Abstract
Background
The aim of this study was to define blood flow characteristics of multiple types of flaps and the theoretical basis of flap axis design.
Material/Methods
Sixty Sprague-Dawley (SD) rats were randomly divided into 6 groups: a normal skin group, and 5 groups with different types of flap: abdominal flap group, dorsal flap group, single-perforator flap group, double-perforators flap group, and delayed cutaneous nerve flap group. The vascular distribution characteristics of normal skin and various flap types were observed by gross morphology of specimens and X-ray after perfusion.
Results
There were distinct differences in vascular anastomosis and density in dorsal and ventral SD rats. The area of flap survival in the dorsal flap group was superior to that in the abdominal flap group, but the flap axis of the 2 groups passed straight through the middle of the pedicle. The flap surviving area in the double-perforators flap group was remarkably larger than in the single-perforator flap group, while the flap axis in the single-perforator flap group passed straight through the perforators, and in the double-perforators flap group there was a linking vessel between the 2 perforators. There were linking and reticulate vessels, in addition, linking vessels and cutaneous nerves were concomitant in the delayed cutaneous nerve flap group. The flap axis was the travel route of the cutaneous nerve.
Conclusions
Variations in flap blood supply patterns and axes with alterations based on flap types have implications for flap survival. Understanding blood flow characteristics within each flap type and accurately designing the flap axis is essential for flap survival.
MeSH Keywords: Axis, Research Design, Surgical Flaps
Background
A surgical flap is a composite structure of skin and tissue containing its own blood supply. Flap surgery has become one of the most widespread surgical procedures in reconstructive surgery. However, despite improvements in surgical technology, necrosis of postoperative skin flaps still regularly occurs [1–3]. There are many factors that affect flap survival, such as the characteristics of blood flow, physiology within multiple types of flaps, anticipated anatomical and dynamic territories of the flap, preoperative flap design, and surgical skills. A solid understanding of blood flow characteristics and physiology, as well as an accurate preoperative design, is essential for ensuring flap survival in various types of flaps [4,5]. Currently, there remains a paucity of evidence regarding characteristics of flap blood flow and preoperative axis design.
Flap surgery has a protracted history in clinical medicine. While it developed slowly in its early stage, advancements in flap surgery rapidly progressed in the 1970s due to better understanding of blood flow characteristics and physiology within flaps. In 1973, McGregor and Morgan proposed the concepts of the axial pattern flap and the random pattern flap based on the direct cutaneous vessels and the musculocutaneous perforator vessels in the skin [6]. This was the first recognition of the unique blood flow characteristics and physiology within the flap. Afterwards, flaps began to be applied extensively in clinical practice. With increasing comprehension of flap blood supply, additional concepts for types of flaps were proposed, often without streamlining information. Fascia flaps, also named super flaps, with a 5: 1 length/width ratio were reported by Ponten et al. [7] in 1981; neurocutaneous flaps are based on the blood vessels that supply cutaneous nerves and were originally reported by Bertelli et al. [8] in 1993. Additional studies confirmed that linking vessels can also supply blood to neurocutaneous flaps [9,10].
Various studies confirmed that flaps may be classified into axial pattern flaps, linking-pattern flaps, reticulate-pattern flaps, and compound vessel pattern flaps, depending on the vessels that provide blood supply to the flap [11,12]. These vessels are the axial vessels, linking vessels, reticulate vessels, and compound vessels, respectively. All current methods of performing flap procedures utilize one of these 4 classifications. The classification describes blood flow characteristics and physiology of multiple types of flaps and is applicable in clinical applications to effectively increase flap survival [13–16]. However, systematic theoretical support of the classification has not been well documented.
In applied anatomy, the concept of “flap axis” refers to the surface projection line that is composed of primary blood vessels within a flap. The flap axis is used as the axis of preoperative flap design; to guarantee flap blood supply during a surgical operation, approximately 1 cm on each side of this line under the deep fascia should be protected. It is essential in the operating room to be familiar with the characteristics of blood flow and vessel distribution within the flap and the flap axis design. Additionally, with changes in classification of flaps, flap axis design varies at times and thus should be described.
Hence, this study established different types of flap models in SD rats, including abdominal and dorsal flap models, single-perforator and double-perforators flap models, and a cutaneous nerve delayed flap model. These flap models were equivalent to the random pattern flap, perforator flap, and neurocutaneous nutrition vessels flap in clinical models. We investigated the vascular distribution of deep and superficial blood vessels and flap survival area, as well as exploring the theoretical flap axis design through perfusion, dissection, and X-ray film. We hope that our results will provide valuable information that will clarify blood flow characteristics of multiple types of flaps and the theoretical basis of flap axis design, and will promote the clinical application of appropriate flap types to reduce surgical failure. To the best of our knowledge, this is the first article that systematically reviews the blood supply characteristics and axis design of skin flaps.
Material and Methods
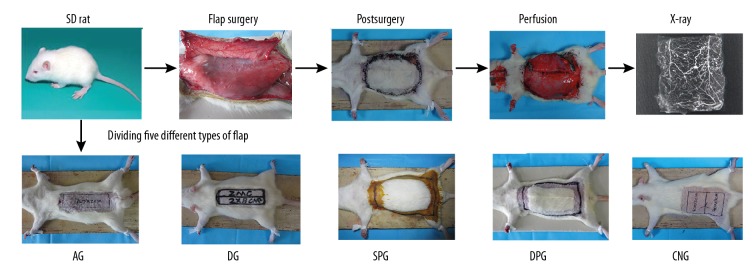
Sixty healthy male SD rats (sanitation degree, 2–3 months old, 250–300 g, mean weight, 275 g) were provided by the Experimental Animal Center of Zunyi Medical College in Guizhou Province, China. The rats were randomly divided into 6 groups with 10 in each group. The groups were: 1) normal skin group, 2) abdominal flap group, 3) dorsal flap group, 4) single-perforator flap group, 5) double-perforators flap group, and 6) cutaneous nerve delayed flap group. In the normal skin group, the rats underwent normal skin perfusion by red lead oxide. In the abdominal flap group, the rats were given an abdominal cranial pedicle reticulate-pattern flap. In the dorsal flap group, a rat dorsal cranial pedicle reticulate-pattern flap was established. In the single-perforator flap group, the rats were perfused through the first perforating branch on the cranial side of the right superior epigastric artery. In the double-perforators group, the rats were perfused through the first and posterior perforating branch on the cranial side of the right superior epigastric artery. In the cutaneous nerve delayed flap group, a right lateral femoral neurocutaneous vascular flap was established (Figure 1).
Figure 1.
Schematic diagram of the experiment. The rats were randomly divided into 6 groups: a normal skin group (NG), an abdominal flap group (AG), a dorsal flap group (DG), a single perforator flap group (SPG), a double perforators flap group (DPG), and a cutaneous nerve-delayed flap group (CNG). During surgery, the flap was designed, incised, sutured in situ, and bred for 7 days. Each group was subjected to surgery and perfusion, lastly, then vascular distribution was observed by X-ray.
Before surgery, all rats were conventionally fasted for 12 h, weighed, labeled, intraperitoneally injected with a 3% pentobarbital sodium (0.1 mL/100 g) (Pharmacy Department of Zunyi Medical College Affiliated Hospital, Guizhou, China), anesthetized, and fixed to an operating table. During surgery, the flap was designed, incised, sutured in situ, and bred for 7 days. (The neural flap was performed by delaying the skin flap 7 days, then excising it, suturing it in situ, and breeding it for 7 days). Gross photos were taken of all flaps. The appropriate perfusing vessels for each respective group were excised after the rats were sacrificed. Lastly, the flaps were dissected and the vascular distribution within each flap was observed by X-ray. This study was approved by the Animal Ethics Committee of the Affiliated Hospital of Zunyi Medical College (Number.201401). All experiments were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Observational indexes
The vascular density
The skin vascular density of SD rats was analyzed using Image J software (National Institutes of Health, Bethesda, MD). Skin vascular density was recorded as follows: Vascular development area/Total skin area × 100%.
Flap survival area
Flap survival area was analyzed using Image J software (National Institutes of Health, Bethesda, MD). Flap survival area was recorded as follows: (Original flap area – necrotic flap area)/Original flap area×100%.
Statistical analysis
Results are expressed as mean ± standard deviation (SD). Data were analyzed with the independent-samples t test using SPSS ver. 19.0 (Armonk, NY, USA). P<0.05 was considered statistically significant.
Results
Normal skin group
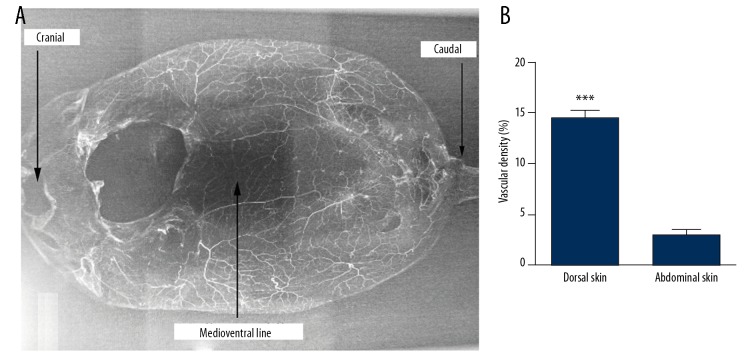
In the dorsal skin of SD rats, there were many perforators originating from deeper arteries, as well as sufficient direct anastomosis with true or choke anastomosis, and the vascular density was 14.53±0.74%. Conversely, in the abdominal skin, there were few perforators originating from deeper arteries and little direct anastomosis with true or choke anastomosis, and the vascular density was 2.86±0.58% (P<0.001) (Figure 2). Each perforator had a zone of vascular network in an inverted cone-like distribution, with the piercing point of the perforator as a cone vertex (Figure 3).
Figure 2.
Perfusion X-ray films from normal rat skin. (A) In the dorsal skin of SD rats, there were sufficient direct anastomoses with true or choke anastomosis, and intensive vascular density. Conversely, in the abdominal skin, especially at the medioventral line, there were little direct anastomosis and lower vascular density. (B) Quantification of the vascular density of the different area of SD rat skin. *** P<0.001.
Figure 3.
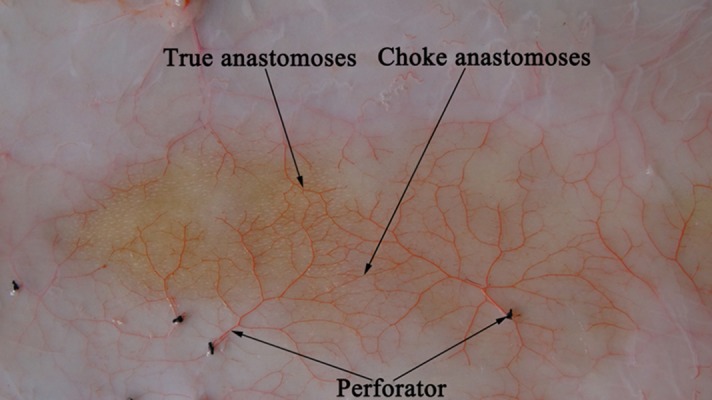
Perforasome (perfusion specimens). Each perforator had a zone of vascular network and there were some true or choke anastomoses among the perforators.
Abdominal flap group
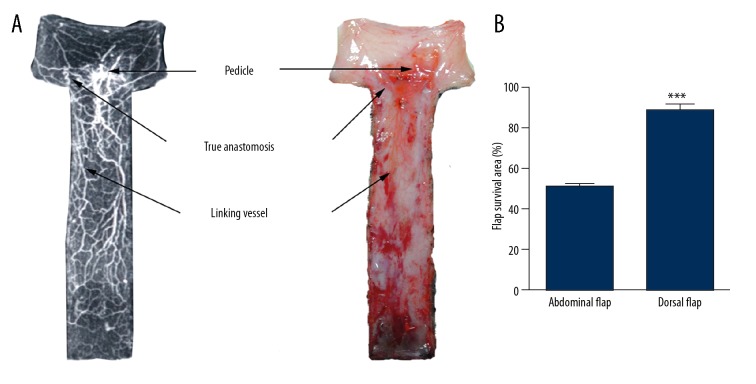
Within flaps in the abdominal flap group, there was lower vascular density with no apparent direct anastomosis or linking vessels. The incision areas of the flap were 7×2 cm, with the distal flaps being mostly necrotic. The proximal surviving area ranged from 2.5×2 cm to 3×2 cm. The surviving area was rectangular with a 1.25: 1–1.5: 1 length/width ratio of flap survival. The flap survival area was 50.38±2.04%. The flap axis was a straight line that that passed through the middle of the pedicle (Figure 4).
Figure 4.
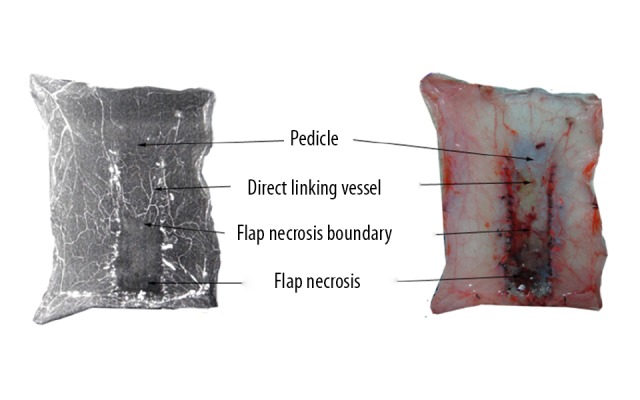
Abdomen reticulate vessel flap (left: perfusion X-ray films; right: perfusion specimens). The proximal flap containing a few direct linking vessels survived and the distal flaps were mostly necrotic. The surviving area was rectangular, with a 1.25: 1–1.5: 1 length/width ratio of flap survival.
Dorsal flap group
Flaps in the dorsal flap group had higher vascular density, definite direct anastomosis, and linking vessels within the flap. The flap areas were 8×2 cm, with the distal parts of the flaps being partly necrotic. The flap survival area was 88.58±3.13%, and the surviving area was markedly larger than that in the abdominal flap group (P<0.001). The flap axis was a straight line that passed through the middle of the pedicle (Figure 5).
Figure 5.
(A) Dorsal reticulate vessel flap (left: perfusion X-ray films; right: perfusion specimens). The proximal flap containing definite true anastomosis and direct linking vessels survived and the distal flaps were partly necrotic. (B) Quantification of the flap survival area of the different flap types. *** P<0.001.
The single perforator flap group
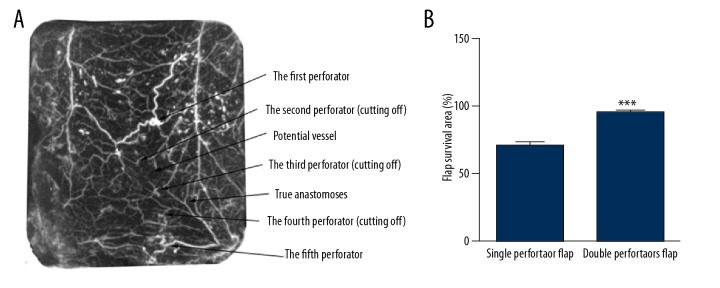
Compared with the normal skin group, there were more true and choke anastomoses between the perforator and the surrounding vessels in the single perforator flap group. The flap survived completely in the area with an adjacent perforator (the second perforator) but was partly necrotic in the area with 2 adjacent consecutive perforators (the third perforator). The flap necrosis boundaries were located between the second perforator and the third perforator, and the flap survival area was 71.74±1.64%. The flap axis was a straight line that passed through the perforators (Figure 6).
Figure 6.
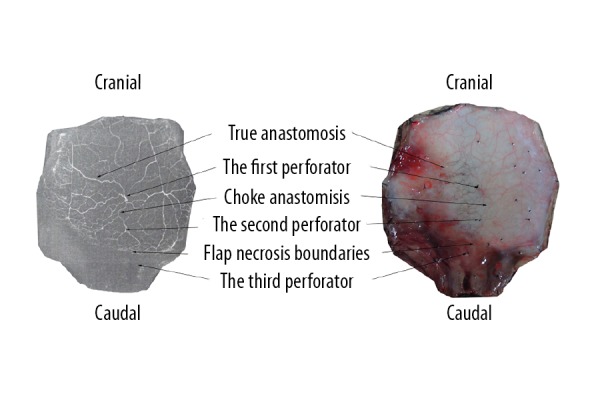
Single perforator flap (left: perfusion X-ray films; right: perfusion specimens). The flap survived completely in the area with an adjacent perforator (the second perforator) but was partly necrotic in the area with 2 adjacent consecutive perforators (the third perforator). The flap necrosis boundaries were located between the second perforator and the third perforator, and the surviving area of the flap was round in shape.
The double perforators flap group
Compared with the normal skin group, the true and choke anastomoses of the double perforators flap group were more evident, and there was a linking vessel between 2 perforators from the flap pedicle. The flap survived completely in the area where 3 or 4 adjacent perforators were present, and the flap survival area was 96.87±1.18%; the surviving area was markedly larger than that in the single perforator flap group (P<0.001). The flap axis was the linking vessel or a straight line connecting the 2 perforators (Figure 7).
Figure 7.
(A) Double perforators flap (perfusion X-ray films). The flap survived completely in the area where 3 or 4 adjacent perforators (the fourth and fifth perforator) were present, and the surviving area was markedly larger than that in the single perforator flap. (B) Quantification of the flap survival area of the different flap types. *** P<0.001.
The cutaneous nerve delayed flap group
The immediate neurocutaneous flaps were complete necrosed. However, the delayed neurocutaneous flap completely survived. The surviving area was 4×3 cm. There were linking and reticulate vessels coming from the proximal and distal areas of the flap. Additionally, the linking vessel and cutaneous nerve were concomitant. The flap axis was the travel line of the cutaneous nerve (Figure 8).
Figure 8.
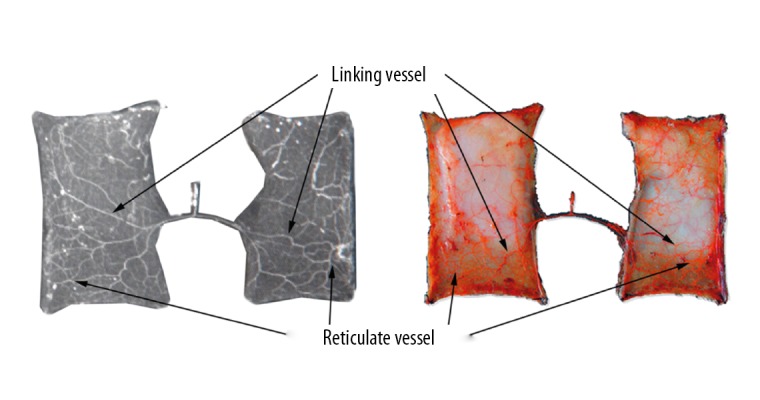
Delayed cutaneous nerve flap (left: perfusion X-ray films; right: perfusion specimens). The delayed neurocutaneous flap completely survived. There were linking and reticulate vessels coming from the proximal and distal areas of the flap.
Discussion
Microvascular anatomy of the flap
The accumulation of knowledge regarding microvascular anatomy has played a vital role in increasing the use of multiple types of flaps in clinical practice. Grade I perforators originated from the deep original arteries and included bone perforators, tendon perforators, muscle perforators, nerve perforators, and cutaneous perforators [17]. Grade I perforators included mostly choke and potential anastomoses and few true anastomoses. Grade I perforators penetrated deep fascia and formed grade II perforators with true or choke anastomoses between adjacent grade II perforators. Grade III perforators traveled under superficial fascia and originated from ascending and descending branches of grade II perforators; they pierced superficial fascia and gave off grade IV perforators, which entered into the dermal layer and formed a subdermal vascular network that created a perforator vascular tree, which was also termed the anatomical territory of the flap [18,19].
Grade II perforators might generate chain-like vessels among 2 or more consecutive true or choke anastomoses in the deep fascia. The linking vessel was often accompanied by the cutaneous nerve. In this study, we confirmed this again with the results seen in the cutaneous nerve delayed flap group. Clinical anatomy also corroborated the phenomenon that the linking vessels and cutaneous nerve run concomitantly, as was seen with the linking vessels located in the anteromedial lower leg from the posterior tibial artery perforators that were accompanied by the saphenous nerve, the linking vessels located lateral lower leg from the peroneal artery perforators accompanied by the lateral sural cutaneous nerve, and the linking vessels located the posterior lower leg from the perforators of the lateral or medial sural artery and peroneal artery accompanied by the sural nerve. In these areas, the linking-pattern flap can be raised [9].
Grade III perforators generated a superficial plexus through true, chock, and potential anastomoses in the superficial fascia. Grade IV perforators pierced the superficial fascia and entered into subdermal and dermal layers and formed vascular networks, making the crown of the perforator vessel tree. The adjacent grade IV perforator vessels also formed a vascular network through true, chock and potential anastomosis.
Pelissier et al. [20] reported on flaps being able to retain dual vascularization from perforators originating from the underlying muscular tissue and the superficial vascular network. We hypothesized that the deep vessels, as well the grade I perforators or axial vessels, entered the flap and formed a superficial vascular network. The superficial vascular network was composed of grade II–IV perforators and included 3 vascular networks from the dermis, superficial fascia, and deep fascia. The linking vessels within the deep fascia layer that were generated by grade II perforators were a special type among the deep fascia vascular network [21]. Unquestionably, the entirety of the blood supply of the skin was from the deep vessels. Only when the deep vessels were destroyed during the flap surgery did the superficial vascular network became the only vascular source for the flap. The blood supply within any skin flap was created by 1, 2, or 3 vascular networks in the 3 described types.
Flap axis design for reticulate-pattern flap
The reticulate vessel was actually a vascular network formed by grade III and IV perforators, which dominated the reticulate-pattern flaps. In areas of poor vascularization, such as the anterior sternum and the tibia, the reticulate vessels were the most important because of the lack of subcutaneous tissue and direct linking vessels. This was representative of reticulate-pattern flaps where the optimal length-to-width ratio to guarantee survival of the flap was 1: 1 [22]. In other poor vascular areas, (e.g., the back, abdomen, medial, posterior and lateral lower leg, hand and forearm) [23–25] there was well-developed subcutaneous tissue, relatively well-developed grade II and III perforators, and fewer shorter linking vessels. The length-to-width ratio of these flaps without long chain-like blood can reach 1.5: 1 or greater. Although subcutaneous tissue in the head and face region was not very well-developed, the length-to-width ratio of incised flaps reached 5: 1 because of profuse reticulate vessels, also called a continuous vascular network, composed of abundant true/direct anastomosis from grade II, III, and IV perforators [9].
In the present study, rat abdominal and dorsal flaps corresponded to reticulate-pattern flaps, which were equivalent to the random pattern flap in clinical practice. Compared with rat abdominal skin, we found more perforators from deeper originating arteries, many direct anastomoses with true, choke, or potential anastomosis, and intensive vascular density in the dorsal skin of the normal skin group. These results are consistent with those reported by Saint-Cyr et al. [22]. The length/width ratio of flap survival in the abdominal flap group was 1.25: 1 to 1.5: 1, while the length/width ratio in the dorsal flap group was 3: 1 to 3.5: 1, which was consistent with that of the multi-territory perforator flaps [26]. Interestingly, unlike multi-territory perforator flaps, there were no prominent perforator vessels within the flap pedicle, such as the thoracodorsal vessel (TD). This suggests that survival of the reticulate-pattern flaps was positively correlated with vascular density under natural physiological conditions. In other words, the length/width ratio of flap survival can be increased through increasing vascular density. Similarly, in the clinical setting, the length/width ratio of the reticulate-pattern flap in the head and face region reached 3–5: 1 because of high vascular density, but the optimal ratio in the anterior tibialis with lower vascular density was 1: 1 and usually did not exceed 1.5: 1. The vessels contained within the reticulate-pattern flap were disorganized. The flap axis should be a straight line passing through the middle of the pedicle; otherwise, the flap survival area can be negatively affected.
Flap axis design for axis-pattern flap
Deep axial vessels gave out multiple grade I perforators, which pierced the sarcolemma, entered into the deep fascia, and gave off grade II, III, and IV perforators. These perforators formed multiple perforator vascular trees known as anatomical donor sites. The anatomical donor sites created an axial vessel angiosome through true, chock, and potential anastomoses, such as the radial artery, the fibular artery, and the posterior tibial artery angiosomes. The axis of an axial vessel flap traveled a route of deep axial vessels regardless of route variation of the axial vessel. For example, the donor site of the posterior tibial artery flap might be the medial leg or the planta pedis; however, the axis was always the travel route of the posterior tibial artery, and a combined flap might be incised. In addition, the axis of a prefabricated flap belonging to axis-pattern flap was always the travel route of prefabricated vessels [27].
Flap axis design for perforator flap
The single grade I perforator pierced the sarcolemma and entered into the deep fascia and formed grade II perforators, then penetrated superficial fascia and gave out grade III and IV perforators into the dermal layer. These grade III and IV perforators formed subdermal vascular networks with a perforator vessel tree, and an anatomical territory was created. The perforator dynamic territory, also called the clinical territory, was the area where blood flows from a perforator anatomical territory and reaches the branches of adjacent perforators to nourish the territory via true or chock anastomosis. In other words, the clinical territory contained the anatomical territory located in the center region and the potential territory from the peripheral area. As described by Taylor et al. [9], the anatomical territory is a circular area covering the anastomosis line between the adjacent perforators; this area was also known as the cutaneous angiosome or perforator angiosome. However, Saint-Cyr et al. [22] proposed a new concept of a perforasome by infusing contrast agent into a single perforator and observing the vascular territory it covered.
In this study, rat single perforator and double perforators flaps were equivalent to human skin perforator flaps. Our results indicated that the vascular territory from a single perforator flap did, in fact, form a circular vascular network based on the perforator of the flap pedicle. This vascular territory possessed true and choke anastomoses between adjacent perforators. A potential anastomosis was even converted into a chock anastomosis after compensation compared with the vascular network from normal adjacent angiosomes. The incision area of a single perforator flap should not exceed that of an adjacent perforator. Otherwise, the flap would die and the necrotic boundary would be equal to the area between the 2 adjacent perforators in the pedicle located in the same axis. These findings are consistent with the multi-territory perforator flap belonging to a single perforator flap based on the deep circumflex iliac vessel (DCI) [26,28], which also corresponds to the theory described by Taylor et al. [9]. In general, the clinical territory of the single perforator flap resembled an inverted cone, with the perforator located at the tip of the cone and the surface of the skin being the top of the cone. In the single perforator flap design, the flap surface should be the top of the cone with the flap axis making up the diameter of cone through the tip. When the clinical territory of the flap is raised, the chain-like vessels that originated from deep vessels should be selected as the flap axis beyond the area of the anatomical territory, especially for flaps located in the vicinity of the limbs and torso. Compound blood supply from the perforator pedicle can develop on the flap. An example of this is the peroneal artery perforator pedicle flap with compound blood supply, which possesses linking vessels of 2 flap axes along with the sural nerve and the lateral sural nerve linking vessels [11,12].
The double perforator group was able to retain observable true and chock anastomoses between adjacent perforators in the pedicle and a potential anastomosis was even converted into a chock anastomosis after compensation. The flap was able to survive when there was an interval of 3 or 4 perforators between the perforators in the pedicle. Interestingly, the combined flap with an interval of 4 perforators between the adjacent perforators was able to survive, and the survival area was considerably larger than the clinical territory of the single perforator flap. Therefore, the clinical application of the flap with combined perforators may cause external pressurization through vessel anastomoses of the distal flap with a receiving site to ensure flap survival in case the incision area of the single perforator flap was beyond the clinical territory [29,30]. There appeared to be linking vessels between the 2 perforators in the double perforator flap, and the flap axis was the linking vessels or a straight line connecting the 2 perforators in the pedicle.
Flap axis design for linking-pattern flap
As previously mentioned, the linking vessels were usually formed via anastomoses of the ascending and descending branches of grade II perforators originating from deep vessels and frequently accompanied by the cutaneous nerve [31]. However, this phenomenon can also be found in other tissues, especially in the muscles and nerve trunk, as well as in delayed flaps [32–35]. The vascular territory of the linking vessels was named the linking vascular angiosome. The flap axis was the linking vessels, which were generally located traveling along the same route of the cutaneous nerve. The neurocutaneous flap was also a type of linking-pattern flap with a blood supply generally originating from a deep arterial perforator, such as the peroneal artery perforator-pedicle sural neurovascular flap or the posterior tibial artery perforator-pedicle saphenous nerve flap. Indeed, the type of blood supply to these flaps were a compound vascular network that supplies blood to the proximal flap from the perforator artery and to the distal flap from the linking vessels and reticulate vessels that emerge beyond the territory of the incised flap. Therefore, it was difficult to find a standard linking-pattern flap. However, Akyürek et al. [36] and Sönmez et al. [37] reported a new flap design, called the neural-island flap, in which the blood supply to the flap was from permanently chain-like vessels from the pedicle. The chain-like vessels were accompanied by the cutaneous nerve and could be enlarged by flap delay. After 1 week, the entire flap pedicle was supplied by the cutaneous nerve and vessels.
Based on the literature, we established a cutaneous nerve delayed flap model that corresponded to a human neurocutaneous nutrition vessels flap. Subsequently, we found that the immediate neurocutaneous island flaps were completely necrotic, but the delayed neurocutaneous flaps all survived. Additionally, we found that there were linking vessels between the pedicle and the flap, confirming that the linking vessels were accompanied by the cutaneous nerve. The linking vessels were outstretched through compensatory expansion to nourish the flap after delay, and were consistent with the previous results. Hence, the proximal and distal areas of the delayed neurocutaneous flap were supplied by linking and reticulate vessels, respectively, suggesting that the incised area of this flap may exceed the anatomical territory and form a compound vascular-pattern flap. Consequently, the donor site of the delayed neurocutaneous flap can be incised beyond the anatomical territory of the axial flap, and the flap axis is the travel line of the cutaneous nerve.
Conclusions
Variations in flap blood supply patterns and axes with alterations based on flap types have implications for flap survival. Understanding blood flow characteristics within each flap type and accurately designing the flap axis is essential for flap survival.
Footnotes
Source of support: This work was supported by the National Natural Science Foundation of China (grant 81360295) and the Scientific and Technological Foundation of Youth Talents of Guizhou Province [2013(12)]
Conflict of interest
None.
References
- 1.Hallock GG. Partial failure of a perforator free flap can be salvaged by a second perforator free flap. Microsurg. 2014;34:177–82. doi: 10.1002/micr.22166. [DOI] [PubMed] [Google Scholar]
- 2.Lie KH, Barker AS, Ashton MW. A classification system for partial and complete DIEP flap necrosis based on a review of 17,096 DIEP flaps in 693 articles including analysis of 152 total flap failures. Plast Reconstr Surg. 2013;132:1401–8. doi: 10.1097/01.prs.0000434402.06564.bd. [DOI] [PubMed] [Google Scholar]
- 3.Regmi S, Gu JX, Zhang NC, Liu HJ. A systematic review of outcomes and complications of primary fingertip reconstruction using reverse-flow homodigital island flaps. Aesthetic Plast Surg. 2016;40:277–83. doi: 10.1007/s00266-016-0624-y. [DOI] [PubMed] [Google Scholar]
- 4.Schierle CF, Rawlani V, Galiano RD, et al. Improving outcomes of the distally based hemisoleus flap: Principles of angiosomes in flap design. Plast Reconstr Surg. 2009;123:1748–54. doi: 10.1097/PRS.0b013e3181a65a74. [DOI] [PubMed] [Google Scholar]
- 5.Chih-Hsun L, Ma H. Use of perforator-based fasciocutaneous flaps for pressure sore reconstruction: Single-perforator-based versus multiple-perforator-based flaps. Aesthetic Plast Surg. 2016;40:540–48. doi: 10.1007/s00266-016-0662-5. [DOI] [PubMed] [Google Scholar]
- 6.McGregor IA, Morgan G. Axial and random pattern flaps. Br J Plast Surg. 1973;26:202–13. doi: 10.1016/0007-1226(73)90003-9. [DOI] [PubMed] [Google Scholar]
- 7.Ponten B. The fasciocutaneous flap: Its use in soft tissue defects of the lower leg. Br J Plast Surg. 1981;34:215–20. doi: 10.1016/s0007-1226(81)80097-5. [DOI] [PubMed] [Google Scholar]
- 8.Bertelli JA. Neurocutaneous axial island flaps in the forearm: Anatomical, experimental and preliminary clinical results. Br J Plast Surg. 1993;46:489–96. doi: 10.1016/0007-1226(93)90223-x. [DOI] [PubMed] [Google Scholar]
- 9.Taylor GI, Corlett RJ, Dhar SC, Ashton MW. The anatomical (angiosome) and clinical territories of cutaneous perforating arteries: Development of the concept and designing safe flaps. Plast Reconstr Surg. 2011;127:1447–59. doi: 10.1097/PRS.0b013e318208d21b. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Q, Qiao Q, Gould LJ, et al. Study of the neural and vascular anatomy of the anterolateral thigh flap. J Plast Reconstr Aesthet Surg. 2010;63:365–71. doi: 10.1016/j.bjps.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 11.Dusseldorp JR, Pham QJ, Ngo Q, et al. Vascular anatomy of the medial sural artery perforator flap: A new classification system of intra-muscular branching patterns. J Plast Reconstr Aesthet Surg. 2014;67:1267–75. doi: 10.1016/j.bjps.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Molina AR, Jones ME, Hazari A, et al. Correlating the deep inferior epigastric artery branching pattern with type of abdominal free flap performed in a series of 145 breast reconstruction patients. Ann R Coll Surg Engl. 2012;94:493–95. doi: 10.1308/003588412X13171221592050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei ZR, Sun GF, Wang DL, Tang XJ. Reconstruction of the Achilles tendon and overlying skin defect: 3 case reports. Ann Plast Surg. 2014;73:325–29. doi: 10.1097/SAP.0b013e31827a3007. [DOI] [PubMed] [Google Scholar]
- 14.Lee SY, Pakela JM, Helton MC, et al. Compact dual-mode diffuse optical system for blood perfusion monitoring in a porcine model of microvascular tissue flaps. J Biomed Opt. 2017;22:1–14. doi: 10.1117/1.JBO.22.12.121609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin R, Chen H, Callow D, et al. Multifaceted effects of astragaloside IV on promotion of random pattern skin flap survival in rats. Am J Transl Res. 2017;9:4161–72. [PMC free article] [PubMed] [Google Scholar]
- 16.Li S, Liu Y, Zang M, et al. Rectus femoris branch: An alternative blood supply for a distally based anterolateral thigh flap. J Plast Reconstr Aesthet Surg. 2018;71:232–38. doi: 10.1016/j.bjps.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Boyd JB, Taylor GI, Corlett R. The vascular territories of the superior epigastric and the deep inferior epigastric systems. Plast Reconstr Surg. 1984;73:1–16. doi: 10.1097/00006534-198401000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Palmer JH, Taylor GI. The vascular territories of the anterior chest wall. Br J Plast Surg. 1986;39:287–99. doi: 10.1016/0007-1226(86)90037-8. [DOI] [PubMed] [Google Scholar]
- 19.Taylor GI, Palmer JH. The vascular territories (angiosomes) of the body: Experimental study and clinical applications. Br J Plast Surg. 1987;40:113–41. doi: 10.1016/0007-1226(87)90185-8. [DOI] [PubMed] [Google Scholar]
- 20.Pelissier P, Santoul M, Pinsolle V, et al. The keystone design perforator island flap. Part I: Anatomic study. J Plast Reconstr Aesthet Surg. 2007;60:883–87. doi: 10.1016/j.bjps.2007.01.072. [DOI] [PubMed] [Google Scholar]
- 21.Taylor GI. The angiosomes of the body and their supply to perforator flaps. Clin Plast Surg. 2003;30:331–42. doi: 10.1016/s0094-1298(03)00034-8. [DOI] [PubMed] [Google Scholar]
- 22.Saint-Cyr M, Wong C, Schaverien M, et al. The perforasome theory: Vascular anatomy and clinical implications. Plast Reconstr Surg. 2009;124:1529–44. doi: 10.1097/PRS.0b013e3181b98a6c. [DOI] [PubMed] [Google Scholar]
- 23.Lecours C, Saint-Cyr M, Wong C, et al. Freestyle pedicle perforator flaps: Clinical results and vascular anatomy. Plast Reconstr Surg. 2010;126:589–603. doi: 10.1097/PRS.0b013e3181f02ee3. [DOI] [PubMed] [Google Scholar]
- 24.Minabe T, Harii K. Dorsal intercostal artery perforator flap: Aatomical study and clinical applications. Plast Reconstr Surg. 2007;120:681–89. doi: 10.1097/01.prs.0000270309.33069.e5. [DOI] [PubMed] [Google Scholar]
- 25.Schaverien M, Saint-Cyr M, Arbique G, Brown SA. Arterial and venous anatomies of the deep inferior epigastric perforator and superficial inferior epigastric artery flaps. Plast Reconstr Surg. 2008;121:1909–19. doi: 10.1097/PRS.0b013e31817151f8. [DOI] [PubMed] [Google Scholar]
- 26.Tao XY, Wang L, Gao WY, et al. The effect of inducible nitric oxide synthase on multiterritory perforator flap survival in rats. J Reconstr Microsurg. 2016;32:643–49. doi: 10.1055/s-0036-1584808. [DOI] [PubMed] [Google Scholar]
- 27.Xu H, Feng S, Xia Y, et al. Prefabricated flaps: Identification of microcirculation structure and supercharging technique improving survival area. J Reconstr Microsurg. 2017 doi: 10.1055/s-0036-1593770. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Zhou ZW, Yang LH, et al. Vasculature characterization of a multiterritory perforator flap: An experimental study. J Reconstr Microsurg. 2017;33:292–97. doi: 10.1055/s-0036-1598011. [DOI] [PubMed] [Google Scholar]
- 29.Hallock GG. The complete nomenclature for combined perforator flaps. Plast Reconstr Surg. 2011;127:1720–29. doi: 10.1097/PRS.0b013e31820a662b. [DOI] [PubMed] [Google Scholar]
- 30.Hallock GG. Branch-based conjoined perforator flaps. Plast Reconstr Surg. 2008;121:1642–49. doi: 10.1097/PRS.0b013e31816aa022. [DOI] [PubMed] [Google Scholar]
- 31.Taylor GI, Gianoutsos MP, Morris SF. The neurovascular territories of the skin and muscles: Anatomic study and clinical implications. Plast Reconstr Surg. 1994;94:1–36. doi: 10.1097/00006534-199407000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Callegari PR, Taylor GI, Caddy CM, Minabe T. An anatomic review of the delay phenomenon: I. Experimental studies. Plast Reconstr Surg. 1992;89:397–407. 417–18. [PubMed] [Google Scholar]
- 33.Morris SF, Taylor GI. Predicting the survival of experimental skin flaps with a knowledge of the vascular architecture. Plast Reconstr Surg. 1993;92:1352–61. [PubMed] [Google Scholar]
- 34.Taylor GI, Corlett RJ, Caddy CM, Zelt RG. An anatomic review of the delay phenomenon: II. Clinical applications. Plast Reconstr Surg. 1992;89:408–16. 417–18. [PubMed] [Google Scholar]
- 35.Dhar SC, Taylor GI. The delay phenomenon: The story unfolds. Plast Reconstr Surg. 1999;104:2079–91. doi: 10.1097/00006534-199912000-00021. [DOI] [PubMed] [Google Scholar]
- 36.Akyurek M, Safak T, Sonmez E, et al. A new flap design: neural-island flap. Plast Reconstr Surg. 2004;114:1467–77. doi: 10.1097/01.prs.0000138749.47015.e8. [DOI] [PubMed] [Google Scholar]
- 37.Sonmez E, Ozdemir H, Safak T, Kecik A. A modification of the neural-island flap: ‘Split neural-island flap’. J Plast Reconstr Aesthet Surg. 2009;62:85–92. doi: 10.1016/j.bjps.2007.10.013. [DOI] [PubMed] [Google Scholar]