Abstract
Background
Berberine, a natural isoquinoline alkaloid derived from Berberis genus plants, has been reported to have anti-cancer effects. While cell behavior can be modulated by long non-coding RNAs (lncRNAs), the contributions of lncRNAs in progression and berberine effects on colorectal cancer are largely unknown. Therefore, the present study investigated the involvement and regulatory function of lncRNA cancer susceptibility candidate 2 (CASC2) during the treatment of human colorectal cancer using berberine.
Material/Methods
Reverse transcription-quantitative PCR (RT-qPCR) was performed to detect the expression levels of lncRNA CASC2 and Bcl-2 mRNA in colorectal cancer cells. MTT assay was performed to evaluate cell viability. Flow cytometry and TUNEL assay were used to analyze the apoptosis of cancer cells. RNA immunoprecipitation (RIP) assay was done to verify the interaction between lncRNA CASC2 and (AU-binding factor 1) AUF1, or AUF1 and B-cell CLL/lymphoma 2 (Bcl-2).
Results
Treatment with berberine suppressed cell viability of colorectal cancer by promoting apoptosis level. LncRNA CASC2 was upregulated in cells treated with berberine, and knockdown of lncRNA CASC2 reversed the berberine-induced apoptosis. In addition, anti-apoptotic gene Bcl-2 was suppressed by berberine treatment and lncRNA CASC2, inducing the pro-apoptotic effects. Moreover, lncRNA CASC2 binds to AUF1, which sequestered AUF1 from binding to Bcl-2 mRNA, thus inducing the inactivation of Bcl-2 translation.
Conclusions
Our study reveals that lncRNA CASC2 mediates the berberine-induced pro-apoptotic effect via inhibition of Bcl-2 expression at the post-transcriptional level.
MeSH Keywords: Apoptosis; Barbering; Colorectal Neoplasms, Hereditary Nonpolyposis; RNA, Long Noncoding
Background
Colorectal cancer is the most common gastrointestinal cancer worldwide and the third leading cause of cancer deaths worldwide [1]. Despite great achievements in surgery and chemotherapy and the development of novel molecular-targeted drugs, the incidence of colorectal cancer continues to increase [2,3]. Early diagnostic and therapeutic strategies can improve the overall survival of patients with colorectal cancer. However, for patients diagnosed with advanced-stage disease, cancer is often irreversible [4]. Currently, wide use of adjunct chemotherapy following surgery resection has dramatically improved clinical outcomes. However, many patients with colorectal cancer become chemo-resistant [5]. The 5-year survival rate for colorectal cancer patients that showed chemo-resistance is 10–15%, compared to 40–90% for responding patients [6]. Early screening and novel therapeutic approaches are essential for improved prognosis of colorectal cancer.
Herbal medicines have long been accepted as useful adjunct therapeutic regimens for various diseases [7]. Berberine is a natural isoquinoline alkaloid derived from Berberis genus plants, such as Coptis chinensis, which has been used to treat intestinal infections, particularly bacterial diarrhea, for thousands of years in China [8]. Recently, it has been reported to have anti-tumor effects on various cancers, including melanoma, glioma, lung cancer, breast cancer, and colorectal cancer [9–13]. Cai et al. revealed that berberine inhibits the growth of human colorectal adenocarcinoma both in vitro and in vivo [14]. However, the underlying regulatory mechanism of berberine still needs more investigation.
Long non-coding RNAs (lncRNAs) are a major group of ncRNAs that contain more than 200 nucleotides [15]. Recently, thousands of reports showed that lncRNAs serve as critical biological regulators during the functions of cellular and molecular signaling pathways. The long non-coding RNA cancer susceptibility candidate 2 (lncRNA CASC2), located at chromosome 10q26, was originally identified as a downregulated gene in endometrial cancer and acted as a tumor suppressor gene [16]. They demonstrated that CASC2 can suppress cell growth with epigenetic and genetic alterations concurring to gene inactivation. In colorectal cancer, Huang et al. found that CASC2 exerts an anti-cancer effect via serving as a competing endogenous RNA by sponging miR-18a [17]. However, the functional influence of CASC2 in chemotherapy and resistance is still not well known.
In this study, we verified the anti-cancer effect of berberine in colorectal cancer cells by promotion of apoptosis. Moreover, we investigated the underlying regulatory mechanism of lncRNA CASC2 in the cancer-suppressive role of berberine. We identified that upregulation of lncRNA CASC2 promotes berberine-induced apoptosis by inhibiting B-cell CLL/lymphoma 2 (Bcl-2) expression at the post-transcriptional level.
Material and Methods
Cell culture
The human colorectal cancer cell lines HT29 and HCT116 were purchased from the Chinese Type Culture Collection, Chinese Academy of Sciences (Shanghai, China). Both cell lines were cultured in RPMI 1640 medium (BioWhittaker, Lonza, USA) supplemented with 10 mM Hepes, 1 mM L-glutamine, 100 U/ml penicillin/streptomycin (BioWhittaker, Lonza), and heat inactivated 10% fetal bovine serum (FBS, Gibco) at 37°C in a humidified incubator with 5% CO2. Berberine (>98% purity), DMSO, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were obtained from Sigma-Aldrich (St. Louis, MO, USA).
RNA oligoribonucleotides and cell transfection
The small-interfering RNA against lncRNA CASC2 (si-CASC2) was synthesized and prepared by GenePharma (Shanghai, China). Negative control siRNA was purchased from Invitrogen (CAT#12935-110, Shanghai, China). The AUF1 overexpression vector (p-AUF1) was generated by cloning the full coding sequences of AUF1 into a PcDNA 3.1 vector. All the vectors were labeled with green fluorescence protein (GFP). Cells were transfected with DNA plasmids using TransFast transfection reagent Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer’s instructions. A total of 5 x 105 cells was seeded into each well of a 6-well plate and transfected with respective oligoribonucleotides upon reaching 70–80% confluence. The final transfection concentration was 100 nM. The expression change of target genes was determined by RT-qPCR after transfection for 24 h to confirm the transfection effects. The cells were then subjected to RNA/protein extraction and further functional assays. The sequences of small-interfering RNAs are shown in Table 1.
Table 1.
Information on the RT-qPCR primer sequence and siRNA sequence.
| RT-qPCR primer name | Primer sequences (5′-3′) |
|---|---|
| CASC2 (forward) | GCACATTGGACGGTGTTTCC |
| CASC2 (reverse) | CCCAGTCCTTCACAGGTCAC |
| Bcl-2 (forward) | TCT TCCAGGAACCTCTGTGATG |
| Bcl-2 (reverse) | CAATGCCGCCATCGCTTACACC |
| GAPDH (forward) | GCACCGTCAAGGCTGAGAAC |
| GAPDH (reverse) | ATGGTGGTGAAGACGCCAGT |
| siRNA name | siRNA sequences (5′-3′) |
| si-CASC2-1 | UGAAAAGAGCCGUGAGCUAdTdT |
| si-CASC2-2 | AAATAAAGATGGTGGAATGdTdT |
| si-CASC2-3 | CUGCAAGGCCGCAUGAUGAdTdT |
| si-NC | GGCUACGUCCAGGAGCGCACCdTdT |
RNA extraction and reverse transcription-quantitative PCR (RT-qPCR)
RNA extraction from cell fraction was performed using Trizol regent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. RNA was reverse-transcribed using SuperScript III® (Invitrogen; Thermo Fisher Scientific, Inc.) and amplified by qPCR based on the SYBER GREEN method. Briefly, 2 μL of diluted RT product was mixed with 12.5 μL of 2×SYBR®Premix Ex Taq™, 0.5 μL of 50×ROX reference Dye II (Takara, Dalian, China), 2 μL forward and reverse primers (10μM), and 8 μL nuclease-free water to a final volume of 25 μL. qPCR was performed by using the ABI 7500 system (Applied Biosystems). The expression of CASC2 and Bcl-2 mRNA was quantified relative to endogenous GAPDH expression, analyzed and expressed relative to quantification cycle values [18]. The primer sequences are shown in Table 1.
Cell viability assay
The altered cell viability after treatment with berberine was assayed using an MTT kit (Dojindo, Rockville, MD, USA). In brief, cells were seeded into a 96-well plate and then treated with berberine for 12–72 h at the concentration of 0–100 μM. Then, cells were treated with the MTT reagent and cultured for 2 h. The optical density at 450 nm was measured with a spectrophotometer (Thermo Electron Corporation, MA, USA).
Flow cytometry for apoptosis analysis
Cells were collected after berberine treatment or transient transfection for 48 h, then washed with PBS and trypsin containing 0.025% trypsin-EDTA to get the single-cell suspensions. They were then fixed in ice-cold 70% ethanol and with Annexin-V FITC and propidium iodide (PI) solution (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Apoptosis was detected on a BD FACSCalibur instrument.
TUNEL assay
TUNEL staining was performed to evaluate cell apoptosis. In brief, cells were fixed by using 4% formaldehyde followed by staining with a TUNEL kit according to the manufacturer’s instructions (Vazyme, TUNEL Bright-Red Apoptosis Detection Kit, A113). TUNEL-positive cells were counted under fluorescence microscopy (DMI4000B, Leica, Mannheim, Germany).
Immunofluorescence
Cell on slides were permeabilized with 0.3% Triton X-100 for 15 min after being fixed with 4% paraformaldehyde. Then, goat serum was used for blocking and cells were incubated with anti-Ki67 antibody (Abcam, ab15580, 1: 500, Cambridge, MA) overnight at 4°C. The slides were then incubated with anti-rabbit Alexa Fluor 488 (Jackson Immunoresearch, West Grove, PA, USA) for 1 h at room temperature. DAPI was used for nuclear counterstaining. The samples were observed under a fluorescence microscope (DMI4000B, Leica). Positive cells were counted blindly at ×20 magnification.
Fluorescence in situ hybridization (FISH) analysis
For FISH assay, fluorescence-conjugated lncRNA CASC2 probes were generated according to the manual of Biosearch Technologies. Colorectal cancer cells were treated in a non-denaturing condition, followed by hybridization with lncRNA CASC2 probes. After RNA hybridization, samples were incubated in 4′,6-diamidino-2-phenylindole (DAPI, 1: 1000) for 5 min of counterstaining, and observed by confocal microscopy (DMI4000B, Leica). All experiments were performed according to the manuals of Biosearch Technologies.
Nucleocytoplasmic separation
Nuclear and cytosolic fraction separation was performed using a PARIS kit (Life Technologies, Thermo Fisher, Inc.). Briefly, 5×106 cells were resuspended in 0.6 ml resuspension buffer for 15-min incubation, followed by homogenization. After centrifugation at 400×g for 15 min, the cytoplasmic fraction was obtained from the supernatant content. The pellet was then resuspended in 0.3 ml PBS, 0.3 ml nuclear isolation buffer, and 0.3 ml RNase-free H2O, followed by 20-minincubation on ice. The pellet underwent nuclear fractionation after centrifugation. lncRNA CASC2 expression was determined by qPCR (ABI 7500).
RNA immunoprecipitation (RIP)
RIP was performed using the Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore, Cambridge, MA). For RIP assay, the supernatant was incubated overnight with beads conjugated with anti-AUF1 (Abcam, ab61193, 1: 50) or anti-Bcl-2 antibody (Abcam, ab32124, 1: 50) or negative control mouse IgG (Millipore). The beads were then rinsed with cold NT2 buffer and cultured with proteinase K at 10 mg/ml (Sigma-Aldrich, USA).
Western blot and antibodies
Cell lysates were prepared with RIPA buffer containing protease inhibitors (Sigma). Cell lysates were separated by 10% SDS-PAGE and transferred to nitrocellulose membranes (GE Healthcare, USA). Then, the membrane was blocked with 5% (5 g/100 mL) nonfat dry milk in tri-buffered saline plus Tween (TBS-T) buffer for 2 h at room temperature and incubated with specific primary antibodies (1: 1000 solution) at 4°C overnight, followed by horseradish peroxidase-conjugated (HRP) secondary antibodies at room temperature for 1 h. The primary antibodies used were: anti-Bcl-2 (Abcam, ab32124), anti-AUF1 (Abcam, ab61193), and anti-GAPDH (#5174, Cell Signaling Technology, Cambridge, MA).
Statistical analysis
The Kolmogorov-Smirnov test was applied for data analysis with the distribution of each group of samples. Data are presented as median (interquartile range). Comparisons between 2 groups were performed by nonparametric Mann-Whitney U tests. The Kruskal-Wallis test (post hoc Mann-Whitney U test with Bonferroni correction) was used to evaluate the differences among multiple treatment groups. A two-sided P<0.05 was considered as statistical significance. All statistical data were analyzed using the SPSS 18.0 software (SPSS, Chicago, IL) and GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA).
Results
Berberine suppresses colorectal cancer progression by promoting apoptosis
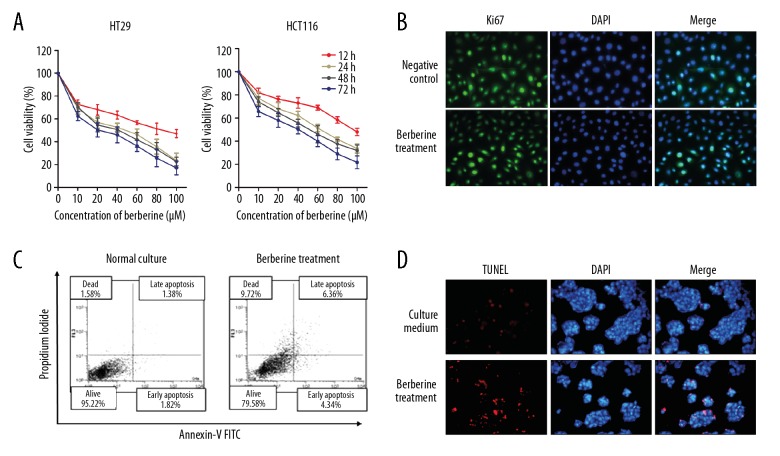
We investigated the functional influence of berberine treatment in colorectal cancer. MTT assay showed that berberine treatment suppressed cell viability in both dose-dependent and time-dependent manners (Figure 1A). To further identify how berberine exerts an anti-cancer effect, we determined cell proliferation and apoptosis via treatment of berberine at 40 μM for 48 h. Immunofluorescence analysis of proliferation marker Ki67 showed that Ki67 was little influenced by treatment of berberine in HT29 cells (Figure 1B). FACS apoptosis assay suggested an increased proportion of apoptotic cells after berberine treatment (Figure 1C), which is further confirmed by TUNEL assay (Figure 1D). Therefore, our data reveal that berberine suppresses cell survival by promoting apoptosis.
Figure 1.
Berberine suppresses colorectal cancer viability by promoting apoptosis. (A) MTT analysis of cell viability after treatment with berberine at indicated concentrations and treatment duration. (B) Immunofluorescence analysis of Ki67 protein level in HT29 cells treated with berberine. (C) Apoptosis was detected by using FACS apoptosis assay in HT29 cells. (D) TUNEL assay was performed to verify the nuclear apoptosis in HT29 cells after treatment with berberine.
LncRNA CASC2 is essential for the berberine-induced pro-apoptotic effect
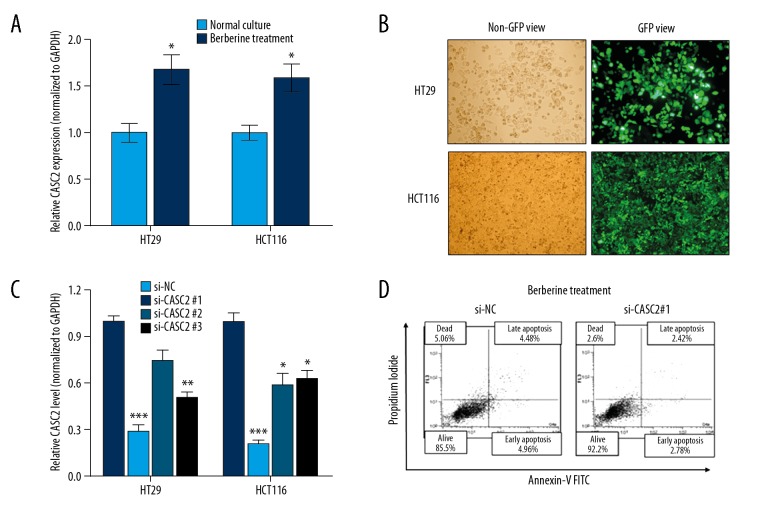
Next, we investigated whether lncRNA CASC2 is involved in the function of berberine in colorectal cancer. RT-qPCR showed that lncRNA CASC2 was upregulated in colorectal cancer cells after berberine treatment when compared to controls (Figure 2A). Then, lncRNA CASC2 was silenced by transient transfection of specific silencing oligonucleotides (Figure 2B), and si-CASC2#1 showed the best silencing efficiency and was used for the subsequent experiments (Figure 2C). After knockdown of lncRNA CASC2 in HT29 cells treated with berberine simultaneously, we found that the promoted apoptosis was dramatically reversed in CASC2-knockdown cells when compared to the cells treated with si-NC (Figure 2D), which strongly suggests that berberine promotes apoptosis via directly targeting lncRNA CASC2.
Figure 2.
lncRNA CASC2 is upregulated in berberine-treated cancer cells. (A) RT-qPCR analysis of lncRNA CASC2 expression in colorectal cancer cells after treatment with berberine, * P<0.05 compared to Normal Culture group. (B) The oligonucleotides labeled with GFP green fluorescence were transfected as described in Methods. (C) The silencing effects were identified as by RT-qPCR, * P<0.05; ** P<0.01; *** P<0.001 compared to si-NC group. (D) FACS apoptosis analysis for the effect of CASC2 knockdown in HT29 cells treated with berberine simultaneously.
Bcl-2 is a functional target for lncRNA CASC2 and berberine treatment in colorectal cancer cells
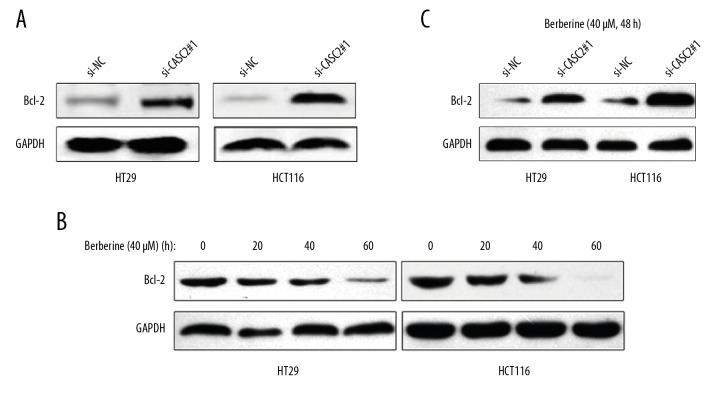
We performed mass spectrometry to determine the expression of several apoptosis-relevant proteins, which may be critical for berberine/CASC2-induced apoptosis. As shown in Table 2, we observed significant differences in several apoptosis-relevant targets between cells silenced with CASC2 and non-silencing cells, among which B-cell lymphoma 2 (Bcl-2) was mostly affected. Western blot analysis also showed that Bcl-2 was upregulated in CASC2-silencing cells when compared to non-silencing cells (Figure 3A). In addition, treatment with berberine (40μM) decreased the expression of Bcl-2 in a dose-dependent manner (Figure 3B). As expected, knockdown of lncRNA CASC2 reversed the suppression of Bcl-2 induced by berberine treatment (40 μM, 48 h) (Figure 3C). Collectively, we verified that Bcl-2 is a direct functional target of berberine/CASC2 in colorectal cancer cells.
Table 2.
lncRNA CASC2-regulated targets that are related to apoptosis.
| Gene symbol | Gene title | Location | Fold change (si-CASC2/si-NC) |
|---|---|---|---|
| Bcl-2 | B-cell CLL/lymphoma 2 | Chr18q21.33 | 22.31 |
| Bcl-2A1 | BCL2-related protein A1 | Chr15q25.1 | 6.84 |
| PARP2 | Poly (ADP-ribose) polymerase 2 | Chr14q11.2 | 4.31 |
| BIRC3 | Baculoviral IAP repeat containing 3 | Chr11q22.2 | 2.63 |
| Bax | BCL2-associated X protein | Chr19q13.33 | 0.24 |
| Mcl1 | Myeloid cell leukemia sequence 1 | Chr1q21.2 | 0.30 |
| Bad1 | BCL2 antagonist/killer 1 | Chr6p21.31 | 0.38 |
| Casp9 | Caspase 9, apoptosis-related cysteine peptidase | Chr1p36.21 | 0.56 |
| Casp3 | Caspase 3, apoptosis-related cysteine peptidase | Chr4p35.1 | 0.71 |
Figure 3.
Bcl-2 is the functional target of berberine and lncRNA CASC2. (A) Western blot analysis of Bcl-2 protein level after knockdown of lncRNA CASC2 in colorectal cancer cells, GAPDH as control. (B) Western blot analysis of Bcl-2 protein level in colorectal cancer cells treated with berberine for different times. (C) Western blot analysis of Bcl-2 protein in colorectal cancer cells treated with si-CASC2 and berberine simultaneously.
lncRNA CASC2 regulates Bcl-2 expression at post-transcriptional level
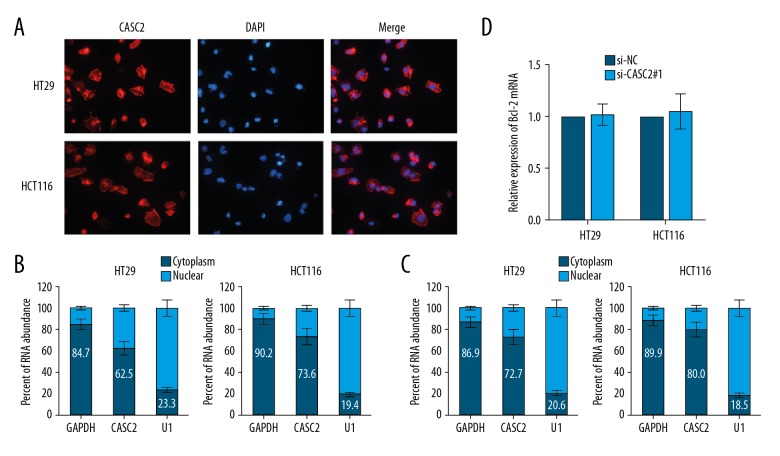
To investigate how lncRNA CASC2 regulates Bcl-2 expression, we identified the cellular localization of lncRNA CASC2 in colorectal cancer cells. Fluorescence in situ hybridization (FISH) assay with specific probe of lncRNA CASC2 confirmed that CASC2 was mainly distributed in the cytoplasm section (Figure 4A), which is consistent with previous studies [17,19,20]. Then, we performed a nucleocytoplasmic separation experiment followed by RT-qPCR analysis and verified that lncRNA CASC2 was located in both nuclear (less then 50%) and cytoplasm sections (more than 50%) (Figure 4B). Moreover, berberine treatment increased the proportion of lncRNA CASC2 in cytoplasm (Figure 4C). In addition, RT-qPCR showed that Bcl-2 mRNA was not influenced by knockdown of lncRNA CASC2 in colorectal cancer cells (Figure 4D), indicating that lncRNA CASC2 regulates Bcl-2 expression at the post-transcriptional level.
Figure 4.
LncRNA CASC2 regulates Bcl-2 expression at post-transcriptional level. (A) FISH analysis of the subcellular location of lncRNA CASC2 with specific probe in colorectal cancer cells. (B) The expression level of lncRNA CASC2 in nuclear and cytoplasm of colorectal cancer cells. U1 (nuclear retained) and GAPDH (exported to cytoplasm) were used as controls. (C) Berberine treatment promoted the proportion of CASC2 expressed in cytoplasm. (D) RT-qPCR analysis of Bcl-2 mRNA level in cells silenced with lncRNA CASC2.
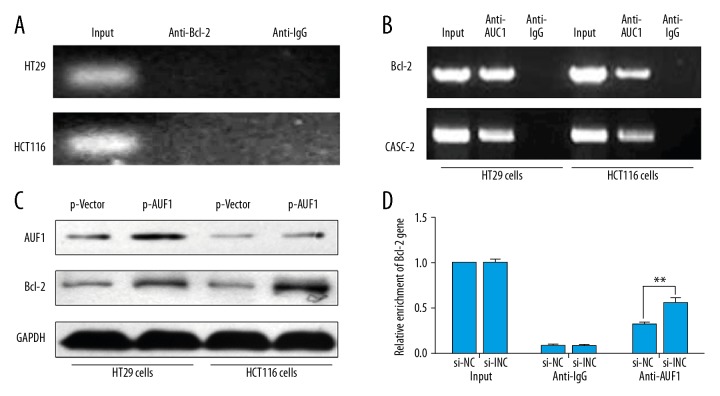
lncRNA CASC2 sequesters AUF1 from binding to Bcl-2
Based on the above results, we used RNA immunoprecipitation (RIP) assay to investigate whether lncRNA CASC2 interacts with Bcl-2 protein. As shown in Figure 5A, CASC2 was not decreased by anti-Bcl-2 antibody, suggesting that lncRNA CASC2 is not directly associated with Bcl-2 protein. Previously, Lapucci et al. reported that AU-binding factor 1 (AUF1) is a Bcl-2 (A+U)-rich element-binding protein involved in activation of Bcl-2 translation without influencing its mRNA level [21]. RIP assay using anti-AUF1 antibody also confirmed that AUF1 was associated with Bcl-2 gene and lncRNA CASC2 (Figure 5B). In addition, overexpression of AUF1 enhanced Bcl-2 protein levels (Figure 5C), indicating that AUF1-binding activates the translation of Bcl-2. More importantly, knockdown of CASC2 increased the interaction between AUF1 and Bcl-2 mRNA (Figure 5D), which strongly suggests that CASC2 serves as a competing endogenous RNA (ceRNA) for AUF1 and sequesters AUF1 from binding to Bcl-2, thus inducing the inactivation of Bcl-2 translation.
Figure 5.
LncRNA CASC2 sequesters AUF1 from binding to Bcl-2. (A) RNA immunoprecipitation (RIP) analysis by using anti-Bcl-2 antibody was performed to pull down CASC2, anti-IgG as negative control. (B) RIP assay by using anti-AUF1 antibody was done to pull down Bcl-2 and CASC2. (C) Western blot analysis was performed to verify the protein expression level of AUF1 and Bcl-2 after transfection of AUF1 overexpression vector. (D) RIP assay was performed to detect the influence of CASC2 knockdown on the interaction between AUF1 and Bcl-2, ** P<0.01.
Discussion
Advanced colorectal cancer patients who develop resistance to chemotherapy have limited therapeutic options in the clinic at present. Many patients therefore turn to alternative treatment [22]. In recent years, the interest in herbal remedies has grown rapidly in the industrialized world, since these drugs are increasingly considered as effective and safe alternatives to synthetic drugs. In this study, we focused on berberine, one of the few well-established plant products supported by clinical trials is the standardized special extract from Coptis chinensis. Our study revealed that berberine treatment inhibited cancer viability via the promotion of apoptosis. In addition, lncRNA CASC2 mediated the berberine-induced apoptosis by suppressing Bcl-2 protein level at post-transcriptional level.
Alkaloids are used in traditional medicine for the treatment of many diseases. These compounds are synthesized in plants as secondary metabolites and have multiple effects on cellular metabolism [23,24]. Berberine is a medicinal alkaloid isolated from Coptis chinensis and has been used orally for decades in China as an over-the-counter (OTC) drug to treat diarrhea, with good safety, and was shown to possess a potent inhibitory activity against bacteria [25,26]. Moreover, several clinical and preclinical studies demonstrated an ameliorative effect of berberine against several disorders, including metabolic, neurological, and cardiological problems [27–29]. More recently, the anti-cancer effect of berberine was recognized and studied [30]. Wang et al. demonstrated that berberine causes colon cancer cell apoptosis in a caspase-mediated manner through activating several apoptosis-relevant factors [31]. Hsu et al. revealed that berberine treatment can induce cell death in colorectal cancer through modulation of reactive oxygen species and activating the MAPK/JNK/p38 pathway and Fasl pathway [32]. A consistent conclusion was reached in this study, which revealed a pro-apoptotic role of berberine.
The roles of lncRNAs in cancer progression have long been researched and lncRNA CASC2 is widely accepted as a tumor-suppressor gene in gastric cancer, non-small cell lung cancer, endometrial cancer, hepatocellular carcinoma, and breast cancer [33]. As for the role of lncRNA CASC2 in colorectal cancer, Huang et al. revealed that CASC2 suppressed cell proliferation and tumor growth in vitro and in vivo by extending the G0/G1-S phase transition [17]. In this study, we identified that CASC2 is upregulated upon berberine treatment and is essential for the berberine-induced anti-cancer effect. Hence, we constructed a direct interaction between berberine treatment and CASC2 in colorectal cancer cells.
Further, we explored the underlying regulatory mechanism by which berberine/CASC2 promotes apoptosis. The well-known anti-apoptosis protein Bcl-2 was identified as the most dysregulated protein upon silencing of lncRNA CASC2. Moreover, lncRNA CASC2 modulates Bcl-2 protein expression but not the mRNA level, indicating that lncRNA CASC2 regulates Bcl-2 expression at the post-transcriptional level. To further investigate how lncRNA CASC2 regulates Bcl-2, we focused on AUF1. AUF1 is one of the RNA-binding proteins (RBPs) specialized in binding to select target mRNAs, also known as heterogeneous nuclear ribonucleoprotein D (hnRNP D) [34,35]. Through its interaction with AREs in specific cellular mRNAs, AUF1 can target the RNAs for degradation via ARE-mediated decay. There are also a few examples of AUF1 acting to activate transcripts, such as c-Myc, and parathyroid hormone and estrogen receptor α mRNAs [36,37]. Expression of the Bcl-2 gene is regulated transcriptionally by a negative regulatory element [38]. Two estrogen-responsive elements within the coding region involved in translational regulation of Bcl-2 have also been characterized. The above evidence suggests that AUF1 may be involved in and activate the translation of Bcl-2 gene. By performing a series of RIP assays, we confirmed that AUF1 protein is associated with both lncRNA CASC2 and Bcl-2 genes. Furthermore, lncRNA CASC2 can sequester AUF1 from binding to the Bcl-2 gene, thus inducing the inactivation of Bcl-2 translation.
Conclusions
We demonstrated that berberine treatment induced apoptosis of colorectal cancer cells via the upregulation of lncRNA CASC2 and subsequent suppression of Bcl-2 protein. Therefore, lncRNA CASC2 is a promising therapeutic target for colorectal cancer treatment.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Liska D, Stocchi L, Karagkounis G, et al. Incidence, patterns, and predictors of locoregional recurrence in colon cancer. Ann Surg Oncol. 2017;24(4):1093–99. doi: 10.1245/s10434-016-5643-z. [DOI] [PubMed] [Google Scholar]
- 3.Rosenblatt KA, Gao DL, Ray RM, et al. Contraceptive methods and induced abortions and their association with the risk of colon cancer in Shanghai, China. Eur J Cancer. 2004;40(4):590–93. doi: 10.1016/j.ejca.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 4.Tol J, Koopman M, Miller MC, et al. Circulating tumour cells early predict progression-free and overall survival in advanced colorectal cancer patients treated with chemotherapy and targeted agents. Ann Oncol. 2010;21(5):1006–12. doi: 10.1093/annonc/mdp463. [DOI] [PubMed] [Google Scholar]
- 5.Tenbaum SP, Ordonez-Moran P, Puig I, et al. beta-catenin confers resistance to PI3K and AKT inhibitors and subverts FOXO3a to promote metastasis in colon cancer. Nat Med. 2012;18(6):892–901. doi: 10.1038/nm.2772. [DOI] [PubMed] [Google Scholar]
- 6.Smith JJ, Deane NG, Wu F, et al. Experimentally derived metastasis gene expression profile predicts recurrence and death in patients with colon cancer. Gastroenterology. 2010;138(3):958–68. doi: 10.1053/j.gastro.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohnishi S, Takeda H. Herbal medicines for the treatment of cancer chemotherapy-induced side effects. Front Pharmacol. 2015;6:14. doi: 10.3389/fphar.2015.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C, Lu M, Pan Q, et al. Berberine improves intestinal motility and visceral pain in the mouse models mimicking diarrhea-predominant irritable bowel syndrome (IBS-D) symptoms in an opioid-receptor dependent manner. PLoS One. 2015;10(12):e0145556. doi: 10.1371/journal.pone.0145556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh T, Vaid M, Katiyar N, et al. Berberine, an isoquinoline alkaloid, inhibits melanoma cancer cell migration by reducing the expressions of cyclooxygenase-2, prostaglandin E(2) and prostaglandin E(2) receptors. Carcinogenesis. 2011;32(1):86–92. doi: 10.1093/carcin/bgq215. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Chen TC, Lai KC, Yang JS, et al. Involvement of reactive oxygen species and caspase-dependent pathway in berberine-induced cell cycle arrest and apoptosis in C6 rat glioma cells. Int J Oncol. 2009;34(6):1681–90. doi: 10.3892/ijo_00000299. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Liu F, Jiang S, et al. Berberine hydrochloride inhibits cell proliferation and promotes apoptosis of non-small cell lung cancer via the suppression of the MMP2 and Bcl-2/Bax signaling pathways. Oncol Lett. 2018;15(5):7409–14. doi: 10.3892/ol.2018.8249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim S, Han J, Kim NY, et al. Effect of berberine on p53 expression by TPA in breast cancer cells. Oncol Rep. 2012;27(1):210–15. doi: 10.3892/or.2011.1480. [DOI] [PubMed] [Google Scholar]
- 13.Liu H, Huang C, Wu L, Wen B. Effect of evodiamine and berberine on miR-429 as an oncogene in human colorectal cancer. Onco Targets Ther. 2016;9:4121–27. doi: 10.2147/OTT.S104729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai Y, Xia Q, Luo R, et al. Berberine inhibits the growth of human colorectal adenocarcinoma in vitro and in vivo. J Nat Med. 2014;68(1):53–62. doi: 10.1007/s11418-013-0766-z. [DOI] [PubMed] [Google Scholar]
- 15.Zhang S, Qin C, Cao G, et al. Systematic analysis of long noncoding RNAs in the senescence-accelerated mouse prone 8 brain using RNA sequencing. Mol Ther Nucleic Acids. 2016;5(8):e343. doi: 10.1038/mtna.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baldinu P, Cossu A, Manca A, et al. CASC2a gene is down-regulated in endometrial cancer. Anticancer Res. 2007;27(1a):235–43. [PubMed] [Google Scholar]
- 17.Huang G, Wu X, Li S, et al. The long noncoding RNA CASC2 functions as a competing endogenous RNA by sponging miR-18a in colorectal cancer. Sci Rep. 2016;6:26524. doi: 10.1038/srep26524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu SY, Huang X, Cheong KL. Recent advances in marine algae polysaccharides: Isolation, structure, and activities. Mar Drugs. 2017;15(12) doi: 10.3390/md15120388. pii: E388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao W, Lin S, Cheng C, et al. Long non-coding RNA CASC2 regulates Sprouty2 via functioning as a competing endogenous RNA for miR-183 to modulate the sensitivity of prostate cancer cells to docetaxel. Arch Biochem Biophys. 2018 doi: 10.1016/j.abb.2018.01.013. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Baldinu P, Cossu A, Manca A, et al. Identification of a novel candidate gene, CASC2, in a region of common allelic loss at chromosome 10q26 in human endometrial cancer. Hum Mutat. 2004;23(4):318–26. doi: 10.1002/humu.20015. [DOI] [PubMed] [Google Scholar]
- 21.Lapucci A, Donnini M, Papucci L, et al. AUF1 Is a bcl-2 A + U-rich element-binding protein involved in bcl-2 mRNA destabilization during apoptosis. J Biol Chem. 2002;277(18):16139–46. doi: 10.1074/jbc.M201377200. [DOI] [PubMed] [Google Scholar]
- 22.Pretner E, Amri H, Li W, et al. Cancer-related overexpression of the peripheral-type benzodiazepine receptor and cytostatic anticancer effects of Ginkgo biloba extract (EGb 761) Anticancer Res. 2006;26(1A):9–22. [PubMed] [Google Scholar]
- 23.Wink M. Quinolizidine alkaloids: Biochemistry, metabolism, and function in plants and cell suspension cultures. Planta Med. 1987;53(6):509–14. doi: 10.1055/s-2006-962797. [DOI] [PubMed] [Google Scholar]
- 24.Han J, Lin H, Huang W. Modulating gut microbiota as an anti-diabetic mechanism of berberine. Med Sci Monit. 2011;17(7):RA164–67. doi: 10.12659/MSM.881842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Habtemariam S. Berberine and inflammatory bowel disease: A concise review. Pharmacol Res. 2016;113(Pt A):592–99. doi: 10.1016/j.phrs.2016.09.041. [DOI] [PubMed] [Google Scholar]
- 26.Cicero AF, Baggioni A. Berberine and its role in chronic disease. Adv Exp Med Biol. 2016;928:27–45. doi: 10.1007/978-3-319-41334-1_2. [DOI] [PubMed] [Google Scholar]
- 27.Yan HM, Xia MF, Wang Y, et al. Efficacy of berberine in patients with non-alcoholic fatty liver disease. PLoS One. 2015;10(8):e0134172. doi: 10.1371/journal.pone.0134172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Yang JQ, He BC, et al. Berberine and total base from rhizoma coptis chinensis attenuate brain injury in an aluminum-induced rat model of neurodegenerative disease. Saudi Med J. 2009;30(6):760–66. [PubMed] [Google Scholar]
- 29.Zhao L, Cang Z, Sun H, et al. Berberine improves glucogenesis and lipid metabolism in nonalcoholic fatty liver disease. BMC Endocr Disord. 2017;17(1):13. doi: 10.1186/s12902-017-0165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diogo CV, Machado NG, Barbosa IA, et al. Berberine as a promising safe anti-cancer agent – is there a role for mitochondria? Curr Drug Targets. 2011;12(6):850–59. doi: 10.2174/138945011795528930. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Liu L, Shi Y, et al. Berberine induces caspase-independent cell death in colon tumor cells through activation of apoptosis-inducing factor. PLoS One. 2012;7(5):e36418. doi: 10.1371/journal.pone.0036418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu WH, Hsieh YS, Kuo HC, et al. Berberine induces apoptosis in SW620 human colonic carcinoma cells through generation of reactive oxygen species and activation of JNK/p38 MAPK and FasL. Arch Toxicol. 2007;81(10):719–28. doi: 10.1007/s00204-006-0169-y. [DOI] [PubMed] [Google Scholar]
- 33.Palmieri G, Paliogiannis P, Sini MC, et al. Long non-coding RNA CASC2 in human cancer. Crit Rev Oncol Hematol. 2017;111:31–38. doi: 10.1016/j.critrevonc.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Loflin P, Chen CY, Shyu AB. Unraveling a cytoplasmic role for hnRNP D in the in vivo mRNA destabilization directed by the AU-rich element. Genes Dev. 1999;13(14):1884–97. doi: 10.1101/gad.13.14.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang W, Wagner BJ, Ehrenman K, et al. Purification, characterization, and cDNA cloning of an AU-rich element RNA-binding protein, AUF1. Mol Cell Biol. 1993;13(12):7652–65. doi: 10.1128/mcb.13.12.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao ZD, Han L, Lee H, et al. Energy stress-induced lncRNA FILNC1 represses c-Myc-mediated energy metabolism and inhibits renal tumor development. Nat Commun. 2017;8(1):783. doi: 10.1038/s41467-017-00902-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sela-Brown A, Silver J, Brewer G, Naveh-Many T. Identification of AUF1 as a parathyroid hormone mRNA 3′-untranslated region-binding protein that determines parathyroid hormone mRNA stability. J Biol Chem. 2000;275(10):7424–29. doi: 10.1074/jbc.275.10.7424. [DOI] [PubMed] [Google Scholar]
- 38.Cheema SK, Mishra SK, Rangnekar VM, et al. Par-4 transcriptionally regulates Bcl-2 through a WT1-binding site on the bcl-2 promoter. J Biol Chem. 2003;278(22):19995–20005. doi: 10.1074/jbc.M205865200. [DOI] [PubMed] [Google Scholar]