Abstract
Hematopoietic stem cell transplantation (HSCT) using unrelated cord blood (CB) donors is a suitable approach when an HLA-matched donor is not available. However, one important drawback is the risk of life-threatening viral infections prior to immune reconstitution, particularly from adenoviruses (AdVs). Although adoptive therapy with ex vivo expanded virus-reactive donor T cells has proven effective to treat these infections in HSCT recipients, the manufacturing process is complex and requires large numbers of cells, which is incompatible with CB donor units. Here, we have adapted our previous accelerated co-cultured dendritic cell (acDC) method, which allows to efficiently and rapidly expand peripheral blood T cells reactive to a given antigen, for use on limited CB material. Selected cytokine cocktails induced DC differentiation and maturation from unfractionated CB mononuclear cell cultures and simultaneously stimulated and expanded, within 10 days, functional CD8+ T cells specific for the model antigen MelanA or AdV immunodominant peptides. In addition, the use of G-Rex cultures yielded numbers of AdV-reactive CD8+ T cells compatible with adoptive cell therapy applications. Our acDC strategy, which uses reagents compatible with good manufacturing practices, may be promptly translated into the clinic for treating intercurrent infections in CB HSCT recipients.
Keywords: cord blood, expansion, virus-specific T cells, dendritic cells, cytokine cocktail, stem cell transplantation
Introduction
For the many patients who lack a suitable HLA (human leukocyte antigen)-matched donor, the use of alternative donors—i.e., mismatched unrelated donors, unrelated cord blood (CB) donors, and haplo-identical family donors—has extended the possibility of hematopoietic stem cell transplantation (HSCT).1 The use of CB has some major advantages: it can be more readily available; it tolerates some degree of HLA mismatch, which greatly helps in finding suitable CB units; and it yields a reduced risk of graft-versus-host disease (GvHD) and relapse.2 Partially HLA-matched CB HSCT affords similar long-term leukemia-free survival compared to fully matched unrelated bone marrow HSCT in both children3 and adults.4 However, CB HSCT is frequently associated with a delayed immune reconstitution, which leaves recipients vulnerable to severe viral infections, e.g., cytomegalovirus (CMV), Epstein-Barr virus (EBV) and adenovirus (AdV, mainly AdV5). While antiviral agents (e.g., ganciclovir and foscarnet) and anti-CD20 treatment can control CMV reactivation and EBV-associated lymphoproliferation, respectively, such agents are toxic and not always effective, and no adequate treatment (with the limited exception of cidofovir) is available for AdV.5 AdV infection is particularly daunting in children, occurring in 10%–30% of cases and in up to 80% in children under 5 years of age.5
Adoptive T cell therapy, using in vitro expanded virus-reactive T cells, can be used to control these infections in adult donor HSCT recipients.6, 7 Such virus-reactive T cells are usually produced from the donor’s peripheral blood mononuclear cells (PBMCs) by cell culture over 4 to 10 weeks8, 9 or by direct selection through interferon (IFN)-γ capture assays10, 11, 12, 13 or HLA multimers (MMrs).14, 15 This procedure is mainly limited by its high PBMC needs, which make it unfeasible in the setting of CB HSCT, in which the CB donor is not available and the CB units are limited in amount. One further challenge is that the CB T cell repertoire is largely immature, hence requiring the expansion of antigen (Ag)-reactive T cells from naive precursors.16, 17 These drawbacks currently limit both wider applicability of CB HSCT and the success rate in patients on whom the procedure is performed.
Hence, techniques to generate viral-reactive T cells in quantities suitable for adoptive cell therapies are needed, starting from the limited cell numbers available in the CB unit(s), without jeopardizing the success of concomitant HSCT using the same unit(s). In the few previous works addressing this issue,18, 19 CB T cells were successfully expanded in 8–14 days, starting from a negligible (3%–5%) fraction of a single CB unit, but a polyclonal anti-CD3/CD28 bead stimulation was used, which yields a higher risk of GvHD. On the other hand, successful generation of viral Ag-reactive T cells from CB was obtained by using large numbers of starting cells (≥40 × 106, i.e., ≥20% of a typical CB unit) stimulated with crude Ag sources such as CMV lysates, B-EBV lines, and transduced Ag-presenting cells (APCs),20, 21, 22, 23 quenching enthusiasm toward clinical application.
We have previously developed an accelerated co-cultured dendritic cell (acDC) methodology,24, 25, 26, 27, 28 which may be more suitable for clinical translation. Using appropriate cytokine cocktails, this culture system allows DCs to differentiate and mature directly in situ within 48 h, using unfractionated PBMCs cultured without preliminary purification of monocytes or other DC precursors. When whole proteins or peptides are added at the start of culture, cognate T cell precursors are stimulated and can be efficiently expanded over the next few (9–11) days and sorted for further use. We have here applied this acDC method to CB samples and obtained numbers of viral Ag-reactive T cells that are suitable for therapeutic applications.
Results
acDC Cytokine Cocktails Induce Equivalent APC Populations in CB and PB
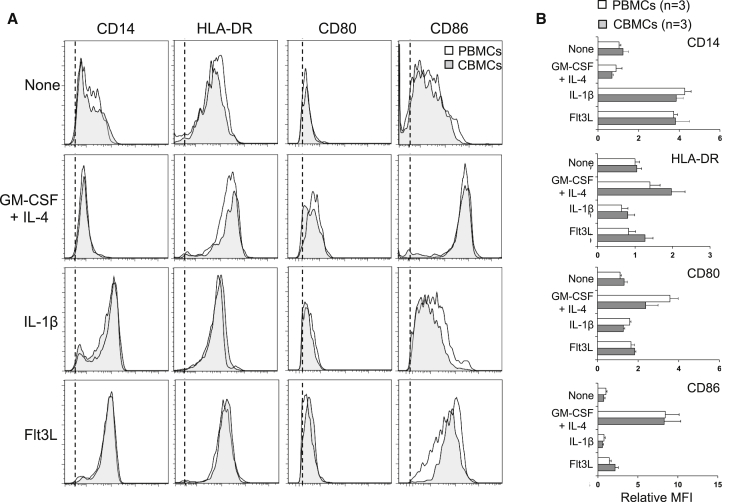
CB harbors immune cells with an immature phenotype29, 30 that are less prone to induce productive immune responses. Therefore, we asked whether suitable APCs could be induced in a cord blood mononuclear cell (CBMC) mixture, as previously obtained with PBMCs,24, 26 by exposing them to different cytokines for 48 h. Exposure to granulocyte-macrophage colony-stimulating factor (GM-CSF)/interleukin (IL)-4, IL-1β-, or fms-like tyrosine kinase 3 ligand (Flt3L) followed by pro-inflammatory cytokines led to identical phenotypic changes when comparing CBMCs with PBMCs (Figures 1A and 1B; gating strategy shown in Figure S1). The GM-CSF/IL-4 cytokine cocktail led to the differentiation of DCs, as evidenced by CD14 downregulation and upregulation of HLA-DR and of the costimulatory molecules CD80 and, to a larger extent, CD86. Conversely, both IL-1β and Flt3L led to CD14 upregulation, without major changes in the expression of HLA-DR, CD80, or CD86, consistent with the induction of different APC populations. Collectively, these results show that acDC cytokine cocktails can be used to differentiate APCs from both CBMCs and PBMCs, with similar results.
Figure 1.
acDC Cytokine Cocktails Induce Equivalent APC Populations in CB and PB
PBMCs or CBMCs (2 × 106 cells per well in 48-well plates) were cultured for 48 h with the indicated cytokine cocktails, namely, GM-CSF/IL-4, IL-1β, Flt3L, or no cytokines during 24 h followed by the addition of TNF-α, PGE2, IL-1β, and low-dose IL-7 for another 24 h. At the end of this 48-h culture, the phenotype of CD3−CD19−CD11c+ cells was assessed by flow cytometry using the indicated cell surface markers. (A) Representative staining obtained from one PBMC (white profiles) and one CBMC sample (gray profiles), as compared to isotype control (dotted line indicates the mean fluorescence intensity; MFI). (B) Cumulative data obtained from 3 CBMC and 3 PBMC donors, represented as relative MFI ± SD for each of the indicated markers, normalized to the MFI registered for PBMC samples in the absence of cytokines.
Efficient Priming of Ag-Reactive CD8+ T Cells from acDC-Stimulated CBMCs Requires the Presence of Homeostatic Cytokines
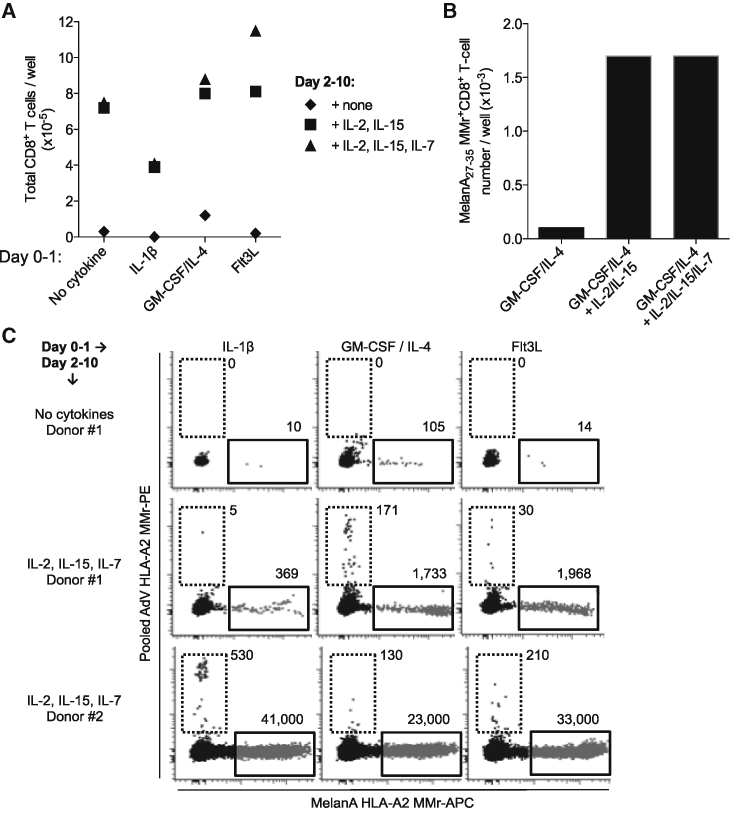
CB T cells mostly harbor a naive phenotype and are exquisitely sensitive to apoptosis, a feature that can be corrected by supplementation of homeostatic cytokines such as IL-2, IL-7, and IL-15.18 We therefore explored the requirement for these common γ-chain receptor cytokines to support the survival and expansion of CD8+ T cells. We focused on CD8+ T cells in light of their role as final effectors in viral clearance, consistent with the therapeutic application envisaged. The previous 48-h acDC stimulation was extended for an additional 8 days (i.e., 10 days total), with homeostatic cytokines added from day 2 and replenished every 2–3 days. Previous kinetics studies documented that 10-day cultures provide the optimal time point to expand Ag-reactive CD8+ T cells.26 As shown in Figure 2A, the number of total CD8+ T cells that could be retrieved after these acDC cultures in the presence of different cytokines was significantly increased by the addition of IL-2 and IL-15, with higher yields obtained when IL-7 was further included. This was also true when acDC cultures were performed in the presence of an immunodominant MelanA26–35 epitope peptide, here used as a model Ag in light of the high precursor frequencies of cognate CD8+ T cells present in a majority of HLA-A2+ healthy individuals (the most prevalent HLA variant in the Caucasian population).26, 31 The number of MelanA26–35-reactive CD8+ T cells retrieved at day 10 of culture following acDC stimulation with GM-CSF/IL-4 was 17-fold higher in the presence of IL-2/IL-15 or IL-2/IL-15/IL-7 (Figure 2B).
Figure 2.
Efficient Priming of Ag-Reactive CD8+ T Cells from acDC-Stimulated CBMCs Requires the Presence of Homeostatic Cytokines
(A) acDC cultures were performed as described previously (2 × 106 CBMCs per well in 48-well plates) in the presence of the indicated cytokines (followed 24 h later by TNF-α, PGE2, IL-1β, and low-dose IL-7), alone (diamonds), with IL-15 plus IL-2 (squares), or with IL-15 plus IL-2 and IL-7 (triangles) from day 2 of culture. The stimulation lasted for a total of 10 days, and the number of total CD8+ T cells obtained was counted by flow cytometry using CountBright beads. (B) Cultures (2 × 106 CBMCs per well in 48-well plates) were performed using the GM-CSF/IL-4 acDC cocktail followed by TNF-α, PGE2, IL-1β, and low-dose IL-7, along with MelanA26–35 peptide after 24 h, and by the indicated homeostatic cytokines from day 2 of culture. The number of MelanA26–35-reactive CD8+ T cells obtained at the end of the 10-d culture was counted as described earlier, using MelanA26–35-loaded HLA-A2 MMrs, and absolute numbers were determined with CountBright beads. Results refer to a representative experiment from one donor out of two tested. (C) HLA-A2+ CBMCs (2 × 106 cells per well in 48-well plates) were cultured using the indicated acDC cytokine cocktails followed by TNF-α, PGE2, IL-1β, and low-dose IL-7, along with MelanA26–35 and a pool of 3 AdV5 peptides (Hexon542–550, Hexon892–901, and Hexon916–925) after 24 h, and by the indicated homeostatic cytokines from day 2 of culture. The number of MelanA26–35-reactive (x axis; APC fluorochrome) and of AdV5-reactive (y axis; PE fluorochrome) CD8+ T cells obtained at the end of the 10-day culture was counted using fluorochrome-labeled HLA-A2 MMrs loaded with the corresponding peptides. Numbers show the absolute counts of MMr+CD8+ T cells, as determined with CountBright beads. Results refer to two representative donors out of four tested.
These experiments were then repeated by analyzing the expansion obtained with the MelanA26–35 model Ag and with relevant viral epitopes; namely, the HLA-A2-restricted AdV5 Hexon542–550, Hexon892–901, and Hexon916–925. Also, in this case, the expansion was superior when the homeostatic cytokines IL-2, IL-15, and IL-7 were included in the acDC cocktails (Figure 2C). This addition of homeostatic cytokines was, therefore, retained for further experiments. While the supporting effect of these cytokines was highly reproducible, different acDC cocktails induced expansion of Ag-reactive CD8+ T cells with different efficiencies, depending on the Ag targeted and on the CB donor. An example is shown in Figure 2C, in which the hierarchies of the expansion of AdV5-reactive CD8+ T cells was GM-CSF/IL-4 > Flt3L > IL-1β for donor 1 and IL-1β > Flt3L > GM-CSF/IL-4 for donor 2. Similarly, MelanA26–35-reactive CD8+ T cell expansion was higher for Flt3L in one case and for IL-1β in the second case.
More Complex acDC Cytokine Cocktails Induce APCs of Similar Phenotype and Ag-Reactive CD8+ T Cells in Similar Numbers but Producing More Cytokines
Given this variability in the expansion yield of Ag-reactive CD8+ T cells, different acDC cytokine combinations were tested to verify whether a synergistic effect could be achieved, thus maximizing the odds for a given CBMC sample and Ag specificity to respond to stimulation.
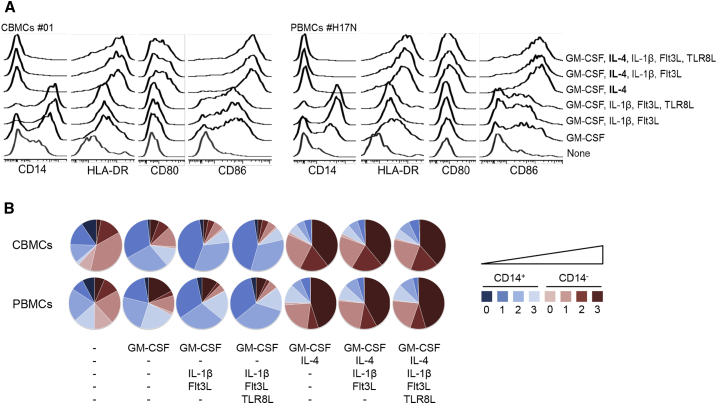
First, the phenotype of the APCs obtained with different acDC cytokine combinations was studied in parallel in CBMC and PBMC samples. These combinations further included the Toll-like receptor 8 ligand (TLR8L) sRNA40, which we previously showed to prime qualitatively superior CD8+ T cells in terms of cytotoxic potency, functionality, and Ag sensitivity.26 First, we observed that IL-4 needed to be combined with GM-CSF to achieve complete CD14 downregulation, both in CBMCs and PBMCs (Figures 3A and 3B). Second, further enrichment of this GM-CSF/IL-4 cocktail with IL-1β and Flt3L—alone or in combination with TLR8L, added along with tumor necrosis factor (TNF)-α, prostaglandin (PG)E2, and IL-7 at day 1—did not significantly change this phenotype, with equivalent CD14 downregulation accompanied by similar upregulation of HLA-DR, CD86, and CD80, both in CBMCs and PBMCs. Cumulative results are shown in Figure 3B, where the fractions of CD14+ and CD14− cells are represented along a color scale, depending on the number of stimulatory molecules expressed (0, 1, 2, or 3 among HLA-DR, CD80, and CD86), reflecting different degrees of maturation and stimulatory potency. While CD14+ cells represent cells of the monocyte lineage, the CD14− fraction represents bona fide DCs. This representation also highlights that, while the APC composition in the absence of IL-4 is mostly made of CD14+ cells, the CD14− fraction becomes predominant once IL-4 is added, with a substantial fraction (>60%) of these cells displaying a mature phenotype expressing 2 to 3 molecules among HLA-DR and co-stimulatory receptors CD80 and CD86. Collectively, these results show that enrichment of the GM-CSF/IL-4 cocktail with additional acDC cytokines does not significantly modify the APC phenotype compared to what was obtained with GM-CSF/IL-4 alone.
Figure 3.
More Complex acDC Cytokine Cocktails Induce APCs of Similar Phenotype
(A) CBMCs and PBMCs (2 × 106 per well in 48-well plates) were cultured using the indicated acDC cytokine cocktails followed by TNF-α, PGE2, IL-1β, and low-dose IL-7 after 24 h. At the end of this 48-h culture, the phenotype of adherent CD3−CD19−CD11c+ cells was assessed by flow cytometry using the indicated cell surface markers. A representative staining obtained from one CBMC sample (left) and one PBMC sample (right) is shown. (B) Cumulative data obtained from 3 CBMC donors (top) and 3 PBMC donors (bottom), displayed as the relative representation of CD14+ and CD14− cells among adherent CD3−CD19−CD11c+ cells expressing 0 to 3 different markers among HLA-DR, CD80, and CD86.
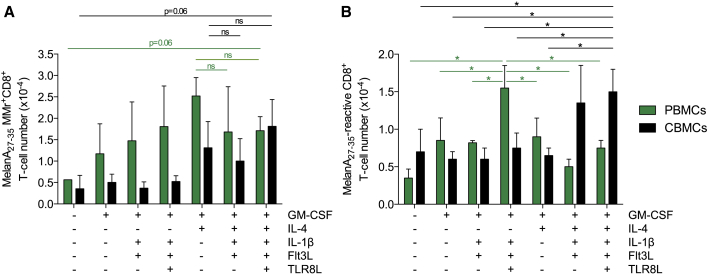
The number and cytokine response of MelanA26–35-reactive CD8+ T cells obtained with these different acDC cocktails was then analyzed in parallel with APC phenotypes (Figure 4). For both PBMCs and CBMCs, yields increased with GM-CSF/IL-4 but were not different when this cocktail was enriched with additional acDC cytokines, as assessed by MMr staining (Figure 4A). Interestingly, the number of CD8+ T cells expressing one or more effector cytokines among IFN-γ, TNF-α, IL-2, and/or macrophage inflammatory protein (MIP)-1β in response to a MelanA26–35 recall was the highest for PBMCs using the GM-CSF/IL-1β/Flt3L/TLR8L cocktail (no IL-4), which induced CD14+ monocytes and/or macrophages rather than DCs (Figure 3). This was not observed for CBMCs. The cytokine production was significantly higher in CB T cells obtained with the combination of GM-CSF/IL-4, IL-1β, Flt3L, and TLR8L, as compared to all other cytokine cocktails except GM-CSF/IL-4/IL-1β/Flt3L, which displayed a higher variability in the induction of functional MelanA26–35-reactive CD8+ T cells (Figure 4B). The GM-CSF/IL-4/IL-1β/Flt3L/TLR8L cocktail was, therefore, retained for further experiments.
Figure 4.
More Complex acDC Cytokine Cocktails Induce Ag-Reactive CD8+ TC in Similar Numbers but Produce More Cytokines
(A) PBMCs and CBMCs (3 donors each, same donors as in Figure 3; 2 × 106 CBMCs per well in 48-well plates) were cultured using the indicated acDC cytokine cocktails followed by TNF-α, PGE2, IL-1β, and low-dose IL-7 along with MelanA26–35 peptide after 24 h and by IL-2, IL-15, and IL-7 from day 2 of culture. Absolute numbers of MelanA26–-35-reactive CD8+ T cells per million PBMCs (green) or CBMCs (black) obtained at the end of the 10-day culture are plotted, as determined with MelanA26–35-loaded HLA-A2 MMrs and CountBright beads. (B) The same cultures were tested at day 13 during a 6-h recall assay in the presence of HLA-A2+ LCL cells pulsed with MelanA26–35 or no peptide. The graph displays absolute numbers of CD8+ T cells from PBMCs (green) or CBMCs (black) producing at least one cytokine among IFN-γ, TNF-α, IL-2, and MIP-1β in response to MelanA26–35-pulsed LCL cells after background subtraction, i.e., the number of cytokine-positive CD8+ T cells detected in response to unpulsed LCL cells. Results are expressed as mean ± SD (*p < 0.05).
acDC Stimulation in G-Rex Devices Further Increases the Yield of Ag-Reactive CD8+ T Cells
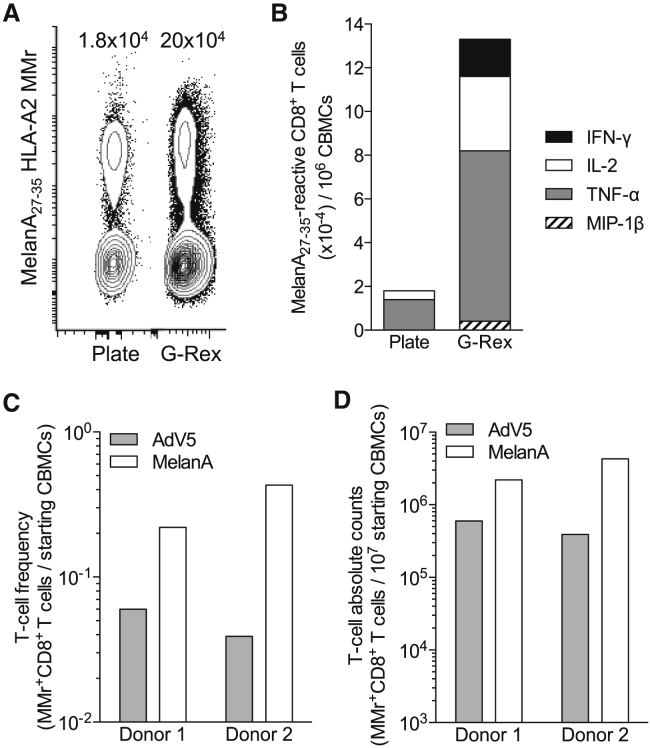
Last, we explored strategies to maximize the yield of Ag-reactive CD8+ T cells obtained during the acDC culture. G-Rex flasks have been recently described to increase cell culture yields by maximizing gas exchanges from the bottom rather than the top of the vessel, i.e., in closer contact with cells. Using the acDC cytokine cocktail selected earlier, supplemented with IL-2, IL-7, and IL-15 and the model Ag MelanA26–35, we observed that acDC stimulation in G-Rex flasks led to an 11-fold increase in Ag-reactive CD8+ T cell expansion compared to a standard plate culture (Figure 5A). In addition, expanded cells consistently showed higher numbers of CD8+ T cells producing TNF-α, IL-2, IFN-γ, and/or MIP-1β for G-Rex cultures (Figure 5B), the two latter cytokines being undetectable in plate cultures.
Figure 5.
acDC Stimulation in G-Rex Devices Further Increases the Yield of Ag-Specific CD8+ T Cells
(A) CBMCs were cultured in 12-well plates (10 × 106 CBMCs per well) or G-Rex10 flasks (10 × 106 CBMCs per flask) using a combination of GM-CSF, IL-4, IL-1β, and Flt3L, followed by TLR8L, TNF-α, PGE2, and low-dose IL-7 along with MelanA26–35 peptide after 24 h and by IL-2, IL-15, and IL-7 from day 2 of culture. The number of MelanA26–35-specific CD8+ T cells obtained at the end of the 10-day culture is represented after gating on viable CD8+ T cells and was counted using MelanA26–35-loaded HLA-A2 MMrs. The absolute numbers of MMr+ cells per million CBMCs obtained are indicated, as determined with CountBright beads. (B) The same culture was tested at day 13 during a 6-h recall assay in the presence of LCL cells pulsed with MelanA26–35 or no peptide. The graph displays absolute numbers of CD8+ cells per million CBMCs producing the indicated cytokines in response to MelanA26–35-pulsed LCL cells after background subtraction, i.e., the number of cytokine-positive CD8+ T cells detected in response to unpulsed LCL cells. (C and D) CBMCs from two representative donors were cultured as described earlier in G-Rex10 flasks (10 × 106 CBMCs per flask) with MelanA26–35 or pooled AdV5 Hexon542–550, Hexon892–901, and Hexon916–925 peptides. The number of peptide-reactive CD8+ T cells obtained at the end of the 10-day culture was analyzed after gating on viable CD8+ T cells using HLA-A2 MMrs loaded with the corresponding peptides. (C) The frequency of MMr+ cells out of total CBMCs. (D) The absolute numbers of MMr+ cells obtained from the same cultures, as determined with CountBright beads.
We then used the G-Rex culture system to expand AdV5-reactive CD8+ T cells from CBMCs, which were stimulated by adding a pool of three AdV5 peptides (Hexon542–550, Hexon892–901, Hexon916–925) along with the MelanA26–35 peptide. As shown fo 2 representative donors (Figures 5C and 5D), a significant T cell expansion was observed: T cell yields obtained for these AdV specificities, starting from a single aliquot of 10 × 106 frozen-thawed CBMCs, were 3.9 × 105 and 6.0 × 105 CD8+ T cells, which would meet the cell numbers required for adoptive cell transfer into adult hosts.
Discussion
This study provides a novel methodology adaptable to the standards of good manufacturing practice (GMP) to efficiently generate functional virus-reactive CD8+ T cells from CB units after a simple and short (10-day) in vitro culture. Indeed, the use of acDC stimulation protocols and suitable culture vessels enhanced the expansion of the viral Ag-reactive fractions of interest to the amounts needed for adoptive T cell transfer therapies using minimal CBMC numbers.
The use of virus-reactive T cells generated from the donor’s PBMCs has emerged as a reliable therapeutic strategy for preventing and treating intercurrent infections in immunocompromised HSCT patients.7 The cell needs for this type of procedure are of ∼109 PBMCs to obtain 1–50 × 103 Ag-reactive T cells per kilogram to infuse into patients.10 This approach is, however, difficult to apply in the setting of CB transplantation due to the low number of T cells available and to their naive phenotype, which requires priming and multiple expansion rounds.
To overcome these limitations and to reduce the cost and complexity of product manufacture, we used a modified version of our previously described acDC protocol, which allows the Ag-driven stimulation of very low-frequency naive CD8+ T cells.24, 26 The three key advantages of this technology are: (1) reduced CBMC needs to obtain Ag-reactive T cells (typically only 1–2 million per Ag specificity); (2) efficient amplification of Ag-reactive T cells, as the procedure lines up the three critical steps of APC differentiation and maturation, Ag presentation, and T cell triggering both spatially, within a small culture vessel, and temporally (10 days); and (3) the use of professional APCs such as DCs, which are permissive for a variety of Ag stimuli (e.g., proteins or polypeptides), without prior knowledge of the precise epitopes recognized or the selection of donors based on specific HLA haplotypes.24
We optimized the cytokine cocktails utilized for DC differentiation and maturation through the use of GM-CSF, IL-4 IL-1β, Flt3L, and TLR8L during the first 48 h of culture. Moreover, the addition of IL-2, IL-7, and IL-15 during the following 8 days was instrumental for promoting T cell survival, expansion, and function (production of multiple inflammatory cytokines) in response to peptide stimulation. Of importance, the use of G-Rex devices yielded numbers of AdV-reactive T cells that are compatible with their in vivo application. Indeed, the targeted number of freshly isolated virus-specific T cells currently used for adoptive transfer is of ∼5 × 103 cells per kilogram of body weight. Thus, the AdV-specific CD8+ T cells obtained here (3.9–6.0 × 105) would be sufficient to treat adult individuals, provided that they maintain potent in vivo proliferative capacities, which should be the case after this short-term (10-day) culture period. The in vivo persistence and expansion rate of these in vitro expanded CB CD8+ T cells remains, however, to be investigated. An extended survival (up to 10 years) of cytotoxic T lymphocytes (CTLs) generated from the donor’s PBMCs has been reported in HSCT patients.9, 14, 32 In contrast, a shorter persistence (14–90 days) of banked third-party, partially HLA-matched CTLs, which are currently being explored as an alternative strategy to restore the anti-viral immunity in bone marrow or CB HSCT recipients, has, so far, been reported.32, 33, 34, 35 Indeed, in the context of CB transplantation, Barker et al. showed that EBV-specific third-party CTLs expanded in vivo after infusion and persisted for 7–10 days but rapidly disappeared afterward, possibly due to apoptosis or rejection.33 Multiple infusions were required for effective treatment. Encouraging results were, however, recently observed following infusion of AdV-specific T cells generated from a related haplo-identical third-party donor in CB HSCT recipients.36 In vivo expansion was observed for up to 3 months post-infusion, along with potent anti-viral responses, although some cases of GvHD occurred during the first month after infusion. Thus, CB-derived CTLs of the donor’s origin may achieve a better and durable engraftment and provide long-term outcomes without repeated infusions.
Our results show that CD8+ T cells reactive against AdV or MelanA Ags can be simultaneously stimulated and expanded in the same culture, thus supporting the feasibility of producing multivirus-reactive or mixed virus- and tumor-reactive T cells from a CB unit. This is similar to what was obtained with PB-derived CTLs,8, 34, 35 using a mixture of immunodominant peptides from the major viral pathogens (AdV, CMV, and EBV) involved in the infectious complications of HSCT. Along the same line, another approach used DCs and EBV-transformed lymphoblastoid cell lines (EBV-LCLs) generated from donor CBMCs and transduced with recombinant AdV vectors encoding CMV peptides to stimulate CB T cells (10 days with DCs and then 2 rounds of 7 days with EBV-LCLs).21 It may, however, be difficult to implement this procedure on a large scale. More recently, Dave et al. reported the production of CB-derived multivirus-reactive T cells using overlapping peptide pools.37 However, this approach required purification and pulsing of mature DCs for the first round of stimulation and the use of genetically modified K562 cells expressing co-stimulatory molecules as feeders for subsequent stimulations, for a total manufacturing time of 28 days. In comparison, our approach allows DCs to mature directly in the well and to generate virus-reactive CD8+ T cells within a 10-day culture period using unfractionated frozen CBMCs, which provides a more feasible, GMP-compatible, and cost-effective method. After volume reduction, CB units contain approximately 80% of mononuclear cells,38 thus further supporting the clinical feasibility of our acDC approach directly on banked cryopreserved CB units. Furthermore, expansion of CD8+ T cells reactive to each virus can be evaluated by our combinatorial flow cytometry assays that use HLA class I MMrs loaded with the same peptides used for stimulation.39 Lastly, acDC cultures may be easily upgraded by performing a concomitant stimulation with both HLA class-I- and HLA class-II-restricted peptides derived from the same viral Ags, as both CD8+ and CD4+ T cell subsets are needed for optimal viral clearance.10 In this regard, we have previously shown that virus-specific CD4+ and CD8+ T cells, identified by HLA-DR4 and HLA-A2 MMrs, respectively, efficiently expanded when primed by their cognate peptides24 and that expansion of both CD4+ and CD8+ T cells can also be obtained when acDC cultures are stimulated with protein Ags.24
With the advent of the chimeric Ag receptor (CAR) technology, it would be appealing to use our acDC culture system for generating virus-reactive T cells that can be later engineered with a tumor-specific CAR. Infusion of such bi-specific T cells derived from PB demonstrated encouraging results in the context of neuroblastoma40 or B cell malignancy relapses after HSCT.41 Of note, Micklethwaite et al. demonstrated the feasibility of generating bi-specific T cells from CB by transfecting multivirus (CMV, EBV, and AdV)-reactive T cells with a CAR.CD19-28ζ construct.22 Our acDC methodology may, thus, be considered for generating virus-reactive CAR T cells endowed with anti-viral (through the native TCR) and anti-tumor (through the introduced CAR) activity.
In conclusion, we propose a simple, rapid, and GMP-compatible method to produce virus-reactive T cells from small fractions of banked CBMCs, with the potential to adoptively provide immunity against opportunistic infections and to increase graft-versus-leukemia activity in CB HSCT recipients.
Materials and Methods
Blood Samples
CB units for research purposes were obtained from the AP-HP Cord Blood Bank and provided by Prof. J. Larghero (Cell Therapy Unit, Saint Louis Hospital, Paris, France). PB samples were obtained from healthy donors. All donors gave written informed consent, and the study was approved by the local ethics committee (Ile de France II, study number 2009-11-03). Mononuclear cells (CBMCs and PBMCs) were separated by density gradient centrifugation and stored frozen in liquid nitrogen as described previously.42, 43, 44 HLA class I typing was performed with AmbiSolv primers (Thermo Fisher Scientific).
acDC Cytokine Treatment of PBMCs and CBMCs
We adapted a methodology that we previously developed to generate Ag-reactive T cells from PBMCs.24, 25, 26, 27, 28 Frozen-thawed PBMCs or CBMCs were plated at the following densities in AIM-V medium (Thermo Fisher Scientific): 2 × 106 cells per 500 μL per well in 48-well plates; 5 × 106 cells per 1 mL per well in 24-well plates; 10 × 106 cells per 2 mL per well in 12-well plates; and 10 × 106 cells per 20 mL per flask in G-Rex10 flasks (Wilson Wolf). The following cytokines were used for acDC stimulation, added sequentially as detailed for each figure: for day 0, GM-CSF (R&D Systems; 1,000 U/mL), IL-4 (R&D Systems; 500 U/mL), IL-1β (R&D Systems; 10 ng/mL), and Flt3L (R&D Systems; 50 ng/mL); for day 1, TLR8L (ssRNA40, Invivogen; 0.5 μg/mL), TNF-α (R&D Systems; 1,000 U/mL), PGE2 (Merck Calbiochem; 1 μM), and IL-7 (R&D Systems; 0.5 ng/mL); and for day 2, IL-2 (Proleukin, Novartis; 100 U/mL), IL-15 (R&D Systems; 25 ng/mL), and IL-7 (R&D Systems; 5 ng/mL), which were added by replacing the half medium volume with AIM-V and 10% human serum containing these cytokines at the indicated final concentrations calculated for the whole culture volume. When IL-1β was added at day 0, it was not further added at day 1. Half medium was replenished every 2–3 days with AIM-V and 10% human serum, supplemented with 100 U/mL IL-2, 25 ng/mL IL-15, and 5 ng/mL IL-7 when indicated.
APC Phenotyping
Cells were collected after 48 h of acDC stimulation and phenotyped using the following antibodies: CD80-FITC (clone BB1), CD86-PE (clone IT2.2), CD14 PerCP-Cy5 (clone M5E2), HLA-DR-APC (clone G46-6), CD3-V450 (clone UCHT1), CD19-V450 (clone HIB19), all from BD Biosciences; CD11c-Alexa Fluor 700 (clone 3.9; eBioscience) and Live/Dead Aqua (Life Technologies). Cells were acquired using a 16-color BD LSR Fortessa flow cytometer and analyzed with FlowJo software (TreeStar).
Expansion of Ag-Reactive CD8+ T Cells by acDC Stimulation
CBMCs and PBMCs from HLA-A2+ (HLA-A*02:01+) donors were used and analyzed at day 10 of acDC cultures. The following HLA-A2-restricted peptides (synthesized at >85% purity; ChinaPeptides) were added after the first 24 h of acDC culture at a 10-μM final concentration: Melan-A26-35 (A27L variant; ELAGIGILTV), AdV5 Hexon542–550 (GLRYRSMLL),45 AdV5 Hexon892–901 (LLYANSAHAL),46 and AdV5 Hexon916–925 (YVLFEVFDVV).46 HLA-A2 MMrs were synthesized using the one-pot, mix-and-read technology,47 and staining was performed in the presence of 50 nM dasatinib,48 as described previously.44 Cells were gated on viable (live/dead−) CD8+ events for analysis. Absolute numbers of MMr+ cells retrieved from each culture were determined with CountBright beads (Thermo Fisher Scientific) following manufacturer’s instructions.
Ag Recall Assays
EBV-transformed HLA-A2+ LCL cells39 were used as APCs and labeled with CellTrace CFSE (Thermo Fisher Scientific) to separate them from cells retrieved from acDC cultures. They were then pulsed for 2 h with the indicated peptide at a 10-μM final concentration. After washing, 0.5 × 106 LCL cells were incubated 1:1 with CBMCs or PBMCs from acDC cultures for 6 h in the presence of 10 μg/mL brefeldin A in 96-well flat-bottom plates. Intracellular cytokine staining was performed using BD Cytofix/Cytoperm reagents and analyzed on a BD LSR Fortessa flow cytometer after gating on live CFSE−CD8+ events with the following antibodies: MIP-1β-Fluorescein (clone 24006, R&D Systems), IFN-γ-PE (clone 4S.B3, eBioscience), IL-2-PE-Cy7 (clone MQ1-17H12, eBioscience), and TNF-α-APC (clone MAb11, BD Biosciences).
Statistical Analysis
Statistical tests were performed using GraphPad Prism 6. Results were analyzed using Student’s t tests. A p value < 0.05 was considered significant.
Author Contributions
Conceptualization, S.C.-Z. and R.M.; Methodology, K.K. and R.M.; Investigation, K.K. and R.M.; Resources, S.C.-Z.; Data Curation, K.K., S.Y., and R.M.; Writing – Original Draft, S.Y. and R.M.; Writing – Review & Editing, K.K., S.C.-Z., S.Y., and R.M.; Visualization, K.K., S.Y., and R.M.; Supervision, S.Y. and R.M.; Funding Acquisition, S.C.-Z. and R.M.
Acknowledgments
The authors would like to thank Prof. J. Larghero (Cell Therapy Unit, Hôpital Saint Louis, Paris, France) for providing CB units and Prof. J.H. Dalle (Service d’Hématologie, Hôpital R. Debré, Paris, France) for discussion and advice. This work was supported by grants from the Agence de la Biomédecine (Recherche et Greffe 2013), INSERM-Transfert, and the International Research Group on Unrelated Hematopoietic Stem Cell Transplantation (IRGHET).
Footnotes
Supplemental Information includes one figure and can be found with this article online at https://doi.org/10.1016/j.omtm.2018.12.010.
Supplemental Information
References
- 1.Shim Y.J., Lee J.M., Kim H.S., Jung N., Lim Y.T., Yang E.J., Hah J.O., Lee Y.H., Chueh H.W., Lim J.Y., Study Alliance of Yeungnam Pediatric Hematology-oncology (SAYPH) Comparison of survival outcome between donor types or stem cell sources for childhood acute myeloid leukemia after allogenic hematopoietic stem cell transplantation: A multicenter retrospective study of Study Alliance of Yeungnam Pediatric Hematology-oncology. Pediatr. Transplant. 2018;22:e13249. doi: 10.1111/petr.13249. [DOI] [PubMed] [Google Scholar]
- 2.Barker J.N., Kurtzberg J., Ballen K., Boo M., Brunstein C., Cutler C., Horwitz M., Milano F., Olson A., Spellman S. Optimal practices in unrelated donor cord blood transplantation for hematologic malignancies. Biol. Blood Marrow Transplant. 2017;23:882–896. doi: 10.1016/j.bbmt.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eapen M., Rubinstein P., Zhang M.J., Stevens C., Kurtzberg J., Scaradavou A., Loberiza F.R., Champlin R.E., Klein J.P., Horowitz M.M., Wagner J.E. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369:1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 4.Laughlin M.J., Barker J., Bambach B., Koc O.N., Rizzieri D.A., Wagner J.E., Gerson S.L., Lazarus H.M., Cairo M., Stevens C.E. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N. Engl. J. Med. 2001;344:1815–1822. doi: 10.1056/NEJM200106143442402. [DOI] [PubMed] [Google Scholar]
- 5.Feuchtinger T., Lang P., Handgretinger R. Adenovirus infection after allogeneic stem cell transplantation. Leuk. Lymphoma. 2007;48:244–255. doi: 10.1080/10428190600881157. [DOI] [PubMed] [Google Scholar]
- 6.Roddie C., Peggs K.S. Immunotherapy for transplantation-associated viral infections. J. Clin. Invest. 2017;127:2513–2522. doi: 10.1172/JCI90599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrett A.J., Prockop S., Bollard C.M. Virus-specific T cells: broadening applicability. Biol. Blood Marrow Transplant. 2018;24:13–18. doi: 10.1016/j.bbmt.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leen A.M., Myers G.D., Sili U., Huls M.H., Weiss H., Leung K.S., Carrum G., Krance R.A., Chang C.C., Molldrem J.J. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat. Med. 2006;12:1160–1166. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- 9.Heslop H.E., Slobod K.S., Pule M.A., Hale G.A., Rousseau A., Smith C.A., Bollard C.M., Liu H., Wu M.F., Rochester R.J. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115:925–935. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feuchtinger T., Matthes-Martin S., Richard C., Lion T., Fuhrer M., Hamprecht K., Handgretinger R., Peters C., Schuster F.R., Beck R. Safe adoptive transfer of virus-specific T-cell immunity for the treatment of systemic adenovirus infection after allogeneic stem cell transplantation. Br. J. Haematol. 2006;134:64–76. doi: 10.1111/j.1365-2141.2006.06108.x. [DOI] [PubMed] [Google Scholar]
- 11.Qasim W., Gilmour K., Zhan H., Derniame S., McNicol A.M., Ip W., Hiwarkar P., Veys P., Gaspar H.B. Interferon-γ capture T cell therapy for persistent Adenoviraemia following allogeneic haematopoietic stem cell transplantation. Br. J. Haematol. 2013;161:449–452. doi: 10.1111/bjh.12251. [DOI] [PubMed] [Google Scholar]
- 12.Feucht J., Opherk K., Lang P., Kayser S., Hartl L., Bethge W., Matthes-Martin S., Bader P., Albert M.H., Maecker-Kolhoff B. Adoptive T-cell therapy with hexon-specific Th1 cells as a treatment of refractory adenovirus infection after HSCT. Blood. 2015;125:1986–1994. doi: 10.1182/blood-2014-06-573725. [DOI] [PubMed] [Google Scholar]
- 13.Creidy R., Moshous D., Touzot F., Elie C., Neven B., Gabrion A., Leruez-Ville M., Maury S., Ternaux B., Nisoy J. Specific T cells for the treatment of cytomegalovirus and/or adenovirus in the context of hematopoietic stem cell transplantation. J. Allergy Clin. Immunol. 2016;138:920–924.e3. doi: 10.1016/j.jaci.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 14.Cobbold M., Khan N., Pourgheysari B., Tauro S., McDonald D., Osman H., Assenmacher M., Billingham L., Steward C., Crawley C. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J. Exp. Med. 2005;202:379–386. doi: 10.1084/jem.20040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neuenhahn M., Albrecht J., Odendahl M., Schlott F., Dössinger G., Schiemann M., Lakshmipathi S., Martin K., Bunjes D., Harsdorf S. Transfer of minimally manipulated CMV-specific T cells from stem cell or third-party donors to treat CMV infection after allo-HSCT. Leukemia. 2017;31:2161–2171. doi: 10.1038/leu.2017.16. [DOI] [PubMed] [Google Scholar]
- 16.Li H.W., Sykes M. Emerging concepts in haematopoietic cell transplantation. Nat. Rev. Immunol. 2012;12:403–416. doi: 10.1038/nri3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ballen K.K., Koreth J., Chen Y.B., Dey B.R., Spitzer T.R. Selection of optimal alternative graft source: mismatched unrelated donor, umbilical cord blood, or haploidentical transplant. Blood. 2012;119:1972–1980. doi: 10.1182/blood-2011-11-354563. [DOI] [PubMed] [Google Scholar]
- 18.Davis C.C., Marti L.C., Sempowski G.D., Jeyaraj D.A., Szabolcs P. Interleukin-7 permits Th1/Tc1 maturation and promotes ex vivo expansion of cord blood T cells: a critical step toward adoptive immunotherapy after cord blood transplantation. Cancer Res. 2010;70:5249–5258. doi: 10.1158/0008-5472.CAN-09-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okas M., Gertow J., Uzunel M., Karlsson H., Westgren M., Kärre K., Ringden O., Mattsson J., Uhlin M. Clinical expansion of cord blood-derived T cells for use as donor lymphocyte infusion after cord blood transplantation. J. Immunother. 2010;33:96–105. doi: 10.1097/CJI.0b013e3181b291a4. [DOI] [PubMed] [Google Scholar]
- 20.Park K.D., Marti L., Kurtzberg J., Szabolcs P. In vitro priming and expansion of cytomegalovirus-specific Th1 and Tc1 T cells from naive cord blood lymphocytes. Blood. 2006;108:1770–1773. doi: 10.1182/blood-2005-10-006536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanley P.J., Cruz C.R., Savoldo B., Leen A.M., Stanojevic M., Khalil M., Decker W., Molldrem J.J., Liu H., Gee A.P. Functionally active virus-specific T cells that target CMV, adenovirus, and EBV can be expanded from naive T-cell populations in cord blood and will target a range of viral epitopes. Blood. 2009;114:1958–1967. doi: 10.1182/blood-2009-03-213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Micklethwaite K.P., Savoldo B., Hanley P.J., Leen A.M., Demmler-Harrison G.J., Cooper L.J., Liu H., Gee A.P., Shpall E.J., Rooney C.M. Derivation of human T lymphocytes from cord blood and peripheral blood with antiviral and antileukemic specificity from a single culture as protection against infection and relapse after stem cell transplantation. Blood. 2010;115:2695–2703. doi: 10.1182/blood-2009-09-242263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanley P.J., Melenhorst J.J., Nikiforow S., Scheinberg P., Blaney J.W., Demmler-Harrison G., Cruz C.R., Lam S., Krance R.A., Leung K.S. CMV-specific T cells generated from naïve T cells recognize atypical epitopes and may be protective in vivo. Sci. Transl. Med. 2015;7:285ra63. doi: 10.1126/scitranslmed.aaa2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinuzzi E., Afonso G., Gagnerault M.C., Naselli G., Mittag D., Combadière B., Boitard C., Chaput N., Zitvogel L., Harrison L.C., Mallone R. acDCs enhance human antigen-specific T-cell responses. Blood. 2011;118:2128–2137. doi: 10.1182/blood-2010-12-326231. [DOI] [PubMed] [Google Scholar]
- 25.Alanio C., Nicoli F., Sultanik P., Flecken T., Perot B., Duffy D., Bianchi E., Lim A., Clave E., van Buuren M.M. Bystander hyperactivation of preimmune CD8+ T cells in chronic HCV patients. eLife. 2015;4:e07916. doi: 10.7554/eLife.07916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lissina A., Briceño O., Afonso G., Larsen M., Gostick E., Price D.A., Mallone R., Appay V. Priming of qualitatively superior human effector CD8+ T cells using TLR8 ligand combined with FLT3 ligand. J. Immunol. 2016;196:256–263. doi: 10.4049/jimmunol.1501140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Briceño O., Lissina A., Wanke K., Afonso G., von Braun A., Ragon K., Miquel T., Gostick E., Papagno L., Stiasny K. Reduced naïve CD8(+) T-cell priming efficacy in elderly adults. Aging Cell. 2016;15:14–21. doi: 10.1111/acel.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bassani-Sternberg M., Bräunlein E., Klar R., Engleitner T., Sinitcyn P., Audehm S., Straub M., Weber J., Slotta-Huspenina J., Specht K. Direct identification of clinically relevant neoepitopes presented on native human melanoma tissue by mass spectrometry. Nat. Commun. 2016;7:13404. doi: 10.1038/ncomms13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chalmers I.M., Janossy G., Contreras M., Navarrete C. Intracellular cytokine profile of cord and adult blood lymphocytes. Blood. 1998;92:11–18. [PubMed] [Google Scholar]
- 30.Marchant A., Goldman M. T cell-mediated immune responses in human newborns: ready to learn? Clin. Exp. Immunol. 2005;141:10–18. doi: 10.1111/j.1365-2249.2005.02799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zippelius A., Pittet M.J., Batard P., Rufer N., de Smedt M., Guillaume P., Ellefsen K., Valmori D., Liénard D., Plum J. Thymic selection generates a large T cell pool recognizing a self-peptide in humans. J. Exp. Med. 2002;195:485–494. doi: 10.1084/jem.20011658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Reilly R.J., Prockop S., Hasan A.N., Koehne G., Doubrovina E. Virus-specific T-cell banks for ‘off the shelf’ adoptive therapy of refractory infections. Bone Marrow Transplant. 2016;51:1163–1172. doi: 10.1038/bmt.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barker J.N., Doubrovina E., Sauter C., Jaroscak J.J., Perales M.A., Doubrovin M., Prockop S.E., Koehne G., O’Reilly R.J. Successful treatment of EBV-associated posttransplantation lymphoma after cord blood transplantation using third-party EBV-specific cytotoxic T lymphocytes. Blood. 2010;116:5045–5049. doi: 10.1182/blood-2010-04-281873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leen A.M., Bollard C.M., Mendizabal A.M., Shpall E.J., Szabolcs P., Antin J.H., Kapoor N., Pai S.Y., Rowley S.D., Kebriaei P. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood. 2013;121:5113–5123. doi: 10.1182/blood-2013-02-486324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tzannou I., Papadopoulou A., Naik S., Leung K., Martinez C.A., Ramos C.A., Carrum G., Sasa G., Lulla P., Watanabe A. Off-the-shelf virus-specific T cells to treat BK virus, human herpesvirus 6, cytomegalovirus, Epstein-Barr virus, and adenovirus infections after allogeneic hematopoietic stem-cell transplantation. J. Clin. Oncol. 2017;35:3547–3557. doi: 10.1200/JCO.2017.73.0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qian C., Campidelli A., Wang Y., Cai H., Venard V., Jeulin H., Dalle J.H., Pochon C., D’aveni M., Bruno B. Curative or pre-emptive adenovirus-specific T cell transfer from matched unrelated or third party haploidentical donors after HSCT, including UCB transplantations: a successful phase I/II multicenter clinical trial. J. Hematol. Oncol. 2017;10:102. doi: 10.1186/s13045-017-0469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dave H., Luo M., Blaney J.W., Patel S., Barese C., Cruz C.R., Shpall E.J., Bollard C.M., Hanley P.J. Toward a rapid production of multivirus-specific T cells targeting BKV, adenovirus, CMV, and EBV from umbilical cord blood. Mol. Ther. Methods Clin. Dev. 2017;5:13–21. doi: 10.1016/j.omtm.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naing M.W., Gibson D.A., Hourd P., Gomez S.G., Horton R.B., Segal J., Williams D.J. Improving umbilical cord blood processing to increase total nucleated cell count yield and reduce cord input wastage by managing the consequences of input variation. Cytotherapy. 2015;17:58–67. doi: 10.1016/j.jcyt.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Culina S., Lalanne A.I., Afonso G., Cerosaletti K., Pinto S., Sebastiani G., Kuranda K., Nigi L., Eugster A., Østerbye T. Islet-reactive CD8+ T cell frequencies in the pancreas, but not in blood, distinguish type 1 diabetic patients from healthy donors. Sci, Immunol, 2018;3:eaao4013. doi: 10.1126/sciimmunol.aao4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pule M.A., Savoldo B., Myers G.D., Rossig C., Russell H.V., Dotti G., Huls M.H., Liu E., Gee A.P., Mei Z. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cruz C.R., Micklethwaite K.P., Savoldo B., Ramos C.A., Lam S., Ku S., Diouf O., Liu E., Barrett A.J., Ito S. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood. 2013;122:2965–2973. doi: 10.1182/blood-2013-06-506741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Afonso G., Scotto M., Renand A., Arvastsson J., Vassilieff D., Cilio C.M., Mallone R. Critical parameters in blood processing for T-cell assays: validation on ELISpot and tetramer platforms. J. Immunol. Methods. 2010;359:28–36. doi: 10.1016/j.jim.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 43.Mallone R., Mannering S.I., Brooks-Worrell B.M., Durinovic-Belló I., Cilio C.M., Wong F.S., Schloot N.C., T-Cell Workshop Committee, Immunology of Diabetes Society Isolation and preservation of peripheral blood mononuclear cells for analysis of islet antigen-reactive T cell responses: position statement of the T-Cell Workshop Committee of the Immunology of Diabetes Society. Clin. Exp. Immunol. 2011;163:33–49. doi: 10.1111/j.1365-2249.2010.04272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scotto M., Afonso G., Østerbye T., Larger E., Luce S., Raverdy C., Novelli G., Bruno G., Gonfroy-Leymarie C., Launay O. HLA-B7-restricted islet epitopes are differentially recognized in type 1 diabetic children and adults and form weak peptide-HLA complexes. Diabetes. 2012;61:2546–2555. doi: 10.2337/db12-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leen A.M., Christin A., Khalil M., Weiss H., Gee A.P., Brenner M.K., Heslop H.E., Rooney C.M., Bollard C.M. Identification of hexon-specific CD4 and CD8 T-cell epitopes for vaccine and immunotherapy. J. Virol. 2008;82:546–554. doi: 10.1128/JVI.01689-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang J., Olive M., Pulmanausahakul R., Schnell M., Flomenberg N., Eisenlohr L., Flomenberg P. Human CD8+ cytotoxic T cell responses to adenovirus capsid proteins. Virology. 2006;350:312–322. doi: 10.1016/j.virol.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 47.Leisner C., Loeth N., Lamberth K., Justesen S., Sylvester-Hvid C., Schmidt E.G., Claesson M., Buus S., Stryhn A. One-pot, mix-and-read peptide-MHC tetramers. PLoS ONE. 2008;3:e1678. doi: 10.1371/journal.pone.0001678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lissina A., Ladell K., Skowera A., Clement M., Edwards E., Seggewiss R., van den Berg H.A., Gostick E., Gallagher K., Jones E. Protein kinase inhibitors substantially improve the physical detection of T-cells with peptide-MHC tetramers. J. Immunol. Methods. 2009;340:11–24. doi: 10.1016/j.jim.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.