Abstract
Background
Surgery for rotator cuff disease is usually used after non‐operative interventions have failed, although our Cochrane Review, first published in 2007, found that there was uncertain clinical benefit following subacromial decompression surgery.
Objectives
To synthesise the available evidence of the benefits and harms of subacromial decompression surgery compared with placebo, no intervention or non‐surgical interventions in people with rotator cuff disease (excluding full thickness rotator cuff tears).
Search methods
We searched CENTRAL, MEDLINE, Embase, Clinicaltrials.gov and WHO ICRTP registry from 2006 until 22 October 2018, unrestricted by language.
Selection criteria
We included randomised and quasi‐randomised controlled trials (RCTs) of adults with rotator cuff disease (excluding full‐thickness tears), that compared subacromial decompression surgery with placebo, no treatment, or any other non‐surgical interventions. As it is least prone to bias, subacromial decompression compared with placebo was the primary comparison. Other comparisons were subacromial decompression versus exercises or non‐operative treatment. Major outcomes were mean pain scores, shoulder function, quality of life, participant global assessment of success, adverse events and serious adverse events. The primary endpoint for this review was one year. For serious adverse events, we also included data from prospective cohort studies designed to record harms that evaluated subacromial decompression surgery or shoulder arthroscopy.
Data collection and analysis
We used standard methodologic procedures expected by Cochrane.
Main results
We included eight trials, with a total of 1062 randomised participants with rotator cuff disease, all with subacromial impingement. Two trials (506 participants) compared arthroscopic subacromial decompression with arthroscopy only (placebo surgery), with all groups receiving postoperative exercises. These trials included a third treatment group: no treatment (active monitoring) in one and exercises in the other. Six trials (556 participants) compared arthroscopic subacromial decompression followed by exercises with exercises alone. Two of these trials included a third arm: sham laser in one and open subacromial decompression in the other.
Trial size varied from 42 to 313 participants. Participant mean age ranged between 42 and 65 years. Only two trials reported mean symptom duration (18 to 22 months in one trial and 30 to 31 months in the other), two did not report duration and four reported it categorically.
Both placebo‐controlled trials were at low risk of bias for the comparison of surgery versus placebo surgery. The other trials were at high risk of bias for several criteria, most notably at risk of performance or detection bias due to lack of participant and personnel blinding. We have restricted the reporting of results of benefits in the Abstract to the placebo‐controlled trials.
Compared with placebo, high‐certainty evidence indicates that subacromial decompression provides no improvement in pain, shoulder function, or health‐related quality of life up to one year, and probably no improvement in global success (moderate‐certainty evidence, downgraded due to imprecision).
At one year, mean pain (on a scale zero to 10, higher scores indicate more pain), was 2.9 points after placebo surgery and 0.26 better (0.84 better to 0.33 worse), after subacromial decompression (284 participants), an absolute difference of 3% (8% better to 3% worse), and relative difference of 4% (12% better to 5% worse). At one year, mean function (on a scale 0 to 100, higher score indicating better outcome), was 69 points after placebo surgery and 2.8 better (1.4 worse to 6.9 better), after surgery (274 participants), an absolute difference of 3% (7% better to 1% worse), and relative difference of 9% (22% better to 4% worse). Global success rate was 97/148 (or 655 per 1000), after placebo and 101/142 (or 708 per 1000) after surgery corresponding to RR 1.08 (95% CI 0.93 to 1.27). Health‐related quality of life was 0.73 units (European Quality of Life EQ‐5D, −0.59 to 1, higher score indicating better quality of life), after placebo and 0.03 units worse (0.011 units worse to 0.06 units better), after subacromial decompression (285 participants), an absolute difference of 1.3% (5% worse to 2.5% better), and relative difference of 4% (15% worse to 7% better).
Adverse events including frozen shoulder or transient minor complications of surgery were reported in approximately 3% of participants across treatment groups in two randomised controlled trials, but due to low event rates we are uncertain if the risks differ between groups: 5/165 (37 per 1000) reported adverse events with subacromial decompression and 9/241 (34 per 1000) with placebo or non‐operative treatment, RR 0.91 (95% CI 0.31 to 2.65) (moderate‐certainty evidence, downgraded due to imprecision). The trials did not report serious adverse events.
Based upon moderate‐certainty evidence from two observational trials from the same prospective surgery registry, which also included other shoulder arthroscopic procedures (downgraded for indirectness), the incidence proportion of serious adverse events within 30 days following surgery was 0.5% (0.4% to 0.7%; data collected 2006 to 2011), or 0.6% (0.5 % to 0.7%; data collected 2011 to 2013). Serious adverse events such as deep infection, pulmonary embolism, nerve injury, and death have been observed in participants following shoulder surgery.
Authors' conclusions
The data in this review do not support the use of subacromial decompression in the treatment of rotator cuff disease manifest as painful shoulder impingement. High‐certainty evidence shows that subacromial decompression does not provide clinically important benefits over placebo in pain, function or health‐related quality of life. Including results from open‐label trials (with high risk of bias) did not change the estimates considerably. Due to imprecision, we downgraded the certainty of the evidence to moderate for global assessment of treatment success; there was probably no clinically important benefit in this outcome either compared with placebo, exercises or non‐operative treatment.
Adverse event rates were low, 3% or less across treatment groups in the trials, which is consistent with adverse event rates reported in the two observational studies. Although precise estimates are unknown, the risk of serious adverse events is likely less than 1%.
Plain language summary
Surgery for rotator cuff disease
Background
The rotator cuff is a group of tendons that holds the shoulder joint in place allowing people to lift their arm and reach overhead. Some people can develop pain in their shoulder related to wear and tear of the rotator cuff. There may also be inflammation of the shoulder tendons or bursa (another part of the shoulder that helps it move), and pressure on the tendons by the overlying bone when lifting the arm up (impingement). Often the pain is made worse by sleeping on the affected shoulder and moving the shoulder in certain directions.
Surgery on your rotator cuff may include removing part of your bone to take the pressure off the rotator cuff tendons (acromioplasty), removing any swollen or inflamed bursa (the small sack of fluid that cushions the shoulder joint), and removing any damaged tissue or bone to widen the space where the tendons pass (subacromial decompression). Most rotator cuff surgery is now performed arthroscopically (surgical instruments are inserted through a small incision or key hole to perform surgery).
Study characteristics
This Cochrane Review is current to 22 October 2018. Trials were performed in hospitals in Denmark, Finland, Germany, Norway, Sweden and the UK. We included eight trials (1062 participants), comparing surgery with placebo (fake) surgery or other non‐operative treatment, such as exercise in people with impingement of the shoulder rotator cuff tendons.
The number of participants ranged from 42 to 313, mean age from 42 to 65 years, and duration of follow‐up from one year up to 12 to 13 years. Five trials failed to report funding sources, three received funding from non‐commercial foundations, and one trial author was paid by an instrument company.
Key results
Two trials (506 participants) met our criteria for inclusion for our main comparison, surgery versus placebo. Subacromial decompression resulted in little benefit to people at one‐year follow‐up.
Pain (lower scores mean less pain):
improved by 3% (3% worse to 8% better), or 0.26 points on a zero to 10 scale
• People who had placebo rated their pain as 2.9 points
• People who had surgery rated their pain as 2.6 points
Function (0 to 100; higher scores mean better function):
improved by 3% (1% worse to 7% better) or 3 points on a zero to 100 scale
• People who had placebo rated their function as 69 points
• People who had surgery rated their function as 72 points
Treatment success (much better or no problems at all):
5% more people rated their treatment a success (5% fewer to 16% more), or five more people out of 100
• 66 out of 100 people considered treatment as successful after placebo procedure
• 71 out of 100 people considered treatment as successful after surgery
Health‐related quality of life (higher scores mean better quality of life):
worsened 2% (8% worse to 4% better) or 0.02 points on a −0.59 to 1 scale
• People who had placebo rated their quality of life as 0.73 points
• People who had surgery rated their quality of life 0.71 points
Adverse events
1% fewer people (4% fewer to 3% more) had adverse events with surgery
• 4 out of 100 people reported adverse events after placebo
• 3 out of 100 people reported adverse event after surgery
Serious adverse events
No serious adverse events were reported in the trials. In observational studies the rate of serious adverse events was between 0.5% and 0.6%.
• 5 or 6 out of 1000 people had a serious adverse event after surgery
Certainty of the evidence
In people with painful shoulder impingement, high‐certainty evidence shows that subacromial decompression surgery does not improve pain, function or health‐related quality of life compared with placebo surgery, and moderate‐certainty evidence (downgraded due to imprecision), shows no improvement in the number of people reporting treatment success. We are uncertain if surgery is associated with more adverse events compared with no surgery.
Serious adverse events including deep infection, pulmonary embolism, nerve injury, and death can occur following shoulder surgery. Although precise estimates are unknown, the risk of serious adverse events is likely less than 1% (moderate‐certainty evidence, downgraded due to imprecision).
Summary of findings
Summary of findings for the main comparison. Subacromial decompression compared to placebo surgery.
| Subacromial decompression compared to placebo surgery for people with impingement syndrome without full‐thickness rotator cuff tears | ||||||
| Patient or population: people with impingement syndrome without full‐thickness rotator cuff tears Setting: hospitals in Finland and UK Intervention: subacromial decompression Comparison: placebo surgery (diagnostic arthroscopy) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo surgery | Risk with subacromial decompression | |||||
| Paina (scale from 0‐10, 0 is no pain) Follow‐up: 1 year | The mean pain was 2.9 pointsb | The mean pain was 0.26 points better (0.84 better to 0.33 worse) | ‐ | 284 (2 RCTs) | ⊕⊕⊕⊕ High | Absolute difference 3% better (8% better to 3% worse); relative difference 4% better (12% better to 5% worse)c |
|
Functional outcome (Constant score from 0‐100, 100 is best) Follow‐up: 1 year |
The mean functional outcome was 69b | MD 2.76 higher (1.36 lower to 6.87 higher) | 274 (2 RCTs) | ⊕⊕⊕⊕ High | Absolute difference 3% better (7% better to 1% worse); relative difference 9% better (22% better to 4% worse)c | |
| Global assessment of treatment success | 655 per 1000 | 708 per 1000 (610 to 832) | RR 1.08 (0.93 to 1.27) | 290 (2 RCTs) | ⊕⊕⊕⊝ Moderated | Absolute difference 5% more reported success (5% fewer to 16% more); relative difference 8% more reported success (7% fewer to 27% more) |
| Health‐related quality of life (scale from −0.59 to 1, 1 is perfect health) Follow‐up: 1 year | The mean health‐related quality of life was 0.73b | MD 0.03 lower (0.11 lower to 0.06 higher) | 285 (2 RCTs) | ⊕⊕⊕⊕ High | SMD 0.09 worse (0.39 worse to 0.21 better) Absolute difference 2% worse (7% worse to 4% better); relative difference 5% worse (20% worse to 11% better)c |
|
| Adverse events | 37 per 1000 | 34 per 1000 (11 to 98) | RR 0.91 (0.31 to 2.65) | 406 (2 RCTs)d | ⊕⊕⊕⊝ Moderatee | Absolute difference of 1% fewer events with surgery (4% fewer to 3% more); relative difference 9% fewer events with surgery (69% fewer to 165% more) |
| Serious adverse events | No events | No events | No estimate | 331 (2 RCTs) | ⊕⊕⊕⊝ Moderatef | Although precise estimates are unknown, serious adverse event rates in observational studies are reported as less than 1%g |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aPain measured with numeric rating scale (NRS) or visual analogue scale (VAS). bMedian value in placebo groups after one‐year follow‐up. cRelative changes calculated relative to baseline in control group (i.e. absolute change (mean difference) divided by mean at baseline in the placebo group from Paavola 2018 (values were: 7.23 points on 0 to 10‐point VAS pain; 31.7 points on 0 to 100‐point Constant score) and Beard 2018 (0.55 points on EQ‐5D quality‐of‐life scale). Absolute change calculated as mean difference divided by scale of the instrument, expressed as percentage.
dPooled both placebo and non‐operative (exercise or no treatment) comparisons from randomised controlled trials in the analysis of adverse events eDowngraded due to imprecision (due to low event rates, or 95% confidence intervals that included both benefits and harms) in the randomised trials.
fDowngraded due to indirectness as arthroscopic procedures other than subacromial decompression were included in the surgery registry observational data gSerious adverse events as reported in observational studies, 7 per 1000 (95% CI 6 to 8 per 1000) include: deep infection; pulmonary embolism; uncontrolled bleeding; myocardial infection; acute renal failure; ventilation more than 48 hours; cerebral vascular incident; septic shock; cardiac arrest; wound dehiscence; deep venous thrombosis; pneumonia; bleeding requiring transfusion; nerve injury; death; organ space infection.
Summary of findings 2. Subacromial decompression compared to exercises.
| Subacromial decompression compared to exercises for people with impingement syndrome without full‐thickness rotator cuff tears | ||||||
| Patient or population: people with impingement syndrome without full‐thickness rotator cuff tears Setting: hospitals or home Intervention: subacromial decompression Comparison: exercises | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with exercise | Risk with subacromial decompression | |||||
| Paina (scale from: 0‐10, 0 is no pain) Follow‐up: 1 year | The mean pain was 3.7 pointsb | MD 1.01 better (1.6 better to 0.42 better) | ‐ | 316 (3 RCTs) | ⊕⊕⊝⊝ Lowc | Absolute difference 10% better (4% better to 16% better); relative difference 14% better (6% better to 22% better)d |
|
Functional outcomee (scale from 0‐100, 100 is best) Follow‐up: 1 year |
The mean functional outcome was 58b | MD 3.24 better (8.08 worse to 14.55 better) | ‐ | 259 (3 RCTs) | ⊕⊕⊝⊝ Lowc | Absolute difference 3% better (8% worse to 15% better); relative difference 9% better (23% worse to 41% better)d |
| Global assessment of treatment success | 598 per 1000 | 723 per 1000 (574 to 902) | RR 1.21 (0.96 to 1.51) | 158 (2 RCTs) | ⊕⊕⊝⊝ Lowc | Absolute difference 13% more reported success (2% fewer to 30% more); relative difference 21% more reported success (4% fewer to 51% more) |
|
Health‐related quality of life (15D; scale from: 0‐1, 1 is perfect health) Follow‐up: 1 year |
The mean health‐related quality of life was 0.91b | MD 0.01 better (0.01 worse to 0.03 better) | ‐ | 116 (1 RCT) | ⊕⊕⊝⊝ Lowc | Absolute difference 1% better (1% worse to 3% better); relative difference 1% better (1% worse to 3% better)d |
| Adverse events | 37 per 1000 | 34 per 1000 (11 to 98) | RR 0.91 (0.31 to 2.65) | 406 (2 RCTs)f | ⊕⊕⊕⊝ Moderateg | Absolute difference of 1% fewer events with surgery (4% fewer to 3% more); relative difference 9% fewer events with surgery (69% fewer to 165% more) |
| Serious adverse events | No events | No events | Not estimable | ⊕⊕⊕⊝ Moderateh | Although precise estimates are unknown, serious adverse events rates in observational studies are reported as less than 1%i | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aPain measured with numeric rating scale (NRS) or visual analogue scale (VAS). bMedian value in exercise groups at one‐year follow‐up. cDowngraded due to risk of bias and imprecision. dRelative changes calculated as mean difference divided by mean at baseline in the exercise group from Paavola 2018 (mean (standard deviation) values were: 7.24 (2.08) points on 0 to 10‐point VAS pain scale; 35.2 (16.2) points on 0 to 100‐point Constant score); and 0.88 (0.08) points on 0 to 1 scale in health‐related quality of life. Absolute difference calculated as mean difference divided by scale of the instrument, expressed as percentage. eFunctional outcome measured with various measures (Constant score, Shoulder Disability Questionnaire, Subjective Shoulder Rating scale, or Neer score).
fPooled both placebo and non‐operative (exercise or no treatment) comparisons from randomised controlled trials in the analysis of adverse events
gDowngraded due to imprecision (due to low event rates) in the randomised trials hDowngraded due to indirectness as arthroscopic procedures other than subacromial decompression were included in the surgery registry observational data. iSerious adverse events as reported in observational studies, 7 per 1000 (95% CI 6 to 8 per 1000) include: deep infection; pulmonary embolism; uncontrolled bleeding; myocardial infection; acute renal failure; ventilation more than 48 hours; cerebral vascular incident; septic shock; cardiac arrest; wound dehiscence; deep venous thrombosis; pneumonia; bleeding requiring transfusion; nerve injury; death; organ space infection.
Background
This Cochrane Review is one of an updated series of Cochrane Reviews of interventions for shoulder disorders. The original review on all interventions for shoulder pain (Green 1998), has been split into a series of reviews that examine interventions for different shoulder disorders separately. The last review on surgery for rotator cuff disease was published in Issue 1, 2008 (up to date to 3 September 2006; Coghlan 2008). For this update we have split the surgery for rotator cuff disease review into three reviews: 1) subacromial decompression surgery for rotator cuff disease (the topic of this review); 2) surgery for full‐thickness rotator cuff tears; and 3) surgery for calcific rotator cuff tendinopathy. A parallel systematic review was performed by an overlapping team of authors (Lähdeoja 2019), and both reviews informed the 13th BMJ Rapid Recommendations on the same topic (Vandvik 2018). Estimates of the lifetime and monthly prevalence of shoulder pain in the general population vary between 6.7% and 66.7% and 18% to 31% respectively (Luime 2004). Shoulder pain is the third most common musculoskeletal complaint presenting to primary care (Rekola 1993). Each year about 1% of the population 45 years and older presents with shoulder pain to primary care settings (Royal College of General Practitioners 1980‐81). The direct annual healthcare costs attributable to shoulder disorders was estimated to be USD 7 billion in the USA in 2000 (Johnson 2004). Rotator cuff disorders are the most common underlying cause, with estimates varying between 65% and 85% depending upon the setting and age of the study population (Chard 1991; Ostör 2005; Vecchio 1995). Subacromial decompression surgery is increasingly performed for rotator cuff disorders (Vitale 2010). For example, a UK study reported a seven‐fold increase in people undergoing this procedure between 2000 (2523 people) and 2010 (21,355 people; Judge 2014), while in the USA an estimated 257,541 (95% CI 185,268 to 329,814) shoulder arthroscopies, excluding those for cuff repairs, were performed in 2006 (Jain 2014).
Description of the condition
A confusing array of diagnostic labels are used for pathology affecting the rotator cuff and related structures (Whittle 2015). We prefer to use the umbrella term 'rotator cuff disease' as a simple categorisation that encompasses all symptomatic disorders of the rotator cuff, regardless of mechanism (inflammatory, degenerative or acute injury), or precise anatomical location (e.g. supraspinatus tendon versus subacromial bursa; Buchbinder 1996). Diagnoses included within this umbrella term include rotator cuff tendinopathy or tendinitis, the impingement syndrome, partial and complete rotator cuff tears and complete rotator cuff tear, calcific tendinitis, and subacromial bursitis.
People with symptomatic rotator cuff disease present with shoulder pain, often described as pain in the upper outer arm. It is aggravated by overhead activities and is often worse at night and lying on the affected side, leading to disrupted sleep. The pain is accompanied by loss of function and often significant disability. A painful arc (as the arm is passively abducted away from the body, pain occurs between 60° and 120°) is nearly always present. Its presence is associated with a positive likelihood ratio of 3.7 (CI 1.9 to 7.0), while its absence is associated with a negative likelihood ratio of 0.36 (CI 0.23 to 0.54; Hermans 2013).
The current tenet proposes that rotator cuff disease is an interaction between mechanistic and biological factors. Mechanistic theory postulates that the mechanical impingement occurs between undersurface of anterior acromion, coracoacromial ligament, and humerus during shoulder flexion or abduction. According to the theory, pathophysiology starts from oedema and thickening of bursa (stage 1). It progresses to fibrosis and inflammatory changes (stage 2), and eventually to partial or complete tear of the tendon (stage 3; Neer 1983). Trauma may also cause the tear but often it is absent. A tear causes imbalance in the forces moving the shoulder joint, which may further aggravate the pathology and symptoms (Nam 2012). Once a tear develops, it does not heal spontaneously (Yamaguchi 2001). The shape of the acromion and fatigue or imbalance of muscular strength in the rotator cuff muscles has also been postulated to predispose to subacromial impingement (Chen 1999). Several observations support the mechanistic theory including the location of tears and their increasing prevalence with increasing age (Neer 1983). Anatomical and imaging studies have shown an association between the shape of the acromion and presence of a tear (Moor 2014). Finally, higher prevalence in the dominant side (Shiri 2007) and experimental pressure measurements (Hyvonen 2003) imply that rotator cuff disease is associated with mechanical factors. Biological studies also offer a framework to explain the condition. Ageing predisposes tendons to tendinopathy, which could explain the observed increasing prevalence in middle age (Teunis 2014). Histological studies have associated rotator cuff tears with several cellular and extracellular changes affecting the structure of the tendon (Dean 2012), but the exact biological mechanism causing the pain remains elusive. Taken together, current evidence suggest that the cause for rotator cuff disease appears to be an interplay between degenerative, metabolic and mechanical factors. It is so common after middle age (Minagawa 2013; Yamamoto 2010), that some consider it part of normal ageing.
Description of the intervention
Surgical procedures that may be used to treat rotator cuff disease include subacromial decompression (acromioplasty/bursectomy), or debridement of partial tears or a rotator cuff repair, or both. Operation may be performed by an open approach, arthroscopic‐assisted (mini‐open) technique, or as an arthroscopy only procedure (Nho 2007). Arthroscopic surgery may result in less morbidity and shorter recovery time enabling earlier return to work or sport (Coghlan 2008; Hata 2001).
Patients typically wear a sling after surgery for one to three weeks and undergo postoperative rehabilitation for three to six months (Hertling 1990; Millett 2006; Van der Meijden 2012). The principles of postoperative physical therapy are similar to those for physical therapy alone except for use of the sling, and the exercise programme must often be adjusted due to postoperative pain in the immediate postoperative period.
Potential risks of surgery include complications related to the anaesthesia or comorbidities, infection, postoperative adhesive capsulitis (or frozen shoulder), peripheral nerve injury, ongoing pain, and even death.
Non‐operative treatment includes physical therapies such as muscle strengthening, scapular stabilisation, and stretching and flexibility exercises (Bennell 2007; Hertling 1990; Kuhn 2009; Misamore 1995; Page 2016), glucocorticoid injection, nonsteroidal anti‐inflammatory drugs (NSAIDs), acupuncture, iontophoresis, phonophoresis, transcutaneous electrical nerve stimulation (TENS), pulsed electromagnetic field (PEMF), topical glyceral trinitrate and ultrasound (Buchbinder 2003; Buchbinder 2011; Cumpston 2009; Engebretsen 2009; Gialanella 2011; Green 2005; Page 2016a; Pedowitz 2012). The benefits of many of these treatments have not been established in high‐quality, randomised, placebo‐controlled trials.
How the intervention might work
As described above, the mechanistic theory contends that impingement symptoms occur primarily due to repetitive compressive and shearing forces on the rotator cuff tendons. Subacromial decompression therefore aims to remove the inflamed subacromial bursa and reduce compressive forces by removing bone from the anterior/lateral undersurface of the acromion. Widening of the space for the traversing tendons in this way is believed to halt the pathological process.
Why it is important to do this review
As we have outlined, rotator cuff disease has substantial economic and quality‐of‐life implications for the patient and healthcare systems, and the numbers of people undergoing subacromial decompression surgery are rapidly rising. Surgery predisposes the patient to risks related to surgery, thus its use has to be supported by evidence of its benefit. Despite a mechanistic theory supporting surgery, improvements can also occur in the absence of surgery.
Our 2008 Cochrane Review identified 14 randomised controlled trials (RCTs) involving 829 participants (Coghlan 2008). Eleven trials included participants with impingement, two trials included participants with rotator cuff tear and one trial included participants with calcific tendinitis. The trials examined heterogeneous interventions and were all susceptible to bias, limiting our ability to draw firm conclusions about the benefits and harms of surgery for rotator cuff disease. For the treatment of impingement, there was moderate‐certainty evidence, based upon three trials, of no significant differences in outcome between open or arthroscopic subacromial decompression versus active non‐operative treatment (exercise programme, physiotherapy regimen of exercise and education, or graded physiotherapy strengthening program) for the treatment of impingement. There was moderate‐certainty evidence from six trials that there were no clinically important differences in outcome between arthroscopic and open subacromial decompression although four trials reported earlier recovery with arthroscopic decompression.
Since the last published version of this review, two additional RCTs assessing the benefits and harms of surgery for rotator cuff disease have been published. Both trials investigated decompression for people with rotator cuff disease excluding full‐thickness tears, and both included a placebo surgery control group (Beard 2018; Paavola 2018). Therefore an updated review of the available evidence is timely.
Objectives
To synthesise the available evidence of the benefits and harms of subacromial decompression surgery compared with placebo, no intervention or non‐surgical interventions in people with rotator cuff disease (excluding full thickness rotator cuff tears).
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs of any design (e.g. parallel, cross‐over, factorial), controlled clinical trials using a quasi‐randomised method of allocation (methods of allocating participants to a treatment that are not strictly random, e.g. date of birth, hospital record number or alternation). Reports of trials were eligible regardless of the language, date of publication, or publication status.
For harms, we also included prospective observational studies from surgery regsitries designed to record harms from subacromial decompression or shoulder arthroscopy for mixed diagnoses including impingement symptoms.
Types of participants
We included trials that enrolled adults (aged 18 years and over) with rotator cuff disease, confirmed by clinical history, physical examination, magnetic resonance imaging (MRI), ultrasound or arthrogram. We excluded trials that included participants with full‐thickness tears, unless it was a minority of participants (< 20%). We excluded studies of adults undergoing surgery for benign or malignant tumours, adhesive capsulitis, shoulder instability, joint replacement or fractures. For the harms, there were no restrictions regarding the diagnoses of participants.
Types of interventions
Subacromial decompression surgery (open or arthroscopic bursectomy and/or acromioplasty) versus placebo, non‐operative treatment, or no treatment were included. For this update, as the benefit of surgery over placebo, or non‐surgical treatment is not yet established, we excluded studies comparing one type of surgical technique to another. We also excluded studies only assessing different surgical devices (such as comparing two types of suture materials or techniques) or biologics.
Comparators could include the following.
Placebo surgery
Non‐operative treatments, including physical therapy, exercises, pharmacologic interventions such as NSAIDs and/or glucocorticoid or other injections
Wait and see/no or delayed treatment
Types of outcome measures
We ensured that the outcomes in our review were consistent with The Outcome Measures in Rheumatology (OMERACT) draft core domain set for clinical trials of shoulder disorders (Buchbinder 2017).
Major outcomes
We included the following outcomes.
Overall pain (mean or mean change measured by visual analogue scale (VAS), numeric or categorical rating scale). If trials did not measure overall pain, we planned to include other pain measures highest on the following hierarchy: unspecified pain, pain with activity, pain at night or at rest.
-
Physical function. Where trial authors reported outcome data for more than one function scale, we extracted data on the scale that was highest on the pre‐defined list below. These questionnaires generally include several domains, such as pain, function, range of motion and strength, and provide a shoulder‐specific composite score. Our hierarchy was based upon the most commonly used scores used in trials assessing surgery, given that there is a paucity of research to inform us which measure is the gold standard (Page 2015).
Constant Murley Score
Shoulder Pain and Disability Index (SPADI)
Oxford Shoulder Score (OSS)
American Shoulder and Elbow Surgeons Standardized Form (ASES‐SF)
UCLA Shoulder Score
Disabilities of the Arm, Shoulder and Hand (DASH)
Shoulder Disability Questionnaire (SDQ)
any other shoulder function scale.
Participant global assessment of treatment success as defined by the trial authors (e.g. proportion of participants with significant overall improvement). See also Differences between protocol and review.
Health‐related quality of life, measured by generic tools (such as components of the Short Form‐36 (SF‐36), SF‐12, EQ‐5D, 15D) or disease‐specific tools.
Number of participants experiencing adverse events, extracted from randomised trials (including neurovascular complications, infections, postoperative shoulder stiffness/adhesive capsulitis (frozen shoulder)
Number of participants experiencing a serious adverse event, extracted from surgical registries. We defined serious harms as death, bleeding (uncontrolled or requiring transfusion), cardiac arrest requiring cardiopulmonary resuscitation, myocardial infarction, cerebrovascular accident, acute renal failure, unplanned intubation, requiring ventilator for more than 48 hours, deep infection (surgical site or organ/space), sepsis, septic shock, pneumonia, wound dehiscence, pulmonary embolism, deep vein thrombosis or peripheral nerve injury.
Minor outcomes
Participation (recreation and work)
Treatment failure (e.g. progression to full‐thickness tear)
Timing of outcome assessment
We extracted outcomes at the following time points.
Up to and including three months
Three months up to six months
Greater than six months up to one year
Greater than one year up to two years
Greater than two years up to five years
Greater than five years
We extracted the latest time point within the time period if there were multiple time points at which outcomes were measured (i.e. if a study reported outcomes at 6 weeks and 4 months and 12 months, we extracted outcomes at 4 months (to 6‐month analysis), and 12 months. The primary time point was one year.
Search methods for identification of studies
Electronic searches
This current review update includes studies published between March 2006 and 22 October 2018. We searched the following databases for randomised or quasi‐randomised trials.
Cochrane Central Register of Controlled Trials (CENTRAL, 2018, Issue 10) via Cochrane Library; Appendix 1
OVID MEDLINE, 2006 to 22 October 2018; Appendix 2
OVID Embase, 2006 to 22 October 2018; Appendix 3
ClinicalTrials.gov for ongoing trials; Appendix 4
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal (apps.who.int/trialsearch/) for ongoing trials to 22 October 2018; Appendix 5
Searching other resources
We used the data from Lähdeoja 2019, who summarised serious adverse events from arthroscopic shoulder surgery registries.
We also reviewed the reference lists of the included trials and any relevant review articles retrieved from the electronic searches, to identify any other potentially relevant trials.
Data collection and analysis
Selection of studies
Three review authors (TK, NBJ and CP) independently selected trials for possible inclusion against a predetermined checklist of inclusion criteria (see Criteria for considering studies for this review). We screened titles and abstracts and initially categorised studies into the following groups.
Possibly relevant: trials that met the inclusion criteria and trials from which it was not possible to determine whether they met the criteria either from their title or abstract
Excluded: those clearly not meeting the inclusion criteria
If a title or abstract suggested that the trial was eligible for inclusion, or we could not tell, we obtained a full‐text version of the article, and two to three review authors (TK, NBJ and CP) independently assessed it to determine whether it met the inclusion criteria. The review authors resolved discrepancies through discussion or adjudication by a fourth author (RB).
For harms, we included the studies identified in the parallel systematic review (Lähdeoja 2019).
Data extraction and management
Two of three review authors (TK NBJ, CP) independently extracted the following data from the included trials.
Trial characteristics, including design (e.g. parallel or cross‐over), country, sample size calculation, primary analysis, source of funding, and trial registration status (with registration number recorded if available)
Number of participants, inclusion/exclusion criteria, participant characteristics, including age, sex, duration of symptoms, outcomes at baseline and details regarding the cuff tear if present
Intervention characteristics for each treatment group, and use of co‐interventions
Outcomes reported, including the measurement instrument used and timing of outcome assessment
When additional data were required, we contacted the trial authors to obtain this. Where data were imputed or calculated (e.g. standard deviations calculated from standard errors, P values, confidence intervals, imputed from graphs, from standard deviations in other trials), we reported this in the Notes section of Characteristics of included studies. We resolved any disagreements and issues by consultation with RB.
To prevent selective inclusion of data based on the results, we used the following a priori defined decision rules to select data from trials.
Where trial authors reported both final values and change from baseline values for the same outcome, we extracted final values.
Where trial authors reported both unadjusted and adjusted values for the same outcome, we extracted unadjusted values.
Where trial authors reported data analysed based on the intention‐to‐treat (ITT) sample and another sample (e.g. per‐protocol, as‐treated), we extracted ITT‐analysed data.
For cross‐over RCTs, we preferentially extracted data from the first period only.
We used a priori hierarchies (see Types of outcome measures) to choose the outcome for each domain if the trial measured one outcome with several instruments.
When trial authors had used different scales, we transformed the scales to match the most commonly used instrument scale before pooling, and reversed the scale if needed to make it comparable to the most commonly used instrument (see Measures of treatment effect).
Serious adverse events from surgical registries: two review authors (TL and CA) extracted the study characteristics and the event rates. We solved any discrepancies by consensus.
Assessment of risk of bias in included studies
Pairs of authors (TK, RJ, CP, NBJ, TL or CA), assessed the risk of bias of each included trial and resolved any disagreements by consensus, or consultation with RB where necessary.
We assessed the following methodological domains, as recommended by Cochrane (Higgins 2017):
sequence generation;
allocation sequence concealment;
blinding of participants and study personnel;
blinding of outcome assessment (assessed separately for self‐reported and objectively assessed outcomes);
incomplete outcome data;
selective outcome reporting;
other potential source of bias: in this bias we judged whether the number of cross‐overs from placebo or from exercise therapy to surgery might bias the analysis.
We rated each item as being at 'low risk', 'unclear risk' or 'high risk' of bias.
For observational studies reporting serious adverse events, for assessing risk of bias we used methods described in Hayden 2013:
study participation;
study attrition;
prognostic factor measurement;
outcome measurement;
study confounding;
statistical analysis and reporting
Measures of treatment effect
We used the Cochrane statistical software, Review Manager 5.3 to perform data analysis (Review Manager 2014). For dichotomous outcomes, we expressed the difference as risk ratios (RRs) with 95% CIs. For continuous data, we expressed results as mean differences (MD) with 95% confidence intervals when the same measurement tool was used across studies or standardised mean difference (SMD) when the same outcome was measured using different instruments.
Where trials used different measures for the same outcome or concept, we used the most common outcome measure as an index outcome measure. We transformed MDs and standard deviations (SDs) of other outcome measures to the scale of the index instrument and pooled the data using MD as the summary estimate, according to the methods of Thorlund 2011. For pain, we assumed VAS and numeric rating scale (NRS) were comparable scales, and transformed 1 to 9 (Haahr 2005) and 1 to 10 (Brox 1993) to a zero to 10 scale. The trials used various functional measures (Constant score, shoulder disability score, subjective shoulder rating scale, and Neer score), but as these were all measured in 0 to 100 scale, no transformation was necessary except for reversal of shoulder disability score used by Ketola 2009.
When large variations in SDs led to problematic weights in the meta‐analysis, we pooled SMDs. In this case, we back‐transformed SMDs to a typical scale (e.g. 0 to 100 for function), by multiplying the SMD by a typical among‐person standard deviation (e.g. the SD of the control group at baseline from the most representative trial; as per Chapter 12 of theCochrane Handbook for Systematic Reviews of Interventions (Schünemann 2017a)). This method was only required for the quality‐of‐life outcome. For this outcome, we used an SD of 0.28 (EQ‐5D index), taken from Beard 2018 in the primary analysis (Analysis 1.4) and SD of 0.07 (15D) taken from Paavola 2018 in the secondary analysis (Analysis 2.4).
1.4. Analysis.
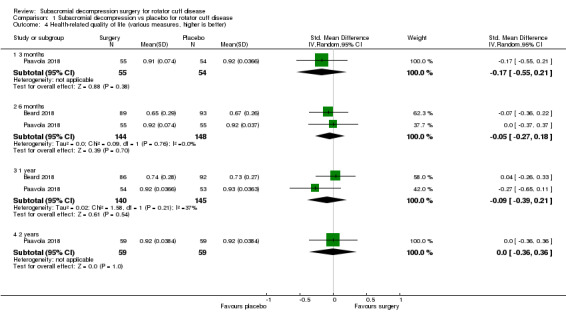
Comparison 1 Subacromial decompression vs placebo for rotator cuff disease, Outcome 4 Health‐related quality of life (various measures, higher is better).
2.4. Analysis.
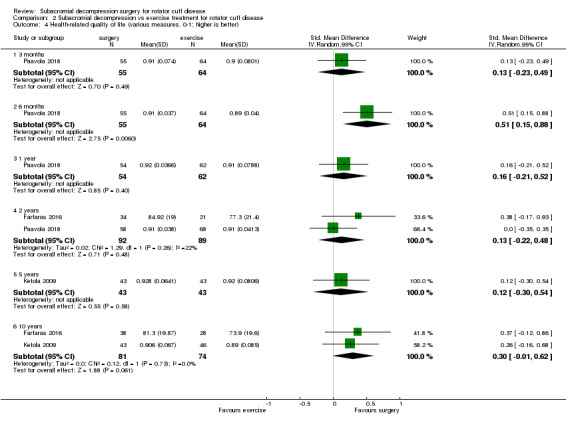
Comparison 2 Subacromial decompression vs exercise treatment for rotator cuff disease, Outcome 4 Health‐related quality of life (various measures, 0‐1; higher is better).
In the Comments column of the 'Summary of findings' tables, we reported the absolute percent difference, the relative percent change from baseline, and for outcomes that show a clinically important difference between treatment groups, we reported the number needed‐to‐treat for an additional beneficial outcome (NNTB), or number needed‐to‐treat for an additional harmful outcome (NNTH). For dichotomous outcomes we planned to calculate the NNTB or NNTH from the control group event rate and the risk ratio using the Visual Rx NNT calculator (Cates 2008). As there were no clinically important differences in the analyses, we did not calculate the NNTB for continuous measures. For dichotomous outcomes, we calculated the absolute difference from the difference in the risks between the intervention and control group, as calculated in GRADEpro GDT (GRADEpro GDT 2015), and expressed as a percentage. We calculated the relative percent change as the RR minus 1 and expressed as a percentage. For continuous outcomes, we calculated the absolute difference as the MD divided by the scale and expressed as percentage. We calculated the relative difference (RD) as the absolute benefit (MD) divided by the baseline mean of the control group, expressed as a percentage.
For harms, we calculated incidence proportions using a generalised linear model, using a binomial distribution and an identity link function.
Unit of analysis issues
The unit of analysis was the participant for all trials. For studies containing more than two intervention groups, making multiple pair‐wise comparisons between all possible pairs of intervention groups possible, we included the same group of participants only once in the meta‐analysis
Dealing with missing data
When required, we contacted trial authors to obtain data that were missing from the trial reports. For continuous outcomes (pain and disability), we calculated the weight of the trial using the number of participants analysed at that time point. If the number of participants analysed was not presented for each time point, we used the number of randomised participants in each group at baseline. For dichotomous outcomes, we used the final data for the events reported in each trial.
For continuous outcomes with no SD reported, we calculated SDs from standard errors (SEs), 95% confidence intervals (CIs) or P values. If we could not obtain any measurement of variance from the trial reports or by contacting the authors, we imputed the SD from the most representative trial. Where we imputed or calculated data (e.g. SDs calculated from SEs, 95% CIs or P‐values, or imputed from graphs or from SDs in other trials), we reported this in the Characteristics of included studies tables.
Assessment of heterogeneity
We assessed clinical diversity by determining whether the characteristics of participants, interventions, outcome measures and timing of outcome measurement were similar across trials. We assessed statistical heterogeneity using the I2 statistic (Higgins 2003). We interpreted the I2 statistic using the following as an approximate guide:
0% to 40% might not be important;
30% to 60% may represent moderate heterogeneity;
50% to 90% may represent substantial heterogeneity;
75% to 100% considerable heterogeneity (Deeks 2017).
Assessment of reporting biases
To assess small study effects, we planned to generate funnel plots for meta‐analyses including at least 10 trials of varying size. If we detected asymmetry in the funnel plots, we planned to review the characteristics of the trials to assess whether the asymmetry was likely due to publication bias or other factors, such as methodological or clinical heterogeneity of the trials (Sterne 2011).
To assess outcome reporting bias, we compared the outcomes specified in trial protocols with the outcomes reported in the corresponding trial publications; if trial protocols were unavailable, we compared the outcomes reported in the methods and results sections of the trial publications (Dwan 2011; Kirkham 2010).
Data synthesis
We defined the following review questions.
For people with rotator cuff disease (without full‐thickness tears):
is subacromial decompression surgery more effective than placebo surgery?
is subacromial decompression surgery more effective than physical therapy or rehabilitation or exercises alone?
is subacromial decompression surgery more effective than no treatment?
Surgery could be followed by postoperative physical therapy or rehabilitation or an exercise program.
For benefit, we considered the first comparison, subacromial decompression versus placebo, to be the least prone to bias and it was therefore the primary comparison for addressing the objectives of this review.
For adverse events, as we expected the results to be similar for both placebo and non‐operative comparisons (exercise or no treatment), to simplify the presentation we presented these data in the same analyses.
We combined results of trials with similar characteristics (participants, interventions, outcome measures and timing of outcome measurement) to provide estimates of benefits and harms. We pooled outcomes using the random‐effects model as a default based on the assumption that clinical and methodological heterogeneity was likely to exist and to have an impact on the results.
GRADE and 'Summary of findings' tables
We presented the six major outcomes (pain, function, global assessment of success, health‐related quality of life, adverse events, serious adverse events) of the review in 'Summary of findings' tables, which summarise the certainty of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the outcomes as recommended by Cochrane. The summary of findings table includes an overall grading of the evidence related to each of the main outcomes, using the GRADE approach (Schünemann 2017b).
We planned two tables, one for surgery versus placebo and one for surgery versus exercise.
Pairs of review authors (TK, TL, CA, RJ and RB), assessed the certainty of the evidence as high, moderate, low, or very low using the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the body of evidence that contributes data to the meta‐analyses for the prespecified outcomes (Schünemann 2017a). We used GRADEpro software to prepare the 'Summary of findings' tables (GRADEpro GDT 2015). We justified decisions to downgrade the certainty of evidence in the footnotes.
A parallel systematic review provided the best estimates of the minimally important differences (MIDs) for each of the measures in the trials where available (Hao 2019). In pain (VAS/NRS), we considered 1.5 points (0 to 10 scale; Tashjian 2009; Hao 2019); in function (Constant score) 8.3 points (Hao 2019); on EQ‐5D‐3L index (UK version; −0.59 to 1) 0.07 (Hao 2019); and on 15D 0.015 points (Alanne 2015).
Subgroup analysis and investigation of heterogeneity
We did not plan subgroup analyses.
Sensitivity analysis
We performed sensitivity analyses to investigate the robustness of the treatment effect by performing an analysis that included all trials combined, that is, trials with placebo and exercise groups, to see if inclusion of trials that did not blind participants changed the overall treatment effect. These were performed for the outcomes of overall pain and function at the six‐month and one‐year time points.
We also planned a sensitivity analysis to assess the impact of including studies with imputed SDs for the outcomes of pain and function.
Results
Description of studies
Results of the search
Only three of the 14 trials included in the previous Cochrane Review (Coghlan 2008), met the inclusion criteria for this updated review due to the restriction in scope from the original review (Brox 1993; Haahr 2005; Rahme 1998). We excluded 11 trials because they compared one type of surgery to another. An additional trial that we had previuosly excluded due to uncertainty about its design, we have now included, following correspondence from the trial authors confirming that it was an RCT (Peters 1997).
The results of the updated search are shown in Figure 1. The updated search returned 3913 records. After duplicates were removed and the titles and abstracts screened for eligibility, we retrieved 29 full texts. Five new RCTs (Beard 2018; Farfaras 2016; Paavola 2018; Peters 1997; Ketola 2009) met the inclusion criteria for this review. In total, we included eight trials in the current review.
1.
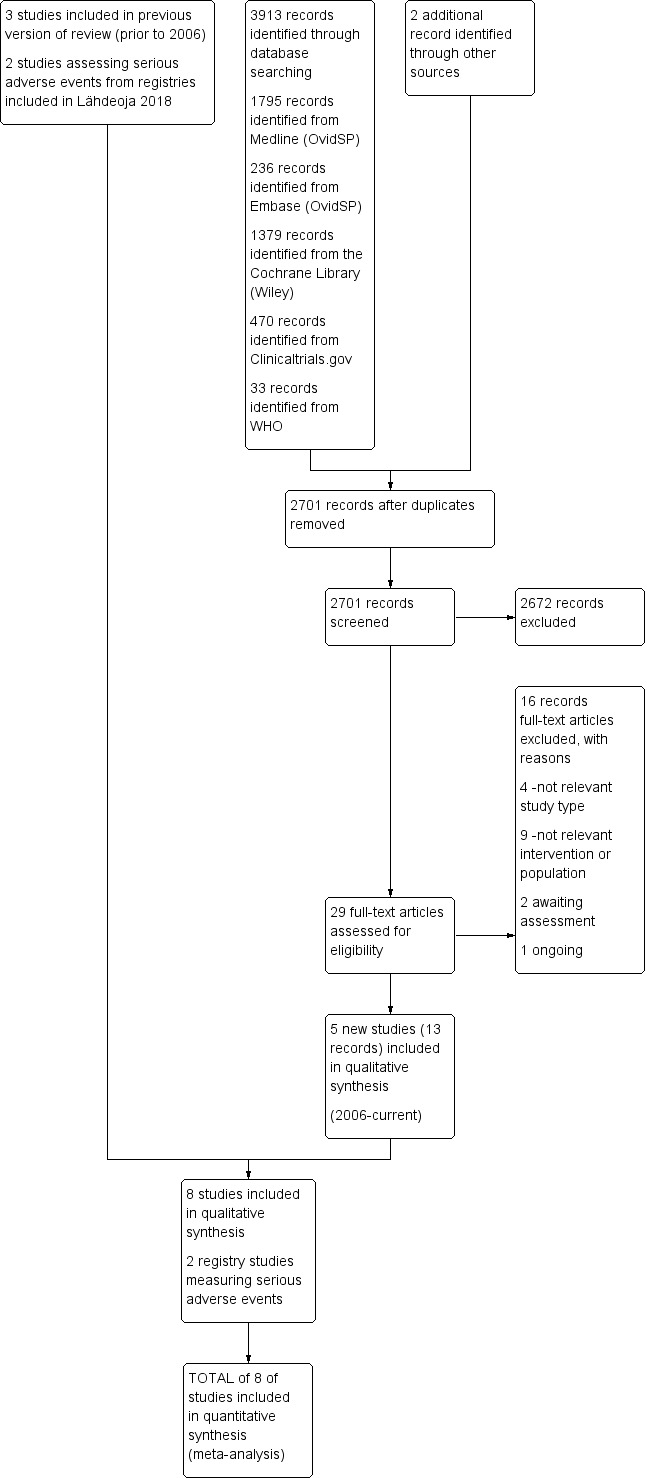
Study flow diagram
For serious adverse events, we included two studies from a single, prospective registry collecting outcomes of arthroscopic shoulder surgery including subacromial decompression (Hill 2017; Shields 2015) as identified in the co‐published review (Lähdeoja 2019).
We identified one ongoing trial meeting the inclusion criteria and its characteristics are presented in Characteristics of ongoing studies table (Paloneva 2008). One trial (TRANSIT 2006), was reported to be completed, but we could not identify published results and the trial authors did not respond to queries. This trial is awaiting classification, along with Schulze 2017, which is awaiting translation from German.
We could find no trial registration for four of the included trials (Brox 1993; Farfaras 2016; Haahr 2005; Ketola 2009), noting that one trial was published before trial registration became mandatory (Brox 1993).
Included studies
We have provided a full description of the eight included trials in the Characteristics of included studies table, and a summary of trial features and participant characteristics in Table 3.
1. Baseline demographic and clinical characteristics of the trial participants.
| Trial | Country | Groups (number randomised) | Mean age, years | Mean symptom duration in months (duration specified in inclusion criteria) | Mean pain | Mean shoulder‐specific score | Mean HRQoL | Treatment delivered by |
| Beard 2018 | UK | Subacromial decompression (106) | 53 | Not reported (≥ 3 months) | Not reported | 39a | 0.52 | 38 different surgeons |
| Placebo surgery (103) | 54 | 43a | 0.55 | |||||
| No treatment (104) | 53 | 38a | 0.50 | Not specified | ||||
| Brox 1993 | Norway | Subacromial decompression (45) | 48 | Not reported (≥ 3 months) | Not reported | 64b | Not measured | 2 surgeons |
| Exercise therapy (50) | 47 | 66 | 1 physiotherapist | |||||
| Placebo‐laser (30) | 48 | 65 | 1 physiotherapist | |||||
| Farfaras 2016 | Sweden | Open subacromial decompression (24) | 52 | Not reported (≥ 6 months) | Not reported | 48a | 69.6 (SF‐36 General Health) | Not specified |
| Arthroscopic subacromial decompression (29) | 49 | 56a | 60.1 | |||||
| Exercise therapy (34) | 50 | 56a | 67.3 | |||||
| Haahr 2005 | Denmark | Subacromial decompression (45) | 45 | Not reported (6 months‐3 years) | 5.9 | 35a | Not measured | 2 surgeons |
| Exercise therapy (45) | 44 | 6.5 | 34a | 2 physiotherapists | ||||
| Ketola 2009 | Finland | Subacromial decompression (70) | 46 | 31 (≥ 3 months) | 6.5 | 78c | Not measured | One surgeon |
| Exercise therapy (70) | 48 | 30 (≥ 3 months) | 6.5 | 83c | Physiotherapist | |||
| Paavola 2018 | Finland | Subacromial decompression (59) | 51 | 18 (≥ 3 months) | 7.1 | 32a | 0.89 (15D) | Not specified |
| Placebo surgery (63) | 51 | 18 (≥ 3 months) | 7.2 | 32a | 0.89 | |||
| Exercise therapy (71) | 50 | 22 (≥ 3 months) | 7.2 | 35a | 0.88 | |||
| Peters 1997 | Germany | Subacromial decompression (32) | 56 | Not reported (not reported) | Not measured | 54d | Not measured | Not specified |
| Exercise therapy (40) | 59 | 59d | ||||||
| Rahme 1998 | Sweden | Subacromial decompression (21) | 42 | Not reported (≥ 12 months) | Not reported | Not measured | Not measured | Not specified |
| Exercise therapy (21) | 42 |
aConstant score. bNeer score. cShoulder Disability Questionnaire. dSubjective Shoulder Rating Scale.
Randomised controlled trials
Trial design, setting and characteristics
Two trials compared arthroscopic subacromial decompression surgery with arthroscopy only (placebo surgery; Beard 2018; Paavola 2018). The surgery was followed by postoperative exercises in all treatment groups. Both trials also included a third treatment group comprising no treatment (active monitoring), in Beard 2018 and an exercise therapy program in Paavola 2018.
Six trials compared arthroscopic or open subacromial decompression followed by exercises with exercises alone (Brox 1993; Farfaras 2016; Haahr 2005; Ketola 2009; Peters 1997; Rahme 1998). One of these trials, Farfaras 2016, included two surgery groups (open or arthroscopic decompression), while one other trial, Brox 1993, also included a third treatment group comprising placebo laser.
The included trials were conducted in six different countries: Denmark (Haahr 2005), Finland (Ketola 2009; Paavola 2018), Germany (Peters 1997), Norway (Brox 1993), Sweden (Farfaras 2016; Rahme 1998), and the UK (Beard 2018).
The total duration of the trials varied between 3 and 14 years and the duration of follow‐up ranged from one year (Beard 2018; Rahme 1998), up to a mean of 12‐13 years in two trials (Ketola 2009; Farfaras 2016).
Three trials reported receiving funding from foundations unrelated to commercial purposes (Beard 2018; Brox 1993; Paavola 2018). Five trials did not report funding sources (Farfaras 2016; Haahr 2005; Ketola 2009; Peters 1997; Rahme 1998). One study reported that one of its authors had received remuneration from an instrument company but the trial itself was not funded by the company (Farfaras 2016).
All studies with an exercise therapy treatment arm allowed cross‐over from exercise therapy to surgery. In the placebo‐controlled trials, the blinded participants could be unblinded if they desired other interventions due to poor outcome or were hospitalised due to a complication (Beard 2018; Paavola 2018). The number of participants not receiving their allocated treatment, having surgery although allocated to exercises, or who were unblinded during follow‐up are presented in Table 4.
2. Deviations from allocated treatment.
| Trial | Group | Did not receive allocated treatment | Crossed over to active surgery | Re‐operated | Side interventions in surgery | Unblinded |
| Beard 2018 | Subacromial decompression | 19 (18%) | N/Aa | 0 | None reported | 0 (0%) |
| Placebo surgery | 35 (34%) | 10 (10%) | 0 | None reported | 1 (1%) | |
| No treatment | 26 (25%) | 25 (24%) | 0 | No surgery | No blinding | |
| Brox 1993 | Subacromial decompression | 13 (29%) | N/Aa | 0 | None reported | No blinding |
| Eexercise therapy | 7 (14%) | 1 (2%) | 0 | No surgery | ||
| Placebo‐laser | 4 (13%) | 2 (7%) | 0 | No surgery | ||
| Farfaras 2016 | Open subacromial decompression | 6 (25%) | N/Aa | 0 | None reported | No blinding |
| Arthroscopic subacromial decompression | 5 (29%) | N/Aa | 0 | None reported | ||
| Exercise therapy | 0 | 3 (9%) | 0 | No surgery | ||
| Haahr 2005 | Subacromial decompression | 4 (9%) | N/Aa | 0 | None reported | No blinding |
| Eercise therapy | 2 (4%) | 6 (13%) by 1 year 11 (24%) by 4‐8 years | 0 | No surgery | ||
| Ketola 2009 | Subacromial decompression | 13 (19%) | N/Aa | 0 | 14 (20%) labrum repair | No blinding |
| Exercise therapy | 0 | 5 (7%) by 1 year 14 (20%) by 2 years 18 (26%) by 5 years |
0 | No surgery | ||
| Paavola 2018 | Subacromial decompression | 0 | N/Aa | 2 (3%) | 0 (0%) | 6 (10%) |
| Placebo surgery | 0 | 8 (13%) | 8 (13%) | 0 (0%) | 9 (14%) | |
| Exercise therapy | 0 | 15 (21%) | 3 (4%) | No surgery | No blinding | |
| Peters 1997 | Subacromial decompression | 0 | N/Aa | 0 | None reported | No blinding |
| Exercise therapy | 0 | 0 (0%) | 0 | None reported | ||
| Rahme 1998 | Subacromial decompression | 0 | N/Aa | 0 | 5 rotator cuff tears were sutured | No blinding |
| Exercise therapy | 0 | 13 (62%) | 0 | No surgery |
aN/A (not applicable), participants in subacromial decompression group could not cross over to surgery.
Trial participants
All participants were recruited from secondary/tertiary care hospitals offering surgical care. Across all included trials there were a total of 1062 participants allocated to either operative or non‐operative treatments. The two placebo‐controlled trials included 506 participants; 331 were randomised to either subacromial decompression or placebo surgery and 175 were randomised to unmasked exercise or no treatment. In the open‐label trials, 376 participants were randomised to subacromial decompression (open or arthroscopic) or exercise therapy and 30 were randomised to unmasked placebo laser in one trial. The number of participants per trial ranged from 42 to 313 and their mean age varied from 42 to 65 years, and almost all had a slight female predominance (other than Peters 1997).
Inclusion criteria for all trials were comparable, requiring clinical features consistent with impingement syndrome, including painful abduction and positive impingement test. Three trials explicitly reported exclusion of full‐thickness rotator cuff tears (Beard 2018; Ketola 2009; Paavola 2018). Brox 1993 excluded "rotator cuff rupture" and Farfaras 2016 "total rotator cuff rupture"; Haahr 2005 excluded participants who had, "signs of a rupture of the cuff", and Peters 1997 excluded participants if they had, "sonographic evidence of complete rupture of the rotator cuff". One trial did not explicitly report exclusion of tears (Rahme 1998).
Two trials used MRI (Ketola 2009; Paavola 2018), two used ultrasound (Farfaras 2016; Haahr 2005), and one used MRI or ultrasound (Beard 2018), to identify rotator cuff tears. Two trials did not specify exclusion on the basis of imaging (Brox 1993; Rahme 1998). In Rahme 1998, 3 of 21(14%) participants in the surgery group were found to have full‐thickness rotator cuff tears during surgery and these were repaired. The corresponding number in the non‐operative treatment group is unknown.
Four trials required symptoms to have been present for at least three months (Beard 2018; Brox 1993; Ketola 2009; Paavola 2018), two trials required symptoms to have been present for six months (Farfaras 2016; Haahr 2005), one trial required symptoms to have been present for 12 months (Rahme 1998), and one trial did not specify a time (Peters 1997). Six trials did not report mean symptom duration, or reported it in categories, and we could not extract the mean duration (Beard 2018; Brox 1993; Farfaras 2016; Haahr 2005; Peters 1997; Rahme 1998). Mean symptom duration was 30 to 31 months across treatment groups in Ketola 2009 and 18 to 22 months across the three treatment groups in Paavola 2018.
Mean baseline pain scores were comparable across the trials, varying between 5.9 and 7.2 (0 to 10 scale). Mean baseline function measured by the Constant‐score (possible range 0 to 100, higher is better) varied between 31 and 58 in the four trials that included this measure (Beard 2018; Farfaras 2016; Haahr 2005; Paavola 2018). Ketola 2009 measured function with the SDQ (possible range 0 to 100, higher is worse), and baseline scores were 78 and 83 (reversed scores 22 and 17) in surgery and exercise groups, respectively. Brox 1993 measured function using the Neer score (possible range 0 to 100, higher is better), and the baseline scores were 64, 66 and 65 in the surgery, exercises and placebo‐laser groups, respectively.
Health‐related quality of life was assessed in three trials, all using different measures. Beard 2018 reported a baseline of 0.50 to 0.55 measured by the EQ‐5D index (possible range −0.59 to 1 scale, higher is better); Paavola 2018 reported a baseline of 0.88 to 0.89, measured by the 15D (possible range 0 to 1 scale, higher is better); and Farfaras 2016 reported all SF‐36 subdomains (possible range 0 to 1, higher score indicates lower disability), at baseline with a baseline score of 74.3 (open subacromial decompression), 65.2 (arthroscopic subacromial decompression), and 73.5 (exercise therapy), in the SF‐36 mental health score.
Paavola 2018 excluded participants if the surgeon deemed that pain was not due to impingement during arthroscopy but before participants were randomised (which occurred intra‐operatively). Beard 2018 randomised participants before the procedure and did not exclude patients if other pathologies were found at surgery. In trials comparing subacromial decompression surgery to exercise therapy, the participants in the exercise group did not undergo arthroscopy to rule out other pathologies (Brox 1993; Farfaras 2016; Haahr 2005; Ketola 2009; Peters 1997; Rahme 1998).
Interventions
Details of the interventions in each trial are presented in the Characteristics of included studies table. One to 38 surgeons performed operations, depending on the trial. The operations were performed arthroscopically in all trials except the ope‐ surgery group in Farfaras 2016 and the surgery group in Rahme 1998.
Arthroscopic subacromial decompression appeared to have been performed similarly across the studies. It included bursectomy, followed by removal of bone from the anterior/lateral undersurface of acromion and release of the acromioclavicular ligament. In Ketola 2009, the acromioclavicular ligament was released only if the operating surgeon deemed it to be tight; in this trial the surgeon also repaired labrum injuries in 14 participants. Other trials did not specifically report other surgical co‐interventions in the operative treatment groups.
Physiotherapists instructed and supervised exercises, which included home exercises. In the exercise groups, the exercises focused on active strengthening and correction of balance and humeroscapular kinematics. One trial reported specific details of the exercises (Paavola 2018).
Most of the studies did not explicitly report whether NSAIDs or glucocorticoid injections were permitted during the trial. However Ketola 2009 indicated that participants could receive up to three injections during the trial and reported a mean of 1 injection (range 1 to 10), in the exercise group and 0.3 injections (range 0 to 3), in the surgery group by two years' follow‐up. The details of the no‐treatment group in Beard 2018 and placebo‐laser group in Brox 1993 are displayed in the Characteristics of included studies table.
Outcomes
Pain
Two trials did not report a pain outcome (Farfaras 2016; Peters 1997). Five trials measured and reported pain in various continuous scales, all with higher scores indicating worse pain (Beard 2018; Brox 1993; Haahr 2005; Ketola 2009; Paavola 2018). Rahme 1998 measured pain using a continuous scale but only reported it categorically. Two trials included dichotomous assessment of pain (Ketola 2009; Paavola 2018).
Beard 2018 measured pain using the PainDETECT questionnaire, which assesses current, strongest and average pain intensity on a NRS from 0 to 10. Brox 1993 assessed pain with activity, as well as at rest and at night, on a 1 to 9 scale. Haahr 2005 measured pain using the Constant pain subscore, and also measured worst and average pain and discomfort in the last three months, and average pain and discomfort in the past seven days (all on 0 to 9 scales), using the Project on Research and Intervention in Monotonous work (PRIM) questionnaire. Ketola 2009 measured pain (unspecified) as well as pain at night on a 0 to 10 scale and also reported the proportion of pain‐free participants (VAS < 3), and pain‐free days during the last three months. Paavola 2018 measured pain at rest and with arm activity on a 0 to 100 scale and also reported the proportion of participants who exceeded the threshold for minimal clinically important improvement and the proportion who had reached the patient‐acceptable symptom state. Rahme 1998 measured pain at rest on a 0 to 10 scale but only reported the results as the proportion of participants reaching more than 50% reduction in pain.
Function
Seven trials included a composite multidimensional shoulder score, which could include pain, disability, range of motion and strength (Beard 2018; Brox 1993; Farfaras 2016; Haahr 2005; Ketola 2009; Paavola 2018; Peters 1997). These included the Constant score (Beard 2018; Farfaras 2016; Haahr 2005; Paavola 2018), Oxford Shoulder Score (Beard 2018), Simple Shoulder Test (Paavola 2018), Shoulder Disablity Questionnaire (Ketola 2009), Watson‐Sonnabend score (Farfaras 2016), and Neer score (Brox 1993). In addition, Ketola 2009 included a self‐reported assessment of function/disability measured on a VAS/NRS 0 to 10 scale, and Haahr 2005 reported 'impaired activity' (0 to 9 scale, higher is worse) as a PRIM sub score. Rahme 1998 assessed function by rating 'pour out of pot' and 'hand in neck' manoeuvres, and Peters 1997 used the subjective shoulder rating scale, a questionnaire developed and validated by the same authors (Kohn 1997).
Participant global assessment of treatment success
Five trials included varying measures of global assessment of treatment success (Beard 2018; Brox 1993; Ketola 2009; Paavola 2018; Rahme 1998).
Beard 2018 assessed global assessment of satisfaction with three questions: 1) How are the problems now compared to before randomisation? (7‐step Likert scale from no problems to much worse); 2) How pleased are you with the results of the treatment? (5‐step Likert scale from very pleased to very disappointed); and 3) Would you choose the same treatment again? (yes/no/not sure). Brox 1993 defined treatment success as those who had more than 80 out of 100 on the Neer score (but only reported this outcome for those who continued follow‐up until 2.5 years, at six months and 2.5 years). Ketola 2009 assessed proportion of participants whose overall state of health was better compared with before treatment on a 5‐point scale ranging from a lot worse to a lot better at a mean of 12 years' follow‐up. Paavola 2018 assessed global satisfaction on a VAS scale and also reported proportion of participants who were 'responders' (satisfied or very satisfied with treatment outcome used. Rahme 1998 defined success as relative reduction of pain by more than 50% compared with baseline.
Health‐related quality of life
Four trials included a measure of health‐related quality of life (Beard 2018; Farfaras 2016; Ketola 2009; Paavola 2018). Beard 2018 used the European Quality of Life with five dimensions index (EQ‐5D index) and EQ‐VAS index, Farfaras 2016 used the SF‐36, and two trials used the 15D (Ketola 2009; Paavola 2018), although Ketola 2009 only reported this outcome at more than 10 years in.
Adverse events and serious adverse events
Only three trials reported adverse events (Beard 2018; Ketola 2009; Paavola 2018). No studies reported serious adverse events.
Minor outcomes
Three trials included a measure of participation (recreation and work; Brox 1993; Ketola 2009; Paavola 2018). Paavola 2018 reported the number of participants at work and able to perform sports or leisure activities without difficulties. Brox 1993 reported the number of participants absent from work due to shoulder problems. Ketola 2009 measured self‐reported working ability on a VAS scale and sick leave due to shoulder reasons in three categories (1 to 7 days per year; 8 to 14 days per year; > 14 days per year), and whether or not the participant was retired due to shoulder condition (at a mean of 12 years' follow‐up only).
None of the trial authors included a definition of treatment failure. Ketola 2009 used MRI to identify cuff tears at five‐year follow‐up and Farfaras 2016 used ultrasound at 13 years and we considered full‐thickness tears as failures. Cross‐overs could occur in one direction (from exercise to surgery) and we did not consider these as treatment failures. The deviations from allocated treatment by trial are presented in Table 4.
Observational studies
The two included observational studies included samples from a single surgical registry in the USA over two separate time periods, using a systematic sampling process to minimise risk of selection bias, and investigating 30‐day postoperative complication rates (Hill 2017; Shields 2015). Hill 2017 included 15,015 participants undergoing arthroscopic shoulder surgery from 258 participating centres for the years 2005 to 2011, and Shields 2015 included 10,255 participants from more than 600 centres undergoing surgery from 2011 to 2013 (Table 5).
3. Types and numbers of surgical procedures included in the two registry studies.
| Procedure | N (%) Hill 2017 | N (%) Shields 2015 |
| Rotator cuff repair | 6399 (43) | 3439 (33.5) |
| Subacromial decompression | 2542 (16.9) | 3362 (32.8) |
| Superior labrum lesion repair | 1175 (7.8) | 976 (9.5) |
| Capsuloraphy | 1000 (6.7) | 726 (7) |
| Distal clavicle resection | 1029 (6.9) | 544 (5.3) |
| Extensive debridement | 1130 (7.5) | 461 (4.5) |
| Limited debridement | 1029 (6.9) | 379 (3.7) |
| Lysis and resection of adhesion | 279 (1.9) | 149 (1.5) |
| Biceps tenodesis | 263 (1.8) | 105 (1) |
| Synovectomy | 137 (0.9) | 76 (0.7) |
| Foreign body removal | 62 (0.4) | 38 (0.4) |
| All | 15,015 | 10,255 |
Excluded studies
We excluded 14 trials from this update and specify reasons for exclusion in the Characteristics of excluded studies table. Four trials recruited mainly participants with full‐thickness rotator cuff tears or calcific tendinopathy and these trials will be included in other updates in this series of Cochrane Reviews of interventions for shoulder disorders (Kukkonen 2014; Lambers Heerspink 2015; Maugars 2009; Moosmayer 2010). In comparison to our previous version of this review (Coghlan 2008), we also excluded trials comparing one type of surgery to another from this update.
Risk of bias in included studies
The summary of the risk of bias assessment is presented in Figure 2.
2.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study
Two trials comparing subacromial decompression with placebo met all methodological low risk of bias criteria for this comparison (Beard 2018; Paavola 2018). The other trials had various sources of bias, most notably detection and performance bias arising from lack of blinding of participants and personnel (Brox 1993; Farfaras 2016; Haahr 2005; Ketola 2009; Peters 1997; Rahme 1998). These same biases applied to the comparisons of surgery to no treatment (Beard 2018), or to exercises (Paavola 2018), of the placebo‐controlled trials. The assessment of each domain of risk of bias for the included trials is summarised in the Characteristics of included studies table.
Allocation
Four trials reported adequate random sequence generation and allocation concealment, and we therefore deemed them to have low risk of selection bias (Beard 2018; Haahr 2005; Ketola 2009; Paavola 2018), while we deemed a fifth trial at unclear risk due to failure to explicitly report allocation concealment, although there was likely adequate random sequence generation (Brox 1993).
We judged two trials to be at unclear risk of selection bias due to failure to adequately report their methods of randomisation and allocation concealment (Peters 1997; Rahme 1998), while we judged one at high risk for both of these domains (Farfaras 2016).
Blinding
Both placebo‐controlled trials were at low risk of performance and detection bias for the comparison of decompression and placebo surgery as they blinded participants and all study personnel other than those in the operating room (Beard 2018; Paavola 2018). For the comparison of surgery to no treatment in Beard 2018 and exercises alone in Paavola 2018, we judged there to be high risk of performance and detection bias, as the participants in the non‐operative treatment groups were not blinded. This may have resulted in an overestimate of the benefit of surgery for these comparisons.
Similarly we judged all trials that compared surgery to non‐operative treatment to be at high risk of performance and detection bias as participants were aware of their treatment allocation (Brox 1993; Farfaras 2016; Haahr 2005; Ketola 2009; Peters 1997; Rahme 1998). We assigned a high risk of bias even if trialists had blinded outcome assessors because all major outcomes were subjective. The trials did not use outcomes that were completely objective and for the imaging outcomes, the radiologists could not be reliably blinded to treatment allocation.
Incomplete outcome data
Risk of attrition bias was low in four trials (Beard 2018; Brox 1993; Haahr 2005; Paavola 2018), high in two trials (Farfaras 2016; Ketola 2009), and unclear in two trials (Peters 1997; Rahme 1998).
In Beard 2018, the number of participants and reasons for loss to follow‐up were similar across the groups (six months: 16/106 (15%) and 9/103 (9%) in the decompression and placebo surgery groups respectively; one year: 18/106 (17%) and 10/103 (10%) in the decompression and placebo surgery groups respectively). In Brox 1993, 4 out of 45 (9%) and 1 out of 50 (2%) participants were lost to follow‐up at six months in the surgery and exercise groups respectively, and the corresponding loss to follow‐up was 6 out of 45 (13%) and 5 out of 50 (10%) participants at 2.5 years. In Haahr 2005 4 out of 45 (9%) and 2 out of 45(4%) participants dropped out or were lost to follow‐up at 12 months in the surgery and exercise groups respectively. In Paavola 2018, missing data were also low and comparable between the groups: for pain and function zero to four participants in the decompression group (0% to 7%); two to seven participants (3 to 11%) in the placebo‐surgery group, and three to seven participants (4% to 10%) in the exercise therapy group in follow‐up points up to 24 months.
Risk of attrition bias was high in Farfaras 2016 as data from 9 out of 24 participants in the open decompression group, 10 out of 29 participants in the arthroscopic decompression group and 13 out of 34 participants in the exercise group were not included in the analysis (only a per‐protocol analysis was performed). Risk of attrition bias was high in Ketola 2009 as there were large and differing proportions of missing data at 3, 6 and 12 months' follow‐up (three months: 27/70 (39%) in the decompression group versus 13/70 (19%) in the exercise group; six months: 26/70 (37%) in the decompression group versus 14/70 (20%) in the exercise group; 12 months: 19/70 (27%) in the decompression group versus 18/70 (26%) in the exercise group). Risk of attrition bias was unclear in Peters 1997 as there was an imbalance in loss to follow‐up at one year (decompression: 6/32 (19%) versus 4/40 (10%) in the exercise group), and the reasons were not provided. It was also unclear in Rahme 1998 as 3 out of 21 (14%) participants in the exercise group were lost to follow‐up and no reasons were provided.
Selective reporting
Risk of reporting bias was low in two trials (Beard 2018; Paavola 2018), high in three trials (Haahr 2005; Peters 1997; Rahme 1998), and unclear in three trials (Brox 1993; Farfaras 2016; Ketola 2009).
We judged Haahr 2005 to be at high risk of reporting bias as there was no trial protocol or trial registration and some outcomes appeared to have been added post hoc and others were incompletely reported. No protocol or trial registration was available for Peters 1997 to confirm whether they had collected any measures other than the subjective shoulder rating score. Rahme 1998 did not report the outcomes specified in the methods consistently and at all time points. For example, they only reported six‐month and 12‐month results in pain but they also specified them as collected at eight and 16 weeks.
We assigned unclear risk for reporting bias for Brox 1993 mainly as participation in work was only reported at two to five years for a subset of participants. It also had no trial protocol or trial registration but this predated mandatory trial registration. We also assigned unclear risk to Farfaras 2016 as we could not find a published protocol and they did not report some outcomes pertinent to this review (pain and adverse events). We also judged Ketola 2009 to be at unclear risk of selective reporting, mainly because some outcomes reported to be measured were not reported (passive movement and strength), and they reported adverse events only for the surgery group.
Other potential sources of bias
Four trials had no other identified potential sources of bias (Beard 2018; Haahr 2005; Paavola 2018; Peters 1997).
We deemed three trials at high risk of other bias. Brox 1993 performed an unplanned interim analysis at six months after recruiting 68 out of 125 participants, and as this showed no benefit of placebo laser, they terminated planned recruitment to this group. Farfaras 2016 stopped recruitment early and there was an unexplained imbalance in the number of participants across the three treatment arms. In Rahme 1998, 12 out of 21 (57%) participants originally allocated to the exercises group crossed over to surgery after six months. The trial authors analysed them as a separate group. In our analysis, we included them in the exercise group as allocated, to conform to intention‐to‐treat principles (Analysis 2.3).
2.3. Analysis.
Comparison 2 Subacromial decompression vs exercise treatment for rotator cuff disease, Outcome 3 Global assessment of treatment success.
We judged Ketola 2009 to be at unclear risk of other bias as nine (13%) participants in the surgery group had an unplanned labral repair during the operation, which may have biased the estimate of the effect of surgery (in either direction). Both treatment groups also received glucocorticoid injections over the two‐year follow‐up (mean of 0.3, range 0 to 3; and 1.0, range 0 to 10 in the surgery and exercise groups, respectively). This may also have biased the estimates.
Risk of bias in the observational studies
The 'Risk of bias' assessment from the co‐published, parallel systematic review (Lähdeoja 2019), is reproduced in Table 6. In general these studies were at low risk of most biases.
4. Risk of bias for registry studies of serious adverse events.
| Domain | Hill 2017 | Shields 2015 | Judgement |
| Study participation | Unsure, but judged unlikely to incur significant bias | Yes, large number of centres, judged likely to be representative | Unclear |
| Study attrition | Probably low risk given the tracking of participants who went elsewhere for care, and given follow‐up was 30 days | Probably low risk given the tracking of participants who went elsewhere for care, and given follow‐up was 30 days | Low |
| Prognostic factor measurement | Yes: arthroscopic procedure is the prognostic factor | Yes: arthroscopic procedure is the prognostic factor | Low |
| Outcome measurement | Yes: based on hospital record + participant contact call | Yes: based on hospital record + participant contact call | Low |
| Study confounding | Yes: total harms are of interest, no proper confounders | Yes: total harms are of interest, no proper confounders | Low |
| Statistical analysis and reporting | Unclear, judged not likely to lead to overestimation of harms | Unclear, judged not likely to lead to overestimation of harms | Low |
Effects of interventions
Benefits
1. Subacromial decompression versus placebo
We considered that both placebo‐controlled trials (Beard 2018; Paavola 2018) recruited clinically comparable participants with respect to inclusion criteria and baseline characteristics of pain, disability and quality of life, to allow pooling of data. We were able to pool data for pain, function (both trials used the Constant score), participant global assessment of treatment success, health‐related quality of life and adverse events. Statistical heterogeneity was unimportant across all outcomes and end points. The certainty of evidence was high for pain, function and health‐related quality of life and moderate for participant global assessment of treatment success (downgraded for imprecision; Table 1).
Pain
Based upon the two trials, there were no clinically important differences between subacromial decompression and placebo surgery with respect to mean pain at six months or at one year (high‐certainty evidence). Pain was 4 points with placebo on a zero to 10‐point scale (lower score indicating less pain), and 0.07 points worse (95% CI 0.51 better to 0.64 worse, 299 participants), with subacromial decompression at six months, or 0.7% worse (5% better to 6% worse; Analysis 1.1; Table 1). At one year, mean pain was 2.9 points with placebo and 0.26 points better (95% CI 0.84 better to 0.33 worse, 284 participants), with subacromial decompression, an absolute improvement of 3% (3% worse to 8% better).
1.1. Analysis.
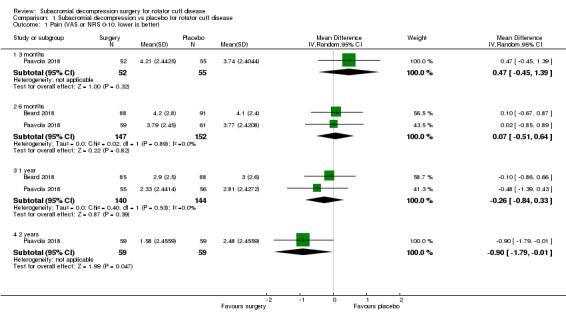
Comparison 1 Subacromial decompression vs placebo for rotator cuff disease, Outcome 1 Pain (VAS or NRS 0‐10, lower is better).
Based upon one trial (Paavola 2018), at three months and two years, there were also no clinically important between‐group differences in pain with subacromial decompression versus placebo surgery but we downgraded the evidence to moderate certainty due to imprecision (one trial, 95% CI overlaps MID at two years). At three months, pain was 3.7 points with placebo and 0.47 points worse (95% CI 0.45 better to 1.39 worse, 1 trial, 117 participants), with subacromial decompression. At two years, pain was 2.5 points with placebo and 0.90 points better ( 95% CI 1.79 better to ‐0.01 worse, 118 participants), with surgery.
Function
There was no evidence of clinically important between‐group differences with respect to mean function at any time point (2 trials at 6 months and 1 year and 1 trial at 2 years). Mean function on a zero to 100 scale (higher indicates better function), was 61 points with placebo and 3.7 points worse (95% CI 8.7 worse to 1.3 better, 286 participants) with subacromial decompression at six months; 69 points with placebo and 2.8 points better (95% CI 1.4 worse to 6.9 better, 274 participants) with subacromial decompression at 1‐2 years; and 74 points with placebo and 4.2 points better (95% CI 1.61 worse to 10.01 better), with subacromial decompression at two years (Analysis 1.2). At two years, the 95% CIs do not exclude a clinically important difference.
1.2. Analysis.
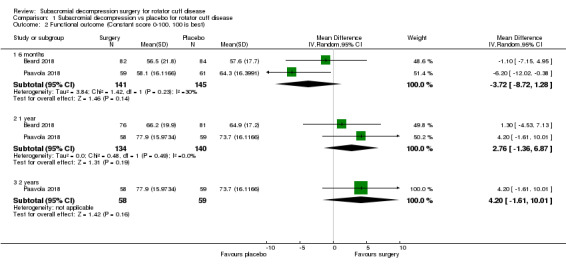
Comparison 1 Subacromial decompression vs placebo for rotator cuff disease, Outcome 2 Functional outcome (Constant score 0‐100, 100 is best).
Participant global assessment of treatment success
Based upon moderate‐certainty evidence (downgraded for imprecision), from two trials, we found no evidence of clinically important between‐group differences in the proportion of participants who rated treatment as successful at six months (surgery: 82/143 (57%) versus placebo: 72/150 (48%), RR 1.17, 95% CI 0.89 to 1.54) or at one year (surgery: 101/142 (71%) versus placebo: 97/148 (66%); RR 1.08, 95% CI 0.93 to 1.27; Analysis 1.3). Based on a single study (Paavola 2018), the success rates were also comparable at two years (surgery: 46/58 (79%) versus placebo 47/58 (81%), RR 0.98, 95% CI 0.82 to 1.17).
1.3. Analysis.
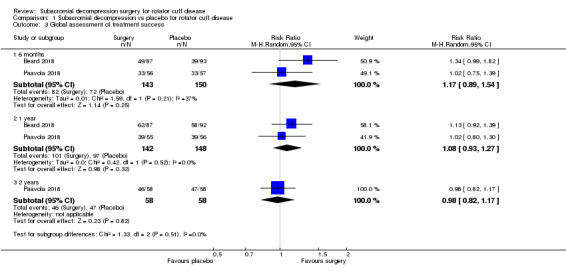
Comparison 1 Subacromial decompression vs placebo for rotator cuff disease, Outcome 3 Global assessment of treatment success.
Health‐related quality of life
We pooled EQ‐5D data from Beard 2018 with 15D data from Paavola 2018 at six months and one year.
Based upon two trials, subacromial decompression did not improve quality of life more than placebo at six months (SMD ‐0.05 (95% CI ‐0.27 to 0.18, 292 participants). When this is back‐transformed, the EQ‐5D index was 0.67 with placebo and 0.01 points worse (0.08 worse to 0.05 better, 292 participants), with subacromial decompression. At one year the SMD was ‐0.09 (95% CI ‐0.39 to 0.21, 285 participants), and back‐transformed to the EQ‐5D index, EQ‐5D was 0.73 with placebo and 0.03 points worse (95% CI 0.11 worse to 0.06 better; 285 participants), with subacromial decompression (Analysis 1.4).
Based upon one trial there was also no between‐group clinically important difference (downgraded to moderate certainty due to imprecision), in health‐related quality of life at three months. 15D was 0.92 with placebo and 0.01 worse (95% CI 0.03 worse to 0.01 better; 118 participants), with subacromial decompression. At two years, 15D was 0.92 with placebo and 0 points (95% CI 0.01 worse to 0.01 better, 118 participants) better with subacromial decompression (high‐certainty evidence).
Minor outcomes
Based upon one trial (Paavola 2018), there were no important differences in participation in work or return to sport or leisure activities up to two years (Analysis 1.5; Analysis 1.6).
1.5. Analysis.
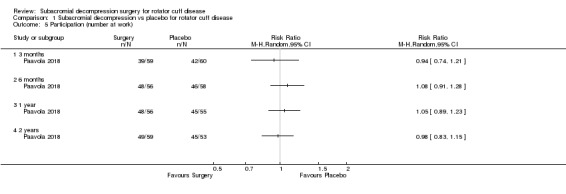
Comparison 1 Subacromial decompression vs placebo for rotator cuff disease, Outcome 5 Participation (number at work).
1.6. Analysis.
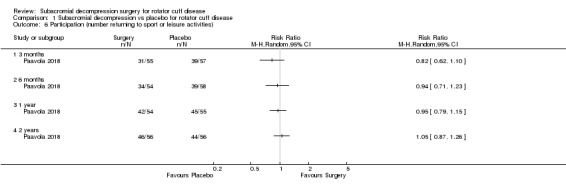
Comparison 1 Subacromial decompression vs placebo for rotator cuff disease, Outcome 6 Participation (number returning to sport or leisure activities).
2. Subacromial decompression versus exercise therapy
Of the seven trials that compared subacromial decompression followed by exercises to exercises alone (Brox 1993, Farfaras 2016, Haahr 2005, Ketola 2009; Paavola 2018; Peters 1997; Rahme 1998), we were able to pool outcome data from one or more of the seven trials across the outcomes of interest. The trials recruited clinically similar participants except that three out of 21 (14%) participants in the decompression group in Rahme 1998 were identified as having full‐thickness rotator cuff tears during surgery and the number in the exercise group is unknown.
Pain
Four trials reported pain at three and six months (Brox 1993; Haahr 2005; Ketola 2009; Paavola 2018); three trials at one year (Haahr 2005; Ketola 2009; Paavola 2018), and at two years (Brox 1993; Ketola 2009; Paavola 2018); two trials at five years (Haahr 2005; Ketola 2009), and one trial at ten years (Ketola 2009). Statistical heterogeneity was unimportant at all time points except at two (I2 = 63%), and five years (I2 = 73%).
Moderate‐certainty evidence (downgraded due to the risk of detection and performance bias), indicates that pain did not differ between surgery and exercises at three months, six months or two years. At three months, pain was 4.4 with exercise and 0.55 better (95% CI 1.24 better to 0.14 worse, 361 participants); at six months, 3.9 with exercise and 0.56 points better (95% CI 0.02 better to 1.09 better, 399 participants) with surgery; at two years, 2.8 points after exercise and 0.44 points better (95% CI 0.49 worse to 1.37 better, 352 participants; Analysis 2.1).
2.1. Analysis.
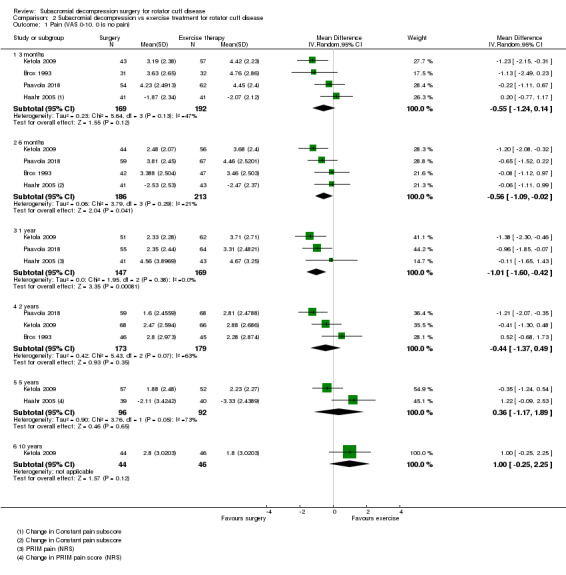
Comparison 2 Subacromial decompression vs exercise treatment for rotator cuff disease, Outcome 1 Pain (VAS 0‐10, 0 is no pain).
At one year, the evidence was low certainty due to bias and imprecision. The 95% CIs included a clinically important change in favour of surgery: pain was 3.7 with exercise and 1.01 points better (95% CI 0.42 better to 1.60 better, 316 participants) with surgery.
At five and ten years, we downgraded the evidence once for bias to moderate certainty; the 95% CIs exclude a clinically important benefit with surgery. At five years, pain was 2.7 with exercise and 0.36 points worse (95% CI 1.17 better to 1.89 worse, 188 participants) with surgery; at ten years, pain was 1.8 with exercises and 1.0 point worse (95% CI 0.25 better to 2.25 worse, 90 participants) with surgery.
Function
Three trials reported function at three months (Brox 1993; Haahr 2005; Ketola 2009); four trials at six months (Brox 1993; Haahr 2005; Ketola 2009; Paavola 2018); three trials at one year ( Haahr 2005; Ketola 2009; Peters 1997); five trials at two years (Brox 1993; Farfaras 2016; Ketola 2009; Paavola 2018; Peters 1997), three trials at five years (Haahr 2005; Ketola 2009; Peters 1997), and two trials at 10 years (Farfaras 2016; Ketola 2009).
Statistical heterogeneity ranged from I2 = 0% to I2 = 81% across different time points, but as there were only few studies this has to be interpreted with care. At six months and one year, the heterogeneity seemed to be largely driven by Ketola 2009; the outcome appears to favour surgery but the confidence intervals overlap with the other studies.
Surgery did not appear to improve function more than exercise at any time point up to five years, but the evidence was low certainty due to bias and imprecision. The 95% CIs include both no change and a clinically important change in favour of surgery. At three months, the mean function was 55 points (on a 0 to 100 scale, higher indicates better function), with exercise and 6.1 points better (95% CI 5.57 worse to 17.79 better, 257 participants), with surgery; at six months mean function was 57 points with exercise and 3.7 points better (95% CI 2.25 worse to 9.58 better, 398 participants), with surgery; at one year, mean function was 58 with exercise and 3.2 points better (95% CI 8.08 worse to 14.55 better, 259 participants), with surgery; at two years mean function was 71 with exercise and 4.9 points better, (95% CI 0.77 better to 9.11 better, 467 participants), with surgery; and at five years, function was 76 with exercise and 7.6 points better (95% CI 0.17 better to 15.09 better, 157 participants) with surgery. At 10 years, there was low‐certainty evidence (downgraded due to bias and imprecision), that surgery was better than exercise with respect to mean function; it was 69 with exercise and 9.54 points better (95% CI 1.93 better to 17.15 better, 156 participants) with surgery (Analysis 2.2).
2.2. Analysis.
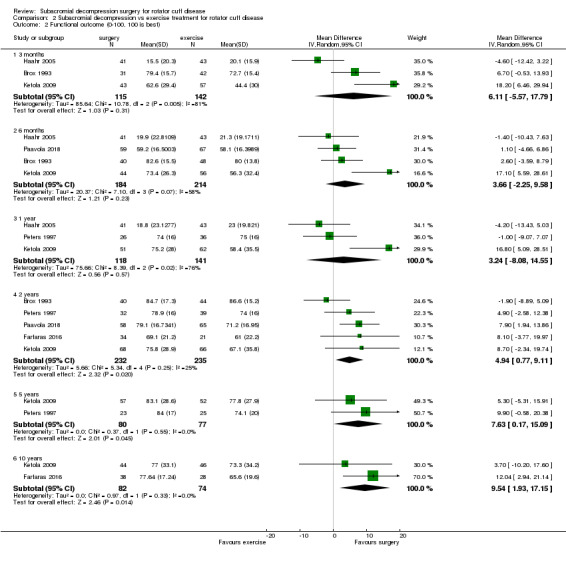
Comparison 2 Subacromial decompression vs exercise treatment for rotator cuff disease, Outcome 2 Functional outcome (0‐100, 100 is best).
Using SMD and back‐transformation to Constant score (0 to 100) in analysis yielded generally narrower CIs (data not shown in tables or forest plots). At three months: MD 4.2 points (95% CI −4.2 to 12.6); at six months: MD 3 points (95% CI −1.3 to 7.2); at one year: MD 1.6 points (95% CI −5.8 to 9.0); at two years: MD 4.2 points (95% CI 1.1 to 7.4); at five years: MD 4.6 points (95% CI −0.48 to 9.6); at 10 years: MD 5.8 points (95% CI −2.7 to 14.2).
Participant global assessment of treatment success
Paavola 2018 and Rahme 1998 reported global assessment of success at six months and one year, Paavola 2018 reported this outcome at two years, Haahr 2005 at five years and Ketola 2009 at 10 years. The statistical heterogeneity was unimportant (I2 = 29% at six months and I2 = 0% at one year).
In the secondary comparison we downgraded this outcome to low certainty due to bias and low event rates (imprecision). The success rates were: 40 out of 77 (52%) with surgery versus 33 out of 84 (39%) with exercises (RR 1.47, 95% CI 0.74 to 2.91), at six months; 55 out of 76 (72%) with surgery versus 49 out of 82 (60%) with exercises (RR 1.21, 95% CI 0.96 to 1.51), at one year; 46 out of 58 (79%) with surgery and 43 out of 68 (63%) with exercises (RR 1.25, 95% CI 1.00 to 1.57), at two years; 23 out of 39 (59%) for surgery and 27 out of 40 (68%) for exercises (RR 0.87, 95% CI 0.62 to 1.23), at five years; 23 out of 44 (52%) with surgery and 24 out of 46 (52%) with exercises (RR 1.00, 95% CI 0.67 to 1.49), at 10 years (Analysis 2.3).
Health‐related quality of life
Paavola 2018 measured quality of life with the 15D (0 to 1) at three months, six months, one year and two years. Farfaras 2016 measured it with the SF‐36 (0 to 100) at two years and at 10 years; and Ketola 2009 measured it with 15D at 10 years. The statistical heterogeneity was unimportant at two years (I2 = 22%) and there was no heterogeneity at 10 years.
Surgery appeared to improve health‐related quality of life more than exercise at some time points (six months and 10 years), but the evidence was low certainty due to bias and imprecision. At six months, mean quality of life was 0.89 with exercise and 0.02 points better (95% CI 0.01 better to 0.03 better, 119 participants), with surgery; at one year, mean quality of life was 0.91 with exercise and 0.01 points better (95% CI 0.01 better to 0.03 better, 116 participants) with surgery; at five years, mean quality of life was 0.92 with exercises and 0.01 points better (95% CI 0.02 worse to 0.04 better, 86 participants). At 10 years, SMD was 0.30 (95% CI −0.01 to 0.62, 155 participants). Back‐transformed to 15D, the mean quality of life was 0.89 with exercise and 0.02 points better (95% CI 0 better to 0.04 better) with surgery (Farfaras 2016; Ketola 2009; Analysis 2.4).
In other time points, we found no clinically important between‐group differences (low‐certainty evidence, downgraded due to bias and imprecision). At three months, mean quality of life was 0.9 with exercises and 0.01 points better (95% CI 0.02 worse to 0.04 better; 119 participants) with surgery; at two years, SMD was 0.13, (95% CI ‐0.22 to 0.48, 118 participants; Farfaras 2016; Paavola 2018). When back‐transformed to15D, the mean quality of life was 0.91 with exercises and 0.01 points better (0.01 worse to 0.03 better), with surgery. At five years, mean quality of life was 0.92 with exercises and 0.01 points better (95% CI 0.02 worse to 0.04 better, 86 participants). The 95% CIs do not exclude a clinically important benefit of surgery at these time points.
Minor outcomes
Brox 1993 and Paavola 2018 reported participation in work at six months' and two years' follow‐up. Paavola 2018 also reported this outcome at three months and one year, and Haahr 2005 at four to eight years' follow‐up. Ketola 2009 reported number of participants who retired for shoulder‐related reasons at five and 10 years. The statistical heterogeneity was substantial (I2 = 60%) at six months and unimportant (I2=0%) at two years. We downgraded the evidence to moderate certainty at six months and one year ( due to serious imprecision). At other time points, we downgraded the evidence to low (twice for very serious imprecision)
We found no important between‐group differences in participation in work. At three months, 39 out of 59 participants in the surgery group and 47 out of 68 in the exercise group were at work (RR 0.96, 95% CI 0.75 to 1.22); at six months, 67 out of 87 participants in surgery group and 73 out of 100 in exercise group (RR 1.05, 95% CI 0.81 to 1.36), were at work; at one year, 48 out of 56 in surgery group and 55 out of 63 were at work (RR 0.98, 95% CI 0.85 to 1.13); at two years, 67 out of 90 in surgery group versus 80 out of 93 in the exercise group (RR 0.87; 95% CI 0.70 to 1.07; Analysis 2.5). At five years 72 out of 96 (75%) participants in the surgery group and 62 out of 92 (67%) participants in the exercise group were working (RR 1.13, 95% CI 0.97 to 1.32). At 10 years, 43 out of 44 (98%) and 42 out of 46 (91%) participants were at work in the surgery group and exercise group respectively (RR 1.07, 95% CI 0.97 to 1.18).
2.5. Analysis.
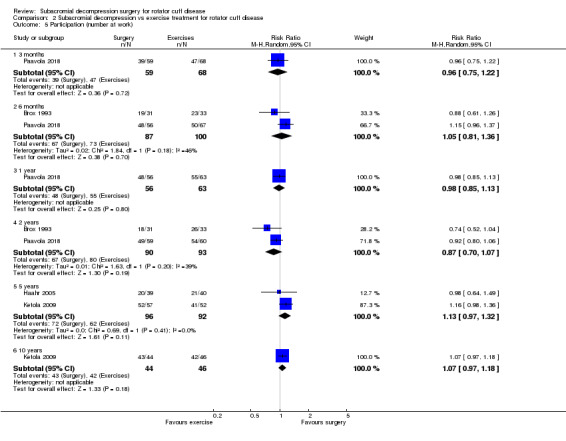
Comparison 2 Subacromial decompression vs exercise treatment for rotator cuff disease, Outcome 5 Participation (number at work).
One trial reported participation in recreational activities (Paavola 2018). There was no important differences between the groups. The participation rate was 31 out of 55 participants for surgery group versus 28 out of 65 in the exercise group (RR 1.31, 95% CI 0.91 to 1.88), at three months; 34 out of 54 for surgery versus 35 out of 62 for exercises (RR 1.12, 95% CI 0.83 to 1.50), at six months; 42 out of 54 for surgery versus 46 out of 64 for exercises (RR 1.08, 95% CI 0.88 to 1.33), at one year; and 46 out of 56 for surgery versus 48 out of 62 (RR 1.06, 95% CI 0.88 to 1.27), at two years (Analysis 2.6).
2.6. Analysis.
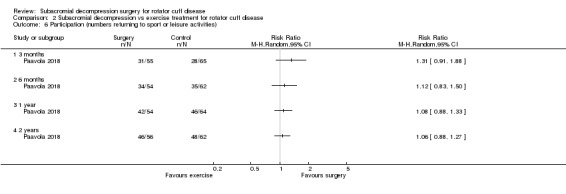
Comparison 2 Subacromial decompression vs exercise treatment for rotator cuff disease, Outcome 6 Participation (numbers returning to sport or leisure activities).
Two trials performed imaging to identify rotator cuff tears during follow‐up, one at five years (Ketola 2009), and the other at a mean of 13 years (Farfaras 2016). Full‐thickness tears in the supraspinatus tendon were identified in 8 out of 48 (17%) participants in the surgery group versus 7 out of 42 (17%) in the exercise group in Ketola 2009 (RR 1.00, 95% CI 0.40 to 2.52), at five years, and 2 out of 38 (5%) participants in the surgery group versus 4 out of 28 (14%) participants in the exercise group in Farfaras 2016 (RR 0.37, 95% CI 0.07 to 1.87), at 13 years (Analysis 2.7).
2.7. Analysis.
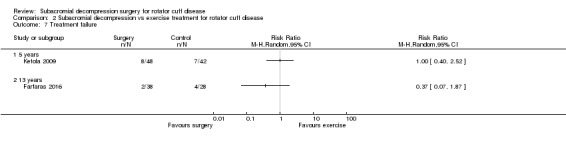
Comparison 2 Subacromial decompression vs exercise treatment for rotator cuff disease, Outcome 7 Treatment failure.
3. Subacromial decompression versus no treatment
One trial included a control group that did not receive any active intervention (active monitoring; Beard 2018). The evidence was downgraded to low certainty due to bias and imprecision (95% CI overlaps minimal important difference) for all outcomes.
Pain
At six months, mean pain was 5 points (on a zero to 10 scale), with no treatment and 0.80 points better (95% CI 0.00 better to 1.60 better; 177 participants), with surgery. At one year,the mean pain was 4.1 points with no treatment and 1.20 points better (0.36 better to 2.04 better; 166 participants), with surgery. The CIs do not exclude a clinically important difference between the groups (Analysis 3.1).
3.1. Analysis.
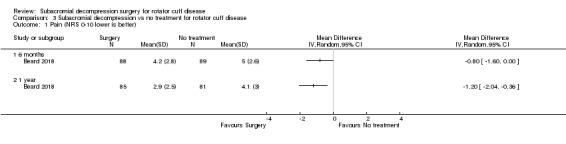
Comparison 3 Subacromial decompression vs no treatment for rotator cuff disease, Outcome 1 Pain (NRS 0‐10 lower is better).
Function
At six months, mean function was 45.4 points (on a zero to 100 scale), with no treatment and 11.10 points better (95% CI 4.52 better to 17.68 better, 165 participants), with surgery. At one year, mean function was 57 points with no treatment and 9.5 points better (95% CI 2.66 better to 16.34 better, 146 participants), with surgery (Analysis 3.2). The CIs do not exclude a clinically important difference between the groups.
3.2. Analysis.
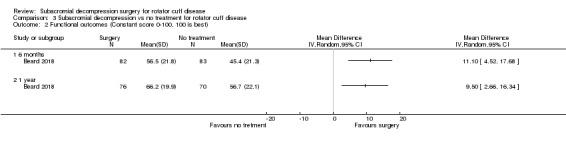
Comparison 3 Subacromial decompression vs no treatment for rotator cuff disease, Outcome 2 Functional outcomes (Constant score 0‐100, 100 is best).
Global assessment of success
More participants had treatment success after surgery compared with no treatment: 49 out of 87 (56%) participants with surgery versus 27 out of 80 (34%) participants with no treatment (RR 1.67, 95% CI 1.17 to 2.39), at six months, and 62 out of 87 (71%) participants with surgery versus 43 out of 80 (54%) participants with no treatment (RR 1.33, 95% CI 1.04 to 1.69). The differences correspond with a NNTB of 4 at six months and 6 at one year (Analysis 3.3).
3.3. Analysis.
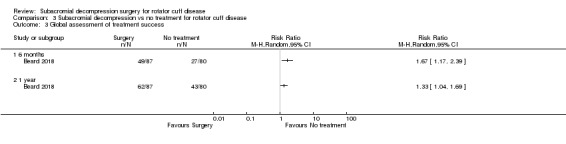
Comparison 3 Subacromial decompression vs no treatment for rotator cuff disease, Outcome 3 Global assessment of treatment success.
Health‐related quality of life
We found no clinically important difference in quality of life between surgery and no treatment but the 95% CIs do not exclude important difference. At six months, quality of life measured with the EQ‐5D index was 0.52 (−0.59 to 1 scale), with no treatment and 0.13 points better (95% CI 0.03 better to 0.23 better), with surgery. At one year, EQ‐5D was 0.66 with no treatment and 0.08 points better (95% CI 0.01 worse to 0.17 better), with surgery (Analysis 3.4).
3.4. Analysis.
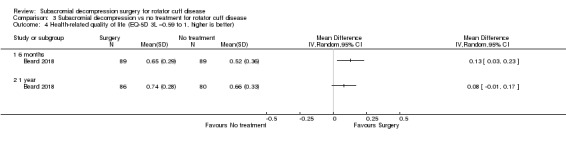
Comparison 3 Subacromial decompression vs no treatment for rotator cuff disease, Outcome 4 Health‐related quality of life (EQ‐5D 3L −0.59 to 1, higher is better).
Minor outcomes
Beard 2018 did not report any participation or treatment failure outcomes.
Harms
Subacromial decompression versus non‐operative control
Adverse events
Adverse events were reported in two randomised controlled trials (Beard 2018; Paavola 2018). Both trials included a placebo control group and also included a third treatment group comprising no treatment (active monitoring) in Beard 2018 and an exercise therapy program in Paavola 2018. A third trial (Ketola 2009) reported an absence of adverse events in the surgery group but did not explicitly report whether or not there were adverse events in the exercise therapy group. Evidence from the randomised trials was downgraded to moderate‐certainty due to imprecision from low event rates (Table 1; Table 2).
Beard 2018 reported that two out of 106 participants in the decompression group, two out of 103 in the placebo group and two out of 104 in the no treatment group had frozen shoulder. Paavola 2018 reported frozen shoulder in three out of 59 participants in the subacromial decompression group, one out of 63 participants in the placebo group and two out of 71 participants in the exercise group. One participant in the placebo group had temporary swelling in the brachial area related to a brachial plexus block, while one participant in the exercise group also developed aggravation of low back pain. The RR for adverse events was 0.91 (95% CI 0.31 to 2.65; 406 participants) (Analysis 4.1).
4.1. Analysis.
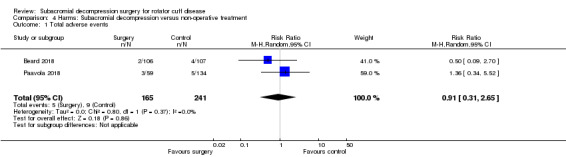
Comparison 4 Harms: Subacromial decompression versus non‐operative treatment, Outcome 1 Total adverse events.
Serious adverse events
There were no reports of serious adverse events in any randomised trials. Reports of serious adverse events came from two observational reports from a surgery registry recording 30‐day morbidity after shoulder arthroscopic surgery. Overall, we are uncertain if there is an increased risk of serious adverse events with decompression surgery (moderate‐certainty evidence, downgraded for indirectness as observational studies included other arthroscopic shoulder surgeries in addition to subacromial decompression procedures).
The incidence of serious harms following mixed shoulder arthroscopic procedures was 0.5% (0.4% to 0.7%) during 2006 to 2011 (Shields 2015), and 0.6% (0.5% to 0.7%), during 2011 to 2013 (Hill 2017). The adverse events were not reported separately by procedure (Table 7). The co‐published parallel review provides the full report of these events (Lähdeoja 2019).
5. Serious adverse events in registry studies.
| Eventa | N (%) Hill 2017 | N (%) Shields 2015 |
| Mortality | 2 (0.01) | 4 (0.04) |
| Bleeding requiring transfusion | 7 (0.05 | 5 (0.05) |
| Sepsis | 0 (0) | 1 (0.01) |
| Septic shock | 3 (0.02) | 2 (0.02) |
| Deep infection | 1 (0.01) | 1 (0.01) |
| Organ or space surgical site infection | 3 (0.02) | 2 (0.02) |
| Wound dehiscence | 1 (0.01) | 1 (0.01) |
| Deep vein thrombosis | 21 (0.14) | 8 (0.08) |
| Pulmonary embolism | 20 (0.13) | 7 (0.07) |
| Myocardial infarction | 3 (0.02) | 4 (0.04) |
| Cardiac arrest requiring cardiopulmonary resuscitation |
1 (0.01) | 2 (0.02) |
| Cerebral vascular event | 4 (0.03) | 2 (0.02) |
| Acute renal failure | 2 (0.01) | 1 (0.01) |
| Pneumonia | 13 (0.09) | 7 (0.07) |
| Unplanned intubation | 7 (0.05) | 3 (0.03) |
| Ventilator > 48 hours | 2 (0.01) | 1 (0.01) |
| Peripheral nerve injury | 2 (0.01) | 2 (0.02) |
aThese serious adverse events were recorded across all procedures in the registry and were not reported separately by procedure.
Sensitivity analyses
To assess the robustness of the findings in our primary analysis, we pooled data from both the placebo‐controlled trials and open‐label trials (subacromial decompression versus exercises), in sensitivity analyses. We did the analyses for pain at the six‐month and one‐year time points and for function at six‐month and one‐to‐three‐year time points.
Pooling the unblinded trials with the placebo‐controlled trials did not change the estimates of treatment effects to a clinically important level. Statistical heterogeneity was unimportant (0%) in all pain comparisons. With respect to function, statistical heterogeneity was larger when combining open‐label trials compared with the placebo‐controlled trials (I2 = 0% combining the two placebo‐controlled trials versus I2 = 50% to 63% when combining the open‐label trials).
At six months the mean pain was 3.9 (0 to 10 scale, higher is worse), with exercise or placebo and 0.31 points better (95% CI 0.12 worse to 0.75 better, 639 participants), with surgery; at one year, the mean pain was 3.3 with exercise or placebo and 0.58 points better (95% CI 0.12 better to 1.05 better, 532 participants), with surgery. The CIs exclude the clinically important difference (Analysis 5.1; Analysis 5.2).
5.1. Analysis.
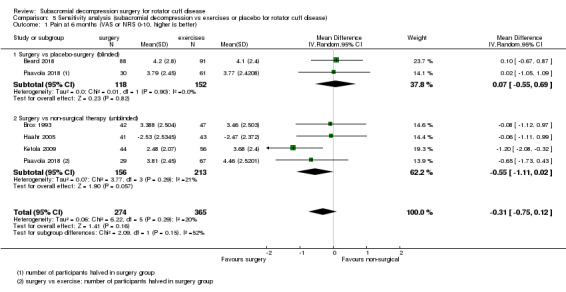
Comparison 5 Sensitivity analysis (subacromial decompression vs exercises or placebo for rotator cuff disease), Outcome 1 Pain at 6 months (VAS or NRS 0‐10, higher is better).
5.2. Analysis.
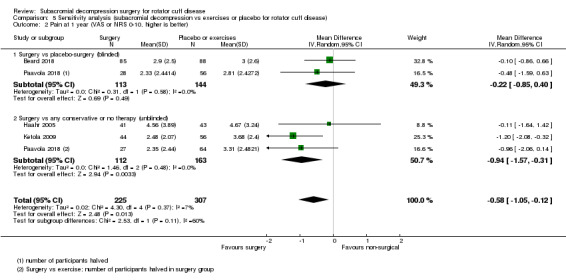
Comparison 5 Sensitivity analysis (subacromial decompression vs exercises or placebo for rotator cuff disease), Outcome 2 Pain at 1 year (VAS or NRS 0‐10, higher is better).
At six months, mean function was 58 points (0 to 100 scale, higher indicates better), with exercise or placebo and 1.05 points better (95% CI 3.77 worse to 5.87 better, 625 participants), with surgery; at one to three years, mean function was 68 points with exercise or placebo and 3.2 points better (95% CI −0.81 to 7.23, 737 participants), with surgery. The CIs exclude an important difference at both time points (Analysis 5.3; Analysis 5.4).
5.3. Analysis.
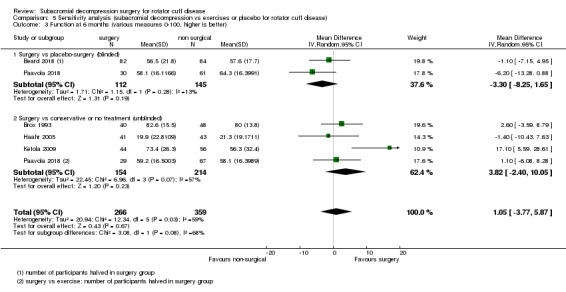
Comparison 5 Sensitivity analysis (subacromial decompression vs exercises or placebo for rotator cuff disease), Outcome 3 Function at 6 months (various measures 0‐100, higher is better).
5.4. Analysis.
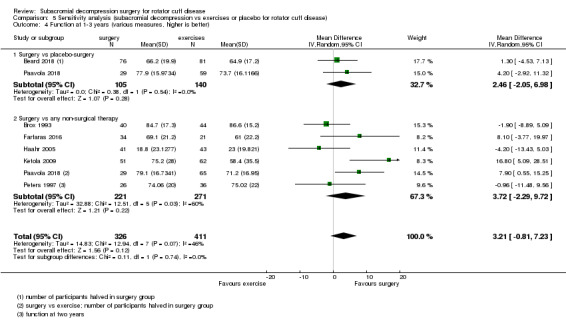
Comparison 5 Sensitivity analysis (subacromial decompression vs exercises or placebo for rotator cuff disease), Outcome 4 Function at 1‐3 years (various measures, higher is better).
Removing data with imputed SD for function (Peters 1997), in the decompression surgery versus exercises comparisons where relevant, resulted in wider CIs but no clinically important change in the estimate (one year: MD 3.24, 95% CI −8.08 to 14.55, including data from Peters 1997, versus MD 5.98, 95% CI −14.59 to 26.55, excluding these data; two years: MD 4.94, 95% CI 0.77 to 9.11, including data from Peters 1997, versus MD 5.07, 95% CI −0.55 to 10.70, excluding these data; five years: MD 7.63, 95% CI 0.17 to 15.09, including data from Peters 1997, versus MD 5.30, 95% CI −5.31 to 15.91 excluding these data.
Discussion
Summary of main results
Two trials compared arthroscopic decompression surgery with placebo surgery (arthroscopy without decompression). Compared with placebo surgery, high‐certainty evidence indicates that subacromial decompression provides no clinically important benefits with respect to pain, function, or quality of life at one year. There was probably no important difference in participant global assessment of treatment success (moderate‐certainty evidence, downgraded for imprecision) (Table 1). Sensitivity analyses including results from open‐label trials (with high risk of bias) did not change the estimates considerably.
Seven trials compared subacromial decompression with exercise therapy. Low‐certainty evidence available from three trials at one year indicates that there were no clinically important benefits in terms of pain, function, global assessment of success or health‐related quality of life (Table 2). We downgraded the evidence due to bias and imprecision. Trials were subject to performance and detection biases because the participants were aware of which treatment they received. The 95% CIs around the treatment estimates do not exclude clinically important differences between exercise and surgery.
Approximately three percent of trial participants report adverse events across the randomised controlled trials, although due to low event rates, we are uncertain if surgery is associated with increased risk in adverse events compared to control groups (Table 1).
Serious adverse events including death, deep venous thrombosis, pneumonia, and peripheral nerve damage have been observed in a surgery registry recording 30‐day morbidity after shoulder arthroscopic surgery. Although the precise estimates are unknown, based on National Surgical Quality Improvement Program (NSQIP) registry, the risk of serious adverse events is likely less than 1%
Overall completeness and applicability of evidence
This review included two placebo‐controlled trials of subacromial decompression at low risk of bias. Both trials also included a third control group of either no treatment or exercises, and participants in these groups were aware of their treatment allocation. We included an additional six trials comparing subacromial decompression to exercise therapy and these were all at high risk of performance and detection bias. One of these trials also included an additional control group that received detuned (placebo) laser treatment. We did not identify any trials comparing subacromial decompression surgery to other non‐operative treatments such as NSAIDs, or glucocorticoid or other injections.
Trials were conducted in six countries and trial participants had typical clinical features of rotator cuff disease (with impingement and without full‐thickness or complete rotator cuff tears or calcification). Most trials excluded full‐thickness tears by MRI, ultrasound or arthroscopy before inclusion. Participants were also similar in terms of age (mean age 41 to 59 years), gender distribution (slight female predominance), baseline pain and function, duration of symptoms, and failure to improve with conservative treatment (exercise therapy with or without glucocorticoid injections) for at least three months. Thus, the synthesis of this review can be applied to similar patients in clinical practice. The trials did not include elderly participants nor report outcomes in specific subpopulations such as manual workers, therefore we cannot draw any conclusion specific to these subgroups.
Our primary analysis only included the trials at low risk for bias but the results did not change significantly when all available trials were pooled together in sensitivity analysis. Placebo control introduces indirectness because placebo surgery is not a treatment option in clinical practice. However, the largely consistent findings of the unblinded studies leave little doubt of the inference that subacromial decompression provides no important benefit in people with rotator cuff disease manifesting as painful shoulder impingement.
Measurement of pain varied across trials from unspecified pain to pain with activity or average pain, as well a pain at night and at rest. We preferred to extract overall pain data when available, but some trials only reported pain with activity and others did not specify the framing of the question by which they assessed pain.
Regarding function, the trials used different outcome measures. We chose to extract Constant score whenever it was used because it was used by both trials in our primary comparison and by most of the trials in our second comparison (Beard 2018; Farfaras 2016; Haahr 2005; Paavola 2018). Other measures were not used in more than one study. Constant score puts considerable weight on capacity rather than function/disability. However, its validity and responsiveness is acceptable compared to other instruments (Roy 2010), and the parallel systematic review (Lähdeoja 2019) provided high credibility MID estimates (Hao 2019).
Since we expected the trials to provide insufficient data to estimate serious adverse events due to their low incidence, we also performed a separate review of observational studies of subacromial decompression and shoulder arthroscopy for various diagnoses as described in Lähdeoja 2019. The estimates were based on two studies using a large registry with total of 25,270 arthroscopic shoulder procedures in more than 600 centres in the USA.
The follow‐up times across trials varied from one year up to 13 years and most trials reported results after two years or more. There was increased attrition and reporting bias at the longer follow‐up time points. The five‐ and 10‐year comparisons provided low certainty evidence that surgery may improve function but not pain. However, it is possible that other factors confound the treatment effects over longer periods of follow‐up. Paavola 2018 will report five‐year results and that will likely affect our confidence in the estimates at five years.
Quality of the evidence
High certainty evidence from randomised controlled trials shows that subacromial decompression does not provide clinically important benefits over placebo in pain, function or quality of life. Due to imprecision, we downgraded evidence to moderate certainty for global assessment of treatment success; there was probably no important benefit in this outcome over placebo.
We downgraded evidence from randomised trials comparing subacromial decompression with exercise to low certainty for pain, function, global assessment of success and health‐related quality of life, primarily due to detection bias associated with the open‐label design and imprecision; the 95% CIs around the effect estimates did not rule in or out clinically important effects.
For adverse events, evidence from the randomised controlled trials was downgraded to moderate certainty due to low reported event rates, and thus imprecise estimates of the comparative risks.
As there were only a few trials in each comparison, we could not get precise estimates for heterogeneity. The two placebo‐controlled trials reported consistent results for all outcomes. Although the trials comparing subacromial decompression with exercise displayed inconsistency at six months and one year, driven by one study for function, we did not downgrade the evidence, as the CIs overlap with the other studies and there were only three to four trials in the time points that we could pool.
The evidence regarding incidence of serious adverse events was moderate certainty. The registry studies were well designed. Although a few events may occur in this population even without surgery, most observed serious adverse events were likely attributable to the index procedure (Table 6; Table 7), thus the default certainty of evidence started at high. The operations included mixed arthroscopic shoulder procedures, thus we downgraded due to indirectness.
Potential biases in the review process
To the best of our knowledge, we identified all relevant trials meeting our inclusion criteria through searching all major databases without language restrictions. We used up to three independent assessors for article screening, selection and for risk of bias judgement. None of the review authors has been involved with the conduct of the included trials.
There were too few studies to formally assess the presence of publication bias. We identified one ongoing trial comparing surgery to exercise and one trial completed in 2008 with no results available (surgery versus usual care). It is unlikely that results of these trials, if or when available, will alter the conclusions of this review.
Agreements and disagreements with other studies or reviews
In addition to our original Cochrane Review (Coghlan 2008), we identified two other published systematic reviews specifically comparing surgery with exercises in the treatment of impingement syndrome (Saltychev 2015; Toliopoulos 2014). The conclusions in these reviews are in keeping with our updated review. Both concluded that there is no benefit of surgery over conservative treatments for shoulder impingement. None of the previous reviews included placebo‐controlled trials.
Authors' conclusions
Implications for practice.
The synthesis of data in this review does not support use of subacromial decompression surgery in the treatment of symptomatic rotator cuff disease presenting with impingement features and without full‐thickness rotator cuff tears. Subacromial decompression does not provide clinically important benefits compared with placebo surgery with respect to pain, function or quality of life, and this probably also applies to participant global assessment of treatment success. Although adverse events associated with subacromial decompression are probably low, serious adverse events such as deep venous thrombosis, pneumonia, peripheral nerve damage and death following arthroscopic shoulder surgery have been observed.
Participants in the trials experienced moderate pain and noticeable impaired function for up to one year but symptoms seemed to improve over the first two years of follow‐up. Therefore, people with rotator cuff disease should be informed that surgery will probably not improve their symptoms compared with exercises; they will likely experience shoulder pain and impaired function whether they have surgery or not, and this is likely to improve slowly over time irrespective of treatment.
Implications for research.
Further research is unlikely to change the conclusions of this review. However, as one placebo‐controlled trial is still ongoing (Paavola 2018), and five‐year follow‐up will be completed in two years, we plan to update this review once the results are available.
If in the future we identify a clearly defined subpopulation, who may benefit from surgery, this should be tested in a well‐conducted placebo‐controlled trial.
Further trials comparing surgical decompression to exercises or no treatment are unlikely to change the conclusions of this review. Therefore, it is unlikely that we will include results from new or ongoing trials comparing subacromial decompression to exercise or no treatment in future review updates.
What's new
| Date | Event | Description |
|---|---|---|
| 23 October 2018 | New citation required and conclusions have changed | Review updated; four new trials included and eight in total |
| 22 October 2018 | New search has been performed | The original review, 'Surgery for rotator cuff disease', published in 2007 was split into three reviews upon updating: this review, 'Subacromial decompression for rotator cuff disease', and two pending updates, 'Surgery for full‐thickness rotator cuff tears', and 'Surgery for calcific rotator cuff tendinopathy'. We excluded the comparison of one type of surgical technique to another in this update. We included four new trials in this update: Beard 2018; Farfaras 2016; Ketola 2009; Paavola 2018 in addition to three trials included in the 2007 version (Brox 1993; Haahr 2005; Rahme 1998). An additional trial, translated from German was excluded in the 2007 version of the review as it was unclear that it was an RCT, but was included in this update after the author confirmed it was an RCT (Peters 1997). |
History
Protocol first published: Issue 1, 2006 Review first published: Issue 1, 2008
| Date | Event | Description |
|---|---|---|
| 1 December 2008 | Amended | Converted to new review format. CMSG ID: C083‐R |
Acknowledgements
We thank the authors of the previous published version of the review for their contribution: TDW Alta, JA Coghlan, SN Bell, and SF King.
Dr N Jain is supported by funding from National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) 1K23AR059199 and 1U34AR069201
Appendices
Appendix 1. CENTRAL (Cochrane library) search strategy
#1 MeSH descriptor: [Shoulder] this term only
#2 MeSH descriptor: [Rotator Cuff] this term only
#3 #1 or #2
#4 MeSH descriptor: [Calcium] this term only
#5 MeSH descriptor: [Bursitis] 1 tree(s) exploded
#6 #4 or #5
#7 #3 and #6
#8 MeSH descriptor: [Shoulder Pain] this term only
#9 MeSH descriptor: [Shoulder Impingement Syndrome] this term only
#10 MeSH descriptor: [Rotator Cuff Injuries] this term only
#11 rotator cuff:ti,ab or supraspinatus:ti,ab or infraspinatus:ti,ab or subscapular*:ti,ab or teres:ti,ab
#12 ((shoulder*:ti,ab or subacromial:ti,ab or rotator cuff:ti,ab) near/5 (tendon*:ti,ab or tendin*:ti,ab or bursitis:ti,ab or calcium:ti,ab or calcif*:ti,ab or impinge*:ti,ab or tear*:ti,ab or pain:ti,ab))
#13 #7 or #8 or #9 or #10 or #11 or #12
#14 MeSH descriptor: [Surgical Procedures, Operative] explode all trees
#15 (surger*:ti,ab or surgical*:ti,ab or operat*:ti,ab)
#16 decompress*:ti,ab
#17 bursectom*:ti,ab
#18 acromioplast*:ti,ab
#19 (calcium:ti,ab next remov*:ti,ab)
#20 debrid*:ti,ab
#21 MeSH descriptor: [Arthroscopy] this term only
#22 arthroscop*:ti,ab
#23 #14 or #15 or #16 or #17 or #18 or #19 or #22
#24 #13 and #23 Publication Year from 2006 to 2018
Appendix 2. Medline (Ovid) search strategy
1 shoulder/ (11615)
2 rotator cuff/ (5502)
3 1 or 2 (16578)
4 calcium/ (257302)
5 exp bursitis/ (4455)
6 4 or 5 (261746)
7 3 and 6 (732)
8 shoulder pain/ (4075)
9 shoulder impingement syndrome/ (1581)
10 rotator cuff injuries/ (4533)
11 (rotator cuff or supraspinatus or infraspinatus or subscapular$ or teres).tw. (13224)
12 ((shoulder$ or subacromial or rotator cuff) adj5 (tendon$ or tendin$ or bursitis or calcium or calcif$ or impinge$ or tear$ or pain)).tw. (12827)
13 or/7‐12 (22497)
14 exp Surgical Procedures, Operative/ (2847798)
15 su.fs. (1819530)
16 (surger$ or surgical$ or operat$).tw. (1944943)
17 decompress$.tw. (34248)
18 bursectom$.tw. (574)
19 acromioplast$.tw. (463)
20 (calcium adj remov$).tw. (301)
21 debrid$.tw. (19906)
22 ARTHROSCOPY/ (20572)
23 arthroscop$.tw. (21931)
24 or/14‐23 (4145221)
25 13 and 24 (11569)
26 randomized controlled trial.pt. (458491)
27 controlled clinical trial.pt. (92309)
28 randomized.ab. (357908)
29 placebo.ab. (171901)
30 drug therapy.fs. (2009606)
31 randomly.ab. (248101)
32 trial.ab. (371145)
33 groups.ab. (1551065)
34 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 (3870583)
35 exp animals/ not humans.sh. (4446637)
36 34 not 35 (3302687)
37 25 and 36 (2282)
38 limit 37 to yr="2006 ‐Current" (1692)
Appendix 3. Embase (Ovid) search strategy
1 shoulder/ (30424)
2 rotator cuff/ (5433)
3 1 or 2 (33875)
4 calcium/ (273333)
5 exp bursitis/ (4560)
6 4 or 5 (277860)
7 3 and 6 (675)
8 shoulder pain/ (13768)
9 exp shoulder impingement syndrome/ (2426)
10 exp rotator cuff injury/ (9234)
11 (rotator cuff or supraspinatus or infraspinatus or subscapular$ or teres).tw. (18601)
12 ((shoulder$ or subacromial or rotator cuff) adj5 (tendon$ or tendin$ or bursitis or calcium or calcif$ or impinge$ or tear$ or pain)).tw. (19693)
13 or/7‐12 (37559)
14 exp surgery/ (4329852)
15 su.fs. (1974350)
16 (surger$ or surgical$ or operat$).tw. (2921649)
17 decompress$.tw. (50428)
18 bursectom$.tw. (697)
19 acromioplast$.tw. (613)
20 (calcium adj remov$).tw. (369)
21 debridement/ (34049)
22 debrid$.tw. (28473)
23 shoulder arthroscopy/ (1647)
24 arthroscop$.tw. (32269)
25 or/14‐24 (5736356)
26 13 and 25 (19708)
27 random$.tw. (1294111)
28 factorial$.tw. (32556)
29 crossover$.tw. (65747)
30 cross over.tw. (28947)
31 cross‐over.tw. (28947)
32 placebo$.tw. (272731)
33 (doubl$ adj blind$).tw. (188634)
34 (singl$ adj blind$).tw. (21007)
35 assign$.tw. (335768)
36 allocat$.tw. (126659)
37 volunteer$.tw. (231815)
38 crossover procedure/ (55181)
39 double blind procedure/ (148991)
40 randomized controlled trial/ (499001)
41 single blind procedure/ (31120)
42 or/27‐41 (1997713)
43 26 and 42 (2117)
44 limit 43 to exclude medline journals (219)
45 limit 44 to yr="2006 ‐Current" (202)
Appendix 4. Clinicaltrials.gov
Rotator cuff OR impingement in Condition
Appendix 5. WHO ITCRP
Rotator cuff AND
surg* or decompress* or bursectom* acromioplast* or debrid* or arthroscop*
(without synonyms)
Data and analyses
Comparison 1. Subacromial decompression vs placebo for rotator cuff disease.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (VAS or NRS 0‐10, lower is better) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 3 months | 1 | 107 | Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.45, 1.39] |
| 1.2 6 months | 2 | 299 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.51, 0.64] |
| 1.3 1 year | 2 | 284 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.84, 0.33] |
| 1.4 2 years | 1 | 118 | Mean Difference (IV, Random, 95% CI) | ‐0.9 [‐1.79, ‐0.01] |
| 2 Functional outcome (Constant score 0‐100, 100 is best) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 6 months | 2 | 286 | Mean Difference (IV, Random, 95% CI) | ‐3.72 [‐8.72, 1.28] |
| 2.2 1 year | 2 | 274 | Mean Difference (IV, Random, 95% CI) | 2.76 [‐1.36, 6.87] |
| 2.3 2 years | 1 | 117 | Mean Difference (IV, Random, 95% CI) | 4.20 [‐1.61, 10.01] |
| 3 Global assessment of treatment success | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 6 months | 2 | 293 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.89, 1.54] |
| 3.2 1 year | 2 | 290 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.93, 1.27] |
| 3.3 2 years | 1 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.82, 1.17] |
| 4 Health‐related quality of life (various measures, higher is better) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 3 months | 1 | 109 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.55, 0.21] |
| 4.2 6 months | 2 | 292 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.27, 0.18] |
| 4.3 1 year | 2 | 285 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.39, 0.21] |
| 4.4 2 years | 1 | 118 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.36, 0.36] |
| 5 Participation (number at work) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 3 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 1 year | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 2 years | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Participation (number returning to sport or leisure activities) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 3 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 1 year | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 2 years | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Subacromial decompression vs exercise treatment for rotator cuff disease.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (VAS 0‐10, 0 is no pain) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 3 months | 4 | 361 | Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐1.24, 0.14] |
| 1.2 6 months | 4 | 399 | Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.09, ‐0.02] |
| 1.3 1 year | 3 | 316 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐1.60, ‐0.42] |
| 1.4 2 years | 3 | 352 | Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐1.37, 0.49] |
| 1.5 5 years | 2 | 188 | Mean Difference (IV, Random, 95% CI) | 0.36 [‐1.17, 1.89] |
| 1.6 10 years | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐0.25, 2.25] |
| 2 Functional outcome (0‐100, 100 is best) | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 3 months | 3 | 257 | Mean Difference (IV, Random, 95% CI) | 6.11 [‐5.57, 17.79] |
| 2.2 6 months | 4 | 398 | Mean Difference (IV, Random, 95% CI) | 3.66 [‐2.25, 9.58] |
| 2.3 1 year | 3 | 259 | Mean Difference (IV, Random, 95% CI) | 3.24 [‐8.08, 14.55] |
| 2.4 2 years | 5 | 467 | Mean Difference (IV, Random, 95% CI) | 4.94 [0.77, 9.11] |
| 2.5 5 years | 2 | 157 | Mean Difference (IV, Random, 95% CI) | 7.63 [0.17, 15.09] |
| 2.6 10 years | 2 | 156 | Mean Difference (IV, Random, 95% CI) | 9.54 [1.93, 17.15] |
| 3 Global assessment of treatment success | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 6 months | 2 | 161 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.74, 2.91] |
| 3.2 1 year | 2 | 158 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.96, 1.51] |
| 3.3 2 years | 1 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [1.00, 1.57] |
| 3.4 5 years | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.62, 1.23] |
| 3.5 10 years | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.67, 1.49] |
| 4 Health‐related quality of life (various measures, 0‐1; higher is better) | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 3 months | 1 | 119 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.23, 0.49] |
| 4.2 6 months | 1 | 119 | Std. Mean Difference (IV, Random, 95% CI) | 0.51 [0.15, 0.88] |
| 4.3 1 year | 1 | 116 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.21, 0.52] |
| 4.4 2 years | 2 | 181 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.22, 0.48] |
| 4.5 5 years | 1 | 86 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.30, 0.54] |
| 4.6 10 years | 2 | 155 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.01, 0.62] |
| 5 Participation (number at work) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 3 months | 1 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.75, 1.22] |
| 5.2 6 months | 2 | 187 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.81, 1.36] |
| 5.3 1 year | 1 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.85, 1.13] |
| 5.4 2 years | 2 | 183 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.70, 1.07] |
| 5.5 5 years | 2 | 188 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.97, 1.32] |
| 5.6 10 years | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.97, 1.18] |
| 6 Participation (numbers returning to sport or leisure activities) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 3 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 1 year | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 2 years | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Treatment failure | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1 5 years | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 13 years | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Subacromial decompression vs no treatment for rotator cuff disease.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (NRS 0‐10 lower is better) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 1 year | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Functional outcomes (Constant score 0‐100, 100 is best) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 1 year | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Global assessment of treatment success | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 1 year | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Health‐related quality of life (EQ‐5D 3L −0.59 to 1, higher is better) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 1 year | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Harms: Subacromial decompression versus non‐operative treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total adverse events | 2 | 406 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.31, 2.65] |
Comparison 5. Sensitivity analysis (subacromial decompression vs exercises or placebo for rotator cuff disease).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain at 6 months (VAS or NRS 0‐10, higher is better) | 5 | 639 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.75, 0.12] |
| 1.1 Surgery vs placebo‐surgery (blinded) | 2 | 270 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.55, 0.69] |
| 1.2 Surgery vs non‐surgical therapy (unblinded) | 4 | 369 | Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐1.11, 0.02] |
| 2 Pain at 1 year (VAS or NRS 0‐10, higher is better) | 4 | 532 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.05, ‐0.12] |
| 2.1 Surgery vs placebo‐surgery (blinded) | 2 | 257 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.85, 0.40] |
| 2.2 Surgery vs any conservative or no therapy (unblinded) | 3 | 275 | Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐1.57, ‐0.31] |
| 3 Function at 6 months (various measures 0‐100, higher is better) | 5 | 625 | Mean Difference (IV, Random, 95% CI) | 1.05 [‐3.77, 5.87] |
| 3.1 Surgery vs placebo‐surgery (blinded) | 2 | 257 | Mean Difference (IV, Random, 95% CI) | ‐3.30 [‐8.25, 1.65] |
| 3.2 Surgery vs conservative or no treatment (unblinded) | 4 | 368 | Mean Difference (IV, Random, 95% CI) | 3.82 [‐2.40, 10.05] |
| 4 Function at 1‐3 years (various measures, higher is better) | 7 | 737 | Mean Difference (IV, Random, 95% CI) | 3.21 [‐0.81, 7.23] |
| 4.1 Surgery vs placebo‐surgery | 2 | 245 | Mean Difference (IV, Random, 95% CI) | 2.46 [‐2.05, 6.98] |
| 4.2 Surgery vs any non‐surgical therapy | 6 | 492 | Mean Difference (IV, Random, 95% CI) | 3.72 [‐2.29, 9.72] |
| 5 Pain at 1 year | 5 | 733 | Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.17, ‐0.39] |
5.5. Analysis.
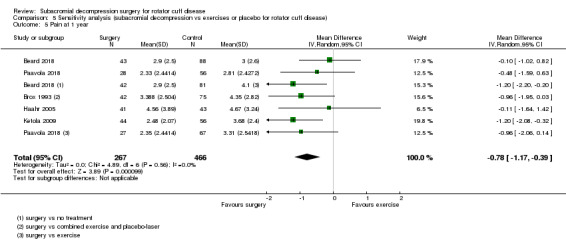
Comparison 5 Sensitivity analysis (subacromial decompression vs exercises or placebo for rotator cuff disease), Outcome 5 Pain at 1 year.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Beard 2018.
| Methods |
Design: multicentre (30 sites), parallel‐group, three‐arm (1:1:1) assessor‐ and participant‐blinded, randomised placebo‐controlled trial Setting: NHS hospitals in UK Timing: September 2012‐July 2016 Interventions: ASD, placebo surgery (arthroscopy only), and no treatment Sample size: 85 participants per group required to have 90% power to detect a difference in the Oxford Shoulder Score of 4.5 (SD 9.0), with a 2‐sided 5% level of significance (α) Analysis: ITT analysis and per‐protocol analysis |
|
| Participants |
Number of participants 2975 patients screened for eligibility 740 eligible (232 followed in observational cohort due to strong preference, 195 did not partake but reasons not available) 313 randomised (106 to ASD; 103 to arthroscopy only; 104 to no treatment) 24 (23%), 43 (42%), and 12 (12%) of the ASD, arthroscopy only, and no‐treatment groups, respectively, did not receive their assigned treatment by 6 months 19 (18%), 35 (3%), and 26 (25%) of the ASD, arthroscopy only, and no‐treatment groups, respectively, did not receive their assigned treatment by 1 year Data for 274 (90 (85%) for ASD; 94 (91%) for arthroscopy only; and 90 (87%) for no treatment) available at the 6‐month follow‐up Data for 265 (88 (83%) for ASD; 93 (90%) for arthroscopy only; and 84 (81%) for no treatment) available at the final 12‐month follow‐up Inclusion criteria:
Exclusion criteria:
Baseline characteristics ASD group: Mean (SD) age: 52.9 (10.3) 54 female 52 male Previously received injections: 2 (range: 1‐3) Mean (SD) Oxford Shoulder Score: 25.2 (8.5) Mean (SD) Constant‐Murley Shoulder Score: 39.4 (13.9) Mean (SD) PainDETECT score: 11.7 (6.6) Mean (SD) EQ‐VAS: 65.8 (19.4) Mean (SD) EQ‐5D Index: 0.52 (0.30) Mean (SD) HADS Depression score: 5.0 (3.8) Mean (SD) HADS Anxiety Score: 6.3 (4.3) Placebo surgery group: Mean (SD) age: 53.7 (10.5) 52 female 51 male Previously received injections: 2 (range: 1‐3) Mean (SD) Oxford Shoulder Score: 26.7 (8.8) Mean (SD) Constant‐Murley Shoulder Score: 43.1 (15.5) Mean (SD) PainDETECT score: 11.0 (5.9) Mean (SD) EQ‐VAS: 69.7 (19.2) Mean (SD) EQ‐5D Index: 0.55 (0.29) Mean (SD) HADS Depression score: 5.0 (3.7) Mean 8SD) HADS Anxiety Score: 6.3 (4.2) No treatment group: Mean (SD) Age: 53.2 (10.2) 52 female 52 male Previously received injections: 2 (range: 1‐3) Mean (SD) Oxford Shoulder Score: 25.5 (8.3) Mean (SD) Constant‐Murley Shoulder Score: 38.3 (14.2) Mean (SD)PainDETECT score: 11.9 (6.6) Mean (SD)EQ‐VAS: 64.4 (23.2) Mean (SD)EQ‐5D‐3L Index: 0.50 (0.33) Mean (SD)HADS Depression score: 5.7 (4.2) Mean (SD) HADS Anxiety Score: 6.9 (4.5) |
|
| Interventions | 38 surgeons performed the operations in 30 sites. ASD The procedure was performed with the participant under general anaesthesia. Skin incisions were made for the introduction of the arthroscope and required instruments. The procedure involved insertion of the arthroscope into the GHJ, where the joint surface was inspected along with the intraarticular portion of the long head of biceps and the joint surface of the rotator cuff tendons. Once this was performed, the arthroscope was removed and inserted into the subacromial bursa, which lies outside the rotator cuff tendons and beneath the acromion process of the scapula. In the bursa, the acromion and superior surface of the rotator cuff were assessed to ensure that the coracoacromial ligament and the AC joint were intact. The ASD included removal of bursa and soft tissue within the subacromial space as well as resection of projecting undersurface of the distal part of the acromion. Postoperative physiotherapy involved advice and between 1 and 4 routine treatment sessions. Placebo (arthroscopy only) The procedure was performed with the participant under general anaesthesia. Participants underwent a routine investigational arthroscopy of the joint and rotator cuff tendon. The operation was performed in exactly the same manner as that in the ASD group. Investigational arthroscopy has all the same essential operative components (and risks) of ASD, but it does not involve surgical removal of any spurs or bursal tissue or release of the coracohumeral ligament. The procedure involved the GHJ and the subacromial bursa being inspected and irrigated. Structures assessed for integrity and damage included the rotator cuff, which was assessed for evidence of full‐thickness tears; and the synovium and lining of the shoulder for evidence of capsulitis and arthritis. Postoperative physiotherapy involved advice and between one and four routine treatment sessions. No treatment (active monitoring) Participants were advised that they would undergo active monitoring with a reassessment appointment with a specialist shoulder surgeon 3 months after entering the study. At that appointment, they were asked to complete questionnaires related to their shoulder pain and undergo a clinical assessment of the shoulder, including a record of any further conservative treatment. If, at the end of the 6‐month assessment period, participants remained sufficiently symptomatic to require further intervention (based on clinical judgement), then additional treatment options were discussed. The participants in the no‐treatment group had no prescribed physiotherapy or steroid injections. |
|
| Outcomes | Outcomes were reported at baseline, 6 and 12 months. Primary end point was at 12 months Primary outcome
Secondary outcomes
Outcomes included in this review
|
|
| Source of funding | The study was funded by Arthritis Research UK via a clinical studies grant. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. | |
| Notes |
Trial registration: NCT01623011 Data analysis: the primary analysis compared ASD and placebo surgery (arthroscopy only) groups (Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4; Analysis 1.7). Had not received allocated treatment by 6 months: 24 (23%), 43 (42%), and 12 (12%) of the decompression, arthroscopy only, and no‐treatment groups, respectively Had not received allocated treatment by 1 year: 19 (18%), 35 (34%), and 26 (25%) of the decompression, arthroscopy only, and no‐treatment groups, respectively The per‐protocol analysis reported no between‐group differences at 6 months or 1 year for the primary outcome of mean Oxford Shoulder Score. Cross overs: 9/103 participants in the placebo surgery group had decompression surgery by 6 months and 10/103 by 12 months. Two participants in the placebo surgery group underwent further surgery (decompression in one and superior labrum debridement in the other). AEs: Two frozen shoulders reported in each of the three groups SAEs: none reported Risk of bias: we separately note the risk of bias for the 2 surgery groups and the monitoring group in the 'Risk of bias' table and authors' judgements are for the comparison of the 2 surgical groups (decompression and placebo). Prof Beard confirmed that postoperative personnel including nurses on the ward were unaware of treatment allocation and postoperative care was identical in the surgery groups. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was generated using an automated computer‐generated minimisation system, minimising for age (< 40, 40–55, or ≥ 56 years), sex, baseline Oxford Shoulder Score (< 19, 19–26, 27–33, or ≥ 34 years), and recruiting site |
| Allocation concealment (selection bias) | Low risk | Quote: "Study site staff who were authorised to perform telephone randomisation contacted the Oxford Clinical Trials Research Unit providing the following information: participant details, research site details, name of caller, name of treating consultant, confirmation of eligibility, confirmation of written informed consent and its date, and stratification factors." Quote: "The surgical group to which the patient was allocated was concealed from the surgical team and revealed only on the day of planned surgery." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants randomly assigned to surgery were not told which procedure they had undergone, decompression or arthroscopy only. Personnel other than the surgical team were blinded to treatment intervention. The time spent in the operating theatre and all postoperative care was identical in both groups. Participants randomised to the monitoring group received a letter stating that a specialist reassessment appointment would be scheduled for a date 3 months hence (the timing of that letter was not specified). We judged blinding for the monitoring group was at high risk of bias. |
| Blinding of outcome assessment for self‐reported outcomes including pain, function and global assessment (detection bias) | Low risk | The participants were blinded to allocation in the two surgery groups (low risk) but not the monitoring‐only group (high risk). |
| Blinding of outcome assessment for outcome assessor reported outcomes (detection bias) | Low risk | Postintervention assessors were blinded to treatment allocation across all 3 treatment groups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of participants lost to follow‐up was similar across the 2 surgical groups at 6 months: 15/106 for decompression surgery (7 participants did not respond, 6 withdrew, and 2 were lost for other reasons) and 9/103 for placebo surgery (6 participants did not respond, 2 withdrew, and 1 was lost for other reasons). At 12 months, 18/106 in the decompression surgery group (10 did not respond, 6 withdrew and 2 were lost for other reasons) and 10/103 in the placebo surgery group (6 did not respond, 3 withdrew and 1 was lost for other reasons) were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | The trial authors report all prespecified outcomes except quantitative sensory testing. As this is a tool for assessing disturbances in somatosensory processing and would not be included in the outcomes of this review, we judged this trial to be at low risk of selective reporting bias. |
| Other bias | Low risk | At 6 and 12 months, 9 (9%) participants and 10 (10%) participants in the placebo surgery group respectively, had crossed over to receive ASD. This may have underestimated any potential benefit of decompression. 24 (23%), 43 (42%), and 12 (12%) of the decompression, arthroscopy‐only, and no‐treatment groups, respectively at 6 months, and 19 (18%), 35 (34%), and 26 (25%) of the decompression, arthroscopy‐only, and no‐treatment groups, respectively at 1 year had not received their assigned treatment. However the analysis was performed on an ITT basis irrespective of whether or not the allocated treatment was received. |
Brox 1993.
| Methods | Design: single‐centre, 3‐arm, randomised trial Setting: public hospital surgery and physiotherapy departments in Norway, patients referred from general practitioners in the hospital catchment area Timing: not reported, trial reported in 1993 Interventions: ASD + exercise vs exercise therapy vs sham laser Sample size: the study was planned to detect a difference of 10 points between groups, which equals a reduction from moderate to mild pain. After a pilot study the SD was estimated at 13 points. With α set at 0 05 and, ß 0.10 36 participants were required for each treatment group to complete the trial. Analysis: ITT analysis | |
| Participants |
Number of participants 444 considered for inclusion 195 fulfilled eligibility criteria 125 consented and randomised (45 ASD; 30 placebo laser; 50 exercises) Data for 31 (69%) for ASD, 27 (90%) for placebo and 42 (84%) for exercises at 3 months; and 41 (91%) for ASD, 30 (100%) for placebo and 49 (98%) for exercises at 6 months Inclusion criteria
Exclusion criteria
Baseline characteristics Surgery (ASD) group Mean age: 48 years Number (%) female: 16 (36%) Duration of symptoms: < 6 months 8 (18%); 6‐12 months 8 (18%); 1‐3 years 9 (20%); > 3 years 20 (44%) Number (%) participants with bilateral pain: 11 (24%) Number (%) participants with dominant affected: 28 (62%) Number (%) participants on sick leave: 27 (60%) Number (%) participants on analgesics: 30 (67%) Mean Neer score: 63.6 (pain 13.8; function 22.3; ROM 17.5) Mean Hopkins symptoms checklist score: 1.6 Exercise therapy group Mean age: 47 years Number (%) female: 28 (56%) Duration of symptoms: < 6 months 6 (12%); 6‐12 months 6 (12%); 1‐3 years 13 (26%); > 3 years 25 (50%) Number (%) participants with bilateral pain: 12 (24%) Number (%) participants with dominant affected: 31 (62%) Number (%) participants on sick leave: 27 (54%) Number (%) participants with on analgesics: 39 (77%) Mean Neer score: 66.2 (pain 14.7; function 23.0; ROM 18.5) Mean Hopkins symptoms checklist score: 1.6 Placebo laser group Mean age: 48 Number (%) female: 15 (50%) Duration of symptoms: < 6 months 5 (17%); 6‐12 months 5 (17%); 1‐3 years 5 (17%); > 3 years 14 (48%) Number (%) participants with bilateral pain: 7 (23%) Number (%) participants with dominant side affected: 16 (53%) Number (%) participants with on sick leave: 18 (60%) Number (%) participants with on analgesics: 23 (75%) Mean Neer score: 64.7 (pain 14.8; function 22.1; ROM 17.8) Mean Hopkins symptoms checklist score: 1.6 |
|
| Interventions | 2 experienced surgeons performed the operations and several physiotherapists supervised the postoperative exercises For the exercise group, 1 experienced physiotherapist supervised the exercises. ASD ASD by removing subacromial bursa and anterior and lateral part of acromion as well as cutting AC ligament. Postoperative rehabilitation was started at first postoperative day. Physiotherapy started 1 week after the procedure and was supervised by a physiotherapist where the participant lived. The postoperative regimen did not follow exactly the same protocol as in the exercise group. Exercise therapy The purpose of the exercises were to "normalise the dysfunctional neuromuscular patterns" and "increase the nutrition of the collagen in the rotator cuff". The participants performed exercises 1 h/day; twice weekly with a physiotherapist and other days at home. Resistance was increased gradually. Exercise regimen continued over 3‐6 months Placebo‐laser 12 sessions of detuned soft laser treatment twice a week for 6 weeks |
|
| Outcomes | The outcomes were assessed at baseline, 3, 6 months and 2.5 years
Primary outcome measure
Secondary outcomes:
Outcomes used in this review
|
|
| Source of funding | Norwegian Research Council | |
| Notes |
Trial registration: not available Data analysis: we included only the comparison ASD versus exercise in the surgery versus exercises analysis. Placebo laser was not relevant to our review question. Trial authors reported median values in the original publications, but provided full data regarding pain and Neer score upon request. Pain was measured on a 1‐9 scale and we transformed pain values and SD to a 0‐10 scale before analyses using the formula from Thorlund 2011. 2.5 year results were used in sensitivity analysis 1‐3 years' time point (Analysis 5.4). Participation to work per protocol data used Withdrawals: 4/45 in arthroscopic surgery group did not attend follow‐up, reasons unclear Cross‐overs: 1/50 participant in exercise group had surgery. 3/45 participants in the surgery group had only exercises AEs: none reported SAEs: none reported Trial authors performed an interim analysis of 68 participants who completed 6 months' follow‐up and found that surgery or exercises were superior to placebo laser, and thus stopped allocating participants to placebo laser (hence the smaller number of participants). The trial authors did not appear to statistically adjust for the interim analysis in the final analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation not described. Random permuted blocks to ensure allocation concealment. Probably done |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants in the surgery and exercise groups were not blinded. Participants in the placebo‐laser arm were told they were receiving a new type of laser treatment so were blinded to being allocated to placebo laser |
| Blinding of outcome assessment for self‐reported outcomes including pain, function and global assessment (detection bias) | High risk | Participants were aware of whether or not they received surgery or exercise. Participants in the placebo laser arm were blinded to having received placebo laser therapy. |
| Blinding of outcome assessment for outcome assessor reported outcomes (detection bias) | Low risk | Outcome assessors were blinded to treatment intervention. Quote: "At follow up the patients wore a T‐shirt to hide a possible scar from surgery. They were carefully told not to talk about their treatment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were 4/45 participants in the arthroscopic group and 1/50 in the supervised exercise group who were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available to confirm all measured outcomes were reported. AEs were not reported and it is unclear if these were measured. Length of sick leave was measured but incompletely reported. Change in the main symptom and disability to carry 5 kg object and take down something was only reported at 2.5 years and as proportion 50% improved. Global success was also not reported as an outcome in the first paper but included as an outcome at 6 months and 2.5 years. This measure was based on Neer score and unclear if post hoc definition of cut‐off caused bias. |
| Other bias | High risk | We judged other bias to be at high risk due to an unplanned interim analysis performed after 68 participants had been followed up for 6 months. At this point randomisation to the placebo‐laser group was ceased, which led to unbalanced numbers across the 3 treatment groups. 9/45 (20%) allocated to surgery did not undergo ASD, and 7/50 (14%) allocated to exercises did not complete the planned exercises and 1 of these had ASD. However the analysis was performed on an ITT basis irrespective of whether or not the allocated treatment was received |
Farfaras 2016.
| Methods | Design: parallel‐group, 3‐arm RCT Setting: Uddevalla, Sweden Timing: November 1998–January 2002 Interventions: open subacromial decompression vs ASD vs physiotherapy Sample size: the study design was planned to include 40 participants in each treatment group. A 10‐point difference in Constant score was considered to be of clinical importance. A SD of 15, significance level at P < 0.05 and a power level of 80% give an estimated sample size of 36 per group Analysis: per protocol analysis | |
| Participants |
Number of participants Number of screened unclear 95 eligible 87 randomised (24 open surgery; 29 arthroscopic surgery; 34 physiotherapy) Data available for 15 (63%) for open surgery; 19 (66%) for arthroscopic surgery; for physiotherapy 21 (62%) at mean follow‐up of 31 months 20 (83%) for open surgery, 18 (62%) and 28 (82%) at mean follow‐up of 13.9 years Inclusion criteria
Exclusion criteria
Baseline characteristics Open surgery group: Mean (SD) age: 52 (9.5) years Number (%) female: 8 (53) Duration of symptoms: 6‐12 months: 2; 13‐36 months: 7; > 36 months 6 Mean (SD) Constant score: 48 (16) Mean (SD) SF‐36 scores: physical functioning 57.3 (13.1); role physical 23.3 (35.9); bodily pain 30.9 (11.8); global health 69.6 (27); vitality 49.3 (26.8); social functioning 80.8 (25.8); role emotional 71.1 (27.5); mental health 74.3 (21.4) Arthroscopic surgery group: Mean (SD) age: 49 (8.9) years Number (%) female 12 (63) Duration of symptoms: 6‐12 months: 2; 13‐36 months: 8; > 36 months 9 Mean (SD) Constant score: 56 (11) Mean (SD) SF‐36 score: physical functioning 61.2 (15.6); role physical 24.7 (33.9); bodily pain 31.9 (15.1); global health 60.1 (22.8); vitality 43.3 (23); social functioning 68.2 (22.5); role emotional 37.9 (44.5); mental health 65.2 (24.8) Exercise group Mean (SD) age: 50 (9.3) years Number (%) female 8 (38) Duration of symptoms: 6‐12 months: 0; 13‐36 months: 7; > 36 months: 14 Mean (SD) Constant score: 56 (13) Mean (SD) SF‐36 scores: physical functioning 69.7 (11.1); role physical 18.6 (25.2); bodily pain 33.2 (14.5); global health 67.3 (21.8); vitality 44.1 (17.2); social functioning 76.1 (18.6); role emotional 63.6 (38.9); mental health 73.5 (20.5) |
|
| Interventions | Open surgery group The procedure started with an anterior, lateral 5‐cm skin incision. The deltoid muscle was split and detached from the anterior third of the acromion and the AC joint capsule. After exposing the anterior edge of the acromion, the tendinous anterior third of the acromion was elevated dorsally prior to removing bone. This manoeuvre exposed the coracoacromial ligament. An osteotome was used to remove the anterior edge and the lateral portion of the undersurface of the acromion. The removed bone included the attachment of the coracoacromial ligament. The piece of bone was about 6–9 mm wide and 20 mm long. Proximal to the coracoid, the coracoacromial ligament was cut. Palpation of the undersurface of the acromion was performed to detect any fragments of bone or prominences. The undersurface of the AC joint was palpated and inspected. If osteophytes were present, they were excised. No AC joint resections were performed. Finally, the medial flap of the deltoid was sutured to the capsule of the AC joint, and the lateral flap was sutured to the origin of the deltoid before closure of the wound. Arthroscopic surgery group A traction device was applied to the arm, and a tension to the arm corresponding to 40 N was applied. The shoulder was in 10° of flexion and 40° of abduction. The bony landmarks of the shoulder (the acromion, the clavicle, the AC joint, the coracoid and the coracoacromial ligament) were marked with a pen. A portal for the arthroscope was created on the dorsal side of the shoulder. The GHJ was first evaluated for cartilage changes, disorder of the biceps tendon, labrum and the rotator cuff. Using the same arthroscopic portal, the subacromial space was visualised and a bursectomy was performed with a shaver introduced from a lateral portal. A resection of the anterior edge of the acromion of about 5–8 mm was then carried out, followed by a resection of about 5–8 mm of the anterior–inferior third of the undersurface of the acromion all the way to the AC joint. Postoperative rehabilitation was similar to physiotherapy group. Exercise group The purpose of the physiotherapy treatment was to let the participants find their normal kinematics of the shoulder, without experiencing pain. The gravitational forces on the arm were removed by suspending the arm in a sling fixed to the ceiling. The training programme started with rotational movements of the arm. As soon as the participant was able to perform these motions without pain, flexion/extension movements were added, followed by abduction/adduction exercises. The training programme postulates everyday practice of at least 60 min. The load was gradually increased in order to strengthen the rotator cuff and the scapula‐stabilising muscles. In the final stage of the programme, the participants replaced some exercises with corresponding leisure activities. The programme was performed twice a week under the supervision of a physiotherapist and the rest of the days at home for a period of 3‐6 months. In order to secure similar treatment, all the participants were trained at 5 local physiotherapy centres by physiotherapists using the same standardised protocol | |
| Outcomes | Outomes were assessed at mean of 30.7 months and 13.9 years after the start of the therapy
Primary outcome
Secondary outcomes
Outcomes included in this review
|
|
| Source of funding | One or more of the authors has declared the following potential conflict of interest or source of funding: remuneration for lecturing for Linvatec | |
| Notes |
Trial registration: not registered Data analysis: 31‐month data was used in > 12‐month time point. We combined open and arthroscopic surgery groups to 1 surgery group because the method of decompression was irrelevant in this review (Analysis 2.2; Analysis 5.4). Withdrawals: at 31 months 1 participant had died, 1 participant missing data and 1 participant no follow‐up in open surgery; 2 participants missing values and 2 participants no follow‐up in arthroscopic surgery group; 6 participants missing data and 4 participants no follow‐up in exercise group Cross‐overs: trial authors excluded participants who crossed over to surgery from exercise group or did not have surgery in surgery groups. In surgery groups, 11/53 (21%) declined operation and in exercises 3/34 (9%) were operated. AEs: not reported SAEs: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No random sequence generation was described. Quote: "One hundred and twenty envelopes were placed in four boxes: males <55 years, males ≥55 years, females <55 years and females ≥55 years. Each box contained 30 envelopes, 10 for each treatment group." |
| Allocation concealment (selection bias) | High risk | Quote: "The patients were asked to choose one envelope from the box corresponding to their age and gender." Comment: It is unclear if the envelopes were in random order or if the envelopes were adequately concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or personnel was attempted |
| Blinding of outcome assessment for self‐reported outcomes including pain, function and global assessment (detection bias) | High risk | Participants were aware of their treatment allocation |
| Blinding of outcome assessment for outcome assessor reported outcomes (detection bias) | Low risk | Participants wore T‐shirts to hide the scars from outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data from 9/24 in the open surgery group, 10/29 in the arthroscopic and 13/34 in the exercise group were not included in the analysis (as only a per‐protocol analysis was performed) |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. It is unclear if the trial authors reported all measured outcomes |
| Other bias | High risk | There was imbalance in the number of participants across the 3 treatment arms that was not explained. Recruitment was stopped early due to recruitment difficulties |
Haahr 2005.
| Methods | Design: single‐centre, parallel, 2‐arm, RCT Setting: public hospital, surgery and physiotherapy departments in Denmark Timing: 1996‐2001 Interventions: ASD + exercise vs exercises alone Sample size: sample size was set at a minimum of 40 participants in each group based on an expected improvement of 30% in the physiotherapy group (mean (SD) expected baseline Constant score, 55 (14), an α set at 0.05 (type I error), and ß at 0.20 (type II error), and a MCID of 50% between the 2 groups in favour of surgery (corresponding to 9‐10 points in Constant score). Thus, a priori, trialists intended to include 100 participants in expectation of a number of dropouts Analysis: ITT analysis | |
| Participants |
Number of participants Number screened not available 90 participants randomised (45 to surgery; 45 to exercise therapy) 41 received surgery; 43 received exercise therapy Data available for 41 for surgery group (91%) and 43 (96%) for exercise group at 12 months Inclusion criteria
Exclusion criteria:
Baseline data Surgery (ASD n = 41) Mean (SD) age: 44.3 (8.3) years Number (%) female: 29 (71%) Number (%) labour compensation claim: 29 (71%) Number (%) received passive physiotherapy: 24 (59%) Number (%) received active physiotherapy: 14 (34%) Number (%) on NSAID: 20 (49%) Number (%) on sick leave: 34 (83%) Number (%) bilateral symptoms: 14 (34%) Duration of symptoms: < 6 months 4; 6‐12 months 3; > 12 months 34 Mean (SD) Constant score 33.7 (14.7) Mean (95% CI) worst pain in past 3 months: 7.6 (7.2‐8.1) Mean, (95% CI) average pain past 3 months: 5.8 (5.3‐6.3) Mean, (95% CI) average pain past 7 days: 5.9 (5.2‐6.6) Mean (95% CI) impaired activity: 6.5 (5.7‐7.2) Mean (95% CI) PRIM score: 25.8 (23.9‐28.8) Exercise group Mean (SD) age: 44.5 (7.9) years Number (%) female: 29 (67%) Number (%) labour compensation claim: 32 (74%) Number (%) received passive physiotherapy: 29 (67%) Number (%) received active physiotherapy: 17 (40%) Number (%) NSAID: 20 (47) Number (%) on sick leave: 27 (63) Number (%) bilateral symptoms: 16 (37) Duration of symptoms: < 6 months 3; 6‐12 months 10; > 12 months 29 Mean (SD): Constant score: 34.7 (14.4) Mean (95% CI) worst pain in past 3 months: 7.3 (6.9‐7.7) Mean, (95% CI) average pain past 3 months: 6.0 (5.5‐6.5) Mean, (95% CI) average pain past 7 days: 6.5 (5.9‐7.0) Mean (95% CI) impaired activity: 6.2 (5.6‐6.7) Mean (95% CI) PRIM score: 25.8 (24.1‐27.5) |
|
| Interventions | 2 experienced therapists supervised the exercise treatments and 2 experienced surgeons undertook all procedures Surgery (ASD) Investigation of shoulder stability, followed by ASD (bursectomy with partial resection of the antero‐inferior acromion and coracoacromial ligament). After surgery, physiotherapist instructed increasingly active exercises including exercises for strengthening the rotator cuff muscles. Exercises 19 sessions of 60 min 3 times/week for 2 weeks, twice a week for 3 weeks and once a week for 7 weeks. Treatment consisted of heat and cold packs or soft tissue treatments, followed by active training of the periscapular muscles and strengthening of the stabilising muscles of the shoulder joint |
|
| Outcomes | The outcomes were assessed at baseline, 3, 6, 12 months and 4‐8 years after randomisation
Primary outcome
Secondary outcomes
Outcomes used in this review
|
|
| Source of funding | Medical Research Unit of Ringkjoebing County, Denmark | |
| Notes |
Trial registration: not available Data analysis: average pain and discomfort in past 7 days was measured in 0‐9 scale. We transformed the score to 0‐10 scale before analyses (Thorlund 2011). Trial authors did not report PRIM at 3 months and 6 months, so we used Constant pain score, which was reported at those time points (Analysis 2.1) Withdrawals: in exercise group, 1 withdrew from participation because of work problems. In the surgery group, 4 participants withdrew before the start of the study (1 because of work problems, 1 with a tumour in the humerus, 1 because his wife advised against participation, and 1 for unknown reasons) Cross‐overs: in the exercise group, 6 participants were operated on within the 12 months of the study (5 because of unsatisfactory improvement during exercises and in 1 case because a labral lesion was suspected) AEs: not reported SAEs: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer program was used to generate a random sequence of allocation." |
| Allocation concealment (selection bias) | Low risk | Quote: "The same specialist (SØ)... randomised the patients into one of two intervention groups by opening a sealed envelope containing the result of randomisation, which was unknown to SØ." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and study personnel were not blinded |
| Blinding of outcome assessment for self‐reported outcomes including pain, function and global assessment (detection bias) | High risk | Participants were aware of their treatment allocation |
| Blinding of outcome assessment for outcome assessor reported outcomes (detection bias) | High risk | Outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/45 (11%) in surgery group and 2/45 (4%) in exercise group dropped out or were lost to follow‐up by12 months, which probably did not bias the outcomes. |
| Selective reporting (reporting bias) | High risk | No trial protocol in trial registry. Some outcomes have been added post hoc and some are incompletely reported. AEs were not reported, unclear if they were measured |
| Other bias | Low risk | 6/42 participants in the exercise group received decompression before 12 months' follow‐up and 11/42 before final 4‐8 years' follow‐up. However the analysis was performed on an ITT basis irrespective of whether or not the allocated treatment was received |
Ketola 2009.
| Methods | Design: 2‐centre, parallel, 2‐arm, RCT Setting: 2 public hospitals in Finland Timing: June 2001‐July 2004 Interventions: ASD + exercise versus exercise therapy alone Sample size: power calculations were performed using self‐reported pain (0‐10 scale VAS) at 24 months as the outcome measure. Using 1.5 (SD 2.5) as a clinically important between‐group difference, the sample size was estimated to be 45 participants per group, if 5% type I (α) and 20% type II (β) errors were allowed. As the SD of the outcome measure was only a rough estimate, 70 participants were included in both groups Analysis: ITT analysis | |
| Participants |
Number of participants Number screened not reported 140 eligible 140 randomised (70 to decompression + exercise and 70 to exercise alone) Data available for 99 (43/70 (61%) for surgery and 56/70 (80%) for exercises) at 6 months; 113 (51/70 (73%) for surgery and 62/70 (89%) for exercises) at 12 months; 134 (68/70 (97%) for surgery and 66/70 (94%) for exercise group) at 2 years; 109 (52/70 (74%) for surgery and 57/70 (81%) for exercise group) at 5 years; and 90 (44 (63%) for surgery and 46 (66%) for exercise group) at 10 years Inclusion criteria
Exclusion criteria
Baseline data Surgery group (ASD) Mean (range) age: 46.4 (23.3‐60.0) years Number (%) of female: 47 (67%) Mean (range) BMI: 27.4 (19.5 – 46.3) Dominant hand affected n (%): 45 (64%) Duration of symptoms, years (range): 2.6 (0.25–20) Mean (range) pain VAS: 6.5 (1‐10) Mean (range) night pain: 6.2 (0–10) Mean (range) disability: 6.2 (1–10) Mean (range) working ability (range): 5.7 (0–9) Mean (range) SDQ score (range): 78.0 Exercise group Age, mean (range): 47.8 (26.8‐59.2) years Number (%) female: 41 (59%) Mean (range) BMI: 27 (15.2‐41.2) Dominant hand affected: 46 (66%) Duration of symptoms, years (range): 2.5 (0.25–17) Mean (range) pain VAS: 6.5 (1.0–10) Mean (range) night pain: 6.4 (0–10) Mean (range) disability (range): 6.5 (2–10) Mean (range) working ability (range): 5.9 (0–9) Mean (range) SDQ score: 82.5 |
|
| Interventions | 1 surgeon performed all operations Surgery (ASD) Acromioplasty + ASD and debridement +/‐ coracoacromial ligament release (participants with thick or tight ligament). Supervised exercise treatment, overnight in hospital, ibuprofen, collar+cuff, mobilisation permitted and free active movement. After 7‐10 days participants commenced on similar programme as exercise group with 6 physiotherapy visits Exercise therapy group Supervised exercise treatment (physiotherapist), individual home programme, sessions 4 times/week, 9 different exercises with 30‐40 repetitions 3 times. 7 control visits by physiotherapist. As self‐assessed ability and strength increased, repetitions diminished. NSAIDs were permitted. Subacromial corticosteroid injections were permitted in both groups if pain interfered with the exercise programme |
|
| Outcomes | Outcomes were assessed at baseline, 3, 6, 12, 24 months, 5 years and > 10 years.
Primary outcome
Secondary
Outcomes used in this review
|
|
| Source of funding | Not stated | |
| Notes |
Trial registration: not available Data analysis: original reports do not include 3‐12 months' results but the trial authors provided pain and SDQ score upon request. We inverted SDQ before entering the data (so that higher indicated better). 15D data at 5 years also received from the trial authors. Imbalance in available data between groups at 6 months, probably due to delay in surgery (data not reported for participants not operated before follow‐up point) Withdrawals: in surgery group, 2/70 (3%); In exercise group, 3/70 (4%) Cross‐overs: in surgery group, 13 (19%) cancelled operation: 6 because lack of symptoms; 2 due to work; 1 fear of operation, 2 other reasons, 1 withdrew, 1 underwent manipulation only. 9 (13% also received labral repair during the operation). 14 (20%) participants in the exercise group received surgery. Over the 2‐year follow‐up a mean of 0.3 (range 0‐3) and 1.0 (range 0‐10) glucocorticoid injections were given to the surgical and exercise groups AEs: no major surgical complications reported, AEs in exercise group not specifically reported SAEs: no major surgical complications |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation (using 14 as the block size) |
| Allocation concealment (selection bias) | Low risk | Computer‐generated numbers sealed in opaque envelopes prepared by an independent statistician not otherwise involved in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither participants nor study personnel were blinded |
| Blinding of outcome assessment for self‐reported outcomes including pain, function and global assessment (detection bias) | High risk | Participants were not blinded |
| Blinding of outcome assessment for outcome assessor reported outcomes (detection bias) | Low risk | A single independent physiotherapist was blinded to treatment allocation as participants wore T‐shirts to cover scars and were asked not to reveal any information regarding their allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were missing data at 3, 6 and 12 months and the proportions differed between groups at 3 and 6 months (3 months: 27/70 (39%) in surgery group and 13/70 (19%) in the exercise group; 6 months: 26/70 (37%) in surgery group and 14/70 (20%) in exercise group; 12 months: 19/70 (27%) in surgery group and 18/70 (26%) in exercise group; 24 months: 2/70 (3% ) in the surgery group and 4/70 (6%) in the exercise group. No reasons for missing data were reported |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Trial authors reported only 24‐month, 5‐year and 10‐year results in the paper but we received pain in VAS and SDQ‐score at 3, 6 and 12 months from the trial authors. Trial authors stated that there were no major surgical complications but AEs in the exercise arm were not reported. Passive movement and strength were measured but not reported. |
| Other bias | Unclear risk | 9 (13%) participants in the surgery group also received labral repair during the operation, which was an unplanned co‐intervention and may have biased the estimate of the effect of surgery (in either direction). Both treatment groups received glucocorticoid injections over the 2‐year follow‐up (mean 0.3 (range 0‐3) and 1.0 (range 0‐10) glucocorticoid injections in the surgical and exercise groups). This may also have biased the estimate of the effect of surgery. The exercise group 14/70 (20%) had decompression by 24 months. In the surgery group, 13 (18%) did not receive planned surgery. However the analysis was performed on an ITT basis irrespective of whether or not the allocated treatment was received. |
Paavola 2018.
| Methods | Design: multicentre, 3‐group, randomised, assessor‐ and patient‐blinded, placebo‐controlled trial Setting: 3 hospitals in Finland Timing: 1 February 1 2005‐25 June 2015 Interventions: ASD vs placebo (arthroscopy only) vs exercise therapy Sample size: trial authors powered the study to detect a difference of at least the MCID (15 points) in the 2 primary outcomes between the decompression and placebo groups. For the study to have 90% power to show a minimal clinically important advantage of decompression over placebo, under the assumption of a 2‐sided type 1 error rate of 5%, study planned to recruit 70 participants per group. Analysis: ITT analysis | |
| Participants |
Number of participants 281 participants screened for eligibility 71 excluded before randomisation: rotator cuff tear 47; AC‐joint osteoarthritis 2; calcifications 2; became asymptomatic while waiting MRA 7; MRA could not be performed 4; declined 3; other intra‐articular pathology 6 210 randomised (71 exercise therapy; 139 to surgery) 5 excluded before surgery (AC‐joint osteoarthritis 2; declined 2; not suitable for outpatient operation 1) 12 excluded during arthroscopy (full‐thickness rotator cuff tear 6; slap or long head of biceps 5; Instability 1) 122 randomised after arthroscopy (63 to placebo and 59 to ASD) Data for 186 (68 (96%) for exercise therapy and 59 (94%) for placebo and 59 (100%) for ASD groups available) at 24‐month follow‐up Inclusion criteria
Exclusion criteria
Baseline characteristics ASDgroup Mean (SD) age: 50.5 (7.3) Sex female N(%): 42 (71%) Mean (SD) duration of symptoms, months: 18 (14) Mean (SD) VAS at rest: 41.3 (25.8) Mean (SD) VAS activity: 71.2 (23.6) Mean (SD) Constant score: 32.2 (15.8) Mean (SD) Simple shoulder test score: 4.9 (2.9) Mean (SD) 15D score: 0.89 (0.06) Placebo group (Arthroscopy and exercises) Age, mean (SD): 50.8 (7.6) Sex female N(%): 46 (73%) Mean duration of symptoms, months (SD): 18 (19) Mean (SD) VAS at rest: 41.6 (25.5) mean (SD) VAS at activity: 72.3 (21.7) Mean (SD) Constant‐Murley Score: 31.7 (14) Mean (SD) Simple shoulder test score: 4.9 (2.9) Mean (SD) 15D score: 0.89 (0.07) Exercise therapy Mean (SD) Age: 50.4 (6.6) Sex female N(%): 47 (66%) Mean (SD) duration of symptoms, months: 22 (23) Mean (SD) VAS at rest: 41.7 (27.5) Mean (SD) VAS activity: 72.4 (20.8) Mean (SD) Constant‐Murley Score: 35.2 (16.2) Mean (SD) Simple shoulder test score: 4.8 (2.7) Mean (SD) 15D score: 0.88 (0.08) |
|
| Interventions |
Placebo surgery group
Arthroscopic examination of the GHJ and subacromial space was performed with the use of standard posterior and lateral portals and a 4‐mm arthroscope with the participant under general anaesthesia, usually supplemented with an interscalene brachial plexus block. The surgeon performed an intra‐articular and subacromial assessment of the rotator cuff integrity. If the rotator cuff insertion could not be otherwise visualised, subacromial bursal tissue was bluntly stretched with a trochar or resected, keeping the resection to a minimum. If arthroscopic examination revealed any pathology requiring intervention other than decompression, the participant was excluded from the trial. For those allocated to the placebo group, the operation was terminated. The placebo participants were kept in the operating theatre for the time required to perform ASD. In both the ASD and the placebo groups, the postoperative rehabilitation was identical. All surgically treated participants had 1 visit to an independent physiotherapist for guidance and instructions for home exercises. Subsequent rehabilitation was carried out according to the standardised rehabilitation protocols of the participant centres. Since the initial rehabilitation after a surgery needed to be ‘tempered’ due to joint irritation, the rehabilitation protocol of the placebo and arthroscopic decompression groups was not identical to the exercise alone group ASD group After arthroscopic examination of the shoulder (i.e. diagnostic arthroscopy as described above), the surgeon debrided the entire subacromial bursa (bursectomy) and resected the bony spurs and the projecting anterolateral undersurface of the acromion. The removal of tissue was carried out with a shaver, burr and/or electrocoagulation Exercise therapy group Supervised, progressive, individually‐designed physiotherapy was started within 2 weeks of randomisation using a standardised protocol that relied primarily on daily home exercises as well as 15 visits to an independent physiotherapist. Programme was divided into 4 phases each consisting of active and passive exercises |
|
| Outcomes | Outcomes were collected at 3, 6, 12 and 24 months. Study primary endpoint was at 24 months
Primary outcomes
Secondary outcomes
Outcomes used in this review
|
|
| Source of funding | The trial was supported by the Sigrid Juselius Foundation, the State funding for university‐level health research (Tampere and Helsinki University Hospitals), the Academy of Finland, and the Jane and Aatos Erkko Foundation. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. Sponsors had no access to the data and did not perform any of the study analysis. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. | |
| Notes |
Trial registration: ClinicalTrials.gov NCT00428870 Data analysis: our primary analysis was decompression versus placebo (Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4; Analysis 1.7). In surgery vs exercises we used surgery and exercise therapy (not masked) group (Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 2.4; Analysis 2.8. Satisfaction was assessed with a Likert scale and VAS scale. Regarding Likert scale, the trial authors report adjusted percentages. The trial authors provided unadjusted values upon request and we used them in global assessment of success (Analysis 1.3; Analysis 2.3) The trial authors measured pain in 0‐100 scale; we transformed values to 0‐10 scale. Adjusted values reported in publication for pain, function and HRQoL and we extracted from relevant comparison (from decompression vs placebo comparison in Analysis 1.1; Analysis 1.2; Analysis 1.4; from ASD vs exercise therapy in Analysis 2.1; Analysis 2.2; Analysis 2.4). Regarding function, we used 24‐month results in 12 months in Analysis 1.2 Withdrawals: of 139 originally allocated to surgery arm, 17 were excluded before the 2nd randomisation (to either decompression or placebo) due to findings at arthroscopy. In the exercise group, 3/71 (4%) participants withdrew; in placebo surgery group 2/63 (3%) participants withdrew; and in ASD group 1 died. Cross‐overs: 9 (14%) participants in placebo group were unblinded before 24 months' follow‐up; 8 of them received surgery (7 ASDs and 1 ASD and rotator cuff repair. 6 (10%) participants in the ASD group were unblinded before 24‐month follow‐up, of whom 2 were re‐operated (1 resection of distal head of clavicle and 1 manipulation under anaesthesia) 15 (21%) participants in the exercise group had surgery before 24 months' follow‐up (13 ASD; 1 ASD and manipulation; 1 ASD + resection of distal head of the clavicle; 1 ASD and arthroscopic capsular release) AEs: in placebo surgery group, 1 participant had temporary swelling in the brachial area related to a brachial plexus block. 3 participants who received decompression and 1 participant who received placebo developed symptoms consistent with a frozen shoulder. SAEs: none Risk of bias: we separately note the risk of bias for the 2 surgery groups and the exercise group in the 'Risk of bias' table and review authors' judgements are for the comparison of the 2 surgical groups (decompression and placebo). Trial authors provided SF‐36 data that were not reported in the results paper upon request. There were no between‐group differences at any time point. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent statistician with no involvement in the execution of the trial prepared separate randomisation lists for each study centre using a computer‐generated algorithm. Block randomisation of varying sizes were used |
| Allocation concealment (selection bias) | Low risk | The envelopes were sealed and opaque and were kept in a secure, agreed location at each centre. The first allocation occurred after inclusion (surgery or exercise) and the second allocation (placebo or ASD) occurred in the operating theatre once the arthroscopy was performed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "To ensure concealment of the participants and the staff other than those in the operating theatre, the participants were kept in the operating theatre for the required time to perform ASD. Personnel other than the surgical team were blinded to treatment intervention. All postoperative care was identical in both groups." Participants in the exercise group alone were aware of their treatment allocation (high risk). At 3 months, 42% in the placebo surgery group and 39% in the decompression group believed they had received placebo treatment. |
| Blinding of outcome assessment for self‐reported outcomes including pain, function and global assessment (detection bias) | Low risk | Participants in both surgery groups were blinded to treatment allocation. 9/63 participants in the placebo surgery group and 6/59 in the decompression group requested unblinding by 24 months. Participants in the exercise group were not blinded so assessment of treatment in this group was at high risk of bias. |
| Blinding of outcome assessment for outcome assessor reported outcomes (detection bias) | Low risk | Assessors were blinded to treatment allocation for all 3 treatment groups. Outcome assessors were instructed not to ask about treatment received. Participants wore a t‐shirt on all follow‐up examinations |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were low and comparable between the groups: for pain and function 0‐4 participants in the decompression group (0%‐7%); 2‐7 participants (3%‐11%) in placebo surgery; and 3‐7 participants (4%‐10%) in exercise therapy group in follow‐up points up to 24 months |
| Selective reporting (reporting bias) | Low risk | The results for the SF‐36 were not reported in the publication but were pre‐specified in the protocol. Trial authors provided these data upon request. |
| Other bias | Low risk | 2/63 participants in the placebo group had decompression by 6 months, 8/63 by 12 months and 8/63 by 24 months. This may have underestimated any potential benefit of decompression. The trial authors excluded 17 participants in the operative arm due to findings at arthroscopy, which caused imbalance between the decompression and exercise treatment groups. |
Peters 1997.
| Methods | Design: RCT Setting: single‐centre study in Germany Timing: not reported, published 1997 Interventions: ASD or open subacromial decompression vs physiotherapy Sample size: 72 participants, no power analysis reported Analysis: unclear; likely per protocol | |
| Participants |
Number of participants Participants screened for eligibility: not reported Participants randomised: not explicitly reported (40 in exercise therapy and 32 in surgery group analysed) Data available for 62 (26/32 (81%) in surgery group and 36/40 (90%) in exercise group) at 1 year; 71 (32/32 (100%) in surgery group and 39/40 (98%) in exercise group) at 2 years; 65 (28/32 (88%) in surgery group 37/40 (93%) in exercise group) at 3 years; 48 (23/32 (72%) in surgery group and 25/40 (63%) in exercise group) at 4 years Inclusion criteria
Exclusion criteria
Baseline characteristics Surgery group Mean (range) age: 56 (37‐78) years Sex female n (%): 14 (44%) Median Constant score: 54 Exercise therapy group Mean (range) age: 59 (37‐82) years Sex female n (%): 12 (30%) Median Constant score: 59 |
|
| Interventions |
ASD group Depending on the preference of the respective surgeon the operation was performed either arthroscopically (15 cases) or open (17 cases). Postoperatively, a Gilchrist dressing was applied. From this bandage, movement exercises were started beginning on the day of the operation. From the 4th postoperative week strengthening exercises were added against resistance. Exercise therapy group All participants were hospitalised for 2 weeks to perform the conservative treatment. A treatment programme with intensive physical therapy was performed. NSAIDs (e.g. ibuprofen 2 × 400 mg) were given as support, provided that there were no gastrointestinal problems. Furthermore, ≤ 3 glucocorticoid injections (triamcinolone 5 mg in 10 mL physiological NaCl solution) were also given into the subacromial space. |
|
| Outcomes | The outcomes were measured at 1, 2, 3 and 4 years Outcomes
Outcomes used in this review
|
|
| Source of funding | Not reported | |
| Notes | This paper was translated from German. Trial registration: not registered Data analysis: the Shoulder Rating Scale score was reported in medians without variance. We requested mean and SD from the trial author who responded but did not have the data stored. We assumed normal distribution and used medians as means and imputed SD (15.9) from Paavola 2018. We used 4‐year data at 5‐year time point (Analysis 2.2) Withdrawals: data missing in surgery group: 6/32 (19%) at 1 year, 0/32 (0%) at 2 years, 4/32 (13%) at 3 years and 9/32 (28%) at 4 years; and in exercise group: 4/40 (10%) at 1 year, 1/40 (3%) at 2 years, 3/40 (8%) at 3 years, 15/40 (38%) at 4 years Cross‐overs: none reported. AEs: none reported, unclear if they were measured SAEs: none reported, unclear if they were measured |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were pre‐randomised to two groups". Comment: the sequence generation method was not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel was not reported and we assumed that it was not attempted |
| Blinding of outcome assessment for self‐reported outcomes including pain, function and global assessment (detection bias) | High risk | The participants were not blinded |
| Blinding of outcome assessment for outcome assessor reported outcomes (detection bias) | Low risk | No outcome‐assessor reported outcomes were reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if participants dropped out before first follow‐up and that caused imbalance between the groups. Data missing in surgery group: 6/32 (19%) at 1 year, 0/32 (0%) at 2 years, 4/32 (13%) at 3 years and 9/32 (28%) at 4 years; and in exercise group: 4/40 (10%) at 1 year, 1/40 (3%) at 2 years, 3/40 (8%) at 3 years, 15/40 (38%) at 4 years. Reasons for missing data were not given |
| Selective reporting (reporting bias) | High risk | No protocol available to confirm if any measures other than the Subjective Shoulder Rating score were collected. Only medians were reported without measures of variance |
| Other bias | Low risk | No other biases apparent |
Rahme 1998.
| Methods | Design: RCT Setting: orthopedic department in a public hospital in Sweden Timing: 1986‐1988 Interventions: open subacromial decompression versus physiotherapy Sample size: 42 participants, power analysis not reported Analysis: as‐treated analysis | |
| Participants |
Number of participants Participants screened for eligibility: not reported 42 randomised (21 to exercise therapy; 21 to surgery) Data for 39 (18 (86%) for exercise therapy and 21 (100%) for ASD) at 6‐month and 12‐month follow‐up points Inclusion criteria
Exclusion criteria
Baseline characteristics Group data not reported separately Mean age (range): 42 (28‐63) years female n (%): 23 (55%) |
|
| Interventions |
ASD Open anterior acromioplasty (Neer technique) with any portion of the acromion which extended beyond the anterior border of the clavicle being osteotomised vertically before removing the area of the anteroinferior surface of the acromion; followed by a physiotherapy regime including exercise and education, starting about 3 months after surgery Exercise therapy The physiotherapy regime was based mainly on the principles of Bohmer: information to the participant on functional anatomy and biomechanics of the shoulder; advice on how to avoid positions for 'wear and tear' of the subacromial structures; unloaded movements of the shoulder; measures to normalise the scapulohumeral rhythm and to increase postural awareness; strengthening of the shoulder muscles and endurance training. Submaximal training of the rotator cuff was started about 3 months after the operation in group 1 and when pain had subsided in group 2. Initially all participants were seen 2‐3 times per week and the intervals between treatments were successively increased as they became more familiar with the object of the exercises. |
|
| Outcomes | The outcomes were measured at 8 weeks, 16 weeks, 6 months and 12 months Outcomes
Outcomes used in this review
|
|
| Source of funding | Not reported | |
| Notes |
Trial registration: not registered Data analysis: pain values or variance not reported. We requested absolute values from the trial author, but did not receive additional data. We included data for the participants in the exercise group who received surgery in the exercise group for the purpose of meta‐analysis (only data for participant global success available from the published report). Withdrawals: 3 participants in exercise group dropped out Cross‐overs: participants were allowed cross‐over from exercise to surgery after 6 months. In exercise group, 12 (57%) participants were operated before 1‐year follow‐up. 6 participants in surgery group had full‐thickness tears, which were repaired in conjunction with ASD AEs: none reported, unclear if they were measured SAEs: none reported, unclear if they were measured |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The participants were randomised to 2 treatment groups using blocked randomisation. Sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants and personnel were likely not blinded to treatment allocation (not explicitly reported) |
| Blinding of outcome assessment for self‐reported outcomes including pain, function and global assessment (detection bias) | High risk | The participants were not blinded |
| Blinding of outcome assessment for outcome assessor reported outcomes (detection bias) | Low risk | There were no outcome assessor‐reported outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3/21 (14%) participants dropped out from the exercise group and reasons were not provided |
| Selective reporting (reporting bias) | High risk | The outcomes specified in the methods were not reported consistently and at all time points. Only 6‐month and 12‐month results for pain were reported |
| Other bias | High risk | 3/21 (14%) participants had full‐thickness tears identified at arthroscopy in the operative group while the number of participants with similar tears in the exercise group is unknown. 12/21 (57%) of participants originally allocated to exercises crossed over to surgery after 6 months. The trial authors analysed them as a separate group (not an ITT analysis). |
AC: acromioclavicular; ADL: activities of daily living; AE: adverse event; ASD: arthroscopic subacromial decompression; GHJ: glenohumeral joint;HADS: Hospital Anxiety and Depression Scale; HRQoL: health‐related quality of life; ITT: intention‐to‐treat; MCID: minimum clinically important difference; MRA: magnetic resonance arthrography; MRI: magnetic resonance imaging; NRS: numeric rating scale; NSAID: non‐steroidal anti‐inflammatory drug; PRIM: Project on Research and Intervention in Monotonous work score: QoL: quality of life; RCT: randomised controlled trial; ROM: range of motion; SAE: serious adverse event; SD: standard deviation; SDQ: Shoulder Disability Questionnaire score; VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bjornsson 2017 | Not the relevant intervention. Comparing a “specific and unspecific exercise group” |
| Hallgren 2014 | Not the relevant intervention. Arthroscopic patient population (awaiting arthroscopic subacromial decompression) randomised to specific or unspecific exercise programme and then provided the option of surgery. Specific exercise programme versus unspecific exercise programme (control). |
| Itoi 2016 | Non‐randomised study. |
| Kukkonen 2014 | Not relevant population; includes participants with tears |
| Kukkonen 2015 | Not relevant population; includes participant with full‐thickness tears |
| Kweon 2015 | Non‐randomised study. A “prospective cohort study” |
| Lamas 2015 | Not the relevant population or intervention. “Collagen type1 implant” versus “collagen type 1 membrane combined with autologous bone marrow‐MSCs”. |
| Lambers Heerspink 2015 | Not relevant population; includes participants with tears |
| Maugars 2009 | Not relevant population; includes participants with tears |
| Mohtadi 2006 | Not a randomised study. Commentary on Haahr 2005 |
| Moosmayer 2010 | Not relevant population; includes participants with tears |
| Moosmayer 2017 | Not the relevant intervention. “Ultrasound guided needling and lavage (barbotage) with steroid injection versus sham barbotage with and without steroid injection” |
| Saggini 2010 | Non‐randomised study. 2 groups were formed “in relation to the choice of each subject to undergo conservative treatment or the surgical one” |
Characteristics of studies awaiting assessment [ordered by study ID]
Schulze 2017.
| Methods | Prospective clinical comparative study |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting translation |
TRANSIT 2006.
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention group ASD performed within 6 weeks after randomisation Control group "Usual medical care", which consists of treatment in general practice according to the Guidelines for Shoulder Complaints of the Dutch College of General Practitioners (issued in 1999). Both groups will be followed for 1 year post‐randomisation |
| Outcomes | Study data will be collected at inclusion, at randomisation, and 3, 6 and 12 months after randomisation Primary
Secondary
|
| Notes | Estimated completion date: November 2008 Status is "planned" . Target recruitment = 70 participants A brief Internet search showed no results or publications associated with this study at this time Above information was copied from the WHO ITCRP database, last updated on 21 July 2014: apps.who.int/trialsearch/Trial2.aspx?TrialID=NTR586 Recruitment finished, no results available. Contacted the trial authors by email, no response by 7 June 2018 |
ASD: arthroscopic subacromial decompression; NSAID: nonsteroidal anti‐inflammatory drug; RCT: randomised controlled trial; SDQ: Shoulder Disability Questionnaire
Characteristics of ongoing studies [ordered by study ID]
Paloneva 2008.
| Trial name or title | Operative versus non‐operative management of subacromial impingement |
| Methods | Randomised trial |
| Participants |
Inclusion criteria
Exclusion criteria
Age minimum: 35 Years Age maximum: N/A Gender: both |
| Interventions | Acromioplasty Physiotherapy |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | June 2008 |
| Contact information | Ilkka Kiviranta, MD, PhD Helsinki University |
| Notes | Target sample size: 100 Status: active, not recruiting Above information copied from ClinicalTrials.gov database 18 June 2018. Last refreshed 7 September 2018 |
AC: acromioclavicular; GHJ: glenohumeral joint;MRI: magnetic resonance imaging; N/A: not applicable
Differences between protocol and review
In the original review we extracted outcomes at all time points. For this update we planned to extract data at six months, one, two and five years. We considered the one‐year time point to be the primary time point, and thus we reported it in the 'Summary of findings' tables (unless the study did not specifically report on one‐year outcome). To harmonise the review with the parallel, rapid recommendation systematic review and meta‐analysis, we also included the three‐month (as this was requested by the rapid recommendation panel members), and 10‐year time points.
We chose the main comparison for the update as subacromial decompression surgery versus placebo surgery, the secondary comparison was surgery versus exercises and the third comparison was subacromial decompression versus no treatment. We excluded the comparison of one type of surgical technique to another in this update.
We extracted all outcomes in the original review, but restricted outcomes in this update to what we considered the most clinically important outcomes (pain, function, quality of life, participant global assessment of success, adverse events, and serious adverse events). Minor outcomes were participation and rotator cuff tears at follow‐up. For this update, we created a hierarchy for the pain and function outcomes.
Subgroup analyses: we excluded the subgroup analysis based on age (people older than 65 years compared with those aged 65 years or less), as there was no clinical reason for these subgroups and the trials did not provide the relevant separate outcome data for these trial subgroups.
Differences between the updated Cochrane Review and the co‐published parallel systematic review
The co‐published rapid review (Lähdeoja 2019) differs in the following ways:
It is up to date to 23 July 2018. No additional trials were identified in the latest search.
The Cochrane Review includes Rahme 1998, which was excluded from the rapid recommendation review due to stricter application of inclusion criteria (three participants in the surgery group had full‐thickness rotator cuff tears). Rahme 1998 reported only one outcome that could be included in the meta‐analysis (participant global assessment of treatment success), and this did not change the findings of this review (effect estimate excluding Rahme 1998 RR 1.19, 95% CI 0.92 to 1.56; including Rahme 1998 RR 1.21, 95% CI 0.96 to 1.51; Analysis 2.3).
Furthemore, the co‐published review subtracted patients who reported much worse from those reporting completely resolved or much better in their global perceived effect outcome. The estimates for risk ratios were comparable: RR 1.17, 95% CI 0.89 to 1.54 in Cohrane Review versus RR 1.04, 95% CI 0.81 to 1.34 in the co‐published review at six months; and RR 1.08, 95% CI 0.93 to 1.27 in Cochrane Review and RR 1.10, 95% CI 0.94 to 1.30 in the co‐published review at one year.
The Cochrane Review includes a comparison of subacromial decompression versus no treatment, which is not included in the co‐published review.
The two review processes were initially started separately and the co‐published review used different search strategy because of different inclusion criteria (surgical comparators to subacromial decompression were accepted); there was no difference in the included studies due to search strategy.
Contributions of authors
TK, TL, NBJ: selected trials, extracted data, interpreted results and contributed to the writing of the updated review
PS, LK and AA: study selection and data extraction of observational arthroscopic shoulder surgery registry studies
CA: data extraction and analysis of harm studies, interpretation of results
PV: interpretation of results
CP: selecting trials, data extraction and analysis and contributed to the writing of the updated review
RJ: interpreted results and contributed to writing of the updated review
RB: conceived the review, supervised the updated review and interpreted the results and contributed to writing the updated review. RB is the guarantor for the review.
Sources of support
Internal sources
Monash Department of Clinical Epidemiology, Cabrini Institute, Australia.
Department of Epidemiology and Preventive Medicine, Monash University, Melbourne, Australia.
External sources
-
Teemu Karjalainen, Finland.
TK is supported by funding from the Finnish Medical Foundation and the Finnish Centre for Evidence Based Orthopedics (FICEBO)
-
Nitin Jain, USA.
NJ is supported by funding from National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) 1K23AR059199 and 1U34AR069201
-
Rachelle Buchbinder, Australia.
RB is supported by an Australian National Health and Medicial Research Council (NHMRC) Senior Principal Research Fellowship
-
Rachelle Buchbinder and Renea Johnston, Australia.
The Cochrane Musculoskeletal Australian Editorial base receives support from the NHMRC The Cochrane Collaboration Round 7 Funding Program
-
Cochrane Musculoskeletal, Canada.
Provided editorial support for this review
Declarations of interest
TK: is one of the authors on a parallel systematic review performed to inform a BMJ Rapid Recommendation on this topic; is one of the authors on the BMJ Rapid Recommendation paper
NBJ: is supported by funding from National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) 1K23AR059199 and 1U34AR069201
CP: no known conflict of interest
TL: is one of the authors on a parallel systematic review performed to inform a BMJ Rapid Recommendation on this topic; is one of the authors on the BMJ Rapid Recommendation paper
RJ: received an NHMRC Cochrane Collaboration Round 7 Funding Program Grant, which supports the Cochrane Musculoskeletal Australian Editorial base, but the funding source did not participate in the conduct of this review.
PS: is one of the authors on a parallel systematic review performed to inform a BMJ Rapid Recommendation on this topic
LK: is one of the authors on a parallel systematic review performed to inform a BMJ Rapid Recommendation on this topic
CA: is one of the authors on a parallel systematic review performed to inform a BMJ Rapid Recommendation on this topic; is one of the authors on the BMJ Rapid Recommendation paper
AA: is one of the authors on a parallel systematic review performed to inform a BMJ Rapid Recommendation on this topic
PV: is one of the authors on a parallel systematic review performed to inform a BMJ Rapid Recommendation on this topic; is one of the authors on the BMJ Rapid Recommendation paper
RB: received an NHMRC Cochrane Collaboration Round 7 Funding Program Grant, which supports the Cochrane Musculoskeletal Australian Editorial base, but the funding source did not participate in the conduct of this review; is one of the authors on a parallel systematic review performed to inform a BMJ Rapid Recommendation on this topic; is one of the authors on the BMJ Rapid Recommendation paper
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Beard 2018 {published data only}
- Beard D, Rees J, Rombach I, Cooper C, Cook J, Merritt N, et al. The CSAW Study (Can Shoulder Arthroscopy Work?) ‐ a placebo‐controlled surgical intervention trial assessing the clinical and cost effectiveness of arthroscopic subacromial decompression for shoulder pain: study protocol for a randomised controlled trial. Trials 2015;16:210. [PUBMED: 25956385] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard DJ, Rees JL, Cook JA, Rombach I, Cooper C, Merritt N, et al. Arthroscopic subacromial decompression for subacromial shoulder pain (CSAW): a multicentre, pragmatic, parallel group, placebo‐controlled, three‐group, randomised surgical trial. Lancet 2018;391(10118):329‐38. [PUBMED: 29169668] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brox 1993 {published data only}
- Brox JI, Staff PH, Ljunggren AE, Brevik JI. Arthroscopic surgery compared with supervised exercises in patients with rotator cuff disease (stage 1 impingement syndrome). BMJ 1993;307:899‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brox JI, Uppheim G, Skagseth A, Brevik JI, Ljunggren AE, Staff PH. Arthroscopic surgery versus supervised exercises in patients with rotator cuff disease (stage 1 impingement syndrome): a prospective, randomized, controlled study in 125 patients with a 21/2 year follow‐up. Journal of Shoulder and Elbow Surgery 1999;8(2):102‐11. [DOI] [PubMed] [Google Scholar]
Farfaras 2016 {published data only}
- Farfaras S, Sernert N, Hallstrom E, Kartus J. Comparison of open acromioplasty, arthroscopic acromioplasty and physiotherapy in patients with subacromial impingement syndrome: a prospective randomised study. Knee Surgery, Sports Traumatology, Arthroscopy 2016;24(7):2181‐91. [PUBMED: 25385527] [DOI] [PubMed] [Google Scholar]
- Farfaras S, Sernert N, Rostgard Christensen L, Hallstrom EK, Kartus JT. Subacromial decompression yields a better clinical outcome than therapy alone: a prospective randomized study of patients with a minimum 10‐year follow‐up. American Journal of Sports Medicine 2018;46(6):1397‐407. [PUBMED: 29543510] [DOI] [PubMed] [Google Scholar]
Haahr 2005 {published data only}
- Haahr JP, Andersen JH. Exercises may be as efficient as subacromial decompression in patients with subacromial stage II impingement: 4–8‐years’ follow‐up in a prospective, randomized study. Scandinavian Journal of Rheumatology 2006;35:224‐8. [DOI] [PubMed] [Google Scholar]
- Haahr JP, Ostergaard S, Dalsgaard J, Norup K, Frost P, Lausen S, et al. Exercises versus arthroscopic decompression in patients with subacromial impingement: a randomised, controlled study in 90 cases with a one year follow up. Annals of the Rheumatic Diseases 2005;64(5):760‐4. [PUBMED: 15834056] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ketola 2009 {published data only}
- Ketola S, Lehtinen J, Arnala I, Nissinen M, Westenius H, Sintonen H, et al. Does arthroscopic acromioplasty provide any additional value in the treatment of shoulder impingement syndrome?: a two‐year randomised controlled trial. Journal of Bone and Joint Surgery. British Volume 2009;91(10):1326‐34. [DOI] [PubMed] [Google Scholar]
- Ketola S, Lehtinen J, Elo P, Kortelainen S, Huhtala H, Arnala I. No difference in long‐term development of rotator cuff rupture and muscle volumes in impingement patients with or without decompression. Acta Orthopaedica 2016;87(4):351‐5. [PUBMED: 27348693] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketola S, Lehtinen J, Rousi T, Nissinen M, Huhtala H, Arnala I. Which patients do not recover from shoulder impingement syndrome, either with operative treatment or with nonoperative treatment?. Acta Orthopaedica 2015;86(6):641‐6. [PUBMED: 25809315] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketola S, Lehtinen J, Rousi T, Nissinen M, Huhtala H, Konttinen YT, et al. No evidence of long‐term benefits of arthroscopic acromioplasty in the treatment of shoulder impingement syndrome: five‐year results of a randomised controlled trial. Bone and Joint Research 2013;2(7):132‐9. [PUBMED: 23836479] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketola S, Lehtinen JT, Arnala I. Arthroscopic decompression not recommended in the treatment of rotator cuff tendinopathy: a final review of a randomised controlled trial at a minimum follow‐up of ten years. Bone and Joint Journal 2017;99‐B(6):799‐805. [PUBMED: 28566400] [DOI] [PubMed] [Google Scholar]
Paavola 2018 {published and unpublished data}
- Paavola M, Malmivaara A, Taimela S, Kanto K, Inkinen J, Kalske J, et al. Subacromial decompression versus diagnostic arthroscopy for shoulder impingement: randomised, placebo surgery controlled clinical trial. BMJ 2018;362:k2860. [PUBMED: 30026230] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavola M, Malmivaara A, Taimela S, Kanto K, Jarvinen TL. Finnish Subacromial Impingement Arthroscopy Controlled Trial (FIMPACT): a protocol for a randomised trial comparing arthroscopic subacromial decompression and diagnostic arthroscopy (placebo control), with an exercise therapy control, in the treatment of shoulder impingement syndrome. BMJ Open 2017;7(5):e014087. [PUBMED: 28588109] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peters 1997 {published data only}
- Peters G, Kohn D. Medium‐term clinical results after operative and non‐operative treatment of subacromial impingement. Der Unfallchirurg 1997;100:623‐9. [DOI] [PubMed] [Google Scholar]
Rahme 1998 {published data only}
- Rahme H, Solem‐Bertoft E, Westerberg CE, Lundberg E, Sorensen S, Hilding S. The subacromial impingement syndrome. A study of results of treatment with special emphasis on predictive factors and pain‐generating mechanisms. Scandinavian Journal of Rehabilitation Medicine 1998;30:253‐62. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bjornsson 2017 {published data only}
- Bjornsson Hallgren HC, Adolfsson LE, Johansson K, Oberg B, Peterson A, Holmgren TM. Specific exercises for subacromial pain. Acta Orthopaedica 2017;88(6):600‐5. [PUBMED: 28812398] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hallgren 2014 {published data only}
- Hallgren HC, Holmgren T, Oberg B, Johansson K, Adolfsson LE. A specific exercise strategy reduced the need for surgery in subacromial pain patients. British Journal of Sports Medicine 2014;48(19):1431‐6. [PUBMED: 24970843] [DOI] [PubMed] [Google Scholar]
Itoi 2016 {published data only}
- Itoi E. Surgical repair did not improve functional outcomes more than conservative treatment for degenerative rotator cuff tears. Journal of Bone and Joint Surgery. American Volume 2016;98(4):314. [PUBMED: 26888679] [DOI] [PubMed] [Google Scholar]
Kukkonen 2014 {published data only}
- Kukkonen J, Joukainen A, Lehtinen J, Mattila KT, Tuominen EKJ, Kauko T, et al. Treatment of non‐traumatic rotator cuff tears ‐ a randomised controlled trial with one‐year clinical results. Bone and Joint Journal 2014;96‐B:75–81. [DOI] [PubMed] [Google Scholar]
Kukkonen 2015 {published data only}
- Kukkonen J, Joukainen A, Lehtinen J, Mattila KT, Tuominen EK, Kauko T, et al. Treatment of nontraumatic rotator cuff tears: a randomized controlled trial with two years of clinical and imaging follow‐up. Journal of Bone and Joint Surgery. American Volume 2015;97(21):1729‐37. [PUBMED: 26537160] [DOI] [PubMed] [Google Scholar]
Kweon 2015 {published data only}
- Kweon C, Gagnier JJ, Robbins CB, Bedi A, Carpenter JE, Miller BS. Surgical versus nonsurgical management of rotator cuff tears: predictors of treatment allocation. American Journal of Sports Medicine 2015;43(10):2368‐72. [PUBMED: 26268847] [DOI] [PubMed] [Google Scholar]
Lamas 2015 {published data only}
- Lamas JR. A double‐blind, randomized, placebo‐controlled trial of mesenchymal stem cells for the treatment of patients with full‐thickness rotator cuff tears [abstract]. American College of Rhematology. 2015; Vol. 67:(suppl 10).
Lambers Heerspink 2015 {published data only}
- Lambers Heerspink F, Raay J, Koorevaar R, Eerden P, Westerbeek RE, Van’t RE, et al. Comparing surgical repair with conservative treatment for degenerative rotator cuff tears: a randomized controlled trial. Journal of Shoulder and Elbow Surgery 2015;24:1274‐81. [DOI] [PubMed] [Google Scholar]
Maugars 2009 {published data only}
- Maugars Y, Varin S, Gouin F, Huguet D, Rodet D, Nizard J, et al. Treatment of shoulder calcifications of the cuff: a controlled study. Joint, Bone, Spine 2009;76(4):369‐77. [DOI] [PubMed] [Google Scholar]
Mohtadi 2006 {published data only}
- Mohtadi N. Exercises or arthroscopic decompression for subacromial impingement?. Clinical Journal of Sports Medicine 2006;16(2):193‐4. [PUBMED: 16685773] [DOI] [PubMed] [Google Scholar]
Moosmayer 2010 {published data only}
- Moosmayer S, Lund G, Seljom U, Svege I, Hennig T, Tariq R, et al. Comparison between surgery and physiotherapy in the treatment of small and medium‐sized tears of the rotator cuff: a randomised controlled study of 103 patients with one‐year follow‐up. Journal of Bone and Joint Surgery. British Volume 2010;92‐B:83‐91. [DOI] [PubMed] [Google Scholar]
Moosmayer 2017 {published data only}
- Moosmayer S, Ekeberg OM, Hallgren HB, Heier I, Kvalheim S, Blomquist J, et al. KALK study: ultrasound guided needling and lavage (barbotage) with steroid injection versus sham barbotage with and without steroid injection ‐ protocol for a randomized, double‐blinded, controlled, multicenter study. BMC Musculoskeletal Disorders 2017;18(1):138. [PUBMED: 28376756] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saggini 2010 {published data only}
- Saggini R, Cavezza T, Pancrazio L, Pisciella V, Saladino G, Zuccaro MC, et al. Treatment of lesions of the rotator cuff. Journal of Biological Regulators and Homeostatic Agents 2010;24(4):453‐9. [PUBMED: 21122285] [PubMed] [Google Scholar]
References to studies awaiting assessment
Schulze 2017 {published data only}
- Schulze C, Kohler HC, Kaltenborn A, Gutcke A, Tischer T. Influence of operative and conservative therapy on the ability to work of patients with subacromial impingement: a prospective clinical comparative study [Einfluss von operativer und konservativer therapie auf die arbeitsfahigkeit bei patienten mit subakromialem impingement ‐ eine prospektive klinische vergleichsstudie]. Zeitschrift fur Orthopadie und Unfallchirurgie 2017;155(4):450‐6. [PUBMED: 28454194] [DOI] [PubMed] [Google Scholar]
TRANSIT 2006 {published data only}
- Diercks RL. Transmural project for subacromial impingement syndrome: a randomized controlled trial comparing a new transmural treatment strategy (TRANSIT) with usual medical care. http://www.isrctn.com/ISRCTN58108023 Trial start date: June 2006. Trial end date: November 2008. [ISRCTN58108023]
- Dorrestijn O, Stevens M, Diercks RL, Meer K, Winters JC. A new interdisciplinary treatment strategy versus usual medical care for the treatment of subacromial impingement syndrome: a randomized controlled trial. BMC Musculoskeletal Disorders 2007;8(15):doi: 10.1186/1471‐2474‐8‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Paloneva 2008 {unpublished data only}
- NCT00637013. The effectiveness and cost‐effectiveness of operative versus non‐operative management of subacromial impingement. https://clinicaltrials.gov/ct2/show/NCT00637013 First registered March 17, 2008. [NCT00637013]
Additional references
Alanne 2015
- Alanne S, Roine RP, Rasanen P, Vainiola T, Sintonen H. Estimating the minimum important change in the 15D scores. Quality of Life Research 2015;24(3):599‐606. [PUBMED: 25145637] [DOI] [PubMed] [Google Scholar]
Bennell 2007
- Bennell K, Coburn S, Wee E, Green S, Harris A, Forbes A, et al. Efficacy and cost‐effectiveness of a physiotherapy program for chronic rotator cuff pathology: a protocol for a randomised, double‐blind, placebo‐controlled trial. BMC Musculoskeletal Disorders 2007;8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buchbinder 1996
- Buchbinder R, Goel V, Bombardier C, Hogg‐Johnson S. Classification systems of soft tissue disorders of the neck and upper limb: do they satisfy methodological guidelines?. Journal of Clinical Epidemiology 1996;49:141‐9. [DOI] [PubMed] [Google Scholar]
Buchbinder 2003
- Buchbinder R, Green S, Youd JM. Corticosteroid injections for shoulder pain. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD004016] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buchbinder 2011
- Buchbinder R, Johnson MP, Roos JF. Shock wave therapy for rotator cuff disease with or without calcification. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD008962] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buchbinder 2017
- Buchbinder R, Page MJ, Huang H, Verhagen AP, Beaton D, Kopkow C, et al. for the Shoulder Core Outcomes Set Special Interest Group. A preliminary Core Domain Set for clinical trials of shoulder disorders: a report from the OMERACT 2016 Shoulder Core Outcome Set Special Interest Group. Journal of Rheumatology 2017;44(12):1880‐3. [DOI] [PubMed] [Google Scholar]
Cates 2008 [Computer program]
- Dr. Christopher Cates EBM website. Visual Rx. Version 3. Dr. Christopher Cates EBM website, 2008.
Chard 1991
- Chard MD, Hazleman R, Hazleman BL, King RH, Reiss BB. Shoulder disorders in the elderly: a community survey. Arthritis and Rheumatology 1991;34:766‐9. [DOI] [PubMed] [Google Scholar]
Chen 1999
- Chen SK, Simonian PT, Wickiewicz TL, Otis JC, Warren RF. Radiographic evaluation of glenohumeral kinematics: a muscle fatigue model. Journal of Shoulder and Elbow Surgery 1999;8:49‐52. [DOI] [PubMed] [Google Scholar]
Cumpston 2009
- Cumpston M, Johnston RV, Wengier L, Buchbinder R. Topical glyceryl trinitrate for rotator cuff disease. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD006355.pub2] [DOI] [PubMed] [Google Scholar]
Dean 2012
- Dean BJ, Franklin SL, Carr AJ. A systematic review of the histological and molecular changes in rotator cuff disease. Bone and Joint Research 2012;1:158‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG (editors), on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Dwan 2011
- Dwan K, Altman DG, Cresswell L, Blundell M, Gamble CL, Williamson PR. Comparison of protocols and registry entries to published reports for randomised controlled trials. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.MR000031.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Engebretsen 2009
- Engebretsen K, Grotle M, Bautz‐Holter E, Sandvik L, Juel NG, Ekeberg OM, et al. Radial extracorporeal shockwave treatment compared with supervised exercises in patients with subacromial pain syndrome: single randomised study. BMJ 2009;339:b3360. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gialanella 2011
- Gialanella B, Prometti P. Effects of corticosteroids injection in rotator cuff tears. Pain Medicine 2011;12(10):1559‐65. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version Accessed 14th December, 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Green 1998
- Green S, Buchbinder R, Glazier R, Forbes A. Systematic review of randomised controlled trials of interventions for painful shoulder: selection criteria, outcome assessment, and efficacy. BMJ 1998;316:345‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Green 2005
- Green S, Buchbinder R, Hetrick S. Acupuncture for shoulder pain. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD005319] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hao 2019
- Hao Q, Devji T, Zeraatkar D, Wang Y, Qasim A, Siemieniuk R, et al. Minimal important differences in shoulder condition patient‐reported outcomes: a systematic survey to inform a BMJ Rapid Recommendation. BMJ Open 2019;submitted:not known. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hata 2001
- Hata Y, Saitoh S, Murakami N, Seki H, Nakatsuchi Y, Takaoka K. A less invasive surgery for rotator cuff tear: mini‐ open repair. Journal of Shoulder and Elbow Surgery 2001;10(1):11‐6. [DOI] [PubMed] [Google Scholar]
Hayden 2013
- Hayden JA, Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Annals of Internal Medicine 2013;158(4):280‐6. [PUBMED: 23420236] [DOI] [PubMed] [Google Scholar]
Hermans 2013
- Hermans J, Luime JJ, Meuffels DE, Reijman M, Simel DL, Bierma‐Zeinstra SMA. Does this patient with shoulder pain have rotator cuff disease? The rational clinical examination systematic review. JAMA 2013;310(8):837‐47. [DOI] [PubMed] [Google Scholar]
Hertling 1990
- Hertling D, Kessler RM. The shoulder and shoulder girdle. Management of common musculoskeletal disorders: physical therapy principles and methods. Philadelphia: Lippincott, 1990. [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Hill 2017
- Hill JR, McKnight B, Pannell WC, Heckmann N, Sivasundaram L, Mostofi A, et al. Risk factors for 30‐day readmission following shoulder arthroscopy. Arthroscopy 2017;33(1):55‐61. [PUBMED: 27641638] [DOI] [PubMed] [Google Scholar]
Hyvonen 2003
- Hyvonen P, Lantto V, Jalovaara P. Local pressures in the subacromial space. International Orthopaedics 2003;27(6):373‐7. [PUBMED: 12856153] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jain 2014
- Jain NB, Higgins LD, Losina E, Collins J, Blazar PE, Katz JN. Epidemiology of musculoskeletal upper extremity ambulatory surgery in the United States. BMC Musculoskeletal Disorders 2014;15:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2004
- Johnson MP, Crossley KL, O'Neil ME, Al‐Zakwani IS. Estimates of direct healthcare expenditures among individuals with shoulder dysfunction in the United States. American Society of Shoulder and Elbow Therapists Annual Meeting. 2004.
Judge 2014
- Judge A, Murphy RJ, Maxwell R, Arden NK, Carr AJ. Temporal trends and geographical variation in the use of subacromial decompression and rotator cuff repair of the shoulder in England. Bone and Joint Journal 2014;96‐B:70‐4. [DOI] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Altman DG, Williamson PR. Bias due to changes in specified outcomes during the systematic review process. PloS One 2010;5(3):e9810. [PUBMED: 20339557] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kohn 1997
- Kohn D, Geyer M. The subjective shoulder rating system. Archives of Orthopaedic and Trauma Surgery 1997;116(6‐7):324‐8. [PUBMED: 9266033] [DOI] [PubMed] [Google Scholar]
Kuhn 2009
- Kuhn JE. Exercise in the treatment of rotator cuff impingement: a systematic review and a synthesized evidence‐based rehabilitation protocol. Journal of Shoulder and Elbow Surgery 2009;18(1):138‐60. [DOI] [PubMed] [Google Scholar]
Luime 2004
- Luime JJ, Koes BW, Hendriksen IJ, Burdorf A, Verhagen AP, Miedema HS, et al. Prevalence and incidence of shoulder pain in the general population; a systematic review. Scandinavian Journal of Rheumatology 2004;33:73‐81. [DOI] [PubMed] [Google Scholar]
Lähdeoja 2019
- Lähdeoja T, Karjalainen TV, Jokihaara J, Kavaja L, Salamh P, Agarwal A, et al. Subacromial decompression surgery for adults with shoulder pain – a systematic review with meta‐analysis. British Journal of Sports Medicine 2019;0:1‐10. [DOI: 10.1136/bjsports-2018-100486] [DOI] [PubMed] [Google Scholar]
Millett 2006
- Millett PJ, Wilcox RB 3rd, O'Holleran JD, Warner JJ. Rehabilitation of the rotator cuff: an evaluation‐based approach. Journal of the American Academy of Orthopaedic Surgeons 2006;14(11):599‐609. [DOI] [PubMed] [Google Scholar]
Minagawa 2013
- Minagawa H, Yamamoto N, Abe H, Fukuda M, Seki N, Kikuchi K, et al. Prevalence of symptomatic and asymptomatic rotator cuff tears in the general population: from mass screening in one village. Journal of Orthopaedics 2013;10:8‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Misamore 1995
- Misamore GW, Ziegler DW, Rushton JL 2nd. Repair of the rotator cuff. A comparison of results in two populations of patients. Journal of Bone and Joint Surgery 1995;77(9):1335‐9. [DOI] [PubMed] [Google Scholar]
Moor 2014
- Moor BK, Wieser K, Slankamenac K, Gerber C, Bouaicha S. Relationship of individual scapular anatomy and degenerative rotator cuff tears. Journal of Shoulder and Elbow Surgery 2014;23(4):536‐41. [PUBMED: 24480324] [DOI] [PubMed] [Google Scholar]
Nam 2012
- Nam D, Maak TG, Raphael BS, Kepler CK, Cross MB, Warren RF. Rotator cuff tear arthropathy: evaluation, diagnosis, and treatment: AAOS exhibit selection. Journal of Bone and Joint Surgery. American Volume 2012;94(6):e34. [PUBMED: 22438007] [DOI] [PubMed] [Google Scholar]
Neer 1983
- Neer CS. Impingement lesions. Clinical Orthopaedics and Related Research 1983;173:70‐7. [PubMed] [Google Scholar]
Nho 2007
- Nho SJ, Shindle MK, Sherman SL, Freedman KB, Lyman S, MacGillivray JD. Systematic review of arthroscopic rotator cuff repair and mini‐open rotator cuff repair. Journal of Bone and Joint Surgery. American Volume 2007;89(Suppl 3):127‐36. [DOI] [PubMed] [Google Scholar]
Ostör 2005
- Ostör AJK, Richards CA, Prevost AT, Speed CA, Hazleman BL. Diagnosis and relation to general health of shoulder disorders presenting to primary care. Rheumatology 2005;44:800‐5. [DOI] [PubMed] [Google Scholar]
Page 2015
- Page MJ, McKenzie JE, Green SE, Beaton DE, Jain NB, Lenza M, et al. Core domains and outcome measurement sets for shoulder pain trials are needed: systematic review of physical therapy trials. Journal of Clinical Epidemiology 2015;68:1270‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2016
- Page MJ, Green S, McBain B, Surace SJ, Deitch J, Lyttle N, et al. Manual therapy and exercise for rotator cuff disease. Cochrane Database of Systematic Reviews 2016, Issue 6. [DOI: 10.1002/14651858.CD012224] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2016a
- Page MJ, Green S, Mrocki MA, Surace SJ, Deitch J, McBain B, et al. Electrotherapy modalities for rotator cuff disease. Cochrane Database of Systematic Reviews 2016, Issue 6. [DOI: 10.1002/14651858.CD012225] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pedowitz 2012
- Pedowitz RA, Yamaguchi K, Ahmad CS, Burks RT, Flatow EL, Green A, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: optimizing the management of rotator cuff problems. Journal of Bone and Joint Surgery. American Volume 2012;94(2):163‐7. [PubMed] [Google Scholar]
Rekola 1993
- Rekola KE, Keinänen‐Kiukaanniemi S, Takala J. Use of primary health services in sparsely populated country districts by patients with musculoskeletal symptoms: consultations with a physician. Journal of Epidemiology and Community Health 1993;47:153‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roy 2010
- Roy JS, MacDermid JC, Woodhouse LJ. A systematic review of the psychometric properties of the Constant‐Murley score. Journal of Shoulder and Elbow Surgery 2010;19(1):157‐64. [PUBMED: 19559630] [DOI] [PubMed] [Google Scholar]
Royal College of General Practitioners 1980‐81
- Royal College of General Practitioners, Office of Populations, Census, Surveys. Third national morbidity survey in general practice 1980‐1981. Department of Health and Social Security. Series MB5 No 1. London: HMSO.
Saltychev 2015
- Saltychev M, Aarimaa V, Virolainen P, Laimi K. Conservative treatment or surgery for shoulder impingement: systematic review and meta‐analysis. Disability and Rehabilitation 2015;37(1):1‐8. [PUBMED: 24694286] [DOI] [PubMed] [Google Scholar]
Schünemann 2017a
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. on behalf of the Cochrane Applicability and Recommendations Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Schünemann 2017b
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Akl E, et al. on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Shields 2015
- Shields E, Thirukumaran C, Thorsness R, Noyes K, Voloshin I. An analysis of adult patient risk factors and complications within 30 days after arthroscopic shoulder surgery. Arthroscopy 2015;31(5):807‐15. [PUBMED: 25661861] [DOI] [PubMed] [Google Scholar]
Shiri 2007
- Shiri R, Varonen H, Heliovaara M, Viikari‐Juntura E. Hand dominance in upper extremity musculoskeletal disorders. Journal of Rheumatology 2007;34(5):1076‐82. [PUBMED: 17343320] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [PUBMED: 21784880] [DOI] [PubMed] [Google Scholar]
Tashjian 2009
- Tashjian RZ, Deloach J, Porucznik CA, Powell AP. Minimal clinically important differences (MCID) and patient acceptable symptomatic state (PASS) for visual analogue scales (VAS) measuring pain in patients treated for rotator cuff disease. Journal of Shoulder and Elbow Surgery 2009;18(6):927‐32. [DOI] [PubMed] [Google Scholar]
Teunis 2014
- Teunis T, Lubberts B, Reilly BT, Ring D. A systematic review and pooled analysis of the prevalence of rotator cuff disease with increasing age. Journal of Shoulder and Elbow Surgery 2014;23(12):1913‐21. [PUBMED: 25441568] [DOI] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Walter SD, Johnston BC, Furukawa TA, Guyatt GH. Pooling health‐related quality of life outcomes in meta‐analysis‐a tutorial and review of methods for enhancing interpretability. Research Synthesis Methods 2011;2(3):188‐203. [PUBMED: 26061786] [DOI] [PubMed] [Google Scholar]
Toliopoulos 2014
- Toliopoulos P, Desmeules F, Boudreault J, Roy JS, Fremont P, MacDermid JC, et al. Efficacy of surgery for rotator cuff tendinopathy: a systematic review. Clinical Rheumatology 2014;33(10):1373‐83. [PUBMED: 24682606] [DOI] [PubMed] [Google Scholar]
Van der Meijden 2012
- Meijden OA, Westgard P, Chandler Z, Gaskill TR, Kokmeyer D, Millett PJ. Rehabilitation after arthroscopic rotator cuff repair: current concepts review and evidence‐based guidelines. International Journal of Sports Physical Therapy 2012;7(2):197‐218. [PMC free article] [PubMed] [Google Scholar]
Vandvik 2018
- Vandvik PO, Lähdeoja T, Ardern C, Buchbinder R, Moro J, Brox JI, et al. Subacromial decompression surgery for adults with shoulder pain: a clinical practice guideline. BMJ open 2019;Submitted:online version not yet available. [DOI] [PubMed] [Google Scholar]
Vecchio 1995
- Vecchio P, Kavanagh R, Hazleman BL, King RH. Shoulder pain in a community‐based rheumatology clinic. British Journal of Rheumatology 1995;34:766‐9. [DOI] [PubMed] [Google Scholar]
Vitale 2010
- Vitale MA, Arons RR, Hurwitz S, Ahmad CS, Levine WN. The rising incidence of acromioplasty. Journal of Bone and Joint Surgery. American Volume 2010;92:1842‐50. [DOI] [PubMed] [Google Scholar]
Whittle 2015
- Whittle S, Buchbinder R. In the clinic: rotator cuff disease. Annals of Internal Medicine 2015;162(1):ITC1. [DOI] [PubMed] [Google Scholar]
Yamaguchi 2001
- Yamaguchi K, Tetro A, Blam O, Evanoff B, Teefey S, Middleton W. Natural history of asymptomatic rotator cuff tears: a longitudinal analysis of asymptomatic tears detected sonographically. Journal of Shoulder and Elbow Surgery 2001;10:199‐203. [DOI] [PubMed] [Google Scholar]
Yamamoto 2010
- Yamamoto A, Takagishi K, Osawa T, Yanagawa T, Nakajima D, Shitara H, et al. Prevalence and risk factors of a rotator cuff tear in the general population. Journal of Shoulder and Elbow Surgery 2010;19:116‐20. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Coghlan 2008
- Coghlan JA, Buchbinder R, Green S, Johnston RV, Bell SN. Surgery for rotator cuff disease. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD005619] [DOI] [PMC free article] [PubMed] [Google Scholar]


