Abstract
The increasing prevalence of inflammatory bowel disease and the high costs associated with biologic therapies suggest that biologics with lower costs, but no compromise on efficacy and safety, should be considered when developing a treatment plan for inflammatory bowel disease. Biosimilars offer a more cost-effective alternative, and although the European Medicines Agency has approved the use of biosimilars for many indications, including inflammatory bowel disease, patients may be concerned about the safety and efficacy of these agents. The updated Nurses–European Crohn’s and Colitis Organisation statements, published in March 2018, recommend that inflammatory bowel disease nurses facilitate patient choice of biologic or biosimilar therapy. Nurses are pivotal in managing the challenges associated with patients transitioning to biosimilars. However, there is limited information available on how inflammatory bowel disease nurses can communicate the concept of biosimilars to patients and also on how best to support them before and during the switch from originators. This review article will focus on patients’ concerns regarding biosimilars and describe considerations for nurses when supporting patients transitioning from originators to biosimilars. Through nurse-led patient education and the use of structured communication strategies, as well as investment in managed switching programmes, patients will become more confident and adherent to their biosimilar therapy, and this may lead to overall reductions in health-care expenditure for inflammatory bowel disease.
Keywords: Biosimilar, education and communication, nurses
1. Introduction
Inflammatory bowel disease [IBD] is a global disease with a prevalence exceeding 0.3% in westernised societies.1 A recent population-based systematic review showed that the highest reported prevalence rates of IBD were in Europe (Crohn’s disease [CD] 322 per 100 000 in Germany; ulcerative colitis [UC] 505 per 100 000 in Norway) and North America.1 These data indicate that there is an increased need for research into preventative therapies and/or innovations in health-care systems to manage IBD1 and that the current treatment options are not sufficient to reduce its prevalence. European long-term follow-up studies suggest that treatment costs, particularly for biologics, are the main driver of high health-care costs associated with IBD.2,3 This suggests that IBD poses an economic burden to society2,4 and that less expensive treatment options may help to reduce health-care expenditure.
Patent expirations have opened the possibility for introducing biosimilar versions of high-cost originator biologics.5 Biosimilars are biologic medicinal products that contain a version of the active substance of an already approved biologic medicinal product [originator].6 Biosimilars must be similar to the originator product in terms of quality characteristics, biologic activity, efficacy and safety.6 The process for developing a biosimilar product is outlined in Figure 1. For example, two infliximab biosimilars have been approved by the European Medicines Agency for the treatment of all indications authorised by the originator product, including CD and UC,7,8 and infliximab biosimilars [CT-P13 and SB2] have shown comparable efficacy and safety in patients with IBD.9,10 Moreover, a number of switch studies have demonstrated maintained efficacy/non-inferiority and similar safety of infliximab biosimilar relative to infliximab originator in patients with CD or UC.10–17 A stochastic economic model simulating the introduction of biosimilars in IBD in The Netherlands calculated potential cost savings [relative to originator products] of €9850 and €2250 per patient for CD and UC, respectively, over a 5-year period.4 This suggests that the use of biosimilars could have a significant impact on the cost profile of IBD; however, the extent of these cost savings will not only depend upon local pricing and procurement policies, but also on the willingness of health-care professionals [HCPs] to start patients on biosimilars or switch them to biosimilars.4
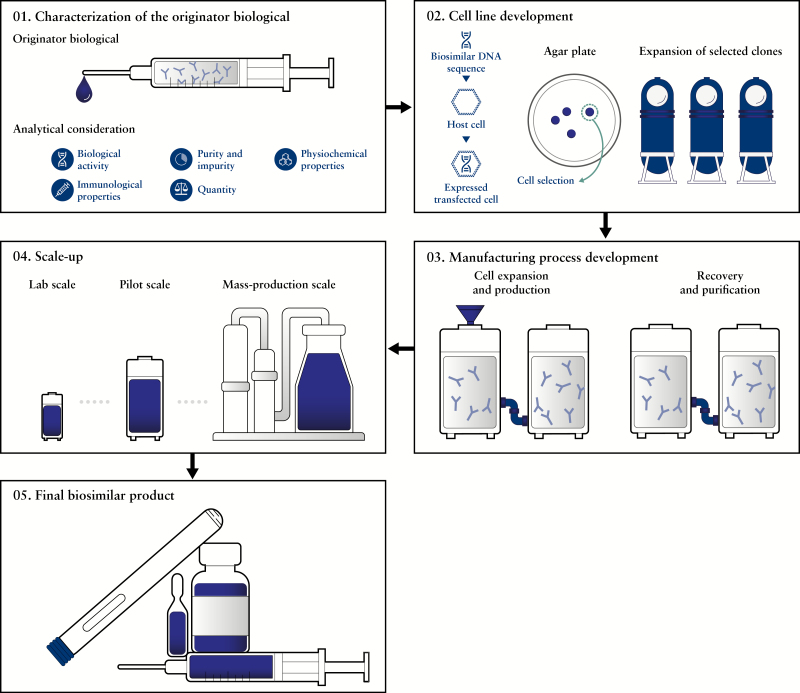
Figure 1.
Development process of biosimilarsa. aFigure adapted from ‘Science behind biosimilars’, Samsung Bioepis Available at: http://www.samsungbioepis.com/file/Science_of_Biosimilars.pdf [Accessed May 2018] 01. The originator biologic first undergoes analyatical consideration to identify its quality attritubes. The data collected are then used to define the critical quality attributes (CQAs) of a biosimilar. 02. The DNA of a biosimilar is incorporated into an expression vector, which is transfected into a host cell to produce a stable expression of the biosimilar. Greater than 15 000 single clones are typically screened to generate the lead cell line that will be the production source of the biosimilar. 03. The lead cell line of a biosimilar is manufactured at lab scale, and the cell culture conditions are refined to increase yield, reduce impurities and maintain optimal growth conditions as a means of achieving consistency and quality. 04. Pilot studies are conducted to maintain real-time monitoring of biosimilar quality control from lab scale to mass-production scale and ensure that the essential quality attributes of the biosimilar are maintained. 05. Final biosimilar products are manufactured under cGMP regulations and must meet the requirements of various regulatory agencies. The final form of biosimilars can be a vial, a pre-filled syringe or an auto-injector. cGMP, current Good Manufacturing Practice; CQA, Critical Quality Attributes; DNA, deoxyribonucleic acid
Nurses are pivotal in the care of patients with IBD, which is a complex and chronic condition requiring expert nursing care and management within the context of a multidisciplinary team.18 The Nurses–European Crohn’s and Colitis Organisation [N-ECCO]18 aims to provide education and networking opportunities for nurses across Europe as a means of sharing best practice guidelines and increase quality of care for patients with IBD.18 The N-ECCO statements were updated and published in March 2018 [2nd N-ECCO]18 and indicate that a patient’s choice of biological or biosimilar therapy should be facilitated by IBD nurses following thorough discussion of effectiveness and safety characteristics.19,20 All EMA-approved biosimilars have shown similar efficacy and safety to their biologic originator product;6 nevertheless, there are differences in the quantity and type of data available in the public domain,21 and patients may have reservations about switching from originator products to biosimilars.22–24 A recent survey conducted by the N-ECCO found that the top priorities for IBD nursing research included patient education to improve self-management of IBD, as well as research into the role of IBD nurses in improving patient outcomes and quality of life.25 There is limited information on how nurses could best communicate the concept of biosimilars to patients with IBD, despite the fact that [for example] infliximab biosimilars have been approved for CD and UC.7 This review article will focus on patients’ concerns regarding biosimilars, with emphasis on how nurses are integral to helping patients transitioning to biosimilars from other treatments, as well as considerations for nurses supporting patients’ switching from originators to biosimilars.
2. Considerations for Nurses 1: The challenges associated with transitioning patients to biosimilars—addressing patients’ concerns
The introduction of any new medicinal product often raises questions and concerns in the minds of patients, and may relate to the approval process, effectiveness, and/or safety of such products.26 This has been observed when examining the attitude of patients towards generic drugs versus branded therapy.27–29 A study conducted in Germany found that 37% of patients expressed general skepticism towards generic drugs due to their lower price, and believed that the introduction of generic prescribing was created to offset costs in the German health insurance system at the perceived expense of patients.28 Changes in formulation from branded to generic treatments may be associated with the perception of reduced effectiveness and increased perceived adverse reactions.27 Consequently, it is evident that changes to treatment can be viewed negatively by patients.29 Patients might be concerned about their status if they are well controlled with their treatment at the time switching is suggested, particularly for patients who went through a long road to achieve that control.30 Although confidence in biosimilars is growing among HCPs,31,32 it is feasible that patients may adopt the same attitude towards biosimilars analogous to that of generic versus branded medication. This may mean that if patient awareness of biosimilars is low, and there are misconceptions about these products, then acceptance, adherence, and therefore treatment outcomes may be affected,33 and this has already been described in a previous publication as the ‘nocebo effect’.34
An international survey conducted in the USA and the European Union [EU] in 2014 involving patients with IBD, patient advocacy groups, caregivers, and the general population, showed that awareness of biosimilars was low, with only 6% of the general population reporting a general knowledge of biosimilars, and many respondents [including patients with IBD] unclear about access to, effectiveness and safety of these agents.22 Moreover, the results from a survey conducted by the European Federation of Crohn’s and Ulcerative Colitis Associations [EFCCA] in 2015 suggested that patients with IBD may not be familiar with biosimilars; in particular, of the 1181 patients who responded to the survey, only 38% had heard of biosimilars.23 Many of these patients were also concerned about the molecular basis, effectiveness and safety of biosimilars [35%, 40%, 47%, respectively].23 A more recent, real-world, cross-sectional study, undertaken in 2015–2016 in Germany, showed that, although patients with IBD exhibited some reluctance to accept biosimilars, 69% were satisfied with the control of their symptoms, and 79% of patients receiving a biosimilar therapy were satisfied with their current treatment and condition.24 These numbers show a similar trend to the proportion of patients who are satisfied with infliximab originator [75%],35 although they are not directly comparable. Since patients and patient organisations should be entitled to reliable up-to-date information to allow them to make informed choices regarding their treatment options and care,26 the results, from the abovementioned surveys, suggest that patient education about biosimilars ensures that informed decisions can be made about future biosimilar use.22–24
3. Considerations for Nurses 2: The importance of patient education and patient empowerment
Informed shared decision-making between patients and HCPs [including nurses] is becoming increasingly advocated in clinical practice to determine the best treatment options for patients.19 The aim of this process is to educate patients about their options so that they are confident about their treatment plan and adherent to their chosen therapy.36 Results from a patient-empowerment study completed by Dutch patients with IBD [617 CD, 450 UC] highlighted that 81% of respondents considered active involvement in the decision-making process ‘very important’.19 Despite this, patients have knowledge deficits about IBD [e.g. anatomy, complications, diet, and therapy], and many patients are unaware of such deficits.37,38 Furthermore, when patients with IBD were questioned across eight different hospitals in the UK, no patients reported a dissatisfaction with their level of IBD knowledge.37,38 Delivery of educational programmes in patients with IBD has been shown to improve disease knowledge and patient satisfaction with regard to educational information and medical care, which ultimately leads to greater treatment adherence and lowers health-care use.39 An educational programme [designed and provided by a nurse practitioner over four consecutive weeks in 3-h sessions] plus standard of care was trialled in patients with IBD, and compared with those who only received standard of care [physician-directed ad hoc teaching during visits and pamphlets on IBD]. The patient group who received nurse-directed education about IBD had significantly higher knowledge scores and perceived knowledge ratings, and therefore reported greater patient satisfaction with the educational programme.39 In addition to providing comprehensive information relating to disease pathology and resultant symptomatology, the nurse-directed education programme also delivered information on current therapies, including the purpose of the treatment, how it works, the most common adverse reactions, and how to manage them.39 Although this study did not investigate the use of biosimilars, considering nurse-directed education programmes have shown similar beneficial impact to patients with IBD40 as well as heart failure,41 it does highlight the importance of nurses in facilitating patient education, and thereby increasing the likelihood of therapy adherence and improved treatment outcomes.
4. Considerations for Nurses 3: Nurses are critical for educating patients with IBD about biosimilars
Nurses are ideally positioned to manage patient concern, and to educate and inform patients regarding their treatment because they work at the interface between patients and the wider health-care team.42 To provide reliable consent, a patient must be well-informed about their treatment regimen and the potential for any adverse reactions.42 Nurses can play a critical role in educating patients by increasing their own knowledge and awareness of biosimilars so that they can relay facts on the effectiveness, safety and development process of biosimilars to patients.43 Nurses should know the difference between biosimilars and small-molecule generics, the specific guidelines applicable to biosimilars, and any potential variations in the administration and handling of a biosimilar compared with the originator product.43 A review outlining considerations for biosimilars for oncology nurses showed that support of nurses by advanced nurse practitioners can assist nurses in achieving these objectives.44 The article also highlights that the principles and policies surrounding biosimilars can be included in the educational planning and needs assessment for all oncology nurse professionals;44 therefore, it is feasible that this could also be implemented into IBD nurse training.
Enhancements in nurse education mean that nurses can advise patients on the correct storage and administration of their biosimilar therapy, which could have a positive impact on the effectiveness of therapy and treatment outcomes.42 Collaboration between nurses and pharmacists is also essential in optimizing delivery and administration of biosimilars in the correct manner, and since nurses are patient advocates, they are ideally placed to maintain pharmacovigilance and monitor adverse reactions.43 An online survey conducted among individual HCPs through the European League Against Rheumatism found that, across Europe, postgraduate rheumatology education was most common in nurses.45 Although the study focused exclusively on rheumatology, the findings suggest that postgraduate education is available for nurses across Europe and that education regarding biosimilars could be implemented during postgraduate training for IBD nurses.
The 2nd N-ECCO statements recommend that IBD nurses provide education to patients with IBD based on individual patient needs, preferences, and coping abilities as a means of enabling patient empowerment.18 There is a wide range of information and videos online that can be used in conjunction with phone calls, written information, and country-specific patient support groups and charities that patients with IBD can be directed to by their nurse practitioner.18 In addition, nurses are advised to encourage patients towards self-management in combination with traditional patient education approaches.18 These strategies can also be used when aiding patients with IBD in their transition to biosimilar therapy.
One recent study investigated the impact of non-mandatory transitioning of patients from etanercept originator to etanercept biosimilar [SB4] on drug survival and effectiveness using a structured communication strategy, with an opt-out option, in adult patients with an inflammatory rheumatic disease [BIO-SPAN study].46 Patients treated with etanercept originator were first informed by letter about the option to transition, then discussions with pharmacy staff and the rheumatologist were available if further help was needed regarding the decision to switch.46 The structured communication strategy involved [i] delivery of a national media item, [ii] reporting that equivalence, lower costs, and potentially fewer site injections were the reasons patients were asked if they would like to transition, and [3] provision of soft-skills training and communication protocol for rheumatology and pharmacy staff about how to address patient concerns regarding biosimilars and how to act if a patient has subjective health complaints, e.g. discuss possible nocebo effect and incorrect attribution effects.47 Of the 642 patients who were contacted, 99% agreed to transition to SB4 and, at 6 months, the persistence rates of SB4 were 90%, which was comparable with the rates observed in a historical cohort of patients treated with etanercept originator in 2014 [persistence rate of 92%].46 The authors hypothesized that the structured communication strategy [including HCP and patient education] led to the high acceptance and persistence rates of SB4, and may have positively influenced patients’ expectations on transitioning to biosimilars.46 Although, non-mandatory transitioning from etanercept originator to SB4 using this structured communication strategy showed a slightly lower persistence rate and smaller decreases in disease activity compared with a historical cohort, this was deemed not clinically relevant.46 These results show that the use of a structured communication strategy may optimize acceptance and persistence rates of patients with respect to biosimilars.46 The benefits of increased education and training have also been observed when comparing patients’ and nurses’ preferences for etanercept biosimilar versus etanercept originator following surveys performed in five EU countries [France, Germany, Italy, Spain and the UK].48,49 In particular, following delivery of an instructional video, device-handling leaflet, a live demonstration on the etanercept biosimilar autoinjector, as well as access to training autoinjectors for both etanercept originator and biosimilar, 74% of patients with rheumatic disease indicated a preference for ‘easy to operate self-injection’ and ‘button-free autoinjector’ attributes, which were features of the biosimilar autoinjector.48 Moreover, most nurses thought that their patients would favour those attributes when choosing a self-administered subcutaneous treatment.49 Although these studies examined the effects of a structured communication strategy and increased training in rheumatic disease, it is feasible that a similar communication strategy could be implemented to aid transitioning of patients with IBD to biosimilars. Moreover, an instructional video, device-handling leaflet, and live demonstration of the devices could not only enhance nurse training, but increase patients’ and nurses’ confidence in biosimilars. A summary of the different communication strategies that can be implemented by IBD nurses to convey the concept of biosimilars to patients and aid the transition to biosimilars is outlined in Table 1.
Table 1.
Summary of different communication strategies that can be implemented by IBD nurses to convey the concept of biosimilars to patients and aid patients’ transitioning to biosimilars
| Aim | Communication strategy |
|---|---|
| Enhance patients’ knowledge about IBD via nurse-led education programmes | • Comprehensive information on disease pathology and symptomatology |
| Aid patients’ understanding of biosimilars | • Development process of biosimilars • Clinical data demonstrating effectiveness and safety • Explanation of the purpose of biosimilars and how they work |
| To aid successful switching, nurses can provide patients with biosimilar information prior to switching | • Letters to inform patients about request to switching • Follow-up telephone calls • Online videos • Provision of written information Directing patients to country-specific support groups and charities |
| Delivery of information of a specific biosimilar | • Use of instructional videos • Provision of biosimilar device-handling leaflet • Live demonstrations on how to use the biosimilar autoinjector • Provision of information on correct storage of biosimilars • Information on the most common adverse reactions and how to manage them |
| Top tips when communicating the concept of biosimilars to patients | • Keep the information simple and use familiar language instead of complex medical terminology • Use visual aids including pictures, graphs and arrays • Check and clarify the patients’ understanding by asking the patient to repeat the information in their own words |
IBD, inflammatory bowel disease
5. Considerations for Nurses 4: Investment in IBD nurse–led services via gain-share agreements
The main driver for using biosimilars is the potential for cost savings to the health economy.4,50 Gain-share agreements have previously been implemented in the UK to invest in IBD services51 and have recently been used to fund the development of a managed switching programme for patients with IBD transitioning to biosimilars.50 The potential role of the IBD biologic clinical nurse specialist in a gain-share agreement is outlined in Table 2. The collaborative arrangement of a gain-share agreement between health commissioners and providers aims to achieve better outcomes for patients and create greater efficiencies in the use of medicinal products that are not reimbursed at national prices in the UK.52 The potential costs savings associated with a gain-share agreement can then be reinvested into patient care and local IBD services.50 In 2013–2014, a gain-share agreement with a local care commissioning group was used to invest £60 000 into IBD nurse-led biologic services at Southampton General Hospital, UK.51 The introduction of the specialist IBD biologics nurse–led services led to substantial improvements in the quality of care and provided support and education for patients as well as their families.51 Moreover, development of the IBD biologic services meant that the initial £60 000 investment was recouped within the first year and led to significant drug-cost savings.51 In the UK, there is no direct incentive for HCPs to switch patients to biosimilars.50 In order to realise the potential cost savings associated with biosimilars, a managed switching and risk-management programme funded by a gain-share agreement in a UK teaching hospital was developed to support patients with IBD through the switching process.50 This programme was developed with input from all key stakeholders, including the local IBD patient panel, gastroenterologists, pharmacists and the IBD nursing team.50 The gain-share agreement was agreed between University Hospital Southampton National Health Service [NHS] Foundation Trust and a local clinical commissioning group to: [i] fund the managed switching programme; [ii] invest in the capacity of the nurse-led IBD biologics services to ensure the continued delivery of high quality and cost-effective patient care; and [iii] develop the inpatient IBD nursing service.50 In this study, patients were approached by an IBD biologics nurse while receiving their originator biologic infusion, and given an information sheet on biosimilars and the opportunity to discuss this with their nurse practitioner.50 Following this, at their next infusion, patients were asked if they would like to switch from infliximab originator to infliximab biosimilar and, if so, they underwent a continuous review at each infusion of the biosimilar.50 Of the 143 patients with IBD who switched to infliximab biosimilar, there were no significant differences in drug persistence, adverse reactions, disease activity, or blood test results compared with infliximab originator in this study.50 Moreover, as a consequence of this managed switching programme to biosimilars, drug acquisition costs decreased by £40 000–60 000 per month, and this could be reinvested into local IBD services.50 As outlined above, reinvestment into IBD biologic services leads to improvements in patient safety and quality of care as well as recruitment into research studies.50,51 Therefore, by investing into biosimilar therapy, the potential cost savings can be leveraged into IBD patient services, including the development of robust data-driven biologics management systems.50
Table 2.
The role of the IBD biologic clinical nurse specialist in the gain–share agreement at Southampton General Hospital, UK45
| Co-ordination and liaison roles | Patient monitoring and support roles |
|---|---|
| Maintaining a database of treated patients | Delivery of patient/carer counselling and education |
| Co-ordination of patient referrals | Demonstration of injection technique and providing support to patients |
| Liaising with infusion day unit | Ensuring screening for opportunistic infections to increase patient safety |
| Liaising with Healthcare at Home for provision and delivery of patient supplies | Blood monitoring of patients |
| Coordination of regular clinical follow-ups to assess therapy response of patients | Involvement in data entry for national biologic therapies audit |
| Coordination of yearly patient reviews to plan for future therapy | |
| Attendance at fortnightly multidisciplinary team reviews | |
| Involvement in research study recruitment |
IBD, inflammatory bowel disease
The Royal Free London NHS Foundation Trust has also used a gain-share agreement53 as a way to reinvest savings associated with the use of biosimilars into increasing the number of health-care staff and improving IBD services.53 The role of nurses was integral to this managed switching programme. The implementation of this plan is outlined in Figure 2.
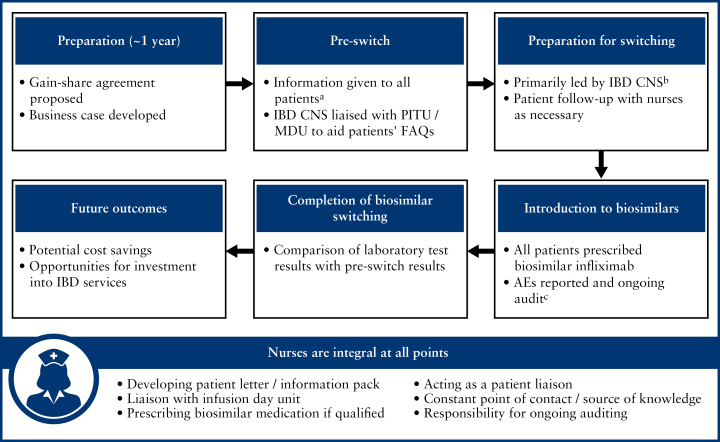
Figure 2.
Biosimilar managed switching programme at the Royal Free London NHS Foundation Trust, UK.47a Letters and information pack posted to patients or daycare nurses distributed information packs at time of patient biologic infusion. All patients had the opportunity to telephone an IBD CNS with questions or arrange a face-to-face conversation at the time of their treatment infusion; patients with further concerns were scheduled to visit their IBD consultant by an IBD CNS or administrator bPreparation for switching was primarily led by the IBD CNS; all patients received the FAQ leaflet, detailed information of the timeline of events, and the planned start date of switching cThe pharmacy and IBD CNS documented the patients switching to biosimilar infliximab and all adverse reactions or prescribing errors were logged. A weekly report was given on each patient at the biologics MDT AE, adverse events; CNS, clinical nurse specialists; FAQs, frequently asked questions; IBD, irritable bowel disease; MDU, Medical Day Unit; MDT, multidisciplinary team; PITU, Planned Investigation Treatment Unit
6. Conclusion
The introduction of biosimilars has the potential to have a significant impact on the cost profile of IBD,4 and as nurses are integral to the care of patients,42 they are ideally placed to aid patients’ transitioning to biosimilars. Many patients with IBD still have concerns about switching to biosimilar therapy;22–24 however, through nurse-led education39,42–44 and a structured communication strategy,46 patients can become more confident in their treatment plan, thereby increasing drug persistence and adherence rates. In addition, use of a managed switching programme50,53 to aid patients’ transitioning to biosimilars, funded by initiatives such as gain-share agreements, may result in potential cost savings that could be reinvested into IBD nurse–led services. This should ultimately lead to overall improvements in patient safety and quality of care.
Funding
This work was supported by Samsung Bioepis Co., Ltd., Incheon, Republic of Korea.
Conflict of Interest
AA has received research funding for his institution from Merck Sharp & Dohme and Takeda and consultancy fees from AbbVie, Allergan, Amgen, Biogen, Celgene, Celltrion, Ferring, Hospira, Janssen, Lilly, Merck Sharp & Dohme, Mundipharma, Pfizer, Samsung Bioepis, Sofar and Takeda. AA has also received payment for lectures including service on speakers’ bureaus from AbbVie, AstraZeneca, Chiesi, Ferring, Hospira, Janssen, Medtronic, Merck Sharp & Dohme, Mitsubishi Tanabe, Mundipharma, Nikkiso, Pfizer, Samsung Bioepis, Takeda, TiGenix and Zambon.
LA has received grants from AbbVie, Atlantic HealthCare, Celgene, Celltrion, Ferring, Janssen, Merck Sharp & Dohme, Mundipharma, Pfizer, Otsuka, Shire, Takeda, TiGenix, and Vifor Pharma. LA has also received personal fees from Celgene, Celltrion, Pfizer and Samsung Bioepis.
KG has received grants from AbbVie and consultancy fees from Takeda, and has received payment for lectures including service speakers’ bureaus from AbbVie and Samsung Bioepis and travel/accommodations/meeting expenses from Janssen.
TK is an employee of Samsung Bioepis Co., Ltd.
Acknowledgments
Medical writing support under the direction of the authors was provided by Gemma McGregor, PhD, of CMC AFFINITY, a division of Complete Medical Communications Ltd, Glasgow, UK funded by Samsung Bioepis, Incheon, Republic of Korea, in accordance with Good Publication Practice [GPP3] guidelines.
Glossary
Abbreviations:
- cGMP
current Good Manufacturing Practice
- CNS
clinical nurse specialists
- CQA
Critical Quality Attributes
- EFCCA
European Federation of Crohn’s and Ulcerative Colitis Associations
- MDU
Medical Day Unit
- N-ECCO
Nurses–European Crohn’s and Colitis Organisation
- PITU
Planned Investigation Treatment Unit.
Author Contributions
AA, LA, KG, and TK all made substantial contributions to all of the following: [i] the conception and design of the study/work, or acquisition of data, or analysis and interpretation of data, [ii] drafting the article or revising it critically for important intellectual content, [iii] final approval of the version to the submitted, and [iv] have agreed to be accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- 1. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2018;390:2769–78. [DOI] [PubMed] [Google Scholar]
- 2. van der Valk ME, Mangen MJ, Leenders M, et al. ; COIN study group and the Dutch Initiative on Crohn and Colitis Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFα therapy: Results from the COIN study. Gut 2014;63:72–9. [DOI] [PubMed] [Google Scholar]
- 3. van der Valk ME, Mangen MJ, Severs M, et al. ; COIN study group and the Dutch Initiative on Crohn and Colitis Evolution of costs of inflammatory bowel disease over two years of follow-up. PLoS One 2016;11:e0142481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Severs M, Oldenburg B, van Bodegraven AA, Siersema PD, Mangen MJ; initiative of Crohn’s and Colitis The economic impact of the introduction of biosimilars in inflammatory bowel disease. J Crohns Colitis 2017;11:289–96. [DOI] [PubMed] [Google Scholar]
- 5. Ben-Horin S, Vande Casteele N, Schreiber S, Lakatos PL. Biosimilars in inflammatory bowel disease: Facts and fears of extrapolation. Clin Gastroenterol Hepatol 2016;14:1685–96. [DOI] [PubMed] [Google Scholar]
- 6. European Medicines Agency Committee for Medicinal Products for Human Use [CHMP]. Guideline on similar biological medicinal products. 2014. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/10/WC500176768.pdf Accessed June 27, 2018. [Google Scholar]
- 7. European Medicines Agency Committee for Medicinal Products for Human Use [CHMP]. Assessment report: Remsima. 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002576/WC500151486.pdf Accessed June 27, 2018. [Google Scholar]
- 8. European Medicines Agency Committee for Medicinal Products for Human Use [CHMP]. Chmp assessment report: Flixabi. 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/004020/WC500208358.pdf Accessed June 27, 2018. [Google Scholar]
- 9. Farkas K, Rutka M, Ferenci T, et al. Infliximab biosimilar CT-P13 therapy is effective and safe in maintaining remission in Crohn’s disease and ulcerative colitis – experiences from a single center. Expert Opin Biol Ther 2017;17:1325–32. [DOI] [PubMed] [Google Scholar]
- 10. Fischer S, Klenske E, Schmitt H, et al. Clinical outcomes and immunogenicity analysis over 6 months following a switch from originator [remicade] to the biosimilar sb2 [flixabi] in inflammatory bowel disease patients. Vienna, Austria: European Crohn’s and Colitis Organisation; 2018. P607 edn. [Google Scholar]
- 11. Argüelles-Arias F, Guerra Veloz MF, Perea Amarillo R, et al. Effectiveness and safety of CT-P13 [Biosimilar Infliximab] in patients with inflammatory bowel disease in real life at 6 months. Dig Dis Sci 2017;62:1305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jørgensen KK, Olsen IC, Goll GL, et al. ; NOR-SWITCH study group Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab [NOR-SWITCH]: a 52-week, randomised, double-blind, non-inferiority trial. Lancet 2017;389:2304–16. [DOI] [PubMed] [Google Scholar]
- 13. Kang B, Lee Y, Lee K, Choi YO, Choe YH. Long-term outcomes after switching to CT-P13 in pediatric-onset inflammatory bowel disease: A single-center prospective observational study. Inflamm Bowel Dis 2018;24:607–16. [DOI] [PubMed] [Google Scholar]
- 14. Schmitz EMH, Boekema PJ, Straathof JWA, et al. Switching from infliximab innovator to biosimilar in patients with inflammatory bowel disease: A 12-month multicentre observational prospective cohort study. Aliment Pharmacol Ther 2018;47:356–63. [DOI] [PubMed] [Google Scholar]
- 15. Smits LJT, Grelack A, Derikx LAAP, et al. Long-term clinical outcomes after switching from Remicade® to biosimilar CT-P13 in inflammatory bowel disease. Dig Dis Sci 2017;62:3117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fiorino G, Manetti N, Armuzzi A, et al. ; PROSIT-BIO Cohort The PROSIT-BIO cohort: A prospective observational study of patients with inflammatory bowel disease treated with infliximab biosimilar. Inflamm Bowel Dis 2017;23:233–43. [DOI] [PubMed] [Google Scholar]
- 17. Armuzzi A, Fiorino G, Variola A, et al. The PROSIT cohort of infliximab biosimilar in IBD: A prolonged follow-up on the effectiveness and safety across italy. Inflamm Bowel Dis. 2017;23:233–43. doi:10.1097/MIB.0000000000000995 [DOI] [PubMed] [Google Scholar]
- 18. Kemp K, Dibley L, Chauhan U, et al. Second N-ECCO Consensus statements on the European nursing roles in caring for patients with Crohn’s disease or ulcerative colitis. J Crohns Colitis 2018;12:760–76. [DOI] [PubMed] [Google Scholar]
- 19. Baars JE, Markus T, Kuipers EJ, van der Woude CJ. Patients’ preferences regarding shared decision-making in the treatment of inflammatory bowel disease: Results from a patient-empowerment study. Digestion 2010;81:113–9. [DOI] [PubMed] [Google Scholar]
- 20. Danese S, Fiorino G, Raine T, et al. ECCO position statement on the use of biosimilars for inflammatory bowel disease—an update. J Crohns Colitis 2017;11:26–34. [DOI] [PubMed] [Google Scholar]
- 21. Jacobs I, Petersel D, Isakov L, Lula S, Lea Sewell K. Biosimilars for the treatment of chronic inflammatory diseases: A systematic review of published evidence. BioDrugs 2016;30:525–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jacobs I, Singh E, Sewell KL, Al-Sabbagh A, Shane LG. Patient attitudes and understanding about biosimilars: An international cross-sectional survey. Patient Prefer Adherence 2016;10:937–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peyrin-Biroulet L, Lönnfors S, Roblin X, Danese S, Avedano L. Patient perspectives on biosimilars: A survey by the European Federation of Crohn’s and Ulcerative Colitis Associations. J Crohns Colitis 2017;11:128–33. [DOI] [PubMed] [Google Scholar]
- 24. Sullivan E, Piercy J, Waller J, Black CM, Kachroo S. Assessing gastroenterologist and patient acceptance of biosimilars in ulcerative colitis and Crohn’s disease across Germany. PLoS One 2017;12:e0175826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dibley L, Bager P, Czuber-Dochan W, et al. Identification of research priorities for inflammatory bowel disease nursing in Europe: a Nurses–European Crohn’s and Colitis Organisation Delphi Survey. J Crohns Colitis 2017;11:353–9. [DOI] [PubMed] [Google Scholar]
- 26. Skingle D. Biosimilars: What do patients need to consider?RMD Open 2015;1:e000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Faasse K, Cundy T, Gamble G, Petrie KJ. The effect of an apparent change to a branded or generic medication on drug effectiveness and side effects. Psychosom Med 2013;75:90–6. [DOI] [PubMed] [Google Scholar]
- 28. Himmel W, Simmenroth-Nayda A, Niebling W, et al. What do primary care patients think about generic drugs?Int J Clin Pharmacol Ther 2005;43:472–9. [DOI] [PubMed] [Google Scholar]
- 29. Kjoenniksen I, Lindbaek M, Granas AG. Patients’ attitudes towards and experiences of generic drug substitution in Norway. Pharm World Sci 2006;28:284–9. [DOI] [PubMed] [Google Scholar]
- 30. Vasudevan A, Gibson PR, van Langenberg DR. Time to clinical response and remission for therapeutics in inflammatory bowel diseases: What should the clinician expect, what should patients be told?World J Gastroenterol 2017;23:6385–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Danese S, Fiorino G, Michetti P. Changes in biosimilar knowledge among European Crohn’s Colitis Organization [ECCO] members: An updated survey. J Crohns Colitis 2016;10:1362–5. [DOI] [PubMed] [Google Scholar]
- 32. Chapman SR, Fitzpatrick RW, Aladul MI. Knowledge, attitude and practice of healthcare professionals towards infliximab and insulin glargine biosimilars: Result of a UK web-based survey. BMJ Open 2017;7:e016730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rezk MF, Pieper B. Treatment outcomes with biosimilars: Be aware of the nocebo effect. Rheumatol Ther 2017;4:209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hahn RA. The nocebo phenomenon: Concept, evidence, and implications for public health. Prev Med 1997;26:607–11. [DOI] [PubMed] [Google Scholar]
- 35. Waters H, Annunziata K, Naim A, Freedman D, Piech C. P-0087: Inflammatory bowel disease patients’ adherence to and satisfaction with treatment. Inflamm Bowel Dis 2008;14:S37-S.18816717 [Google Scholar]
- 36. Siegel CA. Shared decision making in inflammatory bowel disease: Helping patients understand the tradeoffs between treatment options. Gut 2012;61:459–65. [DOI] [PubMed] [Google Scholar]
- 37. Sephton M, Tattersall S, Kemp K, et al. Confirmed: The knowledge of inflammatory bowel disease patients is poor. Vienna, Austria: European Crohn’s and Colitis Organisation; 2013. N009 edn. [Google Scholar]
- 38. Wardle RA, Mayberry JF. Patient knowledge in inflammatory bowel disease: The Crohn’s and Colitis Knowledge Score. Eur J Gastroenterol Hepatol 2014;26:1–5. [DOI] [PubMed] [Google Scholar]
- 39. Waters BM, Jensen L, Fedorak RN. Effects of formal education for patients with inflammatory bowel disease: A randomized controlled trial. Can J Gastroenterol 2005;19:235–44. [DOI] [PubMed] [Google Scholar]
- 40. Nightingale AJ, Middleton W, Middleton SJ, Hunter JO. Evaluation of the effectiveness of a specialist nurse in the management of inflammatory bowel disease [IBD]. Eur J Gastroenterol Hepatol 2000;12:967–73. [DOI] [PubMed] [Google Scholar]
- 41. Kutzleb J, Reiner D. The impact of nurse-directed patient education on quality of life and functional capacity in people with heart failure. J Am Acad Nurse Pract 2006;18:116–23. [DOI] [PubMed] [Google Scholar]
- 42. Wilson P, Wood C. Biosimilar ESAs: A comparative review. J Ren Care 2015;41:53–61. [DOI] [PubMed] [Google Scholar]
- 43. Vizgirda V, Jacobs I. Biosimilars: Considerations for oncology nurses. Clin J Oncol Nurs 2017;21:E54–60. [DOI] [PubMed] [Google Scholar]
- 44. Mayden KD, Larson P, Geiger D, Watson H. Biosimilars in the United States: Considerations for oncology advanced practitioners. J Adv Pract Oncol 2015;6:108–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vliet Vlieland TP, van den Ende CH, Alliot-Launois F, et al. Educational needs of health professionals working in rheumatology in Europe. RMD Open 2016;2:e000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tweehuysen L, Huiskes VJ, van den Bemt BJ, et al. Open-label non-mandatory transitioning from originator etanercept to biosimilar SB4: 6-month results from a controlled cohort study. Arthritis Rheumatol. 2018;70:1408-18. doi:10.1002/art.40516. [Epub 6 August 2018]. [DOI] [PubMed] [Google Scholar]
- 47. Tweehuysen L, Huiskes VJB, van den Bemt BJF, van den Hoogen FHJ, den Broeder AA.. Higher acceptance and persistence rates after biosimilar transitioning in patients with a rheumatic disease after employing an enhanced communication strategy. Annual European Congress of Rheumatology; 2017; Madrid, Spain. FRI1200 edn. [Google Scholar]
- 48. Thakur K, Biberger A, Handrich A, Rezk MF. Patient perceptions and preferences of two etanercept autoinjectors for rheumatoid arthritis: Findings from a patient survey in Europe. Rheumatol Ther 2016;3:245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thakur K, Biberger A, Handrich A, Rezk MF. Perceptions and preferences of two etanercept autoinjectors for rheumatoid arthritis: A new European Union–approved etanercept biosimilar [Benepali®] versus Etanercept [Enbrel®] – findings from a nurse survey in Europe. Rheumatol Ther 2016;3:77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Razanskaite V, Bettey M, Downey L, et al. Biosimilar infliximab in inflammatory bowel disease: Outcomes of a managed switching programme. J Crohns Colitis 2017;11:690–6. [DOI] [PubMed] [Google Scholar]
- 51. Taylor NS, Bettey M, Wright J, et al. The impact of an inflammatory bowel disease nurse–led biologics service. Frontline Gastroenterol 2016;7:283–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. NHS England. Principles for sharing the benefits associated with more efficient use of medicines not reimbursed through national prices. 2014. https://www.england.nhs.uk/wp-content/uploads/2014/01/princ-shar-benefits.pdf Accessed April 2018. [Google Scholar]
- 53. Greveson K. Biosimilars to nurses: Critical communication. Vienna, Austria: European Crohn’s and Colitis Organisation; 2018. [Google Scholar]