Abstract
Bacterial infection is frequently observed in patients with alcoholic liver disease (ALD). We examined a possible role of Porphyromonas gingivalis in the development/progression and severity of disease in patients with acute alcoholic hepatitis (AAH). Plasma specimens from 47 patients with AAH (16 moderate, Model for End‐Stage Liver Disease [MELD] score <20]; 31 severe, MELD score >20) and 22 healthy controls (HCs) were collected. Clinical, drinking history (lifetime drinking history [LTDH]), and demographic data were collected. Antibody tests for immunoglobulin (Ig) G, IgM, and IgA against two P. gingivalis strains were performed. Between‐group comparisons and within‐group association analyses were carried out. Patients with severe AAH showed significantly higher plasma levels of IgG, IgA, and IgM against two P. gingivalis strains (W83 and 33277) compared to HCs. Patients with moderate AAH also had significantly elevated anti‐P. gingivalis IgA concentrations for both strains compared to HCs. Male patients with moderate AAH showed a significant inverse association in LTDH and anti‐P. gingivalis IgM. The aspartate aminotransferase:alanine aminotransferase ratio was positively associated with IgM of both strains in male patients with moderate AAH. Female patients with severe AAH showed a significant association between MELD scores and W83 IgM. Conclusion: Antibody response to P. gingivalis in AAH is elevated. Significantly elevated plasma anti‐P. gingivalis IgG, IgA, and IgM in severe AAH provide preliminary data that P. gingivalis could be a novel risk factor in the development/severity of AAH.
Abbreviations
- AAH
acute alcoholic hepatitis
- ALD
alcoholic liver disease
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CTP
Child‐Turcotte‐Pugh
- DF
Maddrey discriminant function
- HC
healthy control
- HRP
horseradish peroxidase
- Ig
immunoglobulin
- LTDH
lifetime drinking history
- mAAH
moderate acute alcoholic hepatitis
- MELD
Model for End‐Stage Liver Disease
- NAFLD
nonalcoholic fatty liver disease
- NIAAA
National Institute on Alcohol Abuse and Alcoholism
- NOD
nucleotide‐binding oligomerization domain
- OD
optical density
- PBS
phosphate‐buffered saline
- PD
periodontal disease
- sAAH
severe acute alcoholic hepatitis
- TLR
toll‐like receptor
Alcoholic liver disease (ALD) is a major cause of morbidity, mortality, and health care expenditures in the United States and worldwide.1 Acute alcoholic hepatitis (AAH) is an especially severe form of ALD that can carry a high short‐term mortality risk.1 Multiple factors, such as drinking pattern, sex, viral hepatitis infection, iron overload, and malnutrition, are contributing factors associated with the development/progression of ALD.1 However, the role of oral bacterial infections as potential risk factors in the development/progression of ALD has not been thoroughly investigated.
Periodontal diseases (PDs) are induced by dysbiotic oral bacterial communities and affect the supporting structures of the teeth, including the gingiva, alveolar bone, and periodontal ligament. Porphyromonas gingivalis is a major pathogen of severe PD. It functions as a keystone pathogen2 that not only sets the stage for the entire cascade of PD by altering the local immune microenvironment but also enters the blood circulation, is disseminated throughout the body, and contributes to multiple systemic diseases,3 such as diabetes,4 atherosclerosis,5 rheumatoid arthritis,6 and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis (NAFLD/NASH).7, 8 However, its role in ALD, particularly in AAH, is not clear.
P. gingivalis is known to cause alterations in immunoglobulin (Ig) response.9 Changes in serum Ig levels occur frequently in liver disease.10 Patients with ALD frequently show an increase in IgA serum levels, and IgA can be deposited in a continuous pattern along the hepatic sinusoids.11 With the commencement of organ injury, IgM (primary response) rises significantly, whereas the IgG (secondary) response is slower. After several weeks, the IgM levels decrease and IgG rises.12 Detection of serum Ig levels has been used to assist in the diagnosis of liver diseases.13
Because bacterial infections are common in patients with advanced ALD,14 we determined the plasma anti‐P. gingivalis‐specific antibody profiles (IgG, IgM, and IgA) in order to determine whether P. gingivalis is associated with the development/severity of AAH as an independent risk factor. We also evaluated the roles of sex and lifetime drinking history (LTDH) in association with P. gingivalis antibody responses and AAH.15
Participants and Methods
Study Paradigm
This investigation was a single time point evaluation of patients and healthy volunteers. We assessed blood samples, clinical data, relevant medical history, clinical markers of progression, and severity of AAH and drinking history. All authors had access to the study data and had reviewed and approved the final manuscript. We analyzed laboratory markers from plasma samples and compared the factors between patients with severe and moderate AAH and healthy volunteers. We detected anti‐P. gingivalis antibody responses from the plasma samples and examined the association of anti‐P. gingivalis antibody responses with clinical measures of disease severity. This study was approved by the institutional review board (protocol No.12.0427) of the University of Louisville.
Study Participants
Patients with AAH (31 patients with severe AH with Model for End‐Stage Liver Disease [MELD] score ≥20 and 16 patients with moderate AAH with MELD score <20) and 22 healthy volunteers were included in this clinical study. This investigation is part of a large national multisite clinical trial (clinicaltrials.gov: NCT01809132) supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA). All patient participants were diagnosed with AAH. Patients were 21 to 66 years of age, completed the consenting process for participation in the study, and did not have active drug abuse. Healthy controls (HCs) were of similar age and did not have liver disease or any comorbid conditions (heart, kidney, lung, neurologic or psychiatric illness, sepsis), and none had any acute or chronic inflammatory process. Pregnant and lactating women, prisoners, and other individuals with potential vulnerability were excluded from the study.
Specimen Collection
Whole blood (approximately 8 mL) was collected, and plasma was apportioned into 1‐mL aliquots and stored at −80°C until use. Freeze–thaw cycles were avoided to maintain the integrity of the plasma samples.
Clinical, Demographic, and Drinking Data
Collected data consisted of age, sex, body mass index, drinking history (using LTDH), medical assessments at admission (specific for the study to rule out any significant comorbid conditions, e.g., heart, lung, psychiatric illnesses, sepsis), and medical history. Confirmatory tests for AAH (laboratory and imaging) and markers of liver disease severity (Child‐Turcotte‐Pugh [CTP], MELD, and Maddrey discriminant function [DF]) were also performed. The laboratory panel included a comprehensive metabolic panel (including liver panel) and coagulation assessment. Other liver diseases were excluded using blood tests recommended by the NIAAA Alcoholic Hepatitis Consortia.16
Bacterial Strains and Growth Conditions
P. gingivalis strains W83 and ATCC 33277 were purchased from ATCC and cultured anaerobically at 37°C. Bacteria culture medium was trypticase soy broth supplemented with yeast extract (1 mg/mL), hemin (5 μg/mL), and menadione (1 μg/mL).17 Bacteria were harvested in the exponential phase (optical density [OD]600 = 1) and washed 3 times with phosphate‐buffered saline (PBS) before use.
P. gingivalis Whole‐Cell Enzyme‐Linked Immunosorbent Assay for Plasma Antibody Detection
Overnight‐cultured P. gingivalis bacteria were washed 3 times with PBS, and then 2 × 107 P. gingivalis were deposited on a polystyrene enzyme‐linked immunosorbent assay (ELISA) plate in 0.1‐M sodium carbonate buffer (pH 9.5) at room temperature for 2 hours. After three washes, the plate was blocked with PBS (pH 7.0) containing 5% fetal bovine serum for 1 hour. After washing 3 times with 0.05% PBS containing 0.05% Tween 20, the plate was incubated with 100 μL of a patient’s plasma diluted 1:200 for 2 hours. After washing 5 times, the plate was incubated with 100 μL of 1:3,000 horseradish peroxidase (HRP)‐coupled goat anti‐human IgG (catalog No. 62‐8420; Invitrogen) or 1:5,000 HRP‐coupled goat anti‐human IgM (catalog No. A6907; Sigma), or 1:10,000 HRP‐coupled goat anti‐human IgA (catalog No. A0296; Sigma) antibodies, specifically, for 1 hour. After seven washes, color was developed with tetramethylbenzidine for 30 minutes and stopped with 2 M H2SO4. Absorbance was read at OD450.18
Statistical Analysis
Factorial 1‐way analysis of variance was used to determine significance between group differences for clinical, demographic, drinking data, and P. gingivalis antibody levels. Linear regression was used for association analysis. Outcomes for association analyses were presented using significance level and model fit (adjusted R 2). All statistical analyses were performed using IBM SPSS (version 25.0; Chicago, IL). Normally distributed data were expressed as mean ± SD. Statistical significance was set at P ≤ 0.05.
Results
Patient/Healthy Volunteer Characterization
Demographic information and laboratory data of 47 subjects with AAH and 22 healthy volunteers are summarized in Table 1. Subjects with AAH were divided into the following two groups based on MELD scores: moderate subjects (MELD score <20) and severe subjects (MELD score ≥20). There were no differences in age, sex, or ethnicity among the three groups.
Table 1.
Clinical Presentation of AAH and Healthy Controls
| Variables | Healthy Controls | Moderate AAH | Severe AAH | Significant Difference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Male | Female | Total | Male | Female | Total | Male | Female | Total | |
| Numbers (n) | 12 | 10 | 22 | 10 | 6 | 16 | 18 | 13 | 31 | |
| Ages (years) | 43.7 ± 16.4 | 51.1 ± 15.2 | 47.2 ± 15.9 | 47.4 ± 13.2 | 51.3 ± 9 | 48.9 ± 11.6 | 50.1 ± 10.2 | 44.8 ± 7.2 | 47.2 ± 9.6 | |
| Hispanic or Latino (%) | NA | 20% (2/10) | 16.7% (1/6) | 18.8% (3/16) | 33.3% (6/18) | 14.3% (2/14) | 25% (8/32) | |||
| AST (U/L) | 27.77 ± 4.83 | 23.75 ± 7.74 | 25.84 ± 6.58†,‡ | 184.9 ± 97.4 | 116 ± 32.0 | 155.4 ± 82.2† | 138.4 ± 73.8 | 129.7 ± 80 | 134.4 ± 75.6‡ | † P = 0.0001 (Mo vs. HC) |
| ‡ P = 0.0001 (S vs. HC) | ||||||||||
| ALT (U/L) | 27.54 ± 7.24 | 22 ± 4.67 | 24.88 ± 6.65†,‡ | 95.6 ± 57.5 | 46.5 ± 18.9 | 74.6 ± 50.5†,§ | 51.2 ± 28.3 | 37.7 ± 18.7 | 45 ± 25‡,§ | † P = 0.0001 (Mo vs. HC) |
| ‡ P = 0.026 (S vs. HC) | ||||||||||
| § P = 0.005 (Mo vs. S) | ||||||||||
| Alkaline phosphatase (IU/L) | NA | 165.4 ± 105 | 192.5 ± 99.7 | 177 ± 99.7 | 192 ± 98.8* | 172.3 ± 46.5* | 183.1 ± 78.9 | *P = 0.036 (M vs. F)|| | ||
| Serum total bilirubin (μmol/L) | NA | 8.45 ± 6.29 | 7.4 ± 4.1 | 8.1 ± 5.4§ | 20.4 ± 8.2 | 14.9 ± 7.6 | 17.9 ± 8.3§ | § P = 0.0001 (Mo vs. S) | ||
| Creatinine (mg/dL) | NA | 0.75 ± 0.29 | 0.52 ± 0.08 | 0.66 ± 0.26 | 0.98 ± 0.33 | 0.85 ± 0.74 | 0.92 ± 0.55 | |||
| Albumin (g/dL) | NA | 2.62 ± 0.49 | 2.45 ± 0.58 | 2.55 ± 0.51 | 2.49 ± 0.32 | 2.35 ± 0.57 | 2.43 ± 0.45 | |||
| Globulin (g/dL) | NA | 3.65 ± 0.76 | 3.82 ± 1.14 | 3.72 ± 0.9 | 3.44 ± 0.73 | 3.63 ± 0.85 | 3.53 ± 0.8 | |||
| A/G ratio | NA | 0.73 ± 0.14 | 0.68 ± 0.27 | 0.71 ± 0.20 | 0.77 ± 0.19 | 0.72 ± 0.35 | 0.74 ± 0.28 | |||
| WBC (x 109/L) | NA | 9.31 ± 6.54 | 9.88 ± 6.36 | 9.58 ± 6.19 | 14.7 ± 6.85 | 12.75 ± 7.88 | 13.8 ± 7.28 | |||
| INR | NA | 1.4 ± 0.5 | 1.35 ± 0.12 | 1.38 ± 0.39§ | 1.86 ± 0.39 | 1.9 ± 0.36 | 1.88 ± 0.37§ | |||
| AST/ALT | 1.05 ± 0.24 | 1.08 ± 0.24 | 1.06 ± 0.24†,‡ | 2.29 ± 1.35 | 3.13 ± 2.17 | 2.65 ± 1.72† | 2.93 ± 1.14* | 3.79 ± 1.96* | 3.32 ± 1.60‡ | *P = 0.010 (M vs. F)|| |
| † P = 0.002 (Mo vs. HC) | ||||||||||
| ‡ P = 0.0001 (S vs. HC) | ||||||||||
| MELD score | NA | 17.3 ± 1.9 | 16.8 ± 2.3 | 17.1 ± 2.03§ | 24.83 ± 5.23 | 24.13 ± 4.6 | 24.52 ± 4.9§ | § P = 0.0001 (Mo vs. S) | ||
| DF | NA | 19.8 ± 17.9 | 19.2 ± 6.1 | 19.6 ± 14.3§ | 55.3 ± 21.8 | 53.9 ± 17.4 | 54.6 ± 19.6§ | § P = 0.0001 (Mo vs. S) | ||
| CTP | NA | 9 ± 1.41 | 9.17 ± 0.75 | 9.08 ± 1.12§ | 11.11 ± 1.49 | 11 ± 1.41 | 11.06 ± 1.44§ | § P = 0.0001 (Mo vs. S) | ||
Data are means ± SD, analyzed by SPSS 1‐way analysis of variance. *Indicates difference between male and female AAH. †, ‡, § indicate differences between HCs, moderate AAH, and severe AAH. ||M vs. F in severe AAH group.
Abbreviations: F, female; INR, international normalized ratio; M, male; Mo, moderate AAH; S, severe AAH; WBC, white blood cell.
As expected, compared to HCs, patients with severe AAH exhibited significantly higher alanine aminotransferase (ALT) (P = 0.0001), higher plasma total bilirubin (P = 0.0001), higher international normalized ratio (P = 0.0001), higher percentage of ascites (P = 0.0001), higher DF (P = 0.0001), and higher CTP score (P = 0.0001).
Plasma Anti‐P. gingivalis IgG, IgA, IgM Antibody Responses Assessed by AAH Severity
Compared to HCs, plasma IgG levels were significantly higher in the total group of patients with severe AAH and in male patients with severe AAH for both P. gingivalis strains (Fig. 1A‐D) but not in female patients with AAH (Fig. 1E,F). Plasma IgG levels in severe AAH were significantly higher for both P. gingivalis strains (Fig. 1A,B) compared to moderate AAH. We did not find any statistical differences in plasma IgG levels between moderate AAH groups and HCs.
Figure 1.
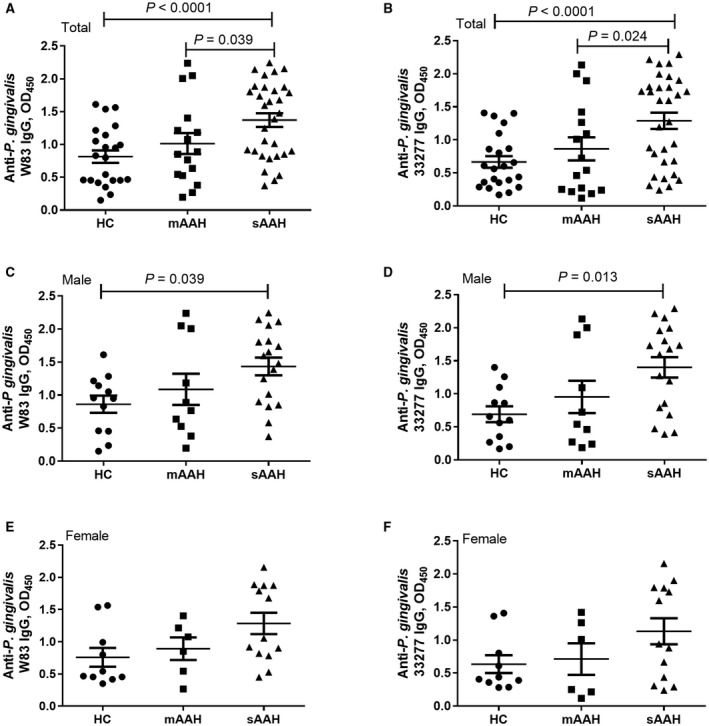
Anti‐P. gingivalis IgG and AAH severity. (A,B) Total AAH. (C,D) Male patients. (E,F,) Female patients. Anti‐P. gingivalis W83 IgG levels in HCs, patients with moderate AAH, and patients with severe AAH in (A) total AAH, (C) male patients, and (E) female patients. Anti‐P. gingivalis 33277 IgG levels in HCs, patients with moderate AAH, and patients with severe AAH in (B) total AAH, (D) male patients, and (F) female patients. Horizontal bars represent mean ± SD.
Compared to HCs, all patients with severe AAH as well as male and female patients with severe AAH separately had significantly increased IgA responses for both P. gingivalis strains (Fig. 2A‐F). The levels were significantly higher in all patients with moderate AAH compared to HCs for both P. gingivalis strains (Fig. 2A,B) and separately in the male group (Fig. 2C) and the female group (Fig. 2E) for the W83 strain but not for the 33277 strain (Fig. 2D,F).
Figure 2.
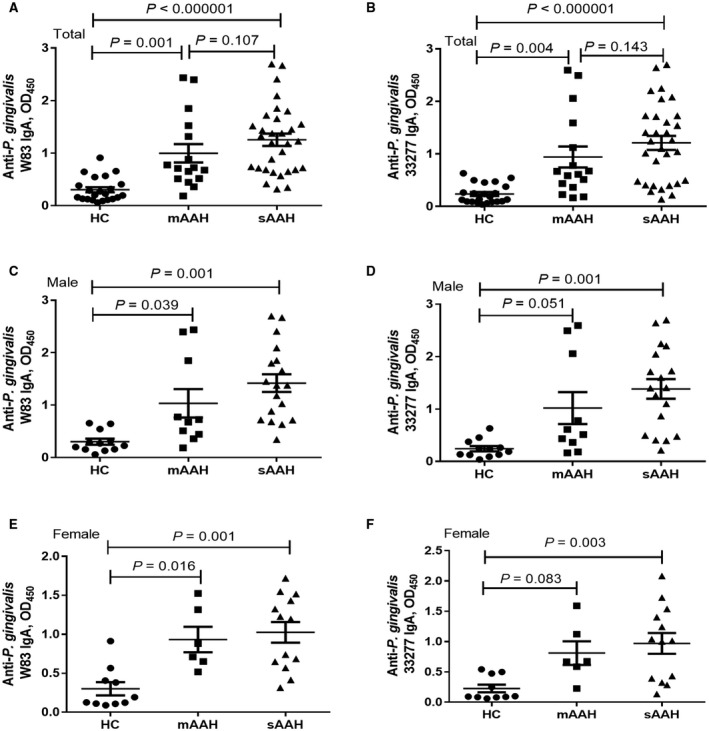
Anti‐P. gingivalis IgA and AAH severity. (A,B) Total AAH. (C,D) Male patients. (E,F) Female patients. Anti‐P. gingivalis W83 IgA levels in HCs, patients with moderate AAH, and patients with severe AAH in (A) total AAH, (C) male patients, and (E) female patients. Anti‐P. gingivalis 33277 IgA levels in HCs, patients with moderate AAH, and patients with severe AAH in (B) total AAH, (D) male patients, and (F) female patients. Horizontal bars represent mean ± SD.
Plasma IgM levels were significantly higher in all patients with severe AAH compared to HCs for both P. gingivalis strains (Fig. 3A,B) but not in male patients (Fig. 3C,D) and female patients (Fig. 3E,F) separately.
Figure 3.
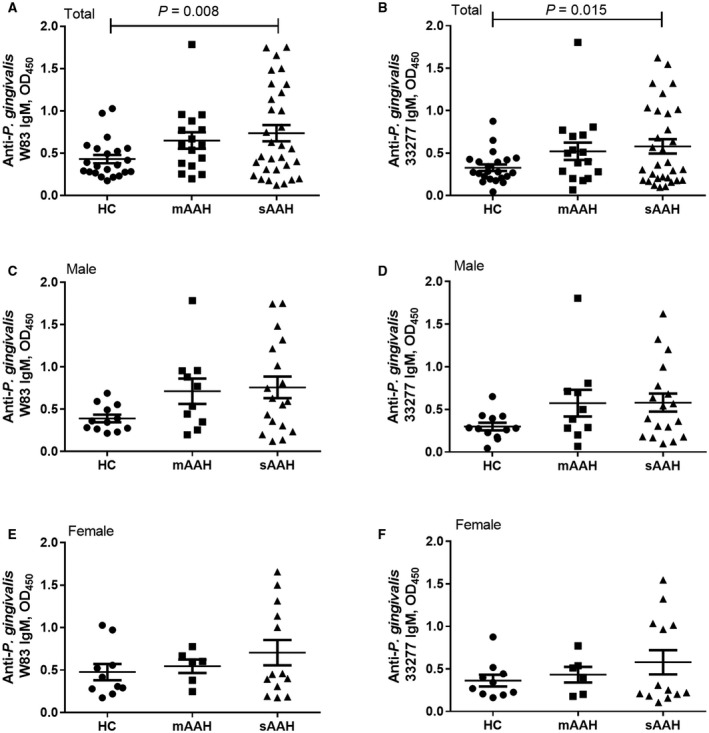
Anti‐P. gingivalis IgM and AAH severity. (A,B) Total AAH. (C,D) Male patients. (E,F) Female patients. Anti‐P. gingivalis W83 IgM levels in HCs, patients with moderate AAH, and patients with severe AAH in (A) total AAH, (C) male patients, and (E) female patients. Anti‐P. gingivalis 33277 IgM levels in HCs, patients with moderate AAH, and patients with severe AAH in (B) total AAH, (D) male patients, and (F) female patients. Horizontal bars represent mean ± SD.
Association of Plasma Anti‐P. gingivalis IgM Responses and LTDH
All patients with AAH showed a significant inverse association in LTDH and IgM antibodies to the two P. gingivalis strains (33277, Fig. 4A; W83, Supporting Fig. S1A). All male patients with AAH but not female patients with AAH (data not shown) showed a significant inverse association (33277, Fig. 4B; W83, Supporting Fig. S1B). We further examined this association within each AAH group. We found that the association between LTDH and IgM antibody levels was only significant in male patients with moderate AAH (33277, Fig. 4C; W83, Supporting Fig. S1C), whereas male patients with severe AAH did not show any significant association between LTDH and IgM antibodies (data not plotted). There was also no association in female patients with AAH.
Figure 4.
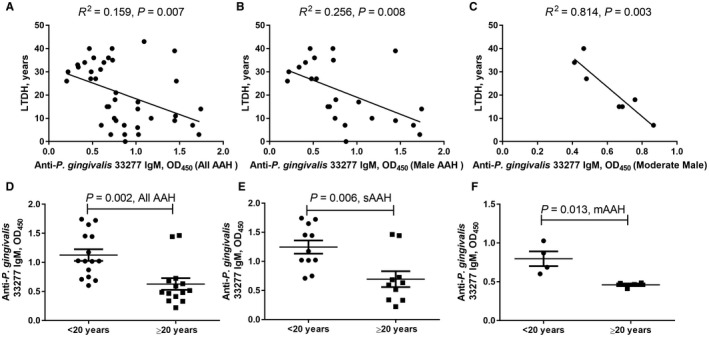
Association of anti‐P. gingivalis 33277 IgM response and LTDH in patients with AAH. IgM response in (A) all AAH, (B) all male AAH, and (C) male moderate AAH. Anti‐P. gingivalis 33277 IgM comparison between LTDH <20 years of drinking and LTDH ≥20 years of drinking in (D) all AAH, (E) severe AAH, and (F) moderate AAH. (D‐F) Horizontal bars represent mean ± SD.
When we separated patients into two groups by LTDH (group 1, <20 years of drinking; group 2, ≥20 years of drinking) and examined IgM, all patients with AAH showed a significantly higher IgM value in group 1 (33277, Fig. 4D; W83, Supporting Fig. S1D). In patients with severe AAH, IgM was also significantly higher among those with fewer years of drinking (33277, Fig. 4E; W83, Supporting Fig. S1E), whereas in patients with moderate AAH, this association was observed for strain 33277 (Fig. 4F) but not for W83 (Supporting Fig. S1F).
Association of Plasma Anti‐P. gingivalis IgM Responses and Aspartate Aminotransferase: ALT Ratio
The aspartate aminotransferase (AST):ALT ratio is considered to be a reliable marker for progression of liver injury.19, 20 The association of the AST:ALT ratio and 33277 IgM was not significant in the total group of patients with AAH (Fig. 5A); however, patients with moderate AAH did show a significant association (Fig. 5B). We also evaluated this correlation by sex. Although the AST:ALT ratio was significantly higher in female patients than in male patients in the severe AAH group (Table 1), we found a significant association in the AST:ALT ratio and 33277 IgM in male patients with AAH (Fig. 5C) and in male patients with moderate AAH (Fig. 5D). Female patients in both disease groups did not show any such association (data not shown).
Figure 5.
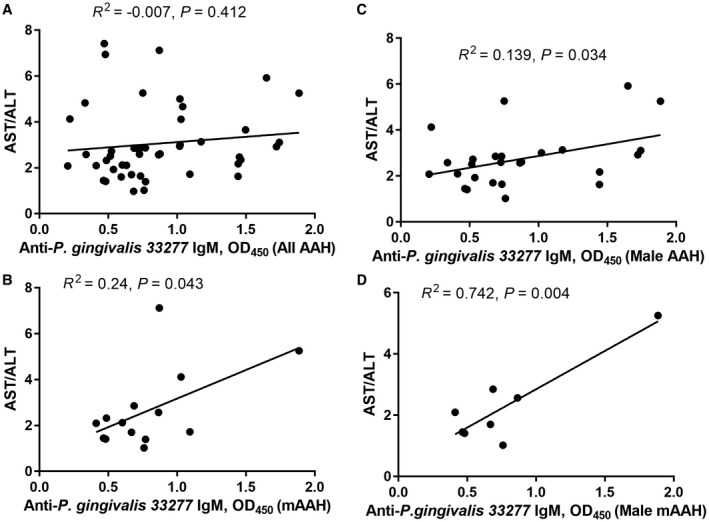
Association of anti‐P. gingivalis 33277 IgM response with progression of liver severity determined by AST:ALT ratio. (A) All patients with AAH. (B) All patients with moderate AAH. (C) All male patients with AAH. (D) All male patients with moderate AAH.
Similarly, there was no significant association between the AST:ALT ratio and W83 IgM antibody in the total group of patients with AAH and patients with moderate AAH (Supporting Fig. S2A,B). Although there was no significant association in all male patients with AAH (Supporting Fig. S2C), a highly significant association in the AST:ALT ratio and W83 IgM in male patients with moderate AAH was observed (Supporting Fig. S2D). This may be because the AST:ALT ratio is more predictive of severity in early liver disease. Female patients in either disease group did not show any such association. Thus, the interactions between IgM responses for both strains and the AST:ALT ratio were similar.
Association of Anti‐P. gingivalis IgM Response and MELD Score
There was a positive association between W83 IgM response and MELD score in the total group of patients with severe AAH (Fig. 6A) and in female patients with severe AAH (Fig. 6B) but not in male patients with severe AAH (Fig. 6C). We did not find an association in male or female patients with moderate AAH (data not shown). There was also no association between IgG or IgA and MELD in either male patients or female patients in the severe or moderate groups.
Figure 6.
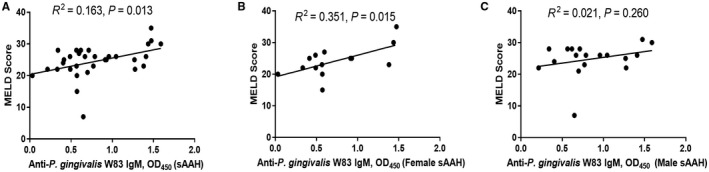
Association of anti‐P. gingivalis W83 IgM response with liver severity determined by MELD score in female patients with severe AAH. (A) All patients with severe AAH. (B) All female patients with severe AAH. (C) All male patients with severe AAH.
Discussion
It is generally well accepted that the gut microbiome plays an important role in the development and progression of several types of liver disease.21 Intestinal dysbiosis has been observed by our group and others in experimental ALD/NAFLD in mice as well as humans.21, 22, 23 There are initial reports suggesting that the oral microbiome may also play a role in liver disease.7, 8, 24, 25, 26 In experimental animals, infection with P. gingivalis worsens steatohepatitis in mice fed a high‐fat diet.27 In humans, periodontitis is associated with increased hepatic fibrosis in subjects with NAFLD.28 Patients with cirrhosis have been reported to have increased mortality if they have periodontitis.29 Importantly, patients with alcoholism have a pathogenic oral microbiome and worse PD than patients without alcoholism,30 and patients with alcoholism with a smoking history have higher odds ratio of PD.31 Thus, we hypothesized that patients with AAH would be a likely population to have their liver disease associated with (and likely negatively impacted by) PD.
We selected two strains of P. gingivalis to perform antibody ELISA detection. Strain 33277 represents the fimbriated/nonencapsulated lineage and possesses both the Mfa1 and FimA fimbriae, which are present in 20 of 21 P. gingivalis strains. Strain W83 represents the nonfimbriated/capsulated lineage.32 Both strains are pathogenic in animal models of infection.33 Detection of the antibodies showed a wide range of concentrations. Because we studied a group with a wide spectrum of alcohol hepatitis disease severity, it was not surprising to find a wide spectrum of antibody levels to P. gingivalis.
Elevated levels of Ig antibodies against P. gingivalis have a detectable protective effect against periodontal infections.34, 35 Elevated serum IgG against P. gingivalis has been reported in patients with chronic adult periodontitis, and levels decrease with appropriate therapy.36 In addition, cardiovascular disease (CVD) and periodontitis are associated with levels of IgG to P. gingivalis. 37 P. gingivalis IgA levels also predict myocardial infarction and stroke independently of established CVD risk factors.38 A significant correlation between fibrosis progression and P. gingivalis IgG titers has been reported in a study evaluating the effect of P. gingivalis infection as a risk factor in the progression of NASH.8 Our data are consistent with this finding, with significantly elevated IgG, IgM, and IgA levels in patients with severe AAH.
P. gingivalis can activate pattern recognition receptors found on cells, such as macrophages/Kupffer cells, and initiate intracellular signaling and an inflammatory response. Receptor types of major importance for P. gingivalis are the toll‐like receptors (TLR2 and TLR4) and nucleotide‐binding oligomerization domains (NODs).39 Activation of either TLR‐2 or NOD1/NOD2 can activate nuclear factor kappa B with subsequent increased expression of many proinflammatory cytokines and possible liver injury. Interestingly, oral administration of P. gingivalis in mice caused intestinal dysbiosis, decreased intestinal tight junction proteins, and endotoxemia, which are all features of ALD.40
The IgM response represents active infection.41 Anti‐P. gingivalis IgM and length of drinking had a strong inverse association, suggesting an important role of heavy prolonged drinking in altering Ig immune responses, with longer drinking showing a lower immune response.15 In the moderate AAH group, the IgM response was strongly correlated with the AST:ALT ratio, suggesting active infection of P. gingivalis was associated with progressive liver damage in these patients (most specifically in male patients).42 There was no correlation between the AST:ALT ratio and IgM in severe AAH, indicating that this association is more meaningful in moderate AAH and is lost in the severe form of AAH.
We found that IgM was highly associated with the MELD score, especially in female patients. Female patients might be especially vulnerable to active infection of P. gingivalis and its negative impact on the liver.
There were some limitations in this study. Importantly, this was an association study and could not assess causality. It would be optimal to verify the specificity of the antibody response to P. gingivalis. In addition, detection of P. gingivalis in liver biopsy specimens from patients with AAH by polymerase chain reaction or immunohistochemistry should be a gold standard in the future, but liver biopsies are not routinely performed for the diagnosis of ALD in the United States.43 Because little is known about the role of P. gingivalis in ALD development/progression, we analyzed immune responses to P. gingivalis as independent factors to estimate their individual effects. In addition, determining the presence or absence of PD in controls and subjects with alcoholic hepatitis would be important in future studies. Studies with larger numbers of participants could further clarify the roles of other confounding factors and their interactions with P. gingivalis, sex, and drinking measures. Moreover, a longitudinal or intervention study could help to better delineate the role of P. gingivalis in the development/progression of AAH.
In summary, P. gingivalis may be associated with ALD and may function as a confounding factor in AAH.25, 26, 44 Our data support the concept that infection with P. gingivalis is associated with both progression and severity of AAH, and this association was modestly impacted by sex. Further studies are indicated to determine whether treatment of PD may help prevent or attenuate ALD.
Potential conflict of interest
Nothing to report.
Supporting information
Acknowledgment
We thank the clinical staff of the University of Louisville Hospital and Outpatient Clinic and the VA Medical Center, Louisville, KY, for their support of this clinical trial. We also acknowledge support from the NIAAA and other sites of the Defeat Alcoholic SteatoHepatitis consortium for this collaborative effort. This includes Dr. Svetlana Radaeva (NIAAA), Dr. Bruce Barton (University of Massachusetts Medical School), Dr. Srinivasan Dasarathy (Cleveland Clinic), Dr. Arthur McCullough (Cleveland Clinic), Dr. Mack Mitchell (University of Texas Southwestern Medical Center), and Dr. Gyongi Szabo (University of Massachusetts Medical School). We also thank Ms. Marion McClain for editorial support for this manuscript.
Supported by the National Institutes of Health (grants U01AA021901, U01AA021893‐01, U01AA022489‐01A1, R01AA023681‐01, P20GM113226, and P50AA024337 to C.J.M.; R21AA020848 and R01AA023190 to W.F.; and P20GM125504 to R.L.) and the U.S. Department of Veteran Affairs (grant 1I01BX002996 to C.J.M.).
ClinicalTrials.gov identifier NCT01922895 and NCT01809132.
Contributor Information
Craig J. McClain, Email: craig.mcclain@louisville.edu
Wenke Feng, Email: wenke.feng@louisville.edu.
References
Author names in bold designate shared co‐first authorship
- 1. Singal AK, Bataller R, Ahn J, Kamath PS, Shah VH. ACG clinical guideline: Alcoholic Liver Disease. Am J Gastroenterol 2018;113:175‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hajishengallis G, Lamont RJ. Dancing with the stars: how choreographed bacterial interactions dictate nososymbiocity and give rise to keystone pathogens, accessory pathogens, and pathobionts. Trends Microbiol 2016;24:477‐489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seymour GJ, Ford PJ, Cullinan MP, Leishman S, Yamazaki K. Relationship between periodontal infections and systemic disease. Clin Microbiol Infect 2007;13(Suppl. 4):3‐10. [DOI] [PubMed] [Google Scholar]
- 4. Makiura N, Ojima M, Kou Y, Furuta N, Okahashi N, Shizukuishi S, et al. Relationship of Porphyromonas gingivalis with glycemic level in patients with type 2 diabetes following periodontal treatment. Oral Microbiol Immunol 2008;23:348‐351. [DOI] [PubMed] [Google Scholar]
- 5. Bartova J, Sommerova P, Lyuya‐Mi Y, Mysak J, Prochazkova J, Duskova J, et al. Periodontitis as a risk factor of atherosclerosis. J Immunol Res 2014;2014:636893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mikuls TR, Payne JB, Yu F, Thiele GM, Reynolds RJ, Cannon GW, et al. Periodontitis and Porphyromonas gingivalis in patients with rheumatoid arthritis. Arthritis Rheumatol 2014;66:1090‐1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yoneda M, Naka S, Nakano K, Wada K, Endo H, Mawatari H, et al. Involvement of a periodontal pathogen, Porphyromonas gingivalis on the pathogenesis of non‐alcoholic fatty liver disease. BMC Gastroenterol 2012;12:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nakahara T, Hyogo H, Ono A, Nagaoki Y, Kawaoka T, Miki D, et al. Involvement of Porphyromonas gingivalis in the progression of non‐alcoholic fatty liver disease. J Gastroenterol 2018;53:269‐280. [DOI] [PubMed] [Google Scholar]
- 9. Akhi R, Wang C, Kyrklund M, Kummu O, Turunen SP, Hyvarinen K, et al. Cross‐reactive saliva IgA antibodies to oxidized LDL and periodontal pathogens in humans. J Clin Periodontol 2017;44:682‐691. [DOI] [PubMed] [Google Scholar]
- 10. Hobbs JR. Immunoglobulins in clinical chemistry. Adv Clin Chem 1971;14:219‐317. [DOI] [PubMed] [Google Scholar]
- 11. van de Wiel A, Delacroix DL, van Hattum J, Schuurman HJ, Kater L. Characteristics of serum IgA and liver IgA deposits in alcoholic liver disease. Hepatology 1987;7:95‐99. [DOI] [PubMed] [Google Scholar]
- 12. Prince HE, Lape‐Nixon M. Role of cytomegalovirus (CMV) IgG avidity testing in diagnosing primary CMV infection during pregnancy. Clin Vaccine Immunol 2014;21:1377‐1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McPherson S, Henderson E, Burt AD, Day CP, Anstee QM. Serum immunoglobulin levels predict fibrosis in patients with non‐alcoholic fatty liver disease. J Hepatol 2014;60:1055‐1062. [DOI] [PubMed] [Google Scholar]
- 14. Gustot T, Fernandez J, Szabo G, Albillos A, Louvet A, Jalan R, et al. Sepsis in alcohol‐related liver disease. J Hepatol 2017;67:1031‐1050. [DOI] [PubMed] [Google Scholar]
- 15. Gonzalez‐Quintela A, Alende R, Gude F, Campos J, Rey J, Meijide LM, et al. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin Exp Immunol 2008;151:42‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crabb DW, Bataller R, Chalasani NP, Kamath PS, Lucey M, Mathurin P; NIAAA . Alcoholic Hepatitis Consortia. Standard definitions and common data elements for clinical trials in patients with alcoholic hepatitis: recommendation from the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology 2016;150:785‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou Y, Sztukowska M, Wang Q, Inaba H, Potempa J, Scott DA, et al. Noncanonical activation of beta‐catenin by Porphyromonas gingivalis . Infect Immun 2015;83:3195‐3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park Y, Simionato MR, Sekiya K, Murakami Y, James D, Chen W, et al. Short fimbriae of Porphyromonas gingivalis and their role in coadhesion with Streptococcusgordonii. Infect Immun 2005;73:3983‐3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Giannini E, Botta F, Fasoli A, Ceppa P, Risso D, Lantieri PB, et al. Progressive liver functional impairment is associated with an increase in AST/ALT ratio. Digest Dis Sci 1999;44:1249‐1253. [DOI] [PubMed] [Google Scholar]
- 20. Vatsalya V, Kong M, Cave MC, Liu N, Schwandt ML, George DT, et al. Association of serum zinc with markers of liver injury in very heavy drinking alcohol‐dependent patients. J Nutr Biochem 2018;59:49‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology 2014;146:1513‐1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bull‐Otterson L, Feng W, Kirpich I, Wang Y, Qin X, Liu Y, et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS One 2013;8:e53028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kirpich IA, Solovieva NV, Leikhter SN, Shidakova NA, Lebedeva OV, Sidorov PI, et al. Probiotics restore bowel flora and improve liver enzymes in human alcohol‐induced liver injury: a pilot study. Alcohol 2008;42:675‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bajaj JS, Betrapally NS, Hylemon PB, Heuman DM, Daita K, White MB, et al. Salivary microbiota reflects changes in gut microbiota in cirrhosis with hepatic encephalopathy. Hepatology 2015;62:1260‐1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Novacek G, Plachetzky U, Potzi R, Lentner S, Slavicek R, Gangl A, et al. Dental and periodontal disease in patients with cirrhosis–role of etiology of liver disease. J Hepatol 1995;22:576‐582. [DOI] [PubMed] [Google Scholar]
- 26. Raghava KV, Shivananda H, Mundinamane D, Boloor V, Thomas B. Evaluation of periodontal status in alcoholic liver cirrhosis patients: a comparative study. J Contemp Dent Pract 2013;14:179‐182. [DOI] [PubMed] [Google Scholar]
- 27. Furusho H, Miyauchi M, Hyogo H, Inubushi T, Ao M, Ouhara K, et al. Dental infection of Porphyromonas gingivalis exacerbates high fat diet‐induced steatohepatitis in mice. J Gastroenterol 2013;48:1259‐1270. [DOI] [PubMed] [Google Scholar]
- 28. Alazawi W, Bernabe E, Tai D, Janicki T, Kemos P, Samsuddin S, et al. Periodontitis is associated with significant hepatic fibrosis in patients with non‐alcoholic fatty liver disease. PLoS One 2017;12:e0185902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grønkjær LL, Holmstrup P, Schou S, Kongstad J, Jepsen P. Vilstrup H. Periodontitis in patients with cirrhosis: a cross‐sectional study. BMC Oral Health 2018;18:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fan X, Peters BA, Jacobs EJ, Gapstur SM, Purdue MP, Freedman ND, et al. Drinking alcohol is associated with variation in the human oral microbiome in a large study of American adults. Microbiome 2018;6:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lages EJ, Costa FO, Lages EM, Cota LO, Cortelli SC, Nobre-Franco GC, et al. Risk variables in the association between frequency of alcohol consumption and periodontitis. J Clin Peiodontol 2005;39:115‐122. [DOI] [PubMed] [Google Scholar]
- 32. Dashper SG, Mitchell HL, Seers CA, Gladman SL, Seemann T, Bulach DM, et al. Porphyromonas gingivalis uses specific domain rearrangements and allelic exchange to generate diversity in surface virulence factors. Front Microbiol 2017;8:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang M, Liang S, Hosur KB, Domon H, Yoshimura F, Amano A, et al. Differential virulence and innate immune interactions of type I and II fimbrial genotypes of Porphyromonas gingivalis . Oral Microbiol Immunol 2009;24:478‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rams TE, Listgarten MA, Slots J. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis subgingival presence, species‐specific serum immunoglobulin G antibody levels, and periodontitis disease recurrence. J Periodontal Res 2006;41:228‐234. [DOI] [PubMed] [Google Scholar]
- 35. Gibson FC 3rd, Gonzalez DA, Wong J, Genco CA. Porphyromonas gingivalis‐specific immunoglobulin G prevents P. gingivalis‐elicited oral bone loss in a murine model. Infect Immun 2004;72:2408‐2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Murayama Y, Nagai A, Okamura K, Kurihara H, Nomura Y, Kokeguchi S, et al. Serum immunoglobulin G antibody to periodontal bacteria. Adv Dent Res 1988;2:339‐345. [DOI] [PubMed] [Google Scholar]
- 37. Damgaard C, Reinholdt J, Enevold C, Fiehn NE, Nielsen CH, Holmstrup P. Immunoglobulin G antibodies against Porphyromonas gingivalis or Aggregatibacter actinomycetemcomitans in cardiovascular disease and periodontitis. J Oral Microbiol 2017;9:1374154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pussinen PJ, Alfthan G, Tuomilehto J, Asikainen S, Jousilahti P. High serum antibody levels to Porphyromonas gingivalis predict myocardial infarction. Eur J Cardiovasc Prev Rehabil 2004;11:408‐411. [DOI] [PubMed] [Google Scholar]
- 39. Amar S, Engelke M. Periodontal innate immune mechanisms relevant to atherosclerosis. Mol Oral Microbiol 2015;30:171‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nakajima M, Arimatsu K, Kato T, Matsuda Y, Minagawa T, Takahashi N, et al. Oral administration of P. gingivalis induces dysbiosis of gut microbiota and impaired barrier function leading to dissemination of enterobacteria to the liver. PLoS One 2015;10:e0134234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Martinez‐Guzman MY, de la Rosa‐Ramirez MA, Arce‐Mendoza AY, Rosas‐Taraco AG. Detection of IgG, IgA and IgM antibodies against Porphyromonas gingivalis in gingival crevicular fluid and saliva in patients with chronic periodontitis. J Infect Dis Immun 2012;4:10‐15. [Google Scholar]
- 42. Deb N, Lahon D, Sharma P. A study on probable correlation of AST/ALT (De Ritis) ratio with the advancement of underlying severity of alcoholic liver disease in the North East Indian population. Asian Pac J Health Sci 2016;3:5‐10. [Google Scholar]
- 43. Mitchell MC, Friedman LS, McClain CJ. Medical management of severe alcoholic hepatitis: expert review from the clinical practice updates Committee of the AGA Institute. Clin Gastroenterol Hepatol 2017;15:5‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Glick M. Medical considerations for dental care of patients with alcohol‐related liver disease. J Am Dent Assoc 1997;128:61‐70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


