date and an event (i.e., incident chronic disease or death) or censoring. Censoring took place 6 years after the interview date; at the study endpoint of March 31, 2015; or, for chronic disease models only, in case of death.
Three models were run to study the association and compare the impact of confounder adjustment. The first model was adjusted for age, sex, and survey cycle. Second, a minimally adjusted model included age, sex, survey cycle, ADG comorbidity score, household income quintile, and immigrant status. Finally, a fully adjusted model was fit that included the terms in the minimally adjusted model plus mood disorder diagnosis, smoking, alcohol consumption, physical activity level, body mass index, and household educational attainment. The proportional hazard assumption was checked and verified for all models.
Bootstrap sampling weights provided by Statistics Canada were applied, using balanced repeated replication, to all analyses to account for the complex survey design of the CCHS (24). All analyses, including descriptive statistics, were based on the weighted cohort, which is representative of the Ontario adult population.
We conducted a sensitivity analysis to further limit the potential influence of reverse causation. We estimated lagged models, excluding cases occurring within 2 years of the interview date, to capture any baseline disease that may have influenced reporting of life satisfaction but was not captured in the diagnostic cohorts. We also estimated models for the age strata younger than 60 years and 60 years or older to assess whether the associations between life satisfaction and chronic disease and death differed by age. All statistical analyses were performed using SAS, version 9.4 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
Cohort characteristics at baseline are shown in Table 1. The cohort had a mean follow-up time of 2,041 days (5.6 years) for chronic disease (minimum of 0 days, maximum of 2,190 days (6 years)) and 2,129 days (5.8 years) for death (minimum of 18 days, maximum of 2,190 days (6 years)).
Table 1.
Baseline Characteristics (Weighted) of Pooled Study Participants With No Chronic Conditions at Baseline According to Life Satisfaction, Surveyed From 2003 to 2007 and Followed to 2015 (n = 73,904), Canadian Community Health Survey Ontario, Canada
| Characteristic | Life Satisfaction | |||
|---|---|---|---|---|
| Very Satisfied or Satisfied, % (n = 67,366)a | Neither Satisfied or Dissatisfied, % (n = 4,105)a | Dissatisfied, % (n = 2,017)a | Very Dissatisfied, % (n = 416)a | |
| Sex | ||||
| Male | 48.4 | 46.6 | 50.3 | 43.8 |
| Female | 51.6 | 53.4 | 49.7 | 56.2 |
| Age group, years | ||||
| <40 | 45.2 | 50.4 | 37.5 | 33.9 |
| 40–49 | 23.2 | 21.0 | 31.1 | 26.9 |
| 50–59 | 15.9 | 15.8 | 19.6 | 24.2 |
| 60–69 | 9.1 | 7.5 | 7.6 | 6.3 |
| 70–79 | 4.8 | 3.8 | 2.9 | 5.2 |
| ≥80 | 1.8 | 1.5 | 1.3 | 3.5 |
| Immigrant | ||||
| No | 69.1 | 62.9 | 60.6 | 64.1 |
| Yes | 30.6 | 36.9 | 38.9 | 35.7 |
| Highest education level in household | ||||
| No postsecondary | 79.0 | 75.1 | 71.2 | 66.1 |
| Postsecondary | 5.8 | 7.5 | 8.0 | 10.3 |
| Household income quintile | ||||
| 1 (lowest) | 13.0 | 24.7 | 31.5 | 37.7 |
| 2 | 16.5 | 20.5 | 17.7 | 17.9 |
| 3 | 18.2 | 17.8 | 14.5 | 14.2 |
| 4 | 21.8 | 14.3 | 15.4 | 10.5 |
| 5 (highest) | 22.3 | 13.6 | 12.3 | 9.8 |
| ADG comorbidity scoreb,c | 2.92 (0.05) | 3.79 (0.24) | 5.06 (0.37) | 6.80 (0.64) |
| Smoking status | ||||
| Current smoker | 22.4 | 32.6 | 38.8 | 40.5 |
| Former smoker | 21.6 | 17.6 | 19.5 | 18.6 |
| Nonsmoker | 55.8 | 49.7 | 41.4 | 40.9 |
| Alcohol consumption | ||||
| Current nondrinker | 17.4 | 21.0 | 24.7 | 28.6 |
| Occasional drinker | 15.9 | 20.2 | 22.6 | 28.4 |
| Regular drinker | 58.8 | 50.4 | 42.4 | 34.6 |
| Occasional binge drinker | 7.6 | 8.1 | 9.6 | 8.1 |
| Physical activity status | ||||
| Active | 26.1 | 18.1 | 17.4 | 17.7 |
| Moderate | 25.6 | 22.1 | 18.7 | 15.1 |
| Inactive | 48.3 | 59.7 | 63.8 | 67.1 |
| Body mass indexd | ||||
| Underweight (<18.5) | 2.7 | 4.1 | 3.9 | 4.9 |
| Normal weight (18.5–24.9) | 47.2 | 47.1 | 43.2 | 39.9 |
| Overweight (25.0–29.9) | 33.3 | 29.4 | 31.2 | 31.0 |
| Moderately obese (30.0–34.9) | 10.4 | 9.7 | 11.9 | 16.7 |
| Very/severely obese (≥35.0) | 6.5 | 9.6 | 9.8 | 7.6 |
| Mood disorder | 4.9 | 16.2 | 34.7 | 41.8 |
| Average annual health care costs, CADb | 1,798.64 (24.35) | 2,272.85 (136.53) | 3,579.23 (325.12) | 5,720.66 (1,067.73) |
Abbreviations: ADG, Aggregated Diagnosis Group; BMI, body mass index; CAD, Canadian dollars.
a Weighted using bootstrap weights provided by Statistics Canada; sampling weights were used to produce population estimates.
b Values are expressed as mean (standard error).
c A weighted score based on an individual’s ADGs. Austin’s weighted ADG score has been described and validated elsewhere (39).
d Weight (kg)/height (m2).
Most respondents (n = 67,366, 91%) reported being satisfied or very satisfied with their life at baseline. Those who reported being very dissatisfied with life had a higher mean ADG comorbidity score when compared with very satisfied or satisfied respondents, indicating a greater burden of overall morbidity (6.80 vs. 2.92) (Table 1). Very dissatisfied respondents were also more likely to belong to the lowest income quintile (37.7% vs. 13.0%) and more likely to report current smoking (40.5% vs. 22.4%) and physical inactivity (67.1% vs. 48.3%) than very satisfied or satisfied respondents (Table 1).
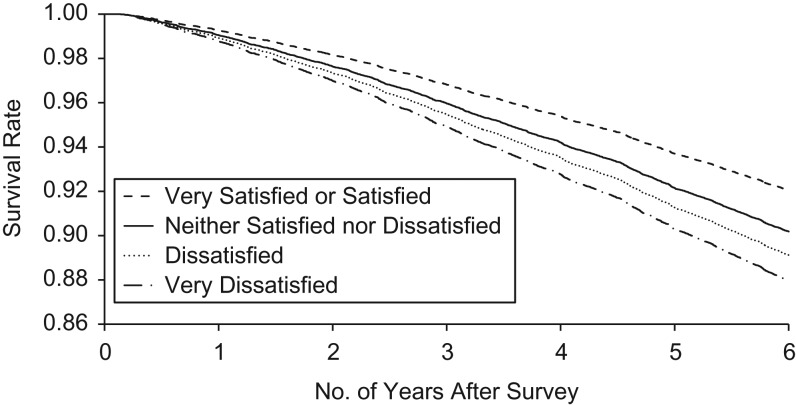
Among those with no prevalent chronic conditions of interest at baseline, poor life satisfaction at baseline was associated with increased risk of a chronic disease endpoint of diabetes, cancer, CHD, or COPD. In the age-sex-cycle adjusted model, the hazard ratio for any chronic disease endpoint was 2.49 for individuals who reported being very dissatisfied at baseline, compared to those who reported being satisfied or very satisfied (95% confidence interval (CI): 1.69, 3.67) (Table 2). Mortality risk also increased with increasing levels of dissatisfaction (Figure 1). In the equivalent age-sex-cycle adjusted model for mortality, those who reported being very dissatisfied at baseline experienced 2.51 times the risk of death of those who were satisfied or very satisfied at baseline (95% CI: 1.81, 3.47) (Table 3). In models adjusted for age, sex, and survey cycle for chronic disease endpoints and death, a dose-response effect was seen for increasing levels of life dissatisfaction, with the highest risk of incident chronic disease and death observed among individuals who were very dissatisfied with their lives at baseline (Tables 2 and 3). Compared with the very satisfied or satisfied group, statistically significant hazards were observed in the model adjusted for age, sex, and survey cycle for all other life-satisfaction groups, for both chronic disease and death (Tables 2 and 3).
Table 2.
Multivariable Adjusted Hazard Models for Life Satisfaction and of Any Incident Chronic Disease for Pooled Participants Surveyed From 2003 to 2007 and Followed to 2015 (n = 73,904), Canadian Community Health Survey, Ontario, Canada
| Life Satisfaction | No.a | Adjusted for Age, Sex, and Survey Cycle | Minimally Adjustedb | Fully Adjustedc | |||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | ||
| Very satisfied or satisfied | 67,366 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Neither satisfied nor dissatisfied | 4,105 | 1.17 | 0.99, 1.38 | 1.07 | 0.90, 1.25 | 0.96 | 0.83, 1.13 |
| Dissatisfied | 2,017 | 1.62 | 1.37, 1.92 | 1.42 | 1.19, 1.69 | 1.19 | 1.00, 1.43 |
| Very dissatisfied | 416 | 2.49 | 1.69, 3.67 | 2.14 | 1.46, 3.15 | 1.70 | 1.16, 2.51 |
| 2-sided P for trend | <0.0001 | <0.0001 | 0.0014 | ||||
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Unweighted.
b Minimally adjusted model includes age, sex, survey cycle, adjusted diagnostic groups comorbidity score, household income quintile, and immigrant status.
c Fully adjusted model includes age, sex, survey cycle, adjusted diagnostic groups comorbidity score, household income quintile, household educational attainment, immigrant status, mood disorder, smoking, alcohol consumption, physical activity level, and body mass index.
Figure 1.
Adjusted survival curves according to baseline life satisfaction among a cohort of 73,904 pooled Canadian Community Health Survey participants surveyed from 2003 to 2007 and followed to 2015 (Ontario, Canada).
Table 3.
Multivariable Adjusted Hazard Models for Life Satisfaction and Risk of Death for Pooled Participants Surveyed From 2003 to 2007 and Followed to 2015 (n = 73,904), Canadian Community Health Survey, Ontario, Canada
| Life Satisfaction | No.a | Adjusted for Sex, Age, and Survey Cycle | Minimally Adjustedb | Fully Adjustedc | |||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | ||
| Very satisfied or satisfied | 67,366 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Neither satisfied nor dissatisfied | 4,105 | 1.36 | 1.15, 1.61 | 1.21 | 1.02, 1.43 | 1.05 | 0.88, 1.25 |
| Dissatisfied | 2,017 | 1.91 | 1.56, 2.35 | 1.50 | 1.22, 1.85 | 1.26 | 1.00, 1.58 |
| Very dissatisfied | 416 | 2.51 | 1.81, 3.47 | 1.81 | 1.30, 2.50 | 1.54 | 1.10, 2.16 |
| 2-sided P for trend | <0.0001 | 0.0002 | 0.0038 | ||||
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Unweighted.
b Minimally adjusted model includes age, sex, survey cycle, adjusted diagnostic groups comorbidity score, household income quintile, and immigrant status.
c Fully adjusted model includes age, sex, survey cycle, adjusted diagnostic groups comorbidity score, household income quintile, household educational attainment, immigrant status, mood disorder, smoking, alcohol consumption, physical activity level, and body mass index.
The observed associations were attenuated by multivariable adjustment for ADG comorbidity score, household income quintile, and immigrant status (minimally adjusted models), and for smoking, alcohol consumption, physical activity level, household educational attainment, and body mass index (fully adjusted models) (Tables 2 and 3). The hazard ratio for incident chronic disease for the very dissatisfied group compared with the very satisfied or satisfied group was 1.70 in the fully adjusted model (95% confidence interval (CI): 1.16, 2.51), compared with hazard ratios of 2.49 (95% CI: 1.69, 3.67) in the model adjusted for age, sex, and survey cycle and 2.14 (95% CI: 1.46, 3.15) in the minimally adjusted model (Table 2). A similar pattern was observed for the same group in the death model, for which the hazard ratio estimate was 1.54 (95% CI: 1.10, 2.16) in the fully adjusted model, compared with hazard ratios of 2.51 (95% CI: 1.81, 3.47) and 1.81 (95% CI: 1.30, 2.50) in the model adjusted for age, sex, and survey cycle and the minimally adjusted model, respectively (Table 3). Despite the attenuation of effect size, the dose response seen in the model adjusted for age, sex, and survey cycle was still observed. For each model, both chronic disease and death risks were highest among individuals who reported being very dissatisfied at baseline and were lowest among those who reported being very satisfied or satisfied (Tables 2 and 3).
When the fully adjusted model was fit for specific chronic conditions, the strongest association between life satisfaction and chronic disease was observed for COPD (Table 4). Individuals who reported being very dissatisfied at baseline experienced a 76% higher risk of having COPD than those who were very satisfied or satisfied at baseline (hazard ratio = 1.76, 95% CI: 0.90, 3.43). The trend in COPD risk was statistically significant across all levels of baseline life satisfaction (Table 4). The trend across life satisfaction groups was also significant for diabetes and CHD risk, though the effect-size estimates for chronic disease risk in the very dissatisfied group were smaller than observed with COPD (for diabetes, hazard ratio = 1.27, 95% CI: 0.82, 1.97; for CHD, hazard ratio = 1.65, 95% CI: 0.73, 3.75). The weakest association was seen in cancer outcomes, which did not differ significantly by baseline life-satisfaction groups (Table 4). In all cases, including for cancer, a dose-response pattern was observed wherein hazard ratio estimates increased with each increasing level of life dissatisfaction (Table 4). Additional disease-specific results from the model adjusted for age, sex, and survey cycle and the minimally adjusted model are shown in Web Table 1.
Table 4.
Fully Adjusteda Hazard Models for Life Satisfaction and of Specific Chronic Disease for Pooled Participants Surveyed From 2003 to 2007 and Followed to 2015 (n = 73,904), Canadian Community Health Survey, Ontario, Canada
| Life Satisfaction | No.b | Risk of Diabetes | Risk of Coronary Heart Disease | Risk of COPD | Risk of Cancer | ||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | ||
| Very satisfied or satisfied | 67,366 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Neither satisfied nor dissatisfied | 4,105 | 0.98 | 0.78, 1.22 | 1.26 | 0.96, 1.67 | 1.02 | 0.77, 1.34 | 0.91 | 0.73, 1.12 |
| Dissatisfied | 2,017 | 1.10 | 0.86, 1.39 | 1.46 | 1.12, 1.92 | 1.46 | 1.07, 1.98 | 0.95 | 0.72, 1.25 |
| Very dissatisfied | 416 | 1.27 | 0.82, 1.97 | 1.65 | 0.73, 3.75 | 1.76 | 0.90, 3.43 | 1.19 | 0.75, 1.89 |
| 2-sided P for trend | 0.025 | 0.001 | 0.0005 | 0.82 | |||||
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; HR, hazard ratio.
a Fully adjusted model includes age, sex, survey cycle, adjusted diagnostic groups comorbidity score, household income quintile, household educational attainment, immigrant status, mood disorder, smoking, alcohol consumption, physical activity level, and body mass index.
b Unweighted.
Sensitivity analysis
The results of our lagged-model sensitivity analysis are listed in Table 5. Excluding cases with chronic disease diagnoses received not more than 2 years after survey response date led to a slight attenuation (<10%) of hazard ratio estimates for CHD, COPD, and any incident chronic disease compared with the primary models (Table 5; for comparison see Tables 2 and 4). However, we observed no notable changes in these models relative to the overall pattern, direction, or statistical significance of the observed associations (Table 5). In the age-stratified sensitivity analysis (<60 years vs. ≥60 years), risk for chronic disease and death was consistently associated in the fully adjusted models with being very dissatisfied. For those younger than 60 years, the adjusted hazard ratios for chronic disease and death were, respectively, 1.78 (95% CI: 1.02, 3.11) and 2.21 (95% CI: 1.19, 4.08). For those age 60 years or older, the respective hazard ratios for chronic disease and death were 2.10 (95% CI: 1.23, 3.57) and 1.39 (95% CI: 0.95, 2.03).
Table 5.
Fully Adjusteda Hazard Models for Life Satisfaction and of Specific Chronic Disease for Pooled Participants Surveyed From 2003 to 2007 and Followed to 2015, Excluding Cases Occurring Within 2 Years of Interview Date (n = 73,904), Canadian Community Health Survey, Ontario, Canada
| Life Satisfaction | No.b | Risk of Diabetes | Risk of Coronary Heart Disease | Risk of COPD | Risk of Cancer | Risk of Any Incident Chronic Disease | Risk of Death | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | ||
| Very satisfied or satisfied | 67,366 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Neither satisfied nor dissatisfied | 4,105 | 1.02 | 0.78, 1.33 | 1.35 | 0.99, 1.85 | 1.06 | 0.77, 1.47 | 0.84 | 0.65, 1.08 | 0.99 | 0.82, 1.21 | 1.00 | 0.83, 1.20 |
| Dissatisfied | 2,017 | 1.26 | 0.93, 1.69 | 1.46 | 1.06, 2.01 | 1.56 | 1.08, 2.25 | 1.08 | 0.80, 1.46 | 1.34 | 1.07, 1.69 | 1.28 | 1.00, 1.64 |
| Very dissatisfied | 416 | 1.47 | 0.84, 2.55 | 1.49 | 0.44, 5.07 | 1.63 | 0.89, 2.98 | 1.22 | 0.70, 2.14 | 1.81 | 1.06, 3.10 | 1.61 | 1.12, 2.31 |
| 2-sided P for trend | 0.014 | 0.0047 | 0.0002 | 0.71 | 0.0049 | 0.0099 | |||||||
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; HR, hazard ratio.
a Fully adjusted model includes age, sex, survey cycle, adjusted diagnostic groups comorbidity score, household income quintile, household educational attainment, immigrant status, mood disorder, smoking, alcohol consumption, physical activity level, and body mass index.
b Unweighted.
DISCUSSION
In this large, population-based cohort study, we demonstrate that poor life satisfaction is associated with increased development of multiple chronic diseases and risk for death. Risk of both death and chronic disease increased for each progressive level of self-reported life dissatisfaction and was highest among individuals who reported being very dissatisfied with their lives at baseline. After adjustment for several demographic, socioeconomic, and behavioral traits, the association between life satisfaction and studied health outcomes was attenuated but not eliminated. When the fully adjusted model was implemented for specific chronic disease outcomes (i.e., diabetes, CHD, COPD, and cancer), significant associations with life satisfaction were seen for diabetes, CHD, and COPD, but not for cancer.
These findings demonstrate that poor life satisfaction is associated with elevated risk of incident chronic disease and death, independent of sociodemographic, health, and behavioral risk factors traditionally conceived as being predictive of health outcomes. Importantly, our findings are also independent of diagnosed mood disorders, providing strong evidence that life satisfaction, and not merely the absence of psychological distress, can have meaningful implications for future chronic disease risk. Because life satisfaction was measured at baseline with up to 6 years of follow-up for chronic disease and death outcomes, and given that baseline comorbidity was accounted for, it is unlikely that the observed association can be explained by reverse causation. The potential influence of reverse causation is further limited by the findings of our sensitivity analysis, which excluded from models incident cases occurring within2 years after interview. These lagged models showed no meaningful differences in the direction or statistical significance of all observed associations and only a slight attenuation of effect size. The results of our work, in concordance with findings from other settings, suggest subjective well-being may directly affect individual health outcomes (7).
The attenuation of the hazards after adjustment suggests that the association between life satisfaction and risk for chronic disease and death may be confounded by lifestyle-related risk factors. It may be that individuals who are most dissatisfied with their life may also engage in unhealthy lifestyle habits, which contribute to their chronic disease risk. This is consistent with research in the literature hypothesizing that the association between life satisfaction and death is mediated by changes in lifestyle factors (e.g., physical activity, smoking, or diet) (10). For instance, negative affect has been correlated with unhealthy behaviors, including initiation and maintenance of smoking (25). These results support the need for analyses to be conducted to understand the nature of the association between life satisfaction and health behaviors and the mechanisms by which they may influence chronic disease and death risk.
Biological mechanisms by which life satisfaction affects chronic disease risk have been proposed in the literature. These proposed mechanisms broadly implicate systemic biological processes. Greater life satisfaction can be linked to restorative processes, such as elevated serum antioxidant levels (26). Simultaneously, life satisfaction may be protective against deteriorative processes and indicators, including dysregulated cortisol output (27), neuroendocrine pathways, and inflammation (28). In the proposed pathway, individuals with positive life satisfaction experience greater expression of health-protective processes and less activity of deleterious biological pathways (4), reducing their risk of chronic disease and death (26). The physiological mechanism connecting life satisfaction and morbidity is best described in the literature for diseases of the cardiovascular system (2). This connection is consistent with the significant associations observed in our analyses between life satisfaction and CHD. Our results suggest that diabetes and COPD outcomes are also strongly linked to life satisfaction.
We did not observe a significant association between life satisfaction and cancer after adjusting for sociodemographic and behavioral covariates, which is inconsistent with other findings (29, 30). However, few studies have been published regarding the association between incident cancer and psychological well-being, which is conceptually distinct from the absence of psychological distress (31). This is an area that warrants more investigation. In the literature on psychological distress and cancer outcomes, the findings are mixed. Null associations between psychological distress traits and incident cancer have been shown in several prospective cohort analyses (32–34). However, site-specific analyses revealed positive associations between psychological distress and pancreatic and prostate cancers (35, 36), and null or negative associations with breast and smoking-related cancers (32, 34, 35, 37). These findings suggest that the association between psychological distress and cancer outcomes may be heterogeneous and dependent on cancer site. It is possible that site-specific analyses of the association between psychological distress and life satisfaction would reveal the same heterogeneity.
Potential limitations of this study include the use of baseline survey data to capture health behavior information, with no ability to capture or account for changes in these behaviors over the study period. For consistency, survival was modelled for 6 years after death for all individuals participating in the CCHS. It is possible that behavioral patterns may have changed during the follow-up time, with implications for chronic disease and death risk. Also, we used a single-item measure of life satisfaction, which is not as comprehensive as a multidimensional scale. Although single-item scales have been shown to perform similarly to multidimensional scales, their scope is somewhat limited (15).
The study findings also may have been affected by reverse causation if survey respondents had prevalent chronic disease at baseline that was not captured by the existing disease cohorts. In this case, self-reported life satisfaction at baseline may have been negatively affected by the symptoms of undiagnosed disease. The disease cohorts have been validated and effectively identify population cohorts with diagnosed disease, which, in a single-payer system, likely represents the majority of symptomatic cases (16, 18, 19, 38). In addition, results from our sensitivity analysis using lagged models did not meaningfully differ from those of our primary analysis, further limiting the risk of reverse causation being the explanation.
This study joins a growing body of evidence characterizing life satisfaction as an important risk factor for future disease and death. By using a large, representative, and population-based cohort and integrating data for a wide variety of disease risk factors, its findings lend compelling support to the idea that life satisfaction can affect chronic disease and death risk independently of traditional physiological pathways and independently of psychological distress. This research supports the need for more upstream interventions and social programs that will ensure all can have an opportunity for maximum life satisfaction. Additional research that elucidates the specific mechanism by which this association takes place can be used to refine where best to intervene and protect individuals and improve population health.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (Laura C. Rosella, Emmalin Buajitti, Vivek Goel); Institute for Clinical Evaluative Sciences, Toronto, Ontario, Canada (Laura C. Rosella, Longdi Fu, Vivek Goel); and Public Health Ontario, Toronto, Ontario, Canada (Laura C. Rosella).
This study was supported by the Institute for Clinical Evaluative Sciences, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. V. G. is supported by University of Toronto research funds and L. C. R. is supported by a Canada Research Chair (CRC-950-23072).
Parts of this material are based on data and information compiled and provided by Immigration, Refugees, and Citizenship Canada.
The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources.
Conflict of interest: none declared.
Abbreviations
- ADG
Aggregated Diagnosis Group
- CCHS
Canadian Community Health Survey
- CHD
coronary heart disease
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- OHIP
Ontario Health Insurance Plan
REFERENCES
- 1. Diener E, Chan MY. Happy people live longer: subjective well-being contributes to health and longevity. Appl Psychol Health Well Being. 2011;3(1):1–43. [Google Scholar]
- 2. Boehm JK, Kubzansky LD. The heart’s content: the association between positive psychological well-being and cardiovascular health. Psychol Bull. 2012;138(4):655–691. [DOI] [PubMed] [Google Scholar]
- 3. Howell RT, Kern ML, Lyubomirsky S. Health benefits: meta-analytically determining the impact of well-being on objective health outcomes. Health Psychol Rev. 2007;1(1):83–136. [Google Scholar]
- 4. Pressman SD, Cohen S. Does positive affect influence health? Psychol Bull. 2005;131(6):925–971. [DOI] [PubMed] [Google Scholar]
- 5. Chida Y, Hamer M. An association of adverse psychosocial factors with diabetes mellitus: a meta-analytic review of longitudinal cohort studies. Diabetologia. 2008;51(12):2168–2178. [DOI] [PubMed] [Google Scholar]
- 6. Feller S, Teucher B, Kaaks R, et al. Life satisfaction and risk of chronic diseases in the European Prospective Investigation Into Cancer and Nutrition (EPIC)-Germany study. PLoS One. 2013;8(8):e73462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chida Y, Steptoe A. Positive psychological well-being and mortality: a quantitative review of prospective observational studies. Psychosom Med. 2008;70(7):741–756. [DOI] [PubMed] [Google Scholar]
- 8. Zaninotto P, Wardle J, Steptoe A. Sustained enjoyment of life and mortality at older ages: analysis of the English Longitudinal Study of Ageing. BMJ. 2016;355:i6267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martín-María N, Miret M, Caballero FF, et al. The impact of subjective well-being on mortality: a meta-analysis of Longitudinal Studies in the General Population. Psychosom Med. 2017;79(5):565–575. [DOI] [PubMed] [Google Scholar]
- 10. Koopmans TA, Geleijnse JM, Zitman FG, et al. Effects of happiness on all-cause mortality during 15 years of follow-up: the Arnhem elderly study. J Happiness Stud. 2010;11(1):113–124. [Google Scholar]
- 11. Roos LL, Wajda A. Record linkage strategies. Part I: estimating information and evaluating approaches. Methods Inf Med. 1991;30(2):117–123. [PubMed] [Google Scholar]
- 12. Statistics Canada Canadian Community Health Survey 2003: User Guide for the Public Use Microdata File Ottawa, ON, Canada: Statistics Canada; 2005. (Catalogue no. 82M0013GPE).
- 13. Bonikowska A, Helliwell JF, Hou F, et al. An assessment of life satisfaction responses on recent statistics Canada surveys. Soc Indic Res. 2014;118(2):617–643. [Google Scholar]
- 14. Pavot W, Diener E. Review of the satisfaction with life scale. Psychol Assess. 1993;5(2):164–172. [Google Scholar]
- 15. Cheung F, Lucas RE. Assessing the validity of single-item life satisfaction measures: results from three large samples. Qual Life Res. 2014;23(10):2809–2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Austin PC, Daly PA, Tu JV. A multicenter study of the coding accuracy of hospital discharge administrative data for patients admitted to cardiac care units in Ontario. Am Heart J. 2002;144(2):290–296. [DOI] [PubMed] [Google Scholar]
- 17. Gershon AS, Wang C, Guan J, et al. Identifying individuals with physcian diagnosed COPD in health administrative databases. COPD. 2009;6(5):388–394. [DOI] [PubMed] [Google Scholar]
- 18. Hux JE, Ivis F, Flintoft V, et al. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care. 2002;25(3):512–516. [DOI] [PubMed] [Google Scholar]
- 19. Schultz SE, Rothwell DM, Chen Z, et al. Identifying cases of congestive heart failure from administrative data: a validation study using primary care patient records. Chronic Dis Inj Can. 2013;33(3):160–166. [PubMed] [Google Scholar]
- 20. Robles SC, Marrett LD, Clarke EA, et al. An application of capture-recapture methods to the estimation of completeness of cancer registration. J Clin Epidemiol. 1988;41(5):495–501. [DOI] [PubMed] [Google Scholar]
- 21. The Johns Hopkins University Bloomberg School of Public Health The Johns Hopkins ACG Case-Mix System: Version 6.0 Release Notes Baltimore, MD: The Johns Hopkins University; 2003.
- 22. Austin PC, van Walraven C, Wodchis WP, et al. Using the Johns Hopkins Aggregated Diagnosis Groups (ADGs) to predict mortality in a general adult population cohort in Ontario, Canada. Med Care. 2011;49(10):932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sibley LM, Moineddin R, Agha MM, et al. Risk adjustment using administrative data-based and survey-derived methods for explaining physician utilization. Med Care. 2010;48(2):175–182. [DOI] [PubMed] [Google Scholar]
- 24. Kolenikov S. Resampling variance estimation for complex survey data. Stata J. 2010;10(2):165–199. [Google Scholar]
- 25. Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129(2):270–304. [DOI] [PubMed] [Google Scholar]
- 26. Smith AW, Baum A. The influence of psychological factors on restorative function in health and illness In: Suls J, Wallston KA, eds. Social Psychological Foundations of Health and Illness. Malden, MA: Blackwell Publishing; 2009:432–457. [Google Scholar]
- 27. McEwen BS. Protective and damaging effects of stress mediators: central role of the brain. Dialogues Clin Neurosci. 2006;8(4):367–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Steptoe A, Wardle J, Marmot M. Positive affect and health-related neuroendocrine, cardiovascular, and inflammatory processes. Proc Natl Acad Sci USA. 2005;102(18):6508–6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chida Y, Hamer M, Wardle J, et al. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol. 2008;5:466–475. [DOI] [PubMed] [Google Scholar]
- 30. Lillberg K, Verkasalo PK, Kaprio J, et al. Stressful life events and risk of breast cancer in 10,808 women: a cohort study. Am J Epidemiol. 2003;157(5):415–423. [DOI] [PubMed] [Google Scholar]
- 31. Pavot W, Diener E. The satisfaction with life scale and the emerging construct of life satisfaction. J Posit Psychol. 2008;3(2):137–152. [Google Scholar]
- 32. Gradus JL. Prevalence and prognosis of stress disorders: a review of the epidemiologic literature. Clin Epidemiol. 2017;9:251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gradus JL, Farkas DK, Svensson E, et al. Posttraumatic stress disorder and cancer risk: a nationwide cohort study. Eur J Epidemiol. 2015;30(7):563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Trudel-Fitzgerald C, Chen Y, Singh A, et al. Psychiatric, psychological, and social determinants of health in the Nurses’ Health Study cohorts. Am J Public Health. 2016;106(9):1644–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Batty GD, Russ TC, Stamatakis E, et al. Psychological distress in relation to site specific cancer mortality: pooling of unpublished data from 16 prospective cohort studies. BMJ. 2017;356:j108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shen CC, Hu YW, Hu LY, et al. The risk of cancer in patients with generalized anxiety disorder: a nationwide population-based study. PLoS One. 2013;8(2):e57399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sawada T, Nishiyama T, Kikuchi N, et al. The influence of personality and perceived stress on the development of breast cancer: 20-year follow-up of 29,098 Japanese women. Sci Rep. 2016;6:32559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gershon AS, Wang C, Guan J, et al. Identifying patients with physician-diagnosed asthma in health administrative databases. Can Respir J. 2009;16(6):183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Austin PC, Walraven CV. The mortality risk score and the ADG score: two points-based scoring systems for the Johns Hopkins aggregated diagnosis groups to predict mortlaity in a general adult population cohort in Ontario, Canada. Med Care. 2011;49(10):940–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.