Abstract
Purpose
To evaluate management strategies and treatment options for patients with ground-glass nodules (GGNs) by using decision-analysis models.
Materials and Methods
A simulation was developed for 1 000 000 hypothetical patients with GGNs undergoing follow-up per the Lung Imaging Reporting and Data System (Lung-RADS) recommendations. The initial age range was 55–75 years (mean, 64 years). Nodules could grow and develop solid components over time. Clinically significant malignancy rates were calibrated to data from the National Lung Screening Trial. Annual versus 3-year-interval follow-up of Lung-RADS category 2 nodules was compared, and different treatment strategies were tested (stereotactic body radiation therapy, surgery, and no therapy).
Results
Overall, 2.3% (22 584 of 1 000 000) of nodules were clinically significant malignancies; 6.3% (62 559 of 1 000 000) of nodules were treated. Only 30% (18 668 of 62 559) of Lung-RADS category 4B or 4X nodules were clinically significant malignancies. The risk of clinically significant malignancy for persistent nonsolid nodules after baseline was higher than Lung-RADS estimates for categories 2 and 3 (3% vs <1% and 1%–2%, respectively). Overall survival (OS) at 10 years was 72% (527 827 of 737 306; 95% confidence interval [CI]: 71%, 72%) with annual follow-up and 71% (526 507 of 737 306; 95% CI: 71%, 72%) with 3-year-interval follow-up (P < .01). At 10 years, OS among patients whose nodules progressed to Lung-RADS category 4B or 4X was 80% after radiation therapy (49 945 of 62 559; 95% CI: 80%, 80%), 79% after surgery (49 139 of 62 559; 95% CI: 78%, 79%), and 74% after no therapy (46 512 of 62 559; 95% CI: 74%, 75%) (P < .01).
Conclusion
Simulation modeling suggests that the follow-up interval for evaluating ground-glass nodules can be increased from 1 year to 3 years with minimal change in outcomes. Stereotactic body radiation therapy demonstrated the best outcomes compared with lobectomy and with no therapy for nonsolid nodules.
© RSNA, 2018
Summary
Our results suggest that the follow-up interval for nonsolid pulmonary nodules can be increased to 3 years with minimal change in outcomes.
Implications for Patient Care
■ The follow-up interval for ground-glass nodules can be raised to 3 years with minimal change in outcomes.
■ The current Lung Imaging Reporting and Data System, or Lung-RADS, estimation of malignancy is likely too low for nonsolid nodules seen at post-baseline examinations.
■ Stereotactic body radiation therapy yielded the best outcomes compared with lobectomy and with no therapy for nonsolid nodules.
Introduction
Nonsolid nodules (sometimes known as subsolid nodules), which include ground-glass nodules (GGNs) and part-solid nodules (PSNs), are commonly identified on chest CT images. These are found in approximately 9% of patients undergoing low-dose CT for lung cancer screening (1,2). Although some nonsolid nodules are transient and likely inflammatory (1,2), many of those that persist range from premalignant lesions to invasive adenocarcinomas (3,4). Nonsolid nodules are more likely to represent lung cancer than solid nodules (5) but are also more likely to exhibit indolent behavior (ie, slow growth and low metastatic potential), which has raised the concern of overdiagnosis and overtreatment of these nodules (6,7). A considerable increase in the number of patients diagnosed with these nodules is expected in the near future, as annual lung cancer screening for high-risk individuals has been recommended by both the U.S. Preventive Services Task Force and the Center for Medicare and Medicaid Services (1,2). Hence, there is an urgent need to address the question of how best to manage nonsolid nodules.
Studies of nonsolid nodule behavior provide a great deal of information that can be used to inform recommendations for nonsolid nodule management. Results of natural history studies evaluating the growth of nonsolid nodules have shown that most of these lesions grow slowly over the course of years, and some never grow at all (8–10). Furthermore, the discrepancy between low lung cancer diagnosis rates among screened patients (1,2,11) and high diagnosis rates among patients undergoing biopsy (12,13) indicates that many nonsolid nodules that are pathologically diagnosed as malignant after biopsy will never manifest clinically. Recurrence-free survival for nonsolid lung cancers after resection is far better than that for solid lung cancers (14), and study results have shown that ground-glass adenocarcinomas in particular rarely metastasize to lymph nodes (2,15). Finally, because ground-glass adenocarcinomas are indolent, the optimal treatment modality is controversial; it remains an open question whether stereotactic body radiation therapy (SBRT) or percutaneous ablation are adequate substitutes for lobectomy, the standard of care for invasive lung cancers.
The American College of Radiology has developed the Lung Imaging Reporting and Data System (Lung-RADS) (16), a classification system providing guidance for the management of solid and nonsolid nodules found in CT screening examinations for lung cancer that is based on a synthesis of nodule malignancy risk estimates from the literature and expert opinion. Because of the potential for overtreatment, slow growth rates, and low risk of metastasis, the Lung-RADS schema has adopted relatively conservative recommendations for nonsolid nodule management (16). However, Lung-RADS is a first-edition guideline that is strongly reliant on expert opinion; hence, some recommendations are controversial, and more data are needed to justify the guidelines (17).
The purpose of this study was to use simulation modeling to analyze the effectiveness of Lung-RADS and treatment options for patients after the discovery of GGNs in screening. We investigated the effect on patient outcomes of extending the follow-up interval for Lung-RADS category 2 nodules—nonsolid nodules that are assigned the lowest probability of malignancy—and we evaluated the change in survival after treatment (lobectomy, SBRT, or no therapy).
Materials and Methods
Consent to access National Lung Screening Trial (NLST) data for this study was obtained from the National Cancer Data Access System of the National Cancer Institute (NCI), through a data transfer agreement between the authors and the NCI. This simulation study used nonidentifiable patient data for secondary data analysis and was approved by our institutional review board. The study analysis complied with the Health Insurance Portability and Accountability Act.
Model Overview
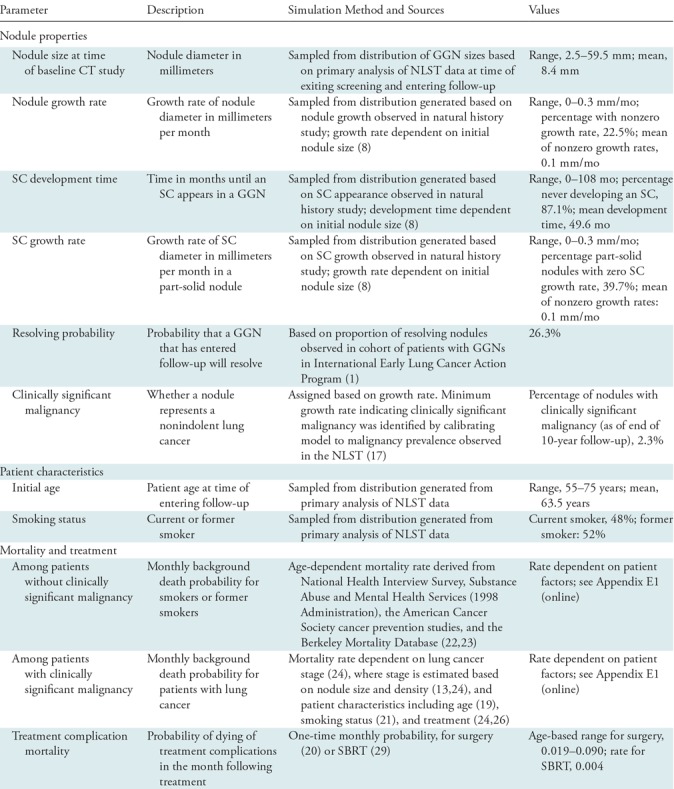
We developed a state-transition Monte Carlo simulation model with a monthly cycle to investigate the effectiveness of the nodule management guidelines for nonsolid nodules. A cohort of current and former smokers with GGNs were simulated as they underwent a follow-up regimen according to Lung-RADS guidelines for up to 10 years. Nodules could grow and develop solid components (SCs) (ie, become PSNs) over time. The model was implemented by using C++. Table 1 presents a summary of the model’s input parameters, which are discussed further below and in Appendix E1 (online).
Table 1:
Model Parameters
Note.—GGN = ground-glass nodule, NLST = National Lung Screening Trial, SBRT = stereotactic body radiation therapy, SC = solid component.
Nodule properties.—We simulated 1 000 000 patients with GGNs (one nodule per patient). At the time of entering the model, each patient undergoes a baseline chest CT examination in which a GGN is discovered. Each GGN is assigned characteristics at the time of discovery that include the following: size, growth rate, whether an SC will develop, and time until an SC develops. Nodule growth rates were related to nodule size at baseline, with larger nodules having a higher chance of fast growth (8). The possibility of a nodule resolving after its discovery at the baseline examination was taken into account (1).
Each nodule was also assigned a status representing whether it was a clinically significant malignancy. In this article, we refer to “clinically significant” malignancies as those that would be discovered through the standard of care, to distinguish them from the indolent types of lung cancer that may never manifest clinically. Clinically significant malignancies were defined in terms of nodule growth rates in the model. Clinically significant malignancy is better tied to growth rate rather than other characteristics such as size, and slowly growing cancers are believed to be a major source of overdiagnosis in the screening setting (7). The range of growth rates corresponding to clinically significant malignancy was derived by calibrating cancer incidence in the model to cancer incidence in nonsolid nodules as calculated by the NLST Nodule Searcher (18). The Nodule Searcher estimates the probability that a lung nodule with a given size and density will be diagnosed as cancer, based on results observed in the NLST (19).
Patient characteristics and mortality.—Secondary analysis of NLST data was used in assigning patient age and smoking status. Patient ages at the time of baseline screening were drawn from a distribution of NLST participant ages. Smoking status was also assigned on the basis of the prevalence of current and former smokers among NLST participants. These characteristics were set so that our patient cohort would be representative of a real-world lung cancer screening cohort. The probability of patient death at any given month depended on age (20,21), smoking status (22–24), presence and stage of clinically significant lung cancer (14,25), and whether the patient had received treatment (26,27). Of note, male and female death rates were averaged to generate the parameter estimates in the model.
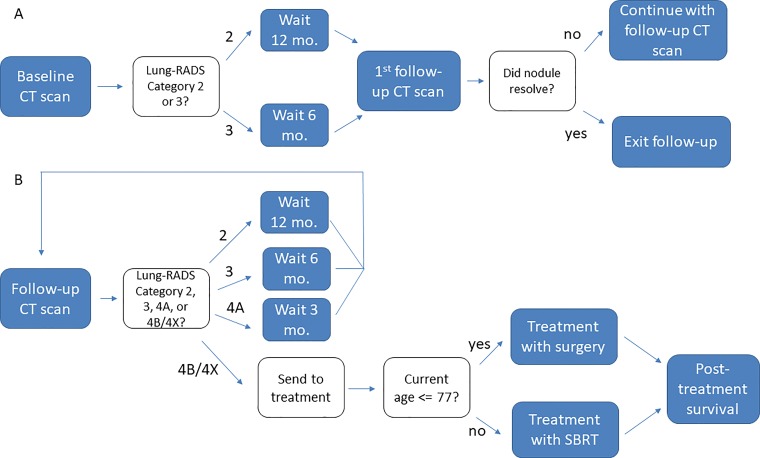
Follow-up schedule.—Depending on the results of the baseline screening examination, the patient was assigned a 6- or 12-month waiting period until the first follow-up CT study. Depending on the results of the follow-up study, the patient will either wait 3, 6, or 12 months for their next follow-up or exit the follow-up process and receive treatment for lung cancer. See Figure 1 for a schematic representation of the model; part A of Figure 1 depicts the model’s protocol for the baseline CT examination, and part B shows post-baseline follow-up. The observed size and/or growth of the nodule dictates the management decision for the patient at the time of each CT examination. Our model assigns a Lung-RADS category to each simulated nodule after each CT study during the 10-year follow-up period.
Figure 1:
Schematic representation of the model. Each state in the model has a 1-month (mo.) duration. A, Patients enter the model on undergoing a baseline CT examination in which a ground-glass nodule is discovered. The number of months a patient spends waiting between follow-up CT examinations depends on the characteristics of the patient’s nodule as it appears in the examination. B, CT results also determine whether a patient is sent to treatment. Lung-RADS = Lung Imaging Reporting and Data System.
Cancer treatment.—Patients with nodules meeting criteria for Lung-RADS category 4B or 4X exit the follow-up process and are assigned a treatment for their nodule. In the simulation, we assumed that once a lesion reached Lung-RADS category 4B or 4X status, it was treated definitively. Note that in our simulation, to reach 4B or 4X status, a nodule must have persisted over time and grown and/or developed an SC (see Appendix E1 [online] for specific criteria for category 4X nodules); transient nodules are benign and would not be treated in our scheme. Both Lung-RADS and Fleischner Society 2017 (high-risk) guidelines recommend that suspicious nodules undergo tissue sampling and/or fluorine 18 fluorodeoxyglucose PET as their initial evaluation. However, both of these approaches have considerable drawbacks for evaluating nonsolid nodules given their greater false-negative rates compared with solid nodules (12,28,29). Therefore, we assumed that once a nodule met criteria for further evaluation (ie, became Lung-RADS category 4B or 4X), it would eventually go on to definitive therapy.
The treatment modalities included in our simulation were surgery and SBRT. For this analysis, the surgical procedure evaluated was lobectomy, which is the current standard of care for invasive lung cancer. For the base-case analysis, procedure selection wasbased on a patient age cutoff of 77 years, above which the patient received SBRT and below which the patient received surgery.
On the basis of results from literature (14), treated GGNs did not result in mortality rates different from general background mortality rates. For PSNs, posttreatment rates of death depended on lung cancer stage (25). Mortality for PSNs was based on observed lung cancer mortality rates from the literature but was scaled down by a factor representing the relative mortality risk of part-solid lung cancers relative to solid cancers (14). Among patients with clinically significant malignancies, survival for those treated with SBRT was lower than for those treated with surgery (26,30).
Incidental cancer.—To estimate the rate of incidental cancer among this high-risk population during follow-up, we used the Lung Cancer Policy Model (LCPM), a well-validated, comprehensive microsimulation model of lung cancer development, progression, detection, treatment, and survival (31). The LCPM has been used in an analysis of the effectiveness of different lung cancer screening strategies that helped to inform the development of recommendations for lung cancer screening with CT by the U.S. Preventive Services Task Force (32). Detection of a nodule in the LCPM may occur during a CT examination or outside of a CT examination because of symptoms. In our study, follow-up studies of nonsolid nodules can result in detection of incident cancer, and hence we used the LCPM to estimate the mortality due to incidental lung cancers that arose over the course of 10 years of nonsolid nodule follow-up and incorporated this mortality into our survival measures. Further details of the LCPM are publicly available, as recorded within a designated National Cancer Institute website (https://cisnet.cancer.gov/resources/profiles.html).
Analysis of Follow-up Intervals and Treatment Options
Our primary analysis examined the outcomes of a simulation of 1 000 000 patients, with initial ages ranging from 55 to 75 years, who are discovered to have a GGN at CT screening and are then assigned to a nodule follow-up regimen based on the Lung-RADS guidelines. The patients are tracked for 10 years from the baseline screening examination or until time of death. A 10-year time frame was used because source studies in the literature for many of our parameters did not follow patients for a longer time than that. We examined the percentage of nodules that were category 2 at baseline and the percentage that were malignant at baseline. We also calculated the percentage of malignant nodules within each Lung-RADS category (2, 3, 4A, or 4B or 4X) over the course of 10 years of follow-up. In addition to the base-case analysis, we performed simulations in which we assigned 2- or 3-year waiting intervals (rather than 1 year, which is the Lung-RADS recommendation) to Lung-RADS category 2 nodules. As in the base-case analysis, overall survival (OS) was measured starting from the time of GGN discovery. We compared the 10-year OS of the cohort of patients with nonresolving nodules in each of these scenarios. We also simulated patient cohorts stratified by selection of treatment procedure and measured overall posttreatment survival. To measure the uncertainty of our primary outcomes of interest, one-way sensitivity analyses were performed by using a previously established method (33) and are described in further detail in Appendix E1 (online). P values for outcome measures were computed by using a Z-test in the case of two proportions, a χ2 test in the case of more than two proportions, and a two-sided t test for independent samples in the case of two means.
Results
Outcomes for Nonsolid Nodules
The vast majority, 96% (957 929 of 1 000 000), of GGNs were assigned a Lung-RADS category of 2 at their baseline CT studies. Of these, 26% (246 470 of 957 929) were transient—that is, they had resolved at the time of the second interval CT examination, and 1.0% (9295 of 957 929) were malignant at baseline. Persistent (nonresolving) nodules were newly assigned a Lung-RADS category at each simulated follow-up CT examination (Table 2). As expected, the rates of clinically significant malignancy were higher in increasing Lung-RADS categories, ranging from 3.0% (21 820 of 722 088) for persistent Lung-RADS category 2 nodules to 30% (18 668 of 62 559) for Lung-RADS category 4B or 4X nodules. Overall, the percentage of treated patients in the model, 6.3% (62 559 of 1 000 000), was about three times larger than the percentage of patients with clinically significant malignancies, 2.3% (22 584 of 1 000 000) (Table 2).
Table 2:
Risk of Clinically Significant Malignancy for Persistent Nonsolid Nodules according to Post-Baseline Lung-RADS Category
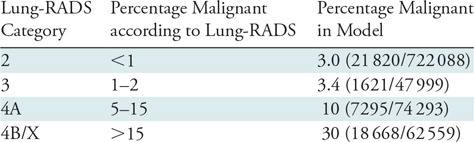
Note.—For this analysis, a nodule was characterized as corresponding to a Lung-RADS category if the nodule was assigned that category at any point during the follow-up process. This analysis excluded some nodules, initially characterized as category 2 or 3, that were found to resolve after being discovered at a baseline screening examination. Lung-RADS = Lung Imaging Reporting and Data System.
Evaluation of Different Follow-up Intervals for Lung-RADS Category 2 Nodules
We evaluated outcomes for patients with persistent Lung-RADS category 2 nodules by assigning them to 1-, 2-, or 3-year follow-up intervals (Table 3). This analysis excluded patients with transient (resolving) nodules but did include mortality from interval lung cancers. There was no clinically meaningful difference in rates of nodule treatment or sizes of nodules at the time of treatment across the three groups. OS at 10 years of follow-up was only minimally different in the three groups: 72% (527 827 of 737 306) in the annual CT group compared with 71% (526 507 of 737 306) in the triennial CT group (P < .01). The average size of the SC at the time of treatment was slightly larger in the triennial CT group, at 3.7 mm, compared with 3.2 mm in the annual CT group (P < .01).
Table 3:
Effect of Changing Follow-up Interval for Persistent Nonsolid Nodules in Lung-RADS Category 2
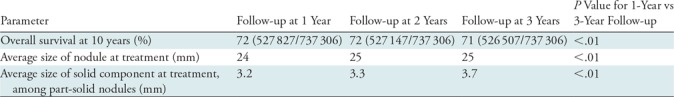
Note.—The current recommendation for a Lung-RADS category 2 nodule is for the patient to undergo a follow-up low-dose CT examination in 1 year. These simulation data show that changing the follow-up interval from 1 year to 3 years does not impact overall survival. Lung-RADS = Lung Imaging Reporting and Data System.
Evaluation of Different Treatment Modalities
We evaluated the outcomes of different treatment options (surgery, SBRT, and no therapy) for patients with persistent nonsolid nodules. No substantial differences in survival were seen among the different treatment modalities within the entire cohort, likely because a very small fraction of patients had clinically significant malignancies. Thus, we focused on the subset of patients who developed nodules that require treatment (Lung-RADS category 4B or 4X). There were small differences in OS across the different treatment modalities. SBRT resulted in the greatest OS (80%; 49 945 of 62 559), followed by surgery (79%; 49 139 of 62 559) and then no therapy (74%; 46 512 of 62 559 [P < .01]).
Sensitivity Analysis
Our major results were generally stable under the sensitivity analysis conditions (see Tables E6A and E6B [online]). In particular, the difference in OS between the 1-year and 3-year follow-up interval conditions remained small (0.14%–0.19%, with 0.18% in the base-case condition). For the three treatment options, seven of eight of our sensitivity analysis conditions resulted in SBRT leading to the highest OS (the difference in OS between SBRT and no therapy ranged from 3.29%–5.49% and was 5.49% in the base-case condition). Only in the extreme scenario of substantially decreasing the mortality rate of untreated patients with malignant nodules did the result change, with the no-therapy arm having a 2.22% higher OS than the SBRT arm.
Discussion
Using empiric data from the literature and secondary analysis of data from the NLST, we built a simulation of nonsolid pulmonary nodules to follow their behavior over time and examine the effects of different follow-up intervals and therapeutic strategies. Our model simulates known behaviors such as resolving inflammatory nodules, slow growth over time, and the development of SCs, with rates matching those in the literature. By evaluating a management strategy derived from Lung-RADS, we have shown that many nodules (70%) that are recommended to undergo treatment do not represent clinically significant malignancies. SBRT resulted in the best OS compared with surgery or no therapy (80% vs 79% and 74%, respectively). Additionally, we showed that Lung-RADS category 2 nodules can be followed every 3 years without substantial impact on patient outcome (71% vs 72% survival at 10 years).
It is well known that nonsolid nodules often represent indolent malignancies (typically a subtype of indolent adenocarcinoma) (Fig 2) (11). This is in contrast to solid nodules, which, while less likely to be malignant, exhibit more aggressive behavior when cancerous (Fig 3). The slow growth of nonsolid lesions raises the possibility of overdiagnosis (6); in other words, many lesions may represent preinvasive adenocarcinoma, but have little impact on patient survival. To estimate the rate of clinically significant malignancies for these nodules, we used cancer rates from similar nodules in the NLST cohort (18); these represent the clinically diagnosed malignancies in nodules of that density and size. In our simulation, thresholds for short-interval follow-up imaging, as well as those for treatment, were based on the Lung-RADS schema (16). In comparing the estimates of malignancy in the Lung-RADS document to those in our study (Table 2), for most categories, the rates were very similar. However, the malignancy rate for Lung-RADS category 2 nodules in our study was 3%, greater than the “<1%” listed in the Lung-RADS document, although similar to the rate in another analysis of NLST data (34). This likely reflects the higher risk of malignancy in large GGNs, as demonstrated in the NLST and other cohorts, as well as the exclusion of transient nodules in follow-up rounds (10). For Lung-RADS category 3, our malignancy rate of 3% was also slightly greater than the “1%–2%” listed in the Lung-RADS document; again, this may reflect exclusion of transient nodules as well as the risk of malignancy in large GGNs.
Figure 2:
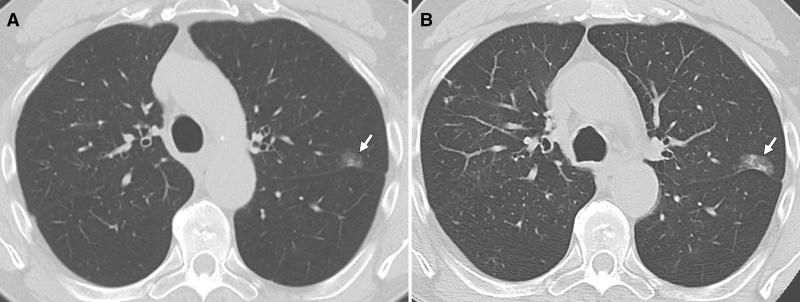
Axial CT images in 77-year-old man who was a former smoker. A, Surveillance chest CT image shows a nonsolid nodule in the left upper lobe with small solid component (SC). B, Follow-up chest CT image obtained 4 years later shows slight growth of the nodule, with a mild increase in the existing SC and the development of a new small SC. At this point, a biopsy was performed and revealed adenocarcinoma, and the patient was subsequently treated with radiation therapy because of poor functional status.
Figure 3:
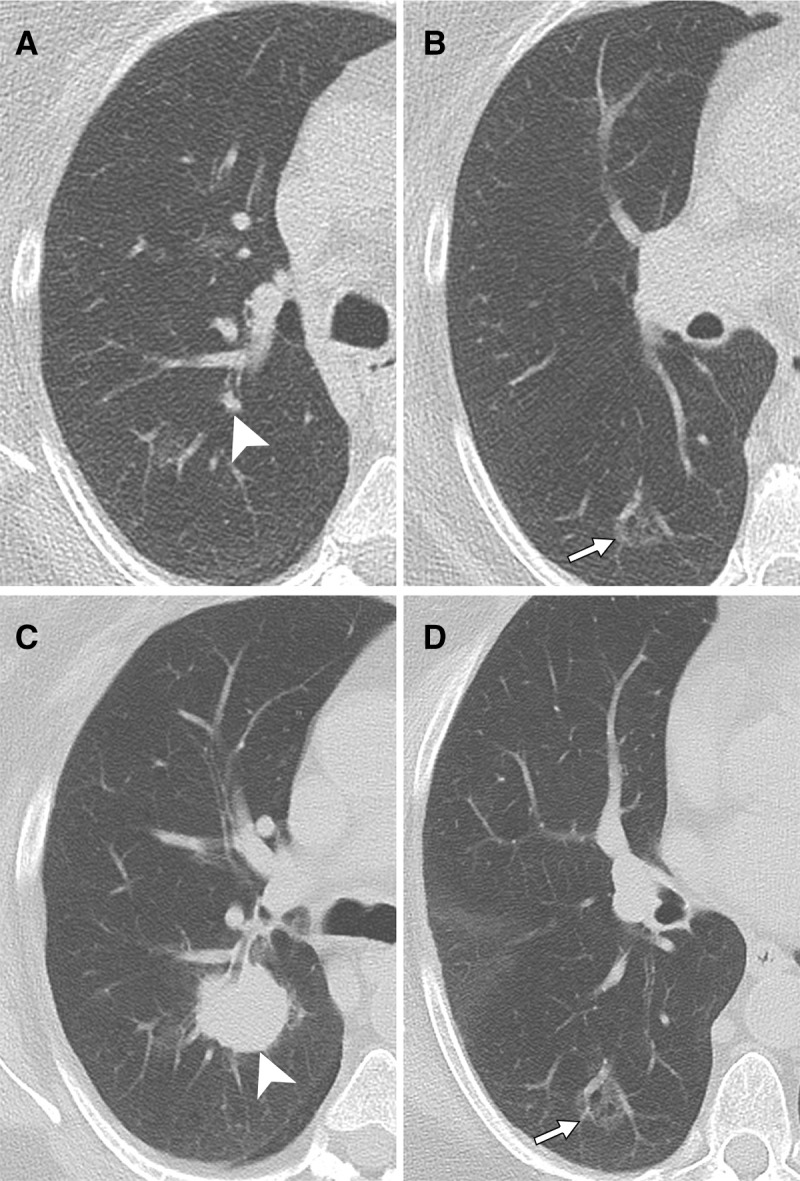
Axial CT images in 69-year-old female former smoker with nodules detected at CT lung cancer screening. A, B, Images obtained at initial chest CT show a small solid right upper lobe nodule (arrowhead), as well as a ground-glass nodule (GGN) in the right lower lobe (arrow). C, D, Images obtained at follow-up chest CT 15 months later show dramatic enlargement of the right upper lobe solid nodule (arrowhead) but minimal change in the GGN (arrow). Surgical resection demonstrated that the right upper lobe nodule was pleomorphic carcinoma, predominantly adenosquamous, while the right lower lobe nodule was lepidic-predominant adenocarcinoma.
We evaluated different follow-up strategies for Lung-RADS category 2 nodules, comparing annual (lung cancer screening/Lung-RADS recommendation), biennial (Fleischner recommendation for GGNs), and triennial follow-up imaging. Because category 2 GGNs grow slowly and are slow to develop SCs, if they ever do, longer follow-up intervals did not show a substantial detrimental effect on patient survival. This was true despite accounting for the development of incidental cancers during follow-up. Although survival was slightly better for the shorter follow-up interval, the difference is not likely to be clinically significant. Because more frequent follow-up studies will lead to accumulation of radiation exposure, a triennial follow-up interval is likely preferable, particularly in younger patients. Moreover, the use of longer follow-up intervals is also potentially beneficial because of reduced costs to patients and health care payers. We do acknowledge that a short initial follow-up interval (eg, 3 months) to confirm persistence of a nodule may have a beneficial psychologic effect on patients if the nodule is inflammatory and then resolves; we did not evaluate such a strategy.
We then evaluated the effects of different treatment strategies for these nodules. In all groups, SBRT yielded better OS than surgery (lobectomy); this is despite our input data of better long-term survival in patients with lung malignancies after lobectomy than SBRT. This paradoxical result reflects the fact that only a minority (30%) of Lung-RADS category 4B or 4X nodules represented clinically significant malignancies. Thus, outcomes in this patient population are driven not by recurrence of malignancy but rather by treatment-related complication rates, which are higher for lobectomy than for SBRT. Our results argue for a “less is more” strategy for treating GGNs and PSNs. Thus, SBRT, which has less morbidity and mortality than surgical resection, may be a better strategy for treating suspicious nonsolid nodules. Please note that percutaneous ablation strategies were not part of the treatment modeling outcomes.
Our study had some limitations. As with all models, ours is a simplification of reality. However, our simulation is based on empiric data from large cohorts, and the results of the simulation match observations in the source literature. One particular limitation was that our model included only three treatment options (lobectomy, SBRT, and no therapy) and excluded, for example, sublobar resection and percutaneous ablation therapy. However, sublobar resection can be viewed as an intermediate treatment option between these two in terms of morbidity. Another limitation was that our simulation does not explicitly model the risk of radiation-induced cancer for patients undergoing CT examinations. However, the radiation-induced cancer risk within an interval of 10 years is estimated to be relatively small (35). Thus, the conclusions will not likely change with the inclusion of radiation-induced cancer risk. The model did not account for sex differences in overall cancer survival.
In conclusion, our results suggest taking a conservative approach to the follow-up and treatment of GGNs. While there is a high rate of malignancy in these nodules at biopsy, the rate of clinically significant malignancy is lower, and, overall, these malignancies demonstrate an indolent course. Our results argue for raising the follow-up interval for GGNs to 3 years. We also found little long-term benefit for aggressive treatment (ie, lobectomy) in these patients. Our results thus suggest the use of treatments that incur less morbidity (eg, radiation therapy) when a nodule has met criteria for treatment. These findings highlight the need for a prospective randomized trial evaluating better treatment thresholds for nonsolid nodules, perhaps based on growth rates rather than size, to avoid overtreatment (7).
APPENDIX
SUPPLEMENTAL FIGURES
Acknowledgments
Acknowledgment
The authors thank the National Cancer Institute (NCI) for access to the NCI’s data collected by the National Lung Screening Trial. The statements contained herein are solely those of the authors and do not represent or imply concurrence or endorsement by the NCI.
L.L.P., A.L.E., and C.Y.K. supported by the National Cancer Institute (U01CA199284).
Disclosures of Conflicts of Interest: M.M.H. disclosed no relevant relationships. L.L.P. disclosed no relevant relationships. A.L.E. disclosed no relevant relationships. E.M.B. disclosed no relevant relationships. C.Y.K. disclosed no relevant relationships.
Abbreviations:
- GGN
- ground-glass nodule
- Lung-RADS
- Lung Imaging Reporting and Data System
- NLST
- National Lung Screening Trial
- OS
- overall survival
- PSN
- part-solid nodule
- SBRT
- stereotactic body radiation therapy
- SC
- solid component
References
- 1.Yankelevitz DF, Yip R, Smith JP, et al. CT screening for lung cancer: nonsolid nodules in baseline and annual repeat rounds. Radiology 2015;277(2):555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henschke CI, Yip R, Smith JP, et al. CT screening for lung cancer: part-solid nodules in baseline and annual repeat rounds. AJR Am J Roentgenol 2016;207(6):1176–1184. [DOI] [PubMed] [Google Scholar]
- 3.Benegas Urteaga M, Rosales-Mayor E, Gutierrez Chacoff J, et al. Lung adenocarcinoma based on IASLC/ATS/ERS International Multidisciplinary Classification: CT and PET findings with pathologic correlation. http://posterng.netkey.at/esr/viewing/index.php?module=viewing_poster&doi=10.1594/ecr2014/C-2159. Published 2014.
- 4.Gardiner N, Jogai S, Wallis A. The revised lung adenocarcinoma classification: an imaging guide. J Thorac Dis 2014;6(Suppl 5):S537–S546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med 2013;369(10):910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mortani Barbosa EJ., Jr. Lung cancer screening overdiagnosis: reports of overdiagnosis in screening for lung cancer are grossly exaggerated. Acad Radiol 2015;22(8):976–982. [DOI] [PubMed] [Google Scholar]
- 7.Veronesi G, Maisonneuve P, Bellomi M, et al. Estimating overdiagnosis in low-dose computed tomography screening for lung cancer: a cohort study. Ann Intern Med 2012;157(11):776–784. [DOI] [PubMed] [Google Scholar]
- 8.Kakinuma R, Noguchi M, Ashizawa K, et al. Natural history of pulmonary subsolid nodules: a prospective multicenter study. J Thorac Oncol 2016;11(7):1012–1028. [DOI] [PubMed] [Google Scholar]
- 9.Kakinuma R, Muramatsu Y, Kusumoto M, et al. Solitary pure ground-glass nodules 5 mm or smaller: frequency of growth. Radiology 2015;276(3):873–882. [DOI] [PubMed] [Google Scholar]
- 10.Lee SM, Park CM, Goo JM, Lee H-J, Wi JY, Kang CH. Invasive pulmonary adenocarcinomas versus preinvasive lesions appearing as ground-glass nodules: differentiation by using CT features. Radiology 2013;268(1):265–273. [DOI] [PubMed] [Google Scholar]
- 11.Scholten ET, de Jong PA, de Hoop B, et al. Towards a close computed tomography monitoring approach for screen detected subsolid pulmonary nodules? Eur Respir J 2015;45(3):765–773. [DOI] [PubMed] [Google Scholar]
- 12.Hur J, Lee H-J, Nam JE, et al. Diagnostic accuracy of CT fluoroscopy-guided needle aspiration biopsy of ground-glass opacity pulmonary lesions. AJR Am J Roentgenol 2009;192(3):629–634. [DOI] [PubMed] [Google Scholar]
- 13.Inoue D, Gobara H, Hiraki T, et al. CT fluoroscopy-guided cutting needle biopsy of focal pure ground-glass opacity lung lesions: diagnostic yield in 83 lesions. Eur J Radiol 2012;81(2):354–359. [DOI] [PubMed] [Google Scholar]
- 14.Hattori A, Matsunaga T, Hayashi T, Takamochi K, Oh S, Suzuki K. Prognostic impact of the findings on thin-section computed tomography in patients with subcentimeter non-small cell lung cancer. J Thorac Oncol 2017;12(6):954–962. [DOI] [PubMed] [Google Scholar]
- 15.Moon Y, Sung SW, Namkoong M, Park JK. The effectiveness of mediastinal lymph node evaluation in a patient with ground glass opacity tumor. J Thorac Dis 2016;8(9):2617–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American College of Radiology . Lung CT Screening Reporting and Data System (Lung-RADS). http://www.acr.org/Quality-Safety/Resources/LungRADS. Published 2014. Accessed June 19, 2016.
- 17.Martin MD, Kanne JP, Broderick LS, Kazerooni EA, Meyer CA. Lung-RADS: pushing the limits. RadioGraphics 2017;37(7):1975–1993. [DOI] [PubMed] [Google Scholar]
- 18.Morrison JJ, Hostetter J, Wang K, Siegel EL. Data-driven decision support for radiologists: re-using the National Lung Screening Trial dataset for pulmonary nodule management. J Digit Imaging 2015;28(1):18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Lung Screening Trial Research Team , Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365(5):395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eguchi T, Bains S, Lee M-C, et al. Impact of increasing age on cause-specific mortality and morbidity in patients with stage i non-small-cell lung cancer: a competing risks analysis. J Clin Oncol 2017;35(3):281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powell HA, Tata LJ, Baldwin DR, Stanley RA, Khakwani A, Hubbard RB. Early mortality after surgical resection for lung cancer: an analysis of the English National Lung cancer audit. Thorax 2013;68(9):826–834. [DOI] [PubMed] [Google Scholar]
- 22.Tammemagi CM, Neslund-Dudas C, Simoff M, Kvale P. Smoking and lung cancer survival: the role of comorbidity and treatment. Chest 2004;125(1):27–37. [DOI] [PubMed] [Google Scholar]
- 23.Jeon J, Meza R, Krapcho M, Clarke LD, Byrne J, Levy DT. Chapter 5: actual and counterfactual smoking prevalence rates in the U.S. population via microsimulation. Risk Anal 2012;32(Suppl 1):S51–S68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenberg MA, Feuer EJ, Yu B, et al. Chapter 3: cohort life tables by smoking status, removing lung cancer as a cause of death. Risk Anal 2012;32(Suppl 1):S25–S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11(1):39–51. [DOI] [PubMed] [Google Scholar]
- 26.Li M, Yang X, Chen Y, et al. Stereotactic body radiotherapy or stereotactic ablative radiotherapy versus surgery for patients with T1-3N0M0 non-small cell lung cancer: a systematic review and meta-analysis. OncoTargets Ther 2017;10:2885–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wao H, Mhaskar R, Kumar A, Miladinovic B, Djulbegovic B. Survival of patients with non-small cell lung cancer without treatment: a systematic review and meta-analysis. Syst Rev 2013;2(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yap CS, Schiepers C, Fishbein MC, Phelps ME, Czernin J. FDG-PET imaging in lung cancer: how sensitive is it for bronchioloalveolar carcinoma? Eur J Nucl Med Mol Imaging 2002;29(9):1166–1173. [DOI] [PubMed] [Google Scholar]
- 29.Heyneman LE, Patz EF. PET imaging in patients with bronchioloalveolar cell carcinoma. Lung Cancer 2002;38(3):261–266. [DOI] [PubMed] [Google Scholar]
- 30.Tramontano AC, Cipriano LE, Kong CY, et al. Microsimulation model predicts survival benefit of radiofrequency ablation and stereotactic body radiotherapy versus radiotherapy for treating inoperable stage I non-small cell lung cancer. AJR Am J Roentgenol 2013;200(5):1020–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMahon PM, Kong CY, Johnson BE, et al. Estimating long-term effectiveness of lung cancer screening in the Mayo CT screening study. Radiology 2008;248(1):278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Koning HJ, Meza R, Plevritis SK, et al. Benefits and harms of computed tomography lung cancer screening strategies: a comparative modeling study for the U.S. Preventive Services Task Force. Ann Intern Med 2014;160(5):311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hur C, Hayeck TJ, Yeh JM, et al. Development, calibration, and validation of a U.S. white male population-based simulation model of esophageal adenocarcinoma. PLoS One 2010;5(3):e9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung K, Jacobs C, Scholten ET, et al. Malignancy estimation of Lung-RADS criteria for subsolid nodules on CT: accuracy of low and high risk spectrum when using NLST nodules. Eur Radiol 2017;27(11):4672–4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pandharipande PV, Eisenberg JD, Avery LL, et al. Journal club: how radiation exposure histories influence physician imaging decisions: a multicenter radiologist survey study. AJR Am J Roentgenol 2013;200(6):1275–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.