Abstract
Background
Bone metastasis of cancer can be a result from systemic blood spreading or vertebral venous plexus spreading. Systemic blood pathway induced bone metastasis can happen in any bone in the body since the spreading is considered to be random. However, it remains unknown whether there is any pattern of vertebral venous plexus related bone metastasis. In this study, we explored bone metastasis patterns in patients whose primary tumors had been well identified.
Methods
We included 290 consecutive cancer patients with bone metastases but no visceral metastases, out of 2559 patients whose bone metastases were diagnosed by positron emission tomography/computed tomography, between Jan 2015 and Oct 2017 at the Fudan University Shanghai Cancer Center. We excluded those with visceral metastasis to ensure that our study focused on metastasis through the vertebral venous plexus. And we analyzed the distribution and pattern of skeletal metastases.
Results
Of the 290 patients, 28 had head and neck tumors, 178 had thorax tumors, 49 had abdominal tumors and 35 had pelvic tumors; 102 (35%) had only one bone containing a metastasis and 188 (65%) had multiple bones containing metastases. Overall, metastases to the thoracic skeleton were more common in patients with thorax tumors than in other patients (81% vs. 67%, P = 0.007); metastases to the cervical spine or thoracic bones were more common in patients with primary tumors above the diaphragm than those below the diaphragm (82% vs. 66%, P = 0.002). Among those with only one bone containing a metastasis (n = 102), patients with head and neck tumors had a higher incidence of cervical spine metastasis than other patients (25% vs. 2%, P = 0.03), those with thorax tumors had a higher incidence of thoracic bone metastasis than other patients (56% vs. 35%, P = 0.035), and those with pelvic tumors had a higher incidence of pelvis bone metastasis than other patients (78% vs. 27%, P = 0.000054).
Conclusions
In patients with only one bone containing a metastasis but no visceral metastasis, bones near the primary were more likely to be first metastasized. This may be a valuable clue to primary tumor sites in patients with cancers of unknown primaries.
Keywords: Bone metastasis, Positron emission tomography/computed tomography (PET/CT), Vertebral venous plexus
1. Introduction
The skeleton is the most common site for distant metastases from malignant tumors, with the prevalence highest in breast and prostate cancers [1], [2]. As many complications of bone metastasis, including severe bone pain, pathological fractures, spinal cord compression, and hypercalcemia, are threats to patients’ wellbeing and quality of life, early diagnosis of bone metastases is of great importance for patients [3], [4], [5].
Bone metastasis of cancer can be a result from systemic blood spreading or vertebral venous plexus spreading [6]. Metastases carried through the circulatory system may spread to any bone in the body, as this pathway is considered to be random [7]. Although numerous studies have been published about systemic circulatory bone metastases, few studies had addressed bone metastasis through the vertebral venous plexus [8], [9], [10], [11], and the pattern of vertebral venous plexus related bone metastasis had not been elucidated.
The vertebral venous plexus is generally described as a valveless network of veins that extends from cranium to pelvis [12]. The vertebral venous plexus, consisting of an external and an internal series which are in free communication [13], anastomose with the vertebral, posterior intercostal and lumbar veins [14]. The intercostal veins anastomose with the veins in the shoulder girdle [15], so tumor cells can metastasize to the shoulder girdle through the vertebral venous plexus. Although vertebral venous plexus spread bone metastasis can affect any bone in the torso, it rarely reaches the extremities. Therefore, our study excluded patients with only metastasis in their extremities, and the patterns of extremities metastasis were not included in our analysis.
Bone scan is a sensitive but not specific method [16]. And in direct comparisons with conventional bone scans, positron emission tomography (PET) revealed more lesions [17], and a combined positron emission tomography/computed tomography (PET/CT) scanner fuses PET image and CT image to depict lesions in sufficient anatomic details [18]. However, as PET/CT is not a full-body scan, but bone scanning is, we added the extra clinical data of bone scans to our analyses.
This study was undertaken to explore bone metastasis pattern in patients whose primary tumors had been well identified, in hope of providing clues to origins of primary tumors for patients with cancers of unknown primaries (CUP).
As visceral metastasis is mainly spread via the circulatory system, we excluded patients with visceral metastasis to preclude those with circulatory system-carried metastasis, and assumed that bone metastases of the remaining patients were through vertebral venous plexus, to ensure that our study was focused on debating about the vertebral venous plexus as an alternative metastatic route.
2. Patients and methods
2.1. Patients
Of 2559 consecutive cancer patients with bone metastases who were diagnosed by 18F-fluoro-2-deoxyglucose (FDG) PET/CT scans between January 1, 2015 and October 19, 2017 at Fudan University Shanghai Cancer Center, we filtered out those with visceral metastases (per pathology confirmation), multiple primary tumors, or with only bone metastases to the extremities (n = 8). Finally, we enrolled 290 patients. All patients involved in this study provided signed informed consent before their bone scan and PET/CT examination. Our study is in accordance with the Code of Ethics of the World Medical Association.
2.2. PET/CT imaging and interpretation
All the patients fasted for 4–6 h before PET/CT and their blood glucose levels were under 10 mmol/L at the time of FDG injection. Examination was initiated 1 h after i.v. injection of FDG (7.4 MBq/kg). FDG PET/CT scanning was performed on Siemens biograph 16HR PET/CT scanner (Knoxville, Tennessee, USA). First, unenhanced low-dose CT scans (120 kV automatic mA, modulation range of 130–370 mA) were acquired. Immediately after CT scans, three-dimensional PET scans were acquired (3–4 min per bed position). PET data were reconstructed iteratively by applying CT data for attenuation correction, and co-registered images were displayed on a workstation.
Images were reviewed and manipulated in a multimodality computer platform (Syngo, Siemens, Knoxville, Tennessee, USA). Two experienced nuclear medicine physicians, unaware of patients’ clinical information, evaluated the images independently. The reviewers reached a consensus in cases of discrepancy. FDG uptakes of lesions were measured as the maximum standardized uptake value (SUVmax).
Criteria for diagnosing of skeletal metastases by FDG PET/CT are increased standardized uptake value (SUV) on PET image, and osteoblastic lesions, osteolytic lesions, mixed osteoblastic/osteolytic lesions, or no demonstrable anatomical change on CT image. Presence of fracture lines or callus formation was interpreted as a fracture.
2.3. Methods
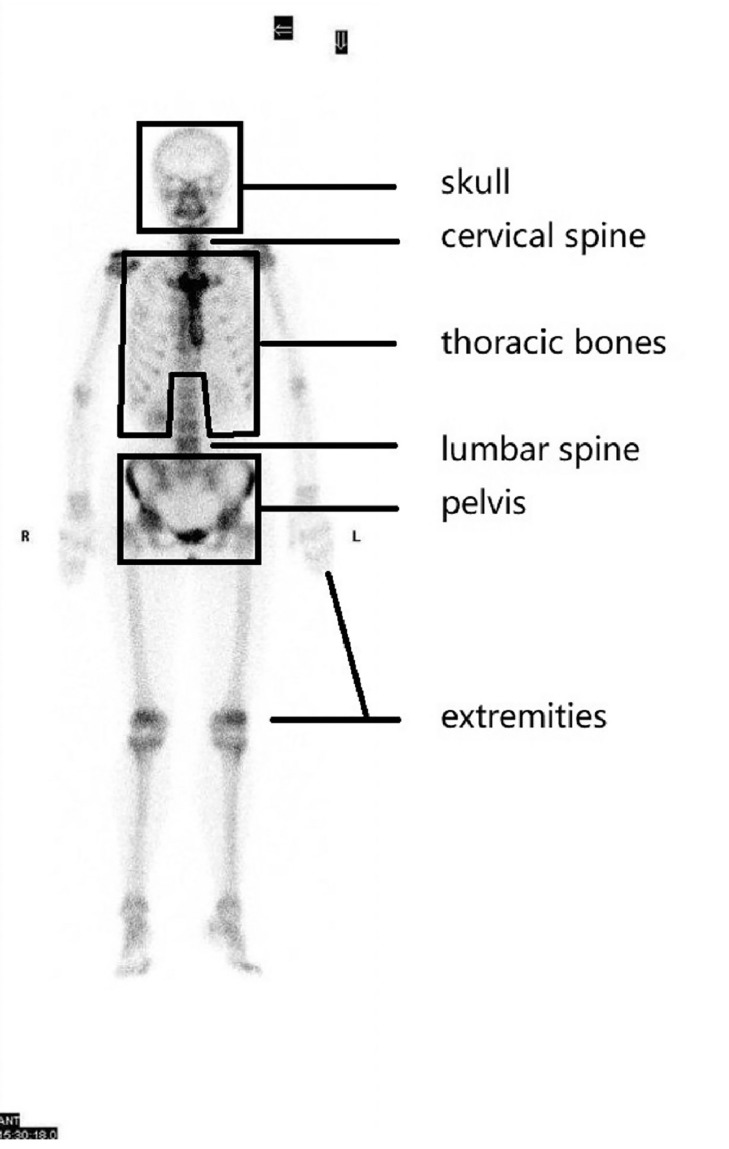
The clinical data of the 290 patients including age, gender, pathological diagnosis reports and the results of PET/CT and bone scanning were retrospectively analyzed. The patients were divided into four groups according to primary tumor regions: head and neck, thorax, abdomen and pelvis. These classifications are shown in Table 1. The skeleton was classified into six regions: skull (including bones of the cerebral cranium and facial cranium); cervical spine; thoracic bones (including ribs, sternum, collarbone, bladebone and thoracic spine); lumbar spine; pelvis (including sacrococcyx, ilium, ischium, and pubis); and extremities (including humerus, femur, radioulnar, and tibiofibular) (Fig. 1). According to the results of PET/CT and bone scanning of the 298 patients, distribution and pattern of skeletal metastases were analyzed.
Table 1.
Characteristics of the patients (N = 290).
| Characteristics | Number | Percentage |
|---|---|---|
| Sex | ||
| Male | 126 | 43.4% |
| Female | 164 | 57.6% |
| Age(years) | ||
| Median | 53 | |
| Range | 14–95 | |
| Primary cancer | ||
| Head and neck tumors (N = 28) | ||
| Nasopharyngeal cancer | 24 | 8.3% |
| Thyroid cancer | 2 | 0.7% |
| Malignant melanoma (behind ear) | 1 | 0.3% |
| Laryngeal cancer | 1 | 0.3% |
| Thorax tumors (N = 178) | ||
| Breast cancer | 98 | 33.8% |
| Lung cancer | 67 | 23.1% |
| Esophageal cancer | 12 | 4.1% |
| Thymus cancer | 1 | 0.3% |
| Abdominal tumors (N = 49) | ||
| Gastric cancer | 28 | 9.7% |
| Liver cancer | 10 | 3.4% |
| Colorectal cancer | 7 | 2.4% |
| Kidney cancer | 4 | 1.4% |
| Pelvic tumors (N = 35) | ||
| Cervical cancer | 21 | 7.2% |
| Prostate cancer | 8 | 2.8% |
| Ovarian cancer | 3 | 1.0% |
| Scrotal cancer | 1 | 0.3% |
| Endometrial cancer | 1 | 0.3% |
| Bladder Cancer | 1 | 0.3% |
Fig. 1.
Six regions of human skeleton: skull, cervical spine, thoracic bones, lumbar spine, pelvis and extremities.
2.4. Statistical analysis
The chi-square test was performed to compare differences in proportions of skeletal metastases between different groups using SPSS statistical software (version 20.0). And P values less than 0.05 was considered statistically significant.
3. Results
Of the enrolled 290 patients in the study, 126 were males and 164 were females. Their average age was 53 years old (range: 14–95 years old). Of the total 290 patients, we included 28 patients (9.7%) with head and neck tumors, 178 (61.4%) with thorax tumors, 49 (16.9%) with abdominal tumors and 35 (12.1%) with pelvic tumors. Overall, 102 (35.2%) patients had only one bone containing a metastasis and 188 (64.8%) had multiple bones containing metastases. Table 1 summarizes their characteristics.
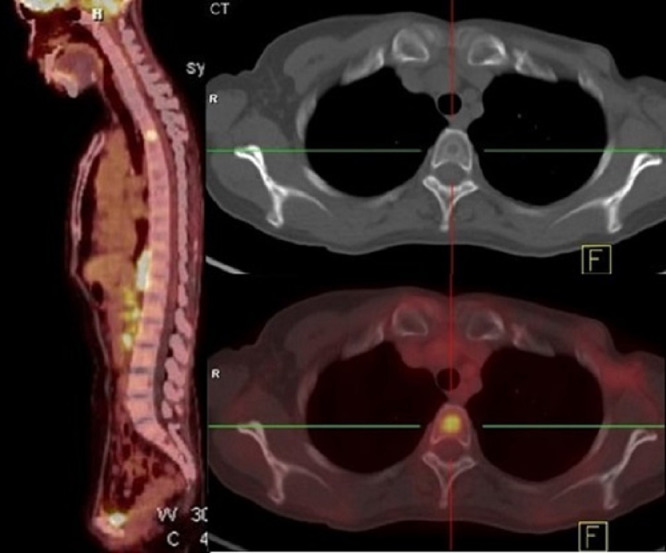
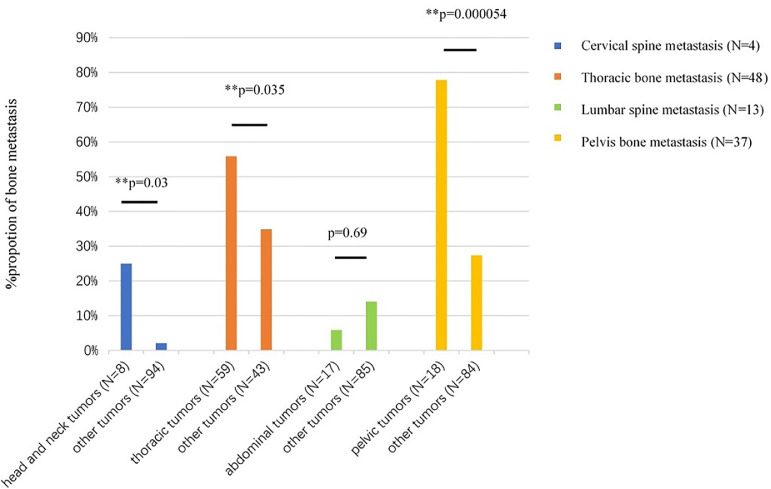
Of the 102 patients with only one bone containing a metastasis, 8 (7.8%) had head and neck tumors, 59 (57.8%) had thorax tumors, 17 (16.7%) had abdominal tumors and 18 (17.6%) had pelvic tumors. As is show in the Fig. 2, one breast cancer patient had only one bone metastasis in the thoracic spine (T3). Among these patients, no patient had only skull metastasis, 4 (3.9%) had only cervical spine metastasis, 48 (47.1%) had only thoracic bones metastasis, 13 (12.7%) had only lumbar spine metastasis, and 37 (36.3%) had only pelvis metastasis.
Fig. 2.
PET/CT images of a patient with breast cancer who had only one bone metastasis, in her thoracic spine (T3).
Of the 102 patients with only one bone containing a metastasis, patients with head and neck tumors had a higher incidence of cervical spine metastasis than other patients (25.0% vs. 2.1%, P = 0.03), patients with thorax tumors had a higher incidence of thoracic bone metastasis than other patients (55.9% vs. 34.9%, P = 0.035), and patients with pelvic tumors had a higher incidence of pelvis bone metastasis than other patients (77.8% vs. 27.4%, P = 0.000054). Cervical spine or thoracic bones were more frequently involved in patients with primary tumors above the diaphragm than those below the diaphragm (61.2% vs. 31.4%, P = 0.004). Lumbar spine or pelvis was more frequently metastasized in patients with primary tumors below the diaphragm than those above the diaphragm (68.6% vs. 38.8%, P = 0.004) (Fig. 3).
Fig. 3.
Metastases of neighboring bones by tumors in different parts of the body among the 102 patients who had only one bone containing a metastasis. **P<0.05.
Among the 290 patients as a whole, distribution of bone metastasis between the four primary-site groups differed significantly. In the head and neck group (n = 28), 8 patients (28.6%) had metastasis of the cervical spine; in the thorax group (n = 178), 144 patients (80.9%) had metastasis of thoracic bones; in the abdominal group (n = 49), 28 patients (57.1%) had metastasis of lumbar spine; and in the pelvic group (n = 35, 12.1%), 29 patients (82.9%) had the metastasis of the pelvis.
Overall, the thoracic skeleton was more frequently metastasized in patients with thorax tumors than the other patients (80.9% vs. 67.0%, P = 0.007). The cervical spine or thoracic bones were more frequently metastasized in patients with primary tumors above the diaphragm than those below the diaphragm (82.0% vs. 65.5%, P = 0.002). Patients with pelvic tumors had a higher incidence of pelvis metastasis than other patients, but not significantly so (82.9% vs. 68.2%, P = 0.077) (Table 2).
Table 2.
The distribution of bone metastases among the total 290 patients.
| Skull metastasis | Cervical spine metastasis | Thoracic bones metastasis | Lumbar spine metastasis | Pelvis metastasis | Extremities metastasis | |
|---|---|---|---|---|---|---|
| Head and neck tumors (N = 28) | 3 | 8 | 20 | 17 | 22 | 9 |
| Thorax tumors (N = 178) | 13 | 56 | 144 | 104 | 116 | 49 |
| Abdominal tumors (N = 49) | 6 | 23 | 41 | 28 | 36 | 23 |
| Pelvic tumors (N = 35) | 2 | 4 | 14 | 15 | 29 | 6 |
As our cohort had many patients with breast cancer (98 cases, 33.8%) and lung cancer (67 cases, 23.1%), we had a large percentage of thorax tumors (178 cases, 61.4%). We therefore compared bone metastasis distributions between breast and lung cancers. Although thoracic bones are predilection sites of these two cancers, we found breast cancer was more likely than lung cancer to metastasize to thoracic bone (88.8% vs. 70.1%, P = 0.003) and lumbar spine (66.3% vs. 49.3%, P = 0.028) (Fig. 4).
Fig. 4.
Distribution of bone metastases among patients with breast cancer (N = 98) and lung cancer (N = 67). **P<0.05.
4. Discussion
Reportedly, about two-thirds of patients with cancer will develop bone metastasis [19], and bone metastasis complications are sometimes the first manifestation of an occult primary tumor [20], [21]. Thus, exploring the distribution features of bone metastasis is important. Although studies on bone metastasis have been published for different tumor types, such as breast cancer [22], prostate cancer [23] and others [24], [25], this study addresses primary tumors in all bodily parts.
Our cohort excluded patients with visceral metastasis to focus on bone metastasis that were likely spread by the vertebral venous plexus, and we found that the metastases are usually found in the bones close to the primary tumor, which is reminiscent of the first-pass organ model for metastatic dissemination. The most frequently involved area was the spine, followed by the pelvis and thoracic bones, which is consistent with previous reports of bone metastases spread by the circulatory system [26], [27].
Especially among patients with only one bone containing a metastasis but no visceral metastases, we observed the characteristics of bone metastatic distribution that neighboring bones to the primary were more likely to be first metastasized. This also correspond to previous findings that the differences in the distribution of bone metastases between pulmonary, breast and prostate cancers [7], [11].
Hematogenous spreading is the most common pathway for bone metastasis, in addition, vertebral venous plexuses play very important roles. Due to the low pressure, large volume, slow blood flow and abundant vessel branches of the vertebral venous plexus, tumor cells can easier transfer to nearby bones. Whether the bone metastasis patterns of cancer patients with bone metastasis but no visceral metastasis are clues to primary tumor locations in patients with CUP warrants further investigation.
Although we found no evidence that indicates the skull and lumbar spine were more frequently metastasized from certain tumors, among patients with only one bone containing a metastasis, the cervical spine was more frequently metastasized in patients with head and neck tumors than in other patients, the thoracic skeleton was more frequently metastasized in patients with thorax tumors than in other patients, and the pelvis was more frequently metastasized in patients with pelvic tumors than in other patients. These distributions may provide valuable clues to primary tumor locations in patients with CUPs, especially those with single or few bone metastases; the CUP is likely to be in a neighboring organ.
Patients whose bones metastases were limited to thoracic region were significantly more likely to have a primary thoracic tumor. Similarly, among patients with only one involved bone, patients with pelvic primary tumors were significantly more likely to have pelvic bone metastases, and those with cervical area primary tumors were significantly more likely to have cervical spine bone metastases; however, this was not true of patients with multiple involved bones. Therefore, single but not multiple bone metastases may be clues to the locations of primary tumors in patients with CUPs.
Differences between bone metastasis distributions of breast cancer and lung cancer may show the discrepancy between the vertebral venous plexus spreading bone metastasis pattern of tumors in thoracic cavity and out of thoracic cavity, which may reflect different drainage routes of vertebral venous plexus from intrathoracic tumors and breast cancer, and this may warrant further research.
Although tumors are generally incurable once they have metastasized to bone [28], [29], exact targeting of the primary tumor can help to develop more individualized treatments [30]. Even if a cure is no longer possible, treating the cancer may help the patient live longer and feel better [31], [32].
Our study had some limitations. Notably, our cohort had a larger proportion of thorax tumors and low proportion of other tumors, which seems to reflect the higher bone metastatic rate of breast cancer and lung cancer; however, this distribution may have biased the results. Therefore, a study based on a larger cohort with more varied primary tumors is warranted.
5. Conclusion
We observed characteristics of bone metastatic distribution in patients with only one bone containing a metastasis but no visceral diseases. And we found that bones near the primary were more likely to be first metastasized. This may provide a valuable clue to the primary tumor in patients with cancers of unknown primaries.
Financial support
None.
Authors' contributions
MYZ, XL, YQ participated in acquiring, analyzing and interpreting data, and drafting the manuscript. XL, SLH, YJZ, WTL, XYZ, HJY, LPZ, QFW, YFH, YC, YLW, YHW, ZWL, ZGL, and XCH contributed to conception and design the study, and enhanced its intellectual content. MYZ, XL, SLH, and XCH revised the manuscript. All authors read and approved the final content of the manuscript.
Conflict of interest statement
The authors declare no conflicts of interest.
Funding
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Contributor Information
Zhiguo Luo, Email: luozhiguo88@aliyun.com.
Xichun Hu, Email: xchu2009@hotmail.com.
References
- 1.Mundy G.R. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 2.Coleman R.E. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001;27:165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 3.Boyce B.F., Yoneda T., Guise T.A. Factors regulating the growth of metastatic cancer in bone. Endocr. Relat. Cancer. 1999;6:333–347. doi: 10.1677/erc.0.0060333. [DOI] [PubMed] [Google Scholar]
- 4.Rubens R.D. Bone metastases – the clinical problem. Eur. J. Cancer. 1998;34:210–213. doi: 10.1016/s0959-8049(97)10128-9. [DOI] [PubMed] [Google Scholar]
- 5.Yin J.J., Pollock C.B., Kelly K. Mechanisms of cancer metastasis to the bone. Cell Res. 2005;15:57–62. doi: 10.1038/sj.cr.7290266. [DOI] [PubMed] [Google Scholar]
- 6.Maccauro G., Spinelli M.S., Mauro S., Perisano C., Graci C., Rosa M.A. Physiopathology of spine metastasis. Int. J. Surg. Oncol. 2011;2011 doi: 10.1155/2011/107969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kakhki V.R., Anvari K., Sadeghi R., Mahmoudian A.S., Torabian-Kakhki M. Pattern and distribution of bone metastases in common malignant tumors. Nucl. Med. Rev. Cent. East. Eur. 2013;16:66–69. doi: 10.5603/NMR.2013.0037. [DOI] [PubMed] [Google Scholar]
- 8.Wilson M.A., Calhoun F.W. The distribution of skeletal metastases in breast and pulmonary cancer: concise communication. J. Nucl. Med. 1981;22:594–597. [PubMed] [Google Scholar]
- 9.Galasko C.S. Skeletal metastases and mammary cancer. Ann. R. Coll. Surg. Engl. 1972;50:3–28. [PMC free article] [PubMed] [Google Scholar]
- 10.Boxer D.I., Todd C.E., Coleman R., Fogelman I. Bone secondaries in breast cancer: the solitary metastasis. J. Nucl. Med. 1989;30:1318–1320. [PubMed] [Google Scholar]
- 11.Wang C.Y., Shen Y., Zhu S.B. Distribution features of skeletal metastases: a comparative study between pulmonary and prostate cancers. Plos One. 2015:10. doi: 10.1371/journal.pone.0143437. ARTNe0143437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckenhoff J.E. The vertebral venous plexus. Can. Anaesth. Soc. J. 1971;18:487–495. [Google Scholar]
- 13.Cooper E.R. The vertebral venous plexus. Acta Anat. (Basel) 1960;42:333–351. doi: 10.1159/000141669. [DOI] [PubMed] [Google Scholar]
- 14.Abrams H.L. The vertebral and azygos venous systems, and some variations in systemic venous return. Radiology. 1957;69:508–526. doi: 10.1148/69.4.508. [DOI] [PubMed] [Google Scholar]
- 15.Batson O.V. The function of the vertebral veins and their role in the spread of metastases. Ann. Surg. 1940;112:138–149. doi: 10.1097/00000658-194007000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franzius C., Sciuk J., Daldrup-Link H.E., Jurgens H., Schober O. FDG-PET for detection of osseous metastases from malignant primary bone tumours: comparison with bone scintigraphy. Eur. J. Nucl. Med. 2000;27:1305–1311. doi: 10.1007/s002590000301. [DOI] [PubMed] [Google Scholar]
- 17.Nakamoto Y., Osman M., Wahl R.L. Prevalence and patterns of bone metastases detected with positron emission tomography using F-18FDG. Clin. Nucl. Med. 2003;28:302–307. doi: 10.1097/01.RLU.0000057556.54046.7A. [DOI] [PubMed] [Google Scholar]
- 18.Hamaoka T., Madewell J.E., Podoloff D.A., Hortobagyi G.N., Ueno N.T. Bone imaging in metastatic breast cancer. J. Clin. Oncol. 2004;22:2942–2953. doi: 10.1200/JCO.2004.08.181. [DOI] [PubMed] [Google Scholar]
- 19.Shaw B., Mansfield F.L., Borges L. One-stage posterolateral decompression and stabilization for primary and metastatic vertebral tumors in the thoracic and lumbar spine. J. Neurosurg. 1989;70:405–410. doi: 10.3171/jns.1989.70.3.0405. [DOI] [PubMed] [Google Scholar]
- 20.Carretero R.G., Brugera M.R., Rebollo-Aparicio N., El Bouayadi Mohamed L. Primary bone metastasis as first manifestation of an unknown primary tumour. BMJ Case Rep. 2015;2015 doi: 10.1136/bcr-2015-211302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pirici E., Pirici A., Patrana N., Recareanu F., Badulescu F., Crisan A.E., Zaharie A.S. Vertebral bone metastasis in breast cancer: a case report. Rom. J. Morphol. Embryol. 2011;52:897–905. [PubMed] [Google Scholar]
- 22.Musat E., Stefanescu C., Rusu V. Whole-body bone scintigraphy in the diagnosis and follow-up of the evolution of breast cancer. Rev. Med. Chir. Soc. Med. Nat. Iasi. 1999;103:163–169. [PubMed] [Google Scholar]
- 23.Wang C., Shen Y. Study on the distribution features of bone metastases in prostate cancer. Nucl. Med. Commun. 2012;33:379–383. doi: 10.1097/MNM.0b013e3283504528. [DOI] [PubMed] [Google Scholar]
- 24.Osorio M., Moubayed S.P., Su H., Urken M.L. Systematic review of site distribution of bone metastases in differentiated thyroid cancer. Head Neck. 2017;39:812–818. doi: 10.1002/hed.24655. [DOI] [PubMed] [Google Scholar]
- 25.Al Tamimi A.S., Zaheer S., Ng D.C., Osmany S. (18)F-Fluorodeoxyglucose-positron Emission tomography/computed tomography imaging of metastatic nasopharyngeal cancer with emphasis on the distribution of bone metastases. World J. Nucl. Med. 2017;16:192–196. doi: 10.4103/1450-1147.207273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tofe A.J., Francis M.D., Harvey W.J. Correlation of neoplasms with incidence and localization of skeletal metastases: an analysis of 1,355 diphosphonate bone scans. J. Nucl. Med. 1975;16:986–989. [PubMed] [Google Scholar]
- 27.Rusu V., Boiculese L.V., Stefanescu C., Hountis D., Costin M. A retrospective study of bone metastases distribution on 420 whole body scans. Rev. Med. Chir. Soc. Med. Nat. Iasi. 2004;108:114–117. [PubMed] [Google Scholar]
- 28.Weilbaecher K.N., Guise T.A., McCauley L.K. Cancer to bone: a fatal attraction. Nat. Rev. Cancer. 2011;11:411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roodman G.D. Mechanisms of bone metastasis. N. Engl. J. Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L., Gong Z. Clinical characteristics and prognostic factors in bone metastases from lung cancer. Med. Sci. Monit. 2017;23:4087–4094. doi: 10.12659/MSM.902971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gdowski A.S., Ranjan A., Vishwanatha J.K. Current concepts in bone metastasis, contemporary therapeutic strategies and ongoing clinical trials. J. Exp. Clin. Cancer Res. 2017;36:108. doi: 10.1186/s13046-017-0578-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y.C., Sosnoski D.M., Mastro A.M. Breast cancer metastasis to the bone: mechanisms of bone loss. Breast Cancer Res. 2010;12:215. doi: 10.1186/bcr2781. [DOI] [PMC free article] [PubMed] [Google Scholar]