Abstract
Hip fracture patients often have comorbid conditions. We investigated whether the combination of comorbidity and hip fracture could explain the previously observed excess mortality among hip fracture patients as compared with the general population. Using a population-based matched study design with 38,126 Norwegian women who suffered a hip fracture during the period 2009–2015 and the same number of women in a matched comparison cohort, we matched participants on prefracture comorbidity, age, and education. We estimated relative survival and additive and multiplicative comorbidity–hip fracture interactions. An additive comorbidity–hip fracture interaction of 4 or 9 additional deaths per 100 patients, depending on Charlson Comorbidity Index (CCI) score, was observed 1 year after hip fracture. Among women with a CCI score of ≥3, 15 additional deaths per 100 patients were observed; of these, 9 deaths could be attributed to the interaction and 6 to the hip fracture per se. On the relative scale, we observed increasing heterogeneity in survival by comorbidity over time; survival was reduced by 39% after 6 years among patients with a CCI score of ≥3, while among women with no comorbidity, survival was reduced by 17% (hip fracture vs. no hip fracture). In summary, prefracture comorbidity was associated with short-term absolute excess mortality and long-term relative excess mortality.
Keywords: cohort studies, comorbidity, hip fracture, interaction, mortality, relative survival
Hip fracture is a serious and costly complication of osteoporosis (1, 2). One-year mortality after hip fracture is more than doubled, with the absolute excess mortality ranging from 8 per 100 persons to 36 per 100 persons (3–5). The increased mortality persists years after the fracture and is higher in men than in women. Risk factors for osteoporosis include female sex, older age, vitamin D deficiency, smoking, use of glucocorticoids, high alcohol intake, lack of exercise, and low body mass index (6). Osteoporosis and hip fracture share risk factors with several chronic diseases, including diabetes mellitus, chronic pulmonary disease, dementia, and cancer, which is why the prevalence of comorbidity among patients with hip fracture is higher than that in the general population (7). Conditions mainly responsible for mortality after hip fracture are the same as those responsible for mortality in the general population, rather than the specific diseases related to osteoporosis (8).
Approximately 45%–60% of men and women who sustain a hip fracture have 1 or more comorbid conditions (1, 9–11). Comorbidity modifies the clinical course of a disease, as seen in patients with hip replacement surgery (12–14) or cancer (15). In previous controlled studies, an association between prefracture conditions and post–hip fracture mortality has been reported (1, 16–18). However, whether comorbidity interacts with hip fracture to produce excess mortality is not well established (11, 19, 20). Therefore, we examined mortality after hip fracture in a nationwide matched population-based cohort study of Norwegian women, comparing survival among women with and without hip fractures according to prefracture comorbidity using a matched cohort design.
METHODS
Setting and data sources
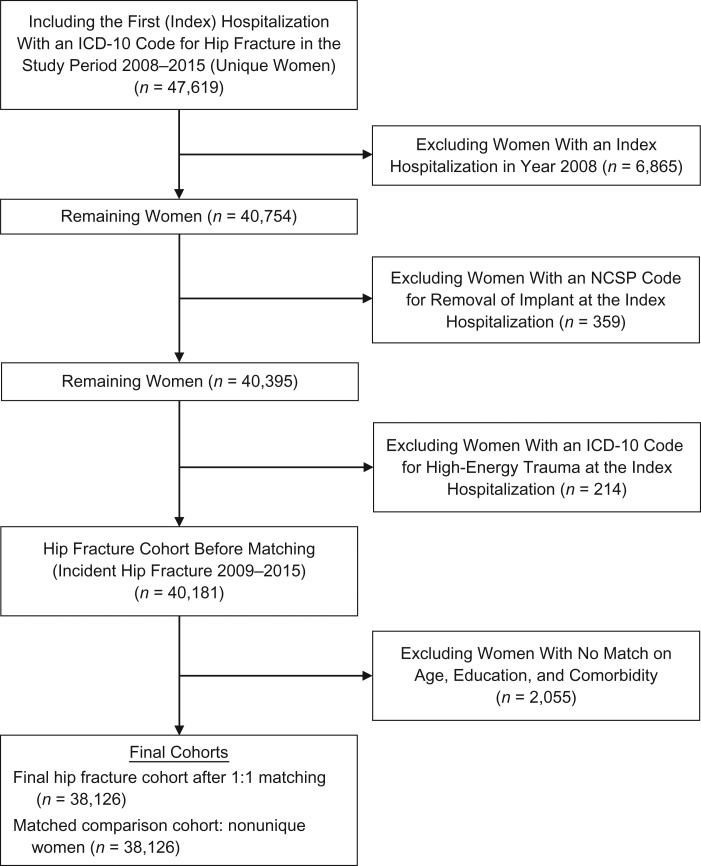
The source population, identified in the Norwegian National Registry, consisted of all 935,897 women in Norway aged 55 years or older during 2008–2015. The unique personal identification number assigned to each Norwegian resident facilitated linkage between the National Registry, the Norwegian Patient Registry (NPR), and the Norwegian Cause of Death Registry. The NPR is a national health registry with mandatory reporting, covering government-funded hospitals and specialty clinics, including data from all inpatient and outpatient hospital encounters starting in 2008 (21). In the NPR, we identified incident hip fractures using codes from the International Classification of Diseases, Tenth Revision (ICD-10) (14) (see Web Table 1, available at https://academic.oup.com/aje). The positive predictive value of the hip fracture diagnosis in the NPR ranges up to 99% and is highest when used together with a surgery code from the NOMESCO [Nordic Medico-Statistical Committee] Classification of Surgical Procedures (22). In this study, to capture patients who died before surgery, we defined hip fracture using ICD-10 codes alone (23). However, patients with hip fracture diagnosed at an outpatient visit not followed by a hospitalization were excluded if they lacked a surgical procedure code (to avoid including possible readmissions—that is, false-positive hip fractures) (Figure 1).
Figure 1.
Selection of postmenopausal women (aged ≥55 years) with hip fracture and a matched comparison cohort for a study of the role of comorbidity in mortality after hip fracture, Norway, 2009–2015. Outpatient visits with no further hospitalization in combination with a missing code for hip fracture surgery (NOMESCO Classification of Surgical Procedures (NCSP)) (22) were excluded. Some women in the matched comparison cohort were resampled: 32,405 were sampled once, 2,431 were sampled twice, 244 were sampled 3 times, and 31 were sampled 4 or more times. ICD-10, International Classification of Diseases, Tenth Revision; NOMESCO, Nordic Medico-Statistical Committee.
Data on education were obtained from the Norwegian Education Database (24). Data on vital status were obtained from the Norwegian Cause of Death Registry, which is 98% complete (25).
Study population
We identified 47,619 women with hip fracture during 2008–2015. After exclusion of women who sustained a hip fracture in 2008 (to ensure at least a 1-year fracture-free period for every woman), women with a surgical procedure code for removal of an implant, and women with an ICD-10 code for trauma (23), the study population included 40,181 women with incident hip fracture during 2009–2015. Among these women, 38,067 (95%) had both a diagnosis code and a procedure code, while the remaining 2,114 women (5%) had only a diagnostic code for hip fracture. The date of the incident hip fracture diagnosis was the index date.
Matching
We used a matched cohort design, with a hip fracture cohort and a comparison cohort, as previously described (26–28). Briefly, for each woman with hip fracture, we sampled (with replacement) 1 matched comparison woman from among those who were alive on the index date and without a previous hip fracture. The matching characteristics included age (in full years) at the index date (±3 years), highest achieved level of education (basic (compulsory), secondary (high school/vocational), or tertiary (college or university)) (29), and each of the 16 comorbid conditions (presence/absence) listed in the Romano modification of the Charlson Comorbidity Index (CCI) (30, 31). All characteristics were assessed from January 1, 2008, to (and including) the index date. Thus, comorbid conditions recorded both on the index date and before the index date were included in the matching criteria.
Follow-up of each matched pair started on the index date and continued until death, emigration, or the end of the study period (December 31, 2015), whichever occurred first. Time from the incident hip fracture to death/censoring was used as the dependent variable. Women with a hip fracture who could not be matched were excluded from the analysis. A woman without a hip fracture could be used as a matched comparison to more than 1 woman with fracture.
Romano CCI
We used the updated Romano modification of the CCI (31), which is a weighted average of 16 conditions representing risk factors relevant to our patient population, with inclusion and updated weights for several of the original 19 CCI diseases, such as dementia, diabetes with complications, and renal disease (32, 33). After matching on the individual diseases in the CCI, we calculated CCI scores for each woman with a hip fracture and her matched cohort member. The validity of administrative diagnoses included as comorbid conditions in this study varies from 82% to 100% (34). ICD-10 codes used in the Romano modification of the CCI are listed in Web Table 2.
Statistical analyses
We computed mortality risk, risk differences, additive interactions, and relative survival, contrasting different combinations of the CCI score categories (0, 1–2, or ≥3) and hip fracture (yes/no). Because of the nature of the data set, with its unequal follow-up times and variation in mortality as a function of time since hip fracture, we used survival analysis. We used Aalen’s additive hazard regression model (35), which estimates the cumulative mortality rates (hazards) B(t) separately for each combination of CCI score categories and hip fracture strata. Within each group, this is equivalent to estimating the cumulative hazards using the Nelson-Aalen estimator. One important advantage of the additive hazards model is that the contrast “hip fracture (yes/no)” for a fixed CCI score category will estimate the excess mortality due to hip fracture for that category, and because of the matched design, the matching factors will balance out in the contrast (excess risk hazards model). Mortality risk (R) and risk differences at time t were calculated as , using the formula for survival. Relative survival (which is expressed as a ratio) was calculated within each CCI score group j as
where the first index refers to the presence (1) or absence (0) of hip fracture. Multiplicative interaction was calculated as . The B(t)’s were estimated using the R package “timereg” (R Foundation for Statistical Computing, Vienna, Austria), which allows for censoring and in particular makes it possible to deal with clustered data (the latter allowing sampling of matched cohort members with replacement). Note also that the matching factors will balance out in the contrasts (under additive excess risk). Mortality risk was calculated cumulatively from the index date to different follow-up dates. This approach avoids stratification of follow-up time, which would have created the need for further covariate adjustment.
As a measure of additive interaction between hip fracture and comorbidity with respect to mortality, we calculated the interaction contrast (IC) (26, 36, 37) for each CCI score category, using the group without comorbidity as the referent. For CCI score j, the IC was calculated as
Thus, IC1–2 measures how much more risky hip fracture is in CCI score group 1–2 than in the group without comorbidity . The latter will be referred to as the risk of death due to hip fracture per se. Thus, the interpretation of an IC1–2 of, for example, 0.02 is that an additional 2 of every 100 patients die because of hip fracture in CCI group 1–2, beyond what would be expected due to hip fracture per se. We further calculated IC and relative survival by type of fracture, stratifying the hip fractures into intracapsular, intertrochanteric, and subtrochanteric fractures (ICD-10 codes listed in Web Table 1).
We calculated a disease-specific IC, using the population without hip fracture and without a given individual disease as the reference group. For example, for myocardial infarction (MI), using the same terminology as for the CCI score, this could be written as . Pointwise confidence intervals for R, risk differences, and additive interactions were obtained using the delta method (38).
RESULTS
The hip fracture and comparison cohorts consisted of 38,126 successfully matched pairs; therefore, the cohorts had the same distributions of age, comorbidity, and education at baseline (Table 1). A total of 2,055 (5%) women with hip fracture who could not be matched were excluded from the analysis. The unmatched women were slightly older, with a median age of 85 years (interquartile range (IQR), 78–90) as compared with 84 years (IQR, 77–89) among the matched women, and had a 96% prevalence of a CCI score of ≥3 as compared with 36% of those matched.
Table 1.
Baseline Characteristics of a Cohort of 38,126 Postmenopausal Women With Hip Fracture and a Nonunique Matched Comparison Cohort, Norway, 2009–2015
| Baseline Characteristic | No. of Persons | % |
|---|---|---|
| Age at index date, yearsa | 84 (77–89) | |
| Index year | ||
| 2009 | 5,863 | 15.4 |
| 2010 | 5,630 | 14.8 |
| 2011 | 5,568 | 14.6 |
| 2012 | 5,512 | 14.5 |
| 2013 | 5,364 | 14.1 |
| 2014 | 5,211 | 13.7 |
| 2015 | 4,978 | 13.1 |
| Individual disease in the CCIb | ||
| Myocardial infarction | 4,809 | 12.6 |
| Congestive heart failure | 4,356 | 11.4 |
| Peripheral vascular disease | 1,761 | 4.6 |
| Cerebrovascular disease | 4,764 | 12.5 |
| Dementia | 7,026 | 18.4 |
| Chronic pulmonary disease | 4,984 | 13.1 |
| Connective tissue disease | 1,693 | 4.4 |
| Ulcer disease | 876 | 2.3 |
| Mild liver disease | 104 | 0.3 |
| Diabetes | 3,456 | 9.1 |
| Diabetes with end organ damage | 1,322 | 3.5 |
| Hemiplegia | 97 | 0.3 |
| Moderate or severe renal disease | 1,776 | 4.7 |
| Any tumor, leukemia, lymphoma | 4,661 | 12.2 |
| Moderate or severe liver disease | 34 | 0.1 |
| Metastatic solid tumor | 804 | 2.1 |
| CCIb score at baseline | ||
| 0 | 14,697 | 38.5 |
| 1 | 3,997 | 10.5 |
| 2 | 5,480 | 14.4 |
| 3 | 6,269 | 16.4 |
| 4 | 2,815 | 7.4 |
| 5 | 2,157 | 5.7 |
| 6 | 1,459 | 3.8 |
| 7 | 571 | 1.5 |
| 8 | 387 | 1.0 |
| 9 | 189 | 0.5 |
| 10 | 75 | 0.2 |
| 11 | 25 | 0.1 |
| 12 | 5 | 0.0 |
| Highest achieved educational level | ||
| Basic education (compulsory) | 18,820 | 49.4 |
| Secondary education (high school/vocational education) | 15,477 | 40.6 |
| Tertiary education (college/university) | 3,545 | 9.3 |
| Unknown | 284 | 0.7 |
Abbreviation: CCI, Charlson Comorbidity Index.
a Value is expressed as median (interquartile range).
b Romano modification of the CCI.
Median age at the index date and IQRs were similar by CCI score group in the 2 cohorts; median ages were 83 years (IQR, 75–89), 84 years (IQR, 77–89), and 85 years (IQR, 80–90) for groups with CCI scores of 0, 1–2, and ≥3, respectively. Median survival was 4.1 years and 5.8 years for the cohorts with and without hip fracture, respectively.
Table 1 shows baseline demographic and clinical characteristics of the hip fracture cohort and the comparison cohort. Dementia was the most prevalent disease (18%), followed by chronic pulmonary disease, myocardial infarction, and cerebrovascular disease (each about 13%). Among the less prevalent diseases were moderate or severe liver disease (0.1%), hemiplegia (0.3%), and mild liver disease (0.3%).
The mortality risk increased with increasing CCI score for both women with hip fracture and women without hip fracture (Table 2). Within each CCI score category, using the cohort without hip fracture as the reference group, the risk difference increased with longer follow-up time for CCI scores of 0 and 1–2, while for CCI scores of ≥3, the risk difference increased up to 365 days following the index date and then decreased. There was a positive additive interaction for CCI score categories of 1–2 and ≥3, first increasing and then decreasing over time. After a maximum of 6 years of follow-up, no additive interaction was observed. Relative survival decreased for increasing CCI score and for increasing follow-up time (Table 2). After 6 years of follow-up, relative survival was 0.83, 0.74, and 0.61 for CCI scores of 0, 1–2 and ≥3, respectively, with multiplicative interactions of 0.89 (CCI score 1–2) and 0.73 (CCI score ≥3).
Table 2.
Mortality Risk, Risk Difference, Additive and Multiplicativea Comorbidity–Hip Fracture Interaction, and Relative Survival (Measured Cumulatively From the Index Date) in a Cohort of 38,126 Postmenopausal Women With Hip Fracture and a Nonunique Matched Comparison Cohort, Norway, 2009–2015b
| CCIc Score | Matched Comparison Cohort (Cohort Without Hip Fracture) | Hip Fracture Cohort | RDd | 95% CI | ICe | 95% CI | Relative Survivald | 95% CI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Deaths | No. of Person-Years | Risk | 95% CI | No. of Deaths | No. of Person-Years | Risk | 95% CI | |||||||
| ≤30 Days of Follow-up | ||||||||||||||
| 0 | 49 | 1,200 | 0.003 | 0.002, 0.004 | 408 | 1,184 | 0.03 | 0.024, 0.030 | 0.02 | 0.021, 0.027 | 0 | Referent | 0.98 | 0.97, 0.98 |
| 1–2 | 51 | 772 | 0.01 | 0.004, 0.007 | 515 | 749 | 0.05 | 0.049, 0.058 | 0.05 | 0.044, 0.054 | 0.03 | 0.018, 0.032 | 0.95 | 0.95, 0.96 |
| ≥3 | 262 | 1,129 | 0.02 | 0.016, 0.021 | 1,499 | 1,068 | 0.11 | 0.102, 0.112 | 0.09 | 0.083, 0.094 | 0.06 | 0.059, 0.070 | 0.91 | 0.90, 0.92 |
| ≤365 Days of Follow-up | ||||||||||||||
| 0 | 721 | 13,571 | 0.05 | 0.048, 0.056 | 1,565 | 12,898 | 0.11 | 0.105, 0.116 | 0.06 | 0.052, 0.064 | 0 | Referent | 0.94 | 0.93, 0.94 |
| 1–2 | 693 | 8,498 | 0.08 | 0.072, 0.084 | 1,610 | 7,782 | 0.18 | 0.169, 0.185 | 0.10 | 0.090, 0.109 | 0.04 | 0.027, 0.055 | 0.89 | 0.88, 0.90 |
| ≥3 | 2,419 | 11,696 | 0.19 | 0.179, 0.194 | 4,522 | 9,841 | 0.34 | 0.330, 0.347 | 0.15 | 0.141, 0.162 | 0.09 | 0.084, 0.103 | 0.81 | 0.80, 0.83 |
| ≤3 Years of Follow-up | ||||||||||||||
| 0 | 2,077 | 33,556 | 0.17 | 0.165, 0.181 | 3,155 | 31,379 | 0.25 | 0.244, 0.261 | 0.08 | 0.069, 0.091 | 0 | Referent | 0.90 | 0.89, 0.92 |
| 1–2 | 1,768 | 19,960 | 0.24 | 0.228, 0.248 | 2,893 | 17,646 | 0.37 | 0.356, 0.379 | 0.13 | 0.115, 0.144 | 0.05 | 0.026, 0.074 | 0.83 | 0.81, 0.85 |
| ≥3 | 5,189 | 24,790 | 0.47 | 0.458, 0.480 | 7,057 | 20,261 | 0.60 | 0.587, 0.606 | 0.13 | 0.113, 0.142 | 0.05 | 0.033, 0.061 | 0.76 | 0.74, 0.79 |
| ≤6 Years of Follow-up | ||||||||||||||
| 0 | 3,192 | 47,316 | 0.36 | 0.342, 0.368 | 4,469 | 43,260 | 0.47 | 0.455, 0.480 | 0.11 | 0.096, 0.129 | 0 | Referent | 0.83 | 0.80, 0.85 |
| 1–2 | 2,578 | 26,534 | 0.47 | 0.456, 0.490 | 3,728 | 22,807 | 0.61 | 0.594, 0.628 | 0.14 | 0.115, 0.161 | 0.03 | −0.019, 0.070 | 0.74 | 0.70, 0.78 |
| ≥3 | 6,505 | 30,256 | 0.75 | 0.732, 0.760 | 8,243 | 24,137 | 0.85 | 0.836, 0.858 | 0.10 | 0.083, 0.119 | −0.01 | −0.031, 0.008 | 0.61 | 0.55, 0.66 |
Abbreviations: CCI, Charlson Comorbidity Index; CI, confidence interval; IC, interaction contrast; RD, risk difference.
a Multiplicative interaction (relative survival): ≤30 days of follow-up—CCI score 1–2: 0.97 (95% CI: 0.97, 0.98); CCI score ≥3: 0.93 (95% CI: 0.93, 0.94); ≤365 days of follow-up—CCI score 1–2: 0.95 (95% CI: 0.93, 0.97); CCI score ≥3: 0.87 (95% CI: 0.85, 0.88); ≤3 years of follow-up—CCI score 1–2: 0.92 (95% CI: 0.89, 0.95); CCI score ≥3: 0.84 (95% CI: 0.81, 0.88); ≤6 years of follow-up—CCI score 1–2: 0.89 (95% CI: 0.83, 0.97); CCI score ≥3: 0.73 (95% CI: 0.66, 0.82).
b All risk measures are dimensionless and have no units.
c Romano modification of the CCI.
d Calculated within each CCI score category, using the cohort without hip fracture as the reference group.
e Additive comorbidity–hip fracture interaction, calculated using the cohort with CCI score equal to zero as the reference group.
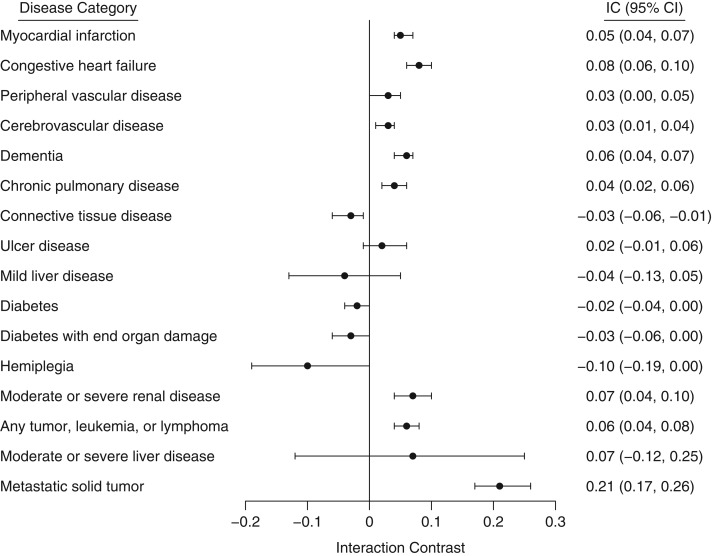
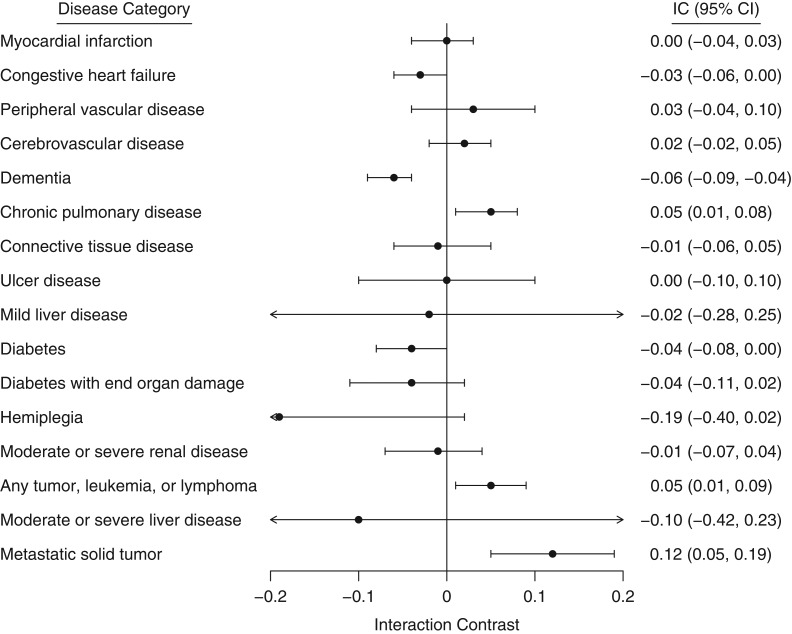
The following diseases showed a positive additive interaction with hip fracture during the first year of follow-up (in declining order of importance): metastatic solid tumor (IC = 0.21); congestive heart failure (IC = 0.08); moderate/severe renal disease (IC = 0.07); dementia (IC = 0.06); any tumor, leukemia, or lymphoma (IC = 0.06); myocardial infarction (IC = 0.05); chronic pulmonary disease (IC = 0.04); and cerebrovascular disease and peripheral vascular disease (IC = 0.03). There was a negative additive interaction for connective tissue disease (IC = −0.03) (Figure 2). After 6 years of follow-up, there was little evidence of any interaction except for dementia (IC = −0.06) and metastatic solid tumor (IC = 0.12) (Figure 3).
Figure 2.
Interaction contrast (IC; additive comorbidity–hip fracture interaction) between hip fracture and individual diseases included in the Romano modification of the Charlson Comorbidity Index at a maximum of 1 year of follow-up, Norway, 2009–2015. Data were available for 38,126 Norwegian women with hip fracture and a matched comparison cohort. Bars, 95% confidence intervals (CIs).
Figure 3.
Interaction contrast (IC; additive comorbidity–hip fracture interaction) between hip fracture and individual diseases included in the Romano modification of the Charlson Comorbidity Index at a maximum of 6 years of follow-up, Norway, 2009–2015. Data were available for 38,126 Norwegian women with hip fracture and a matched comparison cohort. Bars, 95% confidence intervals (CIs).
Stratifying the analysis by type of hip fracture mainly resulted in slightly higher ICs at 1 year of follow-up for intracapsular and subtrochanteric fractures as compared with intertrochanteric fractures (Web Tables 3–5).
DISCUSSION
Main findings
In this large population-based cohort study of postmenopausal women, the absolute excess risk in mortality among women who sustained a hip fracture and had comorbidity was higher than could be explained by the hip fracture per se. The absolute excess risk was observed during the first year of follow-up; thereafter, no excess risk due to the additive interaction was found. The estimated additive interaction increased with number of comorbid conditions (CCI score), ranging between 3 and 9 additional deaths per 100 patients during the first year after a hip fracture. Nevertheless, the mortality risk of hip fracture per se remained increased after 6 years of follow-up. Despite little evidence of any additive interaction between hip fracture and comorbidity after 6 years, we observed an interaction on the multiplicative scale, in the form of decreasing relative survival (hip fracture vs. no hip fracture) as a function of time.
Comparison with other studies
Several clinical studies have found an association between prefracture comorbidity and hip fracture survival (1, 39–42). Prefracture conditions such as cardiac disease, dementia, renal disease, and chronic obstructive pulmonary disease have been identified as predictors of 30-day (41) and 1-year (1) mortality. Previous cohort studies of the association of hip fracture and comorbidity with mortality have matched hip fracture patients and general-population cohort members on age and sex but not on prefracture comorbidity or education (11, 18, 19, 42). An excess risk of myocardial infarction up to 1 year after a hip fracture and for stroke up to 10 years postfracture was found in a recent population-based Danish study (42), where the attributable fraction of any of the outcomes within 30 days due to the interaction between hip fracture and comorbidity was estimated to be 76%. Several cohort studies have indicated an association between prefracture comorbidity and post–hip-fracture mortality (16, 18, 43), but these studies have been small and the investigators have not presented any measures of interaction.
In contrast to our study, Vestergaard et al. (11) found little association between prefracture comorbidity and excess hip fracture mortality in a large population-based Danish study. The authors attributed 70% of deaths to complications that arose during the first 30 days postfracture (the hip fracture per se) and attributed long-term excess mortality to several factors, including income. At the same time, causes contributing to excess mortality included infections, psychiatric disease, pulmonary disease, dementia, and neurological diseases—conditions that may have been present before the hip fracture. A similar conclusion was drawn in a recent Australian study by Lystad et al. (44), where 72% of the 1-year post–hip-fracture mortality was found to be attributed to the hip fracture per se. There could be several reasons for this higher proportion being explained by the hip fracture per se. In the Australian study, hip fracture patients were 3 times more likely to die within 12 months than people without fracture, as compared with a doubling of the risk in our study. Both Vestergaard et al. (11) and Lystad et al. (44) included men in their studies, and the matches (controls) had to be free of hip and femur fracture during the study period. In our study, we did not impose this requirement; we required only that the matches be free of fracture from the index date onward and back to 2008. Further, we matched on comorbidity in the baseline period, including the index hospitalization and going back to 2008, while both Vestergaard et al. and Lystad et al. used a 12-month look-back period.
We found that for many of the comorbid conditions studied, the risk of 1-year mortality was increased beyond that due to the hip fracture per se. Exceptions were hemiplegia and connective tissue diseases, where the additive interaction was negative. Many patients with connective tissue disease are treated with glucocorticoids, which may lead to bone degradation. This often prompts simultaneous treatment with bone-protecting agents, usually bisphosphonates. Intravenous treatment with bisphosphonate may have a preventive effect against mortality, as well as against hip fracture (45). One may speculate as to whether this accounts for some of the negative additive interaction between connective tissue disease and mortality after a hip fracture. The largest negative IC was estimated for patients with hemiplegia 1 year postfracture. In contrast, increased mortality in patients suffering from poststroke hemiplegia has been described previously and was explained by more overall comorbidity (46).
Strengths and weaknesses
This was a nationwide population-based cohort study designed to examine the combined role of hip fracture and comorbidity in mortality among postmenopausal women. Because hip fracture invariably leads to hospitalization and because all hospitalizations in Norway are registered in the NPR, virtually all hip fractures that occurred in Norway during the study period were identified, also including the 5% identified by ICD-10 codes alone. Missing a code for surgery did not change study results substantially, suggesting that risk of selection bias in our study was minimal. Mortality data in the Norwegian Cause of Death Registry have a completeness of 98% (47).
Although we matched women with hip fracture to women without hip fracture by age, education, and comorbidity, the results may be partially explained by potential residual confounding by unmeasured characteristics inherent in a registry-based study, such as smoking, body mass index, use of drugs that may induce osteoporosis, or disease severity (48).
Women with hip fracture for whom we did not find a match were excluded (5%). These women had more comorbidity and shorter survival than the hip fracture cohort, which may potentially have biased the ICs towards zero. The use of duplicates (repeated sampling) in the matched cohort could also potentially have biased our estimated standard deviations. We therefore conducted a bootstrap-type sensitivity study, which showed that the use of duplicate matches had little effect on the estimated standard deviations (less than 5% bias).
Comorbidity was measured for a minimum of 1 year and a maximum of 6 years before the index date and included acute first-time diagnoses made during the index hospitalization. This approach implicitly assumes that acute events may result from an ongoing underlying clinical condition. However, one might argue that the acute condition represents a complication or trauma arising from the hip fracture, rather than part of a preexisting condition, and therefore should not be included as a matching variable (49). Further, use of the Romano modification of the CCI (31) required translation of codes from the International Classification of Diseases, Ninth Revision, to the ICD-10 (34, 50, 51). This modified index may have omitted some acute diseases, such as infections, that could interact with hip fracture to cause excess mortality.
An additional concern is that relatively less severe diseases, such as diabetes, which do not typically lead to hospital encounters, are incompletely captured by the NPR (21). As a consequence, counting the number of comorbid conditions at the index hospitalization has some potential for overmatching. To evaluate the potential bias, we doubled the disease frequency of each of the 16 disease categories in the source population (eligible matches) before matching the hip fracture cohort. The estimated ICs increased approximately 50% at 1-year follow-up to 0.06 and 0.14 for CCI scores of 1–2 and ≥3, respectively, and after 6 years of follow-up the estimated ICs were 0.08 and 0.09, respectively.
Many women had a hospital diagnosis of dementia at the time of the index hospitalization. Nearly 50% of these diagnoses were registered for the first time, which suggests that dementia may not be well captured by the NPR or that many patients are wrongly diagnosed with dementia in an acute-care setting, such as hospitalization due to a hip fracture. If the latter is true, we could expect to see the absolute excess risk change from positive to negative over time, as we actually observed. However, repeating the analyses after excluding all diagnosis made on the index date from the matching did not increase the IC for dementia; rather, the IC decreased slightly, from −0.06 to −0.07.
There was a slight gradual rightward shift in the age distribution with increasing CCI score. This could potentially have biased some of the ICs. We therefore performed a stratified analysis, calculating the IC within 1-year age groups followed by a pro-rata weighting of the ICs. This changed the ICs for some of the CCI scores by up to 0.02 units in either direction.
Given the increasing number of patients with comorbidity due to advanced age and the high mortality among hip fracture patients, optimizing the preoperative and postoperative care of patients with chronic heart, renal, or pulmonary disease, as well as focusing on complications (e.g., infections) known to be related to these diseases, could potentially reduce mortality.
Conclusion
This study confirms that mortality among women with comorbidity is excessively high after a hip fracture.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Global Public Health and Primary Care, Faculty of Medicine, University of Bergen, Bergen, Norway (Astrid Lunde, Grethe S. Tell, Ellen M. Apalset); Department of Clinical Epidemiology, Aarhus University Hospital, Aarhus, Denmark (Alma B. Pedersen, Vera Ehrenstein, Henrik T. Sørensen); Department of Public Health, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (Thomas H. Scheike); and Bergen Group of Epidemiology and Biomarkers in Rheumatic Disease, Department of Rheumatology, Haukeland University Hospital, Bergen, Norway (Ellen M. Apalset).
No specific funding was received for this work.
This study used data from the Norwegian Patient Registry and the Norwegian Cause of Death Registry. Interpretation and reporting of these data are the sole responsibility of the authors, and no endorsement by the Norwegian Patient Registry or the Norwegian Cause of Death Registry is intended or should be inferred.
A.L. is involved in the Denosumab Global Safety Study (Amgen protocol 20090522; Amgen Inc., Thousand Oaks, California). H.T.S. does not report receiving fees, honoraria, grants, or consultancies; however, the Department of Clinical Epidemiology at Aarhus University Hospital is involved in studies that receive funding from various companies as research grants to (and administered by) Aarhus University. None of the other authors have any conflicts of interest to declare.
Abbreviations
- CCI
Charlson Comorbidity Index
- IC
interaction contrast
- ICD-10
International Classification of Diseases, Tenth Revision
- IQR
interquartile range
- NPR
Norwegian Patient Registry
REFERENCES
- 1. Pedersen AB, Ehrenstein V, Szépligeti SK, et al. 35-year trends in first-time hospitalization for hip fracture, one year mortality, and the prognostic impact of comorbidity: a Danish nationwide cohort study, 1980–2014. Epidemiology. 2017;28(6):898–905. [DOI] [PubMed] [Google Scholar]
- 2. Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–1733. [DOI] [PubMed] [Google Scholar]
- 3. Omsland TK, Emaus N, Tell GS, et al. Mortality following the first hip fracture in Norwegian women and men (1999–2008). A NOREPOS study. Bone. 2014;63:81–86. [DOI] [PubMed] [Google Scholar]
- 4. Abrahamsen B, van Staa T, Ariely R, et al. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int. 2009;20(10):1633–1650. [DOI] [PubMed] [Google Scholar]
- 5. Haentjens P, Magaziner J, Colón-Emeric CS, et al. Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med. 2010;152(6):380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Curtis E, Litwic A, Cooper C, et al. Determinants of muscle and bone aging. J Cell Physiol. 2015;230(11):2618–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jackson RD, Mysiw WJ. Insights into the epidemiology of postmenopausal osteoporosis: the Women’s Health Initiative. Semin Reprod Med. 2014;32(6):454–462. [DOI] [PubMed] [Google Scholar]
- 8. Melton LJ 3rd, Atkinson EJ, St Sauver JL, et al. Predictors of excess mortality after fracture: a population-based cohort study. J Bone Miner Res. 2014;29(7):1681–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lawrence VA, Hilsenbeck SG, Noveck H, et al. Medical complications and outcomes after hip fracture repair. Arch Intern Med. 2002;162(18):2053–2057. [DOI] [PubMed] [Google Scholar]
- 10. Roche JJ. Effect of comorbidities and postoperative complications on mortality after hip fracture in elderly people: prospective observational cohort study. BMJ. 2005;331(7529):1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vestergaard P, Rejnmark L, Mosekilde L. Increased mortality in patients with a hip fracture—effect of pre-morbid conditions and post-fracture complications. Osteoporos Int. 2007;18(12):1583–1593. [DOI] [PubMed] [Google Scholar]
- 12. Pedersen AB, Johnsen SP, Sørensen HT. Increased one-year risk of symptomatic venous thromboembolism following total hip replacement: a nationwide cohort study. J Bone Joint Surg Br. 2012;94(12):1598–1603. [DOI] [PubMed] [Google Scholar]
- 13. Pedersen AB, Baron JA, Overgaard S, et al. Short- and long-term mortality following primary total hip replacement for osteoarthritis: a Danish nationwide epidemiological study. J Bone Joint Surg Br. 2011;93(2):172–177. [DOI] [PubMed] [Google Scholar]
- 14. Pedersen AB, Sorensen HT, Mehnert F, et al. Risk factors for venous thromboembolism in patients undergoing total hip replacement and receiving routine thromboprophylaxis. J Bone Joint Surg Am. 2010;92(12):2156–2164. [DOI] [PubMed] [Google Scholar]
- 15. Erichsen R, Horváth-Puhó E, Iversen LH, et al. Does comorbidity interact with colorectal cancer to increase mortality? A nationwide population-based cohort study. Br J Cancer. 2013;109(7):2005–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Magaziner J, Lydick E, Hawkes W, et al. Excess mortality attributable to hip fracture in white women aged 70 years and older. Am J Public Health. 1997;87(10):1630–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lloret A, Coiffier G, Couchouron T, et al. Risk factors of mortality during the first year after low energy osteoporosis fracture: a retrospective case-control study. Clin Cases Miner Bone Metab. 2016;13(2):123–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tosteson AN, Gottlieb DJ, Radley DC, et al. Excess mortality following hip fracture: the role of underlying health status. Osteoporos Int. 2007;18(11):1463–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frost SA, Nguyen ND, Center JR, et al. Excess mortality attributable to hip-fracture: a relative survival analysis. Bone. 2013;56(1):23–29. [DOI] [PubMed] [Google Scholar]
- 20. Kirkland LL, Kashiwagi DT, Burton MC, et al. The Charlson Comorbidity Index score as a predictor of 30-day mortality after hip fracture surgery. Am J Med Qual. 2011;26(6):461–467. [DOI] [PubMed] [Google Scholar]
- 21. Nilssen Y, Strand TE, Wiik R, et al. Utilizing national patient-register data to control for comorbidity in prognostic studies. Clin Epidemiol. 2014;6:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nordic Medico-Statistical Committee NOMESCO Classification of Surgical Procedures (Version 1.16). Copenhagen, Denmark: Nordic Medico-Statistical Committee; 2011. http://norden.diva-portal.org/smash/get/diva2:968721/fulltext01.pdf. Accessed June 1, 2017.
- 23. Høiberg MP, Gram J, Hermann P, et al. The incidence of hip fractures in Norway—accuracy of the national Norwegian patient registry. BMC Musculoskelet Disord. 2014;15:Article 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Statistics Norway Individually Based Education Statistics: Documentation 2005 Oslo, Norway: Statistics Norway; 2006. https://www.ssb.no/a/english/publikasjoner/pdf/nos_d361_en/nos_d361_en.pdf. Accessed June 1, 2018.
- 25. Pedersen AG, Ellingsen CL. Data quality in the Causes of Death Registry. Tidsskr Nor Laegeforen. 2015;135(8):768–770. [DOI] [PubMed] [Google Scholar]
- 26. Rothman J, Greenland S, Lash T. Modern Epidemiology. 3rd ed Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 27. Ording AG, Garne JP, Nyström PM, et al. Comorbid diseases interact with breast cancer to affect mortality in the first year after diagnosis—a Danish nationwide matched cohort study. PLoS One. 2013;8(10):e76013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–549. [DOI] [PubMed] [Google Scholar]
- 29. Igland J, Vollset SE, Nygård OK, et al. Educational inequalities in 28 day and 1-year mortality after hospitalisation for incident acute myocardial infarction—a nationwide cohort study. Int J Cardiol. 2014;177(3):874–880. [DOI] [PubMed] [Google Scholar]
- 30. Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. [DOI] [PubMed] [Google Scholar]
- 31. Schneeweiss S, Wang PS, Avorn J, et al. Improved comorbidity adjustment for predicting mortality in Medicare populations. Health Serv Res. 2003;38(4):1103–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 33. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. [DOI] [PubMed] [Google Scholar]
- 34. Thygesen SK, Christiansen CF, Christensen S, et al. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol. 2011;11:Article 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aalen OO. A linear regression model for the analysis of life times. Stat Med. 1989;8(8):907–925. [DOI] [PubMed] [Google Scholar]
- 36. VanderWeele TJ. Explanation in Causal Inference: Methods for Mediation and Interaction. New York, NY: Oxford University Press; 2015. [Google Scholar]
- 37. VanderWeele TJ, Knol MJ. A tutorial on interaction. Epidemiol Methods. 2014;3(1):33–72. [Google Scholar]
- 38. Li R, Chambless L. Test for additive interaction in proportional hazards models. Ann Epidemiol. 2007;17(3):227–236. [DOI] [PubMed] [Google Scholar]
- 39. Brauer CA, Coca-Perraillon M, Cutler DM, et al. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14):1573–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Björkelund KB, Hommel A, Thorngren KG, et al. Factors at admission associated with 4 months outcome in elderly patients with hip fracture. AANA J. 2009;77(1):49–58. [PubMed] [Google Scholar]
- 41. Khan MA, Hossain FS, Ahmed I, et al. Predictors of early mortality after hip fracture surgery. Int Orthop. 2013;37(11):2119–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pedersen AB, Ehrenstein V, Szépligeti SK, et al. Hip fracture, comorbidity, and the risk of myocardial infarction and stroke: a Danish nationwide cohort study, 1995–2015. J Bone Miner Res. 2017;32(12):2339–2346. [DOI] [PubMed] [Google Scholar]
- 43. Farahmand BY, Michaëlsson K, Ahlbom A, et al. Survival after hip fracture. Osteoporos Int. 2005;16(12):1583–1590. [DOI] [PubMed] [Google Scholar]
- 44. Lystad RP, Cameron CM, Mitchell RJ. Mortality risk among older Australians hospitalised with hip fracture: a population-based matched cohort study. Arch Osteoporos. 2017;12:Article 67. [DOI] [PubMed] [Google Scholar]
- 45. Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357(18):1799–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Feng M, Zhang J, Shen H, et al. Predictors of prognosis for elderly patients with poststroke hemiplegia experiencing hip fractures. Clin Orthop Relat Res. 2009;467(11):2970–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Norwegian Tax Administration National Registry. http://www.skatteetaten.no/en/Person/National-Registry/. Accessed June 1, 2017.
- 48. Nelson HD, Haney EM, Chou R, et al. Screening for Osteoporosis: Systematic Review to Update the 2002 U.S. Preventive Services Task Force Recommendation. (Evidence Synthesis, no. 77). Rockville, MD: Agency for Healthcare Research and Quality; 2010. https://www.ncbi.nlm.nih.gov/pubmed/20722176. Accessed June 1, 2018. [PubMed] [Google Scholar]
- 49. Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25(1):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 51. Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.