Abstract
High birth weight is associated with increased breast cancer risk and, less consistently, with higher mammographic density. In contrast, adolescent body size has been consistently, negatively associated with both MD and breast cancer risk. It is unclear when the direction of these associations changes and whether weight gain in infancy is associated with MD. We evaluated the associations of birth weight and postnatal weight (measured at 4 months, 1 year, and 4 years) by absolute and velocity measures (relative within-cohort percentile changes) with adult mammographic density, assessed using a computer-assisted thresholding program (Cumulus), using linear regression models with generalized estimating equations to account for correlation between siblings in the Early Determinants of Mammographic Density study (1959–2008; n = 700 women with 116 sibling sets; mean age = 44.1 years). Birth weight was positively associated with dense area (per 1-kg increase, β = 3.36, 95% confidence interval (CI): 0.06, 6.66). Weight gains from 0 months to 4 months and 1 year to 4 years were negatively associated with dense area (for 10-unit increase in weight percentile, β = −0.65, 95% CI: −1.23, −0.07, and β = −1.07, 95% CI: −1.98, −0.16, respectively). Findings were similar in the sibling subset. These results support the hypothesis that high birth weight is positively associated with increased breast density and suggest that growth spurts starting in early infancy reduce mammographic dense area in adulthood.
Keywords: birth weight, breast cancer risk, dense area, early life growth, infant growth, mammographic density, percent density
The majority of states in the United States now require notification about mammographic density (MD), a major risk factor for breast cancer (BC), for women undergoing screening mammography (1, 2). Although many adult BC risk factors also are related to MD (3) (e.g., lack of physical activity (4), parity and breast feeding (5), and alcohol consumption (6)), a large portion of MD remains unexplained by adult risk factors, suggesting that MD might be driven by underlying genetic susceptibility. Twin and genome-wide association studies support a role for genetic variants in explaining some variation in MD, with some twin studies suggesting concordance as high as 0.63 in MD in monozygotic twins compared with 0.27 in dizygotic twins (7, 8). Genome-wide association studies have found 9 genetic variants related to MD (9, 10). In addition to genetics, the large portion of MD unexplained by adult risk factors might also be explained by exposures much earlier in life, including environmental and lifestyle exposures and their interactions with underlying genetic susceptibility.
A large body of epidemiologic and animal studies over the past 2 decades has emerged, supporting a role for the early-life and prenatal environment in shaping BC risk (11–22). For example, higher birth weight (15, 18, 23) and earlier breast development (24) have emerged as BC risk factors, independent of long-established indicators of early-life growth and development, including earlier age at menarche (25) and adult height (26). Most, but not all studies have found a positive association between birth weight and BC risk (27, 28), with the greatest consistency seen when data on birth weight are available through records and not recalled retrospectively (27). Even though higher birth weight has been related to increased BC risk, larger adolescent body size, primarily assessed by either body shapes such as recalled somatotypes and/or measured body mass index (BMI), has been consistently related to decreased risk of BC both before and after menopause (29–32). There is some evidence that also suggests that MD mimics these patterns (for review, see Yochum et al. (33)). It is unclear when the direction of these associations changes and whether the positive association seen with birth weight is also seen with large body size in infancy and childhood, particularly given that infancy is also a period when the mammary tissue is rapidly developing (30, 31). Of the studies that examined birth weight and MD (for reviews, see Yochum et al. (33) and Denholm et al. (34)), only a subset also had measures of childhood growth (35–38).
None of the existing studies of MD had measurements of weight gain during the first year of life, a dynamic period when most babies triple their weight in just 12 months (39). Infancy is also a time that has been associated with “minipuberty,” when endogenous steroid and growth hormones rapidly change and breast tissue develops and regresses in many girls (40–42). We recently conducted a study examining prospective measurements of infant weight gain and MD in an urban birth cohort and found negative associations between infant and early childhood weight gain and MD (43). However, given the smaller sample size and the lack of siblings in this cohort, we undertook a prospective study of MD in women of a similar age nested within the Child Health and Development Studies (CHDS) cohort (44) and the National Collaborative Perinatal Project (NCPP) (45), the Early Determinants of Mammographic Density (EDMD) study (46–49). EDMD is a larger study population with prospectively measured infant and childhood weight and height that includes a subgroup of siblings. We evaluated the association between birth weight, absolute weight, and relative change in weight at ages 4 months, 12 months, and 4 years and MD (% density and absolute dense area) in the overall cohort (n = 700) and among the subset of siblings (n = 116 sets). The sibling analyses were used specifically to evaluate the patterns between early-infant weight gain and MD, controlling for family characteristics including socioeconomic status.
METHODS
Study population
The EDMD study is an adult follow-up of women born in 2 US birth cohorts: the CHDS cohort, which was enrolled in California between 1959 and 1967 (44, 50), and 2 sites of the NCPP enrolled in Boston, Massachusetts, and Providence, Rhode Island, between 1959 and 1966 (45). Details of this cohort have been published previously (46–49). Briefly, we attempted to contact 1,925 women randomly selected from the 3,256 eligible women. We successfully traced 1,314 women, of whom 1,134 (86.3%) women participated in the study. The final sample included 521 singletons and 613 individuals in 296 sibling sets. Sibling sets included 277 sets of 2, 17 sets of 3, and 2 sets of 4 siblings (46). The study was approved by the institutional review boards at Columbia University Medical Center, New York, New York; Kaiser Permanente, San Francisco, California; Brigham and Women’s Hospital, Boston, Massachusetts; and Brown University, Providence, Rhode Island.
Baseline maternal and childhood data
At the prenatal clinic visits, study staff collected information on maternal and paternal age at registration, prepregnancy BMI (calculated as weight (kg)/height (m)2), weight gain during pregnancy, smoking during pregnancy, and maternal education at registration. Physicians diagnosed preeclampsia/toxemia at the time of the clinic visit (for details, see previous publications (45, 50, 51)). Gestational age was calculated by subtracting the date of the last menstrual period from the date of delivery. Trained personnel measured birth weight and birth length using standardized procedures on calibrated scales (45, 49–51). In the NCPP, trained clinical staff measured childhood height and weight at 4 months or 12 months, and at 4 years or 7 years of age (45). Serial growth measurements were abstracted from medical records until the age of 5 years in the CHDS (51).
Adult data collection
Women who agreed to participate in the adult follow-up completed a computer-assisted telephone interview (46). The adult follow-up ascertained information on sociodemographic characteristics (age at mammogram, race/ethnicity), BMI (calculated from self-reported height and weight at the time of interview and during ages 20–29 and 30–39 years), smoking status at the time of interview, history of alcohol intake, first degree family history of BC, and reproductive events (age at menarche, menopausal status, hormonal birth control use, and pregnancy history).
MD data
If participants responded in the telephone interview that they had or were planning to have a mammogram, we asked them to report on the facility where they had or planned to have their mammogram. Detailed information on the procurement and assessment of the mammogram data can be found in previous publications (46, 50). Among the 1,134 women in the sample, 87% (n = 981) had a previous mammogram or planned to obtain a mammogram, and 91% of these participants consented to providing their mammogram for density assessments in this study (n = 893). Mammograms for 23 participants could not be retrieved, 51 mammograms were of poor quality, and 119 participants had only digital mammograms available. Because the sensitivities for digital and film mammograms might differ, we restricted our sample to those with film mammograms for a final sample size of 700 women.
We assessed MD through Cumulus (Sunnybrook Health Sciences Centre, Toronto, Canada), a computer-assisted thresholding program (52). We measured total breast area (cm2), total dense area (cm2), and % density (dense area divided by breast area multiplied by 100). We calculated nondense (fat tissue) area as total breast area minus total dense area. All craniocaudal films that were available for a participant were read in one batch, and all films from sibling sets were read within the same batch. Each batch included films from NCPP and CHDS cohorts. Films were read in batches of approximately 50%, and 10% of the films had repeated readings from the same batch. We repeated an additional 10% of films in every batch to estimate batch-to-batch variability. For % density, the overall within-batch correlation coefficient was 0.96, and the between-batch correlation coefficient was 0.95 (46). We used measurements from the mammogram taken closest to the date of interview (median time interval between mammogram and date of interview = 0.8 years, interquartile range, 0.3–1.6 years). We used the left craniocaudal image if available and the right craniocaudal if the left was unavailable (46).
Statistical analysis
Because children were measured at different time points in the NCPP and CHDS, we used individual cubic interpolation splines (49, 53) to interpolate their height and weight measurements at ages 4 months, 1 year, and 4 years given their observed growth paths. We characterized childhood growth by within-cohort percentile changes regarding weight and height between 0 and 4 months, 4 and 12 months, and 1 and 4 years of age. Based on the percentile weight changes, we also categorized the weight change within each age window (i.e., 0–4 months, 4–12 months, and 1–4 years of age) into 3 patterns: rapid, stable, or slow weight change. The definition of growth pattern is based on the Centers for Disease Control and Prevention (CDC) growth chart reference percentiles (53). We defined rapid weight change as a within-cohort percentile rank increase of at least 1 major CDC reference percentile (5th, 10th, 25th, 50th, 75th, and 95th) of weight during the time period. We defined stable weight as within-cohort percentile rank that remained within one major CDC reference percentile, and we used this as the reference group. We defined slow weight change as a within-cohort percentile rank decrease of at least 1 major CDC reference percentile. For example, an increase from 10th percentile to over 25th percentile is considered to be “rapid” weight gain; a decrease from 50th percentile to lower than 25th percentile is considered “slow” weight gain.
We investigated the associations between growth parameters and MD (specifically % density, dense area, and nondense area). We first examined the associations between birth weight and BMI at ages 1, 4, and 20–29 years and MD using linear regression models with generalized estimating equations to account for correlation between siblings. We then examined the associations of the percentile changes in weight from 0 to 4 months, 4 to 12 months, and 1 to 4 years with MD. We then investigated associations between patterns of weight change in each interval (rapid, stable, or slow) with MD. In all models, we adjusted for age at interview, adult BMI, maternal prepregnancy BMI, gestational weight gain, maternal age at registration, maternal education, prenatal smoking exposure, and race/ethnicity. We controlled for weight or weight gain measures in the same or earlier time periods only. For example, when we estimated the association between birth weight and MD, we did not adjust for postnatal weight gain. But when examining the association between weight gain from 1 to 4 years and MD, we adjusted for birth weight, weight gain from 0 to 4 months, and weight gain from 4 to 12 months. Final models did not adjust for birth length or change in height in the 3 time intervals, because these variables were not associated with MD, and their inclusion did not appreciably change the effect estimates for birth weight and postnatal changes in weight. To assess whether associations between postnatal weight gain and MD differed according to size at birth, we stratified the final adjusted weight gain models by birth weight category (<3,000 grams, 3,000–3,854 grams, and ≥3,855 grams). We also performed sensitivity analyses for rapid-weight-gain models excluding infants born before 37 weeks of gestation (n = 27), infants with a birth weight below 2.5 kg (n = 37), or infants born small for gestational age, defined as infants below the within-cohort 10th percentile of birth weight divided by gestational age (n = 70).
We performed a difference-in-difference analysis within sibling sets to examine the associations between birth weight and percentile rank change in weight from 0 to 4 months, 4 to 12 months and 1 to 4 years and MD, controlling for family-level confounding such as socioeconomic status. In families with more than 2 siblings with available MD data, we chose 2 siblings at random for a total of 116 sibling sets (n = 232 individuals) in models with adjustments. For each sibling pair, we calculated their difference in MD (i.e., subtracting the lower MD measure from the higher MD), and measured the within-sibling differences in birth weight and weight changes accordingly. We then modeled the association between differences in growth parameters and difference in MD, while controlling for the differences in age, gestational weight gain, maternal age at pregnancy, and BMI at interview. MD outcomes, % density, dense area, and nondense area were modeled separately. We also stratified by the BMI difference between siblings (n = 80 sibling pairs with a BMI difference ≥2.5 and n = 36 pairs with a BMI difference <2.5) (54).
Participants with missing data on either the size or growth measures or covariates were excluded from relevant models. We performed sensitivity analyses excluding participants with any missing data from all analyses, yielding similar results (Web Table 1, available at https://academic.oup.com/aje).
RESULTS
Descriptive characteristics of the EDMD cohort and sibling subset are presented in Table 1. The overall cohort was primarily non-Hispanic white (78%). The mean age at interview was 44.1 years (range, 39–49 years), and the mean age at mammogram was 43.1 years (range, 30–48 years).
Table 1.
Descriptive Characteristics of Study Sample (n = 700), Early Determinants of Mammographic Density Study, United States, 1959–2008
| Characteristic | Overall | Sibling Subset | ||||
|---|---|---|---|---|---|---|
| No. | % | Mean (SD) | No. | % | Mean (SD) | |
| Maternal variables | ||||||
| Maternal age at registration, years | 700 | 26.2 (5.9) | 254 | 25.8 (5.5) | ||
| Paternal age at registration, years | 672 | 29.4 (6.8) | 248 | 29.1 (6.8) | ||
| Maternal prepregnancy BMIa | 656 | 23.3 (3.8) | 248 | 23.2 (4.0) | ||
| Maternal gestational weight gain, kg | 675 | 9.2 (4.0) | 249 | 8.7 (4.0) | ||
| Maternal education at registration | ||||||
| Less than high school | 180 | 25.9 | 56 | 22.0 | ||
| High-school graduate | 289 | 41.6 | 112 | 44.1 | ||
| Some college, technical/trade school, or college graduate | 225 | 32.4 | 86 | 33.9 | ||
| Prenatal smoking exposureb | ||||||
| Yes | 272 | 40.1 | 112 | 45.2 | ||
| No | 406 | 59.9 | 136 | 54.8 | ||
| Birth variables | ||||||
| Birth weight, kg | 700 | 3.4 (0.5) | 254 | 3.3 (0.5) | ||
| Birth length, cm | 696 | 51.2 (3.0) | 252 | 50.7 (3.0) | ||
| Early growth variables | ||||||
| Weight at 4 months, kg | 700 | 6.4 (0.8) | 254 | 6.2 (0.8) | ||
| Length at 4 months, cm | 696 | 62.4 (2.9) | 250 | 61.8 (3.2) | ||
| Weight at 1 year, kg | 693 | 9.7 (1.3) | 252 | 9.4 (1.3) | ||
| Length at 1 year, cm | 689 | 73.9 (3.2) | 248 | 73.4 (3.2) | ||
| Weight at 4 year, kg | 661 | 16.8 (2.4) | 237 | 16.3 (2.2) | ||
| Length at 4 year, cm | 656 | 100.7 (4.9) | 234 | 100.2 (4.9) | ||
| Adult variables | ||||||
| Race/ethnicityb | ||||||
| Non-Hispanic white | 546 | 78.2 | 201 | 79.5 | ||
| Non-Hispanic black | 86 | 12.3 | 27 | 10.7 | ||
| Hispanic | 40 | 5.7 | 14 | 5.5 | ||
| Non-Hispanic API and other | 26 | 3.7 | 11 | 4.3 | ||
| Weight, kg | 686 | 75.2 (18.6) | 248 | 75.6 (19.0) | ||
| Height, m | 698 | 1.7 (0.1) | 252 | 1.7 (0.1) | ||
| BMIa | 686 | 27.5 (6.4) | 248 | 27.7 (6.8) | ||
| Age at interview, years | 700 | 44.1 (1.8) | 254 | 44.0 (1.8) | ||
| Age at mammogram, years | 700 | 43.1 (2.3) | 254 | 43.1 (2.2) | ||
| % density | 700 | 31.8 (18.7) | 254 | 30.3 (19.2) | ||
| Dense area, cm2 | 700 | 35.8 (22.0) | 254 | 33.6 (22.2) | ||
| Breast area, cm2 | 700 | 137.8 (74.0) | 254 | 138.4 (73.0) | ||
Abbreviations: API, Asians and Pacific Islanders; BMI, body mass index; SD, standard deviation.
a Weight (kg)/height (m)2.
b Values do not sum to total due to missing data.
Age-specific weight
Birth weight was positively associated with dense area (per 1-kg increase in birth weight, β = 3.36, 95% confidence interval (CI): 0.06, 6.66) (Table 2). Postnatal BMI was negatively associated with % density beginning at age 4 years (β = −0.85, 95% CI: −1.62, −0.09) and lasting into ages 20–29 years (β = −0.55, 95% CI: −0.89, −0.20).
Table 2.
Multivariable Models of Birth Weight, Body Mass Index, and Early-Life Weight Change and Mammographic Density, Early Determinants of Mammographic Density Study, United States, 1959–2008
| Variable | % Density | Dense Area | Nondense Area | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Not Adjusted for Adult BMIa | Adjusted for Adult BMIa | Not Adjusted for Adult BMIa | Adjusted for Adult BMIa | Not Adjusted for Adult BMIa | Adjusted for Adult BMIa | |||||||
| β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | |
| Birth weight, kgb | 1.36 | −1.57, 4.30 | 0.96 | −1.46, 3.38 | 3.40 | 0.05, 6.74 | 3.36 | 0.06, 6.66 | 2.97 | −7.93, 13.87 | 5.25 | −2.32, 12.82 |
| Absolute size | ||||||||||||
| BMI at 1 yearc | −0.86 | −1.71, −0.01 | −0.48 | −1.18, 0.22 | −0.47 | −1.37, 0.44 | −0.41 | −1.28, 0.46 | 3.50 | −0.38, 7.39 | 0.81 | −2.32, 3.93 |
| BMI at 4 yearsd | −1.57 | −2.43, −0.70 | −0.85 | −1.62, −0.09 | −0.44 | −1.71, 0.83 | −0.45 | −1.73, 0.82 | 7.28 | 2.84, 11.71 | 3.05 | −0.10, 6.20 |
| BMI at 20–29 yearse | −1.67 | −2.01, −1.34 | −0.55 | −0.89, −0.20 | −0.73 | −1.25, −0.21 | −0.66 | −1.35, 0.03 | 8.80 | 6.51, 11.09 | 2.26 | −0.05, 4.57 |
| Relative change | ||||||||||||
| Percentile rank change in weight from 0 months to 4 months (per 10-unit increase)c | −0.67 | −1.20, −0.13 | −0.41 | −0.85, 0.02 | −0.76 | −1.34, −0.18 | −0.65 | −1.23, −0.07 | 1.94 | −0.09, 3.96 | 0.45 | −0.97, 1.87 |
| Percentile rank change in weight from 4 months to 12 months (per 10-unit increase)f | −0.54 | −1.33, 0.25 | −0.08 | −0.72, 0.55 | 0.31 | −0.67, 1.29 | 0.34 | −0.65, 1.33 | 3.23 | −0.17, 6.64 | 0.70 | −1.64, 3.03 |
| Percentile rank change in weight from 1 year to 4 years (per 10 unit increase)g | −1.52 | −2.27, −0.77 | −0.82 | −1.46, −0.18 | −1.10 | −2.00, −0.19 | −1.07 | −1.98, −0.16 | 5.77 | 2.88, 8.66 | 1.74 | −0.33, 3.81 |
Abbreviations: BMI, body mass index; CI, confidence interval.
a Weight (kg)/height (m)2.
b Original model adjusted for age at interview, maternal prepregnancy BMI, maternal weight gain, maternal age at registration, maternal education, prenatal smoking exposure, race/ethnicity, and adult BMI at interview.
c Adjusted for everything in original model with the addition of birth weight.
d Adjusted for everything in previous model with the addition of BMI at 1 year.
e Adjusted for everything in previous model with the addition of BMI at 4 years.
f Adjusted for everything in previous model with the addition of percentile rank change in weight from 0 months to 4 months.
g Adjusted for everything in previous model with the addition of percentile rank change in weight from 4 months to 12 months.
Weight gain
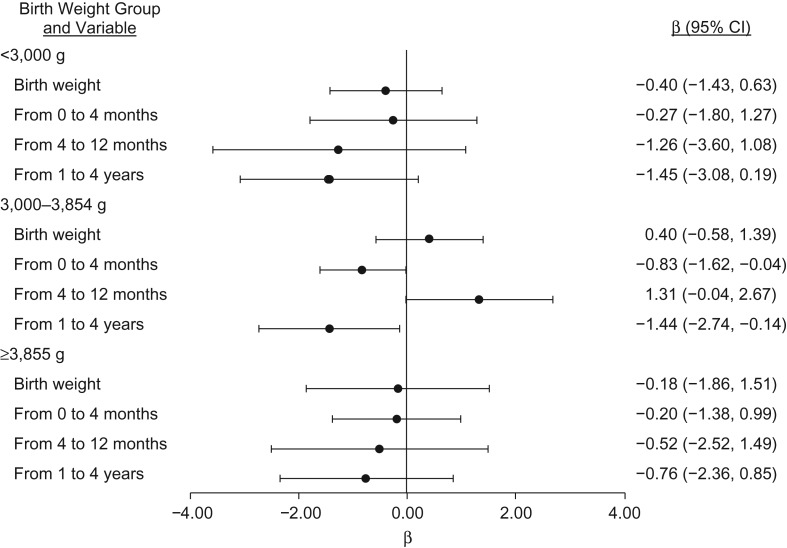
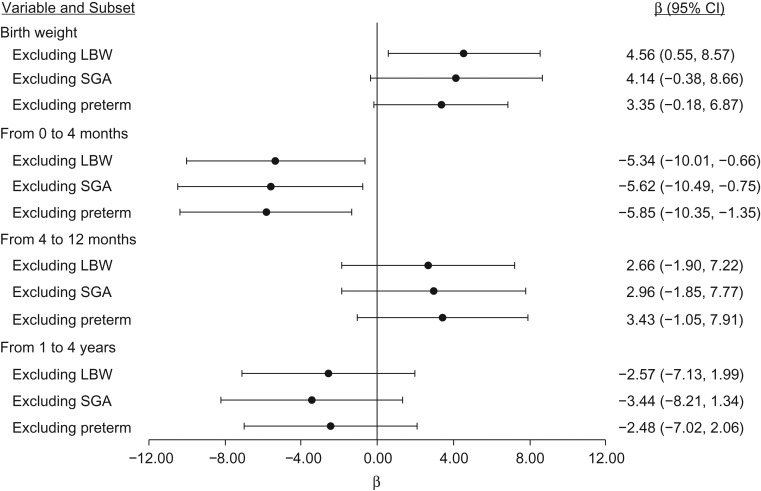
Faster weight gain from 0 to 4 months was negatively associated with % density and dense area (per 10-unit increase in weight percentile, β = −0.41, 95% CI: −0.85, 0.02 and β = −0.65, 95% CI: −1.23, −0.07, respectively) (Table 2). Faster weight gain from 1 to 4 years was negatively associated with % density and dense area (per 10-unit increase in weight percentile, β = −0.82, 95% CI: −1.46, −0.18 and β = −1.07, 95% CI: −1.98, −0.16, respectively), but it was positively associated with nondense area (Table 2). When we stratified by birth weight, the increase in dense area from rapid weight gain from 4 to 12 months was limited to average-weight babies (3,000–3,854 g: β = 1.31, 95% CI: −0.04, 2.67) (Figure 1). Table 3 shows the associations between birth weight, rapid weight gain (defined as an increase of at least 1 major CDC percentile), and dense area. In general, this alternative categorical classification of growth results was consistent with that shown in Table 2. In addition, findings were similar when we excluded preterm, low-birth-weight, or small-for-gestational-age infants (Figure 2), further supporting that infants who were very small at birth do not drive the associations observed for dense area.
Figure 1.
Associations between early-life weight change and dense area (cm2) according to birth weight category, Early Determinants of Mammographic Density study, United States, 1959–2008. β estimates correspond to a 100-g increase in birth weight or a 10-percentile increase in percentile rank change in weight from 0 months to 4 months, 4 months to 12 months, and 1 year to 4 years. Models adjusted for birth weight, percentile change in rank in previous time periods, age at interview, maternal prepregnancy body mass index, maternal weight gain, maternal age at registration, maternal education, prenatal smoking exposure, race/ethnicity, and adult body mass index. CI, confidence interval.
Table 3.
Multivariable Models of Early-Life Weight Patterns and Mammographic Density, Early Determinants of Mammographic Density Study, United States, 1959–2008
| Age Period and Speed of Weight Gain | No. | % Density | Dense Area | Nondense Area | |||
|---|---|---|---|---|---|---|---|
| β | 95% CI | β | 95% CI | β | 95% CI | ||
| 0 months to 4 monthsa | |||||||
| Rapidb | 242 | −0.50 | −3.50, 2.50 | −5.66 | −10.08, −1.24 | −3.74 | −13.61, 6.14 |
| Stablec | 154 | 0 | Referent | 0 | Referent | 0 | Referent |
| Slowd | 218 | 1.52 | −1.57, 4.60 | −0.55 | −5.46, 4.37 | −3.19 | −13.08, 6.71 |
| 4 months to 12 monthse | |||||||
| Rapidb | 162 | −0.61 | −3.67, 2.45 | 3.03 | −1.33, 7.39 | 3.63 | −6.16, 13.43 |
| Stablec | 277 | 0 | Referent | 0 | Referent | 0 | Referent |
| Slowd | 169 | −1.34 | −4.08, 1.40 | 0.23 | −3.71, 4.16 | 1.81 | −8.25, 11.86 |
| 1 year to 4 yearsf | |||||||
| Rapidb | 177 | −0.43 | −3.50, 2.64 | −2.58 | −7.00, 1.83 | −0.01 | −10.58, 10.57 |
| Stablec | 220 | 0 | Referent | 0 | Referent | 0 | Referent |
| Slowd | 181 | 1.29 | −1.58, 4.16 | 0.36 | −3.94, 4.66 | −6.02 | −15.28, 3.25 |
Abbreviations: BMI, body mass index; CDC, Centers for Disease Control and Prevention; CI, confidence interval.
a Original model adjusted for birth weight, age at interview, maternal prepregnancy BMI, maternal weight gain, maternal age at registration, maternal education, prenatal smoking exposure, race/ethnicity, and adult BMI at interview.
b Rapid weight gain was defined as an increase of at least 1 major CDC reference percentile (5th, 10th, 25th, 50th, 75th, 95th) relative to stable.
c Stable weight gain was defined as staying within 1 major CDC reference percentile.
d Slow weight gain was defined as a decrease of at least 1 major CDC reference percentile (5th, 10th, 25th, 50th, 75th, 95th) relative to stable.
e Adjusted for everything in original model with the addition of weight pattern from 0 months to 4 months.
f Adjusted for everything in previous model with the addition of weight pattern from 4 months to 12 months.
Figure 2.
Sensitivity analyses for the association between birth weight, rapid weight gain, and dense area (cm2), Early Determinants of Mammographic Density study, United States, 1959–2008. Low birth weight (LBW) was defined as less than 2.5 kg; being small for gestational age (SGA) was defined as below the 10th percentile of within-cohort birth weight/gestational age; and preterm was defined as less than 37 weeks’ gestational age. β estimate for birth weight corresponds to a 1-kg increase. Rapid weight gain was defined as an increase of at least 1 major Centers for Disease Control and Prevention reference percentile (5th, 10th, 25th, 50th, 75th, 95th) relative to stable (staying within 1 major reference percentile). Models adjusted for birth weight, weight gain pattern in previous time periods, maternal prepregnancy body mass index, maternal weight gain, maternal age at registration, maternal education, prenatal smoking exposure, race/ethnicity, and adult body mass index. CI, confidence interval.
Results of sibling analysis
The overall patterns of these associations with MD (positive for birth weight and negative for postnatal weight gain) were similar in siblings (Table 4). There were some differences, however, when we stratified by sibling discordancy in BMI in adulthood. For example, birth weight was positively associated with dense area in all sibling pairs (for 1-kg difference in birth weight, β = 7.27, 95% CI: −0.12, 14.67), but this association was stronger in magnitude in the majority of siblings (80/116 sets) that differed in adult BMI (BMI difference >2.5) (β = 11.80, 95% CI: 1.63, 21.98).
Table 4.
Relationships Between Difference in Birth Weight, Weight Change, and Mammographic Density in 116 Sibling Pairs, Early Determinants of Mammographic Density Study, United States, 1959–2008
| Variable and Subset | % Density | Dense Area | Nondense Area | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Not Adjusted for Adult BMIa | Adjusted for Adult BMIa | Not Adjusted for Adult BMIa | Adjusted for Adult BMIa | Not Adjusted for Adult BMIa | Adjusted for Adult BMIa | ||||||||
| No. | β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | |
| All sibling pairs | 116 | ||||||||||||
| Birth weight, kgb | 1.07 | −4.48, 6.62 | 0.52 | −5.10, 6.13 | 7.23 | −0.25, 14.71 | 7.27 | −0.12, 14.67 | −8.09 | −27.85, 11.68 | −6.80 | −26.13, 12.53 | |
| Percentile rank change in weight, 0 months to 4 months (per 10-units higher)c | 0.08 | −0.62, 0.78 | 0.00 | −0.65, 0.65 | −0.37 | −1.20, 0.46 | −0.37 | −1.19, 0.45 | −3.17 | −5.88, −0.46 | −2.37 | −4.78, 0.04 | |
| Percentile rank change in weight, 4 months to 12 months (per 10-units higher)d | −0.22 | −1.41, 0.96 | −0.15 | −1.25, 0.95 | −0.69 | −1.94, 0.57 | −0.70 | −1.95, 0.56 | 1.24 | −3.49, 5.97 | 1.52 | −2.55, 5.60 | |
| Percentile rank change in weight, 1 year to 4 years (per 10-units higher)e | −0.36 | −1.33, 0.62 | −0.07 | −0.91, 0.77 | −0.20 | −1.60, 1.19 | −0.29 | −1.72, 1.14 | 2.75 | −1.31, 6.81 | 1.47 | −2.13, 5.07 | |
| Sibling pairs with BMI difference of ≥2.5 | 80 | ||||||||||||
| Birth weight, kgb | 0.30 | −7.00, 7.61 | 0.49 | −6.90, 7.88 | 11.73 | 1.38, 22.08 | 11.80 | 1.63, 21.98 | −21.54 | −46.45, 3.37 | −23.13 | −46.68, 0.42 | |
| Percentile rank change in weight, 0 months to 4 months (per 10-units higher)c | 0.14 | −0.86, 1.13 | 0.14 | −0.75, 1.04 | −0.24 | −1.48, 1.00 | −0.23 | −1.46, 0.99 | −3.16 | −7.15, 0.84 | −3.18 | −6.60, 0.24 | |
| Percentile rank change in weight, 4 months to 12 months (per 10-units higher)d | −0.79 | −2.47, 0.90 | −0.61 | −2.22, 1.00 | −0.57 | −2.22, 1.09 | −0.59 | −2.25, 1.07 | 1.67 | −4.66, 8.01 | 2.08 | −3.46, 7.62 | |
| Percentile rank change in weight, 1 year to 4 years (per 10-units higher)e | −0.45 | −1.55, 0.65 | −0.16 | −1.12, 0.80 | 0.17 | −1.26, 1.61 | 0.06 | −1.43, 1.54 | 2.30 | −2.39, 7.00 | 1.19 | −3.05, 5.43 | |
| Sibling pairs with BMI difference of <2.5 | 36 | ||||||||||||
| Birth weight, kgb | −1.48 | −8.41, 5.46 | −2.33 | −9.77, 5.11 | −2.66 | −14.18, 8.87 | −2.36 | −14.88, 10.16 | 15.55 | −4.87, 35.98 | 16.37 | −2.98, 35.73 | |
| Percentile rank change in weight, 0 months to 4 months (per 10-units higher)c | −0.49 | −1.40, 0.42 | −0.62 | −1.54, 0.31 | −0.60 | −1.49, 0.30 | −0.58 | −1.50, 0.35 | −1.04 | −3.86, 1.79 | −0.91 | −3.92, 2.10 | |
| Percentile rank change in weight, 4 months to 12 months (per 10-units higher)d | 0.90 | −0.52, 2.31 | 0.74 | −0.68, 2.17 | −0.64 | −2.31, 1.03 | −0.61 | −2.28, 1.05 | −0.54 | −4.91, 3.84 | −0.45 | −4.86, 3.97 | |
| Percentile rank change in weight, 1 year to 4 years (per 10-units higher)e | 0.57 | −1.25, 2.40 | 0.48 | −1.42, 2.38 | −1.21 | −4.16, 1.75 | −1.20 | −4.18, 1.78 | 0.54 | −4.52, 5.59 | 0.58 | −4.58, 5.74 | |
Abbreviations: BMI, body mass index; CI, confidence interval.
a Weight(kg)/height(m)2.
b Original model adjusted for within-pair differences in age at interview, maternal weight gain, maternal age at registration, and adult BMI at interview.
c Adjusted for everything in original model with the addition of within-pair difference in birth weight.
d Adjusted for everything in previous model with the addition of within-pair difference in weight gain from 0 months to 4 months.
e Adjusted for everything in previous model with the addition of within-pair difference in weight gain from 4 months to 12 months.
Contribution of adult BMI
Associations between infant weight gain and dense area are largely independent of BMI in adulthood as evident from the point estimates adjusted and not adjusted for adult BMI in the overall cohort (Table 2) and sibling analysis (Table 4).
DISCUSSION
This study extends the previous literature by supporting both a positive association between higher birth weight and MD, specifically dense area, and a negative association between faster infant and childhood weight gain and MD. These associations were observed for both % density and the absolute amount of dense area, and demonstrate that the consistently reported negative association between adolescent body size and MD starts much earlier in the life course. The results were also consistent with and without adjustment for adult BMI, which is viewed as necessary to properly make inference about MD as a construct (55).
In addition to these key findings, we observed a positive association between rapid weight gain in later infancy and dense area that was limited to average-birth-weight babies. The infant period is a period associated with an activation of the hypothalamic-pituitary-gonadal axis, termed “minipuberty” (42). During “minipuberty,” follicle-stimulating hormone and luteinizing hormone both increase in early infancy and peak at 1–3 months. While luteinizing hormone decreases by 6–9 months, follicle-stimulating hormone levels are elevated until ages 3–4 years. Estradiol levels fluctuate during the first year and then decrease (42). Estradiol levels have been positively associated with breast tissue size in 3-month-old female infants (56). Thus, this might suggest that growth during the later infant period, starting around 4 months, has a positive association with MD given that this is when endogenous hormone levels are elevated and might be stimulating infant breast tissue development (41, 42, 56). Unlike large-birth-weight babies, who are more likely to experience “catch down” growth, average-birth-weight babies who grow rapidly in later infancy might have increases in dense area.
While we observed a negative association between weight gain from 0 to 4 months and MD, a nested case-control study of growth patterns from birth to up to 21 days found that infants who lost more than 200 g after delivery and those that gained weight at a rate ≥25 g/day after this initial loss both were at an increased risk of BC compared with infants who lost less weight and grew more slowly (57). Rapid infant weight gain has also been shown to accelerate age at menarche, a risk factor for BC (49, 58–62). Early age at menarche, however, has been associated with lower MD as seen in a recent study of more than 20,000 women, which is consistent with our finding of rapid infant weight gain lowering MD (63). Earlier age at menarche might be associated with increased BC risk through a pathway that is not mediated by MD (64). More research is needed to examine whether MD has opposing patterns with weight gain in infancy than other BC risk factors and whether infant weight gain affects BC risk through multiple, potentially opposing, pathways.
The positive association observed between birth weight and dense area and the negative association observed for early infant and childhood weight gain and dense area was independent of adult BMI, as shown by the similarity of analyses with and without adjustment for adult BMI. The independence of these associations from adult BMI has also been found in other studies of birth weight, later-childhood body size, and MD (34, 65). For example, a nested case-control study of 1,105 mostly postmenopausal women within the Breakthrough Generations Study examined the association between recalled weight at ages 7 and 11 years relative to peers with MD derived from screening mammograms (65). Weight at ages 7 and 11 years was negatively associated with % density and absolute dense area and was positively associated with absolute nondense area. These associations were similar with and without adjustment for adult BMI. Understanding the complex associations between early-life weight gain and MD is important given studies suggest that MD plays a large role in mediating the role between adolescent body size and BC risk (38, 64, 66).
Studies of breast density through additional measures including magnetic resonance imaging (MRI) and dual-energy x-ray absorptiometry prior to the age when mammography is routinely used help shed some light. For example, in the Dietary Intervention Study in Children, childhood BMI z scores measured at ages 9–16 years were negatively associated with both % breast density and absolute dense breast volume as measured by MRI at ages 25–29 years (67). BMI and % body fat were also negatively associated with % fibroglandular volume as assessed by dual-energy x-ray absorptiometry in a study of 113 girls ages 10–16 years from Kaiser Permanente Hawaii (68). Even though other studies of MD have not looked at infant growth, a recent study examining growth trajectories from birth through adolescence and breast tissue composition as assessed by MRI in women at age 21 years observed a positive association between birth weight and MRI % water and inverse associations between pubertal weight growth and % body fat mass in adolescence and MRI % water (69). This analysis supported an association for later childhood and adolescent growth and MRI density measures but was consistent with our findings before adjusting for these later-life measures (70). Thus, while growth trajectories in adolescence might exert a stronger influence on breast tissue composition, this does not preclude a role for infant growth, as we observed in this analysis.
A key strength of our study was that the early-life body size measurements were prospectively collected. Many studies of later childhood and adolescent body size and MD have relied on retrospective reporting of somatotypes (33), with a few key exceptions (e.g., the Tasmanian Longitudinal Health Study, which had prospective measures of body size from ages 7 to 15 years (55)). Another key strength of our study was the ability to examine the overall patterns in the entire cohort and the sibling subset, which largely suggested similar patterns, indicating that family-level factors shared by the siblings such as socioeconomic status did not confound these associations. The consistency between the findings in the sibling analyses and the overall cohort strengthen the overall inferences, but we recognize that even sibling analyses might be confounded by nonshared factors (71). We were also limited by primarily assessing density at a single time point in middle age and the use of an observer-dependent method to assess density, although the within- and between-batch reliability was excellent (≥0.95). Also, our findings were consistent with and without adjustment for adult BMI, which might have more measurement error because it was self-reported.
In conclusion, our study provides further support that the negative association between adolescent body size and breast density has origins in growth starting as early as the first few months of life. Our findings emphasize the importance of considering these early life growth parameters when investigating mechanisms and predictors of MD and BC risk.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, New York (Mary Beth Terry, Mandy Goldberg, Julie D. Flom, Lauren C. Houghton, Parisa Tehranifar, Jasmine A. McDonald, Angeline Protacio); Herbert Irving Comprehensive Cancer Center, Columbia University Medical Center, New York, New York (Mary Beth Terry, Parisa Tehranifar); Imprints Center for Genetic and Environmental Lifecourse Studies, Mailman School of Public Health, Columbia University, New York, New York (Mary Beth Terry); The Child Health and Development Studies, Public Health Institute, Berkeley, California (Barbara A. Cohn, Piera Cirillo); Department of Biostatistics, Mailman School of Public Health, Columbia University, New York, New York (Ying Wei); Department of Epidemiology, Fielding School of Public Health, University of California, Los Angeles, Los Angeles, California (Karin B. Michels); and Institute for Prevention and Cancer Epidemiology, Faculty of Medicine and Medical Center, University of Freiburg, Freiburg, Germany (Karin B. Michels).
This work was funded by the National Cancer Institute (grants R01CA104842-03, K07CA90685, and 5T32CA09529), Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant P01AG023028-01), and National Institute for Environmental Health Sciences (Center Support grant ES009089).
Conflict of interest: none declared.
Abbreviations
- BC
breast cancer
- BMI
body mass index
- CDC
Centers for Disease Control and Prevention
- CHDS
Child Health and Development Studies
- CI
confidence interval
- EDMD
Early Determinants of Mammographic Density
- MD
mammographic density
- MRI
magnetic resonance imaging
- NCPP
National Collaborative Perinatal Project
REFERENCES
- 1. Hooley RJ. Breast density legislation and clinical evidence. Radiol Clin North Am. 2017;55(3):513–526. [DOI] [PubMed] [Google Scholar]
- 2. Green VL. Mammographic breast density and breast cancer risk: implications of the breast density legislation for health care practitioners. Clin Obstet Gynecol. 2016;59(2):419–438. [DOI] [PubMed] [Google Scholar]
- 3. Huo CW, Chew GL, Britt KL, et al. . Mammographic density—a review on the current understanding of its association with breast cancer. Breast Cancer Res Treat. 2014;144(3):479–502. [DOI] [PubMed] [Google Scholar]
- 4. Trinh T, Eriksson M, Darabi H, et al. . Background risk of breast cancer and the association between physical activity and mammographic density. Breast Cancer Res. 2015;17:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yaghjyan L, Colditz GA, Rosner B, et al. . Reproductive factors related to childbearing and mammographic breast density. Breast Cancer Res Treat. 2016;158(2):351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ziembicki S, Zhu J, Tse E, et al. . The association between alcohol consumption and breast density: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2017;26(2):170–178. [DOI] [PubMed] [Google Scholar]
- 7. Boyd NF, Dite GS, Stone J, et al. . Heritability of mammographic density, a risk factor for breast cancer. N Engl J Med. 2002;347(12):886–894. [DOI] [PubMed] [Google Scholar]
- 8. Stone J, Dite GS, Gunasekara A, et al. . The heritability of mammographically dense and nondense breast tissue. Cancer Epidemiol Biomarkers Prev. 2006;15(4):612–617. [DOI] [PubMed] [Google Scholar]
- 9. Lindström S, Thompson DJ, Paterson AD, et al. . Genome-wide association study identifies multiple loci associated with both mammographic density and breast cancer risk. Nat Commun. 2014;5:5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shieh Y, Hu D, Ma L, et al. . Breast cancer risk prediction using a clinical risk model and polygenic risk score. Breast Cancer Res Treat. 2016;159(3):513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sanderson M, Williams MA, Malone KE, et al. . Perinatal factors and risk of breast cancer. Epidemiology. 1996;7(1):34–37. [DOI] [PubMed] [Google Scholar]
- 12. McCormack VA, dos Santos Silva I, De Stavola BL, et al. . Fetal growth and subsequent risk of breast cancer: results from long term follow up of Swedish cohort. BMJ. 2003;326(7383):248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Stavola BL, dos Santos Silva I, Wadsworth MJ. Birth weight and breast cancer. N Engl J Med. 2005;352(3):304–306. [PubMed] [Google Scholar]
- 14. Ahlgren M, Melbye M, Wohlfahrt J, et al. . Growth patterns and the risk of breast cancer in women. N Engl J Med. 2004;351(16):1619–1626. [DOI] [PubMed] [Google Scholar]
- 15. Park SK, Kang D, McGlynn KA, et al. . Intrauterine environments and breast cancer risk: meta-analysis and systematic review. Breast Cancer Res. 2008;10:R8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Trichopoulos D, Adami HO, Ekbom A, et al. . Early life events and conditions and breast cancer risk: from epidemiology to etiology. Int J Cancer. 2008;122(3):481–485. [DOI] [PubMed] [Google Scholar]
- 17. dos Santos Silva I, De Stavola BL, Hardy RJ, et al. . Is the association of birth weight with premenopausal breast cancer risk mediated through childhood growth? Br J Cancer. 2004;91(3):519–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Michels KB, Xue F. Role of birthweight in the etiology of breast cancer. Int J Cancer. 2006;119(9):2007–2025. [DOI] [PubMed] [Google Scholar]
- 19. Forman MR, Cantwell MM, Ronckers C, et al. . Through the looking glass at early-life exposures and breast cancer risk. Cancer Invest. 2005;23(7):609–624. [DOI] [PubMed] [Google Scholar]
- 20. Hertz-Picciotto I, Adams-Campbell L, Devine P, et al. . Breast Cancer and the Environment: A Life Course Approach. Washington, DC: National Acad. Press; 2012. [Google Scholar]
- 21. Fenton SE, Reed C, Newbold RR. Perinatal environmental exposures affect mammary development, function, and cancer risk in adulthood. Annu Rev Pharmacol Toxicol. 2012;52:455–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Michels KB, Trichopoulos D, Robins JM, et al. . Birthweight as a risk factor for breast cancer. Lancet. 1996;348(9041):1542–1546. [DOI] [PubMed] [Google Scholar]
- 23. Xue F, Michels KB. Intrauterine factors and risk of breast cancer: a systematic review and meta-analysis of current evidence. Lancet Oncol. 2007;8(12):1088–1100. [DOI] [PubMed] [Google Scholar]
- 24. Bodicoat DH, Schoemaker MJ, Jones ME, et al. . Timing of pubertal stages and breast cancer risk: the Breakthrough Generations Study. Breast Cancer Res. 2014;16(1):R18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Collaborative Group on Hormonal Factors in Breast Cancer Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118,964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13(11):1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang B, Shu XO, Delahanty RJ, et al. . Height and breast cancer risk: evidence from prospective studies and Mendelian randomization. J Natl Cancer Inst. 2015;107(11):djv219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Silva Idos S, De Stavola B, McCormack V, et al. . Birth size and breast cancer risk: re-analysis of individual participant data from 32 studies. PLoS Med. 2008;5(9):e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang TO, Reeves GK, Green J, et al. . Birth weight and adult cancer incidence: large prospective study and meta-analysis. Ann Oncol. 2014;25(9):1836–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Terry MB. Consistency, now what? Breast Cancer Res. 2017;19:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Okasha M, McCarron P, Gunnell D, et al. . Exposures in childhood, adolescence and early adulthood and breast cancer risk: a systematic review of the literature. Breast Cancer Res Treat. 2003;78(2):223–276. [DOI] [PubMed] [Google Scholar]
- 31. Ruder EH, Dorgan JF, Kranz S, et al. . Examining breast cancer growth and lifestyle risk factors: early life, childhood, and adolescence. Clin Breast Cancer. 2008;8(4):334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xue F, Rosner B, Eliassen H, et al. . Body fatness throughout the life course and the incidence of premenopausal breast cancer. Int J Epidemiol. 2016;45(4):1103–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yochum L, Tamimi RM, Hankinson SE. Birthweight, early life body size and adult mammographic density: a review of epidemiologic studies. Cancer Causes Control. 2014;25(10):1247–1259. [DOI] [PubMed] [Google Scholar]
- 34. Denholm R, De Stavola B, Hipwell JH, et al. . Pre-natal exposures and breast tissue composition: findings from a British pre-birth cohort of young women and a systematic review. Breast Cancer Res. 2016;18:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jeffreys M, Warren R, Gunnell D, et al. . Life course breast cancer risk factors and adult breast density (United Kingdom). Cancer Causes Control. 2004;15(9):947–955. [DOI] [PubMed] [Google Scholar]
- 36. McCormack VA, dos Santos Silva I, De Stavola BL, et al. . Life-course body size and perimenopausal mammographic parenchymal patterns in the MRC 1946 British birth cohort. Br J Cancer. 2003;89(5):852–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lope V, Pérez-Gómez B, Moreno MP, et al. . Childhood factors associated with mammographic density in adult women. Breast Cancer Res Treat. 2011;130(3):965–974. [DOI] [PubMed] [Google Scholar]
- 38. Andersen ZJ, Baker JL, Bihrmann K, et al. . Birth weight, childhood body mass index, and height in relation to mammographic density and breast cancer: a register-based cohort study. Breast Cancer Res. 2014;16:R4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. . CDC growth charts: United States. Adv Data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 40. Chellakooty M, Schmidt IM, Haavisto AM, et al. . Inhibin A, inhibin B, follicle-stimulating hormone, luteinizing hormone, estradiol, and sex hormone-binding globulin levels in 473 healthy infant girls. J Clin Endocrinol Metab. 2003;88(8):3515–3520. [DOI] [PubMed] [Google Scholar]
- 41. Jayasinghe Y, Cha R, Horn-Ommen J, et al. . Establishment of normative data for the amount of breast tissue present in healthy children up to two years of age. J Pediatr Adolesc Gynecol. 2010;23(5):305–311. [DOI] [PubMed] [Google Scholar]
- 42. Kuiri-Hänninen T, Sankilampi U, Dunkel L. Activation of the hypothalamic-pituitary-gonadal axis in infancy: minipuberty. Horm Res Paediatr. 2014;82(2):73–80. [DOI] [PubMed] [Google Scholar]
- 43. Akinyemiju TF, Tehranifar P, Flom JD, et al. . Early life growth, socioeconomic status, and mammographic breast density in an urban US birth cohort. Ann Epidemiol. 2016;26(8):540–545.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van den Berg BJ, Christianson RE, Oechsli FW. The California Child Health and Development Studies of the School of Public Health, University of California at Berkeley. Paediatr Perinat Epidemiol. 1988;2(3):265–282. [DOI] [PubMed] [Google Scholar]
- 45. Broman S. The Collaborative Perinatal Project: an overview In: Mednick S, Harway M, Finello K, eds. Handbook of Longitudinal Research. New York, NY: Praeger Publishers; 1984:185–215. [Google Scholar]
- 46. Terry MB, Schaefer CA, Flom JD, et al. . Prenatal smoke exposure and mammographic density in mid-life. J Dev Orig Health Dis. 2011;2(6):340–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McDonald JA, Michels KB, Cohn BA, et al. . Alcohol intake from early adulthood to midlife and mammographic density. Cancer Causes Control. 2016;27(4):493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Michels KB, Cohn BA, Goldberg M, et al. . Maternal anthropometry and mammographic density in adult daughters. Pediatrics. 2016;138(suppl 1):S34–S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Flom JD, Cohn BA, Tehranifar P, et al. . Earlier age at menarche in girls with rapid early life growth: cohort and within sibling analyses. Ann Epidemiol. 2017;27(3):187–93.e2. [DOI] [PubMed] [Google Scholar]
- 50. Susser E, Buka S, Schaefer CA, et al. . The Early Determinants of Adult Health study. J Dev Orig Health Dis. 2011;2(6):311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. van den Berg BJ. The California Child Health and Development Studies In: Mednick SA, Harway M, Finello KM, eds. Handbook of Longitudinal Research. New York, NY: Praeger Publishers; 1984. [Google Scholar]
- 52. Byng JW, Boyd NF, Fishell E, et al. . The quantitative-analysis of mammographic densities. Phys Med Biol. 1994;39(10):1629–1638. [DOI] [PubMed] [Google Scholar]
- 53. Terry MB, Wei Y, Esserman D, et al. . Pre- and postnatal determinants of childhood body size: cohort and sibling analyses. J Dev Orig Health Dis. 2011;2(2):99–111. [DOI] [PubMed] [Google Scholar]
- 54. Cohn BA, Brand RJ, Hulley SB. Correlates of high density lipoprotein cholesterol in women studied by the method of co-twin control. Am J Epidemiol. 1989;129(5):988–999. [DOI] [PubMed] [Google Scholar]
- 55. Hopper JL, Nguyen TL, Stone J, et al. . Childhood body mass index and adult mammographic density measures that predict breast cancer risk. Breast Cancer Res Treat. 2016;156(1):163–170. [DOI] [PubMed] [Google Scholar]
- 56. Schmidt IM, Chellakooty M, Haavisto AM, et al. . Gender difference in breast tissue size in infancy: correlation with serum estradiol. Pediatr Res. 2002;52(5):682–686. [DOI] [PubMed] [Google Scholar]
- 57. Lagiou P, Hsieh CC, Trichopoulos D, et al. . Neonatal growth and breast cancer risk in adulthood. Br J Cancer. 2008;99(9):1544–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Adair LS. Size at birth predicts age at menarche. Pediatrics. 2001;107(4):E59. [DOI] [PubMed] [Google Scholar]
- 59. dos Santos Silva I, De Stavola BL, Mann V, et al. . Prenatal factors, childhood growth trajectories and age at menarche. Int J Epidemiol. 2002;31(2):405–412. [DOI] [PubMed] [Google Scholar]
- 60. Terry MB, Ferris JS, Tehranifar P, et al. . Birth weight, postnatal growth, and age at menarche. Am J Epidemiol. 2009;170(1):72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ong KK, Emmett P, Northstone K, et al. . Infancy weight gain predicts childhood body fat and age at menarche in girls. J Clin Endocrinol Metab. 2009;94(5):1527–1532. [DOI] [PubMed] [Google Scholar]
- 62. Karaolis-Danckert N, Buyken AE, Sonntag A, et al. . Birth and early life influences on the timing of puberty onset: results from the DONALD (DOrtmund Nutritional and Anthropometric Longitudinally Designed) Study. Am J Clin Nutr. 2009;90(6):1559–1565. [DOI] [PubMed] [Google Scholar]
- 63. Alexeeff SE, Odo NU, Lipson JA, et al. . Age at menarche and late adolescent adiposity associated with mammographic density on processed digital mammograms in 24,840 women. Cancer Epidemiol Biomarkers Prev. 2017;26(9):1450–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rice MS, Bertrand KA, VanderWeele TJ, et al. . Mammographic density and breast cancer risk: a mediation analysis. Breast Cancer Res. 2016;18:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schoemaker MJ, Jones ME, Allen S, et al. . Childhood body size and pubertal timing in relation to adult mammographic density phenotype. Breast Cancer Res. 2017;19:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Harris HR, Tamimi RM, Willett WC, et al. . Body size across the life course, mammographic density, and risk of breast cancer. Am J Epidemiol. 2011;174(8):909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bertrand KA, Baer HJ, Orav EJ, et al. . Body fatness during childhood and adolescence and breast density in young women: a prospective analysis. Breast Cancer Res. 2015;17:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Novotny R, Daida Y, Morimoto Y, et al. . Puberty, body fat, and breast density in girls of several ethnic groups. Am J Hum Biol. 2011;23(3):359–365. [DOI] [PubMed] [Google Scholar]
- 69. Denholm R, De Stavola B, Hipwell JH, et al. . Growth trajectories, breast size, and breast-tissue composition in a British pre-birth cohort of young women. Am J Epidemiol. 2018;187(6):1259–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. De Stavola BL, Denholm R, Dos-Santos-Silva I. Response to the letter to the editor by Goldberg and Terry. Am J Epidemiol. 2018;187(9):2070–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Frisell T, Öberg S, Kuja-Halkola R, et al. . Sibling comparison designs: bias from non-shared confounders and measurement error. Epidemiology. 2012;23(5):713–720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.