Abstract
Background
Medication-related osteonecrosis of the jaw (MRONJ) is due to the direct effects of drug toxicity and the effects on angiogenesis. The aims of this study were to evaluate the effects of treatment with the bisphosphonate, zoledronic acid, on human oral keratinocytes (HOKs) and human umbilical vein endothelial cells (HUVECs) in vitro, and whether epidermal growth factor (EGF) could alter these effects.
Material/Methods
HOKs and HUVECs were incubated with zoledronic acid or EGF. Cell viability was assessed by the cell counting kit-8 (CCK-8), cell apoptosis was studied using Annexin-V conjugated to fluorescein isothiocyanate (FITC). Angiogenesis was studied by observing HUVEC tube formation and cell migrations using a transwell assay. A scratch wound assay investigated cell migration of HOKs. Western blot measured expression levels of phosphorylated epidermal growth factor receptor (EGFR), Akt, phosphoinositide 3-kinase (PI3K), the mechanistic target of rapamycin (mTOR), and endothelial nitric oxide synthase (eNOS).
Results
Zoledronic acid treatment (5 μmol/L) significantly inhibited cell viability and cell migration of HOKs and HUVECs and angiogenesis of HUVECS (P<0.05); EGF partially reversed these effects (P<0.05). Zoledronic acid treatment of HOKs and HUVECs had no significant effects on apoptosis (P>0.05), but significantly reduced expression levels of p-EGFR, p-Akt, p-PI3K, p-mTOR), and p-eNOS (P<0.05); EGF partially reversed these effects and increased the expression levels (P<0.05).
Conclusions
EGF partially reversed the effects of the bisphosphonate, zoledronic acid, on HOKs and HUVECs in vitro via the EGFR/Akt/PI3K signaling pathway. Further studies are required to determine the effects of EGF on MRONJ including bisphosphonate-related osteonecrosis of the jaw.
MeSH Keywords: Bisphosphonate-Associated Osteonecrosis of the Jaw; Endothelial Cells; Epidermal Growth Factor; Keratinocytes; Proto-Oncogene Proteins c-akt; Receptor, Epidermal Growth Factor
Background
Bisphosphonate is used in the treatment of osteoporosis and has also been used for the treatment of malignancy, including prostate cancer and breast cancer [1,2]. Since 2003, bisphosphonate-related osteonecrosis of the jaw (BRONJ has been increasingly reported [3]. In 2014, the American Association of Oral and Maxillofacial Surgeons (AAOMS) renamed BRONJ as medication-related osteonecrosis of the jaws (MRONJ) and is defined as the presence of a necrotic response or fistula formation in the bone of the maxillofacial region for at least eight weeks, in response to therapy with antiresorptive or anti-angiogenic agents, excluding radiotherapy [4]. The mechanism underlying the effects of MRONJ remains unclear [5].
Previously published studies have investigated the influence of bisphosphonate on osteoblasts and osteoclasts and the inhibition of bone remodeling [6,7]. Other mechanisms that have been studied for the effects of bisphosphonate have included an anti-angiogenetic effected by inhibiting endothelial progenitor cells and mature endothelial cells in vitro [8]. Zoledronic acid, a third-generation N-bisphosphonate, is also able to suppress pre-osteoclasts that release platelet-derived growth factor-BB (PDGF-BB), resulting in suppression of angiogenesis [9]. Lu et al. [10] demonstrated that zoledronic acid (Zoledronate) promoted Beclin-1-mediated autophagy to induce endothelial cell apoptosis. Other in vitro studies have examined the toxicity of bisphosphonate oral tissues. Pabst et al. [11] confirmed the negative influences of highly potent N-bisphosphonates on human oral keratinocytes (HOKs), and this effect could be significantly reversed by geranylgeraniol. Kalyan et al. [12] showed that the expression of genes regulating immune and barrier functions was downregulated in patients with MRONJ.
The EGFR/Akt/PI3K signaling pathway is highly correlated with cell proliferation, apoptosis, cell migration, and endothelial cell angiogenesis. Epidermal growth factor receptor (EGFR) is one of the receptor tyrosine kinases (TKs) and is an important driver of growth and differentiation of epithelial cells [13,14]. Extracellular ligands, such as epidermal growth factor (EGF) and transforming growth factor-α (TGF-α), can interact with the EGFR [13], resulting in the stimulation of Akt/PI3K and downstream molecules, including mTOR, eNOS, and the Bcl2-associated antagonist of cell death (BAD). The mammalian target of rapamycin (mTOR) is associated with cell proliferation, survival, migration, and vascular angiogenesis [15]. Also, endothelial nitric oxide synthase (eNOS) acts as a positive regulator of endothelial NOS, and NO can dilate blood vessels and activate the migration and proliferation of vascular cells [16]. BAD is a member of the pro-apoptosis bcl-2 family of proteins. Non-phosphorylated BAD can interact with Bcl-xl, an anti-apoptotic protein belonging to the Bcl-2 family, inducing cell apoptosis, whereas the phosphorylation of BAD results in the loss of pro-apoptotic activity [17].
Previously published studies have shown that the PI3K/Akt signaling pathway was correlated with the adverse impact of bisphosphonates [18,19]. Tang et al. [19] showed that the inhibitory effects of bisphosphonates on the HIF-1α/VEGF axis were associated with the PI3K/Akt/mTOR signaling pathways. Inoue et al. [20] showed that alendronate inhibited the PI3K/Akt/NFκB signaling pathway, which was correlated with the survival of an osteosarcoma cell line.
In view of these previous studies, it is possible to hypothesize that the EGFR/Akt/PI3K signaling pathway might have a role in the anti-angiogenetic effects of bisphosphonate and also in toxicity in the oral mucosa, because EGFR is expressed on the surface of a variety of cells, including epithelial cells and endothelial cells [21,22] (Table 1).
Table 1.
A summary of previously published studies related to the present study.
| Study | Title | Conclusions |
|---|---|---|
| Pabst et al. (2015) [11] | The influence of geranyl geraniol on human oral keratinocytes after bisphosphonate treatment: An in vitro study | Bisphosphonate treatment had negative effects on human oral keratinocytes (HOKs) |
| Ziebart et al. (2011) [8] | Bisphosphonates: restriction for vasculogenesis and angiogenesis: inhibition of cell function of endothelial progenitor cells and mature endothelial cells in vitro | The nitrogen-containing bisphosphonates, pamidronate, and zoledronate, had the greatest impact on human umbilical vein endothelial cells (HUVECs) |
| Kim et al. (2016) [18] | Zoledronic acid is an effective radiosensitizer in the treatment of osteosarcoma | Zoledronic acid decreased the phosphorylation of PI3K-Akt and MAPK pathway proteins |
| Tang et al. (2010) [19] | Bisphosphonates suppress insulin-like growth factor 1-induced angiogenesis via the HIF-1α/VEGF signaling pathways in human breast cancer cells | Inhibitory effects of bisphosphonates on the HIF-1α/VEGF axis are associated with PI3K/Akt/mTOR signaling pathways |
| Treda et al. (2016) [13] | EGFR activation resulted in cell death, ndependent of PI3K/Akt/mTOR in the AD293 cell human embryonic kidney cell line | PI3K, Akt, and mTOR were shown to be downstream proteins of the epidermal growth factor receptor (EGFR) |
| Bhattacharya et al. (2015) [21] | T11TS inhibits angiopoietin-1/Tie-2 signaling, EGFR activation, and the Raf/MEK/ERK pathway in brain endothelial cells, inhibiting angiogenesis in a glioma model | EGFR was expressed by endothelial cells, not only in epithelial cells |
MAPK – mitogen-activated protein kinase; HIF-1 – hypoxia inducible factor-1; VEGF – vascular endothelial growth factor; T11TS – T11-target structure.
Therefore, the aims of this study were to evaluate the effects of treatment with the bisphosphonate, zoledronic acid, on human oral keratinocytes (HOKs) and human umbilical vein endothelial cells (HUVECs) in vitro, and whether epidermal growth factor (EGF) could alter these effects through the EGFR/Akt/PI3K signaling pathway.
Material and Methods
Reagents and antibodies
Zoledronic acid (Zoledronate) was purchased from Sigma-Aldrich (St Louis, MO, USA). Recombinant human epidermal growth factor (EGF) was obtained from Peprotech (Rocky Hill, NJ, USA). Antibodies for p-EGFR (Y845), p-Akt (ser473), p-PI3K (Tyr458), p-mTOR (ser-2448), p-BAD (ser136), and p-eNOS (ser1177) were obtained from Cell Signaling Technology (Boston, MA, USA). Primary antibodies for GAPDH, EGFR, Akt, PI3K, mTOR, BAD, and eNOS were obtained from Hunan Biotechnology (Hangzhou, China).
Cell culture and treatment of human umbilical vein endothelial cells (HUVECs) and human oral keratinocytes (HOKs) and the four treatment groups
Human umbilical vein endothelial cells (HUVECs) and human oral keratinocytes (HOKs) were obtained from the American Type Culture Collection (ATCC) (Manassas, VA, USA) and were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (GE Healthcare, Hyclone, South Logan, UT, USA) with 10% fetal bovine serum (FBS) (GE Healthcare, Hyclone, South Logan, UT, USA) and 1% penicillin and streptomycin (Sigma-Aldrich, St Louis, MO, USA) in a 37°C humidified atmosphere containing 95% air and 5% CO2.
After cell starvation for 24 h, four groups were studied, as follows: a control group of untreated HUVECs and HOKs; HUVECs and HOKs treated with 5, 50, or 100 μmol/L of zoledronic acid; HUVECs and HOKs treated with 10 ng/L of epidermal growth factor (EGF), and; HUVECs and HOKs treated with zoledronic acid combined with EGF (ZA + EGF). The range of concentrations of zoledronic acid was selected based on previous in vivo findings of the plasma levels shortly after zoledronic acid infusion, measured at nearly 5 μmol/L [23]. The concentration of EGF was chosen according to previously published recommendations [24]. Also, according to the findings of Shen et al. [25], 10 ng/ml EGF was the maximum effective concentration for stimulating the proliferation of HUVECs.
Cell viability using the cell counting kit-8 (CCK-8) assay in vitro
Cell viability and cell proliferation analysis were performed using the cell counting kit-8 (CCK-8) assay (Dojindo, Gaithersburg, MD, USA), as previously described [26]. HUVECs or HOKs were plated in 96-well plates (5,000 cells/well) and incubated overnight. After starvation for 24 h, HUVECs were cultured as described above for 24–48 h [8,10]. HOKs were cultured for 48–72 h with the same stimulation conditions [11]. Then, the CCK-8 solution (10 μl) was added to each well, followed by incubation for 1–4 h at 37°C. The absorbance at 450 nm was measured to analyze the cell viability.
Cell apoptosis using immunofluorescence and flow cytometry
Annexin-V conjugated to fluorescein isothiocyanate (FITC) was used in a detection kit (Nanjing KeyGen Biotech. Co. Ltd., Nanjing, China), as previously described [27]. Briefly, HUVECs or HOKs were seeded at a density of 4×105 cells/ml and incubated overnight. After starvation for 24 h, cells were stimulated with 0, 5, 50, or 100 μmol/L of zoledronic acid. HUVECs were collected after 48 h and HOKs were collected after 72 h. The percentage of apoptotic cells was determined using a flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA).
Wound scratch assay
HOKs were seeded into 6-well plates at a density of 5×105 cells/well. After 24 h, a scratch wound was performed in each well using a sterile pipette tip. The size of the scratch wound was measured microscopically and taken as the 100% value. After starvation for 24 h, the cells were treated, as described above. After 72 h, the size of the scratch wounds was measured, as previously described [28].
Angiogenesis assay
HUVECs were seeded and incubated overnight. After starvation for 24 h, the cells were treated, as described above. Matrigel (BD Biosciences, San Jose, USA) was thawed at 4°C overnight and used to coat a 96-well plate, which was then incubated at room temperature for 30 minutes on the gel. After 48 h, HUVECs were plated on the Matrigel (3×104 cells/well). After incubation for 6 h, the newly-formed vascular tube networks were photographed. The number of branching points was counted in a minimum of three fields per image [29].
Cell migration assay
HUVECs were seeded and incubated overnight. After starvation for 24 h, the cells were treated, as described above. After 48 h, HUVECs (105 cells/ml) were cultured in the upper chambers of 24-well transwell chambers with an 8-mm pore size (Costar, Lowell, MA, USA). The lower chambers were supplemented with DMEM containing 10% fetal bovine serum (FBS). After 24 h of incubation at 37°C, the upper chambers were washed with PBS, fixed with paraformaldehyde, and then stained with crystal violet. HUVECs remaining on the upper surface of the transwell membrane were removed. The number of cells that migrated to the lower part of the chamber was evaluated under a microscope at a magnification of ×200, and five fields were randomly chosen [30].
Western blot
HOKs and HUVECs were treated, as described above. Total protein from the cells was extracted using RIPA lysis buffer (Beyotime Biotech, Shanghai, China), containing 1 mM the serine protease inhibitor, phenylmethylsulfonyl fluoride (PMSF), 10 mM PhosStop phosphatase inhibitor (Sigma-Aldrich, St Louis, MO, USA), and 5 mM of protease inhibitors. The total protein concentration was analyzed by the bicinchoninic acid (BCA) protein assay (Beyotime Biotech, Shanghai, China). The proteins were loaded onto 6–10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels at 60–90 V and then electroblotted onto a polyvinylidene difluoride (PVDF) membrane (0.22 μm) (Millipore, Burlington, MA, USA) at 200 mA. After blocking for 1 h with 5% bovine serum albumin (BSA) in TBST, the membranes were incubated with primary antibodies against total and phosphorylated EGFR, Akt, PI3K, mTOR, BAD, and eNOS at 4°C overnight. GAPDH served as the internal control. The membranes were then washed three times with TBST and incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (1: 1,000) (Cell Signaling Technology, Danvers, MA, USA) for 1 h. After washing again with TBST, the proteins were finally detected by SuperSignal enhanced chemiluminescence (ECL) substrate, (Pierce, Rockford IL, USA) [5].
Statistical analysis
Statistical analysis was performed by one-way analysis of variance (ANOVA) using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA) to explore the relationships between the different samples. Fisher’s test of least significant difference (LSD) was used for post hoc analysis. A p-value <0.05 was considered to represent statistical significance.
Results
The effects of zoledronic acid and epidermal growth factor (EGF) on the proliferation of human oral keratinocytes (HOKs) and human umbilical vein endothelial cells (HUVECs) in vitro
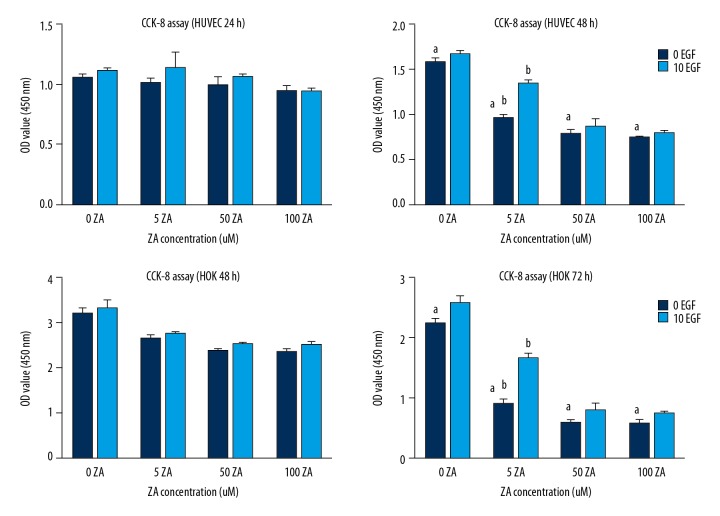
There was no significant difference when human umbilical vein endothelial cells (HUVECs) were stimulated with zoledronic acid for 24 h compared with the control group (P>0.05). Whereas, the cells viability of HUVECs was reduced in a dose-dependent manner following treatment with zoledronic acid for 48 h (P<0.05), but no difference was found between 50 μmol/L and 100 μmol/L of zoledronic acid. The cell viability of human oral keratinocytes (HOKs) was significantly inhibited by 50% following treatment with zoledronic acid for 72 hours, at all three dose concentrations (P<0.05). Also, no significant difference was found between 50 μmol/L of zoledronic acid and 100 μmol/L of zoledronic acid.
Treatment with EGF exerted a significant positive effect when cells were stimulated with 5 μmol/L of zoledronic acid (P<0.05) but had little effect on HUVECs and HOKs with at doses of 50 μmol/L or 100 μmol/L for zoledronic acid (P>0.05) (Figure. 1).
Figure 1.
The effects of zoledronic acid (ZA) and epidermal growth factor (EGF) on the cell viability of human umbilical vein endothelial cells (HUVECs) and human oral keratinocytes (HOKs) measured by the cell counting kit-8 (CCK-8) assay. The results are presented as the average ± standard deviation (SD) of three independent experiments. a: Zoledronic acid treatment showed significant effects on human umbilical vein endothelial cells (HUVECs) after 48 h at the concentrations of 5, 50, and 100 μmol/L. Zoledronic acid significantly affected HOKs after 72 h at these concentrations (P<0.05). b: Treatment with epidermal growth factor (EGF) had a significant positive effect when cells were stimulated with 5 μmol/L of zoledronic acid (P<0.05).
The effects of zoledronic acid on the apoptosis of both HUVECs and HOKs in vitro
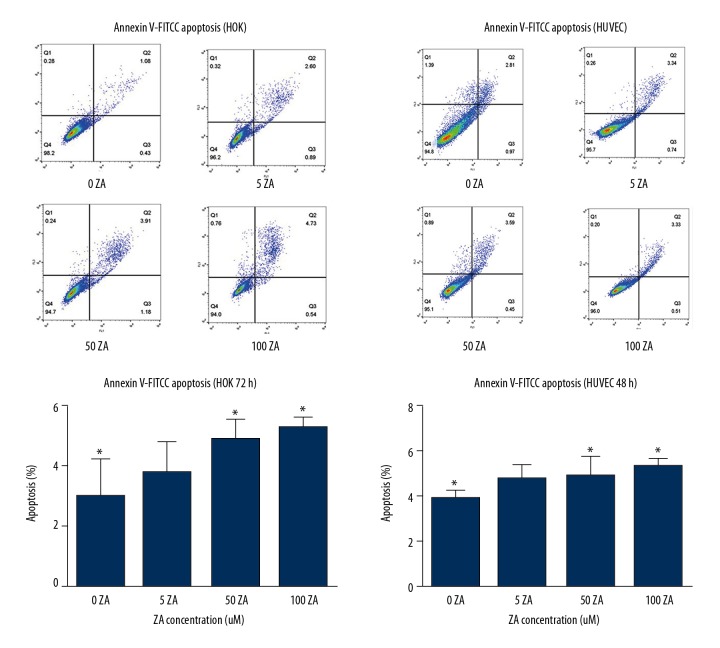
In the present study, zoledronic acid had a small negative influence on apoptosis in HUVECs and HOKs, although there was a significant difference between the control group and 100 μmol/L zoledronic acid (P<0.05) in HUVECs. A significant difference was also found between the control and the HOK cell groups treated with 50 μmol/L and 100 μmol/L of zoledronic acid. However, even the highest concentration (100 μmol/L) of zoledronic acid resulted in less than 10% of cell apoptosis in HUVEC and HOK cells (Figure 2).
Figure 2.
The effects of zoledronic acid on apoptosis in human umbilical vein endothelial cells (HUVECs) and human oral keratinocytes (HOKs) was measured by an Annexin-V conjugated to fluorescein isothiocyanate (FITC) apoptosis assay. The results are presented as the average ± standard deviation (SD) of three independent experiments. There was a significant difference between the control group and the human umbilical vein endothelial cells (HUVECs) treated with 100 μmol/L of zoledronic acid, and a significant difference between the control group and the human oral keratinocytes (HOKs) treated with 50 and 100 μmol/L of zoledronic acid (P<0.05). However, even the highest concentration (100 μmol/L) of zoledronic acid resulted in less than 10% of cell apoptosis. * P<0.05 zoledronic acid-treated vs. control.
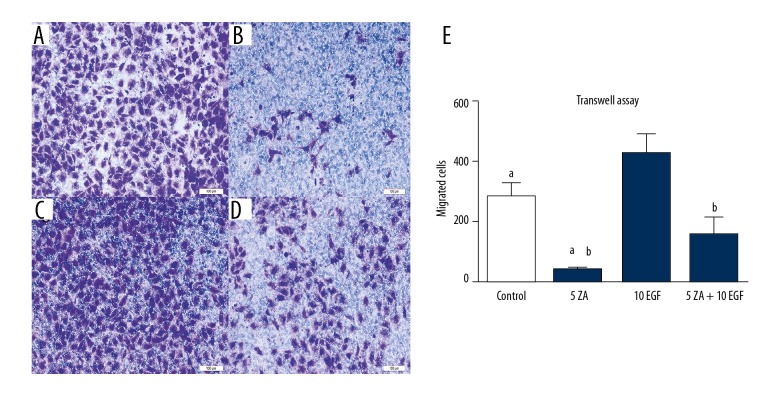
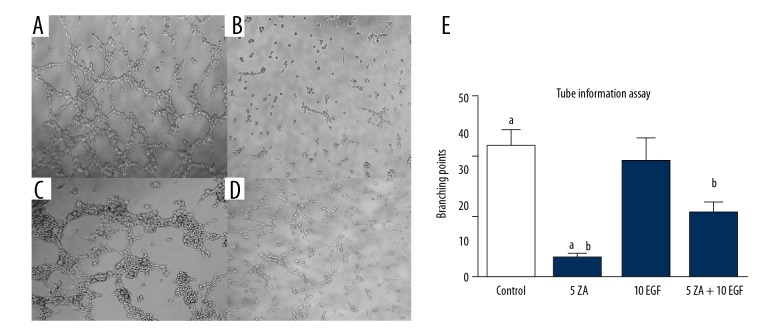
Effects of zoledronic acid and epidermal growth factor (EGFR) on cell migration and angiogenesis of HUVECs in vitro
The migration and angiogenesis of HUVECs were studied when incubated with zoledronic acid and EGF. Zoledronic acid inhibited both cell migration and angiogenesis at a concentration of 5 μmol/L. There were significant differences between the control group and the zoledronic acid-treated groups (P<0.05). Compared with the zoledronic acid-treated group, the combination of EGF (10 ng/L) and zoledronic acid (5 μmol/L) could partially reverse the properties of cell migration and angiogenesis (P<0.05). However, the migration and angiogenesis in the zoledronic acid and EGF-treated group (ZA + EGF) were significantly lower compared with the control and EGF-treated groups (P<0.05) (Figures 3, 4).
Figure 3.
The effects of zoledronic acid and epidermal growth factor (EGF) on the migration ability of human umbilical vein endothelial cells (HUVECs) were measured by a transwell assay. (A) Photomicrograph of the transwell assay of human umbilical vein endothelial cells (HUVECs) treated with Dulbecco’s modified Eagle’s medium (DMEM) without zoledronic acid or epidermal growth factor (EGF) treatment for 48 h. (B) Photomicrograph of the transwell assay of HUVECs, treated with 5 μmol/L zoledronic acid for 48 h. (C) Photomicrograph of the transwell assay of HUVECs, treated with 10 ng/L EGF for 48 h. (D) Photomicrograph of the transwell assay of HUVECs, treated with 5 μmol/L zoledronic acid and 10 ng/L EGF for 48 h. The results are presented as the average ± standard deviation (SD) of three independent experiments. (E) a: Zoledronic significantly inhibited the migration ability at a concentration of 5 μmol/L (P<0.05). b: EGF could partially reverse the inhibitory effects of zoledronic acid (P<0.05).
Figure 4.
The effects of treatment with zoledronic acid (ZA) and epidermal growth factor (EGF) on angiogenesis of human umbilical vein endothelial cells (HUVECs) were measured using an assay for the formation of vascular tubes. (A) Human umbilical vein endothelial cells (HUVECs) were treated with Dulbecco’s modified Eagle’s medium (DMEM) without zoledronic acid or epidermal growth factor (EGF) for 48 h. (B) HUVECs were treated with 5 μmol/L of zoledronic acid for 48 h. (C) HUVECs were treated with 10 ng/L of EGF for 48 h. (D) HUVECs were treated with 5 μmol/L of zoledronic acid and 10 ng/L EGF for 48 h. Results are presented as the average ±SD of three independent experiments. (E) a: Zoledronic acid significantly inhibited angiogenesis at a concentration of 5 μmol/L (P<0.05). b: EGF treatment could partially reverse the negative effects of zoledronic acid (P<0.05).
The effects of zoledronic acid and epidermal growth factor on cell migration of HOKs in vitro
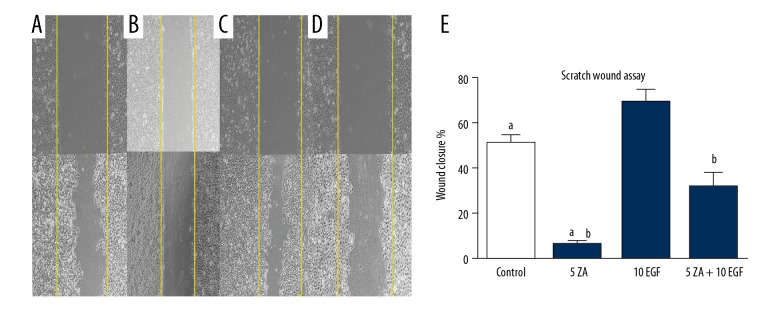
Cell migration of HOKs was evaluated by a scratch wound assay. Treatment with zoledronic acid significantly suppressed the migration of HOKs compared with the control group. (P<0.05), which was partially reversed following treatment with EGF. There was a significant difference between the zoledronic acid-treated group and the zoledronic acid and EGF-treated group (ZA + EGF) groups (P<0.05). Similar to the above assays, the migration ability of HOKs in the ZA + EGF group was significantly lower compared with the control and EGF groups (P<0.05) (Figure 5).
Figure 5.
The effects of treatment with zoledronic acid (ZA) and epidermal growth factor (EGF) on the migration ability of human oral keratinocytes (HOKs) were measured by a scratch wound assay. (A) Human oral keratinocytes (HOKs) were treated with Dulbecco’s modified Eagle’s medium (DMEM) without zoledronic acid or EGF for 72 h. (B) HOKs were treated with 5 μmol/L zoledronic acid for 72 h. (C) HOKs were treated with 10 ng/L EGF for 72 h. (D) HOKs were treated with 5 μmol/L of zoledronic acid and 10 ng/L EGF for 72 h. After 72 h, the sizes of the scratch wounds were measured. The results are presented as the average ± standard deviation (SD) of three independent experiments. (E) a: Zoledronic acid treatment significantly inhibited the migration ability of HOKs at a concentration of 5 μmol/L (P<0.05). b: EGF could partially reverse the negative effects of zoledronic acid (P<0.05).
The effects of zoledronic acid and epidermal growth factor on the EGFR/Akt/PI3K signaling pathway in HOKs and HUVECs in vitro
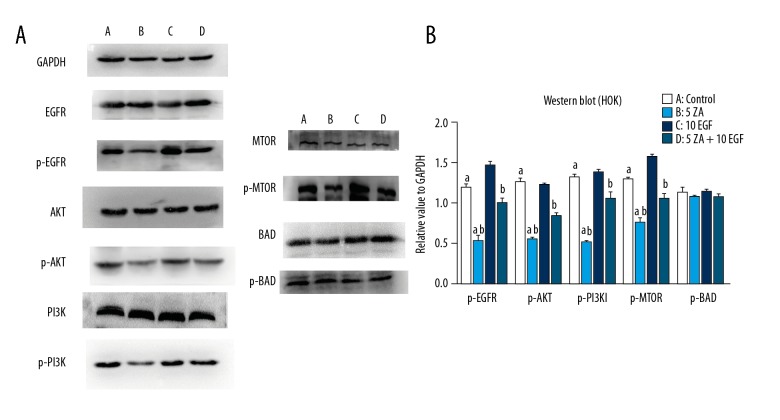
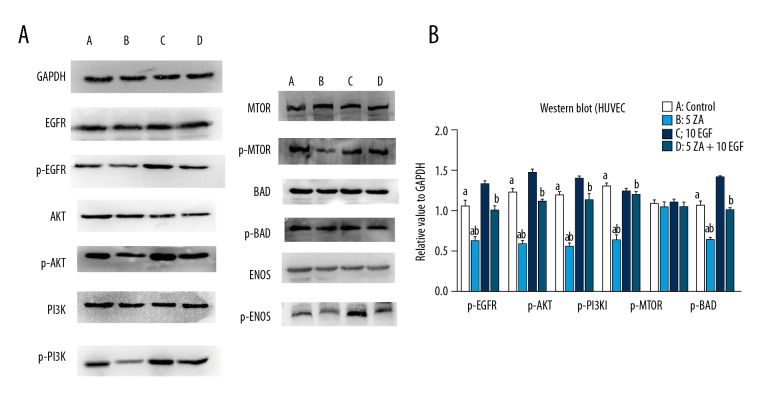
Western blot for the levels of p-EGFR, p-Akt, and p-PI3K in HOKS and HUVECs in the zoledronic acid-treated group were expressed at lower levels compared with the control and EGF groups (P<0.05). Also, the levels of downstream proteins, including p-mTOR and p-eNOS, were also significantly decreased in the zoledronic acid-treated group compared with the control and EGF groups. However, zoledronic acid did not significantly decrease p-BAD levels, and there were no significant differences among all of the groups, which was consistent with the results of the apoptosis assay. In the zoledronic acid and EGF-treated group (ZA + EGF), the relative values of p-EGFR, p-Akt, p-PI3K, p-mTOR, and p-eNOS were significantly increased compared with those of the zoledronic acid-treated group (P<0.05) (Figures 6, 7).
Figure 6.
Western blot determined protein levels in human oral keratinocytes (HOKs). (A) A) Human oral keratinocytes (HOK) cells were treated with Dulbecco’s modified Eagle’s medium (DMEM) without zoledronic acid or epidermal growth factor (EGF) for 72 h. B) HOK cells were treated with 5 μmol/L of zoledronic acid for 72 h. C) HOK cells were treated with 10 ng/L EGF for 72 h. D) HOK cells were treated with 5 μmol/L of zoledronic acid and 10 ng/L EGF for 72 h. The results are presented as the mean ± standard deviation (SD) of three independent experiments. (B) a: Zoledronic acid significantly decreased the expression levels of p-EGFR, p- Akt, p-PI3K, and p-mTOR in HOKs at a concentration of 5 μmol/L (P<0.05). b: EGF could partially reverse the negative effects of zoledronic acid (P<0.05).
Figure 7.
Western blot determined protein levels in human umbilical vein endothelial cells (HUVECs). (A) A) Human umbilical vein endothelial cells (HUVECs) were treated with Dulbecco’s modified Eagle’s medium (DMEM) without zoledronic acid or epidermal growth factor (EGF) for 48 h. B) HUVECs were treated with 5 μmol/L zoledronic acid for 48 h. C) HUVECs were treated with 10 ng/L EGF for 48 h. D) HUVECs were treated with 5 μmol/L of zoledronic acid and 10 ng/L EGF for 48 h. The results are presented as the mean ± standard deviation (SD) of three independent experiments. (B) a: Zoledronic acid significantly decreased the expression levels of p-EGFR, p-Akt, p-PI3K, p-mTOR, and p-eNOS in HUVECs at a concentration of 5 μmol/L (P<0.05). b: EGF could partially reverse the effects of zoledronic acid (P<0.05).
Discussion
The findings of this in vitro study on the effects of treatment with the bisphosphonate, zoledronic acid, on human oral keratinocytes (HOKs) and human umbilical vein endothelial cells (HUVECs), showed a significant negative effect of zoledronic acid on cell viability, cell migration, and angiogenesis. However, these negative effects could be partially reversed by treatment with epidermal growth factor (EGF). with the effects mediated by the EGFR/Akt/PI3K signaling pathway.
This study confirmed the potent inhibitory effects of zoledronic acid on the viability of HOKs and HUVECs at concentrations of 5, 50, and 100 μmol/L, which is similar to previous reports [8,11]. The HOK proliferation ability was reduced by over 50% with zoledronic acid treatment at 72 h in culture and at a concentration of 5 μmol/L (P<0.05), while 50 and 100 μmol/L concentrations of zoledronic acid could inhibited almost 70% of the proliferation ability. This result is higher than those reported by most previous studies, in which the proliferation abilities were reduced to 60–80% at a concentration of 5 μmol/L of zoledronic acid [31,32]. This difference might be due to the cells in this experiment having been starved for 24 h before stimulation. After starvation, cells were maintained under the same conditions, allowing zoledronic acid to fully exert its effect on cells. In addition, few studies have examined the influence of zoledronic acid on HOKs. Pabst et al. [11] reported that the HOK proliferation rate was reduced to nearly 50% in their study, which supports the findings of the present study.
However, there appears to be no direct interaction between the application of zoledronic acid and apoptosis in either HOKs or HUVECs. Even the highest concentration (100 μmol/L) of zoledronic acid could induce less than 10% apoptosis, although there was a statistical difference between the control group and the group treated with 100 μmol/L of zoledronic acid. This result is in contrast to the findings of several reports. Lu et al. [10] found that zoledronic acid could induce apoptosis and autophagy in HUVECs and that the inhibition of autophagy attenuated zoledronic acid-induced apoptosis. Huang et al. [31] showed that zoledronic acid treatment induced apoptosis in osteoblasts in a dose- and time-dependent manner. One reason for this discrepancy might be that methods for assaying apoptosis were different between studies. Other kits were selected in previous experiments, rather than the Annexin-V conjugated to fluorescein isothiocyanate (FITC) apoptosis detection kit [31]. Tai et al. [33] reported that zoledronic acid could activate the p38 MAPK signaling pathway, which would increase the expression of anti-apoptotic Bcl-XL and increase cell survival, which could also explain why zoledronic acid induced less than 10% apoptosis in the present study. Considering that zoledronic acid had little impact on the apoptosis of HOKs and HUVECs, this study did not assay the effects of adding EGF on apoptosis.
The migration ability of HOKs and HUVECs and the angiogenesis ability of HUVECs were also suppressed by zoledronic acid treatment. According to the wound scratch assay, there was little wound healing in HOKs treated with 5 μmol/L of zoledronic acid for 72 h, and a significant difference was found between the zoledronic acid and control groups. In the tube formation and transwell assays, 5 μmol/L of zoledronic acid strongly affected the migration and angiogenesis abilities of HUVECs, in contrast with the control group. These results are in accordance with those reported by with published studies [11,34]. The concentration of 5 μmol/L of zoledronic acid was selected because it is the in vivo concentration found in plasma shortly after zoledronic acid infusion [23], and 5 μmol/L zoledronic acid had a visible impact on HOKs and HUVECs in the viability assay described above.
To examine whether the EGFR/PI3K/Akt signaling pathway was associated with the negative effect of bisphosphonate and whether EGF can reduce the effects of bisphosphonate via the EGFR/PI3K/Akt signaling pathway, Western blot analysis was used to examine the expression of proteins of interest. Based on the results, zoledronic acid treatment downregulated p-Akt and p-PI3K in HOKs and HUVECs. Also, zoledronic acid decreased the expression levels of downstream phosphorylated proteins. The reduced expression level of p-mTOR observed in this study is in accordance with the aforementioned findings that zoledronic acid inhibited proliferation and the migration ability. p-EGFR was also obviously decreased in HOKs. These results suggest that zoledronic acid might inhibit HOKs through the Akt/PI3K signaling pathway after zoledronic acid interacts with the EGFR. In HUVECs, the expression level of p-EGFR was also decreased. This result could be because there are also epidermal growth factor receptors on the surface of endothelial cells, and the activation of EGFRs are required for vascular remodeling and angiogenesis [21,22]. Zoledronic acid also downregulated p-eNOS, which is associated with the angiogenesis ability of HUVECs [35]. This finding supports those from the tube formation assay. The level of p-BAD was not affected by zoledronic acid in both HOKs and HUVECs, which could explain why the apoptosis assay did not show much difference between the zoledronic acid and control groups.
The present study analyzed protein expression levels in the presence of EGF and zoledronic acid simultaneously. Consequently, the expression levels of p-EGFR, p- Akt, p-PI3Kp-mTOR, and p-eNOS were partially elevated by EGF. Although the expression levels were still lower than those in the control and EGF groups, they were significantly higher than those in the zoledronic acid only group. This result is in accordance with the results of the proliferation, migration, and angiogenesis assays described above. When HOKs and HUVECs were treated with both EGF and zoledronic acid, the negative effects of zoledronic acid could be partially reversed by EGF. The outcome of the Western blot analysis illustrates the direct toxicity of bisphosphonate to the oral mucosa, and the anti-angiogenetic effects of bisphosphonate on the endothelium can be partially neutralized by EGF through the EGFR/Akt/PI3K signaling pathway.
This study aimed to investigate neutralization of the in vitro cytotoxic effects of bisphosphonates on oral keratinocytes, as well as the endothelium, based on the theory of an anti-angiogenesis effect. After a series of assays it was found that EGF could attenuate the negative effects of bisphosphonate, and this might be due to the activation of the EGFR/Akt/PI3K signaling pathway. After the systemic or topical application of bisphosphonates, direct contact with the mucosa can lead to cytotoxic effects for the oral mucosa, and high systemic concentrations in the circulating blood can cause angiogenesis inhibition, which can eventually develop into medication-related osteonecrosis of the jaw (MRONJ). Based on these experiments, EGF might represent an option for the prevention and treatment of MRONJ. EGF could be administered to patients by topical delivery systems, such as collagen membranes or mouth rinses [11,36]. Further research should investigate the application of EGF to MRONJ lesions in animal models.
However, several practical considerations remain to be clarified and require further study. In the present study, EGF could only partially neutralize the adverse effects of low-level bisphosphonates. Therefore, other agents should be evaluated to amplify the positive effect of EGF, such as transforming growth factor-α (TGF-α). In addition, the optimal therapeutic EGF concentrations should be confirmed, as this study only analyzed the effects of EGF at a concentration of 10 ng/L. Furthermore, this in vitro study used zoledronic acid, the bisphosphonate most frequently associated with the development of MRONJ. Other bisphosphonates, such as pamidronate and clodronate, and other agents related to MRONJ, including bevacizumab [37], denosumab [38], and sunitinib [39], should be evaluated in further studies.
Conclusions
The present study explored whether the effects of the bisphosphonate, zoledronic acid, on human oral keratinocytes (HOKs) and human umbilical vein endothelial cells (HUVECs) could be partially neutralized with the treatment of epidermal growth factor (EGF) in vitro. EGF reversed the effects of bisphosphonates via the EGFR/Akt/PI3K signaling pathway, which supports that further in vivo studies in animal models are required to investigate the effects of EGF treatment on medication-related osteonecrosis of the jaw (MRONJ).
Footnotes
Source of support: This work was supported by the Youth Scientific Research Foundation of West China Hospital of Stomatology, Sichuan University (Grant number: 2016-05) and Scientific Research Foundation of the Science and Technology Department of Sichuan Province, China (Grant numbers: 2017SZ0094 and 2017SZ0108)
Conflict of interest
None.
References
- 1.Dalle CL, Mottes M, Malerba G, et al. Enhanced osteogenic differentiation in zoledronate-treated osteoporotic patients. Int J Mol Sci. 2017;18(6) doi: 10.3390/ijms18061261. pii: E1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan W, Sun J, Zhou L, et al. Randomized trial comparing efficacies of zoledronate and alendronate for improving bone mineral density and inhibiting bone remodeling in women with post-menopausal osteoporosis. J Clin Pharm Ther. 2016;41(5):519–23. doi: 10.1111/jcpt.12429. [DOI] [PubMed] [Google Scholar]
- 3.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61(9):1115–17. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 4.Ruggiero SL. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw – 2014 update. J Oral Maxillofac Surg. 2014;72(10):1938–56. doi: 10.1016/j.joms.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 5.Aghaloo T, Hazboun R, Tetradis S. Pathophysiology of osteonecrosis of the jaws. Oral Maxillofac Surg Clin North Am. 2015;27(4):489–96. doi: 10.1016/j.coms.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park SH, Kim JY, Cheon YH, et al. Protocatechuic acid attenuates osteoclastogenesis by downregulating JNK/c-Fos/NFATc1 signaling and prevents inflammatory bone loss in mice. Phytother Res. 2016;30(4):604–12. doi: 10.1002/ptr.5565. [DOI] [PubMed] [Google Scholar]
- 7.Ihn HJ, Lee D, Lee T, et al. The 1,2,3-triazole derivative KP-A021 suppresses osteoclast differentiation and function by inhibiting RANKL-mediated MEK-ERK signaling pathway. Exp Biol Med. 2015;240(12):1690–97. doi: 10.1177/1535370215576310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ziebart T, Pabst A, Klein MO, et al. Bisphosphonates: Restrictions for vasculogenesis and angiogenesis: Inhibition of cell function of endothelial progenitor cells and mature endothelial cells in vitro. Clin Oral Investig. 2011;15(1):105–11. doi: 10.1007/s00784-009-0365-2. [DOI] [PubMed] [Google Scholar]
- 9.Gao SY, Zheng GS, Wang L, et al. Zoledronate suppressed angiogenesis and osteogenesis by inhibiting osteoclasts formation and secretion of PDGF-BB. PLoS One. 2017;12(6):e0179248. doi: 10.1371/journal.pone.0179248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu Y, Wang Z, Han W, Li H. Zoledronate induces autophagic cell death in human umbilical vein endothelial cells via Beclin-1 dependent pathway activation. Mol Med Rep. 2016;14(5):4747–54. doi: 10.3892/mmr.2016.5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pabst AM, Krüger M, Ziebart T, et al. The influence of geranylgeraniol on human oral keratinocytes after bisphosphonate treatment: An in vitro study. J Craniomaxillofac Surg. 2015;43(5):688–95. doi: 10.1016/j.jcms.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 12.Kalyan S, Wang J, Quabius ES, et al. Systemic immunity shapes the oral microbiome and susceptibility to bisphosphonate-associated osteonecrosis of the jaw. J Transl Med. 2015;13:212. doi: 10.1186/s12967-015-0568-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cezary T, Marta P, Magdalena K, et al. EGFR activation leads to cell death independent of PI3K/AKT/mTOR in an AD293 cell line. PLoS One. 2016;11(5):e0155230. doi: 10.1371/journal.pone.0155230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.She QB, Gruvberger-Saal SK, Maurer M, et al. Integrated molecular pathway analysis informs a synergistic combination therapy targeting PTEN/PI3K and EGFR pathways for basal-like breast cancer. BMC Cancer. 2016;16:587. doi: 10.1186/s12885-016-2609-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park J-H, Yoon J, Park B. Pomolic acid suppresses HIF1alpha/VEGF-mediated angiogenesis by targeting p38-MAPK and mTOR signaling cascades. Phytomedicine. 2016;23(14):1716–26. doi: 10.1016/j.phymed.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Wu Q, Zhao Y, Duan W, et al. Propofol inhibits high glucose-induced PP2A expression in human umbilical vein endothelial cells. Vascul Pharmacol. 2017;91:18–25. doi: 10.1016/j.vph.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Fu Z, Yang J, Wei Y, Li J. Effects of piceatannol and pterostilbene against beta-amyloid-induced apoptosis on the PI3K/Akt/BAD signaling pathway in PC12 cells. Food Funct. 2016;7(2):1014–23. doi: 10.1039/c5fo01124h. [DOI] [PubMed] [Google Scholar]
- 18.Kim EH, Kim MS, Lee KH, et al. Zoledronic acid is an effective radiosensitizer in the treatment of osteosarcoma. Oncotarget. 2016;7(43):70869. doi: 10.18632/oncotarget.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang X, Zhang Q, Shi S, et al. Bisphosphonates suppress insulin-like growth factor 1-induced angiogenesis via the HIF-1alpha/VEGF signaling pathways in human breast cancer cells. Int J Cancer. 2010;126(1):90–103. doi: 10.1002/ijc.24710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inoue R, Matsuki N, Jing G, et al. The inhibitory effect of alendronate, a nitrogen-containing bisphosphonate on the PI3K-Akt-NFkappaB pathway in osteosarcoma cells. Br J Pharmacol. 2005;146(5):633–41. doi: 10.1038/sj.bjp.0706373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhattacharya D, Chaudhuri S, Singh MK, Chaudhuri S. T11TS inhibits Angiopoietin-1/Tie-2 signaling, EGFR activation and Raf/MEK/ERK pathway in brain endothelial cells restraining angiogenesis in glioma model. Exp Mol Pathol. 2015;98(3):455–66. doi: 10.1016/j.yexmp.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 22.Takayanagi T, Kawai T, Forrester SJ, et al. Role of epidermal growth factor receptor and endoplasmic reticulum stress in vascular remodeling induced by angiotensin II. Hypertension. 2015;65(6):1349–55. doi: 10.1161/HYPERTENSIONAHA.115.05344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen T, Berenson J, Vescio R, et al. Pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with bone metastases. J Clin Pharmacol. 2002;42(11):1228–36. doi: 10.1177/009127002762491316. [DOI] [PubMed] [Google Scholar]
- 24.Miguel JC, Maxwell AA, Hsieh JJ, et al. Epidermal growth factor suppresses intestinal epithelial cell shedding through a MAPK-dependent pathway. J Cell Sci. 2017;130(1):90–96. doi: 10.1242/jcs.182584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen K, Sheng Y, Ji L, Wang Z. Involvement of c-Jun N-terminal kinase and extracellular signal-regulated kinase 1/2 in EGF-induced angiogenesis. Cell Biol Int. 2010;34(12):1213–18. doi: 10.1042/CBI20100185. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Y, Tu C, Zhao Y, et al. Placental growth factor enhances angiogenesis in human intestinal microvascular endothelial cells via PI3K/Akt pathway: Potential implications of inflammation bowel disease. Biochem Biophys Res Commun. 2016;470(4):967–74. doi: 10.1016/j.bbrc.2016.01.073. [DOI] [PubMed] [Google Scholar]
- 27.Sharma D, Hamlet S, Petcu E, Ivanovski S. Animal models for bisphosphonate-related osteonecrosis of the jaws – an appraisal. Oral Dis. 2013;19(8):747–54. doi: 10.1111/odi.12067. [DOI] [PubMed] [Google Scholar]
- 28.Ohlrich EJ, Coates DE, Cullinan MP, et al. The bisphosphonate zoledronic acid regulates key angiogenesis-related genes in primary human gingival fibroblasts. Arch Oral Biol. 2016;63:7–14. doi: 10.1016/j.archoralbio.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Ishii M, Nakahara T, Ikeuchi S, Nishimura M. beta-Amyrin induces angiogenesis in vascular endothelial cells through the Akt/endothelial nitric oxide synthase signaling pathway. Biochem Biophys Res Commun. 2015;467(4):676–82. doi: 10.1016/j.bbrc.2015.10.085. [DOI] [PubMed] [Google Scholar]
- 30.Qi X, Liu G, Qiu L, et al. Marine bromophenol bis(2,3-dibromo-4,5-dihydroxybenzyl) ether, represses angiogenesis in HUVEC cells and in zebrafish embryos via inhibiting the VEGF signal systems. Biomed Pharmacother. 2015;75:58–66. doi: 10.1016/j.biopha.2015.08.033. [DOI] [PubMed] [Google Scholar]
- 31.Huang KC, Cheng CC, Chuang PY, Yang TY. The effects of zoledronate on the survival and function of human osteoblast-like cells. BMC Musculoskelet Disord. 2015;16:355. doi: 10.1186/s12891-015-0818-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pabst AM, Ziebart T, Koch FP, et al. The influence of bisphosphonates on viability, migration, and apoptosis of human oral keratinocytes – in vitro study. Clin Oral Investig. 2012;16(1):87–93. doi: 10.1007/s00784-010-0507-6. [DOI] [PubMed] [Google Scholar]
- 33.Tai TW, Su FC, Chen CY, et al. Activation of p38 MAPK-regulated Bcl-xL signaling increases survival against zoledronic acid-induced apoptosis in osteoclast precursors. Bone. 2014;67:166–74. doi: 10.1016/j.bone.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Pabst AM, Krüger M, Sagheb K, et al. The influence of geranylgeraniol on microvessel sprouting after bisphosphonate substitution in an in vitro 3D-angiogenesis assay. Clin Oral Investig. 2017;21(3):771–78. doi: 10.1007/s00784-016-1842-z. [DOI] [PubMed] [Google Scholar]
- 35.García-Morales V, Luaces-Regueira M, Campos-Toimil M. The cAMP effectors PKA and Epac activate endothelial NO synthase through PI3K/Akt pathway in human endothelial cells. Biochem Pharmacol. 2017;145:94–101. doi: 10.1016/j.bcp.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Ziebart T, Koch F, Klein MO, et al. Geranylgeraniol – a new potential therapeutic approach to bisphosphonate associated osteonecrosis of the jaw. Oral Oncol. 2011;47(3):195–201. doi: 10.1016/j.oraloncology.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Fusco V, Santini D, Armento G, et al. Osteonecrosis of jaw beyond antiresorptive (bone-targeted) agents: New horizons in oncology. Expert Opin Drug Saf. 2016;15(7):925–35. doi: 10.1080/14740338.2016.1177021. [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto A, Sasaki M, Schmelzeisen R, et al. Primary wound closure after tooth extraction for prevention of medication-related osteonecrosis of the jaw in patients under denosumab. Clin Oral Investig. 2017;21(1):127–34. doi: 10.1007/s00784-016-1762-y. [DOI] [PubMed] [Google Scholar]
- 39.Balázs S, László V, József S. [Sunitinib and zoledronic acid-induced osteonecrosis of the jaw]. Orvosi Hetil. 2015;156(46):1865–70. doi: 10.1556/650.2015.30292. [in Hungarian] [DOI] [PubMed] [Google Scholar]