Abstract
Purpose
To compare the effect of autologous blood patch injection (ABPI) with that of a hydrogel plug on the rate of pneumothorax at CT-guided percutaneous lung biopsy.
Materials and Methods
In this prospective randomized controlled trial (https://ClinicalTrials.gov, NCT02224924), a noninferiority design was used for ABPI, with a 10% noninferiority margin when compared with the hydrogel plug, with the primary outcome of pneumothorax rate within 2 hours of biopsy. A type I error rate of 0.05 and 90% power were specified with a target study population of 552 participants (276 in each arm). From October 2014 to February 2017, all potential study participants referred for CT-guided lung biopsy (n = 2052) were assessed for enrollment.
Results
The data safety monitoring board recommended the trial be closed to accrual after an interim analysis met prespecified criteria for early stopping based on noninferiority. The final study group consisted of 453 participants who were randomly assigned to the ABPI (n = 226) or hydrogel plug (n = 227) arms. Of these, 407 underwent lung biopsy. Pneumothorax rates within 2 hours of biopsy were 21% (42 of 199) and 29% (60 of 208); chest tube rates were 9% (18 of 199) and 13% (27 of 208); and delayed pneumothorax rates within 2 weeks after biopsy were 1.4% (three of 199) and 1.5% (three of 208) in the ABPI and hydrogel plug arms, respectively.
Conclusion
Autologous blood patch injection is noninferior to a hydrogel plug regarding the rate of pneumothorax after CT-guided percutaneous lung biopsy.
© RSNA, 2018
Summary
Autologous blood patch injection is noninferior to hydrogel plug regarding the rate of pneumothorax after CT-guided percutaneous lung biopsy.
Implication for Patient Care
■ An autologous blood patch can be used as effectively as hydrogel sealant to reduce the risk of pneumothorax and subsequent chest tube placement after percutaneous needle biopsy of the lung.
Introduction
Percutaneous image-guided needle biopsy of the lung is a well-established and accurate method used to diagnose pulmonary lesions with 93%–95% diagnostic accuracy (1–3). The demand for lung biopsy is increasing, given the increasing rates of lung cancer, the higher detection rate of asymptomatic lung nodules, and the demand for tissue for new molecular profiling and genomic analysis (4).
The most common complication of percutaneous lung biopsy is pneumothorax. Most series report incidences of 20%–25% for pneumothorax and 4%–8% for chest tube placement, although rates as high as 47% and 22%, respectively, have been reported (5–19). The economic burden of a complicated lung biopsy is substantial, with increased costs of 300%–400% (20,21). There is great interest in reducing the occurrence of iatrogenic pneumothorax, which should translate into a lower rate of chest tube placement and subsequent hospital admission.
Pneumothorax is caused by air leaking out of the lung through the needle puncture site at the visceral pleura once the needle is removed (22,23). Several studies have shown that sealing the pleural puncture site with a variety of materials, including autologous blood, hydrogel plug, fibrin glue, gelatin sponge slurry or plug, or saline, reduces the risk of pneumothorax and chest tube placement (9–19). Two of the best-studied sealants are autologous blood patch injection (ABPI) and a manufactured hydrogel plug called BioSentry, which was formerly known as Bio-Seal (Surgical Specialties, Wyoming, Pa), with proven efficacy based on prospective randomized studies (13,14). ABPI uses the participant’s own blood to seal the biopsy track. The hydrogel plug expands on contact with moisture and seals the biopsy track.
We hypothesized that ABPI is noninferior to a hydrogel plug regarding the rate of iatrogenic pneumothorax in CT-guided needle biopsy of the lung. We conducted a prospective single-center randomized controlled trial to test this hypothesis.
Materials and Methods
Study Design
Our institutional review board approved this prospective investigator-initiated study. Written informed consent was obtained from all participants. Study data were collected in a database that was compliant with the Health Insurance Portability and Accountability Act. The authors had full control over data and information submitted for publication.
In this prospective randomized controlled trial (https://ClinicalTrials.gov, NCT02224924), we tested the noninferiority of ABPI as compared with a hydrogel plug with respect to the rate of iatrogenic pneumothorax within 2 hours of CT-guided percutaneous lung biopsy. A noninferiority margin of 10% was used based on historic clinical data of a relatively constant pneumothorax rate of 20%–25% and the results of controlled studies where the difference in the rate of pneumothorax between the two arms ranged from 7.3% to 38% (average, 17.7%; median, 14%) (9–19). The secondary objectives were to compare ABPI and hydrogel plug regarding (a) the rate of iatrogenic pneumothorax within 2 hours of CT-guided lung biopsy on a per-protocol analysis (without intraoperative exclusions), (b) the rate of chest tube placement for pneumothorax up to 2 weeks after lung biopsy, and (c) the rate of delayed pneumothorax up to 2 weeks after lung biopsy. In addition, the Data Safety Monitoring Board (DSMB) recommended an analysis to compare the length of hospital stay after pneumothorax between the two study arms.
Participants
Eligibility was not restricted based on age, sex, race, body habitus, history of smoking or emphysema, indication for biopsy, number of specimens required, or target lesion characteristics, such as size, location, imaging appearance, or planned participant positioning (Table E1 [online]). No attempt was made to grade emphysema, and only biopsies in which the needle path traversed the lung parenchyma with obvious areas of low attenuation (labeled here as bullae and blebs) were ineligible (Fig 1). The hydrogel plug was approved by the Food and Drug Administration to be administered only via a 19-gauge Angiotech introducer needle (Argon Medical Devices, Athens, Tex). All participants in whom the hydrogel plug could not be used were excluded at screening. Beginning in October 2014, all participants referred for percutaneous CT-guided lung biopsy were screened. Eighteen board-certified fellowship-trained interventional radiologists participated in both enrollment and biopsy processes (N.M., H.Y., A.R.D, Y.B., A.J.G., E.Z., and F.E.B. had 2–5 years of experience; J.PE. and R.H.S. had 5–10 years of experience; M.M., K.T.B., G.I.G., C.T.S., A.M.C., L.A.B., W.A., J.C.D., and S.B.S. had more than 10 years of experience). Screening and enrollment were performed at the dedicated outpatient clinic of the interventional radiology service. Medical records and imaging studies were screened by a research study assistant (C.L.Z., M.J.; each with 2–4 years of experience) and at least one interventional radiologist. Medical records were reviewed to explore any history of prior ipsilateral chest interventions. CT images were reviewed at a picture archiving and communication system workstation to identify the safest and most practical percutaneous biopsy approach. Participants who met the inclusion criteria were approached by the consenting interventional radiologist.
Figure 1:
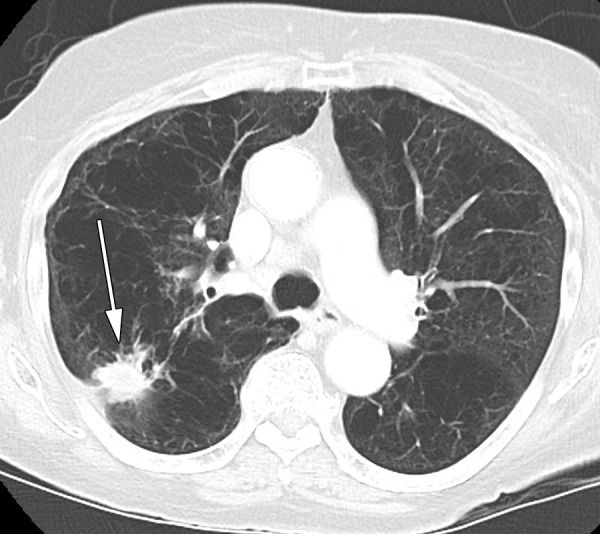
CT image in a patient with emphysema and a new right upper lobe lesion (arrow) was excluded, as no needle path could avoid bullae.
Our study data were reviewed annually by the DSMB and were reported to the institutional review board. In February 2017, the DSMB closed our study to accrual after the second interim analysis for noninferiority of ABPI on the pneumothorax rate within 2 hours of lung biopsy. For an in-depth discussion of procedures we used in this study, please see the Registration, Procedures, Measurements, and Postbiopsy Care sections in Appendix E1 (online).
Statistical Analysis
We assumed the pneumothorax rate with the hydrogel plug was approximately 20% based on a study by Zaetta et al (13), and we calculated that a sample size of 552 participants (276 participants in each study arm) would be sufficient to find ABPI noninferior to hydrogel plug with a margin of 10%, 90% power, one interim analysis for noninferiority, and an overall one-sided type I error rate of 5%. We used the Lan-DeMets spending function and set the trial to terminate early for noninferiority if a one-sided nominal z score of −2.54 (P ≤ .006) was observed. With trial continuation, the final assessment was set to use a z score of −1.662, with an associated P value of .048.
On the basis of the number of lung biopsies performed at our institution, our study was expected to take about 2 years.
The primary analysis was a modified intent-to-treat analysis, in which participants who were randomized but did not undergo biopsy were excluded. All randomized participants who underwent biopsy were analyzed according to the randomized treatment assignment, regardless of actual sealant deployment. The difference between pneumothorax rates for ABPI and those for hydrogel plug was estimated and assessed by using a z test. The same method was used for a secondary analysis in only those participants who had a sealant placed after biopsy without any intraoperative exclusions (per protocol).
The secondary outcomes for comparison of ABPI with hydrogel plug with respect to (a) delayed pneumothorax occurring after discharge and within 2 weeks after lung biopsy and (b) pneumothorax requiring chest tube placement up to 2 weeks after lung biopsy were also assessed with a z test. Superiority between arms was evaluated by using a two-sided χ2 test, and differences in the length of hospital stay were assessed by using a negative binomial regression model. All statistical analyses were performed by using statistical software (SAS, version 9.4, SAS Institute, Cary, NC; East 6, version 6.4, Cytel, Cambridge, Mass; and R, version 3.2.4, www.R-project.org).
Results
From October 2014 to February 2017, 2052 participants were screened, and 850 were interviewed for enrollment at an outpatient interventional radiology clinic. A total of 1598 participants were excluded (Table E2 [online]).
A total of 453 participants (mean age, 66.9 years; age range, 24–93 years; 251 women, 202 men) was randomly assigned to either the ABPI (n = 226; mean age, 66.8 years; age range, 32–92 years; 124 women, 102 men) or hydrogel plug (n = 227; mean age, 67.0 years; age range, 24–93 years; 127 women, 100 men) arm.
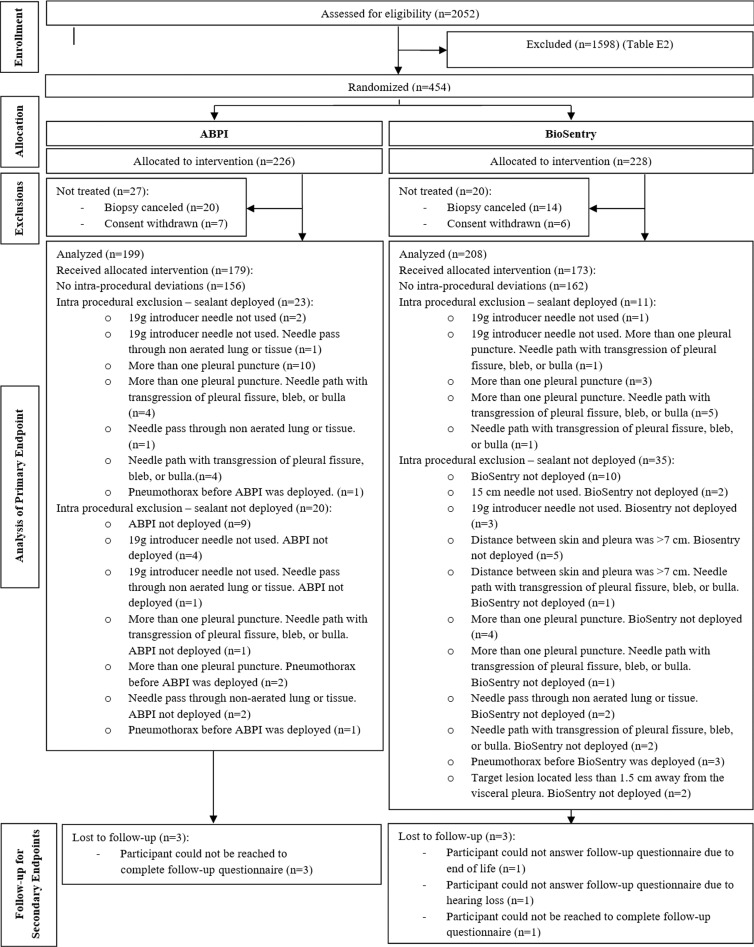
Of these participants, 47 did not undergo treatment because biopsy was cancelled (n = 34) or because consent was withdrawn (n = 13). Intraprocedural exclusions (n = 89) are shown in Table E3 (online). Three participants in each study arm were lost to follow-up for delayed pneumothorax (Fig 2).
Figure 2:
Consort diagram. BioSentry refers to the hydrogel plug. ABPI = autologous blood patch injection.
At the time of interim analysis, the test for noninferiority of ABPI on the pneumothorax rate within 2 hours of biopsy yielded a P value of .004, meeting the criteria to stop our study. The DSMB recommended the trial be stopped early for noninferiority of ABPI on pneumothorax rate within 2 hours of lung biopsy.
Of the 454 randomly assigned participants, 407 underwent lung biopsy and were included in the modified intent-to-treat population. One participant underwent randomization twice in the early stages of our study; therefore, the demographic distribution shows 453 participants in the randomized population and 406 participants in the modified intent-to treat population (Table E4 [online]). The protocol was amended to prevent such an occurrence from happening again. Biopsy target and technical characteristics are shown in Table 1.
Table 1:
Distribution of Target and Technical Characteristics in Per-Protocol Population
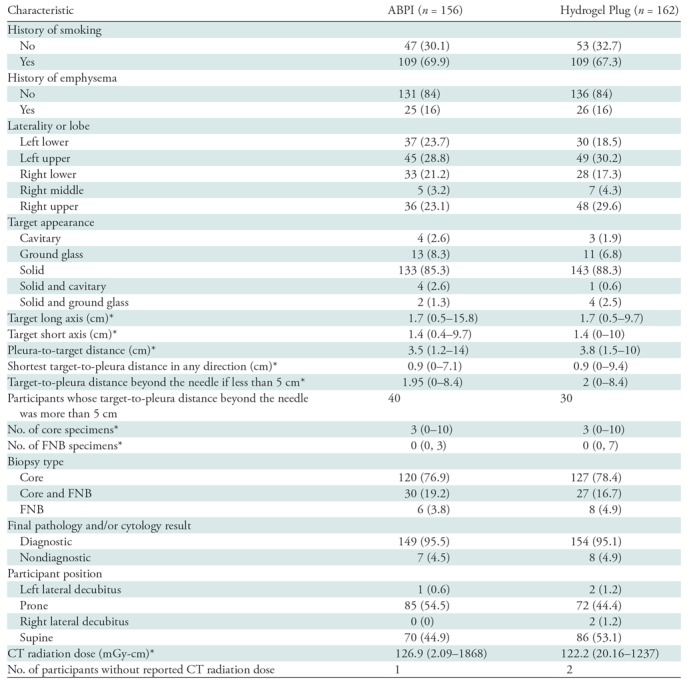
Note.—Unless otherwise indicated, data are number of patients, and data in parentheses are percentages. ABPI = autologous blood patch injection, FNB = fine-needle biopsy.
*Data are the median, and data in parentheses are the range.
The primary aim of this study was to compare the proportion of participants who had a pneumothorax within 2 hours of the procedure. Of the 199 participants in the ABPI arm, 42 (21% [95% confidence interval [CI]: 15%, 27%]) had a pneumothorax within 2 hours of ABPI; 60 of 208 (29%; 95% CI: 23%, 35%) participants had a pneumothorax in the hydrogel plug arm. The difference in proportion between the two arms (hydrogel plug vs ABPI) was −7.7% (95% CI: −16.1%, 0.6%). The primary noninferiority analysis indicated that ABPI was noninferior to hydrogel plug (pNI< 0.0001). While the estimated 2-hour pneumothorax rate was lower with ABPI, in a secondary analysis, we found that ABPI was not statistically significantly superior to hydrogel plug (pS = 0.07 for test of superiority) (Table 2).
Table 2:
Primary Outcome and Per-Protocol Analysis of Primary Outcome according to Treatment Assignment
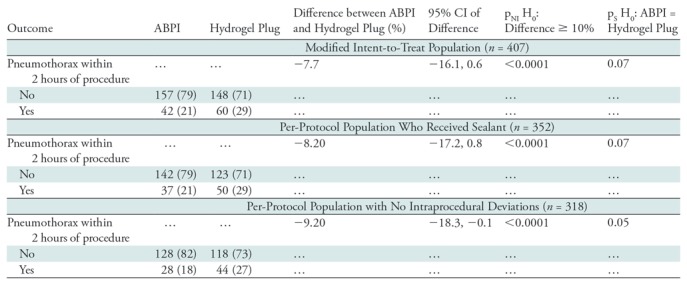
Note.—Unless otherwise indicated, data are number of patients, and data in parentheses are percentages. ABPI = autologous blood patch injection, CI = confidence interval.
A secondary aim of our study was to reanalyze the primary objective by using only participants who had a sealant placed after biopsy (per-protocol analysis). A total of 352 participants underwent the allocated treatment, and 318 underwent the allocated treatment without any intraprocedural deviations. The results were similar to those when assessing the primary aim: Among participants who underwent the allocated treatment, 21% (37 of 179) in the ABPI arm and 29% (50 of 173) in the hydrogel plug arm had a pneumothorax, with a difference of −8.2% (95% CI: −17.2%, 0.8%) (pNI < 0.0001, pS = 0.07). Among participants who underwent the allocated treatment without deviations, 18% (28 of 156) in the ABPI arm and 27% (44 of 162) in the hydrogel plug arm had a pneumothorax, with a difference of −9.2% (95% CI: −18.3%, −0.1%) (pNI < 0.0001, pS = 0.05) (Table 2).
Analyses of the other secondary aims are summarized for the modified intent-to-treat population. The proportion of participants who had a chest tube placed for a pneumothorax anytime within 2 weeks after the procedure was 9% (18 of 199) in the ABPI arm and 13% (27 of 208) in the hydrogel plug arm, with a difference of −3.9% (95% CI: −10%, 2.1%). The proportion of participants who had a delayed pneumothorax within 2 weeks after the procedure was 2% in the ABPI arm (three of 199) and 1% (three of 208) in the hydrogel plug arm, with a difference of 0.1% (95% CI: −2.3%, 2.4%) (Table 3). As per the DSMB recommendation, the length of hospital stay for the 102 participants who had a pneumothorax within 2 hours of the procedure was analyzed by study arm (Table E5 [online]). In the ABPI arm, participants were in the hospital an average of 1.6 days, while in the hydrogel plug arm, participants were in the hospital an average of 0.8 days (P = .03). There were only six participants who had a delayed pneumothorax, and no group comparison was performed. No unanticipated serious adverse events occurred during our study.
Table 3:
Secondary Outcomes according to Treatment Assignment
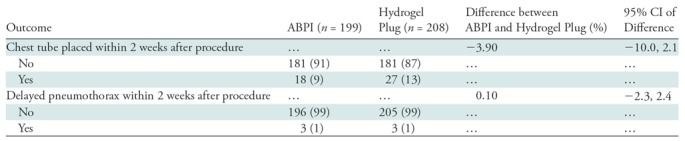
Note.—All data were obtained in the modified intent-to-treat population (n = 407) and, unless otherwise indicated, are number of patients with percentages in parentheses. ABPI = autologous blood patch injection, CI = confidence interval.
Discussion
We performed a prospective randomized controlled trial with a noninferiority design to compare the rate of pneumothorax within 2 hours of CT-guided percutaneous lung biopsy when ABPI or hydrogel plug were used as the track sealant. From October 2014 to February 2017, 2052 potential study participants were assessed for enrollment. A total of 453 participants were randomly assigned to the ABPI (n = 226) or hydrogel plug (n = 227) arm. A total of 407 participants underwent treatment without intraoperative exclusion (ABPI, n = 199; hydrogel plug, n = 208). Pneumothorax rates within 2 hours of biopsy were 21% (42 of 199) and 29% (60 of 208); chest tube rates were 9% (18 of 199) and 13% (27 of 208); and delayed pneumothorax rates within 2 weeks after biopsy were 1.4% (three of 199) and 1.5% (three of 208) in the ABPI and hydrogel plug arms, respectively. The DSMB recommended the trial be closed to accrual after an interim analysis met prespecified criteria for early stopping based on noninferiority. We concluded that ABPI is noninferior to hydrogel plug in regard to the rate of pneumothorax after CT-guided percutaneous lung biopsy.
The most commonly accepted mechanism for iatrogenic pneumothorax from percutaneous needle biopsy is leakage of air from the puncture site at the visceral pleura after needle removal. Since lung biopsies are performed with increasing frequency and because complications such as pneumothorax lead to more costs and resources, substantial interest persists in decreasing the rate of iatrogenic pneumothorax (4,20,21).
In 1974, on the basis of the observation that pneumothorax was rare in patients whose lung lesions “bloomed” (bled) at fluoroscopic-guided biopsy, McCartney et al concluded that bleeding might have sealed the pleural puncture site. They published the first report on the use of ABPI after lung biopsy in 25 patients (25). Early case series and controlled studies showed mixed results but poor study design, including fluoroscopic guidance, participant selection methods, number of operators, and sample size, limited the relevance of their findings (7,8,26). Since 1992, a total of 11 controlled studies on use of track sealant in lung biopsies have shown significant benefits in both pneumothorax and chest tube rates (Table 4).
Table 4:
Controlled Studies on Track Sealants in Image-guided Needle Biopsy of Lung
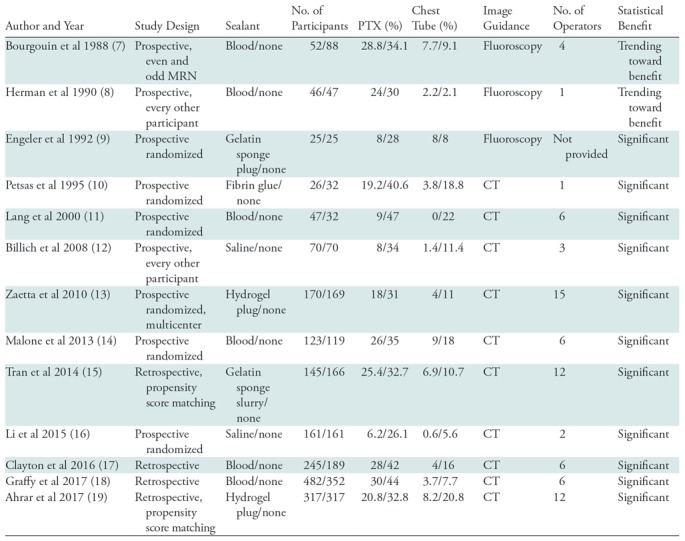
Note.—Blood = autologous blood patch injection, MRN = medical record number, PTX = pneumothorax.
In 2010, Zaetta et al published the results of a prospective multicenter randomized controlled clinical study of 339 study participants allocated to either a hydrogel plug arm or a no sealant arm. They demonstrated significantly fewer pneumothoraxes (18% vs 31%) and fewer chest tube placements (4% vs 11%) in participants in the hydrogel plug arm as compared with those in the no sealant arm (13). In 2013, Malone et al published the results of a prospective randomized controlled clinical study of 242 study participants allocated to either an ABPI arm or a no sealant arm. They showed a trend toward reduction of pneumothorax (26% vs 35%), and a significant reduction in chest tube placement (9% vs 18%) associated with ABPI (14).
ABPI has several advantages over hydrogel plug: it is essentially free; it does not require a specific introducer needle type, gauge, or length; it can be deployed for lesions closer than 1.5 cm to the pleura; and it is proven to be absorbed shortly after deployment.
Our study had limitations. Results are based on an oncologic population in a comprehensive cancer center and may not necessarily be representative of results in other populations. The 10% noninferiority boundary is based on clinical historic data and results of prior relevant studies. We allowed freedom in choosing the type of 19-gauge introducer needle used in the ABPI arm. Such deviation is not expected to change our results significantly, as most controlled studies on this topic restrict only needle size, not needle type, between the two arms (10,14,17–19). A follow-up phone call was used in place of chest radiography to capture late pneumothorax. This may affect accuracy of the rate of delayed pneumothorax in our results, although our rates are comparable to reported rates of around 1% (27).
Further prospective randomized clinical trials will be needed to evaluate the effectiveness of other track sealants. The fact that the overall rates of iatrogenic pneumothorax in percutaneous image-guided needle biopsy of the lung have not grossly changed since earlier reports in the 1970s despite advances in technology indicates potential gaps in our knowledge about risk factors such as emphysema, the physiology of respiration, and the mechanisms of iatrogenic pneumothorax. Until these risk factors are better understood, our study suggests that autologous blood patch injection can be as effective as a hydrogel plug in reducing the risk of pneumothorax and subsequent chest tube placement after percutaneous needle biopsy.
APPENDIX
SUPPLEMENTAL FIGURES
Supported by a National Institutes of Health/National Cancer Institute Cancer Center grant (P30 CA008748).
Disclosures of Conflicts of Interest: M.M. disclosed no relevant relationships. N.M. disclosed no relevant relationships. K.T.B. disclosed no relevant relationships. C.S.M. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant for BioClinica. Other relationships: disclosed no relevant relationships. M.H. disclosed no relevant relationships. C.L.Z. disclosed no relevant relationships. M.J. disclosed no relevant relationships. G.I.G. disclosed no relevant relationships. C.T.S. disclosed no relevant relationships. J.P.E. disclosed no relevant relationships. A.M.C. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is on the advisory board of Accurate Medical; gave lectures for the North American Center for Continuing Medical Education; holds stock in Amgen. Other relationships: disclosed no relevant relationships. L.A.B. disclosed no relevant relationships. H.Y. disclosed no relevant relationships. A.R.D. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant for BTG. Other relationships: disclosed no relevant relationships. Y.B. disclosed no relevant relationships. W.A. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant for, is on the speakers bureau of, and develops educational presentations for Johnson & Johnson. Other relationships: disclosed no relevant relationships. R.H.S. disclosed no relevant relationships. J.C.D. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant for, holds stock in, and is reimbursed for expenses by Adient Medical. Other relationships: disclosed no relevant relationships. A.J.G. disclosed no relevant relationships. E.Z. disclosed no relevant relationships. E.E.B. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: institution received a grant from Guerbet; holds U.S. patent 8,233,586; is the cofounder of Claripacs; is an investor in Labdoor, Qventus, CloudMedx, and Notable Labs; is reimbursed for expenses by Guerbet; received research supplies from Bayer. Other relationships: disclosed no relevant relationships. S.B.S. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant for Astra Zeneca; institution received a grant from GE Healthcare; holds stock in Johnson & Johnson; institution received research support from AngioDynamics. Other relationships: disclosed no relevant relationships.
Abbreviations:
- ABPI
- autologous blood patch injection
- CI
- confidence interval
- DSMB
- data safety monitoring board
References
- 1.Wang Y, Jiang F, Tan X, Tian P. CT-guided percutaneous transthoracic needle biopsy for paramediastinal and nonparamediastinal lung lesions: diagnostic yield and complications in 1484 patients. Medicine (Baltimore) 2016;95(31):e4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takeshita J, Masago K, Kato R, et al. CT-guided fine-needle aspiration and core needle biopsies of pulmonary lesions: a single-center experience with 750 biopsies in Japan. AJR Am J Roentgenol 2015;204(1):29–34. [DOI] [PubMed] [Google Scholar]
- 3.Yang W, Sun W, Li Q, et al. Diagnostic accuracy of CT-guided transthoracic needle biopsy for solitary pulmonary nodules. PLoS One 2015;10(6):e0131373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall D, Laberge JM, Firetag B, Miller T, Kerlan RK. The changing face of percutaneous image-guided biopsy: molecular profiling and genomic analysis in current practice. J Vasc Interv Radiol 2013;24(8):1094–1103. [DOI] [PubMed] [Google Scholar]
- 5.Covey AM, Gandhi R, Brody LA, Getrajdman G, Thaler HT, Brown KT. Factors associated with pneumothorax and pneumothorax requiring treatment after percutaneous lung biopsy in 443 consecutive patients. J Vasc Interv Radiol 2004;15(5):479–483. [DOI] [PubMed] [Google Scholar]
- 6.Moreland A, Novogrodsky E, Brody L, et al. Pneumothorax with prolonged chest tube requirement after CT-guided percutaneous lung biopsy: incidence and risk factors. Eur Radiol 2016;26(10):3483–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourgouin PM, Shepard JA, McLoud TC, Spizarny DL, Dedrick CG. Transthoracic needle aspiration biopsy: evaluation of the blood patch technique. Radiology 1988;166(1 Pt 1):93–95. [DOI] [PubMed] [Google Scholar]
- 8.Herman SJ, Weisbrod GL. Usefulness of the blood patch technique after transthoracic needle aspiration biopsy. Radiology 1990;176(2):395–397. [DOI] [PubMed] [Google Scholar]
- 9.Engeler CE, Hunter DW, Castaneda-Zuniga W, Tashjian JH, Yedlicka JW, Amplatz K. Pneumothorax after lung biopsy: prevention with transpleural placement of compressed collagen foam plugs. Radiology 1992;184(3):787–789. [DOI] [PubMed] [Google Scholar]
- 10.Petsas T, Siamblis D, Giannakenas C, et al. Fibrin glue for sealing the needle track in fine-needle percutaneous lung biopsy using a coaxial system: part II clinical study. Cardiovasc Intervent Radiol 1995;18(6):378–382. [DOI] [PubMed] [Google Scholar]
- 11.Lang EK, Ghavami R, Schreiner VC, Archibald S, Ramirez J. Autologous blood clot seal to prevent pneumothorax at CT-guided lung biopsy. Radiology 2000;216(1):93–96. [DOI] [PubMed] [Google Scholar]
- 12.Billich C, Muche R, Brenner G, et al. CT-guided lung biopsy: incidence of pneumothorax after instillation of NaCl into the biopsy track. Eur Radiol 2008;18(6):1146–1152. [DOI] [PubMed] [Google Scholar]
- 13.Zaetta JM, Licht MO, Fisher JS, Avelar RL; Bio-Seal Study Group . A lung biopsy tract plug for reduction of postbiopsy pneumothorax and other complications: results of a prospective, multicenter, randomized, controlled clinical study. J Vasc Interv Radiol 2010;21(8):1235–1243.e1–e3. [DOI] [PubMed] [Google Scholar]
- 14.Malone LJ, Stanfill RM, Wang H, Fahey KM, Bertino RE. Effect of intraparenchymal blood patch on rates of pneumothorax and pneumothorax requiring chest tube placement after percutaneous lung biopsy. AJR Am J Roentgenol 2013;200(6):1238–1243. [DOI] [PubMed] [Google Scholar]
- 15.Tran AA, Brown SB, Rosenberg J, Hovsepian DM. Tract embolization with gelatin sponge slurry for prevention of pneumothorax after percutaneous computed tomography-guided lung biopsy. Cardiovasc Intervent Radiol 2014;37(6):1546–1553. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Du Y, Luo TY, et al. Usefulness of normal saline for sealing the needle track after CT-guided lung biopsy. Clin Radiol 2015;70(11):1192–1197. [DOI] [PubMed] [Google Scholar]
- 17.Clayton JD, Elicker BM, Ordovas KG, Kohi MP, Nguyen J, Naeger DM. Nonclotted blood patch technique reduces pneumothorax and chest tube placement rates after percutaneous lung biopsies. J Thorac Imaging 2016;31(4):243–246. [DOI] [PubMed] [Google Scholar]
- 18.Graffy P, Loomis SB, Pickhardt PJ, et al. Pulmonary intraparenchymal blood patching decreases the rate of pneumothorax-related complications following percutaneous CT-guided needle biopsy. J Vasc Interv Radiol 2017;28(4):608–613.e1. [DOI] [PubMed] [Google Scholar]
- 19.Ahrar J, Ensor J, Jr, Mahvash A, et al. Efficacy of a self-expanding tract sealant device in the reduction of pneumothorax and chest tube placement rates after percutaneous lung biopsy: a matched controlled study using propensity score analysis. J Vasc Interv Radiol 2015;26(2 Suppl):S149–S150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gurley MB, Richli WR, Waugh KA. Outpatient management of pneumothorax after fine-needle aspiration: economic advantages for the hospital and patient. Radiology 1998;209(3):717–722. [DOI] [PubMed] [Google Scholar]
- 21.Lokhandwala T, Dann R, Johnson M, D’Souza AO. Costs of the diagnostic workup for lung cancer: a medicare claims analysis—diagnosis/staging. Int J Radiat Oncol Biol Phys 2014;90(5 Suppl):S9–S10. [Google Scholar]
- 22.McCartney RL. Further observations on the lung patch technique. with analysis of the first 50 cases. Am J Roentgenol Radium Ther Nucl Med 1975;124(3):397–403. [DOI] [PubMed] [Google Scholar]
- 23.Moore EH, Shelton DK, Wisner ER, Richardson ML, Bishop DM, Brock JM. Needle aspiration lung biopsy: reevaluation of the blood patch technique in an equine model. Radiology 1995;196(1):183–186. [DOI] [PubMed] [Google Scholar]
- 24.Moore EH. Technical aspects of needle aspiration lung biopsy: a personal perspective. Radiology 1998;208(2):303–318. [DOI] [PubMed] [Google Scholar]
- 25.McCartney R, Tait D, Stilson M, Seidel GF. A technique for the prevention of pneumothorax in pulmonary aspiration biopsy. Am J Roentgenol Radium Ther Nucl Med 1974;120(4):872–875. [DOI] [PubMed] [Google Scholar]
- 26.Surprenant EL. Transthoracic needle aspiration biopsy: evaluation of the blood patch technique. Radiology 1988;168(1):285. [DOI] [PubMed] [Google Scholar]
- 27.Choi CM, Um SW, Yoo CG, et al. Incidence and risk factors of delayed pneumothorax after transthoracic needle biopsy of the lung. Chest 2004;126(5):1516–1521. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.