Abstract
Background and Aims
This GEMINI 1 post hoc analysis evaluated vedolizumab efficacy for inducing deep remission in patients with ulcerative colitis and correlation between vedolizumab trough concentrations and deep remission rates.
Methods
Week 6 vedolizumab responders were re-randomized to placebo or vedolizumab every 8 or 4 weeks. Deep remission at Week 52 was measured using four different definitions [from most to least stringent]: [1] Mayo Clinic endoscopic score = 0, rectal bleeding score = 0 and decrease or no change from baseline in stool frequency score; [2] endoscopic score ≤1, rectal bleeding score = 0 and stool frequency score = 0; [3] endoscopic score ≤1, rectal bleeding score = 0, decrease or no change from baseline stool frequency score, and total score [endoscopic score + rectal bleeding score + stool frequency score] ≤1; and [4] endoscopic score ≤1, rectal bleeding score = 0 and stool frequency score ≤1. Steady-state trough vedolizumab serum concentrations were evaluated.
Results
At Week 6, 373 vedolizumab responders were re-randomized to maintenance placebo [n = 126] or vedolizumab every 8 [n = 122] or 4 [n = 125] weeks. Significantly more vedolizumab patients achieved deep remission at Week 52 for the most (placebo 8.7%, every 8 weeks 27.0% [p = 0.0001], every 4 weeks 28.0% [p < 0.0001]) and least (placebo 15.9%, every 8 weeks 43.4% [p < 0.0001], every 4 weeks 43.2% [p < 0.0001]) stringent definitions. Patients with higher vedolizumab trough concentration quartiles had higher deep remission rates [all definitions] compared with those with the lowest quartile or who received placebo.
Conclusion
Vedolizumab was associated with significantly higher deep remission rates than placebo at Week 52, regardless of deep remission definition [NCT00783718].
Keywords: Clinical trials, vedolizumab, ulcerative colitis
1. Introduction
Deep remission is emerging as an important treatment goal for patients with ulcerative colitis [UC] to improve outcomes beyond clinical remission and towards modifying the disease course.1
The currently accepted definition of clinical remission includes normalization of patient-reported symptoms [rectal bleeding and stool frequency] and endoscopic healing.2 Regulatory agencies support such a composite of symptomatic and endoscopic improvement as a clinical trial outcome in the evaluation of treatment for UC. The US Food and Drug Administration [FDA] guidance on clinical remission as an endpoint in clinical trials recommends a definition based on the Mayo Clinic Score consisting of a rectal bleeding score [RBS] of 0, a stool frequency score [SFS] of 0 and an endoscopic score [ES] of 0 or 1, with a score of 0 on the UC Disease Activity Index [UCDAI] as an alternative.3 The European Medicines Agency [EMA] guidance on the definition of remission in UC also cites the Mayo Clinic Score with a preferred definition of remission as an ES of 0, although a score of 0 or 1 on the ES, RBS or SFS is also an acceptable definition.4 However, currently there is no standardized definition for assessing deep remission in UC.5,6 In principle, deep remission is intended to represent complete disease quiescence with ES, RBS and SFS of 0 [although it is debatable whether increased stool frequency is completely reversible, probably due to structural damage in some patients] and should be the clinical target that physicians strive to achieve for their patients. More analyses are needed to assess the utility of deep remission as a clinical outcome in patients with UC.
Moreover, studies of anti-tumour necrosis factor alpha [TNFα] agents have also suggested a positive correlation between high trough serum drug concentrations and favourable therapeutic outcomes, which include endoscopic healing.7,8 A study by Paul et al. showed that mean trough serum concentrations of infliximab were higher in patients with UC in clinical remission [1.9 µg/mL] compared with patients with active disease [0.9 µg/mL; p = 0.01].7 The impact of therapeutic drug monitoring on clinical remission was also demonstrated in a distinct cohort of patients who required dose intensification. An increase in infliximab trough levels was strongly associated with clinical remission after infliximab dose optimization [p = 0.0001].7 A recent analysis confirmed that infliximab trough concentrations during maintenance therapy were associated with endoscopic and histological healing in patients with UC.9 The association between drug trough serum concentrations and improved clinical and endoscopic outcomes suggests that incorporation of therapeutic drug monitoring into clinical practice may allow clinicians to optimize treatment by maintaining effective drug concentrations over time. However, the usefulness of proactive drug monitoring has not been established.
Vedolizumab [ENTYVIO; Takeda Pharmaceuticals] is a gut-selective humanized monoclonal antibody that targets α4β7 integrin. The efficacy of vedolizumab in achieving clinical remission and endoscopic healing in patients with UC has been established,10 with 52-week rates of clinical remission of 41.8% and 44.8% for those receiving vedolizumab 300 mg every 8 weeks [Q8W] and every 4 weeks [Q4W], respectively, and mucosal healing rates of 51.6% and 56.0%, respectively. Vedolizumab has been recommended by recent guidelines from the European Crohn’s and Colitis Organisation [ECCO] as a treatment option for patients with UC11 and is included in the American Gastroenterological Association [AGA] Care Pathway as induction and maintenance therapy for patients with UC at high risk of colectomy.12
In previous studies, higher rates of endoscopic healing were associated with higher vedolizumab trough serum concentrations in patients with UC.13,14 Understanding the relationship between trough serum drug concentrations and deep remission may help to inform clinicians about optimal treatment strategies, thereby increasing the likelihood of achieving this favourable outcome in patients with UC.
Here, we describe a post hoc analysis of the phase 3 GEMINI 1 trial10 to determine the efficacy of vedolizumab in inducing deep remission at Week 52 in patients with UC. The correlation between vedolizumab trough serum concentrations and rates of deep remission was also explored.
2. Materials and Methods
2.1. GEMINI 1 study design and analysis populations
The GEMINI 1 study [NCT00783718] evaluated the efficacy and safety of vedolizumab 300 mg in the treatment of patients with UC as induction and maintenance therapy for up to 52 weeks.10 The design and outcomes of this trial have been described previously10; the protocol was approved by an investigational review board at each study centre, and all patients provided their written informed consent. For the current study, all authors had access to the study data and approved the final manuscript.
Briefly, patients with moderately to severely active UC received vedolizumab induction therapy consisting of either double-blind or open-label vedolizumab 300 mg at Weeks 0 and 2. Patients with a clinical response at Week 6 were then re-randomized to placebo or maintenance vedolizumab 300 mg Q8W or Q4W with no dose escalation, and patients who did not achieve a clinical response by Week 6 received open-label vedolizumab 300 mg Q4W. All patients were followed to Week 52. The induction intention-to-treat [ITT] population consisted of all randomized patients who received any amount of blinded study drug during Weeks 0–6. The maintenance ITT population consisted of all randomized patients who received vedolizumab during the induction phase, met the protocol definition of clinical response at Week 6, were randomized and received any amount of double-blind study drug in the maintenance phase.
This analysis included all vedolizumab-treated patients who were responders at Week 6 [induction ITT population; Supplementary Figure S1, cohort 1] or to open-label induction therapy [Supplementary Figure S1, cohort 2].
2.2. Analysis endpoints
Four definitions of deep remission in the maintenance ITT population were delineated based on the stringency of remission criteria. The four definitions are listed in order from most stringent to least stringent in Table 1. End points were defined using various combinations of endoscopic outcomes [Mayo Clinic ES] and patient-reported outcomes [Mayo Clinic RBS and SFS]. Endoscopic appearance was evaluated at Weeks 0, 6 and 52, and categorized according to Mayo Clinic criteria as: 0 for normal/inactive disease; 1 for mild disease [erythema, decreased vascular pattern, mild friability]; 2 for moderate disease [marked erythema, lack of vascular pattern, friability, erosions]; and 3 for severe disease [spontaneous bleeding, ulceration]. Histological analyses and central evaluation of endoscopies were not included in the GEMINI 1 study protocol; therefore, these data were not available for consideration in the present analyses. Symptomatic data [rectal bleeding and stool frequency] were collected daily via an automated telephone diary system. At Week 52, RBS and SFS were calculated using diaries from the previous 7 days. Scores ranged from 0 to 3, with higher scores indicating greater disease activity. Available scores from the two or three most recent days were averaged and rounded to the nearest integer.
Table 1.
Deep remissiona end points at Week 52
| Deep remission end points | Endoscopic criteria | Patient-reported outcomes |
|---|---|---|
| 1. Endoscopic remission and symptomatic improvement | Mayo Clinic ES = 0 | RBS = 0 and decrease or no change from baseline in SFS |
| 2. Endoscopic improvement and symptomatic remission | Mayo Clinic ES ≤ 1 | RBS = 0 and SFS = 0 |
| 3. Endoscopic and symptomatic improvement with total score [ES + RBS + SFS] ≤ 1 | Mayo Clinic ES ≤ 1 | RBS = 0, decrease or no change from baseline in SFS, and total score [ES + RBS + SFS] ≤ 1 |
| 4. Endoscopic and symptomatic improvement | Mayo Clinic ES ≤ 1 | RBS = 0 and SFS ≤ 1 |
ES, endoscopic score; ITT, intention to treat; RBS, rectal bleeding score; SFS, stool frequency score.
aDeep remission was assessed in the maintenance ITT population of the GEMINI 1 study [NCT00783718].
Blood samples were drawn prior to vedolizumab infusion at Week 46 [steady-state] to determine trough serum concentrations. Vedolizumab concentrations in serum samples were measured using a direct-capture enzyme-linked immunosorbent assay [ELISA] with a lower limit of detection of 0.125 μg/mL.15 Faecal samples were collected at Weeks 0, 6, 30 and 52 to determine faecal calprotectin levels using ELISA [CAL0100 Kit].16
2.3. Statistical analysis
Clinical outcomes were analysed using observed cases at Week 52. Patients with missing data points for the Mayo Clinic score were classified as ‘not in remission’.
Descriptive statistics were calculated for patient demographics and clinical characteristics. The proportion of patients achieving deep remission (with 95% confidence intervals [CIs]) were derived for each definition. In additional analyses, deep remission rates were stratified by vedolizumab trough serum concentration quartiles at Week 46 and by faecal calprotectin levels [≤50 µg/g, >50–150 µg/g, >150–250 µg/g, >250 µg/g]. Intergroup differences were evaluated with the chi-squared test.
A concordance analysis between patient-reported symptoms [RBS and SFS] and ES at Week 52 was carried out by calculating the positive predictive value [PPV], negative predictive value [NPV], sensitivity and specificity for an RBS of 0, SFS subscore of 0 and a composite score [RBS + SFS] of 0.
3. Results
3.1. Baseline demographics and patient characteristics
At Week 6, a total of 373 patients responded to vedolizumab induction therapy and were re-randomized to maintenance placebo (n = 126; median age, 39.9 years [range 18.0–74.0], 45.2% female), vedolizumab Q8W (n = 122; median age, 39.7 years [range 19.0–78.0]; 43.0% female) or vedolizumab Q4W (n = 125; median age, 35.7 years [range 19.0–76.0]; 46.0% female). Baseline demographics and patient characteristics are provided in Table 2.
Table 2.
Baseline demographics and patient characteristics
| Characteristic | Placeboa n = 126 |
VDZ Q8W n = 122 |
VDZ Q4W n = 125 |
|---|---|---|---|
| Age [years], median [range] | 39.9 [18.3–73.7] | 39.7 [18.8–77.7] | 35.7 [18.7–76.3] |
| Female sex, n [%] | 57 [45.2] | 52 [42.6] | 57 [45.6] |
| BMI [kg/m2], mean [SD] | 25.8 [6.1] | 26.8 [6.3] | 24.5 [4.7] |
| UC duration [years], mean [SD] | 7.8 [6.9] | 6.2 [4.8] | 7.6 [7.0] |
| Complete Mayo Clinic score, mean [SD] | 8.4 [1.8] | 8.4 [1.8] | 8.3 [1.7] |
| RBS, mean [SE] | 1.6 [0.1] | 1.6 [0.1]b | 1.5 [0.1] |
| SFS, mean [SE] | 2.1 [0.1] | 2.1 [0.1]b | 2.0 [0.1] |
| Site of disease, n [%] | |||
| Proctosigmoiditis | 9 [7.1] | 18 [14.8] | 14 [11.2] |
| Left-sided colitis | 53 [42.1] | 51 [41.8] | 45 [36.0] |
| Extensive colitis | 17 [13.5] | 14 [11.5] | 14 [11.2] |
| Pancolitis | 47 [37.3] | 39 [32.0] | 52 [41.6] |
| Faecal calprotectin level [µg/g], median [range] | 1070.9 [23.8–20000.0] | 863.7 [34.9–18061.8] | 793.0 [23.8–20000.0] |
| Prior therapies, n [%] | |||
| Anti-TNFα agents | 46 [36.5] | 51 [41.8] | 46 [36.8] |
| Immunosuppressants | 100 [79.4] | 91 [74.6] | 99 [79.2] |
| Corticosteroids only | 120 [95.2] | 118 [96.7] | 122 [97.6] |
| Prior treatment failure, n [%] | |||
| Anti-TNFα agents | 38 [30.2] | 43 [35.2] | 40 [32.0] |
| Immunosuppressants | 61 [48.4] | 56 [45.9] | 60 [48.0] |
| Corticosteroids only | 26 [20.6] | 19 [15.6] | 25 [20.0] |
| Baseline medications, n [%] | |||
| Immunosuppressants | 51 [40.5] | 43 [35.2] | 45 [36.0] |
| Corticosteroids | 72 [57.1] | 70 [57.4] | 73 [58.4] |
BMI, body mass index; RBS, rectal bleeding score; SD, standard deviation; SE, standard error; SFS, stool frequency score; TNFα, tumour necrosis factor alpha; VDZ, vedolizumab.
aPatients received VDZ at Weeks 0 and 2.
b n = 119.
3.2. Deep remission rates
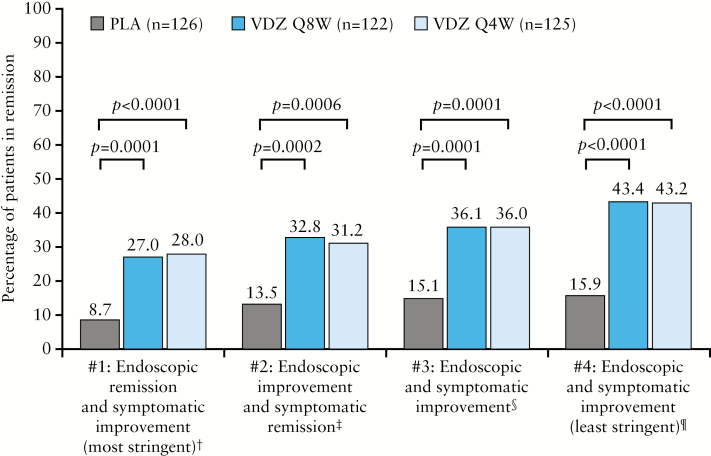
Vedolizumab treatment led to statistically significant and clinically meaningful improvements at Week 52 compared with placebo, according to all four definitions of deep remission [Figure 1]. At Week 52, at least twice as many vedolizumab-treated patients were in deep remission compared with patients who received placebo. For the most stringent definition of deep remission [definition 1: Mayo Clinic ES = 0, RBS = 0 and decrease in SFS or no change from baseline], 8.7% of patients randomized to placebo for the maintenance phase achieved deep remission compared with 27.0% and 28.0% of those randomised to vedolizumab Q8W [p = 0.0001] and vedolizumab Q4W [p < 0.0001], respectively. For the least stringent definition [definition 4: Mayo Clinic ES ≤ 1, RBS = 0 and SFS ≤ 1], 15.9% of those randomized to placebo achieved deep remission compared with 43.4% and 43.2% of those randomised to vedolizumab Q8W and Q4W, respectively [p < 0.0001 for both comparisons].
Figure 1.
Deep remission at Week 52a,b [maintenance ITT population]. One patient randomized to PLA still had active drug concentrations [9 μg/mL] at Week 46 and they were in remission according to all four definitions at Week 52. ES, endoscopic score; ITT, intention to treat; PLA, placebo; Q4W, every 4 weeks; Q8W, every 8 weeks; RBS, rectal bleeding score; SFS, stool frequency score; VDZ, vedolizumab. aPost hoc analyses; ball patients in the maintenance ITT population, including those in the PLA group, had received VDZ at Weeks 0 and 2 and had a clinical response at Week 6; †definition 1: Mayo Clinic ES = 0, RBS = 0 and decrease in SFS or no change from baseline [A]; ‡definition 2: Mayo Clinic ES ≤ 1, RBS = 0 and SFS = 0 [B]; §definition 3: Mayo Clinic ES ≤ 1, RBS = 0, decrease in SFS or no change from baseline, and total score [ES + RBS + SFS] ≤ 1 [C]; ¶definition 4: Mayo Clinic ES ≤ 1, RBS = 0 and SFS ≤ 1 [D].
3.3. Endoscopic score during the maintenance phase
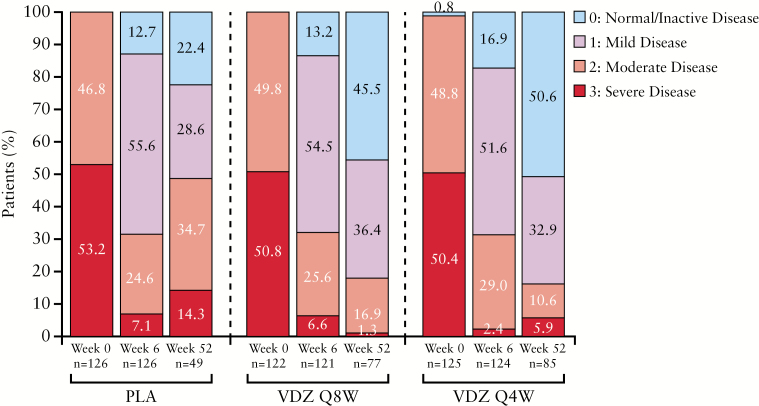
ES during the maintenance phase for the three treatment arms is shown in Figure 2. Among those who had endoscopy at Week 52, 45.5% and 50.6% of patients had an ES of 0 in the vedolizumab Q8W and Q4W groups, respectively, vs 22.4% in the placebo group. For patients with an ES of 0 or 1, the corresponding figures were 81.9% and 83.5%, respectively, vs 51.0%.
Figure 2.
Endoscopic appearance in the maintenance phase for patients with UC in the GEMINI 1 trial.a,b ITT, intention to treat; PLA, placebo; Q4W, every 4 weeks; Q8W, every 8 weeks; UC, ulcerative colitis; VDZ, vedolizumab. aPercentages for each timepoint may not add up to 100% due to rounding; ball patients in the maintenance ITT population, including those in the placebo group, had received vedolizumab at Weeks 0 and 2 and had a clinical response at Week 6.
3.4. Comparison of endoscopic, rectal bleeding and stool frequency subscores
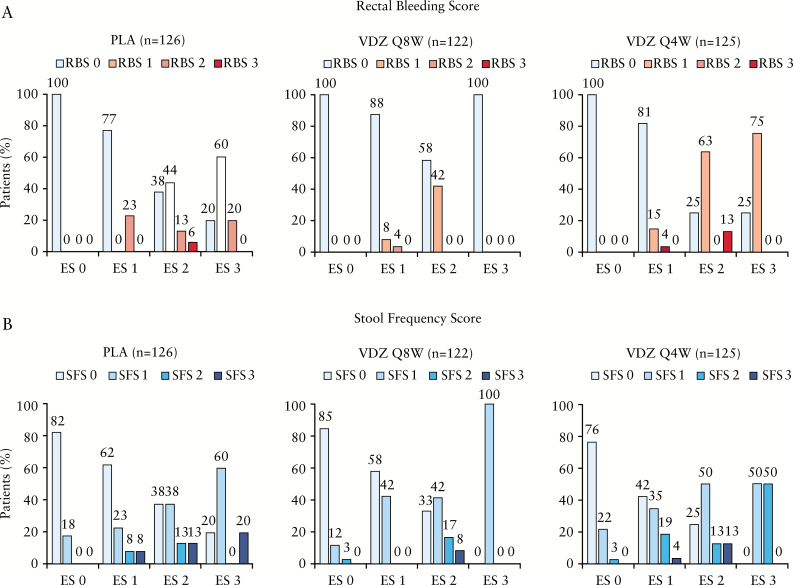
Figure 3 summarizes the percentages of patients with ES 0–3 at Week 52 by increasing RBS [0–3] [Figure 3A] and SFS [0–3] [Figure 3B]. Among patients with an ES of 0, 100% achieved an RBS of 0 in the vedolizumab Q8W and Q4W groups, as well as in the placebo group. None of the patients with an ES of 0 reported an RBS of 1–3.
Figure 3.
ES at Week 52 by RBS [A] and SFS [B]. ES, endoscopic score; PLA, placebo; Q4W, every 4 weeks; Q8W, every 8 weeks; RBS, rectal bleeding score; SFS, stool frequency score; VDZ, vedolizumab.
The proportion of patients with an ES of 0 who reported an SFS of 0 at Week 52 was 85.0% and 76.0% in the vedolizumab Q8W and Q4W groups, respectively, and 82.0% in the placebo group. A similar proportion of patients with an ES of 0 reported an SFS of 1 [12% in the Q8W group; 22% in the Q4W group; 18% in the placebo group] or 2 [3% in the Q8W group; 3% in the Q4W group; no patients in the placebo group].
The results of the concordance analysis between patient-reported symptoms [RBS and SFS] and ES are shown in Table 3 for the pooled ITT population. For an ES of 0 or 1, combining the RBS and SFS offered the highest PPV, the RBS offered the highest NPV and sensitivity, while the SFS offered higher specificity. The associations were less clear when an ES of 0 was considered. The combination of RBS and SFS still offered the highest PPV and specificity, while both the combined and the individual symptom scores offered high NPV and sensitivity.
Table 3.
Comparison of RBS and SFS to endoscopic healing [Mayo Endoscopic subscore]
| Treatment | Symptom score | Number of patients with endoscopic healing | PPV, % [95% CI] | NPV, % [95% CI] | Sensitivity, % [95% CI] | Specificity, % [95% CI] |
|---|---|---|---|---|---|---|
| Endoscopic healing defined as Mayo Endoscopic subscore of 0 or 1 | ||||||
| ITT pooled populationa | RBS 0 | 133 | 88.1 [82.9–93.2] | 71.8 [57.7–85.9] | 92.4 [88.0–96.7] | 60.9 [46.8–75.0] |
| SFS 0 | 98 | 88.3 [82.3–94.3] | 41.8 [30.9–52.6] | 68.1 [60.4–75.7] | 71.7 [58.7–84.8] | |
| RBS + SFS 0 | 96 | 92.3 [87.2–97.4] | 44.2 [33.7–54.7] | 66.7 [59.0–74.4] | 82.6 [71.7–93.6] | |
| Endoscopic healing defined as Mayo Endoscopic subscore of 0 | ||||||
| ITT pooled populationa | RBS 0 | 81 | 53.6 [45.7–61.6] | 100 [100.0–100.0] | 100 [100.0–100.0] | 35.8 [26.8–44.8] |
| SFS 0 | 65 | 58.6 [49.4–67.7] | 79.7 [70.9–88.6] | 80.2 [71.6–88.9] | 57.8 [48.5–67.1] | |
| RBS + SFS 0 | 65 | 62.5 [53.2–71.8] | 81.4 [73.2–89.6] | 80.2 [71.6–88.9] | 64.2 [55.2–73.2] | |
CI, confidence interval; ITT, intention to treat; NPV, negative predictive value; PPV, positive predictive value; RBS, rectal bleeding score; SFS, stool frequency score; VDZ, vedolizumab.
aIncludes all patients who responded to VDZ induction therapy at Week 6 and were subsequently randomized to placebo, VDZ Q4W or VDZ Q8W maintenance therapy.
3.5. Analysis of deep remission by vedolizumab trough serum concentrations
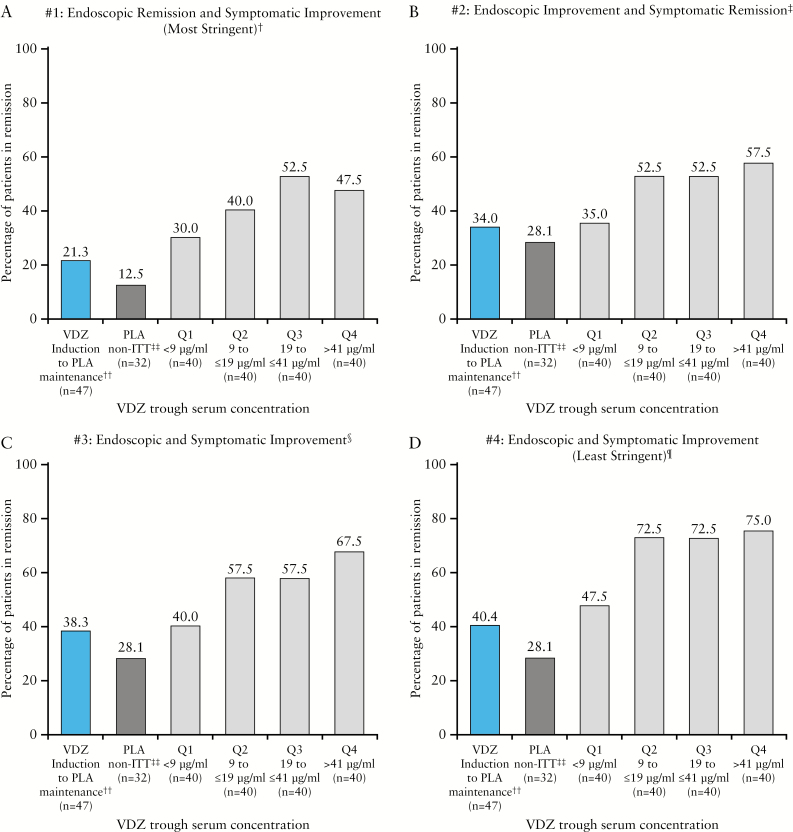
There was a trend towards an association of higher rates of deep remission at Week 52 with higher vedolizumab trough steady-state serum concentrations, for all definitions of deep remission. Rates [95% CI] of deep remission for the lowest [<9.26 µg/mL] vs highest [>41 µg/mL] vedolizumab trough serum quartiles, respectively, were as follows: definition 1 [the most stringent definition]: 30.0% [15.8–44.2] vs 47.5% [32.0–63.0]; definition 2: 35.0% [20.2–49.8] vs 57.5% [42.2–72.8]; definition 3: 40.0% [24.8–55.2] vs 67.5% [53.0–82.0]; definition 4 [the least stringent definition]: 47.5% [32.0–63.0] vs 75.0% [61.6–88.4] [Figure 4; Supplementary Table S1]. The placebo group had deep remission rates similar to those of patients with vedolizumab trough serum concentrations in quartile 1 for all definitions.
Figure 4.
Rates of deep remission by vedolizumab trough serum steady-state concentration quartiles. ES, endoscopic score; ITT, intention to treat; PLA, placebo; RBS, rectal bleeding score; SFS, stool frequency score; VDZ, vedolizumab. †Definition 1: Mayo Clinic ES = 0, RBS = 0 and decrease in SFS or no change from baseline [A]; ‡definition 2: Mayo Clinic ES ≤ 1, RBS = 0 and SFS = 0 [B]; §definition 3: Mayo Clinic ES ≤ 1, RBS = 0, decrease in SFS or no change from baseline, and total score [ES + RBS + SFS] ≤ 1 [C]; ¶definition 4: Mayo Clinic ES ≤ 1, RBS = 0 and SFS ≤ 1 [D]; ††one patient randomized to PLA still had active drug concentrations [9 μg/mL] at Week 46 and was in remission according to all four definitions at Week 52; ‡‡the maintenance PLA non-ITT population received PLA as induction therapy and was not included in the primary maintenance ITT efficacy analyses.
The proportion of patients achieving deep remission by each definition at Week 6 and stratified by vedolizumab trough serum concentrations at Week 6 are shown in Supplementary Table S2 and Supplementary Figure S2, respectively. Numerically higher rates of deep remission at Week 6 for all four definitions were achieved by patients with the highest vedolizumab trough serum concentrations [quartile 4] at Week 6.
3.6. Analysis of deep remission by faecal calprotectin levels
Rates of deep remission at Week 52 were associated with lower faecal calprotectin levels at Week 52, for all definitions of deep remission [Supplementary Figure S3]. Rates [95% CI] of deep remission for the lowest [≤50 µg/g] vs highest [>250 µg/g] faecal calprotectin levels, respectively, were as follows: definition 1: 59.4% [47.3–71.4] vs 18.6% [7.0–30.2]; definition 2: 62.5% [50.6–74.4] vs 23.3% [10.6–35.9]; definition 3: 71.9% [60.9–82.9] vs 27.9% [14.5–41.3]; definition 4: 82.8% [73.6–92.1] vs 34.9% [20.6–49.1]. It was notable that placebo-treated patients and vedolizumab-treated patients with low faecal calprotectin levels [≤50 µg/g] had similar deep remission rates for all definitions.
4. Discussion
The post hoc analysis presented here has shown that patients treated with vedolizumab as maintenance were statistically significantly more likely to achieve deep remission as measured by improvements in endoscopic healing and symptomatic patient-reported outcomes, than were those who received placebo. More than 40% of vedolizumab-treated patients vs up to 15.9% of placebo-receiving patients achieved deep remission after 46 weeks of follow-up. The differences in deep remission rates compared with placebo were statistically significant for each dose regimen of vedolizumab [Q4W and Q8W] and the two dosing regimens were similarly effective. These benefits of vedolizumab treatment were found with respect to all four definitions of deep remission, including the most stringent [definition 1] that required both endoscopic remission and symptomatic improvement. Higher rates of deep remission compared with placebo were also demonstrated using less stringent criteria.
The GEMINI 1 study showed that rates of durable clinical remission and mucosal healing were higher among vedolizumab-treated patients than among placebo-treated patients.
This post hoc analysis of GEMINI 1 data extends these findings by providing an important and clinically relevant perspective on the effectiveness of vedolizumab in achieving deep remission in patients with UC. These data inform a progressive and evolving approach to measuring treatment success that goes beyond the standard phase 3 clinical trial end points. Furthermore, these data support the need for guideline-mediated standardization of criteria for defining deep remission in clinical practice.
Current recommendations for defining clinical remission in patients with UC include both patient-reported measures [rectal bleeding and stool frequency], which reflect the burden of the disease on patients, and endoscopic measures.3,4 The use of both patient-reported and endoscopic measures is supported by the observation that normalization of stool frequency does not always correlate with mucosal healing and, in patients with mucosal healing, the highest quality of life is reported by patients who achieve normalization of stool frequency.17,18 The findings from the current study also support this, with some degree of abnormal stool frequency in a proportion of patients with mucosal healing [ES of 0; normal or inactive disease] in both the vedolizumab and the placebo groups. Although UC is traditionally regarded as a disease that is limited to the mucosa, accumulating evidence suggests that fibrosis is a common occurrence in UC.19 Fibrotic changes are likely to have important clinical consequences, even in the absence of strictures, and may account for the discrepancy between mucosal healing and stool frequency. An analysis of the concordance between the individual symptom scores and ES in the pooled ITT population provides further support for the use of both RBS and SFS in the evaluation of clinical remission. While SFS performed less well in terms of positive prediction and specificity for ES of 0 or of 0/1 [possibly due to irreversibility of increased stool frequency in some patients because of irreversible structural damage], combining SFS with RBS offered the highest PPV, NPV, sensitivity and specificity when considering an ES of 0/1 and the highest PPV and specificity when considering an ES of 0. The importance of including endoscopic healing in definitions of remission in UC is reinforced by studies that have shown endoscopic healing could impact long-term outcomes for patients, such as relapse-free survival, the need for colectomy20,21 and future cancer risk.22,23 Both the FDA3 and the EMA4 accept an ES score of 1 within their criteria for clinical remission. Moreover, professional organizations such as the World Gastroenterology Organization24 and the American College of Gastroenterology25 do not provide explicit criteria for defining deep remission. The most recent definition is that issued by ECCO, which describes it as stool frequency ≤3 per day with no bleeding and no mucosal lesions at endoscopy.5 Consequently, there is a lack of consensus about appropriate definitions of deep remission for use in the evaluation of new treatments for UC or for the evaluation of patients in routine clinical practice.
The current analysis has addressed the issue of a lack of consensus on definitions of deep remission through evaluation of four definitions differing in the stringency of symptomatic and endoscopic criteria. A potential risk of using less stringent criteria to define deep remission is that patients who might benefit from further treatment optimization may be overlooked, with a subsequent negative impact on their long-term outcomes. Such patients may also experience persistent decrements to their health-related quality of life [HRQoL]. A previous post hoc analysis of GEMINI 1 data highlighted that the achievement of clinical remission at Week 52 was associated with improved clinical and HRQoL outcomes.26 The lack of standardization in defining deep remission further complicates the clinical evaluation of new treatments for UC, because it precludes comparison of deep remission rates across studies using different definitions. A patient defined as being in deep remission in one study may not have met the more stringent criteria applied in another study. In this way, the efficacy of some new treatments may be over-estimated in terms of their potential to optimize long-term outcomes for patients.
In the GEMINI 1 study, patients administered vedolizumab Q4W showed a consistent trend of higher trough serum vedolizumab concentrations compared to patients administered vedolizumab Q8W.27 The present analysis demonstrates a relationship between vedolizumab pharmacokinetics and deep remission, suggesting a trend towards higher rates of deep remission at Week 52 with higher trough serum steady-state concentrations. Of patients within the top 50% of vedolizumab trough serum concentrations, approximately half achieved the most stringent deep remission criteria [definition 1]. More than 70% of patients achieving the top 75% of vedolizumab trough serum concentrations also achieved the least stringent deep remission criteria [definition 4]. Patients with UC having vedolizumab trough serum concentrations in the upper quartiles [Q2–4] achieved higher rates of deep remission across all definitions compared with those who received vedolizumab during induction and placebo during maintenance. These results are hypothesis-generating only and require confirmation in a larger, prospective analysis.
However, this relationship is less clear for patients with the lowest vedolizumab trough concentrations [Q1]. The percentages of these patients who achieved deep remission did not differ significantly from those of patients who received vedolizumab during induction and placebo during maintenance across all definitions. Patients with the lowest vedolizumab trough serum concentration may represent a patient subgroup with high drug clearance. Dose optimization may be required to achieve and sustain effective drug concentrations in these patients and is currently being evaluated in a phase 4 open-label study [NCT03029143] in non-responders with moderately to severely active UC.
These observations support another recent evaluation of the relationship between vedolizumab trough concentrations and clinical outcomes utilizing data from the GEMINI 1 study, which found a relationship between higher vedolizumab trough serum concentrations and early [Week 6] clinical remission rates.15 Interestingly, this latter analysis highlighted that patients who were anti-TNFα-naive were more likely to achieve clinical remission than patients who had failed previous anti-TNFα treatment.15 An analysis of Week 6 deep remission rates by vedolizumab trough serum concentrations supports the previous evaluation of clinical remission rates, demonstrating a pattern of numerically higher deep remission rates at Week 6 for those achieving higher vedolizumab serum trough concentrations. The relatively low rates of deep remission at Week 6 across all four definitions evaluated in the current study suggest that deep remission may represent a more appropriate target for longer-term maintenance therapy than for acute, induction therapy.
Faecal calprotectin is a well-studied biomarker of intestinal inflammation in patients with inflammatory bowel disease.28–30 In a recent study of 68 patients with UC, Patel et al.31 showed that faecal calprotectin levels were significantly associated with disease extent based on the Montreal Classification,32 Mayo score and Nancy score.33 These authors also reported that faecal calprotectin levels of ≤60 µg/g predicted deep remission [defined by RBS = 0, SF ≤ 2 and endoscopic Mayo score = 0], with a sensitivity of 86% and a specificity of 87%. The results of the current analysis are consistent with these findings. We have shown that patients with UC who had faecal calprotectin levels ≤50 µg/g had significantly higher rates of deep remission at Week 52 than patients with faecal calprotectin levels >250 µg/g for all definitions of deep remission.
A further potential component of any definition of remission in UC is that of histological remission.34 Preliminary evaluations suggest that histological remission may outperform endoscopic healing as a predictive marker for disease progression and response to therapy.35,36 This may be due to the identification of patients with residual microscopic inflammation who are at increased risk for symptomatic relapse than are those with normal histology.37 Histological remission could represent a more stringent definition for remission in UC for the evaluation of new drug treatments. In this context, it will also be important to understand what differentiates patients who achieve deep remission with therapy from those who do not. Such understanding may inform treatment decisions and expectations, and aid in decisions to modify therapy in patients with persistent symptoms.
The post hoc nature of the analysis and the lack of histological data mandates further evaluation of the ability of vedolizumab to achieve deep and sustained remission in patients with UC. The current analysis is limited by the fact that it did not include an evaluation by prior anti-TNFα antagonist use, which is known to have an impact on other clinical outcomes.38 The strengths of the current analysis lie in the comparison of a range of definitions of deep remission that varied in stringency of criteria, and the analysis of patients who were randomized and blinded to both induction and maintenance therapy regimen.
In conclusion, this post hoc analysis of data from the phase 3 GEMINI 1 trial conducted in patients with moderately to severely active UC has shown that treatment with vedolizumab was associated with significantly higher rates of deep remission than placebo at Week 52, regardless of dosing frequency or definition of deep remission. More than 40% of vedolizumab-treated patients vs up to 15.9% of those who received placebo achieved deep remission, measured by improvements in endoscopic healing and symptomatic patient-reported outcomes.
Funding
This work was supported by Takeda Pharmaceutical Company Ltd. Medical writing support was provided by Oana Coban of Chameleon Communications International Ltd, UK [a Healthcare Consultancy Group Company] and sponsored by Takeda Pharmaceuticals USA, Inc.
Conflict of Interest
William J. Sandborn [WJS] reports consulting fees from University of Western Ontario [owner of Robarts Clinical Trials, Inc.], AbbVie, Akros Pharma, Allergan, Ambrx Inc., Amgen, Ardelyx, Arena Pharmaceuticals, Atlantic Pharmaceuticals, Avaxia, Biogen, Boehringer Ingelheim, Bristol Meyers Squibb, Celgene, Conatus, Cosmo Technologies, Escalier Biosciences, Ferring, Ferring Research Institute, Forward Pharma, Galapagos, Genentech, Gilead Sciences, Immune Pharmaceuticals, Index Pharmaceuticals, Janssen, Kyowa Hakko Kirin Pharma, Lilly, Medimmune, Mesoblast, Miraca Life Sciences, Nivalis Therapeutics, Novartis, Nutrition Science Partners, Oppilan Pharma, Otsuka, Palatin, Paul Hastings, Pfizer, Precision IBD, Progenity, Prometheus Laboratories, Qu Biologics, Regeneron, Ritter Pharmaceuticals, Robarts Clinical Trials, Salix, Seattle Genetics, Seres Therapeutics, Shire, Sigmoid Biotechnologies, Takeda, Theradiag, Theravance, Tigenix, Tillotts Pharma, UCB Pharma, Vascular Biogenics, Vivelix; research grants from Atlantic Healthcare Ltd, Amgen, Genentech, Gilead Sciences, AbbVie, Janssen, Takeda, Lilly, Celgene/Receptos; payments for lectures/speakers bureau from AbbVie, Janssen, Takeda; and holds stock/stock options in Escalier Biosciences, Oppilan Pharma, Precision IBD, Progenity, Ritter Pharmaceuticals. Jean-Frederic Colombel [J-FC] has served as consultant, advisory board member or speaker for AbbVie, Amgen, Boehringer-Ingelheim, Celgene Corporation, Celltrion, Enterome, Ferring, Genentech, Janssen and Janssen, Lilly, Medimmune, Merck & Co., Pfizer, PPM Services, Protagonist, Second Genome, Seres, Shire, Takeda, Theradiag, Theravance Biopharma. Stock options: Intestinal Biotech Development, Genfit. Research grants: AbbVie, Takeda, Janssen and Janssen. Remo Panaccione [RP] has received consultant fees from AbbVie, Alba Therapeutics, Allergan, Amgen, Atlantic Healthcare, Boehringer-Ingelheim, Bristol-Myers Squibb, Celgene, Cosmo Technologies, Coronado Biosciences, Eisai Medical Research, Elan, EnGene, Eli Lilly, Enteromedics, Exagen Diagnostics, Ferring, Genentech, Genzyme, Gilead, Given Imaging, GlaxoSmithKline, Human Genome Sciences, Janssen, Merck & Co., Merck Research Laboratories, MerckSerono, Millennium, Nisshin Kyorin, Novo Nordisk, Pfizer, Receptos, Relypsa, Salient, Salix, Santarus, Shire Pharmaceuticals, Sigmoid Pharma, and Qu Biologics; received speaker’s fees for AbbVie, Celgene, Aptalis, Ferring, Janssen, Merck, Pfizer, Prometheus, Shire, and Takeda; Advisory Board for AbbVie, Abbott, Allergan, Amgen, Aptalis, AstraZeneca, Baxter, Biogen Idec, Eisai, Ferring, Genentech, Janssen, Merck, Shire, Elan, Glaxo-Smith Kline, Hospira, Pfizer, Bristol-Myers Squibb, Takeda, Cubist, Celgene and Salix; research/educational support from AbbVie, Ferring, Janssen, Shire, and Takeda. Parambir S. Dulai [PSD] has received grants and other funding from Takeda Pharmaceuticals, and grants from Pfizer. Maria Rosario [MR] is an employee and stockholder of Takeda Development Center Americas, Inc. Charlie Cao [CC] and Karen Lasch [KL] are employees of Takeda Pharmaceuticals USA, Inc. Morris Barocas [MB] was an employee of Takeda Pharmaceuticals USA, Inc., at the time the study was conducted.
Supplementary Material
Acknowledgments
Medical writing support was provided by Oana Coban of Chameleon Communications International Ltd, UK [a Healthcare Consultancy Group Company] and sponsored by Takeda Pharmaceuticals USA, Inc.
Author Contributions
WJS: study concept and design, acquisition of data, and review of data and the manuscript. J-FC: analysis and interpretation of data, and review of data and the manuscript. RP: interpretation of data, and preparation and critical review of the manuscript. PSD: interpretation of data, and review of data and the manuscript. MR, CC: analysis and interpretation of data. MB: study concept. KL: study concept and design, and review of data and the manuscript. All authors had full access to all study data and had final responsibility for the decision to submit for publication.
References
- 1. Zallot C, Peyrin-Biroulet L. Deep remission in inflammatory bowel disease: looking beyond symptoms. Curr Gastroenterol Rep 2013;15:315. [DOI] [PubMed] [Google Scholar]
- 2. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015;110:1324–38. [DOI] [PubMed] [Google Scholar]
- 3. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Ulcerative colitis: clinical trial endpoints guidance for Industry. 2016. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM515143.pdf. Accessed February 14, 2018. [Google Scholar]
- 4. European Medicines Agency. Draft guideline on the development of new medicinal products for the treatment of ulcerative colitis. 2016. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003266.pdf. Accessed February 14, 2018. [Google Scholar]
- 5. Magro F, Gionchetti P, Eliakim R, et al. ; European Crohn’s and Colitis Organisation [ECCO] Third European Evidence-based Consensus on diagnosis and management of ulcerative colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis 2017;11:649–70. [DOI] [PubMed] [Google Scholar]
- 6. Rogler G, Vavricka S, Schoepfer A, Lakatos PL. Mucosal healing and deep remission: what does it mean?World J Gastroenterol 2013;19:7552–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Paul S, Del Tedesco E, Marotte H, et al. Therapeutic drug monitoring of infliximab and mucosal healing in inflammatory bowel disease: a prospective study. Inflamm Bowel Dis 2013;19:2568–76. [DOI] [PubMed] [Google Scholar]
- 8. Roblin X, Marotte H, Rinaudo M, et al. Association between pharmacokinetics of adalimumab and mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2014;12:80–84.e2. [DOI] [PubMed] [Google Scholar]
- 9. Papamichael K, Rakowsky S, Rivera C, Cheifetz AS, Osterman MT. Infliximab trough concentrations during maintenance therapy are associated with endoscopic and histologic healing in ulcerative colitis. Aliment Pharmacol Ther 2018;47:478–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feagan BG, Rutgeerts P, Sands BE, et al. ; GEMINI 1 Study Group Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 11. Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis 2017;11:769–84. [DOI] [PubMed] [Google Scholar]
- 12. Dassopoulos T, Cohen R, Schert E, Schwartz R, Kosinski L, Regueiro M.. Identification, assessment and initial medical treatment of ulcerative colitis. Clinical care pathway. Available at: http://campaigns.gastro.org/algorithms/UlcerativeColitis/pdf/Ulcerative_Colitis_Care_Pathway.pdf. Accessed February 14, 2018. [Google Scholar]
- 13. Rosario M, Abhyankar B, Sankoh S, Dirks N, Lasch K, Sandborn W. Relationship between vedolizumab pharmacokinetics and endoscopic outcomes in patients with ulcerative colitis. J Crohns Colitis 2015;9:S46. [Google Scholar]
- 14. Ungar B, Kopylov U, Yavzori M, et al. Association of vedolizumab level, anti-drug antibodies, and α4β7 occupancy with response in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2018;16:697–705.e7. [DOI] [PubMed] [Google Scholar]
- 15. Rosario M, French JL, Dirks NL, et al. Exposure–efficacy relationships for vedolizumab induction therapy in patients with ulcerative colitis or Crohn’s disease. J Crohns Colitis 2017;11:921–9. [DOI] [PubMed] [Google Scholar]
- 16. Røseth AG, Aadland E, Grzyb K. Normalization of faecal calprotectin: a predictor of mucosal healing in patients with inflammatory bowel disease. Scand J Gastroenterol 2004;39:1017–20. [DOI] [PubMed] [Google Scholar]
- 17. Colombel JF, Keir ME, Scherl A, et al. Discrepancies between patient-reported outcomes, and endoscopic and histological appearance in UC. Gut 2017;66:2063–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jharap B, Sandborn WJ, Reinisch W, et al. Randomised clinical study: discrepancies between patient-reported outcomes and endoscopic appearance in moderate to severe ulcerative colitis. Aliment Pharmacol Ther 2015;42:1082–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Latella G, Rieder F. Time to look underneath the surface: ulcerative colitis-associated fibrosis. J Crohns Colitis 2015;9:941–2. [DOI] [PubMed] [Google Scholar]
- 20. Arias MT, Vande Casteele N, Vermeire S, et al. A panel to predict long-term outcome of infliximab therapy for patients with ulcerative colitis. Clin Gastroenterol Hepatol 2015;13:531–8. [DOI] [PubMed] [Google Scholar]
- 21. Frøslie KF, Jahnsen J, Moum BA, Vatn MH; IBSEN Group Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology 2007;133:412–22. [DOI] [PubMed] [Google Scholar]
- 22. Rutter MD, Saunders BP, Wilkinson KH, et al. Cancer surveillance in longstanding ulcerative colitis: endoscopic appearances help predict cancer risk. Gut 2004;53:1813–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lukas M. Inflammatory bowel disease as a risk factor for colorectal cancer. Dig Dis 2010;28:619–24. [DOI] [PubMed] [Google Scholar]
- 24. Bernstein CN, Fried M, Krabshuis JH, et al. World Gastroenterology Organization Practice Guidelines for the diagnosis and management of IBD in 2010. Inflamm Bowel Dis 2010;16:112–24. [DOI] [PubMed] [Google Scholar]
- 25. Kornbluth A, Sachar DB; Practice Parameters Committee of the American College of Gastroenterology Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol 2010;105:501–23; quiz 524. [DOI] [PubMed] [Google Scholar]
- 26. Sandborn W, Colombel JF, Panaccione R, et al. Deep clinical remission in patients with ulcerative colitis: evaluating the effects of vedolizumab on various combinations of endoscopic and patient-reported outcomes. 10th Congress of European Crohn’s and Colitis Organisation; 21 February, 2015; Barcelona, Spain. Poster P319a. [Google Scholar]
- 27. Rosario M, Dirks NL, Gastonguay MR, et al. Population pharmacokinetics– pharmacodynamics of vedolizumab in patients with ulcerative colitis and Crohn’s disease. Aliment Pharmacol Ther 2015;42:188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis 2012;18:2218–24. [DOI] [PubMed] [Google Scholar]
- 29. Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin more accurately reflects endoscopic activity of ulcerative colitis than the Lichtiger Index, C-reactive protein, platelets, hemoglobin, and blood leukocytes. Inflamm Bowel Dis 2013;19:332–41. [DOI] [PubMed] [Google Scholar]
- 30. Sipponen T, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Färkkilä M. Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn’s disease activity index and endoscopic findings. Inflamm Bowel Dis 2008;14:40–6. [DOI] [PubMed] [Google Scholar]
- 31. Patel A, Panchal H, Dubinsky MC. Fecal calprotectin levels predict histological healing in ulcerative colitis. Inflamm Bowel Dis 2017;23: 1600–4. [DOI] [PubMed] [Google Scholar]
- 32. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006;55:749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marchal-Bressenot A, Salleron J, Boulagnon-Rombi C, et al. Development and validation of the Nancy histological index for UC. Gut 2017;66:43–9. [DOI] [PubMed] [Google Scholar]
- 34. Chernavskaia O, Heuke S, Vieth M, et al. Beyond endoscopic assessment in inflammatory bowel disease: real-time histology of disease activity by non-linear multimodal imaging. Sci Rep 2016;6:29239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bryant RV, Winer S, Travis SP, Riddell RH. Systematic review: histological remission in inflammatory bowel disease. Is ‘complete’ remission the new treatment paradigm? An IOIBD initiative. J Crohns Colitis 2014;8:1582–97. [DOI] [PubMed] [Google Scholar]
- 36. Bryant RV, Burger DC, Delo J, et al. Beyond endoscopic mucosal healing in UC: histological remission better predicts corticosteroid use and hospitalisation over 6 years of follow-up. Gut 2016;65:408–14. [DOI] [PubMed] [Google Scholar]
- 37. Magro F, Lopes SI, Lopes J, et al. ; Portuguese IBD group [GEDII] Histological outcomes and predictive value of faecal markers in moderately to severely active ulcerative colitis patients receiving infliximab. J Crohns Colitis 2016;10:1407–16. [DOI] [PubMed] [Google Scholar]
- 38. Loftus EV Jr, Colombel JF, Feagan BG, et al. Long-term efficacy of vedolizumab for ulcerative colitis. J Crohns Colitis 2017;11:400–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.