Abstract
Background and Aims
CD71+ erythroid cells are enriched during pregnancy with immuno suppressive properties. We investigated the frequency and functionality of CD71+ erythroid cells in peripheral blood, cord blood, and placenta of inflammatory bowel disease [IBD] patients versus healthy controls [HCs]. We aimed to determine their role in IBD pathogenesis during pregnancy.
Methods
Peripheral blood was collected at preconception, the first, second and third trimesters, and postpartum. Cord blood and placental tissues were collected at the time of birth. Cells from different specimens were subjected to immune-phenotyping and functional assays. CD71+ erythroid cells were purified for quantitative polymerase chain reaction [qPCR] analysis. Using an allogeneic mouse model of pregnancy, the effects of CD71+ erythroid cells depletion on intestinal homeostasis and dysbiosis was studied.
Results
IBD patients had lower CD71+ erythroid cells during pregnancy compared with HCs. Placenta and cord blood CD71+ erythroid cells from IBD patients exhibited impaired functionality and expressed lower inhibitory molecules including VISTA, TGF-β, and reactive oxygen species [ROS]. Lower CD71+ erythroid cells were correlated with reduced regulatory T cells and increased immune-activation in IBD patients. Depletion of CD71+ erythroid cells in an allogeneic pregnancy model resulted in upregulation of TLRs, IL-6, and CXCL-1, and enhanced production of TNF-α, in intestinal tissues. In contrast, TGF-β gene expression was reduced. Excessive inflammatory response in the gut [e.g. TNF-α] affects intestinal integrity and CD71+ erythroid cells impact on the gut’s bacterial composition.
Conclusions
Reduced frequency and/or impaired functionality of CD71+ erythroid cells during pregnancy may predispose IBD patients to a more pro-inflammatory milieu in their gastrointestinal tract, characterised by lower Tregs, higher IL-6, and TNF-α, and dysbiosis.
Keywords: CD71+ erythroid cells, IBD, immunosuppression
1. Introduction
Inflammatory bowel disease [IBD] is characterised into two major types of chronic intestinal disorders: Crohn’s disease [CD], and ulcerative colitis[UC].1 Numerous studies have described an association between IBD immunopathogenesis and dysbiosis of the gut microbiota through the hygiene hypothesis.2,3
During pregnancy, the immune system adopts a tolerogenic state in order to accommodate the semi-allogeneic fetus and still provide the mother with a robust immune response to pathogens when needed.4 This requires a complex interplay of immune cells and a shift to a more tolerogenic phenotype facilitated by the influence of regulatory T cells [Tregs], T helper 2 [Th2] cells, and myeloid-derived suppressor cells [MDSCs].5–7 The tightly regulated and tolerogenic state during pregnancy not only provides a safe environment to nurture the allogeneic fetus, but also can be beneficial in mothers with hyperimmune activations. For instance, in some autoimmune diseases, pregnancy is correlated with amelioration of symptoms; rheumatoid arthritis and multiple sclerosis patients specifically experience lower risk of active disease during pregnancy.8,9 However, individuals with IBD may not always benefit from the favourable impact of pregnancy on the clinical course of disease, as a higher risk of flaring has been reported in some cohorts. For instance, women with UC have a 70% risk of active disease during pregnancy and women with CD have a 54% risk of active disease.10 In contrast, a study conducted in a European cohort indicated improved IBD symptoms during gestation in a number of patients.11 In pregnant IBD patients, pro-inflammatory cytokines [e.g. IFN-γ and TNF-α] are more abundant during the inactive and active stages of disease.12 Elevated levels of serum TNF-α through the second trimester of gestation has been reported to be associated with preterm delivery in an IBD cohort.13 Thus, the active immune status of IBD patients during pregnancy may substantially impact on the health of both the mother and the fetus.8,10,14
Recently, we revealed a previously unappreciated immunological property for immature red blood cells. Immature red blood cells co-express CD71 [transferrin receptor] and CD235a [erythroid lineage marker] in humans, but CD71 and TER119 in mice. We observed that these cells are physiologically abundant in neonatal mice and human cord blood, with distinctive immunosuppressive properties, and allow a swift adaptation of the neonatal gut to their microbiome.15,16 In our studies we have defined them as CD71+ erythroid cells which are abundant in human cord blood and inhibit TNF-α production.15–17 More recently, we have shown that these cells play an important role in feto-maternal tolerance.18 In agreement, lower frequency of cord blood CD71+ erythroid cells in spontaneous preterm labour is reported.19 Since complications, such as increased risk of spontaneous abortion, low birthweight, and preterm birth, are more prevalent in IBD patients than healthy controls [HCs],10,14,20 we proposed to investigate frequency and functionality of CD71+ erythroid cells during pregnancy in HCs versus IBD patients. Here, we demonstrate that IBD patients have lower frequency of CD71+ erythroid cells in their peripheral blood. In addition, CD71+ erythroid cells from IBD patients have different immunological properties which may modulate the host’s immune responses to the microbial communities in the gut. Thus, due to their immunosuppressive nature, decreased frequency or dysfunction of CD71+ erythroid cells in IBD patients may exacerbate inflammatory response in these patients during pregnancy. Finally, we demonstrated that CD71+ erythroid cells may play an important role in intestinal haemostasis, using a mouse model of allogeneic pregnancy.
2. Materials and Methods
2.1. Study population
Blood was collected from healthy or IBD adult women at preconception [PC], first trimester [T1], second trimester [T2], third trimester [T3], and postpartum [PP]. All participants were HIV, HCV, and HBV seronegative. The appropriate institutional review boards at the University of Alberta approved the studies. All study participants gave written informed consent to participation in this study [Table 1].
Table 1.
Clinical information of the study participants.
| Diagnosis | Patient ID | Age | Medications at T3/T2 | % CD71+ erythroid cells at T3/T2 | Active disease at T3/T2 | |
|---|---|---|---|---|---|---|
| UC | 107 | 28 | 5-ASA | 8.56 | No | |
| UC | 69 | 31 | Anti-TNF and Vancomycin | 0.5 | Yes | |
| UC | 54 | 34 | 5-ASA and Imuran | 0.22 | Yes | |
| UC | 123 | 41 | 5-ASA | 1.85 | No | |
| UC | 119 | 28 | 5-ASA | 0.1 | Yes | |
| UC | 128 | 35 | Anti-TNF | 3.48 | No | |
| UC | 113 | 32 | None | 1.02 | No | |
| UC | 37 | 35 | 5-ASA | 7.14 | No | |
| UC | 127 | 32 | Vedolizumab | 11.4 | No | |
| UC | 147 | 26 | Anti-TNF | 1.12 | No | |
| UC | 122 | 36 | Imuran | 12.4 | No | |
| UC | 6 | 43 | 5-ASA | 6.65 | No | |
| UC | 11 | 35 | 5-ASA | N/A | No | |
| UC | 13 | 42 | 5-ASA | N/A | No | |
| UC | 18 | 46 | 5-ASA | N/A | Yes | |
| UC | 19 | 33 | 5-ASA | N/A | No | |
| UC | 31 | 28 | 5-ASA and prednisone | N/A | No | |
| UC | 28 | 40 | N/A | |||
| UC | 38 | 28 | 5-ASA | N/A | No | |
| UC | 53 | 29 | None | N/A | No | |
| UC | 55 | 30 | 5-ASA | N/A | Yes | |
| UC | 66 | 35 | 5-ASA | N/A | Yes | |
| UC | 81 | 34 | Vedolizumab | N/A | No | |
| UC | 114 | 30 | None | N/A | No | |
| UC | 116 | 31 | 5-ASA | N/A | No | |
| UC | 131 | 34 | 5-ASA | N/A | No | |
| UC | 142 | 32 | 5-ASA and imuran | N/A | No | |
| UC | 146 | 26 | None | N/A | No | |
| UC | 143 | 32 | 5-ASA | N/A | No | |
| CD | 75 | 35 | Imuran and anti-TNF | 2.43 | No | |
| CD | 118 | 29 | Anti-TNF | 5.89 | No | |
| CD | 138 | 35 | Anti-TNF | 3.42 | Yes | |
| CD | 88 | 31 | None | 2.52 | No | |
| CD | 130 | 35 | Anti-TNF | 1.05 | No | |
| CD | 108 | 28 | Imuran and anti-TNF | 1.36 | No | |
| CD | 114 | 30 | None | 1.91 | No | |
| CD | 153 | 28 | Anti-TNF | 1.4 | No | |
| CD | 101 | 35 | Anti-TNF | 2.8 | No | |
| CD | 3 | 40 | Anti-TNF | 1.47 | No | |
| CD | 12 | 36 | Imuran and anti-TNF | 2.3 | Yes | |
| CD | 20 | 32 | Anti-TNF | N/A | No | |
| CD | 22 | 31 | 5-ASA and Imuran | N/A | No | |
| CD | 18 | 46 | 5-ASA | N/A | Yes | |
| CD | 27 | 46 | 5-ASA and imuran | N/A | No | |
| CD | 36 | 30 | Anti-TNF | N/A | No | |
| CD | 37 | 35 | 5-ASA | N/A | No | |
| CD | 42 | 31 | 5-ASA | N/A | No | |
| CD | 43 | 29 | None | N/A | Yes | |
| CD | 51 | 33 | Anti-TNF | N/A | No | |
| CD | 58 | 38 | Imuran | N/A | No | |
| HC | 91 | 34 | None | 3.71 | No | |
| HC | 100 | 35 | None | 18.40 | No | |
| HC | 99 | 33 | None | 16.01 | No | |
| HC | 96 | 37 | None | 4.20 | No | |
| HC | 86 | 27 | None | 35.90 | No | |
| HC | 97 | 41 | None | 4.79 | No | |
| HC | 90 | 35 | None | 21.40 | No | |
| HC | 89 | 38 | None | 7.12 | No | |
| HC | 88 | 31 | None | 11.00 | No | |
| HC | 105 | 29 | None | 30.00 | No | |
| HC | 133 | 34 | None | 9.10 | No | |
| HC | 78 | 36 | None | 37.20 | No | |
| HC | 41 | 35 | None | 27.0 | No | |
| HC | 87 | 34 | None | 25.70 | No | |
| HC | 84 | 29 | None | 8.23 | No | |
| HC | 59 | 29 | None | 37.00 | No | |
| HC | 71 | 32 | None | 14.2 | No | |
| UC | CD | HC | ||||
| # of Patients followed during pregnancy | 23 | 20 | 17 | |||
Although 17 HCs were longitudinally studied, another 11 cord blood specimens from HCs were also used for in vitro assays.
HC, healthy control; UC, ulcerative colitis; CD, Crohn’s disease; T3, T2, third and second trimesters; 5-ASA, 5-aminosalicylic acid; TNF, tumour necrosis factor; N/A, not available.
2.2. Animals
BALB/c and C57BL/6 mice were purchased from Charles River Laboratories and bred together to create allogeneic pregnancies. This study was conducted in strict accordance with the recommendations in the Guide for Care and Use of Laboratory animals of the Canadian Council for Animal Care [Protocol # AUP00001021]. Female non-pregnant or pregnant BALB/c mice were used for these studies. For depletion of CD71+ erythroid cells, anti-CD71 antibody [clone 8D3, Bio X cell] ~300 μg or Rat IgG2a isotype control antibodies were administered to pregnant mice at gestation age of E10.5 to E14.5 days via intraperitoneal injection, as we have reported elsewhere,18 and mice were euthanised 3 days later.
2.3. Fluorescein isothiocyanate labelled dextran studies
Control or anti-CD71 treated pregnant mice [E10.5-E14.5] were fed fluorescein isothiocyanate labelled dextran [FITC-dextran] in phosphate-buffered saline [PBS] at 40mg/100g body weight. The mice, 4 h later, were euthanised and the serum was subjected to FITC-dextran quantification. Serum FITC levels were measured by spectrophoto fluorometry with an excitation of 485 nm and an emission wavelength of 528 nm.
2.4. Cell isolation
Peripheral blood mononuclear cells [PBMCs] and cord blood mononuclear cells [CBMCs] were isolated using Ficoll-Paque gradients.16 Extravillous tissues from the maternal side of the human placenta were obtained for cell isolation. Similarly gut tissues from pregnant or non-pregnant mice were collected and subjected to cell isolation, as we reported elsewhere.15,16
2.5. Flow Cytometry
The antibodies used were purchased from BD Bioscience or eBioscience: human anti-CD3 [SP-34-2], anti-CD4 [RPA-T4], anti-CD8 [RPA-T8], anti-CD69 [FN50], anti-CD71 [M-A712], anti-CD235a [GA-R2], anti-CD25 [M-A251], anti-CD127 [HIL-7R-M21], and anti-Foxp3 [236A/E7]; and for mice, anti-CD11b [M1/70], anti-CD11c [N418], anti-IL-6 [MP5-20F3], anti-TGF-β [LAP, TW4-9E7], TNF-α [MP6-XT22], anti-CD71 [R17217], and anti-TER119 [TER119]. Cell viability was assessed using LIVE/DEAD Kit [Life Technologies]. CellTraceTM carboxyfluorescein succinimidyl ester [CFSE] was used for T cell proliferation [Thermo Fisher Scientific], acquired on a LSRFortessa [BD Bioscience] and analysed with FlowJo Version 8.7.3 software. In some experiments, CD235a+ CD71+ cells were isolated from CBMCs, and placenta cells by positive selection, using biotinylated antibodies [eBioscience] and magnetic cell separation [Miltenyi] with purity of ≥96% [Supplementary Figure 1A, available as Supplementary data at ECCO-JCC online].
2.6. Cell culture
For ex vivo cytokine production, PBMCs, CBMCs, and placenta cells were cultured and stimulated with 0.1 µg/mL-1 of anti-human CD3 antibody [Clone UCHT1] in presence or absence of CD71+ erythroid cells, for 72 h. Culture supernatants were collected for enzyme-linked immunosorbent assay [ELISA] [R&D Systems]. In some studies, heat-killed Listeria monocytogenes [HK Lm] was used for cell stimulation, as we have reported elsewhere.16 Proliferation assays were performed according to our previous reports,17,21 using either total PBMCs/CBMCs or CD71-depleted PBMCs/CBMCs. CD71+ erythroid cells were depleted from PBMCs/CBMCs by positive selection using anti-CD71 biotinylated antibody followed by anti-biotin beads, as we have described elsewhere.16 In some cases, CD71+ erythroid cells from PBMCs were removed by using red blood cell [RBC] lysis buffer.
2.7. Reactive oxygen species measurement
The production of intracellular reactive oxygen species [ROS] was measured using 2’,7’-dichlorofluorescein diacetate [DCFH‑DA, Sigma]. The ROS staining was conducted according to the manufacturing protocol and detected by flow cytometry.
2.8. Gene expression analysis
RNA isolation and quantitative polymerase chain reaction [qPCR] were conducted according to our published data.15 The resulting cDNA [5 ng/µl] was used as a template for TaqMan qPCR [Applied Biosystems] with the following gene expression probe assays: TGF-β [Hs00998133_m1], PD-1H [Hs01088398_m1], arginase-2: Hs00982833_m1 and VEGFa [Hs00900055_m1], IL-6 [Mm00446190_m1], CXCL-1 [Mm04207460_m1], TLR-2 [Mm01213946_g1], TLR-3 [Mm01207404_m1], TLR-4 [Mm00445273_m1], and TLR-9 [Mm00446193_m1]. Each sample was run in duplicates on CFX96 TouchTM Real-Time PCR Detection System [BioRad]. Beta actin [Hs01060665_g1] was used as a reference gene, and the gene expression of the targeted genes was calculated by the 2-ΔΔCt method.
2.9. Total DNA extraction from gut contents
Total DNA from ileal, caecal, and colon contents was extracted using the QIAamp DNA Stool Mini Kit [Qiagen]. Zirconium beads with 0.1-mm diameter [500 mg] were used, and the bead-beating step was performed in a FastPrep-24 [MP Biomedical, Solon, OH] in three cycles of 30 s at 4 m/s speed. Samples were then incubated at 95ºC for 15 min and further processed along with the Stool Mini Kit protocol. Total DNA was eluted in 50 µl of water, and the concentration of each sample was checked on a Nano-Drop ND-1000 Spectrophotometer [NanoDrop Technologies].
2.10. Quantification of gut bacteria
Microbiota analysis was carried on CFX96 TouchTM Real-Time PCR detection System as we described previously elsewhere,22 and primers are listed in Supplementary Table 2, available as Supplementary data at ECCO-JCC online.
2.11. Faecal calprotectin measurement
Faecal samples were collected from newborns at 12 weeks of age, and kept frozen until use. The frozen faecal samples were thawed, and a CALEX Cap Stool Extraction Device [Bühlmann Laboratories, AG] was used to dilute the samples to a working concentration. The faecal calprotectin [FCP] was measured using a fCAL ELISA Calprotectin kit [Bühlmann Laboratories, AG].
2.12. Statistical analysis
P-values displayed in cumulative flow cytometry plots were determined by non-parametric Mann-Whitney test. When more than two groups were compared, one-way analysis of variance [ANOVA], followed by Tukey’s test, was used to compare the results. Expression levels of targeted genes between groups were analysed by one-way ANOVA followed by Tukey’s test.
3. Results
3.1 CD71+ erythroid cells are expanded during pregnancy and exhibit immunosuppressive properties
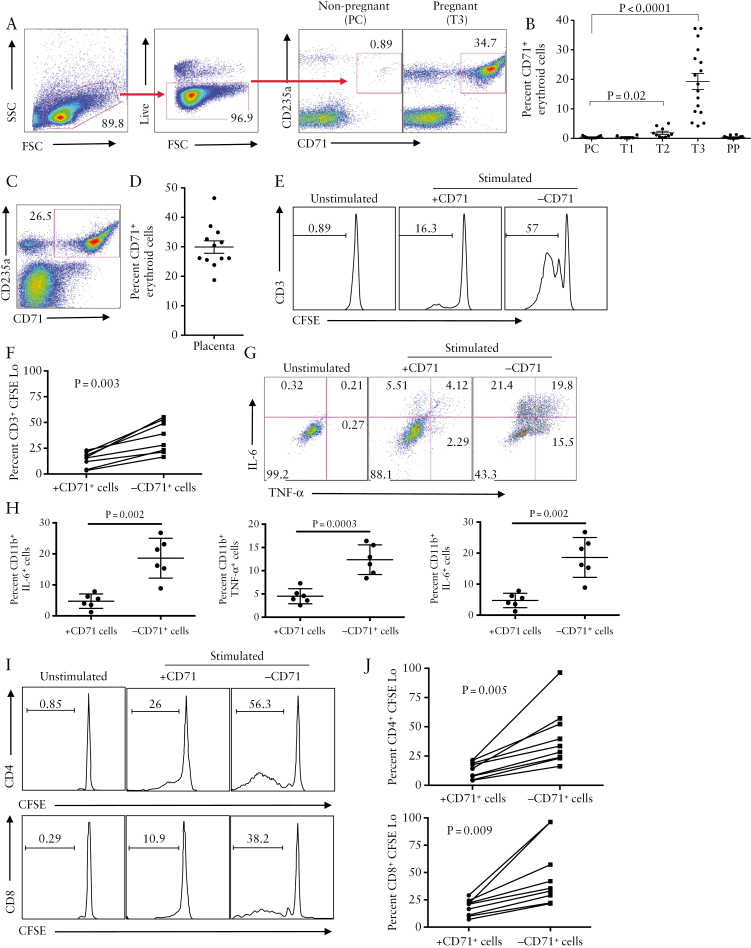
We found that CD71+ erythroid cells were physiologically expanded in the peripheral blood of pregnant women at T2, and peaked at T3, compared with PC and T1 [Figure 1A, B]. These cells were also abundant in placental tissues [Figure 1A, D].
Figure 1.
Expansion of CD71+ erythroid cells in human pregnancy. [A] Representative dot plots showing gating strategy and frequency of CD71+ erythroid cells in blood of pregnant versus non-pregnant women. [B] Cumulative data indicating percentages of CD71+ erythroid cells in preconception [PC], first trimester [T1], second trimester [T2], third trimester [T3], and postpartum [PP]. [C] Representative dot plots showing frequency of CD71+ erythroid cells in placenta tissues of a healthy woman. [D] Percentages of CD71+ erythroid cells in placental tissues of healthy women. [E] Representative dot plots showing proliferation of CD3+ T cells as measured by carboxyfluorescein succinimidyl ester[CFSE] dye in peripheral blood mononuclear cells [PBMCs] in the presence or absence of CD71+ erythroid cells. [F] Percentage of CD3+ T cells proliferation in PBMCs in the presence or absence of CD71+ erythroid cells. [G] Representative dot plots showing IL-6 and TNF-α production among CD11b+ cells in PBMCs in the presence or absence of CD71+ erythroid cells. [H] Percentages of CD11b+ cells producing IL-6, TNF-α or both cytokines, in the presence or absence of CD71+ erythroid cells. [I] Representative dot plots showing proliferation of CD4+ and CD8+ T cells in the presence or absence of CD71+ erythroid cells. [J] Percentages of proliferated CD4+ and CD8+ T cells in placenta cells in the presence or absence of CD71+ erythroid cells.
To determine whether CD71+ erythroid cells from pregnant women exhibited immunosuppressive activities similar to neonatal CD71+ erythroid cells,16,17 CFSE proliferation assay was performed. We found a significant increase in the proliferative capabilities of CD3+ T cells in the absence of CD71+ erythroid cells [Figure 1E, F]. Likewise, we observed that removal of CD71+ erythroid cells from PBMCs unleashes inflammatory cytokine production [e.g. IL-6 and TNF-α] by CD11b+ cells, following stimulation with HK Lm [Figure 1G, H].
Consistently, placental CD71+ erythroid cells inhibited proliferative capacities of both CD4+ and CD8+ T cells [Figure 1I, J]. Overall, these results indicate that CD71+ erythroid cells in PBMCs and placenta of healthy pregnancies exhibit immunosuppressive properties.
3.2. CD71+ erythroid cells are significantly reduced in IBD patients compared with HCs during pregnancy
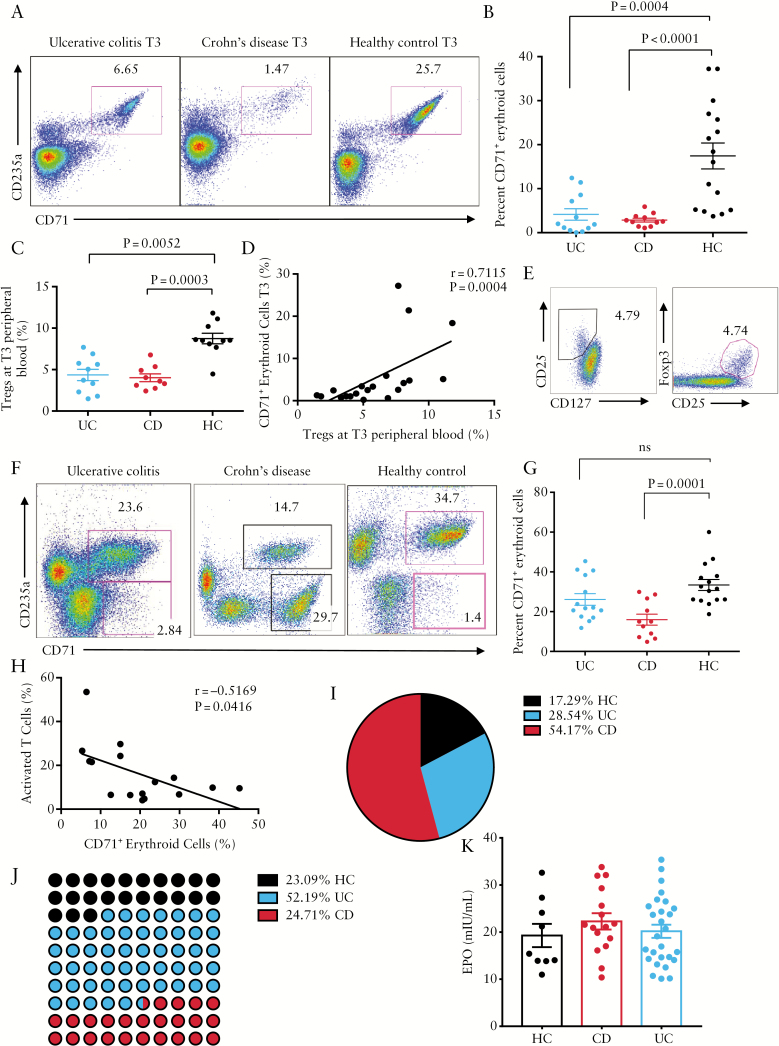
We found significantly lower percentages of CD71+ erythroid cells in the peripheral blood of IBD patients at T3, compared with HCs [Figure 2A, B]. Interestingly, reduced abundance of CD71+ erythroid cells was associated with lower percentages of Tregs in these patients [Figure 2C, D]. Tregs were identified as CD4+CD25hiFOXP3+ or CD4+CD25+CD127- cells [Figure 2E]. In addition, a significant reduction in percentages of CD71+ erythroid cells in the placental tissues of CD patients compared with HCs was noted [Figure 2F, G]. However, we did not observe any significant difference in the frequency of CD71+ erythroid cells in UC patients compared with HCs [Figure 2G]. Lower frequency of immunosuppressive CD71+ erythroid cells in placental tissues of CD patients was associated with hyperimmune activation, exhibited by increased percentages of immune cells expressing CD71 [transferrin receptor] [Figure 2F]. CD71 can be used as an activation marker on non-erythroid cells.23 In agreement, a negative correlation between the percentages of activated immune cells [expressing CD71] and CD71+ erythroid cells was observed in CD patients [Figure 2H]. Reduced percentages of CD71+ erythroid cells and Tregs could be associated with hyperimmune activation and active disease, which may impact on pregnancy outcome. As a result, higher rates of caesarean section in IBD patients was observed in our cohort [Figure 2I]. Similarly, increased numbers of preterm deliveries in IBD patients compared with HCs was also seen [Figure 2J]. We also observed that lower levels of CD71+ erythroid cells in cord blood were correlated with hyperimmune activation in cord blood of newborns of CD mothers [Supplementary Figure 1B, available as Supplementary data at ECCO-JCC online]. We hypothesised that lower CD71+ erythroid cells in IBD patients might be associated with lower serum erythropoietin [EPO].24,25 However, despite previous reports, no difference in the serum EPO levels was observed at T3 among IBD patients or HCs [Figure 2K]. Finally, no association between percentages of CD71+ erythroid cells and the type of treatment in IBD patients was noticed [Table 1].
Figure 2.
Lower frequency of CD71+ erythroid cells in IBD patients during pregnancy. [A] Representative dot plots showing frequency of CD71+ erythroid cells in the peripheral blood of ulcerative colitis [UC], Crohn’s disease [CD]. and healthy controls [HC] in the third trimester [T3]. [B] Percentages of CD71+ erythroid cells in the peripheral blood of pregnant women [T3], either those with UC or CD, or HCs. [C] Percentages of Tregs at T3 in inflammatory bowel disease [IBD] versus HCs. [D] Correlation of % Tregs with % CD71+ cells in IBD patents. [E] Representative dot plots showing how Tregs are defined. [F] Representative dot plots showing frequency of CD71+ erythroid cells in placental tissues of UC, CD, and HC. [G] Percentages of CD71+ erythroid cells in placental tissues of UC, CD or HCs. [H] Correlation of activated cells [CD71+ cells lymphocytes] with frequency of CD71+ erythroid cells in placenta. [I] Percentages of caesarean section [C-section] in HC versus IBD pregnancies in the cohort. [J] Percentages of preterm deliveries by HC versus IBD mothers in the cohort. [K] Detected levels of serum erythropoietin [EPO] by ELISA at T3 in IBD versus HCs.
3.3. Lower abundance of CD71+ erythroid cells in pregnant IBD patients predisposes them to a pro-inflammatory condition
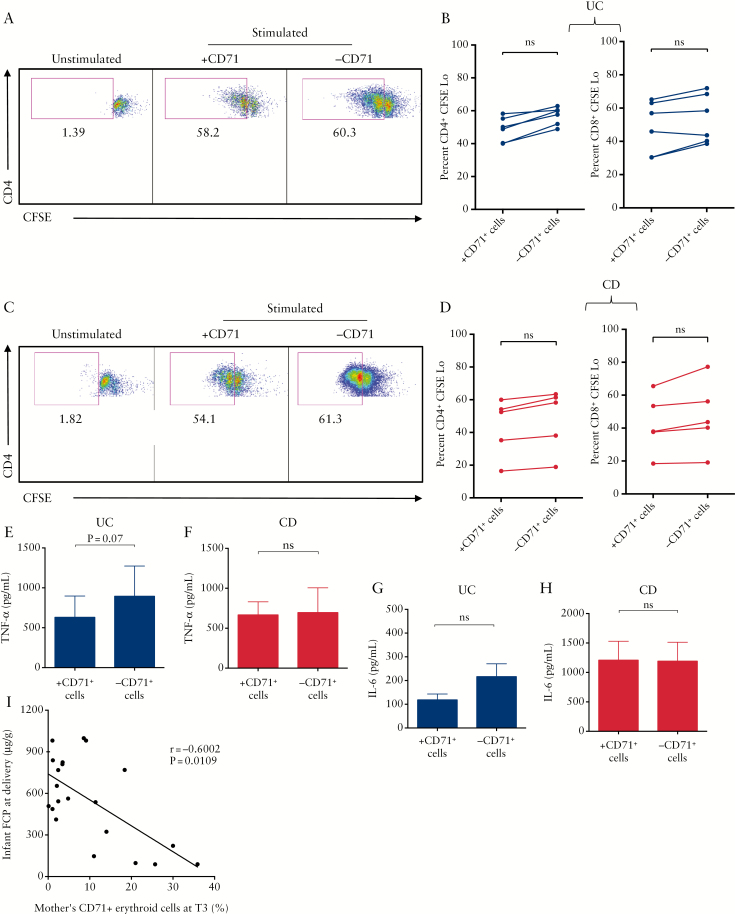
We found that depletion of CD71+ erythroid cells from placenta cells of UC [Figure 3A, B] or CD patients [Figure 3C, D] had no significant impact on the proliferative capacities of either CD4+ or CD8+ T cells. Additionally, depletion of CD71+ erythroid cells did not increase TNF-α production by placenta cells in UC [Figure 3E] or CD patients [Figure 3F]. Similar results were obtained for IL-6 production [Figure 3G, H]. We also measured the expression of activation markers [e.g. CD69, CD38, and HLA-DR] on T cells following stimulation in the presence or absence of CD71+ erythroid cells. Lack of inhibition by IBD placental CD71+ erythroid cells was observed of CD69 expression [Supplementary Figure 1C, available as Supplementary data at ECCO-JCC online]. Similar results was observed for CD38 and HLA-DR expression [data not shown].
Figure 3.
Lack of immunosuppression by CD71+ erythroid cells in inflammatory bowel disease [IBD] patients. [A] Representative dot plots showing proliferation of CD4+ T cells in peripheral blood mononuclear cells [PBMCs] of an ulcerative colitis [UC] patient. [B] Percentages of CD4+ and CD8+ T cells proliferation in PBMCs of UC patients in the presence or absence of CD71+ erythroid cells. [C] Representative dot plots showing proliferation of CD4+ T cells in PBMCs of a Crohn’s disease [CD] patient. [D] Percentages of CD4+ and CD8+ T cells proliferation in PBMCs of CD patients in the presence or absence of CD71+ erythroid cells. [E] TNF-α production by PBMCs of UC patients; or [F] CD patients. [G] TNF-α production by PBMCs of UC patients; or [H] CD patients following in vitro stimulation with anti-CD3 as measured by ELISA. [I] Correlation of CD71+ erythroid cells frequency in mothers in the third trimester [T3] with faecal calprotectin [FCP] levels in meconium.
Taken together, our data indicate that IBD mothers have lower and impaired CD71+ erythroid cells. More interestingly, we found a reverse correlation between percentage of CD71+ erythroid cells at T3 of mothers with FCP levels in meconium [Figure 2I]. Of note, we were unable to obtain meconium from all the newborns, and thus we combined all groups together.
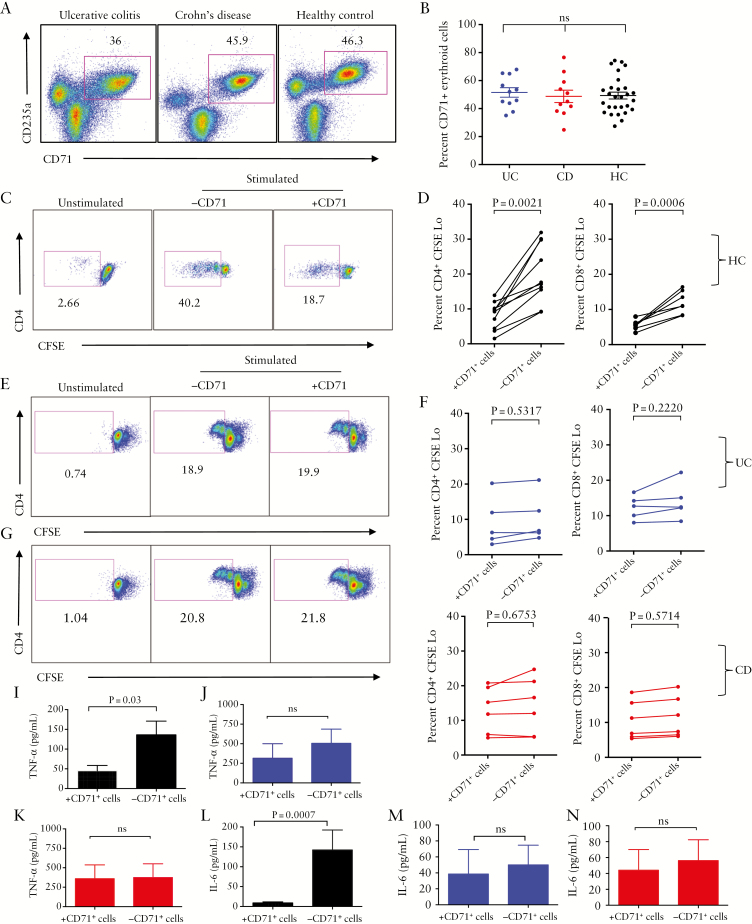
3.4. Functionality of CD71+ erythroid cells in the cord blood of infants born to mothers with IBD is impaired
Surprisingly, we found no significant difference in the abundance of CD71+ erythroid cells among CBMCs obtained from infants born to HCs, UC, or CD mothers [Figure 4A, B] except in emergency deliveries [Supplementary Figure 1B, available as Supplementary data at ECCO-JCC online]. When CD71+ erythroid cells from CBMCs of infants born to HCs were depleted, there was a significant increase in proliferation of both CD4+ and CD8+ T cells , demonstrating their immunosuppressive capabilities [Figure 4C, D]. A similar pattern was observed for the expression of CD69 [Supplementary Figure 1D, available as Supplementary data at ECCO-JCC online].
Figure 4.
Impaired functionality of CD71+ erythroid cells from cord blood of newborns to inflammatory bowel disease [IBD] mothers. [A] Representative dot plots showing frequency of CD71+ erythroid cells in cord blood mononuclear cells [CBMCs] of infants born to ulcerative colitis [UC], Crohn’s disease [CD], and healthy control [HC] mothers. [B] Percentages of CD71+ erythroid cells in CBMCs of infants born to UC, CD, and HC mothers. [C] Representative dot plots showing proliferation of CD4+ T cells in the presence or absence of CD71+ erythroid cells among CBMCs of an infant born to an HC mother. [D] Percentages of proliferated CD4+ T cells and CD8+ T cells in the presence or absence of CD71+ erythroid cells among CBMCs of infants born to HC mothers following stimulation with anti-CD3 in vitro. [E] Representative dot plots showing proliferation of CD4+ T cells in the presence or absence of CD71+ erythroid cells among CBMCs of an infant born to a UC mother. [F] Percentages of proliferated CD4+ T cells and CD8+ T cells in the presence or absence of CD71+ erythroid cells among CBMCs of infants born to UC mothers. [G] Representative dot plots showing proliferation of CD4+ T cells in the presence or absence of CD71+ erythroid cells among CBMCs of an infant born to a CD mother. [H] Percentages of proliferated CD4+ T cells and CD8+ T cells in the presence or absence of CD71+ erythroid cells among CBMCs of infants born to CD mothers. [I] TNF-α and [J] IL-6 production by stimulated CBMCs with anti-CD3 antibody obtained from infants born to HC mothers in the presence or absence of CD71+ erythroid cells. [K] TNF-α and [L] IL-6 production by stimulated CBMCs with anti-CD3 antibody obtained from infants born to UC mothers. [M] TNF-α and [N] IL-6 production by stimulated CBMCs with anti-CD3 antibody obtained from infants born to CD mothers in the presence or absence of CD71+ erythroid cells as measured by ELISA.
In contrast, depletion of CD71+ erythroid cells did not enhance proliferative capacity of either CD4+ or CD8+ T cells from infants born to either UC [Figure 4E, F] or CD mothers [Figure 4G, H]. This also was the case for CD69 expression on T cells [Supplementary Figure 1D, available as Supplementary data at ECCO-JCC online].
Since neonatal CD71+ erythroid cells suppress TNF-α and IL-6 production,16 we investigated suppressive effects of cord blood CD71+ erythroid cells on TNF-α and IL-6 production using cell culture supernatants, by ELISA. We found CD71+ erythroid cells from cord blood of infants born to HCs exhibited significant suppressive effects on TNF-α production [Figure 4I], in contrast to those of UC or CD infants which showed lack of immunosuppression [Figure 4J, K]. Similarly, removal of CD71+ erythroid cells from cord blood of infants born to healthy mothers significantly enhanced IL-6 production [Figure 4L], but this was not the case for the CBMCs of infants born to IBD mothers [Figure 4M, N]. Overall, these results indicate a disparity in the functionality of CD71+ erythroid cells in the cord blood of infants born to mothers with IBD compared with HCs.
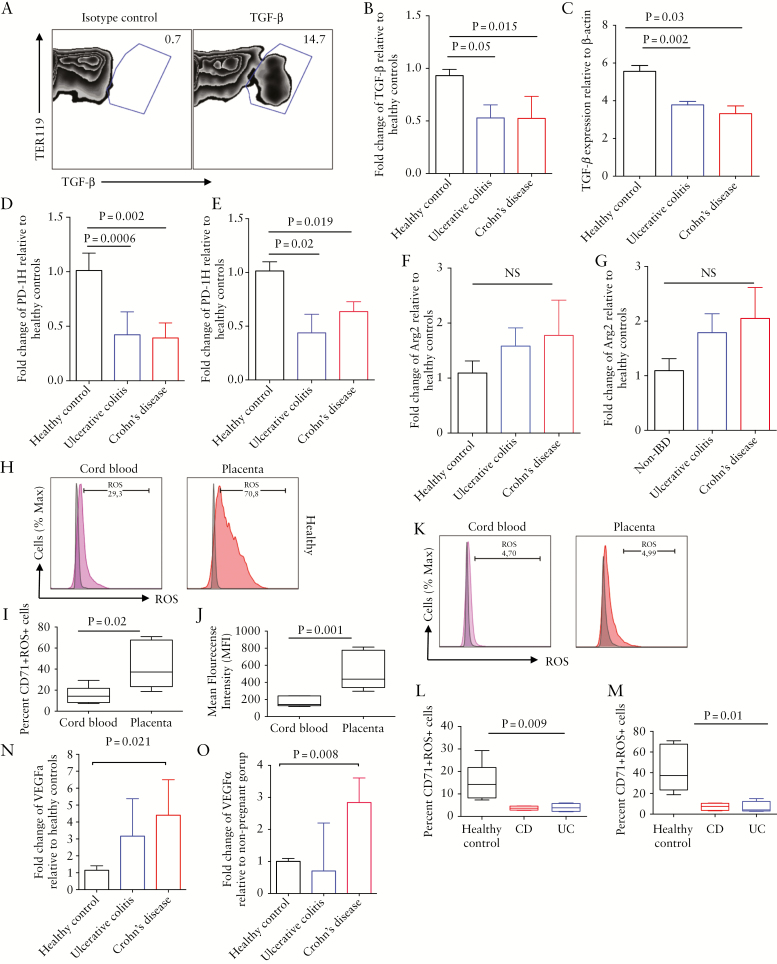
3.5. Cord blood CD71+ erythroid cells from newborns to IBD mothers express lower TGF-β, ROS, and PD-1H but higher vascular endothelial growth factor-α genes
To determine the mechanism behind differential functionality of CD71+ cells obtained from cord blood/placenta of IBD patients versus HCs, these cells were enriched [see Supplementary Figure 1A, available as Supplementary data at ECCO-JCC online] for real-time PCR analysis. Human CD71+ erythroid cells are sensitive to permeabilisation buffer, so measuring cytokines by intracellular cytokine staining [ICS] is impossible for these cells. In contrast, mouse CD71+ erythroid cells are resistant to perm buffer; thus, we analysed TGF-β secretion by these cells and found that they constitutively secrete TGF-β [Figure 5A]. Interestingly, CD71+ erythroid cells from cord blood or placenta of newborns to either CD or UC mothers expressed significantly lower levels of TGF-β mRNA compared with newborns to HCs [Figure 5B, C]. We have found that neonatal CD71+ erythroid cells express PD-1H or V-domain Ig suppressor of T cell activation [VISTA] [under review]. VISTA is a potent negative regulator of T cell function,26 so we investigated the expression levels of VISTA mRNA in CD71+ erythroid cells obtained from cord blood and placenta tissues. We found that PD-1H gene expression levels were significantly lower in CD71+ erythroid cells from cord blood [Figure 5D] and placenta [Figure 5E] of newborns to IBD versus healthy mothers. Although CD71+ erythroid cells express arginase-2 [Arg-2],16 no significant difference in mRNA levels of Arg-2 was observed for either cord blood or placenta-derived CD71+ erythroid cells in IBD versus HCs [Figure 5F, G]. Furthermore, CD71+ erythroid cells from both cord blood and placental tissues of HCs express ROS [Figure 5H]. Importantly, percentages of ROS expressing CD71+ erythroid cells [Figure 5I] and intensity of ROS [Figure 5J] were significantly higher when originating from the placenta compared with the cord blood. Due to the documented inhibitory role of ROS in autoimmune diseases and cancers,27 we aimed to compare the expression of ROS in CD71+ erythroid cells from IBD mothers with HCs. Interestingly, CD71+ erythroid cells from cord blood [Figure 5K, L] and placenta [Figure 5K, M] of IBD patients expressed significantly lower levels of ROS compared with HCs. In contrast, higher VEGFα gene expression was observed in CD71+ erythroid cells obtained from the cord blood and placenta of IBD patients compared with HCs. However, due to variability between samples, only the expression levels of this gene were significantly higher in CD versus UC and HCs [Figure 5N, O].
Figure 5.
Differential gene expression of inhibitory and stimulatory molecules by CD71+ erythroid cells of inflammatory bowel disease [IBD] patients versus healthy controls [HCs]. [A] Representative dot plots showing production of TGF-β by neonatal mice CD71+ erythroid cells. [B] Expression of TGF-β gene by CD71+ erythroid cells from cord blood or [C] placenta of HC compared with ulcerative colitis [UC] and Crohn’s disease [CD] patients. [D] Expression of PD-1H gene by CD71+ erythroid cells from cord blood or [E] placenta of HC compared with UC and CD patients. [F] Expression of arginase-2 gene by CD71+ erythroid cells from cord blood or [G] placenta of HC compared with UC and CD patients. [H] Representative plots showing expression of reactive oxygen species [ROS] among CD71+ erythroid cells from cord blood [pink] or placenta [orange] compared with isotype control [grey] of an HC. [I] Cumulative data showing percentages and [J] mean fluorescence intensity [MFI] of ROS+CD71+ erythroid cells in cord blood versus placenta of HCs. [K] Representative plots showing expression ROS among CD71+ erythroid cells from cord blood [pink] or placenta [orange] compared with isotype control [grey] of an IBD patient. [L] Cumulative data showing percentages of ROS+CD71+ erythroid cells in cord blood and [M] placenta of IBD versus HCs. [N] Expression of VEGF-α gene by CD71+ erythroid cells from cord blood or [O] placenta of HC compared with UC and CD patients. Data are obtained from a minimum of 5–7 patients/group.
3.6. Enriched CD71+ erythroid cells may contribute to the gut homeostasis during pregnancy
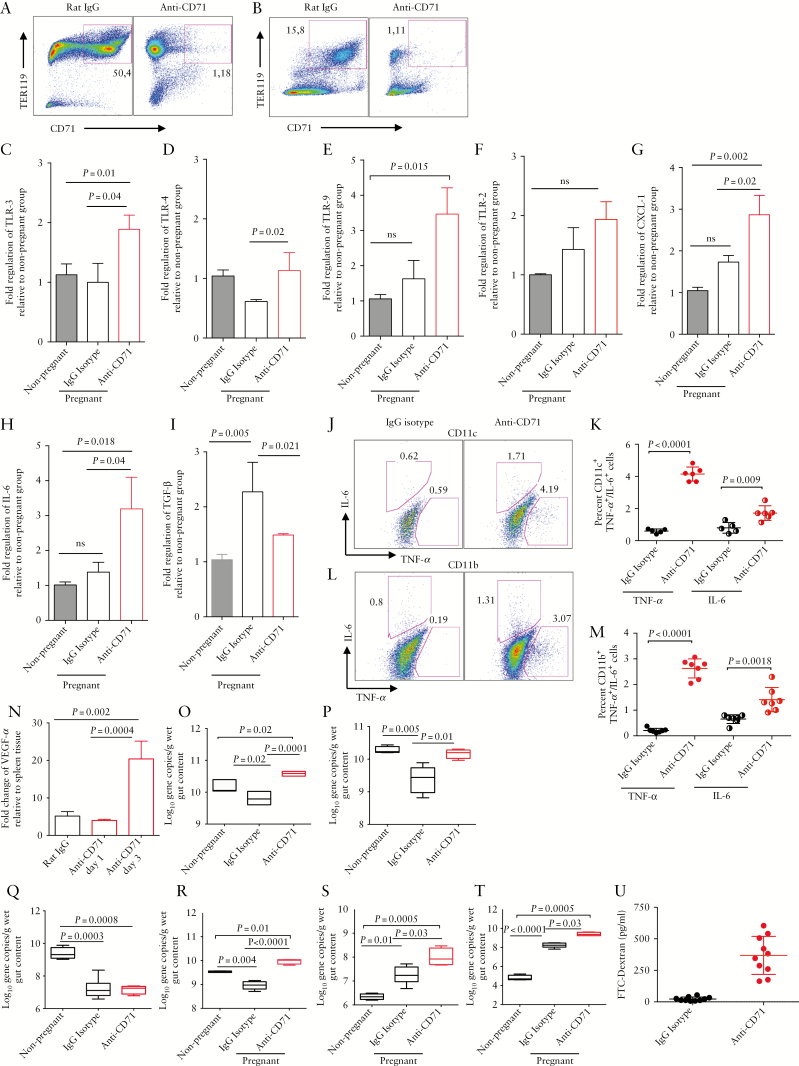
We have previously shown that neonatal CD71+ erythroid cells protect against excessive inflammation triggered by the microbiome.16 Thus, we proposed that lower frequency of and/or dysfunctional CD71+ erythroid cells in IBD patients during pregnancy may affect their digestive health.28 To address the question, we used an allogeneic animal model, which mimics human pregnancy. To further characterise the immunomodulatory role of CD71+ erythroid cells during pregnancy, mice were treated with anti-CD71 antibody which resulted in substantial depletion of CD71+ erythroid cells in both placenta and spleen tissues [Figure 6A, B]. Immunological changes in the gut tissues and modifications in the microbial communities in pregnant mice were then studied. A significant increase in the expression of TLR-3, TLR-4, and TLR-9 levels in the gut tissues [small intestine and colon] of anti-CD71 treated versus isotype control pregnant groups was observed [Figure 6C-E]. However, expression levels of TLR-2 in the gut tissues of the anti-CD71 treated group did not reach a significant level [Figure 6F]. In addition, depletion of CD71+ erythroid cells resulted in a significant increase in the gene expression of CXCL-1 and IL-6, not only compared with the control mice but also with the non-pregnant mice [Figure 6G, H]. Whereas TGF-β expression was significantly upregulated in the gut tissues of pregnant mice, depletion of CD71+ erythroid cells significantly downregulated the expression of this gene in the gut tissues [colon and small intestine] compared with controls [Figure 6I]. We found that depletion of CD71+ erythroid cells unleashed production of pro-inflammatory cytokines IL-6 and TNF-α by gut CD11c+ and CD11b+ cells [Figure 6J-M] and led to T cell activation in gut tissues [Supplementary Figure 1E, F, available as Supplementary data at ECCO-JCC online]. Finally, higher expression of VEGFα mRNA was observed in the intestinal tissues of mice in the absence of CD71+ erythroid cells [Figure 6N].
Figure 6.
CD71+ erythroid cells may impact on gut haemostasis during pregnancy. [A] Representative dot plots showing percentages of CD71+ erythroid cells in placenta and [B] spleen of mice before and after anti-CD71 antibody treatment. [C] Expression of TLR-1, [D] TLR-4, [E] TLR-9, [F] TLR-2, [G] CXCL-1, [H] IL-6 and [I] TGF-β genes by gut tissues of non-pregnant compared with pregnant mice treated with rat IgG isotype control or anti-CD71 antibody three days after treatment. [J] Representative plots and [K] cumulative data showing IL-6 and TNF-α production by intestinal CDllc+ cells. [L] Representative plots and [M] cumulative data showing IL-6 and TNF-α production by intestinal CDllb+ cells. [N] Expression of VEGFα gene in placenta tissues. [O] Treated mice were subjected to 16S rRNA-based polymerase chain reaction [PCR] for total bacteria, [P] Bacteroides-Prevotella-Porphyromonas group [Q] Enterobacteriaceae group, [R] Clostridium cluster XIVa, [S] Clostridium cluster 1, and [T] Clostridium cluster IV. [U] Levels of fluorescein isothiocyanate labelled dextran [FITC-dextran] in the blood of IgG versus anti-CD71 treated mice. Data are obtained from a minimum of five mice/group and at least two independent experiments.
3.7. CD71+ erythroid cells impact on gut microbial communities during pregnancy
In an attempt to reveal whether the gut microbiota during pregnancy is influenced by CD71+ erythroid cells, we investigated changes in the bacterial communities following depletion of these cells in mice. We found that total bacterial communities in the gut of pregnant mice were significantly reduced compared with their non-pregnant counterparts. However, total bacterial gene copies were significantly increased following depletion of CD71+ erythroid cells compared with pregnant controls [Figure 6O]. We further observed that depletion of CD71+ erythroid cells resulted in a significant increase in the Bacteroides-Prevotella-Porphyromonas group [Figure 6P] but a reduction in Enterobacteriaceae group [Figure 6Q]. Furthermore, Clostridium cluster XIVa was significantly reduced in pregnant mice compared with their non-pregnant counterparts; however, this population was significantly increased when CD71+ erythroid cells were depleted [Figure 6R]. Moreover, depletion of CD71+ erythroid cells during pregnancy resulted in a significant increase in Clostridium cluster I and Clostridium cluster IV bacteria in the gut of pregnant mice compared with pregnant control mice [Figure 6S, T]. Although total gene copies for Clostridium cluster I and Clostridium cluster IV were significantly increased following pregnancy, this increase was more pronounced when CD71+ erythroid cells were depleted [Figure 6S, T]. Finally, depletion of CD71+ erythroid cells compromises intestinal integrity, as evidenced by increased intestinal permeability when using FITC-dextran [Figure 6U].
3.8. Remodelling of the gut bacterial composition during pregnancy
CD71+ erythroid cells become physiologically expanded during the second and third trimesters of pregnancy. It is widely reported that pregnancy impacts ongut bacterial communities by either increasing or decreasing their diversity and composition [Supplementary Figure 2, available as Supplementary data at ECCO-JCC online]. Profound changes of gut microbiota take place in the third trimester, which coincides with the expansion of CD71+ erythroid cells. Depletion of CD71+ erythroid cells in a mouse model of pregnancy, which mimics lower frequency of CD71+ erythroid cells in IBD patients, was associated with higher TLRs, IL-6, TNF-α, and CXCL-1 expression and lower expression of TGF-β in the gut tissues. In addition, substantial changes in the gut microbial communities following their depletion was observed. Thus, our data support an immune-regulatory role for CD71+ erythroid cells in gut health during pregnancy.
4. Discussion
Here, we demonstrated that CD71+ erythroid cells are enriched in healthy pregnancy and exhibit distinctive immunosuppressive properties, but they are significantly reduced in IBD patients during pregnancy. Reduced frequency of CD71+ erythroid cells deprives these patients of much needed and beneficial immunosuppression. Although the underlying mechanism of lower CD71+ erythroid cells in IBD patients is unknown, inadequate EPO production has been reported.24,25 Human placenta expresses EPO and its receptors,29,30 and increased EPO production during pregnancy has been reported.31 Since EPO promotes the proliferation and differentiation of erythroid precursors,32 increased production of this hormone during pregnancy may contribute to the induction of CD71+ erythroid cells. Thus, we expected lower levels of EPO in IBD patients. However, we did not see any difference in EPO levels in the plasma of IBD mothers versus HCs. We suggest that inflammatory molecules, such as high mobility group box protein-1 [HMGB1] which is abundant in IBD patients,33 may inhibit EPO’s role in erythropoiesis.34 CD71+ erythroid cells, from peripheral blood, placenta, or cord blood of infants born to healthy mothers, exhibit immunosuppressive properties. In contrast CD71+ erythroid cells, obtained from placenta of IBD mothers or from the cord blood of infants born to IBD mothers, showed impaired immunosuppressive activities. Lower frequency of these cells in the blood of IBD mothers compared with HCs may explain lack of suppression; however, this cannot justify defective immunosuppression by cord blood CD71+ erythroid cells of newborns born to IBD mothers, where percentages of CD71+ erythroid cells were comparable to those of infants born to HCs.
We specifically investigated the mechanism of impaired CD71+ erythroid cell function in IBD patients. In contrast to HCs, CD71+ erythroid cells from IBD patients expressed significantly lower levels of TGF-β gene. Lower production of this cytokine has been reported in active disease, and restoration of TGF-β signalling has therapeutic implications in IBD.35 CD71+ erythroid cells constitutively produce TGF-β during pregnancy, and this cytokine can promote generation of Tregs while induction of Th17 is inhibited. Tregs, due to their immune-regulatory properties, play an important role in the repair of bowel mucosa of IBD patients, but they transform into pathogenic Th17 cells in the presence of IL-6 and other cytokines [e.g. IL-23].36 Thus, impaired TGF-β production due to lower CD71+ erythroid cells in IBD patients may enhance an inflammatory response [e.g. IL-6 and TNF-α production] and reduce Treg frequency, which impedes immune tolerance to luminal bacterial antigens. In agreement, we have demonstrated that CD71+ erythroid cells promote induced Tregs via TGF-β [PLoS Biol, under revision].
Despite our previous reports of arginase-2 as an immunomodulatory molecule produced by neonatal CD71+ erythroid cells,16 no significant difference in arginase-2 gene expression was observed among CD71+ erythroid cells of IBD versus HCs. Interestingly, we discovered that CD71+ erythroid cells obtained from placenta and cord blood of healthy mothers produced higher levels of ROS, similar to MDSCs.37 Defective ROS production by neutrophils is reported in rare pathogenic variants in NCF4 gene which causes Crohn’s disease-like intestinal inflammation.38 Excessive intracellular and extracellular ROS is detrimental to effector T cell responses.39 Consequently, CD71+ erythroid cells like MDSCs use ROS as a major mechanism to execute immunosuppression. However, CD71+ erythroid cells from IDB patients have impaired ROS production. In addition, CD71+ erythroid cells from IBD patients express significantly lower levels of PD-1H gene [VISTA] compared with HCs. In contrast, CD71+ cells from IBD patients tend to express higher VEGFα, which might be associated with hypoxia.40 Taken together, these results demonstrate that CD71+ erythroid cells from IBD patients are different from those in HCs during pregnancy. Although we are unaware of the underlying mechanism for such changes in the gene expression profile of CD71+erythroid cells in IBD patients versus HCs, we suggest that the pro-inflammatory milieu in IBD patients may influence the phenotype/function of these cells. However, further investigations are required to better understand the mechanism[s] associated with compromised functionality of CD71+ erythroid cells in IBD patients.
We further investigated the effects of lower CD71+ erythroid cells on intestinal inflammation and microbial communities, using an allogeneic pregnancy mouse model. In contrast to control pregnant or non-pregnant mice, the intestinal tissues of anti-CD71 treated pregnant mice expressed significantly higher TLR-3, TLR-4, and TLR-9. This is in agreement with upregulation of TLRs in the GI tract of IBD patients compared with HCs.41 Upregulation of TLR signalling results in activation of NF-kB which regulates expression of a variety of inflammatory cytokines such as IL-1β, IL-6, and TNF-α.42 In agreement, we observed overexpression of IL-6 and TNF-α and T cell activation in intestinal tissues when CD71+ erythroid cells were depleted. We suggest that CD71+ erythroid cells prevent improper immune activation against commensal microbial communities during pregnancy, as we have reported in neonates.16 Therefore, reduction/impaired functionality of erythroid CD71+ cells [e.g. in IBD] favours upregulation of TLRs in response to microbiota, which subsequently causes inflammatory response in the intestinal mucosa. We further found that depletion of CD71+ erythroid cells upregulates neutrophil chemoattractant [CXCL-1] which is reported in IBD patients.43 In contrast, depletion of CD71+ erythroid cells reduced expression of TGF-β mRNA in intestinal tissues, which may impact on Tregs induction in this compartment.
A remarkable observation of this study is that depletion of CD71+ erythroid cells results in excessive production of TNF-α by intestinal CD11b+/CD11c+ cells, a well-studied cytokine in IBD pathogenesis,44 which promotes expression of pro-inflammatory cytokines and chemokines mainly via an NF-kB dependent pathway. Associated immune activation following upregulation of TNF-α production may result in excessive tissue damage and disruption of tight junctions.45 In agreement, we observed increased in intestinal epithelial barrier permeability which can result in translocation of bacteria and their products, triggering a vicious cycle of inflammation.
IBD is clearly associated with intestinal dysbiosis and dysfunctional interaction between the microbiota and the gut mucosal immune system, which results in dysregulated immune response against commensal microbial antigens.46 Our animal studies revealed that depletion of CD71+ erythroid cells was associated with increased abundance of Clostridium clusters I, IV and XIVa. However, caecal butyrate concentrations were not affected by the anti-CD71 treatment [data not shown], possibly suggesting that the depletion of CD71+ erythroid cells during the pregnancy period increased non-butyrate producing microbes from the clostridial clusters. Furthermore, anti-CD71 treatment increased the caecal gene copies of the Bacteroides group with a controversial role in IBD.47,48 Such microbial changes following depletion of CD71+ erythroid cells may influence bacterial biodiversity and subsequently impact on immune regulation and differentiation in the gut. For instance, changing the cytokine balance can skew a Th1, Th17, or Treg differentiation. Commensal bacteria can regulate the development of Th1, Th17, and Treg cells, suggesting the relevance of local environment induced by commensal microorganisms in immunological homeostasis of gut-associated lymphoid tissues.
In summary, we have shown that reduced frequency of CD71+ erythroid cells and/or impaired function may predispose IBD patients to pregnancy complications associated with dysbiosis and a pro-inflammatory milieu in the gut. Such an inflammatory milieu not only influences the clinical course of the disease, but also impacts on pregnancy outcome and subsequently the neonate. In agreement, we observed that newborns from mothers with lower CD71+ erythroid cells [T3] had higher FCP in their meconium. Thus, future longitudinal studies are required to determine the impact of lower/dysfunctional CD71+ erythroid cells on gut haemostasis in infants born to IBD mothers. In addition, using animal models of IBD such as IL-10 KO mice, might be another approach to delineate the role of CD71+ erythroid cells during pregnancy.
4.1. Significance of this study
4.1.1. What is current knowledge?
Cord blood CD71+ erythroid cells are reduced in neonates born to women in spontaneous preterm labour.
CD71+ erythroid cells play an important role in feto-maternal tolerance.
CD71+ erythroid cells have immunosuppressive properties.
CD71+ erythroid cells promote conversion of naïve CD4+ T cells into Tregs.
4.1.2. What is new here?
CD71+ erythroid cells are significantly reduced in IBD patients during pregnancy.
CD71+ erythroid cells in placental tissues and cord blood of IBD patients are functionally impaired.
CD71+ erythroid cells from IBD patients exhibit lower VISTA, ROS, and TGF-β expression.
There is direct correlation between CD71+ erythroid cells and Treg frequency in IBD patients.
Depletion of CD71+ erythroid cells in an allogeneic mouse model of pregnancy resulted in upregulation of TLRs, CXCL-1, TNF-α, and IL-6, but downregulation of TGF-β in the gut tissues.
Depletion of CD71+ erythroid cells in a mouse model of pregnancy impaired gut integrity and influenced gut microbiome composition.
Funding
This work was supported by a Foundation Scheme grant from the Canadian Institutes of Health Research [CIHR], a New Investigator Salary Award from CIHR, and a New Investigator grant in Maternal, Reproductive, Child and Youth Health from CIHR/Women and Children’s Health Research Institute [WCHRI] [all to SE]. In addition, WCHRI supported this study through an innovation grant [to VH and SE].
Conflict of Interest
The authors have no financial, professional, or personal conflicts of interest to declare.
Supplementary Material
Acknowledgments
We thank our study volunteers for providing samples and supporting this work.
Author Contributions
GD performed most of the functional assays on human specimens including cord blood, placenta, and peripheral blood, analysed some of the data, wrote the first draft of Introduction, Methods and Results sections. PK performed all of the animal studies, isolated CD71+ erythroid cells, extracted RNA, performed qPCR studies and gut microbiome studies, and analysed the relevant data. NG conducted the ROS studies. RS and LM were actively involved in patient recruitment and sample and clinical data collection. XM performed EPO and other ELISA assays. VN and NH performed FCP assays. KM provided intellectual support and guidance. VH as a clinician recruited all of the study participants, provided all the clinical data, and assisted in clinical data analysis. LD as a clinician also contributed tp patient recruitment. SE proposed the study, supervised all of the research, performed intracellular cytokine assays and some of the CFSE assays, analysed the data, and wrote the manuscript.
References
- 1. Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med 2009;361:2066–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koloski NA, Bret L, Radford-Smith G. Hygiene hypothesis in inflammatory bowel disease: a critical review of the literature. World J Gastroenterol 2008;14:165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saidel-Odes L, Odes S. Hygiene hypothesis in inflammatory bowel disease. Ann Gastroenterol 2014;27:189–90. [PMC free article] [PubMed] [Google Scholar]
- 4. Trowsdale J, Betz AG. Mother’s little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol 2006;7:241–6. [DOI] [PubMed] [Google Scholar]
- 5. Ghaebi M, Nouri M, Ghasemzadeh A, et al. Immune regulatory network in successful pregnancy and reproductive failures. Biomed Pharmacother 2017;88:61–73. [DOI] [PubMed] [Google Scholar]
- 6. Pan T, Liu Y, Zhong LM, et al. Myeloid-derived suppressor cells are essential for maintaining feto-maternal immunotolerance via STAT3 signaling in mice. J Leukoc Biol 2016;100:499–511. [DOI] [PubMed] [Google Scholar]
- 7. Saito S, Nakashima A, Shima T, Ito M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol 2010;63:601–10. [DOI] [PubMed] [Google Scholar]
- 8. Adams Waldorf KM, Nelson JL. Autoimmune disease during pregnancy and the microchimerism legacy of pregnancy. Immunol Invest 2008;37:631–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vukusic S, Marignier R. Multiple sclerosis and pregnancy in the ‘treatment era’. Nat Rev Neurol 2015;11:280–9. [DOI] [PubMed] [Google Scholar]
- 10. Bröms G, Granath F, Linder M, Stephansson O, Elmberg M, Kieler H. Birth outcomes in women with inflammatory bowel disease: effects of disease activity and drug exposure. Inflamm Bowel Dis 2014;20:1091–8. [DOI] [PubMed] [Google Scholar]
- 11. Riis L, Vind I, Politi P, et al. ; European Collaborative study group on Inflammatory Bowel Disease Does pregnancy change the disease course? A study in a European cohort of patients with inflammatory bowel disease. Am J Gastroenterol 2006;101:1539–45. [DOI] [PubMed] [Google Scholar]
- 12. Niessner M, Volk BA. Altered Th1/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reversed transcribed polymerase chain reaction [RT-PCR]. Clin Exp Immunol 1995;101:428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang F, Wang MQ, Chen WY, et al. Relationship between the polymorphism of tumor necrosis factor-alpha-308 G > A and susceptibility to inflammatory bowel diseases and colorectal cancer: a meta-analysis. Eur J Hum Genet. 2011;19:432–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Lima-Karagiannis A, Zelinkova-Detkova Z, van der Woude CJ. The effects of active IBD during pregnancy in the era of novel IBD therapies. Am J Gastroenterol 2016;111:1305–12. [DOI] [PubMed] [Google Scholar]
- 15. Dunsmore G, Bozorgmehr N, Delyea C, Koleva P, Namdar A, Elahi S. Erythroid suppressor cells compromise neonatal immune response against Bordetella pertussis. J Immunol 2017;199:2081–95. [DOI] [PubMed] [Google Scholar]
- 16. Elahi S, Ertelt JM, Kinder JM, et al. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature 2013;504:158–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Namdar A, Koleva P, Shahbaz S, Strom S, Gerdts V, Elahi S. CD71+ erythroid suppressor cells impair adaptive immunity against Bordetella pertussis. Sci Rep 2017;7:7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Delyea C, Bozorgmehr N, Koleva P, et al. CD71+ erythroid suppressor cells promote fetomaternal tolerance through arginase-2 and PDL-1. J Immunol 2018;200:4044–58. [DOI] [PubMed] [Google Scholar]
- 19. Gomez-Lopez N, Romero R, Xu Y, et al. Umbilical cord CD71+ erythroid cells are reduced in neonates born to women in spontaneous preterm labor. Am J Reprod Immunol 2016;76:280–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bengtson MB, Aamodt G, Mahadevan U, Vatn MH. Inadequate gestational weight gain, the hidden link between maternal IBD and adverse pregnancy outcomes: results from the Norwegian mother and child cohort study. Inflamm Bowel Dis 2017;23:1225–33. [DOI] [PubMed] [Google Scholar]
- 21. Elahi S, Dinges WL, Lejarcegui N, et al. Protective HIV-specific CD8+ T cells evade Treg cell suppression. Nat Med 2011;17:989–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koleva PT, Valcheva RS, Sun X, Gänzle MG, Dieleman LA. Inulin and fructo-oligosaccharides have divergent effects on colitis and commensal microbiota in HLA-B27 transgenic rats. Br J Nutr 2012;108:1633–43. [DOI] [PubMed] [Google Scholar]
- 23. Motamedi M, Xu L, Elahi S. Correlation of transferrin receptor [CD71] with Ki67 expression on stimulated human and mouse T cells: The kinetics of expression of T cell activation markers. J Immunol Methods 2016;437:43–52. [DOI] [PubMed] [Google Scholar]
- 24. Gasche C, Waldhoer T, Feichtenschlager T, et al. ; Austrian Inflammatory Bowel Diseases Study Group Prediction of response to iron sucrose in inflammatory bowel disease-associated anemia. Am J Gastroenterol 2001;96:2382–7. [DOI] [PubMed] [Google Scholar]
- 25. Schreiber S, Howaldt S, Schnoor M, et al. Recombinant erythropoietin for the treatment of anemia in inflammatory bowel disease. N Engl J Med 1996;334:619–23. [DOI] [PubMed] [Google Scholar]
- 26. Lines JL, Pantazi E, Mak J, et al. VISTA is an immune checkpoint molecule for human T cells. Cancer Res. 2014;74:3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang I, Eibach D, Kops F, et al. Intestinal microbiota composition of interleukin-10 deficient C57BL/6J mice and susceptibility to Helicobacter hepaticus-induced colitis. PLoS One 2013;8:e70783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Elahi S. New insight into an old concept: role of immature erythroid cells in immune pathogenesis of neonatal infection. Front Immunol 2014;5:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Conrad KP, Benyo DF, Westerhausen-Larsen A, Miles TM. Expression of erythropoietin by the human placenta. FASEB J 1996;10:760–8. [DOI] [PubMed] [Google Scholar]
- 30. Fairchild Benyo D, Conrad KP. Expression of the erythropoietin receptor by trophoblast cellsin the human placenta. Biol Reprod 1999;60:861–70. [DOI] [PubMed] [Google Scholar]
- 31. Cotes PM, Canning CE, Lind T. Changes in serum immunoreactive erythropoietin during the menstrual cycle and normal pregnancy. Br J Obstet Gynaecol 1983;90:304–11. [DOI] [PubMed] [Google Scholar]
- 32. Toth B, Fischl A, Scholz C, Kunze S, Friese K, Jeschke U. Erythropoietin and erythropoietin receptor expression in normal and disturbed pregnancy. Eur J Obstet Gynecol Reprod Biol 2008;140:192–200. [DOI] [PubMed] [Google Scholar]
- 33. Boyapati RK, Rossi AG, Satsangi J, Ho GT. Gut mucosal DAMPs in IBD: from mechanisms to therapeutic implications. Mucosal Immunol 2016;9:567–82. [DOI] [PubMed] [Google Scholar]
- 34. Dulmovits BM, Papoin J, Hale J, et al. Inhibition of human erythropoiesis during inflammation is mediated by high mobility group box protein 1 [HMGB1] through decreased commitment of hematopoietic stem cells to the erythroid lineage and by increased apoptosis of terminally differentiating erythroblasts. Blood 2016;128;702. [Google Scholar]
- 35. Ihara S, Hirata Y, Koike K. TGF-β in inflammatory bowel disease: a key regulator of immune cells, epithelium, and the intestinal microbiota. J Gastroenterol 2017;52:777–87. [DOI] [PubMed] [Google Scholar]
- 36. Kitani A, Xu L. Regulatory T cells and the induction of IL-17. Mucosal Immunol 2008;1[Suppl 1]:S43–6. [DOI] [PubMed] [Google Scholar]
- 37. Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol 2004;172:989–99. [DOI] [PubMed] [Google Scholar]
- 38. Matute JD, Arias AA, Wright NA, et al. A new genetic subgroup of chronic granulomatous disease with autosomal recessive mutations in p40 phox and selective defects in neutrophil NADPH oxidase activity. Blood 2009;114:3309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang B, Liu SQ, Li C, et al. MicroRNA-23a curbs necrosis during early T cell activation by enforcing intracellular reactive oxygen species equilibrium. Immunity 2016;44:568–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ramakrishnan S, Anand V, Roy S. Vascular endothelial growth factor signaling in hypoxia and inflammation. J Neuroimmune Pharmacol 2014;9:142–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cario E. Toll-like receptors in inflammatory bowel diseases: a decade later. Inflamm Bowel Dis 2010;16:1583–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Caamaño J, Hunter CA. NF-kappaB family of transcription factors: central regulators of innate and adaptive immune functions. Clin Microbiol Rev 2002;15:414–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Arijs I, De Hertogh G, Machiels K, et al. Mucosal gene expression of cell adhesion molecules, chemokines, and chemokine receptors in patients with inflammatory bowel disease before and after infliximab treatment. Am J Gastroenterol 2011;106:748–61. [DOI] [PubMed] [Google Scholar]
- 44. Atreya R, Neurath MF. New therapeutic strategies for treatment of inflammatory bowel disease. Mucosal Immunol 2008;1:175–82. [DOI] [PubMed] [Google Scholar]
- 45. Grabinger T, Bode KJ, Demgenski J, et al. Inhibitor of apoptosis protein-1 regulates tumor necrosis factor-mediated destruction of intestinal epithelial cells. Gastroenterology 2017;152:867–79. [DOI] [PubMed] [Google Scholar]
- 46. Dalal SR, Chang EB. The microbial basis of inflammatory bowel diseases. J Clin Invest 2014;124:4190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lucke K, Miehlke S, Jacobs E, Schuppler M. Prevalence of Bacteroides and Prevotella spp. in ulcerative colitis. J Med Microbiol 2006;55:617–24. [DOI] [PubMed] [Google Scholar]
- 48. Waidmann M, Bechtold O, Frick JS, et al. Bacteroides vulgatus protects against Escherichia coli-induced colitis in gnotobiotic interleukin-2-deficient mice. Gastroenterology 2003;125:162–77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.