Abstract
Dysphagia is a common deficit after a stroke, and it is frequently associated with pneumonia, malnutrition, dehydration, and poor quality of life. It is not yet fully clear which brain regions are directly related to swallowing, and how lesions affect swallow physiology. This study aimed to assess the statistical relationship between acute stroke lesion locations and impairment of specific aspects of swallow physiology. We performed lesion symptom mapping with 68 retrospectively recruited, acute, first-ever ischemic stroke patients. Lesions were determined on diffusion weighted MRI scans. Post-stroke swallow physiology was determined using the Modified Barium Swallow Study Impairment Profile (MBSImP©™). The relationship between brain lesion location and 17 physiological aspects of swallowing were tested using voxel-based and region-based statistical associations corrected for multiple comparisons using permutation thresholding. We found that laryngeal elevation, anterior hyoid excursion, laryngeal vestibular closure, and pharyngeal residue were associated with lesioned voxels or regions of interests. All components showed distinct and overlapping lesion locations, mostly in the right hemisphere, and including cortical regions (inferior frontal gyrus, pre- and postcentral gyrus, supramarginal gyrus, angular gyrus, superior temporal gyrus, insula), subcortical regions (thalamus, amygdala) and white matter tracts (superior longitudinal fasciculus, corona radiata, internal capsule, external capsule, ansa lenticularis, lenticular fasciculus). Our findings indicate that different aspects of post-stroke swallow physiology are associated with distinct lesion locations, primarily in the right hemisphere, and primarily including sensory-motor integration areas and their corresponding white matter tracts. Future studies are needed to expand on our findings and thus, support the development of a neuroanatomical model of post-stroke swallow physiology and treatment approaches targeting the neurophysiological underpinnings of swallowing post stroke.
Keywords: Deglutition, Deglutition disorders, Stroke, Magnetic resonance imaging, Lesion analysis
Abbreviations: CCI, Charlson comorbidity index; DICOM, Digital imaging and communications in medicine; DW-MRI, MRI including diffusion weighted sequences; ICC, intraclass correlation coefficients; JHU, Johns Hopkins University; MBSImP, Modified barium Swallow Impairment Profile; MBSS, Modified barium swallow study; N, number; PCA, principal component analysis; ROI, region of interest; SD, standard deviation; VLSM, voxel-based lesion symptom mapping
Highlights
-
•
Different aspects of swallow physiology are associated with distinct stroke lesion locations.
-
•
Sensory-motor integration areas and white matter tracts are crucial for swallow physiology.
-
•
Mostly regions in the right and only few in the left hemisphere contribute to swallow physiology.
1. Introduction
Per year, 795,000 people experience a new or recurrent stroke in the United States (Benjamin et al., 2017). New forms of acute intervention have decreased the morbidity and mortality rates after a stroke (Benjamin et al., 2017; Wilmskoetter et al., 2016) but stroke remains a leading cause of disability (Benjamin et al., 2017; Centers for Disease Control and Prevention (CDC), 2009). A common and disabling condition after stroke is dysphagia, which is defined by impairments in swallowing. Dysphagia is associated with stroke mortality long term (Arnold et al., 2016; Sharma et al., 2001), and post-stroke complications, such as malnutrition and dehydration (Crary et al., 2013), and pneumonia (Marik and Kaplan, 2003; Martino et al., 2005). Furthermore, dysphagia has adverse effects on self-esteem, socialization, and quality of life (Ekberg et al., 2002) and has been directly linked to increased health care associated costs (Bonilha et al., 2014).
Dysphagia affects most stroke survivors, with 55% to 78% of all patients experiencing trouble with swallowing at least during the first days after their stroke (Daniels and Foundas, 1999; Martino et al., 2005). Dysphagia after stroke is very common, because swallowing can be easily disrupted, as it is a fast and complex neuromuscular mechanism, requiring the orchestration of several brain regions to control multiple muscles and structures. Swallowing involves >30 muscles or muscle pairs and five cranial nerves (Shaw and Martino, 2013). The involvement of the brainstem (especially medulla and pons) in post-stroke dysphagia has been investigated in much detail; however, the role of supratentorial regions is less clear (see Flowers et al., 2011 for review). Evidence suggests that swallowing is likely mediated by a complex bilateral neural network (Gonzalez-Fernandez et al., 2008; Leopold and Daniels, 2010; Miller, 2008), but there is no consensus if one hemisphere is more involved in the central control than the other, and if so which hemisphere is dominant (Cola et al., 2010; Daniels et al., 1996, Daniels et al., 2006; Li et al., 2009; Marian et al., 2017; Suntrup et al., 2015). Further, different lesion locations can potentially cause dysphagia, such as lesions in the somatosensory and motor cortices, basal ganglia, insula, and internal capsule (Flowers et al., 2017; Gonzalez-Fernandez et al., 2008).
While many studies have investigated the relationship between lesion locations and post-stroke dysphagia, the current body of evidence does not allow for conclusions about lesion locations and their impact on critical aspects of swallow physiology and safety, such as tongue control, initiation of the pharyngeal swallow, hyoid movement, or laryngeal vestibular closure. The lack of a more definitive association between brain lesion location and specific aspects of swallow physiology likely stems from limitations in both neuroimaging as well as quantitative assessments of swallow physiology. Due to limitations in neuroimaging methods at the time of the execution of previous studies, the segmentations of brain regions were limited to coarser areas (e.g. infratentorial, supratentorial, cortical, subcortical, bulbar) (e.g. Cola et al., 2010; Jeon et al., 2014; Moon et al., 2012), limiting the precision and sensitivity of lesion location. Moreover, studies have focused primarily on the occurrence of (oral or pharyngeal) dysphagia in general, symptoms like aspiration or residue, and have mainly applied clinical bedside or fiberendoscopic assessments (Daniels et al., 2017; Flowers et al., 2017; Galovic et al., 2013, Galovic et al., 2016; Gonzalez-Fernandez et al., 2008; Schmahmann et al., 2004; Steinhagen et al., 2009; Suntrup-Krueger et al., 2017). While these are clinically important outcomes and assessment tools, they lack detail regarding the complex interplay of structural movements and bolus flow that is present during swallowing. An understanding of deficits in specific aspects of swallow physiology, what we will term ‘physiological swallow impairments’ is needed to associate swallow physiology with lesion location.
The most comprehensive assessment of physiological swallow impairments is the modified barium swallow study (MBSS), the recognized gold-standard in determining swallow impairment throughout the continuum of the oropharyngeal swallow. Based on the patient's specific physiological swallow impairments, the MBSS enables clinicians to prescribe individualized rehabilitative exercises, compensatory strategies, and diet recommendations (Daniels and Huckabee, 2008; Logemann, 1998; Martin-Harris et al., 2000).
Only few lesion-symptom studies have been conducted in acute ischemic stroke patients investigating swallow impairments with MBSSs. These studies found an association between parietotemporal or medullary lesions and impaired laryngeal elevation (Moon et al., 2012), insular lesions and delayed initiation of pharyngeal swallow (Daniels and Foundas, 1997; Riecker et al., 2009; Stickler et al., 2003), and non-specific subcortical lesions and slower oral bolus transit (Cola et al., 2010). These studies, however, determined lesion locations with low precision methodology and investigated only a limited set of physiological swallow impairments that did not reflect the complete continuum of the oropharyngeal swallowing mechanism.
Thus, to date, much has been accomplished to understand the association between lesion locations and the occurrence of dysphagia in general. However, the usefulness of lesion locations to predict specific types of physiological swallow impairments is underexplored and, moreover, no evidence exists hitherto to understand if lesion locations can be mapped to swallow impairments across the continuum of the oropharyngeal swallow. The potential impact of the work herein is the ability to predict swallow impairment type at the time of the acute insult and expedited targeted interventions to improve the swallowing deficits, optimize nutrition and airway protection.
The goal of this study was to assess the critical location of supratentorial stroke lesions associated with fine-grained disruptions in swallow physiology defined as the movement of swallow relevant structures in relation to bolus flow patterns. We employed lesion-symptom mapping combined with comprehensive, valid and standardized assessments of MBSSs from a large cohort of acute stroke survivors. We hypothesized that different physiological swallow impairments are associated with distinct cortical and subcortical bilateral lesion locations involving motor-sensory networks primarily in the right hemisphere.
2. Materials and methods
We conducted a retrospective observational cross-sectional study of acute first-ever ischemic stroke patients admitted to the stroke service at the Medical University of South Carolina between 2008 and 2017. Patients were included in this study if they had a diagnostic MRI including diffusion-weighted sequences (DW-MRI) and a modified barium swallow study (MBSS) during their acute hospital stay. Patients with a history of previous strokes or neuroimaging evidence of previous strokes were excluded. They were also excluded if they had a history of diseases known to affect swallowing (e.g. head and neck cancer, Parkinson, dementia), neurological worsening between the MRI and MBSS, or were younger than 21 years.
2.1. Variables
All variables – demographic and medical information, MRI and MBSS data – were collected from electronical medical records. Comorbidities burden was estimated with the validated Charlson comorbidity index (CCI) (Charlson et al., 1987; Quan et al., 2005, Quan et al., 2011).
2.1.1. Lesion locations from DW-MRI
All patients underwent conventional standard of care MRI imaging for the purposes of stroke diagnosis. The neuroimaging data used in this study was obtained from these diagnostic studies. The MRI sequence details varied since the cohort studied here was admitted over many years (2008–2017) and technological improvements in diagnostic radiology during this time led to updates in MRI sequences. Overall, all patients had DW-MRI with whole brain coverage, with voxel-wise resolution ranging from 0.9375 × 0.9375 × 3.0000 mm to 1.4458 × 1.4458 × 6.0000 mm.
MR DICOM images were converted into NIfTI files using the software dcm2niix (Li et al., 2016). One rater (JW) manually drew the acute stroke lesions in the DW-MRI using the software MRIcron (www.mricron.com). All lesion drawings were reviewed for accuracy and precision by a neurologist (LB) with special expertise in voxel-based lesion symptom mapping (VLSM). Both raters were blinded to the MBSS results at the time of lesion drawing. Further details in image processing are described below.
2.1.2. Swallow measures from MBSSs
The dependent variables were various MBSS measurements of physiological swallow impairments. The primary dependent variables were swallow components derived from the Modified Barium Swallow Impairment Profile (MBSImP™©) (Martin-Harris et al., 2008), secondary dependent variables were the MBSImP summative scores (oral total and pharyngeal total sum scores), and airway invasion measured with the Penetration-Aspiration Scale (PAS) (Rosenbek et al., 1996). All MBSImP components were scored on the first swallow in cases where multiple swallows per bolus occurred.
2.1.2.1. Modified barium swallow impairment profile
The MBSImP is a standardized and validated tool to rate 17 different swallow components that are scored on 11 different swallow tasks (such as teaspoon thin, sequential nectar and solid) during the MBSS. Hereby, the MBSImP quantifies physiological swallow impairments. Each of the 17 components are scored on ordinal three- to five-point rating scales. Components C1 to C6 represent oral physiology, components C7 to C16 pharyngeal physiology, and component C17 esophageal physiology (Table 1).
Table 1.
Components of the modified barium swallow impairment profile (MBSImP).
| Oral components |
| C1 – Lip Closure |
| C2 – Tongue Control During Bolus Hold |
| C3 – Bolus Preparation/Mastication |
| C4 – Bolus Transport/Lingual Motion |
| C5 – Oral Residue |
| C6 – Initiation of Pharyngeal Swallow |
| Pharyngeal components |
| C7 – Soft palate elevation |
| C8 – Laryngeal elevation |
| C9 – Anterior hyoid excursion |
| C10 – Epiglottic movement |
| C11 – Laryngeal vestibular closure |
| C12 – Pharyngeal stripping wave |
| C13 – Pharyngeal contraction |
| C14 – Pharyngoesophageal segment opening |
| C15 – Tongue base retraction |
| C16 – Pharyngeal residue |
| Esophageal component |
| C17 – Esophageal clearance in upright position |
We scored each component on each performed swallow that was part of the standard protocol (Hazelwood et al., 2017). The worst score across all swallows was then determined as the overall impression score for each of the 17 components and used as the primary dependent variables. In addition, the sum of the oral components was used as the oral total sum score (min 0, max 22) and the sum of the pharyngeal components as the pharyngeal total sum score (min 0, max 29). The oral and pharyngeal total sum scores had been previously established based on results of a principal component analysis (PCA) in a study conducted on a heterogeneous patient population (Martin-Harris et al., 2008). We confirmed the same two-factor solution in the stroke patient sample of the study presented here by conducting a PCA using varimax rotation with Kaiser Normalization. Thus, this supported the calculation of oral and pharyngeal total sum scores.
2.1.2.2. Penetration-aspiration scale
The PAS is a standardized, valid and reliable eight-point ordinal scale to judge if and how deep bolus material enters the airway and if it is expelled afterwards (Rosenbek et al., 1996). We calculated the worst and median PAS score across all performed swallows that were part of the standard protocol.
2.1.2.3. Reliability
One rater (JW) performed all MBSS measures. Intra- and inter-rater reliability were established on 20% of the MBSSs with the first rater re-rating the MBSSs with a time difference of at least 2 weeks to limit possible recalls, and a second rater (JC) rating the same MBSSs. Both raters received thorough training beforehand. Reliability measures were assessed using two-way mixed intraclass correlation coefficients (ICC). In addition, percent agreements and weighted Kappa coefficients were calculated for all ordinal measures (MBSImP components). We interpreted the size of the ICCs and Kappa coefficients with <0.4 as poor agreement, 0.4–0.75 as good agreement, and >0.75 as excellent agreement (Fleiss, 1986).
2.2. Lesion symptom mapping
Lesion symptom mapping was performed to define the relationship between lesion location and physiological swallow impairments. In order to compare lesion location across individuals, the stroke lesions were spatially normalized to standard space using SPM12 and open source MATLAB scripts developed in-house (Rorden et al., 2012) through the following steps: 1) the lesion maps were smoothed using a 3 mm full-width half maximum Gaussian kernel to remove uneven edges; 2) an enantiomorphic approach (Nachev et al., 2008) using SPM12's unified segmentation-normalization (Ashburner and Friston, 2005) was applied to normalize the DW-MRI onto the standard space (1x1x1mm chimeric T1-weighted image, with the corresponding to the stroke lesion being replaced by the mirrored equivalent region in the intact hemisphere).
We performed VLSM on all swallow measures. In cases where there were no statistically significant voxel-impairment associations, we performed region of interest (ROI) based lesion symptom mapping using the Johns Hopkins University (JHU) neuroanatomical atlas that segments the brain into 189 grey and white matter areas and ventricles (Faria et al., 2012). Only voxels/ROIs lesioned in ≥10% of the patients were included. For voxel-based calculations, lesion status was expressed on a binary scale as lesion vs no lesion; for ROI-based calculations, lesion status was expressed on a continuous scale with percentage lesion of each ROI. We tested all ordinal variables (MBSImP components) for normality with the Shapiro-Wilk test and visual data inspection. Highly skewed variables were dichotomized. Cut-off points for dichotomization, derived from MBSImP guidelines, were used to differentiate between impaired and unimpaired swallow physiology (Martin-Harris et al., 2008; Northern Speech Services, 2010).
2.3. Statistical analysis
For the lesion symptom mapping, lesioned voxels or ROIs were the independent variables, and MBSImP components, oral total and pharyngeal total sum scores the dependent variables. One-tailed statistical tests were applied based on the assumption that lesioned tissue will lead to impairment and not improvement. For all tests, we used a P-threshold of 0.05 corrected for multiple comparisons with permutation thresholding (5000 permutations). After unadjusted lesion symptom mapping, we performed adjusted lesion symptom mapping using the Freedman-Lane multivariable regression approach to model MBSImP components and total sum scores (Winkler et al., 2014) controlling for age and total lesion volume because these factors have previously been shown to impact swallow physiology or stroke outcome in general (Hope et al., 2013; Humbert et al., 2009; Logemann et al., 2000; Rademaker et al., 1998; Turhan et al., 2009; Wu et al., 2015; Yassi et al., 2015). Further, we controlled for number of days between the DW-MRI and MBSS, to control for the potential time-dependent changes in physiological swallow impairment. Thus, with the lesion symptom mapping approach employed in this study, we attempted to elucidate the most critical brain regions for physiological swallow impairments, by modeling the impact of specific lesions locations on the severity of different swallow impairments.
We used the script NiiStat (version 9, released October 2016, Neuroimaging Informatics Tools and Resources Clearinghouse) running on MATLAB (version R2016b) and SPM (version 12) to perform the lesion symptom mapping analyses. For any other statistical analysis, we used SAS statistical software (version 9.4, released 2016, SAS Institute, Inc., Cary, N.C., USA) or IBM SPSS Statistics for Windows (version 24, released 2016, IBM Corp., Armonk, N.Y., USA). Our Institutional Review Board approved the study.
3. Results
3.1. Patient characteristics
We included 68 first-ever ischemic stroke patients with an average age of 68.21 years. The median modified rankin scale score at hospital admission was 0, meaning no disabilities prior to the stroke. The number of days between the MRI and MBSS was 3–4 days. Average lesion volume was 87.57 ml. Table 2 shows the patients' demographic and medical characteristics.
Table 2.
Demographic and medical characteristics of all included stroke patients (N = 68).
| Demographical information | ||
|---|---|---|
| Age, mean (SD; range) | 68.21 (15.23; 28–95) | |
| Gender, N (%) | Female | 36 (53) |
| Male | 32 (47) | |
| Race, N (%) | White or Caucasian | 45 (66) |
| Black or African-American | 21 (31) | |
| Asian | 1 (1.5) | |
| Other/Unknown | 1 (1.5) | |
| Ethnicity, N (%) | Not Hispanic or Latino | 68 (100) |
| Hispanic or Latino | 0 (0) | |
| Status at hospital admission and stroke characteristics | ||
| National institute of health stroke scale, N, mean (SD; range) | 64, 12.75 (6.97; 0–33) | |
| Modified Rankin Scale, N, median (range) | 50, 0 (0–4) | |
| Charlson comorbidity index, mean (SD; range) | 1.00 (1.48; 0–8) | |
| Lesion volume (in ml/cc), mean (SD; range) | 87.57 (95.72; 0.21–360.72) | |
| Hospital course | ||
| Length of hospital stay, mean (SD; range) | 12.04 (12.53; 2–90) | |
| Tissue plasminogen activator N (%) | 33 (49) | |
| Thrombectomy, N (%) | 17 (25) | |
| Intubation, N (%) | 13 (19) | |
| Days of intubation, mean (SD; range) | 6.08 (4.52; 1–18) | |
| Tracheotomy, N (%) | 4 (6) | |
| Percutaneous endoscopic gastrostomy, N (%) | 12 (18) | |
| Time line of MRI and MBSS | ||
| Days between hospital admission and MRI, mean (SD; range) | 1.87 (2.90; 0–17) | |
| Days between hospital admission and MBSS, mean (SD; range) | 5.41 (5.25; 0–23) | |
| Days between MRI and MBSS, mean (SD; range) | 3.47 (4.65; 0–20) | |
MBSS = modified barium swallow study, N = number, SD = standard deviation.
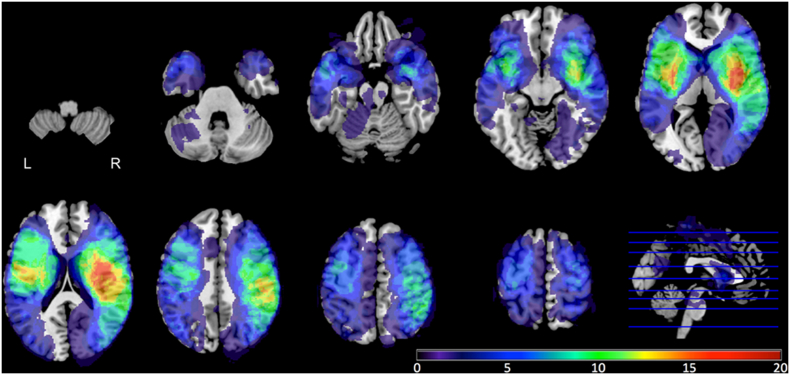
Fig. 1 displays the overlap of the stroke lesions from all 68 patients. Brain regions that were most commonly lesioned were those supplied by the middle cerebral arteries. Only voxels/brain regions that were lesioned in at least seven patients (>10% of 68) were included in the lesion symptom mapping analyses. Regions with the highest average proportional damage across patients were the left external capsule, right external capsule, and right posterior insula.
Fig. 1.
Lesion overlap of all included stroke patients (N = 68). Different colors represent different numbers of patients with lesions in that area.
3.2. Reliability
Intra- and inter-rater reliability measures were established for 14 out of 68 patients (20.6%). Overall, intra-rater reliability was excellent and inter-rater reliability good. Additionally, the average intra-rater agreement across all MBSImP components for overall impression was 85.71% (SD 8.75). For inter-rater reliability, average agreement was 71.85% (SD 14.17).
3.3. MBSImP components and PAS score distributions
None of the MBSImP components or the PAS were normally distributed, and all were dichotomized for neuroimaging analyses. For the MBSImP components a score of “0” was denoted as “not impaired”, scores greater than “0” as “impaired; except for components C1 “lip closure”, C5 “oral residue”, C15 “tongue base retraction” and C16 “pharyngeal residue” where a score of “0” or “1” was denoted as “not impaired”. PAS scores of “2” or less were denoted as “not impaired”, PAS scores of greater than “2” as “impaired”. For seven out of the 17 MBSImP components we did not have sufficient data or data variation for the lesion symptom mapping analyses, leaving ten components for the final analyses. These were components C1 “lip closure”, C4“bolus transport/lingual motion”, C7 “soft palate elevation”, C8 “laryngeal elevation”, C9 “anterior hyoid movement”, C10 “epiglottic movement”, C11 “laryngeal vestibular closure”, C12 “pharyngeal stripping wave”, C15 “tongue base retraction”, and C16 “pharyngeal residue”.
3.4. Lesion symptom mapping results
3.4.1. Modified barium swallow impairment profile
The MBSImP oral total, pharyngeal total sum scores, and the PCA factor scores for the two functional domains of the MBSImP were not associated with lesions to specific voxels or ROIs. When we assessed the MBSImP components separately, four components (C8 “laryngeal closure”, C9 “anterior hyoid excursion, C11 “laryngeal vestibular closure”, C16 “pharyngeal residue”) showed significant results for the adjusted lesion symptom mapping analyses.
3.4.1.1. Component 8 – laryngeal elevation
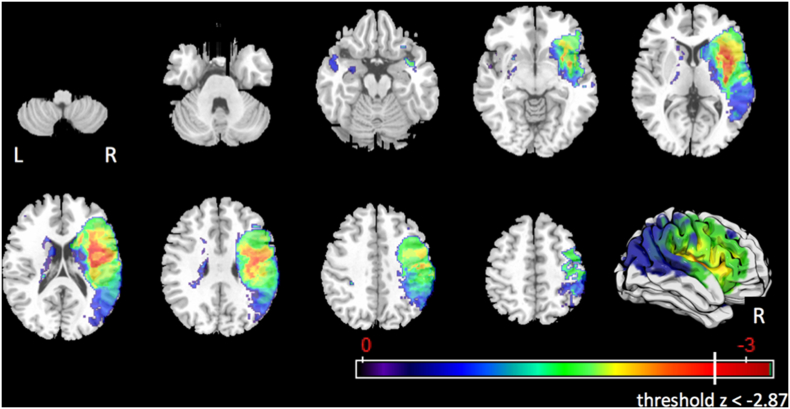
After controlling only for age and number of days between MRI and MBSS, a total of 104 voxels survived the corrected threshold of z < −2.87 for impaired laryngeal elevation (Fig. 2). These voxels were in the right precentral gyrus (0.3%), right anterior insula (4.2%), right posterior insula (2.8%), right external capsule (8.2%), and in addition in the right superior corona radiata (0.2%) and right superior longitudinal fasciculus (0.1%). No voxels or ROIs showed significant associations with impaired laryngeal elevation after controlling for age, number of days between MRI and MBSS, and lesion volume.
Fig. 2.
Statistical map for associations between lesioned voxels and impaired laryngeal elevation after controlling for age and number of days between MRI and MBSS.
3.4.1.2. Component 9 – anterior hyoid excursion
Using ROI-based lesion symptom mapping, three regions survived the corrected threshold of z < −2.78 after controlling for age, number of days between MRI and MBSS, and lesion volume (Fig. 3). These regions were the left amygdala, left ansa lenticularis, and left lenticular fasciculus. There were no significant voxel-impairment relationships for anterior hyoid excursion.
Fig. 3.
Statistical map for associations between lesioned ROIs and impaired anterior hyoid excursion after controlling for age, number of days between MRI and MBSS, and lesion volume.
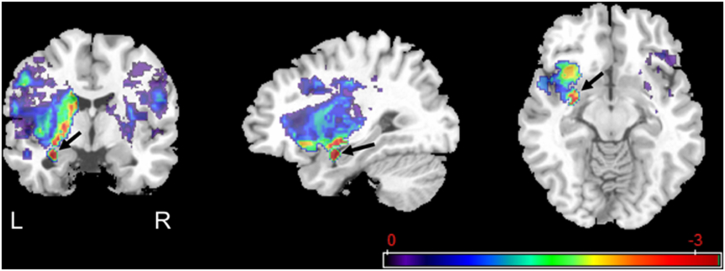
In order to determine the nuclei of the left amygdala that were most likely associated with impaired anterior hyoid excursion, we inspected the location of the lesioned voxels in the left amygdala (Fig. 4). The nuclei of the basolateral and central group showed voxels with z-values closest to the corrected threshold of z < −3.44.
Fig. 4.
Statistical map for associations between lesioned voxels and impaired anterior hyoid excursion after controlling for age and number of days between MRI and MBSS. Arrows are pointing at the left amygdala.
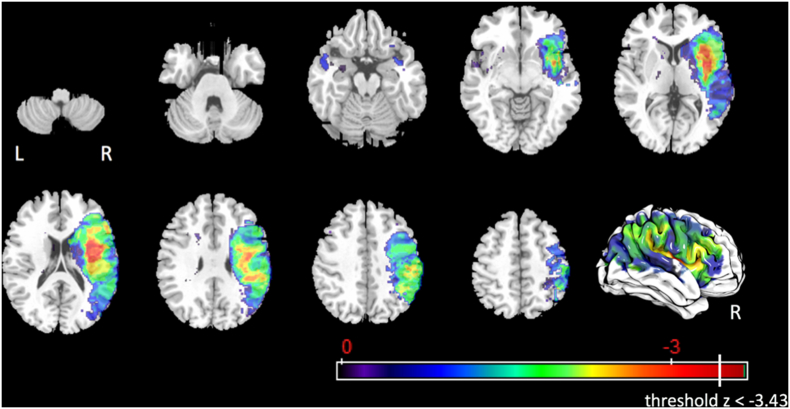
3.4.1.3. Component 11 – laryngeal vestibular closure
In total, 116 voxels survived the corrected threshold of z < −3.43 for impaired laryngeal vestibular closure after controlling for age, number of days between MRI and MBSS, and lesion volume. (Fig. 5). The significant voxels were located in the left postcentral gyrus (1%), left supramarginal gyrus (0.2%), right anterior insula (3.6%), right posterior insula (1.1%), right superior corona radiata (0.1%), and right external capsule (5.5%).
Fig. 5.
Statistical map for associations between lesioned voxels and impaired laryngeal vestibular closure after controlling for age, number of days between MRI and MBSS, and lesion volume.
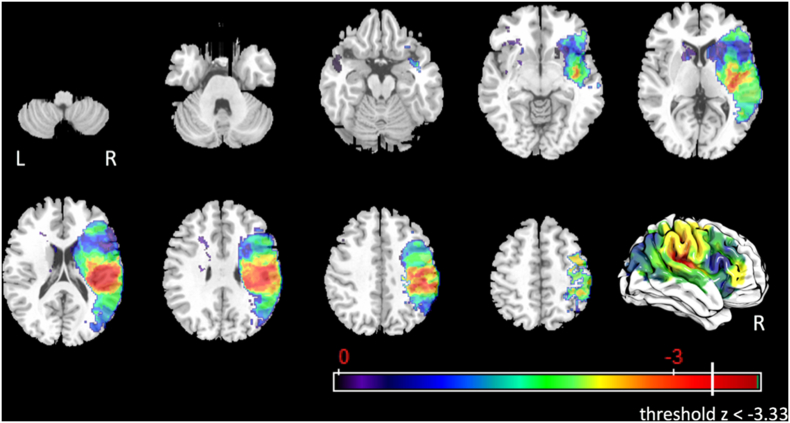
3.4.1.4. Component 16 – pharyngeal residue
In total, 785 voxels survived the corrected threshold of z < −3.33 for pharyngeal residue (Fig. 6) after controlling for age, number of days between MRI and MBSS, and lesion volume. These voxels were in the right postcentral gyrus (3.5%), right supramarginal gyrus (20.2%), right angular gyrus (0.1%), right superior temporal gyrus (1.1%), right superior corona radiata (1.2%), right posterior corona radiata (12.5%), right tapatum (1.7%), posterior limb of right internal capsule (0.2%), retrolenticular part of right internal capsule (3.9%), right superior longitudinal fasciculus (8.8%), right posterior insula (6.0%), and right posterior superior temporal gyrus (1.1%).
Fig. 6.
Statistical map for associations between lesioned voxels and pharyngeal residue after controlling for age, number of days between MRI and MBSS, and lesion volume.
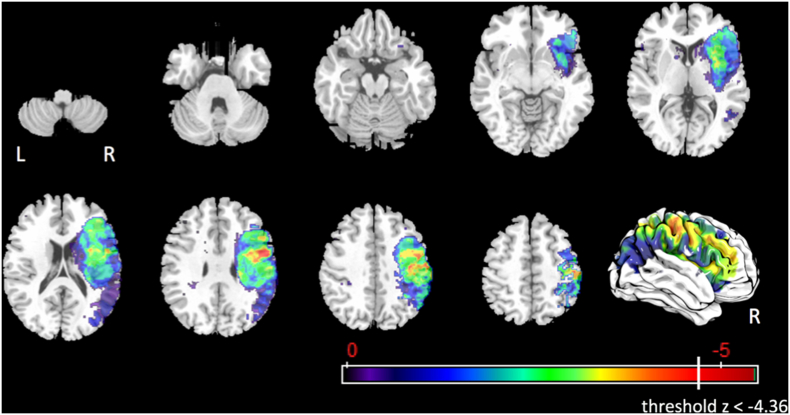
3.4.1.5. Penetration-aspiration scale
After controlling for age, days between MRI and MBSS, and lesion volume, 212 voxels survived the corrected threshold of z < −4.36 for abnormal median penetration-aspiration scores (Fig. 7). These voxels were in the right precentral gyrus (3.1%), right postcentral gyrus (1.1%), right superior longitudinal fasciculus (1.7%), and right supramarginal gyrus (0.1%).
Fig. 7.
Statistical map for associations between lesioned voxels and abnormal median penetration-aspiration scale scores after controlling for age, number of days between MRI and MBSS, and lesion volume.
4. Discussion
The goal of our study was to investigate if there is a direct link between supratentorial stroke lesion locations and impairment in different aspects of swallow physiology, or in other words to dissociate the anatomical locales of lesions with different aspects of swallow impairments and their severity. We sought to provide clinicians with information to improve their ability to diagnose, treat and predict physiological swallow impairment after stroke. Hereby, we responded to recent and urgent requests for further investigations of the complex role of supratentorial brain regions in swallow physiology that can promote a shift of dysphagia diagnostics and treatment from targeting symptoms to neurophysiological underpinnings (Ciucci et al., 2016).
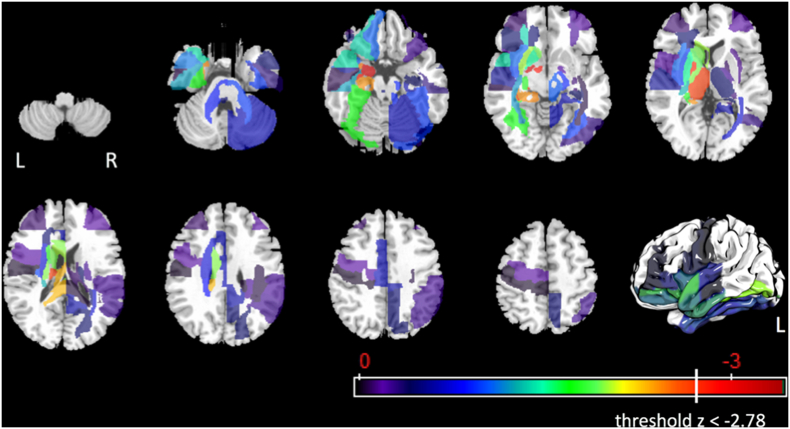
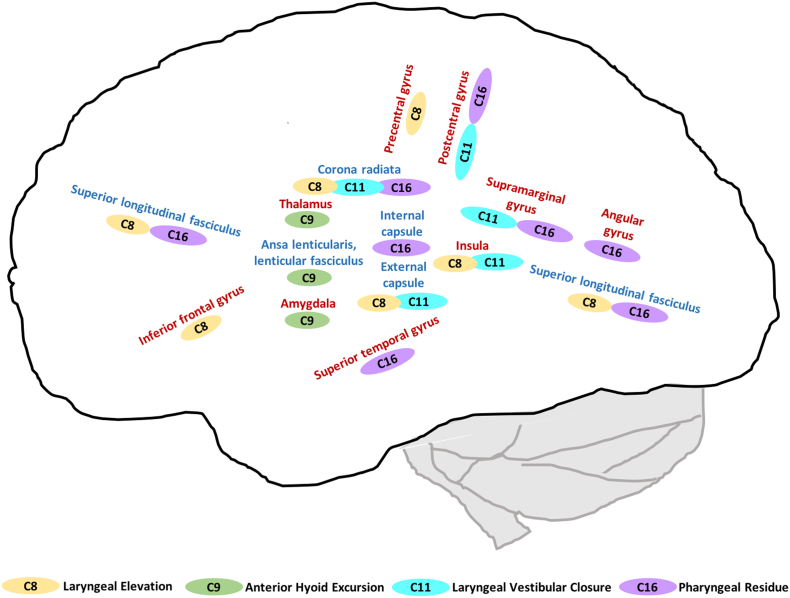
Our primary outcome measures were the 17 components of the MBSImP. For seven MBSImP components, we had either notably missing data or very little data variation. For four of the remaining 10 components we found significant lesion-impairment relationships, these were laryngeal elevation, anterior hyoid excursion, laryngeal vestibular closure and pharyngeal residue. Besides overlapping lesion locations, we also found distinct locations for the four significant physiological swallow impairments, as schematically shown in Fig. 8.
Fig. 8.
Significant lesion-impairment relationships for MBSImP components (red brain regions = grey matter; blue brain regions = white matter; C8 = laryngeal elevation; C9 = anterior hyoid excursion; C11 = laryngeal vestibular closure; C16 = pharyngeal residue).
These four physiological swallow impairments share that they are all pharyngeal components and crucial for swallowing safety. Anterior hyoid excursion is associated with pharyngoesophageal segment opening and inversion of the epiglottis. This movement is based on the traction forces of the submental muscles, especially the geniohyoid muscle (Pearson et al., 2011). Laryngeal elevation comprises an early superior movement of the thyroid cartilage and the subsequent approximation of the arytenoids with the epiglottic petiole. Laryngeal vestibular closure ideally concludes laryngeal elevation and provides airway protection while the bolus is passing the larynx posteriorly and is entering the pharyngoesophageal segment. Interestingly, the components laryngeal elevation and laryngeal vestibular closure shared some of the same significant lesion locations but also showed distinct locations (Fig. 8). Impairment in these two components occurs commonly but not necessarily together (Martin-Harris, 2015); thus, shared but distinct lesion locations support the neurophysiological link between these two components as well as their independence. Oropharyngeal residue is not an impairment per se, but often a consequence or symptom of multiple underlying physiological swallow impairments (e.g., reduced tongue base retraction, pharyngeal contraction, pharyngoesophageal segment opening). This explains the associated multiple brain areas of involvement spanning the fronto-parieto-temporal cortex and subcortical white matter tracts.
4.1. Lesion lateralization
In terms of side of the lesion, most of the significant voxels or ROIs were in the right hemisphere and only comparably few in the left hemisphere. This is in line with previous studies linking right hemisphere strokes to pharyngeal impairment or to more severe dysphagia in general (Daniels et al., 1996; May et al., 2016; Robbins, 1993; Suntrup et al., 2015; Suntrup-Krueger et al., 2017; Wilmskoetter et al., 2018). However, two (anterior hyoid movement, laryngeal vestibular closure) of the four (laryngeal elevation, anterior hyoid movement, laryngeal vestibular closure, pharyngeal residue) significant pharyngeal components were associated with lesions in the left hemisphere; thus, our findings are in line with our previous research emphasizing that lesions in the left hemisphere can also impair pharyngeal swallow physiology (Wilmskoetter et al., 2018). Therefore, our study implicates that bilateral brain networks are crucial for swallow physiology, but that the right hemisphere, especially sensorimotor integration networks, contributes more than the left hemisphere.
4.2. Lesion locations
Several cortical and subcortical grey matter brain regions were linked to one or more MBSImP swallow components. In the following, we will discuss grey and white matter brain regions in descending order of the number of swallow components showing a significant association with that area.
4.2.1. Grey matter lesion locations
Impaired laryngeal elevation and laryngeal vestibular closure were associated with lesions in the insula. Anatomically, the importance of the insula stems from its structural connections to brain areas crucial for swallowing, such as the primary and secondary somatosensory cortex, premotor area, supplementary motor area, frontal operculum, thalamus, anterior cingulate and the nucleus tractus solitarius (Augustine, 1996; Daniels and Foundas, 1997; Humbert and McLaren, 2014; Leopold and Daniels, 2010). Functionally, previous evidence suggests that the insula contributes to processing food taste, texture and temperature (Rolls, 2015). In terms of swallow physiology, the insula has been associated with controlling the timing and synchronization of swallowing motor events by integrating sensory-motor information (Mosier and Bereznaya, 2001), for example during the initiation of the pharyngeal swallow (Daniels and Foundas, 1997; Riecker et al., 2009; Stickler et al., 2003; Watanabe, 2004). Our findings present new information on the role of the insula in distinct mechanisms of airway protection during swallowing.
Impaired laryngeal vestibular closure and pharyngeal residue were associated with lesions in the supramarginal gyrus and pharyngeal residue with angular gyrus lesions. Our results replicate previous findings on the relationship of oropharyngeal residue and lesions in these cortices (Suntrup-Krueger et al., 2017) and also provide new information about lesions in the regions. The functional role of these cortices for swallowing remains speculative, but likely relates to their contribution to sensorimotor integration during swallowing (Suntrup-Krueger et al., 2017). In particular the right supramarginal gyrus has been linked to proprioception (e.g., of the wrist), that might be related to its role in motor control and spatial processing (Ben-Shabat et al., 2015). Swallowing requires a detailed spatial and temporal orchestration of swallow musculature and structures in relation to a bolus that is being propelled through the oropharynx into the esophagus. Thus, lesions to the supramarginal gyrus might result in coordination deficits during swallowing.
Impaired laryngeal vestibular closure and pharyngeal residue were associated with lesions in the postcentral gyrus and impaired laryngeal elevation was associated with lesions in the precentral gyrus. These areas play a major role in decoding and encoding sensory-motor information and regulate the swallowing output of the brainstem through descending and ascending fiber tracts. Previous studies have related lesions or disruptions of the primary motor and sensory cortices to swallow disturbances (Mistry et al., 2007; Suntrup et al., 2015; Suntrup-Krueger et al., 2017; Teismann et al., 2007). Our study emphasizes the importance of afferent sensory information for swallowing safety.
Lesions in the inferior frontal gyrus (pars opercularis) were associated with impaired laryngeal elevation. Previous activation studies found the inferior frontal gyrus to be activated during swallowing (Dziewas, 2003; Humbert et al., 2009; Mosier and Bereznaya, 2001) and lesion studies found a relationship between lesions in the inferior frontal gyrus and risk for aspiration (Galovic et al., 2013) as well as prolonged impaired oral intake after stroke (Galovic et al., 2017). We speculate that laryngeal elevation might be the underlying physiological impairment that resulted in the observed risk for aspiration after lesions to the inferior frontal gyrus as reported by others (Galovic et al., 2013).
Our findings on the association between lesions in the superior temporal lobe and pharyngeal residue are in line with a previous lesion symptom mapping study using fiberendoscopic evaluation of swallowing (Suntrup-Krueger et al., 2017) as well as with functional MRI studies that showed activation in this area during swallowing in healthy individuals (Humbert et al., 2009; Mosier, 1999). The exact role of the temporal lobe in swallowing control remains speculative but is likely related to its rich reciprocal connections with the frontal, parietal and occipital lobe, and nuclei of the thalamus (Kiernan, 2012).
The thalamus is believed to be a central hub to relay and process afferent and efferent signals between subcortical and cortical brain regions and likely contributes to the sensory-motor integration during swallowing (Mosier, 1999). We found a significant association between lesions in the thalamus and impaired anterior hyoid excursion. Further, we identified the amygdala, and more specifically the nuclei of the basolateral and central group of the amygdala, to contribute to impaired anterior hyoid excursion. Besides being involved in gustatory and somatosensory processing, these locations might be crucial for anterior hyoid excursion because of their connections to the striatum (basolateral nuclei), pons and medulla (central nuclei) (Sah et al., 2003).
4.2.2. White matter lesion locations
There were three white matter structures that were related to more than one physiological swallow component. These were the corona radiata (related to three components), the superior longitudinal fasciculus and external capsule (both related to two components). Other white matter structures, the ansa lenticularis and lenticular fasciculus, were associated with only one swallow component.
Lesions to the corona radiata were associated with impaired laryngeal elevation, laryngeal vestibular closure and pharyngeal residue. Lesions in the corona radiata have been strongly associated in previous studies with impaired oral intake after stroke (Galovic et al., 2016, Galovic et al., 2017). The corona radiata carries ascending and descending projection fibers between the cortex and brainstem and thus, lesions here can lead to interruptions of the sensory input to the cortex as well as to interruptions of the cortical control of the motor output of the brainstem.
In our study, lesions in the superior longitudinal fasciculus were related to impaired laryngeal elevation and pharyngeal residue. Previous studies showed a relation to impaired “swallow response” (Suntrup-Krueger et al., 2017) and impaired oral intake (Galovic et al., 2016). The superior longitudinal fasciculus connects areas in the frontal, parietal, temporal and occipital lobe. For example, parts of the superior longitudinal fasciculus connect the supramarginal gyrus with the premotor and prefrontal cortex and transfers somatosensory information. Interestingly, the superior longitudinal fasciculus was significantly associated with the exact same physiological swallow components as the supramarginal gyrus (impaired laryngeal elevation and pharyngeal residue). Thus, it may be that damage to the supramarginal gyrus or the superior longitudinal fasciculus disrupts the input from the parietal sensory-motor brain regions to the frontal motor-coordination and motor-initiation areas (e.g., supplementary motor area, premotor and primary motor cortices). This disruption could be considered a contributor to a possible swallowing dyspraxia syndrome that warrants further investigation (Daniels, 2000).
Prior studies have related lesions in the external capsule with post-stroke oral intake impairment (Galovic et al., 2016, Galovic et al., 2017). Our study adds to the understanding of the role of the external capsule by showing a relationship between lesions in the external capsule and impaired laryngeal elevation and laryngeal vestibular closure. The external capsule includes fibers connecting the primary sensorimotor cortex with the putamen and the supplementary motor area with the caudate nucleus (Schmahmann et al., 2008). Thus, it is believed that the external capsule is a critical link between cortical motor regions and the basal ganglia and contributes to the engagement of the basal ganglia in motor control (Schmahmann et al., 2008).
Lesions to the ansa lenticularis and lenticular fasciculus were associated with impaired anterior hyoid excursion. These tracts form together the pallidothalamic fibers connecting the globus pallidus with the thalamus. The ansa lenticularis and lenticular fasciculus contribute in sending information from the basal ganglia for movement planning – such as swallowing – to the executing motor areas in the frontal lobe. As we speculated earlier, impairment in anterior hyoid excursion might be particularly associated with lesions involving areas responsible in sensory-motor integration, such as the amygdala, thalamus, and pallidothalamic fibers.
4.2.3. Additional analyses
In contrast to significant lesion-impairment relationships for separate swallow components, we did not find any relationships between global swallow impairment scores – MBSImP oral total and pharyngeal total sum scores, PCA factor scores – and lesion locations. This is in line with a recently published study investigating associations between lesion location (supratentorial vs. infratentorial), lesion lateralization (right vs. left vs. bilateral), and MBSImP oral total and pharyngeal total sum scores (Daniels et al., 2017). This supports our hypothesis that lesion locations have a distinct impact on impairment in different aspects of swallow physiology.
To further confirm the validity of our results in using the MBSImP as a tool to determine physiological swallow impairment, we additionally performed lesion symptom mapping analyses on swallow timing, distance, area and speed measures. For up to 5 different swallow types, we obtained 17 different timing measures (e.g. pharyngeal transit time (Logemann et al., 1993), stage transition duration (Daniels et al., 2009)), 10 different distance and area measures (e.g. hyoid excursion (Thompson et al., 2014), pharyngeal constriction ratio (Leonard et al., 2011)), and measures for hyoid speed (Barikroo et al., 2015; Nagy et al., 2015). We found significant lesion-impairment relationships for only three out of 85 timing measures, two out of 50 distance and area measures, and none of the hyoid speed measures. This supports our choice of the MBSImP in order to detect physiological swallow impairments that are linked to neurophysiological correlates.
4.3. Limitations
Our study has limitations that might explain why we were not able to identify lesion-impairment relationships for more than four swallow components. Only a few patients in our study had infratentorial lesions and thus, we were not able to investigate lesion-impairment relationships in the brainstem or cerebellum.
Further, the likelihood to reveal existing lesion-impairment relationships depends on factors such as the variation in lesion locations across subjects and the number of patients with lesions in a specific brain region. For instance, given the constraints of vascular anatomy, two brain regions may be commonly lesioned together, and thus we might have failed to differentiate the impact of each region on the observed impairment. In addition, some regions like the insula are more likely lesioned following a stroke than others. Thus, the statistical power is higher in the insula given the higher number of voxels involved in the lesion-symptom mapping analyses. To some extent, this may bias the insular involvement with behavioral functions such as swallowing, but also language and spatial perception. This dissociation is at times difficult (Bonilha and Fridriksson, 2009) and requires the evaluation of diaschisis or symptoms in the absence of lesions (Hillis et al., 2004). A future, dedicated and focused study on insular lesions would be a natural follow-up to our current and more comprehensive study.
Moreover, we had missing data or little data variation in seven of the 17 MBSImP components impeding the lesion-impairment analyses. Reasons for missing data were based on the retrospective design of our study and on clinical and technical constraints during the MBSS.
4.4. Clinical implications
Clinicians can use our results on the relationships between lesion locations and physiological swallow impairments as an adjunct to their clinical swallowing assessment to identify patients at risk for dysphagia and to determine the underlying physiological swallow impairment before access to a MBSS. Furthermore, our results might open future avenues to develop new treatment approaches that consider lesion-impairment associations. For example, neuromodulation techniques could target specific brain areas depending on the individual patient's physiological swallow impairment.
5. Conclusions
To our knowledge, we present the first study on lesion symptom mapping of physiological swallow impairments across the continuum of the oropharyngeal swallow in acute stroke patients. Our study revealed that associations exist between distinct supratentorial lesion locations and pharyngeal swallow components. Future studies are needed to validate our results and to expand on our findings with complimentary methods to aid to the first-ever development of a neuroanatomical model of physiological swallow impairment after stroke and to support the development of new treatment approaches focusing on the neurophysiological axis of post-stroke dysphagia.
Acknowledgments
Acknowledgement
We would like to thank Dr. William G. Pearson Jr., Ph.D., for providing resources and instructions on conducting the distance and area swallowing measures, and Dr. Grigori Yourganov, Ph.D., for his help with questions regarding the NiiStat software used for the neuroimaging analysis.
Conflict of interest statement
Janina Wilmskoetter receives salary from the Medical University of South Carolina. Leonardo Bonilha reports no disclosures. Bonnie Martin-Harris is funded by NIH/NIDCD grants #1K24DC12801 and #1R01DC011290, receives research support from Bracco Diagnostics, and holds copyright royalties from Northern Speech Services and the Medical University of South Carolina. Jordan J. Elm reports no disclosures. Janet Horn receives a stipend/salary from the Medical University of South Carolina. Heather S. Bonilha is funded by NIH/NIDDK grant #R01 DK098222, by the Center for Biomedical Research Excellence (COBRE) in Stroke Recovery at the Medical University of South Carolina (MUSC) in Charleston, South Carolina, #5P20GM109040, serves as an editorial board member for ASHA journal, serves as a grant reviewer for NIH, and receives salary from the Medical University of South Carolina.
Funding
The project described was supported in part by the National Institutes of Health/National Center for Advancing Translational Sciences (NCATS) through Grant Number UL1 TR001450, SCTR Pilot Project 17254, Center for Biomedical Research Excellence (COBRE) in Stroke Recovery at the Medical University of South Carolina (MUSC) in Charleston, South Carolina through Grant Number 5P20GM109040, and National Institutes of Health/National Institute on Deafness and Other Communication Disorders (NIDCD) through Grant Number T32DC014435.
References
- Arnold M., Liesirova K., Broeg-Morvay A., Meisterernst J., Schlager M., Mono M.L., El-Koussy M., Kagi G., Jung S., Sarikaya H. Dysphagia in acute stroke: incidence, burden and impact on clinical outcome. PLoS One. 2016;11 doi: 10.1371/journal.pone.0148424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Augustine J.R. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res. Brain Res. Rev. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Barikroo A., Carnaby G., Crary M. Effects of age and bolus volume on velocity of hyolaryngeal excursion in healthy adults. Dysphagia. 2015;30:558–564. doi: 10.1007/s00455-015-9637-y. [DOI] [PubMed] [Google Scholar]
- Benjamin E.J., Blaha M.J., Chiuve S.E., Cushman M., Das S.R., Deo R., de Ferranti S.D., Floyd J., Fornage M., Gillespie C., Isasi C.R., Jimenez M.C., Jordan L.C., Judd S.E., Lackland D., Lichtman J.H., Lisabeth L., Liu S., Longenecker C.T., Mackey R.H., Matsushita K., Mozaffarian D., Mussolino M.E., Nasir K., Neumar R.W., Palaniappan L., Pandey D.K., Thiagarajan R.R., Reeves M.J., Ritchey M., Rodriguez C.J., Roth G.A., Rosamond W.D., Sasson C., Towfighi A., Tsao C.W., Turner M.B., Virani S.S., Voeks J.H., Willey J.Z., Wilkins J.T., Wu J.H., Alger H.M., Wong S.S., Muntner P. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shabat E., Matyas T.A., Pell G.S., Brodtmann A., Carey L.M. The right supramarginal gyrus is important for proprioception in healthy and stroke-affected participants: a functional MRI study. Front. Neurol. 2015;6 doi: 10.3389/fneur.2015.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L., Fridriksson J. Subcortical damage and white matter disconnection associated with non-fluent speech. Brain. 2009;132:e108. doi: 10.1093/brain/awn200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha H.S., Simpson A.N., Ellis C., Mauldin P., Martin-Harris B., Simpson K. The one-year attributable cost of post-stroke dysphagia. Dysphagia. 2014;29:545–552. doi: 10.1007/s00455-014-9543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Prevalence and most common causes of disability among adults--United States, 2005. MMWR Morb. Mortal. Wkly Rep. 2009;58:421–426. [PubMed] [Google Scholar]
- Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Ciucci M., Jones C.A., Malandraki G.A., Hutcheson K.A. Dysphagia practice in 2035: beyond fluorography, thickener, and electrical stimulation. Semin. Speech Lang. 2016;37:201–218. doi: 10.1055/s-0036-1584155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cola M.G., Daniels S.K., Corey D.M., Lemen L.C., Romero M., Foundas A.L. Relevance of subcortical stroke in dysphagia. Stroke. 2010;41:482–486. doi: 10.1161/STROKEAHA.109.566133. [DOI] [PubMed] [Google Scholar]
- Crary M.A., Humphrey J.L., Carnaby-Mann G., Sambandam R., Miller L., Silliman S. Dysphagia, nutrition, and hydration in ischemic stroke patients at admission and discharge from acute care. Dysphagia. 2013;28:69–76. doi: 10.1007/s00455-012-9414-0. [DOI] [PubMed] [Google Scholar]
- Daniels S.K. Swallowing apraxia: a disorder of the praxis system? Dysphagia. 2000;15:159–166. doi: 10.1007/s004550010019. [DOI] [PubMed] [Google Scholar]
- Daniels S.K., Foundas A.L. The role of the insular cortex in dysphagia. Dysphagia. 1997;12(3):146–156. doi: 10.1007/PL00009529. [DOI] [PubMed] [Google Scholar]
- Daniels S.K., Foundas A.L. Lesion localization in acute stroke patients with risk of aspiration. J. Neuroimaging. 1999;9(2):91–98. doi: 10.1111/jon19999291. [DOI] [PubMed] [Google Scholar]
- Daniels S.K., Huckabee M.L. Plural Publishing; San Diego: 2008. Dysphagia Following Stroke. [Google Scholar]
- Daniels S.K., Foundas A.L., Iglesia G.C. Lesion site in unilateral stroke patients with dysphagia. J. Stroke Cerebrovasc. Dis. 1996;6:30–34. doi: 10.1016/s1052-3057(96)80023-1. [DOI] [PubMed] [Google Scholar]
- Daniels S.K., Corey D.M., Fraychinaud A., DePolo A., Foundas A.L. Swallowing lateralization: the effects of modified dual-task interference. Dysphagia. 2006;21:21–27. doi: 10.1007/s00455-005-9007-2. [DOI] [PubMed] [Google Scholar]
- Daniels S.K., Schroeder M.F., DeGeorge P.C., Corey D.M., Foundas A.L., Rosenbek J.C. Defining and measuring dysphagia following stroke. Am. J. Speech Lang. Pathol. 2009;18:74–81. doi: 10.1044/1058-0360(2008/07-0040). [DOI] [PubMed] [Google Scholar]
- Daniels S.K., Pathak S., Mukhi S.V., Stach C.B., Morgan R.O., Anderson J.A. The relationship between lesion localization and dysphagia in acute stroke. Dysphagia. 2017;32:777–784. doi: 10.1007/s00455-017-9824-0. [DOI] [PubMed] [Google Scholar]
- Dziewas R. Neuroimaging evidence for cortical involvement in the preparation and in the act of swallowing. NeuroImage. 2003;20(1):135–144. doi: 10.1016/s1053-8119(03)00285-4. [DOI] [PubMed] [Google Scholar]
- Ekberg O., Hamdy S., Woisard V., Wuttge-Hannig A., Ortega P. Social and psychological burden of dysphagia: its impact on diagnosis and treatment. Dysphagia. 2002;17:139–146. doi: 10.1007/s00455-001-0113-5. [DOI] [PubMed] [Google Scholar]
- Faria A.V., Joel S.E., Zhang Y., Oishi K., van Zjil P.C., Miller M.I., Pekar J.J., Mori S. Atlas-based analysis of resting-state functional connectivity: evaluation for reproducibility and multi-modal anatomy-function correlation studies. NeuroImage. 2012;61:613–621. doi: 10.1016/j.neuroimage.2012.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiss J.L. Wikey; New York, NY: 1986. The Design and Analysis of Clinical Experiments. [Google Scholar]
- Flowers H.L., Skoretz S.A., Streiner D.L., Silver F.L., Martino R. MRI-based neuroanatomical predictors of dysphagia after acute ischemic stroke: a systematic review and meta-analysis. Cerebrovasc. Dis. 2011;32:1–10. doi: 10.1159/000324940. [DOI] [PubMed] [Google Scholar]
- Flowers H.L., AlHarbi M.A., Mikulis D., Silver F.L., Rochon E., Streiner D., Martino R. MRI-based neuroanatomical predictors of dysphagia, dysarthria, and aphasia in patients with first acute ischemic stroke. Cerebrovasc. Dis. Extra. 2017;7:21–34. doi: 10.1159/000457810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galovic M., Leisi N., Muller M., Weber J., Abela E., Kagi G., Weder B. Lesion location predicts transient and extended risk of aspiration after supratentorial ischemic stroke. Stroke. 2013;44:2760–2767. doi: 10.1161/STROKEAHA.113.001690. [DOI] [PubMed] [Google Scholar]
- Galovic M., Leisi N., Muller M., Weber J., Tettenborn B., Brugger F., Abela E., Weder B., Kagi G. Neuroanatomical correlates of tube dependency and impaired oral intake after hemispheric stroke. Eur. J. Neurol. 2016;23:926–934. doi: 10.1111/ene.12964. [DOI] [PubMed] [Google Scholar]
- Galovic M., Leisi N., Pastore-Wapp M., Zbinden M., Vos S.B., Mueller M., Weber J., Brugger F., Kagi G., Weder B.J. Diverging lesion and connectivity patterns influence early and late swallowing recovery after hemispheric stroke. Hum. Brain Mapp. 2017;38:2165–2176. doi: 10.1002/hbm.23511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Fernandez M., Kleinman J.T., Ky P.K., Palmer J.B., Hillis A.E. Supratentorial regions of acute ischemia associated with clinically important swallowing disorders: a pilot study. Stroke. 2008;39:3022–3028. doi: 10.1161/STROKEAHA.108.518969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelwood R.J., Armeson K.E., Hill E.G., Bonilha H.S., Martin-Harris B. Identification of swallowing tasks from a modified barium swallow study that optimize the detection of physiological impairment. J. Speech Lang. Hear. Res. 2017;60:1855–1863. doi: 10.1044/2017_JSLHR-S-16-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis A.E., Work M., Barker P.B., Jacobs M.A., Breese E.L., Maurer K. Re-examining the brain regions crucial for orchestrating speech articulation. Brain. 2004;127:1479–1487. doi: 10.1093/brain/awh172. [DOI] [PubMed] [Google Scholar]
- Hope T.M., Seghier M.L., Leff A.P., Price C.J. Predicting outcome and recovery after stroke with lesions extracted from MRI images. Neuroimage Clin. 2013;2:424–433. doi: 10.1016/j.nicl.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert I.A., McLaren D.G. Differential psychophysiological interactions of insular subdivisions during varied oropharyngeal swallowing tasks. Physiol. Rep. 2014;2 doi: 10.1002/phy2.239. (n/a-n/a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert I.A., Fitzgerald M.E., McLaren D.G., Johnson S., Porcaro E., Kosmatka K., Hind J., Robbins J. Neurophysiology of swallowing: effects of age and bolus type. NeuroImage. 2009;44:982–991. doi: 10.1016/j.neuroimage.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon W.H., Park G.W., Lee J.H., Jeong H.J., Sim Y.J. Association between location of brain lesion and clinical factors and findings of videofluoroscopic swallowing study in subacute stroke patients. Brain Neurorehabil. 2014;7:54. [Google Scholar]
- Kiernan J.A. Anatomy of the temporal lobe. Epilepsy Res. Treat. 2012;2012:12. doi: 10.1155/2012/176157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R., Rees C.J., Belafsky P., Allen J. Fluoroscopic surrogate for pharyngeal strength: the pharyngeal constriction ratio (PCR) Dysphagia. 2011;26:13–17. doi: 10.1007/s00455-009-9258-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold N.A., Daniels S.K. Supranuclear control of swallowing. Dysphagia. 2010;25:250–257. doi: 10.1007/s00455-009-9249-5. [DOI] [PubMed] [Google Scholar]
- Li S., Luo C., Yu B., Yan B., Gong Q., He C., He L., Huang X., Yao D., Lui S., Tang H., Chen Q., Zeng Y., Zhou D. Functional magnetic resonance imaging study on dysphagia after unilateral hemispheric stroke: a preliminary study. J. Neurol. Neurosurg. Psychiatry. 2009;80:1320–1329. doi: 10.1136/jnnp.2009.176214. [DOI] [PubMed] [Google Scholar]
- Li X., Morgan P.S., Ashburner J., Smith J., Rorden C. The first step for neuroimaging data analysis: DICOM to NIfTI conversion. J. Neurosci. Methods. 2016;264:47–56. doi: 10.1016/j.jneumeth.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Logemann J.A. 1998. Evaluation and Treatment of Swallowing Disorders. [Google Scholar]
- Logemann J.A., Shanahan T., Rademaker A.W., Kahrilas P.J., Lazar R., Halper A. Oropharyngeal swallowing after stroke in the left basal ganglion/internal capsule. Dysphagia. 1993;8:230–234. doi: 10.1007/BF01354543. [DOI] [PubMed] [Google Scholar]
- Logemann J.A., Pauloski B.R., Rademaker A.W., Colangelo L.A., Kahrilas P.J., Smith C.H. Temporal and biomechanical characteristics of oropharyngeal swallow in younger and older men. J. Speech Lang. Hear. Res. 2000;43:1264–1274. doi: 10.1044/jslhr.4305.1264. [DOI] [PubMed] [Google Scholar]
- Marian T., Schroder J.B., Muhle P., Claus I., Riecker A., Warnecke T., Suntrup-Krueger S., Dziewas R. Pharyngolaryngeal sensory deficits in patients with middle cerebral artery infarction: lateralization and relation to overall dysphagia severity. Cerebrovasc. Dis. Extra. 2017;7:130–139. doi: 10.1159/000479483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marik P.E., Kaplan D. Aspiration pneumonia and dysphagia in the elderly. Chest. 2003;124:328–336. doi: 10.1378/chest.124.1.328. [DOI] [PubMed] [Google Scholar]
- Martin-Harris B. Northern Speech Services, Inc; Gaylord, MI: 2015. Standardized Training in Swallowing Physiology: Evidence-Based Assessment Using the Modified Barium Swallowing Impairment Profile (MBSImP™) Approach. [Google Scholar]
- Martin-Harris B., Logemann J.A., McMahon S., Schleicher M., Sandidge J. Clinical utility of the modified barium swallow. Dysphagia. 2000;15:136–141. doi: 10.1007/s004550010015. [DOI] [PubMed] [Google Scholar]
- Martin-Harris B., Brodsky M.B., Michel Y., Castell D.O., Schleicher M., Sandidge J., Maxwell R., Blair J. MBS measurement tool for swallow impairment--MBSImp: establishing a standard. Dysphagia. 2008;23:392–405. doi: 10.1007/s00455-008-9185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino R., Foley N., Bhogal S., Diamant N., Speechley M., Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36:2756–2763. doi: 10.1161/01.STR.0000190056.76543.eb. [DOI] [PubMed] [Google Scholar]
- May N.H., Pisegna J.M., Marchina S., Langmore S.E., Kumar S., Pearson W.G., Jr. Pharyngeal swallowing mechanics secondary to hemispheric stroke. J. Stroke Cerebrovasc. Dis. 2016;26:952–961. doi: 10.1016/j.jstrokecerebrovasdis.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.J. The neurobiology of swallowing and dysphagia. Dev. Disabil. Res. Rev. 2008;14:77–86. doi: 10.1002/ddrr.12. [DOI] [PubMed] [Google Scholar]
- Mistry S., Verin E., Singh S., Jefferson S., Rothwell J.C., Thompson D.G., Hamdy S. Unilateral suppression of pharyngeal motor cortex to repetitive transcranial magnetic stimulation reveals functional asymmetry in the hemispheric projections to human swallowing. J. Physiol. 2007;585:525–538. doi: 10.1113/jphysiol.2007.144592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H.I., Pyun S.B., Kwon H.K. Correlation between location of brain lesion and cognitive function and findings of videofluoroscopic swallowing study. Ann. Rehabil. Med. 2012;36:347–355. doi: 10.5535/arm.2012.36.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier K.M. Lateralization of cortical function in swallowing: a functional MR imaging study. Am. J. Neuroradiol. 1999;20(8):1520–1526. [PMC free article] [PubMed] [Google Scholar]
- Mosier K., Bereznaya I. Parallel cortical networks for volitional control of swallowing in humans. Exp. Brain Res. 2001;140:280–289. doi: 10.1007/s002210100813. [DOI] [PubMed] [Google Scholar]
- Nachev P., Coulthard E., Jager H.R., Kennard C., Husain M. Enantiomorphic normalization of focally lesioned brains. NeuroImage. 2008;39:1215–1226. doi: 10.1016/j.neuroimage.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A., Molfenter S.M., Peladeau-Pigeon M., Stokely S., Steele C.M. The effect of bolus consistency on hyoid velocity in healthy swallowing. Dysphagia. 2015;30:445–451. doi: 10.1007/s00455-015-9621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northern Speech Services . 2010. Modified Barium Swallow Impairment Profile. [Google Scholar]
- Pearson W.G., Jr., Langmore S.E., Zumwalt A.C. Evaluating the structural properties of suprahyoid muscles and their potential for moving the hyoid. Dysphagia. 2011;26:345–351. doi: 10.1007/s00455-010-9315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan H., Sundararajan V., Halfon P., Fong A., Burnand B., Luthi J.C., Saunders L.D., Beck C.A., Feasby T.E., Ghali W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- Quan H., Li B., Couris C.M., Fushimi K., Graham P., Hider P., Januel J.M., Sundararajan V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011;173:676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- Rademaker A.W., Pauloski B.R., Colangelo L.A., Logemann J.A. Age and volume effects on liquid swallowing function in normal women. J. Speech Lang. Hear. Res. 1998;41:275–284. doi: 10.1044/jslhr.4102.275. [DOI] [PubMed] [Google Scholar]
- Riecker A., Gastl R., Kuhnlein P., Kassubek J., Prosiegel M. Dysphagia due to unilateral infarction in the vascular territory of the anterior insula. Dysphagia. 2009;24:114–118. doi: 10.1007/s00455-008-9164-1. [DOI] [PubMed] [Google Scholar]
- Robbins J. Swallowing after unilateral stroke of the cerebral cortex. Arch. Phys. Med. Rehabil. 1993;74(12):1295–1300. doi: 10.1016/0003-9993(93)90082-l. [DOI] [PubMed] [Google Scholar]
- Rolls E.T. Functions of the anterior insula in taste, autonomic, and related functions. Brain Cogn. 2015;110:4–19. doi: 10.1016/j.bandc.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Rorden C., Bonilha L., Fridriksson J., Bender B., Karnath H.O. Age-specific CT and MRI templates for spatial normalization. NeuroImage. 2012;61:957–965. doi: 10.1016/j.neuroimage.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbek J.C., Robbins J.A., Roecker E.B., Coyle J.L., Wood J.L. A penetration-aspiration scale. Dysphagia. 1996;11:93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- Sah P., Faber E.S., Lopez De Armentia M., Power J. The amygdaloid complex: anatomy and physiology. Physiol. Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D., Ko R., MacMore J. The human basis pontis: motor syndromes and topographic organization. Brain. 2004;127:1269–1291. doi: 10.1093/brain/awh138. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D., Smith E.E., Eichler F.S., Filley C.M. Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann. N. Y. Acad. Sci. 2008;1142:266–309. doi: 10.1196/annals.1444.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma J.C., Fletcher S., Vassallo M., Ross I. What influences outcome of stroke--pyrexia or dysphagia? Int. J. Clin. Pract. 2001;55:17–20. [PubMed] [Google Scholar]
- Shaw S.M., Martino R. The normal swallow: muscular and neurophysiological control. Otolaryngol. Clin. N. Am. 2013;46:937–956. doi: 10.1016/j.otc.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Steinhagen V., Grossmann A., Benecke R., Walter U. Swallowing disturbance pattern relates to brain lesion location in acute stroke patients. Stroke. 2009;40:1903–1906. doi: 10.1161/STROKEAHA.108.535468. [DOI] [PubMed] [Google Scholar]
- Stickler D., Gilmore R., Rosenbek J.C., Donovan N.J. Dysphagia with bilateral lesions of the insular cortex. Dysphagia. 2003;18:179–181. doi: 10.1007/s00455-002-0103-2. [DOI] [PubMed] [Google Scholar]
- Suntrup S., Kemmling A., Warnecke T., Hamacher C., Oelenberg S., Niederstadt T., Heindel W., Wiendl H., Dziewas R. The impact of lesion location on dysphagia incidence, pattern and complications in acute stroke. Part 1: dysphagia incidence, severity and aspiration. Eur. J. Neurol. 2015;22:832–838. doi: 10.1111/ene.12670. [DOI] [PubMed] [Google Scholar]
- Suntrup-Krueger S., Kemmling A., Warnecke T., Hamacher C., Oelenberg S., Niederstadt T., Heindel W., Wiendl H., Dziewas R. The impact of lesion location on dysphagia incidence, pattern and complications in acute stroke. Part 2: Oropharyngeal residue, swallow and cough response, and pneumonia. Eur. J. Neurol. 2017;24:867–874. doi: 10.1111/ene.13307. [DOI] [PubMed] [Google Scholar]
- Teismann I.K., Steinstraeter O., Stoeckigt K., Suntrup S., Wollbrink A., Pantev C., Dziewas R. Functional oropharyngeal sensory disruption interferes with the cortical control of swallowing. BMC Neurosci. 2007;8:62. doi: 10.1186/1471-2202-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson T.Z., Obeidin F., Davidoff A.A., Hightower C.L., Johnson C.Z., Rice S.L., Sokolove R.L., Taylor B.K., Tuck J.M., Pearson W.G., Jr. Coordinate mapping of hyolaryngeal mechanics in swallowing. J. Vis. Exp. 2014;87:51476. doi: 10.3791/51476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turhan N., Atalay A., Muderrisoglu H. Predictors of functional outcome in first-ever ischemic stroke: a special interest to ischemic subtypes, comorbidity and age. NeuroRehabilitation. 2009;24:321–326. doi: 10.3233/NRE-2009-0485. [DOI] [PubMed] [Google Scholar]
- Watanabe Y. Cortical regulation during the early stage of initiation of voluntary swallowing in humans. Dysphagia. 2004;19(2):100–108. doi: 10.1007/s00455-003-0509-5. [DOI] [PubMed] [Google Scholar]
- Wilmskoetter J., Simpson A.N., Simpson K.N., Bonilha H.S. Practice patterns of percutaneous endoscopic gastrostomy tube placement in acute stroke: are the guidelines achievable? J. Stroke Cerebrovasc. Dis. 2016;25:2694–2700. doi: 10.1016/j.jstrokecerebrovasdis.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmskoetter J., Martin-Harris B., Pearson W.G., Jr., Bonilha L., Elm J.J., Horn J., Bonilha H.S. Differences in swallow physiology in patients with left and right hemispheric strokes. Physiol. Behav. 2018;194:144–152. doi: 10.1016/j.physbeh.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu O., Cloonan L., Mocking S.J., Bouts M.J., Copen W.A., Cougo-Pinto P.T., Fitzpatrick K., Kanakis A., Schaefer P.W., Rosand J., Furie K.L., Rost N.S. Role of acute lesion topography in initial ischemic stroke severity and long-term functional outcomes. Stroke. 2015;46:2438–2444. doi: 10.1161/STROKEAHA.115.009643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassi N., Churilov L., Campbell B.C., Sharma G., Bammer R., Desmond P.M., Parsons M.W., Albers G.W., Donnan G.A., Davis S.M., EPITHET, Investigators, DEFUSE The association between lesion location and functional outcome after ischemic stroke. Int. J. Stroke. 2015;10:1270–1276. doi: 10.1111/ijs.12537. [DOI] [PubMed] [Google Scholar]